Abstract
Background
Pharmacovigilance reports have suggested that certain commonly used medications may trigger autoimmune diseases (ADs) and immune-mediated inflammatory diseases (IMIDs). We systematically reviewed the literature to evaluate whether psychiatric medication use is associated with ADs and IMIDs.
Methods
The protocol was registered in PROSPERO (CRD42022296524) before the start of the study. We searched Medline Ovid and Scopus up to November 28th, 2021, for comparative studies, with any psychiatric medication as exposure and ADs and IMIDs as outcomes. Meta-analysis was performed using DerSimonian-Laird random-effects modeling. The PRISMA 2020 guidelines were followed in reporting. Study-level risk of bias was assessed using the Newcastle-Ottawa Scale, and the overall certainty of evidence using GRADE.
Results
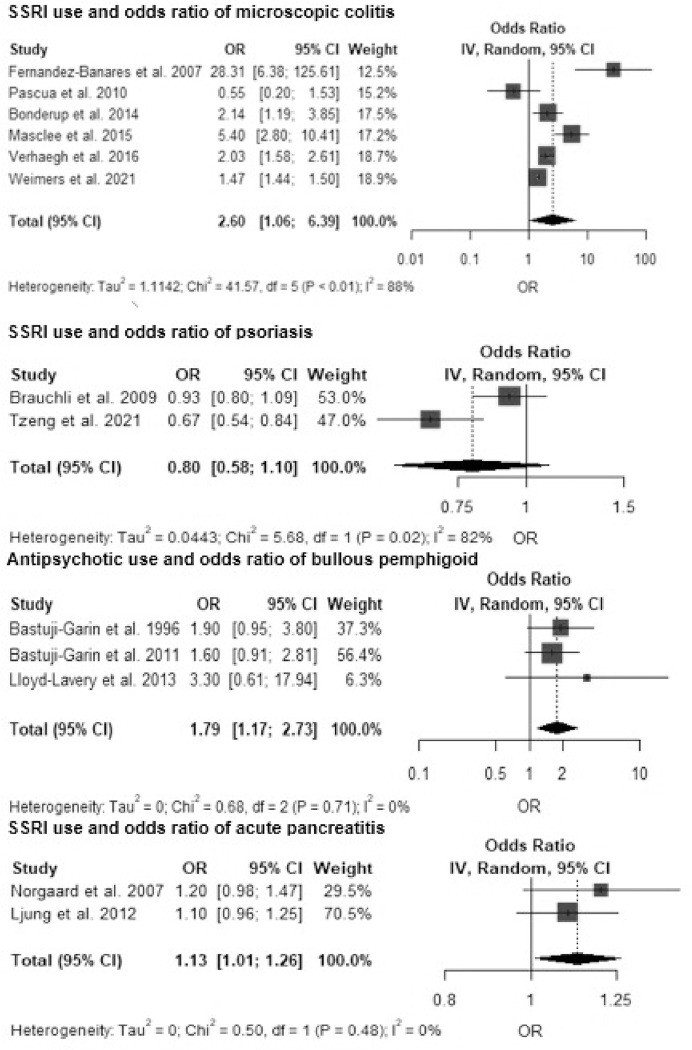
There were 7,265 citations from which 31 studies were eligible, all from high-income countries, covering 15 distinct immune diseases. The evidence for the association between selective serotonin reuptake inhibitor (SSRI) use and higher risk of microscopic colitis (meta-OR 2.60, 95% CI 1.05–6.39, I2 97.5%, 6 studies) was of low certainty. A subgroup analysis by the histological type of microscopic colitis showed a statistically significant association only with lymphocytic colitis (meta-OR 2.88, 95% CI 2.60–3.18, I2 00.00%, three studies). In two case-control studies, SSRI use had no significant association with psoriasis (meta-OR 0.80, 95% CI 0.58–1.10, I2 82.4%). The risk of acute pancreatitis was slightly increased with exposure to SSRIs (meta-OR 1.13, 95% CI 1.01–1.26, I2 00.0%), as was the risk of bullous pemphigoid after exposure to antipsychotics (meta-OR 1.79, 95% CI 1.17–2.73, I2 0%).
Conclusions
We reviewed the literature on whether psychiatric medications associate with the risk of ADs and IMIDs and concluded that, despite several signals, the credibility of evidence remains low at best. Prospective cohort studies would be needed as the next step to confirm the suggestions of increased risk.
Introduction
The causes of most autoimmune diseases (ADs) and immune-mediated inflammatory disorders (IMIDs) are unknown. The etiology and pathophysiology of most of them are probably unrelated, yet some share common genetic and environmental risk factors. The common denominator of these diseases is the dysregulation of immune pathways, leading to mistargeted or excessively activated responses, manifesting in subsequent organ-specific or multisystem damage [1]. Globally, the incidence of many of these diseases is increasing, including celiac disease, rheumatoid arthritis, inflammatory bowel diseases, and type 1 diabetes mellitus [2]. Numerous case reports, case series, and some pharmacovigilance risk signals suggest that certain commonly prescribed medications could trigger immune diseases [3]. Additionally, some observational studies suggest that exposure to antidepressants may be associated with a beneficial course in certain inflammatory diseases [4].
The most widely documented drug-induced autoimmune phenomenon is drug-induced lupus (DIL). A range of medications has been linked with it, including psychiatric drugs such as carbamazepine and levomepromazine [5]. Drug-induced immune thrombocytopenia characteristically occurs within two weeks after initiation of the causative medication and re-occurs rapidly after subsequent exposures [6]. Among inflammatory diseases without autoimmune mechanisms, the United European Gastroenterology organization and the European Microscopic Colitis Group have both stated that the use of selective serotonin reuptake inhibitors (SSRIs) is associated with an increased risk of developing an inflammatory colonic disease called microscopic colitis (MC) [3].
Animal models have associated mental stress with a proinflammatory state [7]. In humans, there is a relationship between having mental disorders and higher levels of proinflammatory cytokines (e.g., TNF-alpha, interleukin-6) that can activate the inflammatory response system [7]. This relationship appears bidirectional, as higher levels of these cytokines also increase the risk of depression [8].
We aimed to assess whether reports from case series could be supported by epidemiological evidence from comparative observational studies. Due to uncertainty in the etiology of the range of these diseases and finding no biological rationale to restrict the search to only a few of these diseases, we conducted a search of all major ADs and IMIDs covered in the literature.
