Background:
Necrotizing fasciitis is a rapidly progressive infection with a high mortality rate. Pathogens evade the host containment and bactericidal mechanisms by hijacking the coagulation and inflammation signaling pathways, leading to their rapid dissemination, thrombosis, organ dysfunction, and death. This study examines the hypothesis that measures of immunocoagulopathy upon admission could aid in the identification of patients with necrotizing fasciitis at high risk for in-hospital mortality.
Methods:
Demographic data, infection characteristics, and laboratory values from 389 confirmed necrotizing fasciitis cases from a single institution were analyzed. A multivariable logistic regression model was built on admission immunocoagulopathy measures (absolute neutrophil, absolute lymphocyte, and platelet counts) and patient age to predict in-hospital mortality.
Results:
The overall in-hospital mortality rate was 19.8% for the 389 cases and 14.6% for the 261 cases with complete measures of immunocoagulopathy on admission. A multivariable logistic regression model indicated that platelet count was the most important predictor of mortality, followed by age and absolute neutrophil count. Greater age, higher neutrophil count, and lower platelet count led to significantly higher risk of mortality. The model discriminated well between survivors and non-survivors, with an overfitting-corrected C-index of 0.806.
Conclusions:
This study determined that measures of immunocoagulopathy and patient age at admission effectively prognosticated the in-hospital mortality risk of patients with necrotizing fasciitis. Given the accessibility of neutrophil-to-lymphocyte ratio and platelet count measurements determined from a simple complete blood-cell count with differential, future prospective studies examining the utility of these measures are warranted.
Level of Evidence:
Prognostic Level III. See Instructions for Authors for a complete description of levels of evidence.
Keywords: Acute phase response, immunocoagulopathy, neutrophil-to-lymphocyte ratio, necrotizing fasciitis, orthopaedics, in-hospital mortality
Necrotizing fasciitis is a rapidly evolving and destructive infection that is one of the most potentially lethal infections of the musculoskeletal system. Causative pathogens evade the host’s protective containment and bactericidal mechanisms by hijacking the coagulation and inflammation signaling pathways during the survival acute phase response (APR). This leads to the rapid dissemination of the infection, eliciting both sepsis-induced coagulopathy (SIC) and systemic inflammatory response syndrome (SIRS)1. Together, these 2 pathologic states are principal causes of complications, such as multiple organ dysfunction syndrome (MODS) and death.
The APR is initiated in proportion to the degree of tissue damage, directing a coordinated response between coagulation factors and the survival inflammatory response to temporarily seal off affected tissue regions with a fibrin and platelet seal2-4. In addition to achieving hemostasis, this sealant promotes the ingress of inflammatory cells, such as neutrophils and lymphocytes, which help to contain and combat the infection4,5. Once survival is ensured, the APR then transitions to a reparative inflammatory response that paves the way for the regeneration of the damaged tissues2,4,6. In cases of necrotizing fasciitis, pathogens have evolved virulence factors that hijack components of the APR, evading containment by fibrin-platelet networks and allowing the pathogen to rapidly disseminate throughout the body1,3,4. As the infection progresses, the recurrent damage to surrounding tissues continually activates the APR, driving inflammation and coagulation to pathologic levels that can lead to SIC, SIRS, MODS, and death1,7.
Given that an exuberant APR is central to this pathologic response, the purpose of this study was to examine if coagulation and inflammation at admission, assessed together as measures of immunocoagulopathy, are predictive of the prognosis, specifically in-hospital mortality, in patients with necrotizing fasciitis. Specifically, this study examined if admission measures of inflammation, assessed by the white blood-cell (WBC) count, absolute neutrophil count, absolute lymphocyte count, and/or neutrophil-to-lymphocyte ratio (NLR), and measures of coagulation, assessed by the platelet count, were predictive of in-hospital mortality.
Materials and Methods
Patient Identification
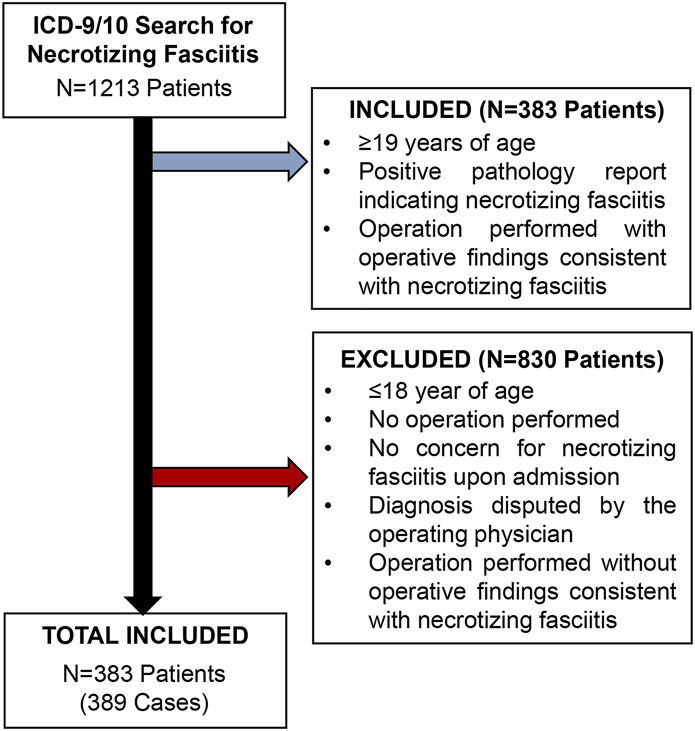
After institutional review board approval (#171361), this retrospective study exclusively utilized de-identified information extracted from the medical records in the Vanderbilt University Medical Center “Synthetic Derivative” (SD) database. All patients from February 1982 to December 2020 with the International Classification of Diseases, 9th Revision (ICD-9) code 728.86 or the ICD-10 code M72.6, indicating necrotizing fasciitis, were reviewed by the research team, to ensure rigor in the selection criteria (n = 1,213 patients). Patients were included if they were ≥19 years of age and met the criteria outlined in Figure 1. Patients who were admitted for an alternative cause and developed necrotizing fasciitis during hospitalization were excluded from this study. These criteria resulted in the inclusion of 389 cases of necrotizing fasciitis across 383 patients in the study.
