Abstract
The kinetics of SARS-CoV-2 reactive IgG antibodies after full vaccination and booster in allogeneic and autologous stem cell transplantation (allo-HSCT, ASCT) and chimeric antigen receptor T-cell therapy (CAR-T) are of utmost importance for estimating risk of infection. A prospective multicenter registry-based cohort study, conducted from December 2020 to July 2022 was used to analyze antibody waning over time, booster effect and the relationship of antibody response and breakthrough infection in 572 recipients (429 allo-HSCT, 121 ASCT and 22 CAR-T cell therapy). A significant decline in antibody titers was observed at 3 and 6 months after full vaccination in recipients without pre-vaccine SARS-CoV-2 infection, whereas recipients infected prior to vaccination showed higher and stable antibody titers over time. In poor responders, a booster dose was able to increase antibody titers in 83% of allo-HSCT and 58% of ASCT recipients but not in CART-T cell recipients [0%] (p < 0.01). One-year cumulative incidence of breakthrough infection was 15%, similar among cell therapy procedures. Immunosuppressive drugs at the time of vaccination [hazard ratio (HR) 1.81, p = 0.0028] and reduced intensity conditioning (HR 0.49, p = 0.011) were identified as the only conditions associated with different risk of breakthrough infection in allo-HSCT recipients. Antibody titers were associated with breakthrough infection and disease severity. No death was observed among the 72 breakthrough infections. Antibody level decay after the first two vaccine doses was common except in recipients with pre-vaccination SARS-CoV-2 infection. Poorly responding allo-HSCT recipients showed a response advantage with the booster as compared to ASCT and, especially, the null response found in CAR-T cell recipients. Antibody titers were positively correlated with the risk of breakthrough SARS-CoV-2 infection which was mainly driven by the immunosuppression status.
Subject terms: Medical research, Risk factors
Introduction
Recipients of autologous (ASCT), allogeneic hematopoietic stem cell transplantation (allo-HSCT) and chimeric antigen receptor T-cell (CAR-T) therapy are at risk of severe coronavirus disease 2019 (COVID-19), with overall mortality exceeding 18% during the first waves [1–3]. The new coronavirus (SARS-CoV-2) is causing major and persistent disruptions in daily clinical practice in transplant centers worldwide. Although vaccination with mRNA vaccines showed a high SARS-CoV2-reactive antibody (any isotype) (SARS-CoV-2-S-RA) response [4] with apparent mitigation of severe COVID-19 in these immunocompromised patients [5, 6], specific concerns such as SARS-CoV-2-S-RA titers waning over time remain underexplored [7].
The emergence of new variants of concern (VOC) such as Omicron variants seems to reduce mRNA vaccine efficacy (VE) which were initially designed against the Wuhan strain [8]. This VE reduction may be mitigated by inducing higher SARS-CoV-2-S-RA titers with additional vaccine doses [8, 9]. This is particularly important in immunocompromised patients since they show lower SARS-CoV-2-S-RA titers after complete vaccination than healthy controls [10], and consequently became the focus of worldwide National Health Authority recommendations for further vaccine booster doses and/or pre-exposure prophylaxis with monoclonal antibodies [11]. However, scarce data is available regarding the effect of further booster doses on either SARS-CoV-2-S-RA titers or breakthrough SARS-CoV-2 infection prevention.
The current study aims to analyze SARS-CoV-2-S-RA kinetics at 3–6 weeks, 3 months, 6 months after a full course of SARS-CoV-2 vaccination and after additional vaccine doses, in a prospective monitored cohort of allo-HSCT, ASCT and CAR-T cell therapy recipients. Additionally, we aim to characterize the incidence, severity and risk factors of breakthrough SARS-CoV-2 infection one year after initiation of mass vaccination in this vulnerable population.
Patients and methods
Study population
This prospective, observational, multicenter registry study was conducted by the Infectious Complications Subcommittee (GRUCINI) of the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH-TC). Characteristics of the registry have been published elsewhere in detail [4, 12]. Briefly, the registry included consecutive hematological disorders (HD) patients who were vaccinated against SARS-CoV-2 between December 30, 2020 and June 30, 2021 in 21 participating Spanish centers. In June 2021, a specific form for booster doses was added to the registry. Patients were monitored and followed for development of SARS-CoV-2-S-RA and breakthrough SARS-CoV-2 infection at different time points (3–6 weeks, 3, 6 and 12 months) after completing the initial vaccination schedule. The status of all included patients was updated on July 28, 2022. All patients included in this registry gave their informed consent in accordance with the declaration of Helsinki. The local Research Ethics Committee of the Hospital Clínico Universitario of Valencia approved the registry and study protocol (reference code 35.21).
Inclusion criteria and cohort selection
As of July 28, 2022, 1783 fully vaccinated HM patients were registered in the GETH-TC database. The 572 cell therapy recipients included in our database were selected with the aim of assessing antibody kinetics over time, the effect of booster dose and cumulative incidence estimates of breakthrough SARS-CoV-2 infection.
Definitions and technical considerations
Complete vaccination (full primary immunization) schedules were defined as per marketing authorization at the time of study conduct and included two doses for full primary immunization (except COVID-19 Vaccine Janssen®). An additional dose after completion of full primary immunization was considered as a booster dose.
We defined antibody detection or seropositivity when SARS-CoV-2-S-RA was above the lower limit level of detection using serological ELISA or chemiluminescence immunoassay assays. As recommended by the Spanish Society of Hematology and Hemotherapy (SEHH), in vaccinated individuals serological testing included antibody IgG detection against nucleocapsid (N) and surface (S) proteins (anti-N and anti-S IgG, respectively) [13], the former to document past SARS-CoV-2 infection. Antibody levels in patients with quantitative assessment were normalized according to WHO standards (NIBSC 20/136) and results were reported as SARS-CoV-2-S-RA binding antibody units per milliliter (BAU/mL). Supplementary Table S1 summarizes the technical characteristics of the serological tests used and normalization of antibody titers to BAU/mL according to WHO standards.
Poor responding patients were define as those whose antibody titers at 3-6 weeks after primary complete vaccination was below 250 BAU/mL according to our previous experience [5, 14].
Pre-vaccination SARS-CoV-2 infection was defined as patients with prior history of PCR-proven COVID-19 and/or positive SARS-CoV-2 serostatus (IgG and/or IgM) before the first vaccine dose.
Breakthrough SARS-CoV-2 infection was defined as molecular (PCR test), antigenic or serological (IgG anti-N seroconversion between two consecutive serological tests) evidence of SARS-CoV-2 infection 7 days after full vaccination and until last follow-up.
The inference of VOC on each episode was based on the likelihood of having a specific VOC according to Spanish sequencing epidemiological data [15]. We inferred a VOC when the infection was diagnosed during the predominance (>50% of all sequenced VOC) of a specific VOC in our country (Supplementary Fig. S1). For example, Alpha-Beta VOC period was considered in SARS-CoV-2 infections diagnosed from April to June 20, 2021. Delta period was considered from June 21 to December 26, 2021 and Omicron period from December 27 to the end of the study.
Endpoints and statistical analysis
The primary objective of the study was to assess SARS-CoV-2-S-RA kinetics over time in HSCT and CAR-T cell therapy recipients at 3-6 weeks, 3 and 6 months after full COVID-19 vaccination and after a booster dose. Secondary endpoints include the cumulative incidence estimates of breakthrough SARS-CoV-2 infections as well as clinical characteristics and factors (including SARS-CoV-2-S-RA titers at different time points) associated with the incidence and severity of breakthrough infection.
The main patient characteristics were reported by descriptive statistics on the total available information: medians and ranges were used for continuous variables, while absolute and percentage frequencies were used for categorical variables. For comparisons Fisher’s exact test or Mann–Whitney’s U test were used when appropriate.
SARS-CoV-2-S-RA titer dynamics over time and after booster doses were analyzed in patients with and without pre-vaccination COVID-19 in ASCT and allo-HSCT separately. Analyses of the kinetics of SARS-CoV-2-S-RA titers were censored at the time of breakthrough SARS-CoV-2 infection. Median test was used to check differences in median SARS-CoV-2-S-RA titers according to different conditions. For these analyses we excluded recipients who developed breakthrough SARS-CoV-2 infection before each serological assessment.
Breakthrough SARS-CoV-2 infection after vaccination was estimated by the cumulative incidence method considering death without SARS-CoV-2 infection as the competing event [16, 17]. Univariate and multivariate analyses of risk factors for breakthrough infection were calculated using the Fine and Gray test [18]. For multivariate analysis, only variables with parameter estimates showing a p ≤ 0.10 in the univariate analysis were finally included. A median test analysis to check the protective effect of the amount of SARS-CoV-2-S-RA was carried out in patients with available quantitative SARS-CoV-2-S-RA titers normalized to BAU/mL according to whether they developed breakthrough infections or not. A p-value <0.05 was considered statistically significant. All p-values are two-sided. Analyses were performed using the statistical software SPSS v. 25 (IBM SPSS Statistics, Armonk, New York, USA) and R software, version 4.0.
Results
Patient characteristics
We included 572 cell therapy recipients (429 allo-HSCT, 121 ASCT and 22 CAR-T cell therapy recipients) with full vaccination schedule and serological tests available at different time points after complete vaccination. Detailed clinical, laboratory and vaccination characteristics by patient category (allo-HSCT, ASCT and CAR-T cell therapy recipients) are summarized in Table 1. The median age of the whole cohort was 56 years (range 16–79), with a predominance of males (57%). Most patients (74%) in this series were vaccinated more than one year after cell therapy. Forty-six patients (8%) had history of prior COVID-19 before vaccination. The most common 2-dose vaccine schedule was mRNA-1273-based (79%%), followed by BNT162b2 (17%).
Table 1.