Methods
The systematic review protocol was registered on PROSPERO (CRD42022296524) before commencing the study. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement was used to guide the reporting of the process [9]. Authors IL and ON searched MEDLINE Ovid and Scopus from inception until 28 November 2021 (non-duplicate process). After this initial search of titles and abstracts, full-texts were read independently and in parallel by IL and ON. Reference lists of eligible articles were browsed for potentially missed studies. Studies were eligible if they satisfied the following inclusion criteria, which are demonstrated under the PICO model for cohort studies in Table 1. The full search strategy and PRISMA checklist are available in the supplement. In short, we listed as exposures all psychiatric medications that have been widely used. We included studies having any immune diseases as outcomes after exposure to psychiatric medications. Primarily allergic immune diseases, immune deficiencies, or diseases with an infectious etiology such as viral hepatitis, were not included as outcomes.
Table 1. Search question in terms of PICO (Population, intervention, comparison, and outcome).
| Population (P) | Human subjects who were exposed to any medication used to treat psychiatric disorders as indexed in the WHO ATC (S4 File). The exception was benzodiazepines, which were not considered. We accepted the drug exposure period as defined by the investigators. Exposure to the medication should have occurred prior to the immune disease diagnosis, and studies with an unclear sequence of events were not considered. |
| Intervention (I) | We included only observational studies. Because immune diseases are rare, we did not expect to find interventional studies on these relationships. |
| Comparison (C) | The observational study should include a comparison group of individuals who have not been exposed to or have been exposed to a lesser degree (e.g., regular vs. non-regular use) to the medication. Case series were not included due to the lack of a comparison group. |
| Outcome (O) | The outcome is the diagnosis of any AD or IMID as has been listed in the search strategy (Supplement). Inflammatory conditions with a clear infectious etiology, such as viral hepatitis, were not included. |
We considered original articles published in any language but excluded conference abstracts and reviews. We managed the citations using the RefWorks legacy edition. All authors participated in the data collection and risk-of-bias assessments, which were performed in duplicate by two authors using a Microsoft Excel sheet. The collected data were checked and synthesized by one author (ON). Data items included basic study details, which diseases were studied as outcomes, which medications were studied as exposures, drug exposure definitions, study country and continent, age and gender distributions, years of recruitment, study design (case-control or cohort), and whether the study was prospective or retrospective. Studies were excluded, if an effect measure was not reported and it was not possible to calculate it based on the reported data. Individual studies were assessed with the Newcastle–Ottawa Scale (NOS) in three domains: selection, comparability, and outcome ascertainment [10]. The NOS score ranges from 0 to 9, where 9 indicates the lowest risk of bias. NOS scores are shown in the supplement, including which studies had a sufficient adjustment for confounders. To evaluate the overall strength of evidence of each meta-analysis, we applied the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) procedure [11].
Statistical analysis
The summary measure for binary outcomes is the odds ratio (OR) in case-control studies, the risk ratio (RR) in cohort studies, and the hazard ratio (HR) in cohort studies with time-to-event data. RStudio (version 2022.02.2) and the meta package with metagen and forest.meta functions were used to perform random-effects meta-analyses (DerSimonian and Laird) and create forest plots. Between-study heterogeneity in estimates is designated by the Tau2 and I2 statistics, and in the case of the latter, 0–40% indicates non-important heterogeneity, and 75–100% indicates very important heterogeneity [12, 13]. We used the harvest plot to visualize the results when it was not possible to conduct a meta-analysis [14]. Reanalyzing the data using different statistical approach was used for the sensitivity analysis in the current study.
Results
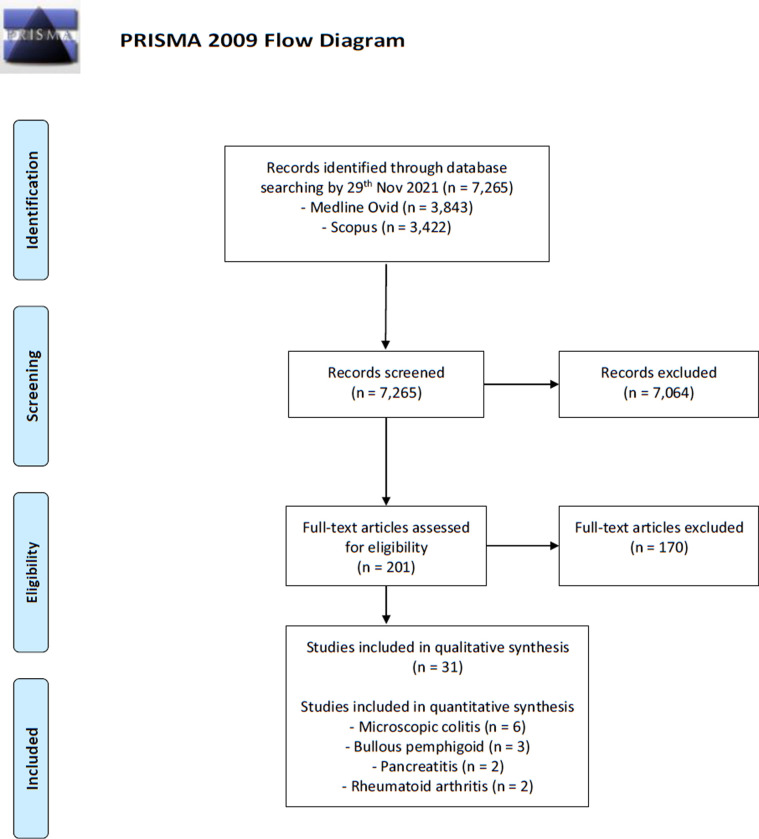
The primary search yielded 7,265 citations, of which 203 were assessed in duplicate (authors IL and ON), eventually contributing 31 articles eligible for narrative review (Fig 1, Table 2) [15–45]; seven on MC [15–21], six on acute pancreatitis (AP) [22–27], four on bullous pemphigoid [28–31], three on lupus erythematosus [32–34], two on alopecia areata [35, 36], psoriasis [37, 38], and rheumatoid arthritis [39, 40], while one on immune thrombocytopenia [41], cutaneous lupus erythematosus [34], interstitial lung disease [42], multiple sclerosis [43], silent thyreoiditis [44], and vitiligo [45]. According to the World Bank income classification, all studies were conducted in countries of high income. Studies from Europe comprised the majority, with 74% (23/31), followed by 19% (6/31) from Northern America, and the two Asian studies were from Taiwan (Table 1).