Fig. 1.
CONSORT diagram showing the retrospective identification and validation of patients with necrotizing fasciitis. Utilizing the deidentified synthetic derivative database, all patients with an ICD-9 or 10 code for necrotizing fasciitis were identified. All charts were individually reviewed to confirm the diagnosis of necrotizing fasciitis by surgeon notes, operative findings, and/or pathology reports.
Data Collection
A database modeled after past large retrospective cohort studies on necrotizing fasciitis was developed8-13. Demographic data including age, sex, race, and comorbidities were collected from the SD. Comorbidities were confirmed through individual chart review and/or the presence of the following disease-associated ICD-9 and 10 codes listed in Appendix Supplemental Index 1. An aggregate comorbidity score of 0 to 7 was generated for each patient; 1 point each was given for confirmed diabetes, hypertension, peripheral vascular disease, kidney disease, history of cancer, cirrhosis, and heart disease.
Characteristics of the infection, including its initial location and cause, were collected. Operational definitions for the location and mechanism of infection are noted in Appendix Supplemental Index 2.
The primary outcome of this study was in-hospital mortality. Laboratory values assessing inflammation and coagulation were collected from the complete blood-cell count (CBC) with differential, both at the time of admission to the tertiary care center and throughout the course of disease. Inflammation was assessed by the WBC count, absolute neutrophil count, absolute lymphocyte count, and neutrophil-to-lymphocyte ratio (NLR). Coagulation was assessed by the platelet count. All of the laboratory values analyzed in the study were the first values obtained in the emergency department or on the floor upon admission.
Statistical Analysis
Descriptive statistics for demographics and infection sequelae were presented using the frequency and proportion. A multivariable logistic regression model was built on immunocoagulopathy measures (absolute neutrophil, absolute lymphocyte, and platelet counts) and patient age to predict in-hospital mortality in cases of confirmed necrotizing fasciitis. Platelet count was natural-logarithm-transformed to be included in the model. We initially assumed a nonlinear relationship for all predictors by including restricted cubic spline terms with 3 knots. The nonlinear term was removed if a Wald test on it gave a p value of >0.2. In the final model, the nonlinear term was only included for the natural-logarithm-transformed platelet count. Predictor importance was measured by degrees-of-freedom-penalized Wald statistics. The model performance (discrimination and calibration) in future patients was evaluated using a bootstrap approach. A sensitivity analysis was performed using multiple imputation for missing immunocoagulopathy measures. Patient demographics, medical history, and other laboratory results were included in the imputation model. Multiple imputation was done using the aregImpute function from the Hmisc package in R (R Foundation for Statistical Computing). Ten imputed data sets were generated, logistic regression models were fitted on each of the data sets, and coefficients and standard errors from the 10 models were pooled using the Rubin rules14. The threshold for significance was set at p < 0.05, and all statistical analyses were performed using IBM SPSS Statistics version 27 or R version 4.1.0.
Source of Funding
Funding was provided by the Caitlin Lovejoy Fund, the Vanderbilt University Medical Center Department of Orthopaedics, and the Vanderbilt School of Medicine Research Immersion program. Creation of the retrospective database utilized in this study was supported by the Clinical and Translational Science Award (CTSA) number UL1 TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Results
Patient Demographics
The retrospective review identified 389 verified cases of necrotizing fasciitis infections treated at our tertiary care center (Fig. 1). The primary mechanisms of infection among these cases were infected wounds (n = 131) and idiopathic (n = 130), accounting for ∼67% of all cases (Table I, Fig. 2-A). The pelvis (n = 170) and extremities (n = 136) were the most common locations of the infection, accounting for ∼79% of all cases (Table I, Fig. 2-B). The median age of the patients was 51 years, and comorbidities including diabetes, obesity, and hypertension were common among the study cohort (Table I). The overall in-hospital mortality rate in this population was 19.8% (Fig. 2-C).
Fig. 2.