Patient characteristics.
| Characteristics | Allo-SCT (n = 429) | ASCT (n = 121) | CAR-T (n = 22) |
|---|---|---|---|
| COVID-19 pre-vaccination, n (%) | 37 (8.5) | 9 (7.4) | 0 |
| Serology pre-vaccination, n (%) | 237 (55) | 35 (29) | 16 (72.7) |
| • Positive, n (%) | 16 (6.7) | 7 (20) | 0 |
| Type of vaccine, n (%) | |||
| • Moderna mRNA-1273 | 349 (81.3) | 87 (72) | 20 (90) |
| • Pfizer-BioNTech BNT162b2 | 71 (16.6) | 24 (20) | 2 (10) |
| • Adenoviral vector-based | 9 (2.1) | 9 (8) | 0 |
| 3rd vaccine and type, n (%) | 357 (83.2) | 106 (87.6) | 19 (86.4) |
| • Moderna mRNA-1273 | 64 (18) | 29 (27) | 4 (21) |
| • Pfizer-BioNTech BNT162b2 | 292 (82) | 75 (73) | 15 (79) |
| • Adenoviral vector-based | 0 | 0 | 0 |
| Age (years), median (range) | 55 (16-78) | 61 (19-79) | 59 (26-78) |
| • 18–40 years, n (%) | 91 (21.2) | 9 (7.4) | 3 (13.6) |
| • 41–60 years, n (%) | 205 (47.8) | 49 (40.5) | 15 (68.3) |
| • 61–70 years, n (%) | 107 (24.9) | 50 (41.4) | 3 (13.6) |
| • >71 years, n (%) | 26 (6.1) | 13 (10.7) | 1 (4.5) |
| Male, n (%) | 246 (57.3) | 69 (57) | 13 (59) |
| Baseline disease, n (%) | |||
| • AML | 151 (35.2) | 5 (4.1) | |
| • MDS | 58 (13.5) | ||
| • NHL | 68 (15.8) | 26 (21.4) | 13 (59.2) |
| • MM | 11 (2.5) | 73 (60.4) | 7 (31.8) |
| • CLL | 8 (2) | 1 (4.5) | |
| • HD | 42 (9.8) | 17 (14.1) | |
| • MPN | 27 (6.3) | ||
| • ALL | 49 (11.4) | 1 (4.5) | |
| • Others | 15 (3.5) | ||
| Status disease at vaccination, n (%) | |||
| • Complete remission | 387 (90.2) | 87 (71.8) | 17 (77.3) |
| • Partial remission | 13 (3) | 22 (18.2) | 2 (9.1) |
| • Not responding | 29 (6.8) | 12 (10) | 3 (13.6) |
| Median time from transplant to COVID-19 vaccine, months (range) | 33 (2–317) | 36 (3–305) | 9.6 (4.4–23.5) |
| • <6 months | 55 (12.8) | 13 (10.7) | 3 (13.6) |
| • ≥6 months to 1 year | 57 (13.3) | 14 (11.6) | 9 (40.9) |
| • ≥1 year | 317 (73.9) | 94 (77.7) | 10 (45.5) |
| CAR-T cell type | |||
| • Axi-cell | 11 | ||
| • Tisa-cell | 4 | ||
| • Anti-BCMA | 5 | ||
| • Academic anti-CD19 | 2 | ||
| Conditioning Regimen, n (%) | |||
| • Melphalan | 73 (60) | ||
| • BEAM | 48 (40) | ||
| • BUCY | 12 (2.8) | ||
| • TBF | 144 (3.6) | ||
| • TBI-based | 40 (9.3) | ||
| • FluBuCy | 41 (9.6) | ||
| • FluBu | 123 (8.7) | ||
| • FluMel | 37 (8.6) (9) | ||
| • FluCy | 7 (1.6) | ||
| • Others | 25 (5.8) | ||
| Allo-SCT, n (%) | |||
| • HLA identical sibling donor | 170 (39.6) | ||
| • URD | 146 (34) | ||
| • Haplo-identical family donor | 106 (24.7) | ||
| • UCBT | 6 (1.7) | ||
| Donor/recipient HLA mismatch, n (%) | 132 (30.7) | ||
| GvHD prophylaxis | |||
| • Post-Cy based | 218 (50.8) | ||
| • Sirolimus based | 152 (35.4) | ||
| • CNI based | 277 (64.6) | ||
| ATG-based conditioning regimen, n (%) | 24 (5.5) | ||
| Conditioning regimen intensity, n (%) | |||
| • MAC | 175 (40.8) | ||
| • RIC | 253 (59.2) | ||
| IS drugs at vaccination, n (%) | 164 (38.2) | 36 (29.8) | 0 |
| IS without corticosteroids, n (%) | 123 (28.7) | 17 (14) | 0 |
| Corticosteroids at vaccination, n (%) | 36 (8.4) | 40 (33) | 0 |
| Active GvHD at vaccination, n (%)* | 121 (28.2) | ||
| • Acute GvHD | 8 (1.9) | ||
| • Chronic GvHD | 113 (26.3) | ||
| Lenalidomide maintenance, n (%) | 3 (0.7) | 150 (41.3) | |
| Ruxolitinib as GvHD therapy | 24 (5.6) | ||
| Blood count before vaccination (x109/mL) | |||
| • Absolute neutrophile counts, median (range) | 2.92 (0.06–11.57) | 2.7 (0.44–15.4) | .12 0.5–6.3) |
| • Absolute lymphocyte counts, median (range) | 1.95 (0.28–19.4) | 1.49 (0.65–5.4) | 1.27 (0.44–4.6) |
| Time from 2° dose to first serologies, median days (range) | 21 (15–51) | 22 (15–53) | 22 (20–46) |
| Median time between 1st and 2nd vaccine doses, median days (range) | 28 (18–88) | 28 (20–76) | 28 (28–31) |
| Time from 2nd dose to 3rd dose, days (range) | 161 (31–390) | 149 (69–538) | 182 (122–253) |
| Median follow-up after 3 doses, days (range) | 187 (9–289) | 194 (8–302) | 172 (36–268) |
| Forth vaccine dose, n (%) | 130 (30.3) | 50 (41) | 7 (31.8) |
| Time from 3rd dose to 4th dose, days (range) | 160 (108–246) | 161 (151–266) | 155 (147–259) |
| Median follow-up after 4 doses, days (range) | 39 (1–143) | 42 (15–107) | 47 (0–86) |
| SARS-CoV-2-S-RA detection at 3-6 weeks after 2 vaccine doses, n/evaluable (%) | 295/373 (79) | 93/112 (83) | 12/21 (57) |
| • Available antibody titers in BAU/mL, n (%) | 309 (82) | 101 (90) | 20 (91) |
| SARS-CoV-2-S-RA detection at 3 months after 2 vaccine doses, n/evaluable (%) | 270/321 (84) | 76/91 (83) | 10/17 (59) |
| • Available antibody titers in BAU/mL, n (%) | 303 (94.3) | 80 (88) | 17 (100) |
| SARS-CoV-2-S-RA detection at 6 months after full vaccination, n/evaluable (%) | 240/262 (92) | 65/75 (86.6) | 13/21 (62) |
| • Available antibody titers in BAU/mL, n (%) | 256 (98) | 53 (81) | 18 (100) |
| SARS-CoV-2-S-RA detection at 1 year after full vaccination, n/evaluable (%) | 210/217 (97) | 52/60 (87) | 9/16 (56) |
| • Available antibody titers in BAU/mL, n (%) | 217 (100) | 59 (98) | 16 (100) |
| COVID-19 after vaccination, n (%) | 53 (12.4) | 23 (19) | 3 (13.6) |
| Median time from 2nd dose of vaccine to SARS-CoV-2 infection, days (range) | 405 (15–517) | 398 (171–477) | 368 (368–420) |
| All-cause mortality n (%) | 9 (2.1) | 5 (4.1) | 0 |
| Median time from vaccination to death, days (range) | 235 (15–286) | 325 (104–416) | |
| Median follow-up from 1st dose, days (range) | 380 (49-517) | 380 (66–561) | 402 (219–436) |
AML means acute myeloid leukemia, MDS myelodysplastic syndrome, NHL non-Hodgkin lymphoma, MM multiple myeloma, CLL chronic lymphocytic leukemia, HD Hodgkin disease, MPN chronic myeloproliferative neoplasm, ALL acute lymphoblastic leukemia, BEAM BCNU, etoposide, cytarabine and melphalan, TBF thiotepa, fludarabine and busulphan, TBI total body irradiation, FluBuCy fludarabine, busulphan and cyclophosphamide, FluMel fludarabine and melphalan, Allo-HSCT allogeneic stem cell transplantation, URD adult unrelated donor, UCBT umbilical cord blood transplantation, Post-Cy post-transplant cyclophosphamide, CNI calcineurin inhibitor, ATG anti-thymocyte globulin, MAC myeloablative conditioning, RIC reduced intensity conditioning, IS Immunosuppressors, GvHD graft versus host disease, SARS-CoV-2-S-RA SARS-CoV-2-reactive antibody.
*121 recipients had active GvHD at the time of vaccination, of them 46 (38%) had mild GvHD with no IS requirement whereas 49 (40%) and 26 (21%) were on IS w/o or with corticosteroids, respectively.
SARS-CoV-2-reactive IgG antibody monitoring and compliance
Antibody titers testing at 3-6 weeks, 3, 6 and 12 months after the complete 2-dose vaccination was available in 506 (89%), 429 (75%), 355 (62%) and 294 (51%) patients, respectively, reflecting a high rate of missing serology data over time. To analyze whether there were relevant unknown biases which led to not having serological testing, we compared the clinical characteristics of patients and the survival of those with and without complete serological data at different time points and we did not find any relevant differences (see supplementary Table S2), suggesting random reasons for missing serological data. In addition, we used a listwise deletion analysis to evaluate the effect of antibody titers on the risk of breakthrough infection at each pre-specified time point. The overall positivity rate at these time points was 79%, 83%, 89.6% and 92.5%, respectively. CAR-T cell therapy recipients showed the lowest positivity rate at each time point assessment as compared to allo-HSCT and ASCT (p < 0.01 for all comparisons) (Table 1). As expected, seropositivity rates increased over time in ASCT and allo-HSCT recipients, reflecting the effect of boosters and/or breakthrough SARS-CoV-2 infections. This is in contrast with CAR-T cell therapy patients, in which no similar gradual increase in the seropositivity rate was observed.