Fig 1. Flowchart of study selection.
Table 2. Characteristics of studies reporting an association between the exposure to psychiatric medications and the risk of autoimmune diseases or immune-mediated inflammatory diseases.
| Author, year | Country, continent | Disease(s) | Individual medications or groups analyzed | Age distribution, years | Years of recruitment | Cohort or case-control | Prospective or retrospective |
|---|---|---|---|---|---|---|---|
| Bonderup 2014 | Denmark, Europe | MC, CC, and LC | (1) SSRI | Mean 64.7 (CC), 63.9 (LC) | 2005–2011 | Case-control | Prospective |
| Fernández-Bañares 2013 | Spain, Europe | MC, CC, and LC | (1) Sertraline | Mean 62.5 ± 1.4 (CC), 62.6 ± 1.9 (LC) | 3/2007–5/2010 | Case-control | Prospective |
| Fernández-Bañares 2007 | Spain, Europe | MC, CC, and LC | (1) SSRI | Mean 61.0 ± 2.02 (CC), 67.4 ± 2.25 (LC) | 1/1993–12/1999 | Case-control | Prospective |
| Masclee 2015 | Netherlands, Europe | MC | (1) SSRI | Mean 45.1 ± 2.1 | 1/1999–3/2012 | Nested case-control | Prospective |
| Pascua 2010 | US, North America | MC | (1) SSRI | Median 68.9, range 18–92 | 2002–2007 | Case-control | Retrospective |
| Verhaegh 2016 | UK, Europe | MC, CC, and LC | (1) SSRI | Mean 63.4 ± 14 | 1/1992–12/2013 | Case-control | Retrospective |
| Weimers 2021 | Denmark, Europe | MC | (1) SSRI | Mean 65.0 ± 14.0 | 1/1995–12/2016 | Case-control | Retrospective |
| Boden 2012 | Sweden, Europe | AP | (1) Clozapine & olanzapine (2) Other antipsychotics: fluphenazine, perphenazine, flupenthixol, thioridazine, chlorpromazine, haloperidol, pimozide, ziprasidone, aripiprazole, quetiapine, risperidone, paliperidone, clozapine, and olanzapine |
Range 40–85 | 1/2006–12/2008 | Nested case-control | Retrospective |
| Gasse 2008 | Denmark, Europe | AP | (1) Conventional, including flupentixol, fluphenazine, haloperidol, pimozide, penfluridol, periciazine, perphenazine, prochlorperazine, zuclopenthixol, chlorpromazine, chlorprothixene, levomepromazine, melperone, and thioridazine (2) Atypical antipsychotics: clozapine, olanzapine, risperidone, quetiapine, ziprasidone, sulpiride, levosulpiride, and amisulpride |
Range 18–89 | 1991–2003 | Case-control | Retrospective |
| Lin 2017 | Taiwan, Asia | AP | (1) SSRI (2) Non-SSRI antidepressants |
Mean 50.9 ± 15.9, range 20–84 | 2000–2013 | Case-control | Retrospective |
| Ljung 2012 | Sweden, Europe | AP | (1) SSRI (2) Non-SSRI |
Range 40–84 | 2006–2008 | Case-control | Retrospective |
| Norgaard 2006 | Denmark, Europe | AP | (1) Valproic acid | Range 18–89 | North Jutland 1991–2003, Aarhus 1996–2003, and Viborg 1998–2003 | Case-control | Retrospective |
| Norgaard 2007 | Denmark, Europe | AP | (2) SSRI | Range 18–89 | North Jutland 1991–2003, Aarhus 1996–2003, and Viborg 1998–2003 | Case-control | Retrospective |
| Bastuji-Garin 1996 | France, Europe | BP | (1) Neuroleptics | Mean 79.2 ± 10.1 | 3/1989–2/1992 | Case-control | Prospective |
| Bastuji-Garin 2011 | France, Europe | BP | (1) Neuroleptics (N05A) (2) Psycholeptic agents (N05) (3) Phenothiazine aliphatic chain (N05AA) (4) Psychoanaleptics (N06) |
Mean 82.4 ± 8.7 | 1/2003–4/2007 | Case-control | Prospective |
| Lloyd-Lavery 2013 | UK, Europe | BP | (1) Antipsychotics (2) Antidepressants (3) SSRI (4) TCA (5) SNRI (6) Mirtazapine (7) Tradozone |
Mean 81.5 ± 9.7 | 1/2004–12/2008 | Case-control | Retrospective |
| Varpuluoma 2019 | Finland, Europe | BP | (1) Carbamazepine (2) Pregabalin (3) Biperiden (4) Levomepromazine (5) Perphenazine (6) Periciazine (7) Haloperidol (8) Melperone (9) Quetiapine (10) Sulpiride (11) Risperidone (12) Hydroxyzine (13) Amitriptyline (14) Doxepin (15) Citalopram (16) Sertraline (17) Escitalopram (18) Mianserin (19) Mirtazapine (20) Venlafaxine (21) Duloxetine |
Mean 76.6 | 1987–2013 | Case-control | Retrospective |
| Grönhagen 2012 | Sweden, Europe | SLE | (1) Antidepressants (N06AX) | Median 65, IQR 48–76 | 01/01/2006–31/12/2009 | Case-control | Retrospective |
| Roberts 2018 | US, North America | SLE | (1) SSRI (2) Tricyclic and other antidepressants |
Range 28–93 | Study I: 1996–2012, study II: 1993–2013 | Cohort | Prospective |
| Schoonen 2010 | UK, Europe | SLE | (1) Carbamazepine (2) Chlorpromazine |
Range 4.5–88.4 | 1987–2001 | Nested case-control | Retrospective |
| Mirza 2021 | US, North America | Alopecia areata | Antidepressants | Range 30–55 | 2002–2012 | Cohort | Prospective |
| Vallerand 2019 | UK, Europe | Alopecia areata | Antidepressants | Range 10–90, median 36.6, IQR 24.0 | 1986–2012 | Cohort | Retrospective |