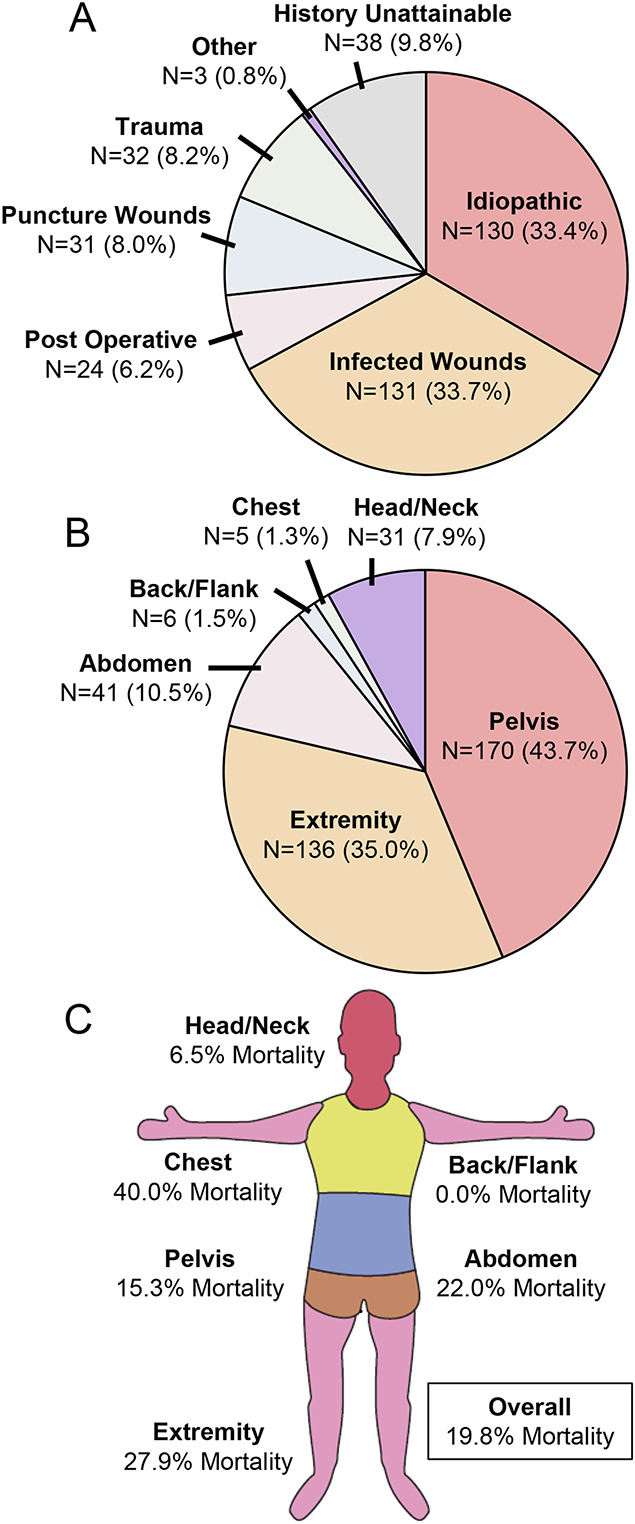
Infection characteristics across the cases of necrotizing fasciitis. Diverse causes of infection (Fig. 2-A) and locations of infection (Fig. 2-B) were observed among the 389 confirmed cases of necrotizing fasciitis obtained through retrospective review, resulting in differences in mortality across anatomical locations (Fig. 2-C).
TABLE I.
Demographics of the 389 Retrospectively Identified Cases of Necrotizing Fasciitis
| Gender (no. [%]) | |
| Male | 214 (55.0) |
| Female | 175 (45.0) |
| Median age (range) (yr) | 51 (19-85) |
| Race (no. [%]) | |
| Caucasian | 302 (77.6) |
| African American | 52 (13.4) |
| Asian/Pacific | 5 (1.3) |
| Native American/other | 12 (3.1) |
| Unknown | 18 (4.6) |
| Mechanism of infection (no. [%]) | |
| Idiopathic | 130 (33.4) |
| Infected wound | 131 (33.7) |
| Postoperative infection | 24 (6.2) |
| Puncture wound | 31 (8.0) |
| Trauma | 32 (8.2) |
| Other | 3 (0.8) |
| History unattainable | 38 (9.8) |
| Infection origin (no. [%]) | |
| Abdomen | 41 (10.5) |
| Back/flank | 6 (1.5) |
| Chest | 5 (1.3) |
| Extremity | 136 (35.0) |
| Head/neck | 31 (7.9) |
| Pelvis | 170 (43.7) |
| Comorbidities (no. [%]) | |
| Diabetes | 221 (56.8) |
| Obesity | 238 (61.1) |
| Hypertension | 229 (58.9) |
| Peripheral vascular disease | 29 (7.5) |
| Kidney disease | 59 (15.2) |
| History of cancer | 55 (14.1) |
| Cirrhosis | 21 (5.4) |
| Heart disease | 93 (23.9) |
| Median comorbidity score (range) | 2 (0-6) |
| Amputation (no. [%]) | 41 (10.5) |
| Multiorgan dysfunction (no. [%]) | 61 (15.7) |
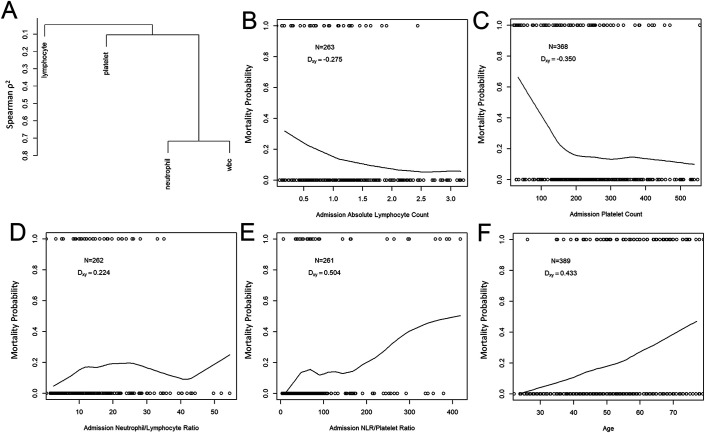
Of the 389 cases of confirmed necrotizing fasciitis, 261 (67.1%) had complete study data obtained from a CBC with differential measured near the time of admission, including absolute neutrophil, absolute lymphocyte, and platelet counts, allowing for assessment of immunocoagulopathy. Univariate correlation between mortality status and each immunocoagulopathy measure is presented in Fig. 3.