SARS-CoV-2-reactive IgG antibody kinetics before booster
We analyzed the median SARS-CoV-2-S-RA levels in allo-HSCT and ASCT, and according to whether they had contracted COVID-19 prior to vaccination (see Fig. 1a–c). For these analyses we excluded cases with antibody levels determined after developing breakthrough infection.
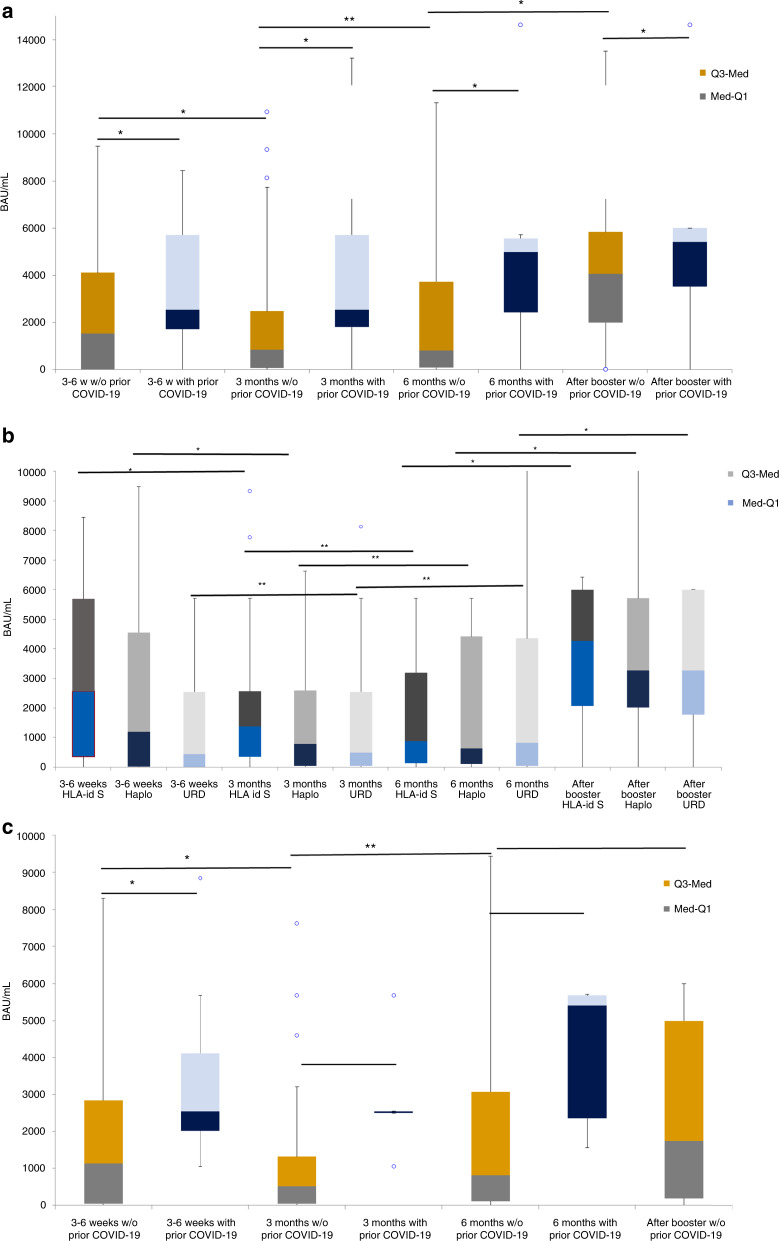
Fig. 1. Antibody titers kinetics.
Antibody titers in BAU/mL at at 3-6 weeks, 3 months and 6 months after complete vaccination and after booster dose a in allo-HSCT, b according to donor type and c in ASCT recipients. a Abbreviations, Q3-med means median interquartil 75%; Med-Q1, median interquartil 25%. * median test p value <0.05. ** median test p value not significant. b Abbreviations, Q3-med means median interquartil 75%; Med-Q1, median interquartil 25%. * median test p value <0.05. ** median test p value not significant. (see Suplementary Table S3 of Fig. 1b). C) Abbreviations, Q3-med means median interquartil 75%; Med-Q1, median interquartil 25%. * median test p value <0.05. ** median test p value not significant. (see Suplementary Table S4 of Fig. 1c).
In allo-HSCT recipients without prior COVID-19, median antibody levels dropped from 1527 BAU/ml at 3–6 weeks after complete vaccination to 843 BAU/mL at 3 months (p = 0.029). However, median antibody levels at 3 months and at 6 months (797.76 BAU/mL) after complete vaccination were not statistically different. Allo-HSCT with history of COVID-19 pre-vaccination showed significantly higher antibody levels at 3–6 weeks (2550 BAU/mL) than those without prior COVID-19 (1527 BAU/mL) [p = 0.02]. Note that the former did not experience a significant decrease in antibody levels from 3-6 weeks to 3 and/or 6 months (Fig. 1a). Analyzing antibody titers at different time points according to donor types (Fig. 1b), recipients allografted from HLA-identical siblings (HLA-id-S) showed higher antibody levels (2565 BAU/mL) at 3–6 weeks after complete vaccination than HLA haplo-identical family donors (Haplo, 1185 BAU/mL) and unrelated donors (URD, 431 BAU/mL) [p < 0.001]. Differences remained significant at 3 months. However, at 6 months after complete vaccination the median antibody titers were similar among groups (see Supplementary Table S3). Regarding antibody titer waning over time, the groups showing a significant decrease at 3 months after full vaccination were HLA-id-S and Haplo. Of note, the only group that still experienced a decrease in antibody titers at 6 months was the HLA-id-S group (p = 0.03). In contrast, antibody levels remained stable between 3 and 6 months in the Haplo group, while they remained low but stable over time in the URD group.
We next analyzed antibody titer waning in the ASCT group and found similar results to the allo-HSCT group. A significant decrease in antibody titers was observed between 3–6 weeks (1127 BAU/mL) and 3 months (509 BAU/mL) after complete vaccination (p = 0.032) in recipients without pre-vaccination COVID-19. Again, antibody titers remained high and stable over time (at 3 and 6 months after two vaccine doses) in those with prior COVID-19 (Fig. 1c). Of note, we observed no significant differences in antibody levels among ASCT and allo-HSCT at any of the time points assessed (see Supplementary Table S4).
Effect of booster vaccine dose on SARS-CoV-2-reactive IgG antibody levels
Out of 572 patients, 482 (84%) recipients (81% of allo-HSCT, 72% of ASCT and 86% of CAR-T cell recipients) received a first booster dose, mostly with BNT162b2 (79%), at a median of 172 days after complete vaccination. Of these, 306 (63%) had antibody testing available after the booster dose at a median of 101 days (range 17–215) after administration (see Table 1).
Figure 1a–c shows median SARS-CoV-2 antibody levels after the booster dose in allo-HSCT and ASCT recipients without pre-vaccination COVID-19. In the allo-HSCT group median antibody titers were significantly higher after booster than median antibody titers at 3–6 weeks, 3 and 6 months after complete vaccination (p < 0.05 for all comparisons). Differences were similar when we compared the median antibody titers by donor type (Fig. 1b). Note that no significant differences were observed by donor type in median antibody titers after booster (HLA-id-S 4256 BAU/mL vs. Haplo 3270 BAU/mL vs. URD 3270 BAU/mL, p = 0.6).
In ASCT recipients, a booster dose (1750 BAU/mL) was able to restore similar antibody levels to those reached at 3–6 weeks (1127 BAU/mL) after complete vaccination (p = 0.7), with a significant increase compared to median antibody levels at 3 (509 BAU/mL) and 6 (806 BAU/mL) months (p < 0.01, for both comparisons). Supplementary Fig. S2A–D illustrates antibody response at 3-6 weeks after booster dose in allo-HSCT (Fig. S2A) and in the subset of poorly responding allo-HSCT recipients (Fig. S2B). The latter comprised 49 allo-HSCT recipients without breakthrough SARS-CoV-2 infection before the booster dose. In total, 41 (83%) patients were able to develop adequate SARS-CoV-2-S-RA titers (>250 BAU/mL). Similar results were observed in ASCT (Fig. S2C). However, among poorly responding ASCT recipients only 11 out of 19 (58%) were able to achieve SARS-CoV-2-S-RA titers >250 BAU/mL (Fig. S2D). Regarding CAR-T cell recipients, 10 out of 21 (48%) did not show detectable SARS-CoV-2-S-RA antibodies at 3–6 weeks after full vaccination. Antibody titers decreased at 3 and 6 months in all responders but one (Fig. 2). Those with detectable antibodies at 3-6 weeks after full vaccination showed an increase in antibody titers after booster (Fig. 2), whereas none of the eight evaluable CAR-T cell therapy recipients with undetectable antibodies at 3–6 weeks after full vaccination showed even minimal serological response after booster.
Fig. 2. Antibody kinetics in CAR-T cell recipients at different time points and after booster.