| Brauchli 2009 | UK, Europe | Psoriasis | (1) SSRI (2) Lithium (3) Atypical antipsychotics (4) Butyrophenones (5) Phenothiazines (6) Other typical antipsychotics (7) Olanzapine |
No figures, assumably adults and elderly | 01/01/1994–31/12/2005 | Case-control | Retrospective |
| Tzeng 2021 | Taiwan, Asia | Psoriasis | (1) Antidepressants (2) SSRI (3) TCAs (4) SNRI (5) Other antidepressants |
20 years and older, mean 48.0 ± 16.7 | 1/2001–12/2010 | Cohort | Retrospective |
| Sparks 2021 | US, North America | Rheumatoid arthritis | Antidepressants | Range 25–55 | Nurses’ Health Study, NHS 1992–2014, NHSII 1993–2015 | Cohort | Prospective |
| Vallerand 2018 | UK, Europe | Rheumatoid arthritis | Antidepressants | Range 10–90, median 36.6, IQR 24.0 | 1986–2012 | Cohort | Retrospective |
| Garbe 2012 | Germany, Europe | Immune thrombocytopenia | (1) Melperone (2) Promethazine |
Outpatients: median 56, range 18–90. Inpatients: median 66, range 29–91 | 10/2000–03/2009 | Case-control | Prospective |
| Rosenberg 2017 | Canada, North America | Interstitial lung disease | SSRI and SNRI together | Range 75–93, cases mean 89.0, control mean 88.7 | Patients active practice members 01/02/2016 | Case-control | Retrospective |
| Nielsen 2015 | Denmark, Europe | Multiple sclerosis | Valproic acid | 18–54 | 1/1/1997–31/12/2011 | Cohort | Retrospective |
| Miller 2001 | US, North America | Silent thyreoiditis | Lithium | Range 24–49, mean 34 ± 6 | 1/1992–7/1996 | Case-control | Retrospective |
| Vallerand 2019 | UK, Europe | Vitiligo | Antidepressants | Range 10–90, median 36.6, IQR 24.0 | 1986–2012 | Cohort | Retrospective |
CC = Collagenous colitis
IQR = Interquartile range
LC = Lymphocytic colitis
MC = Microscopic colitis
NR = Not reported
SD = Standard deviation
Because of the limited number of studies by exposure-outcome pairs, a meta-analysis was possible only for 1) antipsychotics (any type) and bullous pemphigoid, 2) SSRIs and MC (including both histological subtypes CC and LC), 3) SSRIs and psoriasis, and 4) SSRIs and acute pancreatitis (Fig 2). Other single associations with at least 50 patients with the diagnosis and the use of psychiatric medication are visualized in modified harvest plots (Fig 3). The limited number of exposure–outcome pairs also prevented the use of a funnel plot with Egger’s regression to investigate publication bias or any other meta-regression investigation of study-level variables [46]. Among the different methods to control for confounding in eight cohort studies, only one used propensity-score matching [42].
Fig 2. Forest plots for available meta-analyses of the risk of immune-mediated inflammatory diseases by the use of selective serotonin reuptake inhibitors (SSRI) and antipsychotics.
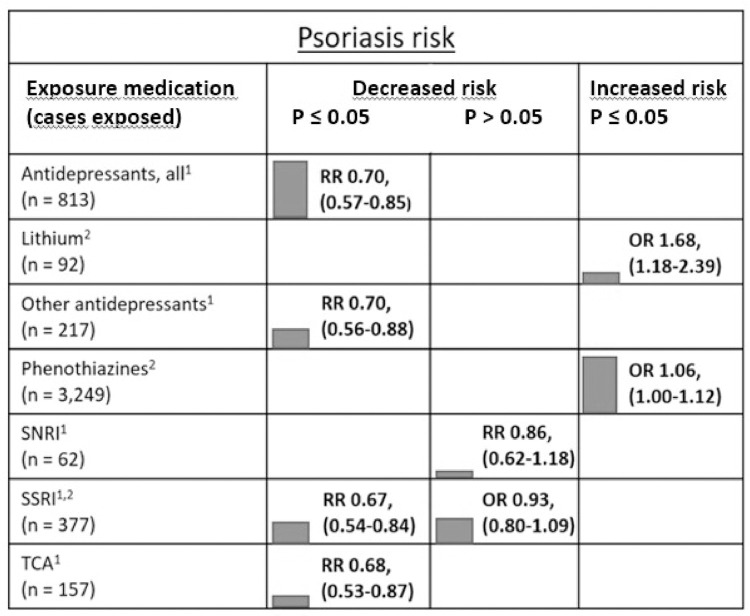
Fig 3. Harvest plot of the associations between the use of psychiatric medication and risk of psoriasis.
1 Tzeng et al. (2021) is a cohort study reporting relative risks (RRs). 2 Brauchli et al. (2009) is a case-control study with odds ratios (ORs) of the association shown. The harvest plot shows the risk of psoriasis by the type of psychiatric medication. Only associations with at least 50 psoriasis patients with the drug of interest are shown. The height of the column indicates the size of the study, and the green color represents a study with a low risk of bias according to the Newcastle–Ottawa Scale.
Microscopic colitis and its subtypes
Seven case-control studies investigated the odds of MC (or CC and LC) by exposure to SSRIs (any) or sertraline [16]. The overall association between SSRIs and MC had a very high heterogeneity with an OR of 2.60 (95% CI 1.06–6.39, I2 97.45%, 6 studies, at least 1,322 exposed cases). The studies by Fernández-Bañares and Pascua had the highest risk of bias (NOS scores 5 and 6, respectively) [17, 19]. In a sensitivity analysis of four studies with the lowest risk of bias, heterogeneity remained high while the meta-OR was only marginally lower, 2.26 (95% CI 1.37–3.73, I2 92.01%, 4 studies) than in the analysis of all SSRI studies. All studies except Weimers et al. reported the numbers of individuals in the analysis [21].
A meta-analysis by histologic subtype of MC showed a statistically significant association between SSRIs and LC (meta-OR 2.88, 95% CI 2.60–3.18, I2 00.00%, 3 studies) but not between SSRIs and CC (meta-OR 2.32, 95% CI 0.55–9.81, I2 95.53%, 3 studies). Based on GRADE, the certainty of the evidence was low, with considerably high heterogeneity.