Fig. 3.
Correlation between admission immunocoagulopathy measures and mortality. Fig. 3-A Hierarchical clustering, using Spearman correlation coefficients as a similarity measure, of values obtained from a CBC with differential in patients with necrotizing fasciitis. Figs. 3-B through 3-F Univariate analyses. Smoothed estimates of mortality probability by individual predictor using LOWESS (locally weighted scatterplot smoothing). Circles are observed values. The number of observations (N) and Someer rank correlation (Dxy) between mortality and each predictor are also presented. Absolute lymphocyte count (Fig. 3-B) and platelet count (Fig. 3-C) each had moderate correlation with mortality status, with Dxy of −0.275 and −0.350, corresponding to an area under the receiver operator characteristic curve (AUC) of 0.638 and 0.675, respectively. NLR (Fig. 3-D) had a moderate correlation with mortality status, with Dxy of 0.224. Performance was further improved by evaluating the ratio of NLR to platelet count (Fig. 3-E), resulting in a relatively strong correlation with mortality, with Dxy of 0.504 and AUC of 0.752. Increased patient age (Fig. 3-F) strongly correlated with mortality, with Dxy of 0.433 and AUC of 0.717. Absolute neutrophil count and WBC correlated poorly with mortality (Dxy of −0.044 and −0.144, respectively, data not shown).
Utilizing Measures of Immunocoagulopathy to Predict In-Hospital Mortality
Guided by the above results, a multivariable logistic regression model was built including the measures of immunocoagulopathy collected from a CBC with differential, specifically absolute neutrophil count and absolute lymphocyte count evaluated as an NLR and a transformed platelet count, along with patient age, to predict in-hospital mortality in cases of confirmed necrotizing fasciitis. Measures from the 261 cases with complete data led to the generation of the following model:
where
and
This model differentiated between survivors and non-survivors well, with an original area under the receiver operating characteristic curve (AUC) of 0.829 (Fig. 4-A). In internal validation, the model has an overfitting-corrected AUC of 0.806 (Fig. 4-B). To facilitate clinical implementation of this prediction model, a user-friendly online risk calculator was developed (https://statcomp2.app.vumc.org/NF_Mortality/).
Fig. 4.
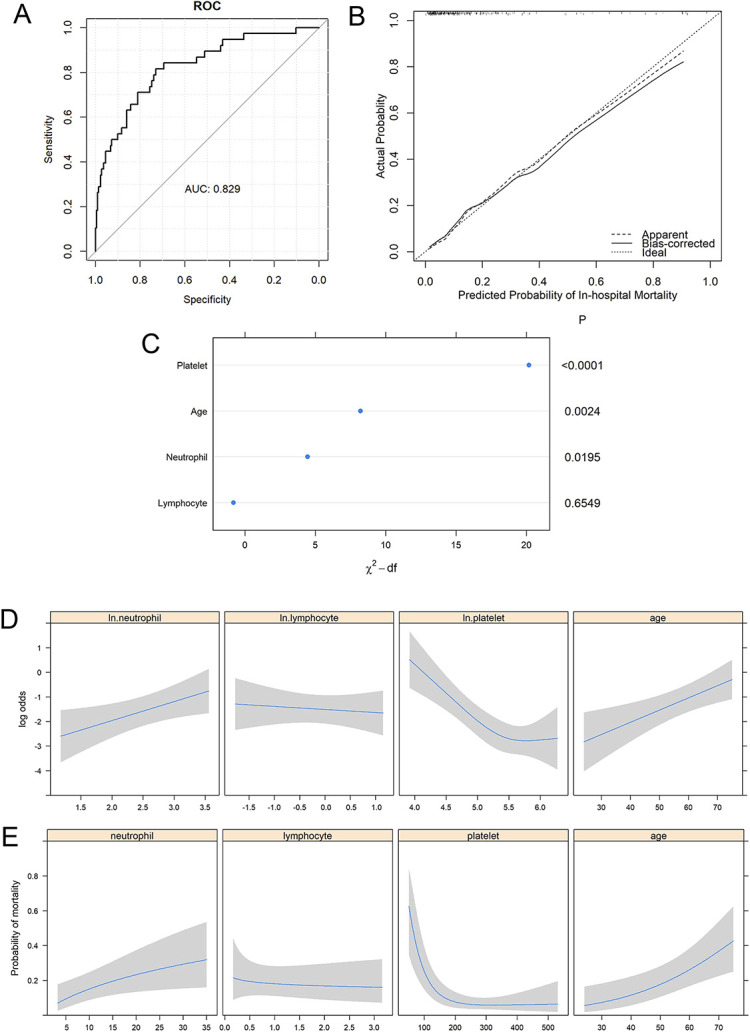
Multivariable logistic regression model using a complete-case approach. Fig. 4-A The model discriminated between survivors and non-survivors well, with an original AUC of 0.829. Fig. 4-B A bootstrap overfitting-corrected LOESS (locally estimated scatterplot smoothing) nonparametric calibration curve had an AUC of 0.806. The curve shows good calibration. Fig. 4-C Predictor importance, measured by degrees-of-freedom (df)-penalized chi-square statistics. P values are listed on the right. Fig. 4-D Predicted log odds of in-hospital mortality as a function of each individual predictor, adjusted for age = 50 years, ln.platelets = 4.8, ln.lymphocytes = 0.207, and ln.neutrophils = 2.552. The shading indicates the 95% CI. Fig. 4-E Predicted probability of in-hospital mortality as a function of each individual predictor on the original scale, adjusted for age = 50 years, platelets = 121.5 × 103 /µL, lymphocytes = 1.23 × 103 /µL, and neutrophils = 12.83 × 103 /µL. The shading indicates the 95% CI.