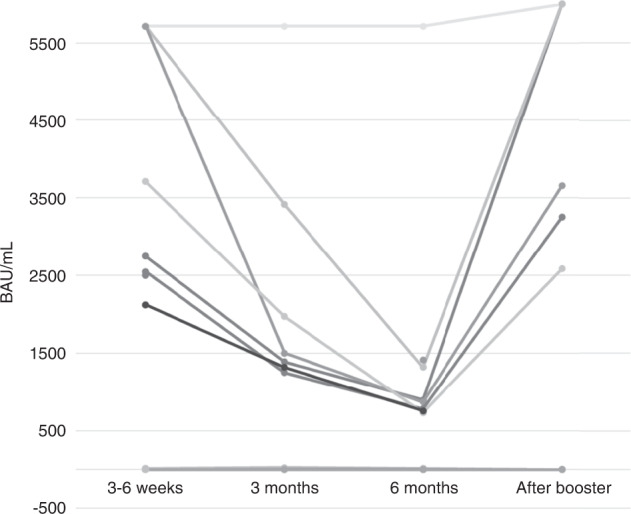
Antibody levels increased significantly after booster vaccine dose (P < 0.05 for all comparisons, median test).
Breakthrough SARS-CoV-2 severity, incidence, risk factors and mortality
Overall, 79 out of 572 recipients (14%) developed SARS-CoV-2 breakthrough infection at a median of 400 days (range 15–517) after complete vaccination (Table 1). Characteristics of SARS-CoV-2 breakthrough infection by cell therapy procedure are detailed in Table 2. Fifty-three (67%) breakthrough infections occurred after booster administration, with a predominance of Omicron VOC (55/79, 70%). Overall, 40 recipients (50.4%) developed COVID-19, whereas the remainder had asymptomatic SARS-CoV-2 infection. Pneumonia was observed in nine cases (11%) whereas hospital admission was required in eight cases (10%) and only one patient required ICU admission. No deaths directly attributed to breakthrough SARS-CoV-2 infection were noted in this series.
Table 2.
Characteristics of patients with breakthrough SARS-CoV-2 infection.
| Characteristics | SARS-CoV-2 infection Allo-SCT (n = 53) | SARS-CoV-2 infection ASCT (n = 23) | SARS-CoV-2 infection CAR-T (n = 3) |
|---|---|---|---|
| Prior COVID-19, n (%) | 0 | 2 (8.7) | 0 |
| Type of 1st and 2nd vaccines, n (%) | |||
| • Moderna mRNA-1273 | 43 (81) | 15 (65.3) | 3 (100) |
| • Pfizer-BioNTech BNT162b2 | 6 (11.3) | 7 (30.4) | 0 |
| • Adenoviral vector-based | 4 (7.7) | 1 (4.3) | 0 |
| Type of 3rd vaccines before SARS-CoV-2 breakthrough, n evaluable (%) | |||
| • Moderna mRNA-1273 | 37 (69.8) | 14 (60.9) | 2 (66.7) |
| • Pfizer-BioNTech BNT162b2 | 4 (7.5) | 8 (34.8) | 0 |
| • Adenoviral vector-based | 0 | 0 | 0 |
| Likely SARS-CoV-2 variants≠, n (%) | |||
| • Alpha or Beta | 6 (11.3) | 3 (13) | 0 |
| • Delta | 8 (15) | 7 (30.4) | 0 |
| • Omicron | 39 (74) | 13 (56.6) | 3 (100) |
| Age (years), median (range) | |||
| • 16–40 years, n (%) | 15 (28.3) | 2 (8.7) | 0 |
| • 41–60 years, n/ (%) | 27 (50.9) | 11 (47.8) | 3 (100) |
| • 61–70 years, n (/%) | 10 (18.9) | 6 (26) | 0 |
| • >71 years, n (%) | 1 (1.9) | 4 (17.5) | 0 |
| Male, n (%)/n (%) | 33 (62.3) | 14 (60.9) | 2 (66.7) |
| Baseline disease, n (%) | |||
| • AML | 21 (39.7) | 1 (4.3) | |
| • ALL | 5 (9.5) | ||
| • MDS | 7 (13.3) | ||
| • B cell NHL | 4 (7.6) | 1 (4.3) | 1 (33.3) |
| • T cell NHL | 3 (5.8) | 1 (4.3) | |
| • Plasma cell disorders | 2 (3.9) | 15 (65.3) | 1 (33.3) |
| • CLL | 0 | 1 (33.3) | |
| • HD | 5 (9.5) | 5 (21.8) | |
| • cMPN | 2 (3.9) | ||
| • Aplastic anemia | 4 (7.7) | ||
| Immunosuppressant drugs at vaccination, n (%) | 28 (52.8) | 16 (69.6) | 0 |
| Active GvHD, n (%) | 21 (39.6) | ||
| Corticosteroids at vaccination, n (%) | 10 (18.9) | 8 (34.8) | 0 |
| Lenalidomide, n (%) | 1 (1.9) | 13 (56.5) | 0 |
| Ruxolitinib therapy, n (%) | 4 (7.5) | 0 | 0 |
| Timing and characteristics of breakthrough SARS-CoV-2 infection | |||
| SARS-CoV-2 infection after 2 vaccine doses and before booster/s, n (%) | 20 (37.7) | 10 (43.5) | 1 (33.3) |
| • Median time, days (range) | 142 (15–364) | 177 (24–362) | 65 |
| SARS-CoV-2 infection after 3 vaccine doses and before the 4th dose, n (%) | 28 (52.8) | 10 (43.5) | 2 (66.7) |
| • Median time, days (range) | 114 (13–221) | 93 (23–215) | 123 (118–129) |
| SARS-CoV-2 infection after 4 vaccine doses, n (%) | 5 (9.4) | 3 (13) | 0 |
| • Median time, days (range) | 15 (3-27) | 21 (5-31) | |
| Symptomatic SARS-CoV-2 infection, n /n evaluable (%) | 29 (54.7) | 9 (39) | 2 (66.7) |
| Pneumonia, n /n evaluable (%) | 5 (9.4) | 3 (13) | 1 (33.3) |
| Hospital admission, n /n evaluable (%) | 5 (9.4) | 2 (1.7) | 1 (33.3) |
| Oxygen requirement, n /n evaluable (%) | 4 (7.5) | 0 | 1 (33.3) |
| ICU admission, n /n evaluable (%) | 1 (1.9) | 0 | 0 |
| COVID-related Death, n /n evaluable (%) | 0 | 0 | 0 |
| All-cause mortality at median follow-up, n /n evaluable (%) | 2 (3.8) | 0 | 0 |
AML acute myeloid leukemia, ALL acute lymphoblastic leukemia, MDS myelodysplastic syndrome, B-cell NHL B-cell non-Hodgkin lymphoma, T cell NHL T cell non-Hodgkin lymphoma, CLL chronic lymphocytic leukemia, HD Hodgkin disease, MPN chronic myeloproliferative neoplasm, Allo-HSCT allogeneic hematopoietic stem cell transplantation, ASCT autologous stem cell transplantation, CAR-T T-cell chimeric antigen receptor, moAb monoclonal antibody, BTK inhibitor Bruton’s tyrosine kinase inhibitor, TKIs tyrosine kinase inhibitors, ICU intensive care unit.
≠According to the Spanish epidemiological data regarding the predominance of each SARS-CoV-2 variant during the time of the study period, we considered Alpha or Beta VOC the episodes of SARS-CoV-2 infection diagnosed between April 1st 2021 and July 26th 2021, Delta VOC between July 27 2021 and Omicron between December 27 2021 to July 31 2022.
The cumulative incidence of SARS-CoV-2 breakthrough infection at one year after full vaccination was 15.3% [95%, confidence interval (C.I.), 12–18]. Cumulative incidence was similar among cell procedures: 21% (95%, C.I. 13–28) in ASCT vs. 14.4% (95%, C.I. 10–17) in allo-HSCT and 14% (95%, C.I. 0–28) in CAR-T cell therapy recipients (p = 0.24) (Fig. 3a). Table 3 shows cumulative incidence univariate analyses for allo-HSCT and ASCT. Regarding allo-HSCT, multivariate analysis identified immunosuppression at vaccination [hazard ratio (HR) 1.81 (95%, C.I. 1.23–2.69, p = 0.0028)] (Fig. 3b), and reduced intensity conditioning [HR 0.49 (95%, C.I., 0.28–0.84, p = 0.011)] (Fig. 3c) were the only risk factors for breakthrough infection. In ASCT no risk factor for breakthrough infection was found.
Fig. 3. Cumulative incidence of breakthrough SARS-CoV-2 infection.
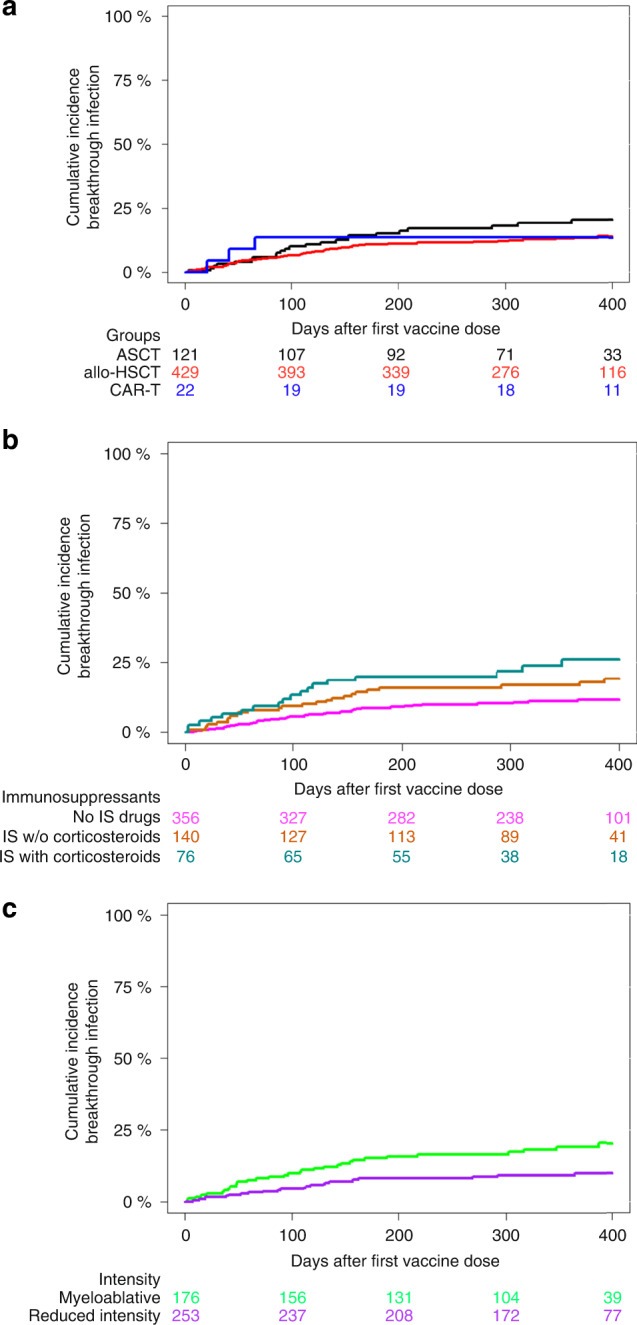
Cumulative incidence of SARS-CoV-2 infection in allo-HSCT according to a cell therapy procedure, b use of immunosuppression at the time of first vaccine dose and c conditioning intensity.
