Abstract
Background.
Cocaine affects not only the central nervous system, but also systemic immunity. The role of cocaine in gut mucosal integrity is not fully understood.
Methods.
Here we evaluated the effect of cocaine use on gut endothelial permeability and system inflammation in rats that self-administered cocaine or saline and in humans using immunohistochemistry, qPCR, ELISA, and Transepithelial/transendothelial electrical resistance (TEER).
Results.
Cocaine administration maintained intact and undisturbed intestinal mucosal structures, increased tight junction claudin 1 and 2 mRNA expression, and decreased plasma TNF-α levels, compared to the control group, at the end of study in rats. Further, cocaine treatment decreased gut endothelial permeability in a dose-dependent manner in human epithelial Caco-2 cells in vitro. Consistently, chronic cocaine users exhibited decreased plasma levels of TNF-α compared with non-drug users in vivo. However, plasma IL-6 levels were similar between cocaine use and control groups both in humans and rats in vivo.
Conclusions.
Our results from both human and rat studies in vivo and in vitro suggest that cocaine use may exert a protective effect on the integrity of gut mucosa and suppresses plasma TNF-α levels. This study may provide information on some beneficial effects of cocaine use on gut endothelial cells integrity and systemic inflammation.
Keywords: cocaine, gut permeability, systemic inflammation, tight junction
Graphical Abstract

Cocaine exerts a protective effect on the integrity of gut mucosa and suppresses circulating TNF-α. Determination of responses to the active cocaine lever during and after cocaine self-administration in rats. Gut mucosa HE staining, tight junction expression, and blood levels of TNF-α and IL-6 were evaluated for cocaine-reduced gut permeability and circulating inflammation presumably by preventing microbial translocation via gut mucosal barrier. In human subjects, blood levels of TNF-α and IL-6 were evaluated in individuals with cocaine use disorder and non-drug controls.
Introduction
While addictive drugs have been reported to exhibit both proinflammatory and anti-inflammatory effects [1–3], systemic inflammation produced by drugs of abuse [4, 5] may stem from monocyte/macrophage activation and oxidative stress [6, 7]. Addictive drugs may activate monocytes/macrophages directly or indirectly, induce a compromised mucosa, and allow microbial translocation (e.g., lipopolysaccharide (LPS)) to stimulate monocytes/macrophages [8–11]. The effects of drugs of abuse on the intestine can be affected by the route, dose, and duration of use. Previous studies have shown that cocaine intraperitoneal administration directly activates macrophages and microglia to induce neuroinflammation and neurotoxicity through the endoplasmic reticulum (ER) stress-TLR2 axis [12]. Activated monocytes, macrophages, and T cells can infiltrate the central nervous system (CNS) and induce a cascade of inflammation (e.g., TNF-α, IFN-γ), which results in the activation of microglia, astrocytes, and perivascular macrophages [13]. In turn, the activation of these cells produces chemokines (e.g., MCP-1, MIP-1) and cytokines (e.g., IL-6, TNF-α), which further accentuate inflammation and recruit peripheral lymphocytes to the CNS, resulting in neuronal and glial cell injury and death [14].
The central and peripheral proinflammatory responses to cocaine are not fully understood and results are not always consistent [15, 16]. One possibility is that cocaine has been reported to increase permeability of the blood–brain barrier (BBB) or gut mucosal barrier [8, 17]. The intestinal mucosal barrier is one of the most important immune defense barriers, separating the body from exogenous substances and microbes. Cocaine addiction has been shown to exacerbate gut permeability and lead to a “leaky” gut [8]. Tight junctions in gut epithelial cells play a pivotal role in the mucosal barrier function. Tight junction proteins include transmembrane proteins occludin and claudins, which seal the paracellular pathways in the epithelium, as well as zona occludens, which exert scaffolding functions [18]. Disrupting gut tight junctions can result in bacterial product translocation and consequently immune activation and inflammation. Moreover, cocaine induces changes in the gut microbiome and cocaine use disorder are reported to associate with gut-barrier dysfunction and dysbiosis [19, 20]. Furthermore, Toll-like receptor (TLR) signaling, which is essential for innate immunity, is tightly modulated to maintain homeostasis in the gut epithelium, given the large number of commensal bacteria present in the intestinal lumen. Particularly, TLR2 and TLR4 are involved in the physiological and pathological processes related to intestinal permeability [21]. In the present study, we investigated whether cocaine abuse influences gut permeability and systemic inflammation using a rat model of cocaine addiction (i.e., intravenous cocaine self-administration) in vivo and human systems in vitro and in vivo.