The most important predictor in this model was the platelet count, which accounted for 66.7% of the total variance explained by the model, followed by age (27.1%) and neutrophil count (14.7%) (Fig. 4-C). The ratio of NLR to platelet count (i.e., a measure of immunocoagulopathy) accounted for 69.9% of the total variance. The mortality risk increased with a decreasing level of platelets (p < 0.001), and the adjusted odds ratio for mortality was estimated to be 5.43 (95% confidence interval [CI], 2.50 to 11.77) for a patient with a platelet count of 100 × 103/µL compared with a patient with a platelet count of 300 × 103. A high level of neutrophils also increased mortality risk (p = 0.020), with an adjusted odds ratio of 2.34 (95% CI, 1.15 to 4.76) for 30 × 103 /µL compared with 10 × 103/µL neutrophils. Older patients were more likely to die in the hospital (p = 0.002), and the odds of mortality for a 60-year-old were 170% (95% CI, 42% to 413%), or 2.7 times, higher than those for a 40-year-old (Figs. 4-D and 4-E).
Because of the retrospective nature of this study, neutrophil and lymphocyte counts were available for only 67.4% of the cases, and the mortality rate was much higher in cases with missing neutrophil and/or lymphocyte counts (29.9%) than those with available neutrophil and lymphocyte counts (14.6%). In the model with multiple imputation, the effects of platelet count and age on in-hospital mortality remained similar, but the association between neutrophil count and mortality was no longer significant (see Appendix Supplemental Figs. 1-A, 1-B, and 1-C), and the predicted mortality risks in the complete-case model agreed with those in the model with multiple imputation (see Appendix Supplemental Fig. 1-D).
Discussion
This retrospective study found that measures of immunocoagulopathy at admission effectively prognosticated in-hospital mortality risk in patients with necrotizing fasciitis and complete data. Immunocoagulopathy can be sensitively quantified in real time through serial measures of coagulation and inflammation. For example, coagulation activation can be assessed by the platelet count. In addition to forming a physical barrier with fibrin, platelets are capable of directly interacting with bacteria15,16, engulfing bacteria17, and releasing bactericidal molecules from their granules. For these reasons, elevated platelet counts have long been examined as a prognostic indicator for infection18. Conversely, an uncontrolled APR may result in thrombocytopenia due to consumption (formation of a fibrin-platelet complex), sequestration (binding to cells in a tissue depot), or clearance (by macrophages in the spleen) of platelets from circulation. Therefore, platelet counts have a bimodal relationship with outcomes, in which both abnormally high as well as abnormally low platelet counts are associated with adverse outcomes following infection3,19,20. In the present study, we observed that non-survivors had lower platelet counts and greater proportion of patients had thrombocytopenia at admission compared with survivors, aligning with recent findings by Chen et al.21.
WBC counts are commonly utilized to assess the presence of an infection. While the total WBC count can be nonspecific3, analysis of specific subtypes of leukocytes, such neutrophils, lymphocytes, and their proportions relative to each other, has been useful for assessing the presence and/or severity of infection4. Neutrophils, in cooperation with the host’s coagulation response, work to trap bacteria in neutrophil extracellular traps (NETs) composed of DNA and in fibrin-platelet webs before releasing chemotoxins to kill the pathogens3,5,22. An elevated neutrophil count has long been utilized as a prognostic indicator of infection; however, the neutrophil count alone does not predict infection severity23, or the risk of inpatient mortality from necrotizing fasciitis as seen in this present study. For these reasons, studies have examined cellular ratios, such as the NLR, as more sensitive predictors of disease severity and prognosticators of patient outcomes4,24. While frequently utilized as a prognostic indicator for patient outcomes in the fields of cardiology25-27, oncology28-30, and infectious disease4,23,31,32, fewer studies to date have examined the utility of the NLR in cases of musculoskeletal infection or emergency general surgery33,34. In a recent study by Ravindhran et al., a preoperative NLR of >7.5 was reported to be a reliable predictor of poor outcomes of necrotizing fasciitis35. A study by Yim et al. illustrated that high NLR (≥8) upon admission positively predicted in-hospital mortality in patients with Fournier gangrene34. In the present study, while the NLR was predictive of in-hospital mortality, a model that also included the patient’s age and platelet count at admission outperformed the simple NLR at predicting in-hospital mortality for necrotizing fasciitis.