Table 3.
Univariate analysis of conditions associated with cumulative incidence of SARS-CoV-2 infection in allo-HSCT and ASCT recipients.
| Characteristics | Allo-SCT Cum. Inc (C.I. (95%) | P Value | ASCT Cum Inc (C.I. 95%) | P value |
|---|---|---|---|---|
| COVID-19 pre-vaccination | 0.01 | 0.6 | ||
| • Yes | 0 | 24 (0–53) | ||
| • No | 14.4 (11–18) | 20.2 (12–28) | ||
| Type of 1st and 2nd vaccines, n (%) | 0.09 | 0.4 | ||
| ➣ Moderna mRNA-1273 | 13 (10–17) | 18 (14–26) | ||
| ➣ Pfizer-BioNTech BNT162b2 | 9 (2–16) | 28 (0–62) | ||
| ➣ Adenoviral vector-based | 33 (3–64) | 12 (0–53) | ||
| Age (years), median (range) | 0.2 | 0.3 | ||
| • 16–40 years | 18 (9–26) | 34 (0–75) | ||
| • 41–60 years | 14 (9–19) | 24 (11–36) | ||
| • 61–70 years | 10 (4–16) | 13 (3–23) | ||
| • >71 years | 4 (0–11) | 31 (6–56) | ||
| Sex | 0.4 | 0.5 | ||
| • Male | 15 (10–19) | 22 (11–32) | ||
| • Female | 11.3 (7–16) | 19 (8–30) | ||
| Baseline disease | 0.5 | 0.2 | ||
| • AML | 15 (9–21) | |||
| • ALL | 10 (2–19) | |||
| • MDS | 14 (5–22) | |||
| • B cell NHL | 10 (0.7–19 | 9 (0-20) | ||
| • T cell NHL | 12 (0–25) | |||
| • Plasma cell disorders | 12 (5–20) | 22 (12–32) | ||
| • CLL | 11 (0–26) | 35 (9–62) | ||
| • HD | 15 (2.1–27) | |||
| • cMPN | 15 (4–27) | |||
| • Aplastic anemia | 14 (4–25) | |||
| Time from transplant to vaccination | 0.8 | 0.8 | ||
| • <6 months | 13 (4–22) | 23 (0.2–46) | ||
| • 6 months to 1 year | 15 (5–25) | 8 (0–22) | ||
| • 1 year | 12.6 (9–16.4) | 22 (13–31) | ||
| Type of donor | 0.4 | |||
| • HLA identical sibling donor | 13 (8–18) | |||
| • URD | 16 (9–23) | |||
| • Haplo-identical family donor | 8 (3–14) | |||
| • UCBT | 17 (0–46) | |||
| Donor/recipient HLA mismatch | 0.3 | |||
| • Yes | 11 (5–16) | |||
| • No | 14.2 (10–18) | |||
| GvHD prophylaxis | 0.08 | |||
| • Post-Cy based | 11 (6–15) | |||
| • Not post-Cy | 15.9 (11–21) | |||
| ATG-based conditioning regimen | 0.5 | |||
| • ATG | 17 (2–32) | |||
| • No ATG | 12.9 (10–16.5) | |||
| Conditioning regimen intensity | 0.01 | |||
| • MAC | 18.1 (12–24) | |||
| • RIC | 10 (6–14) | |||
| IS drugs at vaccination | 0.002 | 0.7 | ||
| • Yes, with corticosteroids | 30 (14-47) | 24 (3–45) | ||
| • Yes, but w/o corticosteroids | 16.9 (10-24) | 22 (8–36) | ||
| • No | 10 (6–13) | 19 (9–29) | ||
| Corticosteroids at vaccination | 0.003 | 0.3 | ||
| • Yes | 30 (14–47) | 22 (8–36) | ||
| • No | 11.6 (8–14) | 20 (11–29) | ||
| Active GvHD at vaccination | 0.047 | |||
| • Yes | 17.6 (11–24) | |||
| • No | 11 (8–15) | |||
| Ruxolitinib | 0.5 | |||
| • Yes | 17 (2–32) | |||
| • No | 13 (9–16) | |||
| Lymphocyte count < 1 × 109/mL | 0.2 | 0.29 | ||
| • Yes | 18 (9–27) | 10 (0–24) | ||
| • No | 13 (9–16) | 22 (13–31) | ||
| Lymphocyte count < 0.5 × 109/mL | 0.2 | |||
| • Yes | 17 (0–35) | |||
| • No | 14 (10–17) |
OR odds ratio, Allo-HSCT allogeneic stem cell transplantation, URD adult unrelated donor, UCBT umbilical cord blood transplantation, HLA human leucocyte antigen, post-Cy post-transplant cyclophosphamide, CNI calcineurin inhibitor, ATG anti-thymocyte globulin, MAC myeloablative conditioning, RIC reduced intensity conditioning, IS Immunosuppressors, GvHD graft versus host disease, NT not tested.
Breakthrough infection rate according to SARS-CoV-2 reactive antibody titers
Table 4 shows the rate of breakthrough infection according to median antibody levels in allo-HSCT and ASCT at different time points after full vaccination. Due to the low number of CAR-T cell therapy recipients and events, such an analysis was not performed in this subset of patients. At each time point we only considered breakthrough infections occurring after each serological assessment. In allo-HSCT recipients, median SARS-CoV-2-S-RA were significantly lower at 3–6 weeks and at 3 and 6 months after full vaccination in those who had breakthrough infection. At 12 months no significant differences were found, likely due to the low number of events. In contrast, in ASCT recipients, we found no significant differences in median SARS-CoV-2-S-RA levels between patients with and without later occurring breakthrough infection.
Table 4.
Median SARS-CoV-2-reactive IgG antibody levels according to the development of breakthrough infection in ASCT and allo-HSCT recipients.
| Variables | SARS-CoV-2 infection | No infection | P value |
|---|---|---|---|
| Allo-HSCT | |||
| SARS-CoV-2-S-RA at 3-6 weeks (n = 315) | 45 | 270 | |
| • Median Ab titers in BAU/mL (range) | 57 (0–5714) | 2191 (0–40000) | 0.018 |
| SARS-CoV-2-S-RA at 3 months (n = 240) | 22 | 218 | |
| • Median Ab titers in BAU/mL (range) | 401 (0–5714) | 1296 (0–45344) | 0.034 |
| SARS-CoV-2-S-RA at 6 months (n = 245) | 30 | 215 | |
| • Median Ab titers in BAU/mL (range) | 202 (0–5714) | 1854 (0–95334) | 0.001 |
| SARS-CoV-2-S-RA at 12 months (n = 191) | 7 | 184 | |
| • Median Ab titers in BAU/mL (range) | 1590 (0–6423) | 3705 (0–26859) | 0.13 |
| ASCT | |||
| SARS-CoV-2-S-RA at 3-6 weeks (n = 101) | 21 | 80 | |
| • Median Ab titers in BAU/mL (range) | 2500 (0–5700) | 1040 (0–41600) | 0.2 |
| SARS-CoV-2-S-RA at 3 months (n = 70) | 10 | 60 | |
| • Median Ab titers in BAU/mL (range) | 1279 (0–4602) | 469 (0–23700) | 0.22 |
| SARS-CoV-2-S-RA at 6 months (n = 64) | 13 | 51 | |
| • Median Ab titers in BAU/mL (range) | 2500 (0–5714) | 1844 (0–23426) | 0.9 |
| SARS-CoV-2-S-RA at 12 months (n = 46) | 3 | 43 | |
| • Median Ab titers in BAU/mL (range) | 4475 (435–5000) | 1820 (0–206568) | 0.7 |
Allo-HSCT refers to allogeneic hematopoietic stem cell transplant, ASCT autologous stem cell transplantation, Ab antibody, SARS-CoV-2-S-RASARS-CoV2-reactive antibodies.
Severity of SARS-CoV-2 infection according to antibody titers, VOC and vaccination doses
We next analyzed the rate and severity of breakthrough SARS-CoV-2 infection according to the antibody level cut-off of 250 BAU/mL, VOC types and vaccination doses across the whole cohort and separately in allo-HSCT recipients. Table 5 shows the rate and severity of breakthrough infection at each serological time point according to the cut-off of 250 BAU/mL. The breakthrough rate was consistently higher in those with <250 BAU/mL in the whole cohort, although statistical significance was only observed at 6 months after full vaccination. In contrast, in the allo-HSCT group this cut-off was significantly protective in all time points (3–6 weeks, 3 and 6 months after full vaccination). Symptomatic SARS-CoV-2 infection was more frequently observed in those with <250 BAU/mL, although differences did not reach statistical significance. Nevertheless, in the whole cohort this cut-off was significantly discriminative for pneumonia at 3 and 6 months, for hospital admission at 3–6 weeks and 6 months, and for supplementary oxygen requirement at 3–6 weeks, albeit no differences were found in allo-HSCT recipients.