Methods and Materials
The study design and workflow are shown in the graphical abstract.
Rat studies
Cocaine self-administration (SA). We used a previously published rodent model of cocaine self-administration and withdrawal [22]. All rat studies were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at the Medical University of South Carolina (MUSC). Adult (200–250 g) male Sprague–Dawley rats (Charles River Laboratories, South Carolina, USA) were housed on a reverse 12-h/12-h light/dark cycle. Food and water were available ad libitum. The rats were trained to self-administer cocaine using surgical and behavioral techniques, as described previously [23]. Briefly, the animals were anesthetized with ketamine/xylazine (100 mg/kg and 7 mg/kg) given intramuscularly (IM) and implanted with jugular catheters. After recovery, the rats underwent a single 2 h food SA session to learn the association between the active lever and reward delivery. The subsequent day, the rats began daily 2 h SA sessions, during which presses on an active lever yielded cocaine delivery (0.2 mg intravenous (IV) infusion per active lever press followed by a 20 s timeout). Each active lever press and cocaine infusion was paired with tone and light cues for 5 s. The animals were maintained on 25 g chow per day during SA. After 10 days of SA, they were placed in the same operant chambers, but lever presses had no consequence. Following 10 days of extinction (EX) training, rats in the EX-group were sacrificed 24 h after the last session. Rats undergoing reinstatement were placed in the operant chamber for the final time and the tone-light pairing was restored to the active lever, but active lever presses yielded no cocaine delivery. Reinstated rats were sacrificed after spending 15 min in the operant chamber. Control yoked rats underwent the same surgical manipulations and behavioral training as SA rats, but received passive infusions of saline paired with tone and light cues. When no differences between EX and reinstatement groups were detected, these groups were combined.
Gut hematoxylin and eosin (H&E) staining. The large intestine was excised and intestine segments of the transverse tissues (1 cm) were fixed in 10% buffered formalin, embedded in paraffin, and stained with H&E. The histological severity was examined by the structure of the intestinal wall.
Quantitative real-time PCR (qRT-PCR) analysis of tight junction mRNA expression. Total RNAs were extracted from rats’ gut endothelial cells using Trizol (Invitrogen, California, USA) in accordance with the manufacturer’s instructions. cDNAs were synthesized from 2 μg of total RNA using a SuperScript™ III First-Strand Synthesis System (Thermo Fisher Scientific, Massachusetts, USA). qRT-PCR was performed with PCR mix (PerfeCTa SYBR Green SuperMix, QuantaBio, Maryland, USA) in a 20 μL reaction mix including 1.5 μL of 5× diluted reverse transcription product, 1.5 μL cDNA, 1 μL Forward and 1 μL Reverse Primer, 10 μL PCR mix, and 6.5 μL double distilled water. qRT-PCR was performed on a C1000™ Touch Thermal Cycler System (Bio-rad, California, USA). Fluorescent signal was captured at each extension step. The data were normalized to expression of a housekeeping gene GAPDH. Each sample was analyzed in triplicate. The relative expression was determined using the 2−ΔΔCT method, where the normalized CT (ΔCT) was calculated by subtracting the CT of a housekeeping control gene. The primer sequences are summarized in Table 1.
Enzyme-linked immunosorbent assay (ELISA) for detection of TNF-α and IL-6. We examined plasma levels of TNF-α and IL-6 in rats by ELISA in accordance with the manufacturer’s instructions (R&D SYSTEMS, Minnesota, USA).
Table 1.
Primer sequences of Occludin-1, Claudin-1, Claudin-2, and GAPDH
| Gene | Sequence |
|---|---|
| Occludin-1 | F: 5’- gggaatgtccagaacgagaaga-3’ R: 5’-cgtggcaatgaacaccatga-3’ |
| Claudin-1 | F:5’-tcccaagccaacaccttctagt-3’ R:5’-gtattcgctccaggaggatctct-3’ |
| Claudin-2 | F:5’-tctgtggtgggcatgagatg −3’ R:5’-ctccacccactacagccactct-3’ |
| GAPDH | F:5’-cctggagaaacctgccaagtat-3’ R:5’-agcccaggatgccctttagt-3’ |
Human subjects
Ethics and human subject information. The human study was approved by the Institutional Review Board (IRB) for Human Research at the Medical University of South Carolina (PRO00062384). All participants were adults over the age of 21 years and provided written consent in a clinical room. Individuals with cocaine use disorder (n = 9) were recruited from the Department of Psychiatry and Behavioral Sciences at MUSC. Healthy individuals (n = 20) were recruited from MUSC campus. Clinical information of the individuals with cocaine use disorder is shown in supplemental material.