Clinical Algorithms
Prior studies have examined the utility of clinical algorithms to aid in the diagnosis and prognostication of patients with suspected necrotizing fasciitis. One of the most utilized systems for aiding in the diagnosis of necrotizing fasciitis, LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis), considers multiple circulating humoral markers of the APR, including C-reactive protein (CRP), hemoglobin, sodium, creatinine, glucose, and WBC count1,36,37. Thus, to fully calculate a LRINEC score, patients must have a CBC, a complete metabolic panel (CMP), and CRP measurement. CRP, a measure of inflammation, has been demonstrated to be a powerful predictor of disease severity and patient prognosis in a variety of musculoskeletal infection studies3,4,38. While the original LRINEC score places marked weight on CRP values, subsequent versions of this scoring system have lessened the impact of CRP in exchange for a greater focus on cellular changes and patient factors39. In this current retrospective database study, one limitation encountered was that only 36.9% (115) of the 312 survivors and 28.6% (22) of the 77 non-survivors had the required CRP values at admission to fully calculate a LRINEC score retrospectively. With the limited data, no difference in the LRINEC score was observed between survivors and non-survivors. Therefore, while a study by El-Menyar et al. suggests that the LRINEC score may likewise be useful as a prognostic measure to identify high-risk patients40, this could not be confirmed in the present retrospective study population.
The lack of available data illustrates a potential limitation to applying the LRINEC score retrospectively. A greater proportion of patients in the present study possessed a CBC with platelet counts and leukocyte differentials, allowing for the assessment of immunocoagulopathy retrospectively: 94.6% (295) of the 312 survivors and 94.8% (73) of the 77 non-survivors had platelets assessed at the time of admission, while 71.5% (223) of the 312 survivors and 50.6% (39) of the 77 non-survivors additionally had the leukocyte differential assessed. This single test (the CBC with differential) can obtain all necessary values to calculate a measure of immunocoagulopathy at admission, thus representing a sensitive, time- and cost-effective prognostic indicator of in-hospital mortality for patients with necrotizing fasciitis. Given the limited LRINEC scores available retrospectively, the present study could conclude that measures of immunocoagulopathy are equivalent or superior to the LRINEC score; however, the ease and availability of assessing immunocoagulopathy make this measure advantageous. Thus, future prospective studies are warranted.
Strength and Limitations
This study analyzed one of the largest retrospective cohorts of necrotizing fasciitis cases from a single center. As a tertiary referral center, our population is likely biased toward severe cases and conditions such as necrotizing fasciitis, thus providing ample patients to be assessed and analyzed. Given the retrospective nature of the study, there were limitations in our ability to regulate the timing and availability of laboratory blood draws, and to assess the impact of the causative pathogen(s) or medication(s) administered, such as anticoagulants, on laboratory values and mortality from necrotizing fasciitis. Furthermore, the missingness of neutrophil and lymphocyte counts cannot be fully accounted for by other variables; therefore, the missing-at-random assumption may not be valid and the model with multiple imputation could introduce bias41. We suggest that our model (with a complete-case approach) should only be applied to the patients with neutrophil and lymphocyte counts measured under a situation similar to our current clinical settings. Finally, while all cases of necrotizing fasciitis were confirmed through evaluation of the medical and surgical records, we likely excluded some positive cases because insufficient records were available retrospectively, and we potentially included some cases involving other forms of necrotizing soft-tissue infections (NSTI).
As part of this study, NLR and the platelet count were evaluated together as a measure of immunocoagulopathy. However, alternative clinical laboratory values that can accurately depict immunocoagulopathy likely exist. Alternative measures of inflammation include CRP, procalcitonin, and cytokines such as interleukin (IL)-6, and coagulopathy can be assessed by the prothrombin time or fibrinogen level. Given the retrospective nature of our data set, alternative measures were not reliably available to determine which measure of immunocoagulopathy is superior. Future prospective studies would be required to determine the most sensitive measures of immunocoagulopathy for predicting patient morbidity and mortality.
Conclusions
This study determined that, in patients with necrotizing fasciitis, age and measures of immunocoagulopathy at admission, specifically the NLR and platelet count assessed from the CBC with differential, can accurately prognosticate a patient’s in-hospital mortality risk, with an overfitting-corrected AUC of 0.806. Paralleling these findings, numerous recent studies have illustrated the utility of similar measures for predicting severe outcomes and death in cases of COVID-194,42-45. Given the accessibility of measures of immunocoagulopathy determined from a simple CBC with differential, future prospective studies examining the utility of these measures in cases of necrotizing fasciitis and other serious musculoskeletal infections are warranted.
Appendix
Supporting material provided by the authors is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJSOA/A477).
Acknowledgments
Note: The authors thank members of the Schoenecker Laboratory, in particular Drs. Andrew Rees and Jacob Schultz, for their review of this work. Additionally, we thank all of the experts who have provided guidance for this study, including Drs. Jackie Pennings and Frank Harrell. Finally, we thank our families and friends for their continued support and understanding.
Footnotes
Disclosure: The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A476).
Contributor Information
Samuel R. Johnson, Email: samuel.r.johnson@vanderbilt.edu.
Teresa Benvenuti, Email: tabenvenuti@gmail.com.
Hui Nian, Email: hui.nian@VUMC.org.
Isaac P. Thomson, Email: isaac.thomsen@vumc.org.
Keith Baldwin, Email: baldwink@chop.edu.
William T. Obremskey, Email: william.obremskey@vumc.org.
Stephanie N. Moore-Lotridge, Email: stephanie.n.moore.1@vumc.org.