Table 5.
Rate of SARS-CoV-2 breakthrough infection and its severity according to SARS-CoV-2-reactive IgG antibody levels cut-off at 3–6 weeks, 3 months and 6 months after complete vaccination in the whole cohort and in allo-HSCT recipients.
| Variable | <250 BAU/mL At 3–6 weeks | ≥250 BAU/mL At 3–6 weeks | P value | <250 BAU/mL At 3 months | ≥250 BAU/mL At 3 months | P value | <250 BAU/mL At 6 months | ≥250 BAU/mL At 6 months | P value |
|---|---|---|---|---|---|---|---|---|---|
| Whole cohort | 149 | 282 | 125 | 265 | 91 | 233 | |||
| SARS-CoV-2 infection, n (%) | 29 (19) | 35 (12.4) | 0.06 | 21 (17) | 30 (11) | 0.1 | 20 (22) | 23 (9.8) | 0.006 |
| Symptomatic SARS-CoV-2 | 18/29 (62) | 15/35 (43) | 0.1 | 14/21 (66.7) | 12/30 (40) | 0.08 | 14/20 (70) | 10/23 (43) | 0.1 |
| Pneumonia | 6/29 (20.6) | 2/35 (5.6) | 0.1 | 4/21 (19) | 2/30 (6.6) | .02 | 4/20 (20) | 0 | 0.039 |
| Hospital admission | 6/29 (20.6) | 1/35 (2.8) | 0.04 | 5/21 (24) | 1/30 (3) | 0.07 | 5/20 (25) | 0 | 0.016 |
| Oxygen requirement | 4/29 (13.7) | 0 | 0.03 | 3/21 (14.2) | 0 | 0.06 | 3/20 (15) | 0 | 0.09 |
| ICU admission | 0 | 0 | ns | 0 | 0 | ns | 0 | 0 | ns |
| Allo-HSCT, n evaluable | 106 | 206 | 90 | 206 | 69 | 175 | |||
| SARS-CoV-2 infection, n (%) | 23 (21.7) | 19 (9.2) | 0.005 | 17 (18.8) | 16 (7.7) | 0.008 | 16 (23) | 13 (7.4) | 0.002 |
| Symptomatic SARS-CoV-2 | 15/23 (65) | 8/19 (42%) | 0.2 | 11/17 (65) | 6/16 (37.5) | 0.16 | 11/16 (69) | 5/13 (38) | 0.14 |
| Pneumonia | 4/23 (17.4) | 0/19 | 0.1 | 2/17 (11.7) | 0 | 0.4 | 2/16 (12.5) | 0 | 0.4 |
| Hospital admission | 4/23 (17.4) | 0 | 0.1 | 3/17 (17.6) | 0 | 0.2 | 3/16 (18.5) | 0 | 0.2 |
| Oxygen requirement | 3/23 (13) | 0 | 0.2 | 2/17 (11.7) | 0 | 0.4 | 2/16 (12.5) | 0 | 0.4 |
Allo-HSCT refers to allogeneic hematopoietic stem cell transplant, ASCT autologous stem cell transplantation, GvHD graft versus host disease, IS immunosuppressor drugs, NC not calculable when the subgroup had less than 5 patients.
We further analyzed the effect of a booster dose and of the different VOCs on the rate of symptomatic breakthrough SARS-CoV-2 infection and its severity in the whole cohort and in allo-HSCT recipients (Table 6). We observed that the third and fourth doses were associated with lower pneumonia rate in the whole cohort but not in allo-HSCT recipients. Regarding VOCs, Omicron was more frequently symptomatic than Alpha-Beta or Delta. However, no ICU admission was observed with Omicron and Delta. Statistical differences were not found in the allo-HSCT setting, probably due to the low number of events.
Table 6.
SARS-CoV-2 breakthrough infection severity according to the timing of vaccine doses and SARS-CoV-2 variant of concern period in the whole cohort and in allo-HSCT recipients.
| Variable | After 2 doses | After 3 doses | After 4 doses | P value | Alpha-beta period | Delta period | Omicron period | P value |
|---|---|---|---|---|---|---|---|---|
| Whole cohort | 31 | 40 | 8 | 9 | 15 | 55 | ||
| Symptomatic SARS-CoV-2 | 14/31 (45) | 20/40 (50) | 6/8 (75) | 0.3 | 2/9 (22) | 2/15 (13) | 36/55 (65) | <0.001 |
| Pneumonia | 7/31 (22.6) | 2/40 (5) | 0 | 0.03 | 1/9 (11) | 2/15 (13) | 6/55 (11) | 0.9 |
| Hospital admission | 6/31 (19.3) | 2/40 (5) | 0 | 0.08 | 1/9 (11) | 1/15 (6.5) | 6/55 (11) | 0.8 |
| Oxygen requirement | 4/31 (13) | 1/40 (2.5) | 0 | 0.1 | 1/9 (11) | 1/15 (6.5) | 3/55 (5.5) | 0.8 |
| ICU admission | 1/31 (3.2) | 0 | 0 | ns | 1/9 (11) | 0 | 0 | 0.01 |
| Allo-HSCT, n evaluables | 17 | 28 | 5 | 6 | 8 | 39 | ||
| Symptomatic SARS-CoV-2 | 10/17 (59) | 14/28 (50) | 4/5 (80) | 0.4 | 2/6 (33) | 2/8 (25) | 25/39 (64) | 0.06 |
| Pneumonia | 3/17 (17.6) | 1/28 (3.5) | 0 | 0.1 | 1/6 (16) | 2/8 (25) | 2/39 (5) | 0.1 |
| Hospital admission | 2/17 (11.7) | 2/28 (7) | 0 | 0.6 | 1/6 (16) | 1/8 (12.5) | 3/39 (7) | 0.7 |
| Oxygen requirement | 2/17 (11.7) | 1/28 (3.5) | 0 | 0.4 | 1/6 (16) | 1/8 (12.5) | 2/39 (5) | 0.5 |
| ICU admission | 0 | 0 | 0 | ns | 1/6 (16) | 0 | 0 | 0.01 |
ICU intensive care unit, Allo-HSCT refers to allogeneic hematopoietic stem cell transplant.
Discussion
The current study provides a comprehensive picture of waning of SARS-CoV-2-S-RA levels over time, as well as the effect of booster administration on antibody levels and the estimate of cumulative incidence and risk factors for breakthrough SARS-CoV-2 infection in a large series of cell therapy recipients at one year after mass vaccination. We report a significant decline in antibody titers at 3 and 6 months after full vaccination in most recipients without pre-vaccine SARS-CoV-2 infection, whereas recipients who had SARS-CoV-2 infection prior to vaccination showed higher antibody titers that remained stable over time. The booster dose significantly increased antibody titers in both allo-HSCT and ASCT, including responses of 83% and 58% among poor responders, respectively. However, poorly responding CAR-T cell therapy recipients did not show any benefit from the booster vaccine dose. Breakthrough SARS-CoV-2 incidence at one year after full vaccination was 15% in the whole cohort and similar among cell therapy procedures. We identified immunosuppressive drugs at the time of vaccination and myeloablative intensity conditioning as the only conditions associated with higher incidence of breakthrough infection in allo-HSCT recipients. Additionally, our data suggest that antibody levels may be linked with the development of breakthrough infection and disease severity.
Although both natural infection and vaccination have good humoral immunogenicity, it is thought that antibody titers will invariably wane, which could be associated with significant loss of protection and increased probability of future infection [19]. In this regard, we observed in our series that antibody decline was seen as early as 3 months after full vaccination, showing similarities with trends reported in the general population [20, 21] and in other series of allo-HSCT recipients [22]. Antibody level decay in allo-HSCT has been associated with immunosuppressive drugs and low lymphocyte counts in peripheral blood [7]. However, antibody levels remained stable over time (at least until 6 months after full vaccination) in some recipients, such as those with pre-vaccination SARS-CoV-2 infection, irrespective of cell therapy procedure. In healthy patients, hybrid immunity (conferred from both natural infection and vaccination) induces serum-binding and -neutralizing antibody responses that are markedly more potent, durable, and resilient to the spike mutations observed in different SARS-CoV-2 variants than those of subjects who received only two vaccine doses [23–25]. However, the duration of hybrid immunity has not been analyzed in detail in immunocompromised patients. Our data indicate that hybrid immunity could also preserve longer stability in antibody titers, like in healthy individuals, suggesting that this group may not require an additional vaccine dose, or at least not as early as those vaccinated without prior SARS-CoV-2 infection.
Although we did not observe significant differences in antibody waning among cell therapy procedures, we noticed subtle differences in the allo-HSCT group. As previously reported, recipients of allo-HSCT from HLA-id-S showed higher antibody titers than Haplo or URD at 3-6 weeks and 3 months after the 2-dose vaccine schedule [4, 26]. This highlights the major role of HLA matching for reaching an adequate humoral response after vaccination, and suggests that recipients allografted from alternative donors may require early booster or different vaccination schedules with additional doses.
VE seems reduced against Omicron VOC, although it has been suggested that increasing antibody titers through additional vaccine doses may reduce the likelihood of developing severe SARS-CoV-2 infection [9]. Similar to other studies [27, 28], the booster vaccine dose was able to increase antibody levels in all cell therapy scenarios, reaching antibody levels even higher than those observed at 3–6 weeks after full vaccination [26, 27]. In contrast to the 2-vaccine dose schedule, the booster dose was able to achieve comparable antibody titers in allo-HSCT recipients with different donor types. However, the booster effect differed significantly between procedures in poorly responding recipients. Most poorly responding allo-HSCT recipients (83%) achieved adequate antibody levels, compared to 56% of poorly responding ASCT recipients and 0% of CAR-T cell recipients with poor response (p < 0.001). This observation is somewhat expected, since most ASCT recipients received either maintenance therapy with an immunomodulator in cases of multiple myeloma, or anti-CD 20 monoclonal antibody during conditioning or as maintenance in low-grade B cell or mantel cell lymphoma, both associated with lower antibody response [29]; in contrast, CAR-T cell therapy recipients experienced long-lasting B cell aplasia, and the seroconversion rate after receiving a third or fourth vaccine dose was only 24.3% in a prior series [30]. Nevertheless, this data could support favoring monoclonal antibody pre-exposure prophylaxis over further vaccine doses while in-vitro neutralization activity is maintained against predominant VOC, specifically in poorly responding CAR-T cell therapy recipients. Of note, most emerging Omicron BA. 4 and BA. 5 subvariants showed a loss of neutralization activity to almost all monoclonal antibodies except bebtelovimab [31].