Drug and psychiatric disorder diagnostic/descriptive assessment using Timeline Follow-Back (TLFB). For individuals with cocaine use disorder, appropriate modules of the Mini-International Neuropsychiatric Interview (M.I.N.I.) were used to assess exclusionary psychiatric diagnoses, as described in our previous study [24, 25]. Substance use in the three months prior to testing was assessed using TLFB [26]. This is a calendar-based instrument used with specific probes to ascertain detailed information about amounts of substance use. Drug screens were performed using the onTrak test cup, an in vitro diagnostic test for the qualitative detection of drug or drug metabolite in the urine.
ELISA for detection of TNF-α and IL-6. We examined plasma levels of TNF-α and IL-6 in humans by ELISA in accordance with the manufacturer’s instructions (R&D SYSTEMS).
Gut permeability assay in vitro. A human epithelial colorectal cancer cell line, Caco-2 (ATCC, Manassas, VA), was used in vitro to test cocaine-mediated permeability, as described in our previous study [27]. Briefly, Caco-2 cells were grown in 24-well plates and seeded at 20,000 cells per insert on 6.5 mm diameter and 0.4 mm pore PET membrane insert (Corning, YN). The medium was changed every 3 days for a total of 21 days. Next, Caco-2 cells were treated with media alone (control) or cocaine. To test transepithelial/transendothelial electrical resistance (TEER), confluent monolayers were washed three times with prewarmed PBS, left for 30 min at 37°C to equilibrate, and then measured using the Millicell-Electrical Resistance System (ERS, Millipore, Billerica, MA, USA).
Statistical analysis
Data from repeated experiments were shown as median. Group medians were compared using unpaired Student’s t-test (parametric) or Mann-Whitney test (non-parametric). One-way ANOVA was used for comparisons using data sets with more than two groups (GraphPad Prism 7.0, San Diego, USA). P values of < 0.05 were considered statistically significant.
Experimental Results
Rat studies: operant responding during cocaine self-administration
We examined the response of rats during 10 days of SA and during the subsequent 10 days of EX training (Figure 1). Based on the results presented in Figure 1, the response of rats to cocaine exhibited a sharp decrease after food training in response to cocaine availability and gradually increased over 10 days of SA. Lever pressing gradually decreased when cues and cocaine were withheld during 10 days of EX, but increased during 15 minutes of reinstatement using cues. In subsequent analyses, cocaine-trained groups (extinction and reinstatement) were combined when no differences were observed between them. The response to cocaine uses in the reinstatement group was maintained at a high level following 10 days of EX training.
Figure 1.

Study design in rats. Determination of responses to the active lever during and after cocaine self-administration in rats. A total of 14 rats were used, including four rats in the control group, five rats in the EX, and five rats in the reinstatement group.
Cocaine administration increases expression of tight junction genes claudin-1 and claudin-2 in rat gut endothelial cells
To evaluate the effect of cocaine on gut permeability, we analyzed tight junction gene claudin-1 and claudin-2 mRNA expression in the control group as well as in cocaine-treated groups. Notably, cocaine treatment resulted in increased claudin-1 expression (Figure 2A), but no difference was observed between cocaine EX and reinstatement groups (Supplemental Figure 1A). Consistently, cocaine treatment significantly increased claudin-2 expression (Figure 2B), but no difference between cocaine EX and reinstatement groups was observed (Supplemental Figure 1B). The median relative claudin-2 mRNA expression was 1.17 ± 0.32 for the control group, 1.78 ± 0.15 for the cocaine EX group, and 1.64 ± 0.12 the cocaine reinstatement group. These results suggest that cocaine administration may decrease gut permeability.
Figure 2.