Psoriasis
Psoriasis was investigated by Brauchli et al. in a case-control study based on the nationwide GPRD patient register in the UK and by Tzeng et al. in a cohort study based on the nationwide health insurance registry in Taiwan [37, 38]. Both studies were considered to have a low risk of bias. The Taiwanese study found a lower psoriasis risk among the users of any kind of antidepressants analyzed together, and also when analyzed by the main antidepressant classes (SNRI, SSRI, TCA) (Fig 3). The incidence of psoriasis was 2.80 (95% CI 2.61–3.00) per 1,000 person-years among users of any antidepressants and 3.82 (95% CI 3.15–4.59) per 1,000 person-years among non-users. A meta-analysis was possible for SSRI use and psoriasis risk, and the difference was not statistically significant (meta-OR 0.80, 95% CI 0.58–1.10, I2 82.39%, 2 studies). In individual studies, only lithium and phenothiazines were associated with a slightly increased psoriasis risk (Fig 3). All associations for psoriasis were considered to be of low certainty based on GRADE.
Bullous pemphigoid
Bullous pemphigoid as outcome was investigated in four case-control studies, all representing studies with a relatively higher risk of bias (NOS score range 5–6) [28–31]. Meta-analysis was possible for three studies with antipsychotics as the exposure (meta-OR 1.79, 95% CI 1.17–2.73, I2 0%). However, these studies were small-scale, together contributing only 48 patients for the meta-analysis. The only reasonably powered study (Varpuluoma et al. 2019) was not included in the meta-analysis, as it reported individual medications instead of broader classes [31]. In the study by Varpuluoma, controls were not from a population-based sample; rather, they were patients with basal cell carcinoma. They reported the highest risk of bullous pemphigoid in relation to hydroxyzine (aOR 17.3, 95% CI 11.5–26.0), periciazine (aOR 7.55, 95% CI 2.91–10.6), melperone (aOR 4.0, 95% CI 2.46–6.49), and risperidone (aOR 3.04, 95% CI 2.38–3.89).
Pancreatitis
AP was the outcome in six case-control studies, all representing a low risk of bias according to NOS (score 9 for all) [22–27]. For reasonably similar medication–outcome pairs, a meta-analysis was possible for only two studies on the current use of SSRIs and acute pancreatitis (meta-OR 1.13, 95% CI 1.01–1.26, I2 00.00%, 649 SSRI-exposed patients) with adjustment for major confounding factors, including alcohol-related diseases. Current SNRI use was associated with a marginally increased pancreatitis risk after the adjustment of relevant confounders (aOR 1.2, 95% CI 1.0–1.4) [25]. The estimates for both SSRIs and SNRIs and the development of pancreatitis represent low-certainty evidence.
Gasse et al. were the only ones to investigate the potency of conventional antipsychotics in blocking dopamine receptors with pancreatitis as the outcome [23]. The current use of preparations with high potency in blocking dopamine receptors was not associated with a statistically significant pancreatitis risk (aOR 1.2, 95% CI 0.7–2.0), whereas the use of intermediate-potency preparations (aOR 1.5, 95% CI 1.0–2.2) and low-potency preparations (aOR 2.8, 95% CI 2.0–3.8) were associated nearly 1.5 and three-fold odds, respectively. Neither the current use of atypical antipsychotics (OR 0.6, 95% CI 0.3–1.1) nor the previous use of clozapine or olanzapine (Boden 2012) had a statistically significant association with pancreatitis.
The current use of valproic acid increased the risk of pancreatitis in an analysis adjusted for, among other factors, alcohol-related diseases and gallstone disease (aOR 1.9, 95% CI 1.1–7.0). However, this estimate was based on only 16 valproic acid-exposed pancreatitis patients and thus constitutes very low-certainty evidence.
Other diseases
Roberts et al. was the only cohort study on the risk of systemic lupus erythematosus (SLE), with a high risk of bias (NOS 5) [33]. They found similarly increased risks of SLE with the use of SSRIs (HR 2.63, 95% CI 1.66–4.14) and other antidepressants (HR 2.85, 95% CI 1.53–5.31) in an analysis adjusted for BMI, smoking, and oral contraceptive and postmenopausal hormone use. Schoonen et al. investigated both SLE and SCLE in a case-control study with a low risk of bias (NOS 9) [34]. Carbamazepine use increased the odds (aOR 1.88, 95% CI 1.09–3.22). Moreover, in a subgroup analysis on the number of prescriptions, the risk increase was related to longer use of carbamazepine (i.e., those with seven or more prescriptions) (aOR 3.04, 95% CI 1.34–6.85). The risk of SCLE was studied by Grönhagen et al., but the analysis involved only eight cases exposed to any antidepressants (OR 1.2, 95% CI 0.5–2.6) [32].
Two cohort studies assessed the risks of alopecia areata after exposure to any antidepressants with contrasting results. Vallerand et al. found a protective effect (HR 0.57, 95% CI 0.53–0.62), and Mirza et al. a modestly increased risk (RR 1.67, 95% CI 1.15–2.44) with the use of antidepressants [35, 36]. Similarly, Vallerand et al. also found a lower risk of rheumatoid arthritis (HR 0.74, 95% CI 0.71–0.76) in antidepressant users, while another study by Sparks et al. found a small increase in risk (HR 0.74, 95% CI 1.16–1.63) [36, 39].
Single studies assessed the risk of interstitial lung disease after SSRI or SNRI use (OR 8.79, 95% CI 2.40–32.2, Rosenberg 2017), silent thyroiditis after lithium use (OR 4.7, 95% CI 1.3–17.1), multiple sclerosis after valproic acid use (HR 1.30, 95% CI 0.44–3.80, Nilsen 2015), and vitiligo after antidepressant use (HR 0.58, 95% CI 0.54–0.63, Vallerand 2019) [42–45].
Discussion
We systematically searched an extensive list of ADs and IMIDs and evaluated whether the use of psychiatric medications can be considered a risk factor for their development. Published associations between any psychiatric medications and immune diseases were considered to represent low to very low certainty of evidence at best. This means that the available literature cannot reliably confirm or refute whether associations really exist, and the true effect sizes might be markedly different from the available estimates.