References
- 1.Hysong AA, Posey SL, Blum DM, Benvenuti MA, Benvenuti TA, Johnson SR, An TJ, Devin JK, Obremskey WT, Martus JE, Moore-Lotridge SN, Schoenecker JG. Necrotizing Fasciitis: Pillaging the Acute Phase Response. J Bone Joint Surg Am. 2020;102(6):526-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benvenuti M, An T, Amaro E, Lovejoy S, Mencio G, Martus J, Mignemi M, Schoenecker JG. Double-edged sword: musculoskeletal infection provoked acute phase response in children. Orthop Clin North Am. 2017;48(2):181-97. [DOI] [PubMed] [Google Scholar]
- 3.An TJ, Benvenuti MA, Mignemi ME, Thomsen IP, Schoenecker JG. Pediatric musculoskeletal infection: Hijacking the acute-phase response. JBJS Rev. 2016. Sep 27;4(9):e4. [DOI] [PubMed] [Google Scholar]
- 4.Moore-Lotridge SN, Gibson BH, Duvernay MT, Martus JE, Thomsen IP, Schoenecker JG. Pediatric Musculoskeletal Infection. J Pediatric Orthopaedic Society North America. 2020;2(2). [Google Scholar]
- 5.Kobayashi SD, Voyich JM, Burlak C, DeLeo FR. Neutrophils in the innate immune response. Arch Immunol Ther Exp (Warsz). 2005;53(6):505-517. [PubMed] [Google Scholar]
- 6.Baker CE, Moore-Lotridge SN, Hysong AA, Posey SL, Robinette JP, Blum DM, Benvenuti MA, Cole HA, Egawa S, Okawa A, Saito M, McCarthy JR, Nyman JS, Yuasa M, Schoenecker JG. Bone Fracture Acute Phase Response-A Unifying Theory of Fracture Repair: Clinical and Scientific Implications. Clin Rev Bone Miner Metab. 2018;16(4):142-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YT, Chou TD, Peng MY, Chang FY. Rapidly progressive necrotizing fasciitis caused by Staphylococcus aureus. J Microbiol Immunol Infect. 2005. Oct;38(5):361-4. [PubMed] [Google Scholar]
- 8.Wall DB, de Virgilio C, Black S, Klein SR. Objective criteria may assist in distinguishing necrotizing fasciitis from nonnecrotizing soft tissue infection. Am J Surg. 2000. Jan;179(1):17-21. [DOI] [PubMed] [Google Scholar]
- 9.Wall DB, Klein SR, Black S, de Virgilio C. A simple model to help distinguish necrotizing fasciitis from nonnecrotizing soft tissue infection. J Am Coll Surg. 2000. Sep;191(3):227-31. [DOI] [PubMed] [Google Scholar]
- 10.Childers BJ, Potyondy LD, Nachreiner R, Rogers FR, Childers ER, Oberg KC, Hendricks DL, Hardesty RA. Necrotizing fasciitis: a fourteen-year retrospective study of 163 consecutive patients. Am Surg. 2002. Feb;68(2):109-16. [PubMed] [Google Scholar]
- 11.Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am. 2003. Aug;85(8):1454-60. [PubMed] [Google Scholar]
- 12.Sarani B, Strong M, Pascual J, Schwab CW. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009. Feb;208(2):279-88. [DOI] [PubMed] [Google Scholar]
- 13.Huang KF, Hung MH, Lin YS, Lu CL, Liu C, Chen CC, Lee YH. Independent predictors of mortality for necrotizing fasciitis: a retrospective analysis in a single institution. J Trauma. 2011. Aug;71(2):467-73, discussion 473. [DOI] [PubMed] [Google Scholar]
- 14.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley and Sons; 2004. [Google Scholar]
- 15.Kerrigan SW. The expanding field of platelet-bacterial interconnections. Platelets. 2015;26(4):293-301. [DOI] [PubMed] [Google Scholar]
- 16.Ali RA, Wuescher LM, Dona KR, Worth RG. Platelets mediate host defense against Staphylococcus aureus through direct bactericidal activity and by enhancing macrophage activities. J Immunol. 2017. Jan 1;198(1):344-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNicol A, Israels SJ. Beyond hemostasis: the role of platelets in inflammation, malignancy and infection. Cardiovasc Hematol Disord Drug Targets. 2008. Jun;8(2):99-117. [DOI] [PubMed] [Google Scholar]
- 18.Riise ØR, Kirkhus E, Handeland KS, Flatø B, Reiseter T, Cvancarova M, Nakstad B, Wathne KO. Childhood osteomyelitis-incidence and differentiation from other acute onset musculoskeletal features in a population-based study. BMC Pediatr. 2008. Oct 20;8(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assinger A, Schrottmaier WC, Salzmann M, Rayes J. Platelets in sepsis: an update on experimental models and clinical data. Front Immunol. 2019. Jul 17;10:1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gafter-Gvili A, Mansur N, Bivas A, Zemer-Wassercug N, Bishara J, Leibovici L, Paul M. Thrombocytopenia in Staphylococcus aureus bacteremia: risk factors and prognostic importance. Mayo Clin Proc. 2011. May;86(5):389-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YC, Liou YT, Tsai WH, Chen LW. Prognostic Role of Subsequent Thrombocytopenia in Necrotizing Fasciitis Without Liver Disease. Ann Plast Surg. 2022. Mar 1;88(1s)(Suppl 1):S99-105. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi SD, Malachowa N, DeLeo FR. Influence of microbes on neutrophil life and death. Front Cell Infect Microbiol. 2017. May 1;7:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Jager CP, van Wijk PT, Mathoera RB, de Jongh-Leuvenink J, van der Poll T, Wever PC. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care. 2010;14(5):R192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwab P, Varady N, Chen A, editors. Novel marker for septic hip and knee arthritis: neutrophil-to-lymphocyte ratio is a strong predictor of treatment failure and postoperative 90-day mortality. Orthopaedic Proceedings. 2019;101-B: No. SUPP_4. [Google Scholar]