Evaluation of tight junction gene expression by qRT-PCR in rats. Expression levels of tight junction genes were examined in the gut with or without cocaine treatment. Levels of claudin-1 (A) and claudin-2 (B) are shown after normalization to GAPDH. Statistics represent non-parametric Mann–Whitney or ANOVA tests. (C) H&E staining of rectum mucosa in control rats, rats receiving cocaine, and rats in the reinstatement group. The stable structures of gut mucosa are indicated with arrows (×200).
Gut mucosa tissue structure is intact in rats after cocaine administration
To further investigate the integrity of gut mucosa, we assessed the rectum mucosa tissue by H&E staining. Histopathological analysis of H&E-stained intestines revealed that the intestinal wall of each group was clear, with no obvious blood pooling or abnormal lymphocyte infiltration (Figure 2C). Examination of the intestine in the cocaine treatment group revealed a complete and stable structure of the intestinal epithelial mucosa, which was most pronounced in the reinstatement group (Figure 2C). These results suggest that cocaine administration may protect gut mucosa.
Cocaine treatment reduces gut epithelial cell permeability in vitro
We have conducted the gut permeability assay using Caco-2 cells in vitro. TEER is the measurement of electrical resistance across cells, which has been conducted in our previous study [27]. Consistent with the results obtained in vivo in mice, cocaine treatment reduced Caco2 cell permeability in a dose-dependent manner in vitro (Figure 3).
Figure 3.
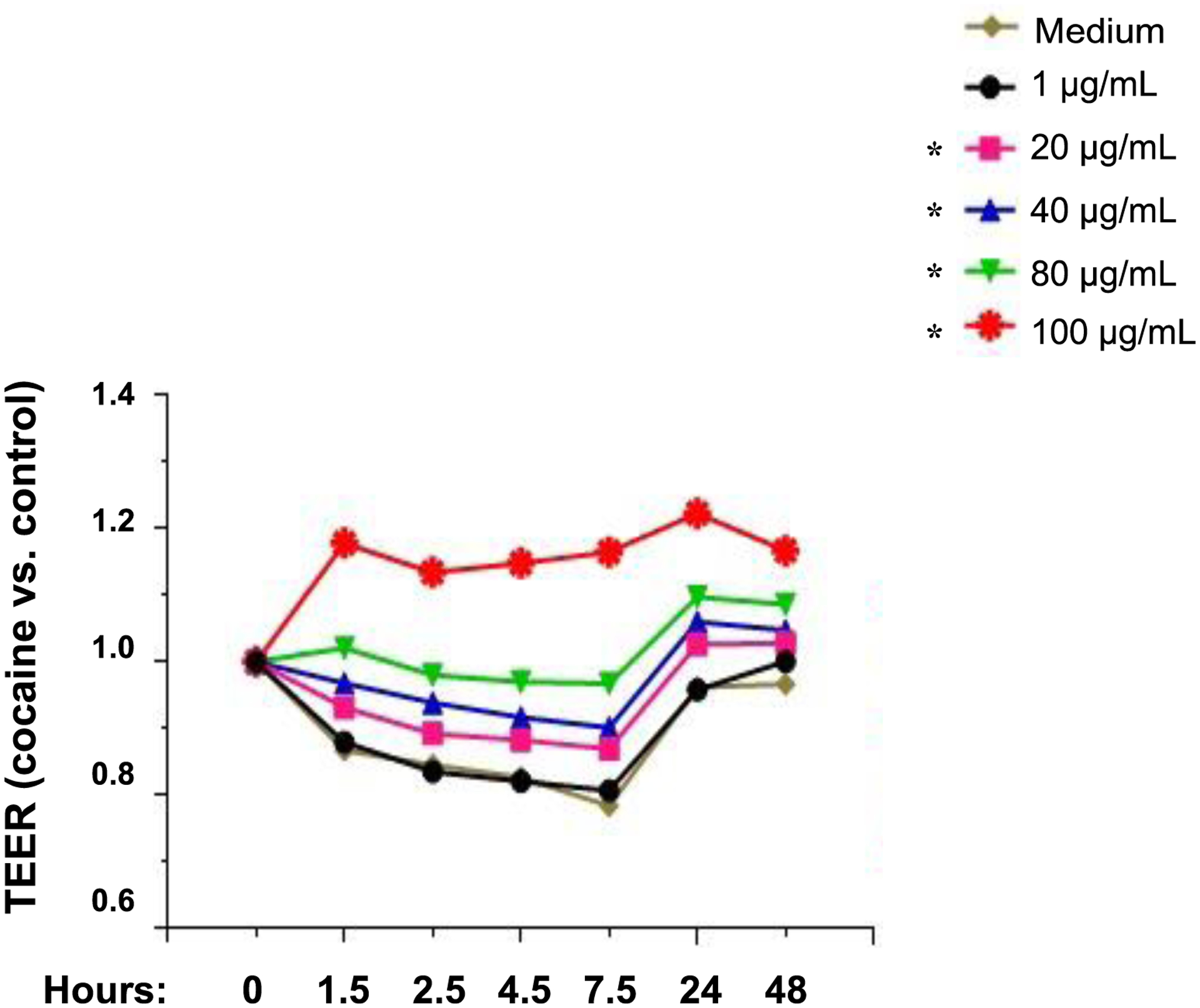
Cocaine reduces gut epithelial cell permeability in Caco2 cells in vitro. Caco-2 cells were cultured for 21 days. Different doses of cocaine were added to the cells. Transepithelial electrical resistance (TEER) was evaluated at different time points and concentrations of cocaine. ANOVA tests. *, P < 0.05.