In a meta-analysis, we found that SSRIs are associated with a 2–3-fold increase in the odds of developing MC. Furthermore, in a subgroup analysis by histological type, this risk appeared to be related to the development of LC rather than CC. However, considering the large imprecision and overall uncertainty, these analyses represent low-certainty evidence, and the estimate is, therefore, likely to change with further research. Serotonin is an important neurotransmitter in the gut, and animal models have shown SSRIs to increase the release of serotonin in the gut (Minami 2003), and that serotonin is involved in the inflammatory reaction of experimental colitis (Ghia 2009). Although common adverse effects of SSRIs include stomach complaints such as diarrhea, it remains uncertain whether SSRIs could trigger chronic inflammation manifesting in diarrhea. Hundreds of medications have been associated with chronic diarrhea, mainly in case reports and uncontrolled case series [47]. In controlled observational studies, however, proton-pump inhibitors (especially lansoprazole) have been linked with moderate certainty of the development of a chronic inflammatory diarrhea disease, namely MC and its subtype CC [48].
There was a non-statistically significant suggestion in two case-control studies that SSRI use would associate with a lower risk of psoriasis. All of the available outcome diseases are rare, and even in hypothetical causal cases, the absolute number of new diseases would be low. For example, using the incidence figures by Tzeng et al. 2021 to illustrate the scale of these risk magnitudes, and assuming the incidences were true, would yield a number needed to treat of 980 (95% CI 656–1936) to prevent the development of one psoriasis case with any antidepressant treatment [38]. SSRIs have been shown to have some anti-inflammatory effects, and a case-control study from Sweden found that patients with psoriasis who used SSRIs had less often need for systemic treatments [49]. Although there are findings of SSRIs having anti-inflammatory effects, how this would translate into changes in both psoriasis severity and incidence requires more epidemiological research preferably with a cohort study design.
BP is the most common bullous autoimmune disease affecting the subepidermal layer of the skin and mainly affects elderly people. Our meta-analysis of three studies found a 1.8-fold increase in the odds of BP after exposure to antipsychotics, representing low certainty of evidence due to these studies’ relatively high risk of bias. Such a modest effect size could be explained by confounding and biases, and larger prospective studies would be needed before antipsychotics could be considered risk factors for BP. The case-control study by Varpuluoma et al. (2019) reported only statistically significant associations, therefore risking reporting bias [31]. They reported 21 psychiatric medications (and additionally seven benzodiazepines, which we did not include in this review) that increase the risk of BP. We consider it biologically implausible that so many psychiatric medications spanning different classes could cause BP. These associations, therefore, most likely reflect systematic bias due to the selection of basal cell carcinoma patients as controls instead of a random sample from the general population. Such distortion would be expected to occur with any exposure associated with a lower risk of basal cell carcinoma but not related to BP, thus appearing in a case-control design as an increase in the odds of BP [50].
Limitations
The limitations of this study mostly reflect limitations in the available literature and especially how results have been reported. Although some individual studies reported the number of prescriptions, the literature was too heterogenous and scarce for a meta-analysis on dose dependence.
Patients with immune diseases generally have higher than expected rates of psychiatric disorders, that are often treated with medications [51]. In studies on the associations between psychiatric medications and immune diseases, protopathic bias would occur if the exposure medication was used to manage the symptoms due to the yet unidentified AD or IMID. Because of the highly somatic manifestation of identified target diseases, we consider in the case of psychiatric medications, the possibility of protopathic bias to be less important than other major sources of bias, although we had no lag-time analyses available to support this assumption. In pharmacoepidemiological studies, confounding by indication is generally an important source of bias. However, it is probably less of an issue with our research question, as psychiatric medications are not used to manage the target diseases.
Assessing drug exposure independently of outcome data using nationwide registers eliminates recall bias and diminishes the impact of selection bias. Other approaches would introduce more uncertainty. For example, Pascua et al. (2010) had only a modest inter-rater agreement (kappa statistic 0.50) when patient-reported medications were compared to those found in electronic databases [19].
Conclusions
Currently, in the case of psychiatric medications, the entire literature of comparative studies represents, at best, low certainty of evidence linking them to any AD or IMID. We have reviewed important sources of bias in the field for future studies to be more reliable. Identified associations, particularly that of SSRIs and the risk of MC, should be investigated in prospective cohort studies.
Supporting information
(RTF)
(RTF)
(RTF)
(RTF)
(RTF)
Acknowledgments
We thank the personnel of the Tampere University library for helping to find some hard-to-find scientific articles for the study.