Although the vaccine booster was able to increase antibody titers in our recipients, most breakthrough SARS-CoV-2 infections occurred after the third dose (51%) and with Omicron VOC predominance (69%). The prospective design of our registry enabled us to estimate the cumulative incidence of breakthrough SARS-CoV-2 infection at one year after the start of mass vaccination (15%), which will invariably be higher with longer follow-up. Although there is no available comparative data in the general population, current incidence shows that breakthrough SARS-CoV-2 infection in cell therapy recipients is high and occurs quickly, supporting the hypothesis that these patients could be more susceptible to breakthrough infections than the general population [32, 33]. While in ASCT we did not identify any condition associated with breakthrough infection, in the allo-HSCT group, recipients receiving immunosuppressive drugs with corticosteroids at the time of vaccination showed the highest incidence (30%), followed by those with immunosuppressants without corticosteroids (16%) and a significantly lower incidence in those without immunosuppressors (10%). It is well-known that immunosuppressive drugs impair both cellular and humoral function [4, 34]. T-cell function, particularly in CD8+T cells, contributed towards protection from SARS-CoV-2 challenge in non-human primates when antibody titers were sub-protective [35], and this was substantially reduced in patients receiving immunosuppressors [10] that likely contributed to the acquisition and spread of SARS-CoV-2 infection. In this regard, median antibody levels at 3–6 weeks after full vaccination were significantly lower in the subset of recipients receiving corticosteroids (48.7 BAU/mL) vs. those with immunosuppressors without corticosteroids (546.6 BAU/mL) or those without immunosuppressors (2550 BAU/mL) (p < 0.001). Furthermore, highly myeloablative conditioning (MAC) was associated with higher SARS-CoV-2 breakthrough infection (18%) compared to reduced intensity conditioning [RIC] (10%) in multivariate analysis. Although MAC could be regarded as a more intense immunosuppressant regimen, we did not observe significant differences between the two procedures in median antibody titers (2550 BAU/mL in MAC vs. 1150 BAU/mL in RIC, p = 0.2). A potential explanation for such differences could be related to social behavior, since recipients of MAC regimens were younger (median age of 46 years compared to 60 years in RIC recipients, p < 0.01). Younger patients are of course more socially active than elderly recipients, resulting in more repeated exposure to the virus.
Analyses of immune correlates from the phase 3 clinical trials of mRNA-1273 and Ad26. COV2.S confirmed that antibody titers correlated with protection against symptomatic COVID-19 infection in non-Omicron VOC [36, 37]. Our prior experience also supports a similar correlate of protection in hematological malignancy patients [5, 14]. In the cell therapy setting, this correlation was more obvious in allo-HSCT recipients, where non-infected recipients showed higher antibody titers at each time point assessed. The fact that poorly responding allo-HSCT recipients show a higher probability of adequate humoral response after booster vaccine dose may enhance this protective effect against breakthrough infection. Indeed, the cut-off of 250 BAU/mL was highly discriminative in terms of breakthrough infection in the allo-HSCT setting only.
Analyzing the effect of booster doses on symptomatic SARS-CoV-2 breakthrough infection, the third and fourth doses were associated with a lower pneumonia rate in the whole cohort. This observation may reflect potential benefit in increasing antibody titers with further vaccine booster dose. Although poor responders may lose the benefits of a booster dose through increasing antibody titers it is likely that cellular immunity could be enhanced and efficiently preventing severe infection. In line with prior findings [38], we also report that Omicron VOC was more frequently symptomatic than Alpha-Beta or Delta VOC in these highly immunosuppressed patients. Finally, no patients died from SARS-CoV-2 breakthrough infection in the current series, confirming a significant reduction in the severity of SARS-CoV-2 VOCs, and notably, that breakthrough SARS-CoV-2 infection did not increase all-cause mortality.
This study should be interpreted in light of certain limitations, such as the absence of neutralizing antibody testing, absence of cellular immune response analyses, the lack of molecular data regarding SARS-CoV-2 variants in patients with breakthrough infections and the use of different serological tests although most of them tend to correlate [39–44]. Another limitation was that only 12% of the current series were recently treated. In addition, a significant drop out of serological assessments after full vaccination over time (49% at 12 month) could be interpreted as a limitation. Although we do not have the exact percentage for each major reason for having missing serologies, from our personal experience and through our interactions with all participating physicians/centers, the most common reasons involved the patients’ and investigators’ exhaustion and/or the difficulties in scheduling serological tests outside the routine schedule for outpatient visits in long term survivors, as well as the institutional refusal for serological monitoring through academic non-funded studies. Thus, logistical reasons were by far the most common reason for lack of long-term serologies, while the patient’s death before the scheduled serological test was a very uncommon reason for missing serological follow-up. In addition, although serologies may not have been performed, all patients alive at the given follow-up time points were indeed clinically assessed, and there were thus no patients who were alive and truly lost to follow-up during the study period. In this sense, the random appearance of serological missing data along with a significant large number of evaluable cases and consistent results at each time point assessment may have limited potential bias.
Conclusion
Early antibody level decline was observed in the setting of all cell therapy procedures. However, antibody titers remained stable for at least 6 months after full vaccination in recipients with pre-vaccination SARS-CoV-2 infection. Among poor antibody responders, allo-HSCT recipients showed a response advantage with booster dose compared to ASCT and CAR-T cell recipients. The quantity of antibody titers, mostly influenced by the immunosuppression status, appears useful for identifying cell therapy recipients at higher risk of breakthrough SARS-CoV-2 infection, whereas in the allo-HSCT setting myeloablative conditioning and immunosuppressors were associated with higher breakthrough infection. Finally, we report a reassuring reduction of SARS-CoV-2 mortality in this vulnerable population.
Supplementary information
Acknowledgements
REDCap is developed and supported by Vanderbilt Institute for Clinical and Translational Research. We thank the Spanish Society of Hematology (SEHH) for its support in study diffusion. We are sincerely grateful for the invaluable aid of microbiology departments in their commitment to SARS-CoV-2-reactive IgG antibody monitoring in these highly immunosuppressed patients from all participating centers: in particular to Santiago Garcia Muñoz from the Microbiology Department of Hospital Clínico Universitario of Salamanca, and to Tamar Talaván from the Microbiology Department of Hospital Infanta Leonor of Madrid. Special thanks to all hematology units from participating centers for their commitment to the current study. Finally, we would also like to thank patients, nurses and study coordinators for their invaluable contributions to this study.
Author contributions
Authors responsible for study conception and the design: JLP, AC and AS. Authors who performed the data analysis and generated the tables and figures: JLP, RM, DN and CS. Authors responsible for patient recruitment: JLP, LV, LL-C, AP, PC, AA-P, M-JP, AS-S, GS-L, MT. O, IA, MT, LV, VC-G, BG, M-JT, MV, VG-G, AC, JÁH-R, EF, IG-C, CS. Authors responsible for manuscript writing: - JLP, RM, DN and CS were responsible for writing and supervising the writing of the manuscript. - All co-authors were responsible for reviewing analysis interpretation, suggesting modifications to the text, critically reviewing the manuscript, and final approval of the manuscript.
Data availability
Data available upon request by email to the Spanish hematopoietic transplant and cell therapy group (GETH-TC).
Competing interests
The authors declare no competing interests.
Ethics approval
The local Research Ethics Committee of the Hospital Clínico Universitario of Valencia approved the registry and study protocol (reference code 35.21)
Consent to participate
All patients included in this registry gave their signed informed consent in accordance with the declaration of Helsinki.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Supplementary information
The online version contains supplementary material available at 10.1038/s41409-023-01946-0.