Rat and human studies: cocaine administration decreases plasma levels of proinflammatory cytokine TNF-α
To evaluate whether cocaine-mediated protection of gut mucosa results in decreased inflammation, we assessed plasma levels of TNF-α and IL-6, proinflammatory cytokines, in rats after cocaine SA and in humans with cocaine use disorder. Notably, cocaine treatment significantly decreased plasma TNF-α levels compared to controls (Figure 4A). Moreover, levels of TNF-α tended to decrease in reinstatement rats compared with cocaine EX rats, but did not reach significance (Supplemental Figure 1C). In addition, plasma TNF-α levels were lower in reinstatement rats than in controls (Supplemental Figure 1C). Importantly, humans with cocaine use disorder had decreased plasma TNF-α levels compared with non-users (Figure 4B). Nonetheless, the levels of IL-6, another cytokine downstream of TLR, were similar in cocaine-treated rats and controls, and also similar in humans with cocaine use disorder and controls (Supplemental Figure 2). These results suggest that cocaine use inhibits TNF-α in vivo, perhaps through decreased gut permeability.
Figure 4.

Determination of TNF-α levels in rat and human plasma. Plasma levels of TNF-α were measured in rats treated with or without cocaine (A) and humans with or without cocaine use disorder (B) by ELISA. Twenty healthy individuals without drug use (non-drug) and nine individuals with cocaine use disorder (cocaine) were tested. Statistics represent non-parametric Mann–Whitney tests.
Discussion
In this work, we evaluated the effect of chronic exposure to cocaine on systemic inflammation in rats after cocaine administration and in human individuals with cocaine use disorder. Furthermore, we measured the intestinal permeability in rats after chronic intravenous cocaine administration, and found that rats who had undergone cocaine SA showed intact gut mucosa relative to the control group. Furthermore, cocaine administration was associated with decreased plasma levels of TNF-α in both rats and human users of cocaine.
Other drug uses and gut permeability.
Opioid receptors are abundant within the digestive tracts, and previous studies have shown that opioid administration can induce a “leaky” gut [11]. Additionally, intermittent morphine, but not sustained morphine treatment, results in a significant increase in gut permeability [28]. Consistently, morphine has been found to increase gut permeability in mouse models with acute pancreatitis in vivo, in rat intestinal epithelial cells cocultured with enteric glial cells in vitro, and in rats treated with both morphine and TNF-α [29–31]. However, morphine alone did not change gut permeability in rats [29].
Cocaine use and gut permeability.
Cocaine has been shown to be associated with altered indigenous gut microbiome, and gut dysbiosis is reported to affect behavioral response to drugs of abuse [19, 32]. Recently, a study conducted in mice intraperitoneally injected with cocaine (20 mg/kg for 7 days) has reported that cocaine administration changed the gut microbiome and increased colon barrier permeability [8]. However, the study also showed that cocaine administration increased tight junction claudin-1, 2, and 3, especially claudin-2 expression in the colon tissues [8]. In the current study, we evaluated gut permeability in a rat system with SA of cocaine and human gut epithelial cell line Caco-2 cells; unexpectedly, results from both in vivo and in vitro indicate that cocaine administration protected the rectum mucosal barrier. The different results between these studies may be due to the different experimental designs (IP vs. IV; mice vs. rats; acute vs. chronic). In addition, cocaine SA was conducted for 10 days in order to mimic sustained cocaine use in our study, which may also account for the difference observed between other studies and ours. Similarly, cocaine treatment was observed to elevate intestinal TEER in Caco-2 cells in vitro dose-dependently. Taken together, our results indicate that cocaine may inhibit small intestinal permeability. The mechanism of this inhibition is currently unknown. As we observed increased claudin-2 mRNA expression, further investigations in a claudin-2 genetic ablation model may help elucidate its role in cocaine-mediated protection of gut mucosa.