- 25.Bhat T, Teli S, Rijal J, Bhat H, Raza M, Khoueiry G, Meghani M, Akhtar M, Costantino T. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013. Jan;11(1):55-9. [DOI] [PubMed] [Google Scholar]
- 26.Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008. Sep 15;102(6):653-7. [DOI] [PubMed] [Google Scholar]
- 27.Núñez J, Núñez E, Bodí V, Sanchis J, Miñana G, Mainar L, Santas E, Merlos P, Rumiz E, Darmofal H, Heatta AM, Llàcer A. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008. Mar 15;101(6):747-52. [DOI] [PubMed] [Google Scholar]
- 28.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014. May 29;106(6):dju124. [DOI] [PubMed] [Google Scholar]
- 29.Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, Widmann WD. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012. Jan;19(1):217-24. [DOI] [PubMed] [Google Scholar]
- 30.Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer. 2012. Aug 7;107(4):695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naess A, Nilssen SS, Mo R, Eide GE, Sjursen H. Role of neutrophil to lymphocyte and monocyte to lymphocyte ratios in the diagnosis of bacterial infection in patients with fever. Infection. 2017. Jun;45(3):299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farah R, Khamisy-Farah R. Association of neutrophil to lymphocyte ratio with presence and severity of gastritis due to Helicobacter pylori infection. J Clin Lab Anal. 2014. May;28(3):219-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yapıcı O, Berk H, Öztoprak N, Seyman D, Tahmaz A, Merdin A. Can Ratio of Neutrophil-to-Lymphocyte Count and Erythrocyte Sedimentation Rate in Diabetic Foot Infecti on Predict Osteomyelitis and/or Amputation? Hematol Rep. 2017. Feb 23;9(1):6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yim SU, Kim SW, Ahn JH, Cho YH, Chung H, Hwang EC, Yu HS, Oh KJ, Kim SO, Jung SI, Kang TW, Kwon DD, Park K. Neutrophil to Lymphocyte and Platelet to Lymphocyte Ratios Are More Effective than the Fournier’s Gangrene Severity Index for Predicting Poor Prognosis in Fournier’s Gangrene. Surg Infect (Larchmt). 2016. Apr;17(2):217-23. [DOI] [PubMed] [Google Scholar]
- 35.Ravindhran B, Rajan S, Kerketta D, Balachandran G, Mohan LN. Neutrophil to Lymphocyte Ratio (NLR) and Platelet to Lymphocyte Ratio (PLR) Versus Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) as Predictors of Outcome in Necrotising Fasciitis. Indian J Surgery. 2020;82(3):325-30. [Google Scholar]
- 36.Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004. Jul;32(7):1535-41. [DOI] [PubMed] [Google Scholar]
- 37.Bechar J, Sepehripour S, Hardwicke J, Filobbos G. Laboratory risk indicator for necrotising fasciitis (LRINEC) score for the assessment of early necrotising fasciitis: a systematic review of the literature. Ann R Coll Surg Engl. 2017. May;99(5):341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amaro E, Marvi TK, Posey SL, Benvenuti MA, An TJ, Dale KM, Lovejoy SA, Martus JE, Johnson ME, Mencio GA, Moore-Lotridge SN, Thomsen IP, Schoenecker JG. C-Reactive Protein Predicts Risk of Venous Thromboembolism in Pediatric Musculoskeletal Infection. J Pediatr Orthop. 2019. Jan;39(1):e62-7. [DOI] [PubMed] [Google Scholar]
- 39.Borschitz T, Schlicht S, Siegel E, Hanke E, von Stebut E. Improvement of a Clinical Score for Necrotizing Fasciitis: ‘Pain Out of Proportion’ and High CRP Levels Aid the Diagnosis. PLoS One. 2015. Jul 21;10(7):e0132775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Menyar A, Asim M, Mudali IN, Mekkodathil A, Latifi R, Al-Thani H. The laboratory risk indicator for necrotizing fasciitis (LRINEC) scoring: the diagnostic and potential prognostic role. Scand J Trauma Resusc Emerg Med. 2017. Mar 7;25(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baldwin KD, Ohman-Strickland P. Missing data in orthopaedic research. U Pennsylvania Orthopaedic Journal. 2009;19. [Google Scholar]
- 42.Lagunas-Rangel FA. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. J Med Virol. 2020. Oct;92(10):1733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Liu C, Mao Z, Xiao M, Wang L, Qi S, Zhou F. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Crit Care. 2020. Nov 16;24(1):647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu J, Kong J, Wang W, Wu M, Yao L, Wang Z, Jin J, Wu D, Yu X. The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: A retrospective study in Suzhou China. Thromb Res. 2020. Aug;192:3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simadibrata DM, Calvin J, Wijaya AD, Ibrahim NAA. Neutrophil-to-lymphocyte ratio on admission to predict the severity and mortality of COVID-19 patients: A meta-analysis. Am J Emerg Med. 2021. Apr;42:60-9. [DOI] [PMC free article] [PubMed] [Google Scholar]