References
- 1.Piñana JL, Martino R, García-García I, Parody R, Morales MD, Benzo G, et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9:21. doi: 10.1186/s40164-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ljungman P, de la Camara R, Mikulska M, Tridello G, Aguado B, Zahrani MA, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021;35:2885–94. doi: 10.1038/s41375-021-01302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spanjaart AM, Ljungman P, de La Camara R, Tridello G, Ortiz-Maldonado V, Urbano-Ispizua A, et al. Poor outcome of patients with COVID-19 after CAR T-cell therapy for B-cell malignancies: results of a multicenter study on behalf of the European Society for Blood and Marrow Transplantation (EBMT) Infectious Diseases Working Party and the European Hematology Association (EHA) Lymphoma Group. Leukemia. 2021;35:3585–8. doi: 10.1038/s41375-021-01466-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piñana JL, López-Corral L, Martino R, Montoro J, Vazquez L, Pérez A, et al. SARS-CoV-2-reactive antibody detection after SARS-CoV-2 vaccination in hematopoietic stem cell transplant recipients: Prospective survey from the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group. Am J Hematol. 2022;97:30–42. doi: 10.1002/ajh.26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piñana JL, López-Corral L, Martino R, Vazquez L, Pérez A, Martin-Martin G, et al. SARS-CoV-2 vaccine response and rate of breakthrough infection in patients with hematological disorders. J Hematol Oncol. 2022;15:54. doi: 10.1186/s13045-022-01275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niemann CU, da Cunha-Bang C, Helleberg M, Ostrowski SR, Brieghel C. Patients with CLL have a lower risk of death from COVID-19 in the Omicron era. Blood. 2022;140:445–50. doi: 10.1182/blood.2022016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leclerc M, Redjoul R, Le Bouter A, Beckerich F, Robin C, Parinet V, et al. Determinants of SARS-CoV-2 waning immunity in allogeneic hematopoietic stem cell transplant recipients. J Hematol Oncol. 2022;15:27. doi: 10.1186/s13045-022-01250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carreño JM, Alshammary H, Tcheou J, Singh G, Raskin AJ, Kawabata H, et al. Activity of convalescent and vaccine serum against SARS-CoV-2 omicron. Nature. 2022;602:682–8. doi: 10.1038/s41586-022-04399-5. [DOI] [PubMed] [Google Scholar]
- 9.Nemet I, Kliker L, Lustig Y, Zuckerman N, Erster O, Cohen C, et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N. Engl J Med. 2022;386:492–4. doi: 10.1056/NEJMc2119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collier AY, Yu J, McMahan K, Liu J, Atyeo C, Ansel JL, et al. Coronavirus Disease 2019 Messenger RNA Vaccine Immunogenicity in Immunosuppressed Individuals. J Infect Dis. 2022;225:1124–8. doi: 10.1093/infdis/jiab569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cesaro S, Ljungman P, Mikulska M, Hirsch HH, von Lilienfeld-Toal M, Cordonnier C, et al. Recommendations for the management of COVID-19 in patients with haematological malignancies or haematopoietic cell transplantation, from the 2021 European Conference on Infections in Leukaemia (ECIL 9) Leukemia. 2022;36:1467–80. doi: 10.1038/s41375-022-01578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piñana JL, Rodríguez-Belenguer P, Caballero D, Martino R, Lopez-Corral L, Terol MJ, et al. Applicability of probabilistic graphical models for early detection of SARS-CoV-2 reactive antibodies after SARS-CoV-2 vaccination in hematological patients. Ann Hematol. 2022;101(Sep):2053–67. doi: 10.1007/s00277-022-04906-8. [DOI] [PubMed] [Google Scholar]
- 13.Piñana JL, Vázquez L, Martino R, de la Cámara R, Sureda A, Rodríguez-Veiga R, et al. Spanish Society of Hematology and Hemotherapy expert consensus opinion for SARS-CoV-2 vaccination in onco-hematological patients. Leuk Lymphoma. 2022;63:538–50. doi: 10.1080/10428194.2021.1992619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piñana JL, Vazquez L, Calabuig M, López-Corral L, Martin-Martin G, Villalon L, et al. One-year breakthrough SARS-CoV-2 infection and correlates of protection in fully vaccinated hematological patients. Blood Cancer J. 2023;13:8. doi: 10.1038/s41408-022-00778-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/COVID19_Actualizacion_variantes_20220523.pdf
- 16.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(SICI)1097-0258(19990330)18:6<695::AID-SIM60>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 2012;94:496–509. doi: 10.1080/01621459.1999.10474144.. [DOI] [Google Scholar]
- 18.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. doi: 10.1214/aos/1176350951. [DOI] [Google Scholar]
- 19.Townsend JP, Hassler HB, Sah P, Galvani AP, Dornburg A. The durability of natural infection and vaccine-induced immunity against future infection by SARS-CoV-2. Proc Natl Acad Sci. 2022;119:e2204336119. doi: 10.1073/pnas.2204336119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N. Engl J Med. 2021;384:80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doria-Rose N, Suthar MS, Makowski M, O’Connell S, McDermott AB, Flach B, et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N. Engl J Med. 2021;384:2259–61. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang A, Cicin-Sain C, Pasin C, Epp S, Audigé A, Müller NJ, et al. Antibody Response to SARS-CoV-2 Vaccination in Patients following Allogeneic Hematopoietic Cell Transplantation. Transpl Cell Ther. 2022;28:214.e1–214.e11. doi: 10.1016/j.jtct.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walls AC, Sprouse KR, Bowen JE, Joshi A, Franko N, Navarro MJ, et al. SARS-CoV-2 breakthrough infections elicit potent, broad, and durable neutralizing antibody responses. Cell. 2022;185:872–880.e3. doi: 10.1016/j.cell.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altarawneh HN, Chemaitelly H, Ayoub HH, Tang P, Hasan MR, Yassine HM, et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N. Engl J Med. 2022;387:21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman LS, Ash N, et al. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N. Engl J Med. 2022;386:2201–12. doi: 10.1056/NEJMoa2118946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura M, Ferreira VH, Kothari S, Pasic I, Mattsson JI, Kulasingam V, et al. Safety and Immunogenicity After a Three-Dose SARS-CoV-2 Vaccine Schedule in Allogeneic Stem Cell Transplant Recipients. Transplant Cell Ther. 2022 Jul:S2666-6367(22)01510-X. 10.1016/j.jtct.2022.07.024 [DOI] [PMC free article] [PubMed]
- 27.Redjoul R, Le Bouter A, Parinet V, Fourati S, Maury S. Antibody response after third BNT162b2 dose in recipients of allogeneic HSCT. Lancet Haematol. 2021;8:e681–e683. doi: 10.1016/S2352-3026(21)00274-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed-Belkacem A, Redjoul R, Brillet R, Ahnou N, Leclerc M, López-Molina DS, et al. Third Early “Booster” Dose Strategy in France of bnt162b2 SARS-CoV-2 Vaccine in Allogeneic Hematopoietic Stem Cell Transplant Recipients Enhances Neutralizing Antibody Responses. Viruses. 2022;14:1928. doi: 10.3390/v14091928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maneikis K, Šablauskas K, Ringelevičiūtė U, Vaitekėnaitė V, Čekauskienė R, Kryžauskaitė L, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8:e583–e592. doi: 10.1016/S2352-3026(21)00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uyemura BS, Abid MA, Suelzer E, Abid MB Efficacy of SARS-CoV-2 primary and booster vaccine doses in CAR-T recipients - targeting the target antigen. Bone Marrow Transplant. 2022 Aug:1–5. 10.1038/s41409-022-01795-3 [DOI] [PMC free article] [PubMed]
- 31.Jian F, Yu Y, Song W, Yisimayi A, Yu L, Gao Y, et al. Further humoral immunity evasion of emerging SARS-CoV-2 BA.4 and BA.5 subvariants. Lancet Infect Dis. 2022 Sep. 10.1016/S1473-3099(22)00642-9 [DOI] [PMC free article] [PubMed]
- 32.Mittelman M, Magen O, Barda N, Dagan N, Oster HS, Leader A, et al. Effectiveness of the BNT162b2mRNA COVID-19 vaccine in patients with hematological neoplasms in a nationwide mass vaccination setting. Blood. 2022;139:1439–51. doi: 10.1182/blood.2021013768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.La J, Wu JT, Branch-Elliman W, Huhmann L, Han SS, Brophy MT, et al. Increased COVID-19 Breakthrough Infection Risk in Patients with Plasma Cell Disorders. Blood. 2022 May:blood.2022016317. 10.1182/blood.2022016317 [DOI] [PMC free article] [PubMed]
- 34.Jiménez M, Roldán E, Fernández-Naval C, Villacampa G, Martinez-Gallo M, Medina-Gil D, et al. Cellular and humoral immunogenicity of the mRNA-1273 SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Adv. 2022;6:774–84. doi: 10.1182/bloodadvances.2021006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–4. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fong Y, McDermott AB, Benkeser D, Roels S, Stieh DJ, Vandebosch A, et al. Immune Assays Team; the Coronavirus Vaccine Prevention Network (CoVPN)/ENSEMBLE Team; and the United States Government (USG)/CoVPN Biostatistics Team. Immune correlates analysis of the ENSEMBLE single Ad26.COV2.S dose vaccine efficacy clinical trial. Nat Microbiol. 2022;7:1996–2010. 10.1038/s41564-022-01262-1 [DOI] [PMC free article] [PubMed]
- 38.Menni C, Valdes AM, Polidori L, Antonelli M, Penamakuri S, Nogal A, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399:1618–24. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Misra A, Theel ES. Immunity to SARS-CoV-2: what do we know and should we be testing for it? J Clin Microbiol. 2022;60:e0048221. doi: 10.1128/jcm.00482-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins V, Fabros A, Kulasingam V. Quantitative measurement of anti-SARS-CoV-2 antibodies: analytical and clinical evaluation. J Clin Microbiol. 2021;59:e03149. doi: 10.1128/JCM.03149-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saker K, Escuret V, Pitiot V, Massardier-Pilonchéry A, Paul S, Mokdad B, et al. Evaluation of commercial anti-SARS-CoV-2 antibody assays and comparison of standardized titers in vaccinated healthcare workers. J Clin Microbiol. 2021;60:e017462. doi: 10.1128/JCM.01746-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swadźba J, Anyszek T, Panek A, Chojęta A, Wyrzykowska K, Martin E. Head-to-head comparison of 5 anti-SARS-CoV-2 assays performance in one hundred COVID-19 vaccinees, over an 8-month course. Diagnostics. 2022;12:1426. doi: 10.3390/diagnostics12061426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danese E, Montagnana M, Salvagno G, Gelati M, Peserico D, Pighi L, et al. Comparison of five commercial anti-SARS- CoV-2 total antibodies and IgG immunoassays after vaccination with BNT162b2 mRNA. J Med Biochem. 2021;40:335–40. doi: 10.5937/jomb0-31475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camacho J, Albert E, Zulaica J, Álvarez-Rodríguez B, Rusu L, Olea B, et al. A performance comparison of two (electro) chemiluminescence immunoassays for detection and quantitation of serum anti-spike antibodies according to SARS-CoV-2 vaccination and infections status. J Med Virol. 2023;95:e28397. doi: 10.1002/jmv.28397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon request by email to the Spanish hematopoietic transplant and cell therapy group (GETH-TC).