Abbreviations
- AD
Autoimmune disease
- aOR
Adjusted odds ratio
- AP
Acute pancreatitis
- CC
Collagenous colitis
- CI
Confidence interval
- DDD
Defined daily dose
- DIL
Drug-induced lupus
- GRADE
Grading of Recommendations, Assessment, Development, and Evaluations
- HR
Hazard ratio
- IMID
Immune-mediated inflammatory disease
- IQR
Interquartile range
- LC
Lymphocytic colitis
- MC
Microscopic colitis
- NOS
Newcastle–Ottawa Scale
- OR
Odds ratio
- PRISMA
Preferred reporting items for systematic review and meta-analysis protocols
- PROSPERO
The International Prospective Register of Systematic ReviewsRR Risk ratio
- SD
Standard deviation
- SLE
Systemic lupus erythematosus
- SNRI
Serotonin and norepinephrine reuptake inhibitor
- SSRI
Selective serotonin reuptake inhibitor
- TCA
Tricyclic antidepressant
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Rosenblum MD, Remedios KA, Abbas AK. Mechanisms of human autoimmunity. J Clin Invest. 2015;125(6):2228–33. doi: 10.1172/JCI78088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lerner A, Patricia J, Torsten M. The World Incidence and Prevalence of Autoimmune Diseases is Increasing. Int J Celiac Dis. 2015;3.4:151–155. doi: 10.12691/ijcd-3-4-8 [DOI] [Google Scholar]
- 3.Miehlke S et al. European guidelines on microscopic colitis: United European Gastroenterology and European Microscopic Colitis Group statements and recommendations. UEG Journal. 2021;22;9(1):13–37. doi: 10.1177/2050640620951905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macer BJ, Prady SL, Mikocka-Walus A. Antidepressants in Inflammatory Bowel Disease: A Systematic Review. Inflamm Bowel Dis. 2017;23(4):534–550. doi: 10.1097/MIB.0000000000001059 [DOI] [PubMed] [Google Scholar]
- 5.Borchers AT, Keen CL, Gershwin ME. Drug-induced lupus. Ann N Y Acad Sci. 2007;1108:166–182. doi: 10.1196/annals.1422.019 [DOI] [PubMed] [Google Scholar]
- 6.Vayne C, Guéry EA, Rollin J, Baglo T, Petermann R, Gruel Y. Pathophysiology and Diagnosis of Drug-Induced Immune Thrombocytopenia. J Clin Med. 2020;9(7):2212. doi: 10.3390/jcm9072212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033 [DOI] [PubMed] [Google Scholar]
- 8.Rengasamy M, Marsland A, Spada M, Hsiung K, Kovats T, Price RB. A chicken and egg scenario in psychoneuroimmunology: Bidirectional mechanisms linking cytokines and depression. J Affect Disord Rep. 2021;6:100177. doi: 10.1016/j.jadr.2021.100177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. doi: 10.1371/journal.pmed.1003583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells G SB, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. 3rd Symposium on Systematic Reviews: Beyond the Basics. 2000. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed September 27, 2021. [Google Scholar]
- 11.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;15;21(11):1539–58. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook. Accessed September 27, 2021. [Google Scholar]
- 14.Ogilvie D, Fayter D, Petticrew M, et al. The harvest plot: a method for synthesising evidence about the differential effects of interventions. BMC Med Res Methodol. 2008;8:8. doi: 10.1186/1471-2288-8-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonderup OK, Fenger-Grøn M, Wigh T, Pedersen L, Nielsen GL. Drug exposure and risk of microscopic colitis: a nationwide Danish case-control study with 5751 cases. Inflamm Bowel Dis. 2014;20(10):1702–1707. doi: 10.1097/MIB.0000000000000143 [DOI] [PubMed] [Google Scholar]
- 16.Fernández-Bañares F, de Sousa MR, Salas A, et al. Epidemiological risk factors in microscopic colitis: a prospective case-control study. Inflamm Bowel Dis. 2013;19(2):411–417. doi: 10.1002/ibd.23009 [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Bañares F, Esteve M, Espinós JC, et al. Drug consumption and the risk of microscopic colitis. Am J Gastroenterol. 2007;102(2):324–330. doi: 10.1111/j.1572-0241.2006.00902.x [DOI] [PubMed] [Google Scholar]
- 18.Masclee GM, Coloma PM, Kuipers EJ, Sturkenboom MC. Increased risk of microscopic colitis with use of proton pump inhibitors and non-steroidal anti-inflammatory drugs. Am J Gastroenterol. 2015;110(5):749–759. doi: 10.1038/ajg.2015.119 [DOI] [PubMed] [Google Scholar]
- 19.Pascua MF, Kedia P, Weiner MG, Holmes J, Ellenberg J, Lewis JD. Microscopic colitis and Medication Use. Clin Med Insights Gastroenterol. 2010;2010(3):11–19. doi: 10.4137/cgast.s4469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhaegh BP, de Vries F, Masclee AA, et al. High risk of drug-induced microscopic colitis with concomitant use of NSAIDs and proton pump inhibitors. Aliment Pharmacol Ther. 2016;43(9):1004–1013. doi: 10.1111/apt.13583 [DOI] [PubMed] [Google Scholar]
- 21.Weimers P, Vedel Ankersen D, Lophaven SN, et al. Microscopic Colitis in Denmark: Regional Variations in Risk Factors and Frequency of Endoscopic Procedures. J Crohns Colitis. 2021;16(1):49–56. doi: 10.1093/ecco-jcc/jjab119 [DOI] [PubMed] [Google Scholar]
- 22.Bodén R, Bexelius TS, Mattsson F, Lagergren J, Lindblad M, Ljung R. Antidopaminergic drugs and acute pancreatitis: a population-based study. BMJ Open. 2012;2(3):e000914. Published 2012 May 11. doi: 10.1136/bmjopen-2012-000914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gasse C, Jacobsen J, Pedersen L, et al. Risk of hospitalization for acute pancreatitis associated with conventional and atypical antipsychotics: a population-based case-control study. Pharmacotherapy. 2008;28(1):27–34. doi: 10.1592/phco.28.1.27 [DOI] [PubMed] [Google Scholar]
- 24.Lin HF, Liao KF, Chang CM, Lin CL, Lai SW. Association of use of selective serotonin reuptake inhibitors with risk of acute pancreatitis: a case-control study in Taiwan. Eur J Clin Pharmacol. 2017;73(12):1615–1621. doi: 10.1007/s00228-017-2328-x [DOI] [PubMed] [Google Scholar]
- 25.Ljung R, Rück C, Mattsson F, Bexelius TS, Lagergren J, Lindblad M. Selective serotonin reuptake inhibitors and the risk of acute pancreatitis: a Swedish population-based case-control study. J Clin Psychopharmacol. 2012;32(3):336–340. doi: 10.1097/JCP.0b013e318253d71a [DOI] [PubMed] [Google Scholar]
- 26.Nørgaard M, Jacobsen J, Ratanajamit C, et al. Valproic acid and risk of acute pancreatitis: a population-based case-control study. Am J Ther. 2006;13(2):113–117. doi: 10.1097/00045391-200603000-00005 [DOI] [PubMed] [Google Scholar]
- 27.Nørgaard M, Jacobsen J, Gasse C, Pedersen L, Mortensen PB, Sørensen HT. Selective serotonin reuptake inhibitors and risk of acute pancreatitis: a population-based case-control study. J Clin Psychopharmacol. 2007;27(3):259–262. doi: 10.1097/JCP.0b013e318058a9c3 [DOI] [PubMed] [Google Scholar]
- 28.Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. A case-control study. Arch Dermatol. 1996;132(3):272–276. doi: 10.1001/archderm.1996.03890270044006 [DOI] [PubMed] [Google Scholar]
- 29.Bastuji-Garin S, Joly P, Lemordant P, et al. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol. 2011;131(3):637–643. doi: 10.1038/jid.2010.301 [DOI] [PubMed] [Google Scholar]
- 30.Lloyd-Lavery A, Chi CC, Wojnarowska F, Taghipour K. The associations between bullous pemphigoid and drug use: a UK case-control study. JAMA Dermatol. 2013;149(1):58–62. doi: 10.1001/2013.jamadermatol.376 [DOI] [PubMed] [Google Scholar]
- 31.Varpuluoma O, Jokelainen J, Försti AK, et al. Drugs used for neurologic and psychiatric conditions increase the risk for bullous pemphigoid: A case-control study. J Am Acad Dermatol. 2019;81(1):250–253. doi: 10.1016/j.jaad.2019.02.017 [DOI] [PubMed] [Google Scholar]
- 32.Grönhagen CM, Fored CM, Linder M, Granath F, Nyberg F. Subacute cutaneous lupus erythematosus and its association with drugs: a population-based matched case-control study of 234 patients in Sweden. Br J Dermatol. 2012;167(2):296–305. doi: 10.1111/j.1365-2133.2012.10969.x [DOI] [PubMed] [Google Scholar]
- 33.Roberts AL, Kubzansky LD, Malspeis S, Feldman CH, Costenbader KH. Association of Depression With Risk of Incident Systemic Lupus Erythematosus in Women Assessed Across 2 Decades. JAMA Psychiatry. 2018;75(12):1225–1233. doi: 10.1001/jamapsychiatry.2018.2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoonen WM, Thomas SL, Somers EC, et al. Do selected drugs increase the risk of lupus? A matched case-control study. Br J Clin Pharmacol. 2010;70(4):588–596. doi: 10.1111/j.1365-2125.2010.03733.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirza MA, Jung SJ, Sun W, Qureshi AA, Cho E. Association of depression and alopecia areata in women: A prospective study. J Dermatol. 2021;48(8):1296–1298. doi: 10.1111/1346-8138.15931 [DOI] [PubMed] [Google Scholar]
- 36.Vallerand IA, Lewinson RT, Parsons LM, et al. Assessment of a Bidirectional Association Between Major Depressive Disorder and Alopecia Areata. JAMA Dermatol. 2019;155(4):475–479. doi: 10.1001/jamadermatol.2018.4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brauchli YB, Jick SS, Curtin F, Meier CR. Lithium, antipsychotics, and risk of psoriasis. J Clin Psychopharmacol. 2009;29(2):134–140. doi: 10.1097/JCP.0b013e31819a4b7c [DOI] [PubMed] [Google Scholar]
- 38.Tzeng YM, Li IH, Kao HH, et al. Protective Effects of Anti-depressants against the Subsequent Development of Psoriasis in Patients with Major Depressive Disorder: a Cohort Study. J Affect Disord. 2021;281:590–596. doi: 10.1016/j.jad.2020.11.110 [DOI] [PubMed] [Google Scholar]
- 39.Sparks JA, Malspeis S, Hahn J, et al. Depression and Subsequent Risk for Incident Rheumatoid Arthritis Among Women. Arthritis Care Res (Hoboken). 2021;73(1):78–89. doi: 10.1002/acr.24441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vallerand IA, Lewinson RT, Frolkis AD, et al. Depression as a risk factor for the development of rheumatoid arthritis: a population-based cohort study. RMD Open. 2018;4(2):e000670. Published 2018 Jul 11. doi: 10.1136/rmdopen-2018-000670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garbe E, Andersohn F, Bronder E, et al. Drug-induced immune thrombocytopaenia: results from the Berlin Case-Control Surveillance Study. Eur J Clin Pharmacol. 2012;68(5):821–832. doi: 10.1007/s00228-011-1184-3 [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg T, Lattimer R, Montgomery P, Wiens C, Levy L. The relationship of SSRI and SNRI usage with interstitial lung disease and bronchiectasis in an elderly population: a case-control study. Clin Interv Aging. 2017;12:1977–1984. Published 2017 Nov 21. doi: 10.2147/CIA.S144263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen NM, Svanström H, Stenager E, et al. The use of valproic acid and multiple sclerosis. Pharmacoepidemiol Drug Saf. 2015;24(3):262–268. doi: 10.1002/pds.3692 [DOI] [PubMed] [Google Scholar]
- 44.Miller KK, Daniels GH. Association between lithium use and thyrotoxicosis caused by silent thyroiditis. Clin Endocrinol (Oxf). 2001;55(4):501–508. doi: 10.1046/j.1365-2265.2001.01381.x [DOI] [PubMed] [Google Scholar]
- 45.Vallerand IA, Lewinson RT, Parsons LM, et al. Vitiligo and major depressive disorder: A bidirectional population-based cohort study. J Am Acad Dermatol. 2019;80(5):1371–1379. doi: 10.1016/j.jaad.2018.11.047 [DOI] [PubMed] [Google Scholar]
- 46.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chassany O, Michaux A, Bergmann JF. Drug-induced diarrhoea. Drug Saf. 2000;22(1):53–72. doi: 10.2165/00002018-200022010-00005 [DOI] [PubMed] [Google Scholar]
- 48.Nevalainen A, Nevalainen O. Autoimmune and immune-mediated inflammatory diseases after exposure to acid-suppressive medication: a systematic review and meta-analysis. Int J Risk Saf Med. 2022;10.3233/JRS-220012. doi: 10.3233/JRS-220012 [DOI] [PubMed] [Google Scholar]
- 49.Thorslund K, Svensson T, Nordlind K, Ekbom A, Fored CM. Use of serotonin reuptake inhibitors in patients with psoriasis is associated with a decreased need for systemic psoriasis treatment: a population-based cohort study. J Intern Med. 2013;274(3):281–287. doi: 10.1111/joim.12093 [DOI] [PubMed] [Google Scholar]
- 50.Grimes DA, Schulz KF. Compared to what? Finding controls for case-control studies. Lancet. 2005;365(9468):1429–1433. doi: 10.1016/S0140-6736(05)66379-9 [DOI] [PubMed] [Google Scholar]
- 51.Marrie RA, Walld R, Bolton JM, et al. Increased incidence of psychiatric disorders in immune-mediated inflammatory disease. J Psychosom Res. 2017;101:17–23. doi: 10.1016/j.jpsychores.2017.07.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(RTF)
(RTF)
(RTF)
(RTF)
(RTF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.