Gut permeability and inflammation.
Proinflammatory cytokines such as TNF-α play an important role in systemic and local inflammation. Proinflammatory cytokines may be associated with barrier impairment in the intestine, and barrier dysfunction can also be modulated by cytokines. For example, several studies have reported that TNF-α induces apoptosis of endothelial cells and decreases tight junction protein expression, resulting in increased intestinal permeability through NF-κB or JNK signaling [33, 34]. In addition, TNF-α was reported to impair gut barrier integrity via myosin light chain phosphorylation and occludin internalization, whereas not affecting the internalization of claudin-1 or zonula occluden-1 [35, 36]. In the current study, we found that plasma levels of TNF-α were lower in rats after cocaine treatment and in humans with cocaine use disorder compared with controls. In contrast, we did not observe any significant differences in the levels of IL-6 between the control and cocaine groups. These results suggest that cocaine use may exert diverse influence on TLR downstream cytokines, or regulate anti-inflammatory responses through other unknown mediators, or stem from acute versus chronic cocaine use. Moreover, the decreased local inflammation by cocaine treatment may further protect the gut mucosa barrier. This is consistent with the proposed positive feedback mechanism reported between gut mucosal barrier function and cytokines such as TNF-α and IFN-γ [36, 37]. The possibility that chronic cocaine treatment may affect the barrier function by altering the response of gut epithelial cells to TLR activation warrants further investigation.
The possible reasons of discrepancy from different studies.
Our finding that cocaine improves integrity of the gut mucosal barrier in rats and humans is unexpected, given the previous reports demonstrating reduced BBB permeability after chronic cocaine use [38–40]. The exact reasons for this apparent discrepancy and detailed mechanisms underlying drug-accelerated cognitive impairment are largely unknown. Distinct experimental conditions might contribute to the differences, such as Caco-2 cell preculturing condition and duration, monolayers vs. non-monolayers, in vitro vs. in vivo, and further experiments may be needed to clarify the effects. Nonetheless, our results from both in vivo and in vitro studies in humans and in rats support the hypothesis that cocaine treatment may protect gut mucosal barrier. Microbial product translocation through the gut mucosa is considered a driver of inflammation. Furthermore, cocaine has been shown to induce changes in the gut or oral indigenous microbiota [19, 20, 24], which exhibited the enrichment of proinflammatory bacterial strains [24]. The proinflammatory bacterial strains may affect BBB permeability [41], the behavioral sensitivity of cocaine, and the levels of synaptic proteins in response to cocaine, suggesting that a cocaine-altered microbiota–gut brain axis may have predominant impact in the absence of increased gut permeability [32]. Future studies using larger group sizes will be important for confirming and extending these results.
There are several limitations of this study. First, we used a rodent model of extinction training and reinstatement as this is our current rodent model for cocaine studies [22]. However, the effects of cocaine self-administration on gut barrier integrity may be best addressed immediately following the last cocaine administration. The additional 10 days for extinction training may indicate any immediate effects of cocaine on gut tight junction proteins may have been resolved by the time tissues were harvested for assessment. Second, the sample size in the control rat group was low, which limited us to draw further conclusions. Third, the effect of cocaine on gut permeability may be more complicated and different using different animal models and experimental systems, and the H&E assessment was from the rectum and the cell line used in vitro study was a colon cancer cell line. Fourth, the sample size in human was low, thus, we cannot compare plasma levels of TNF-α and IL-6 in cocaine users and controls after controlling for potential confounders (e.g., tabaco smoking, alcohol consumption).
CONCLUSION
In summary, the current study suggests that cocaine likely exerts a protective effect on the intestinal mucosa barrier, contributing to decreased mucosal endothelial permeability and systemic TNF-α level. Next, an in-depth analysis of the role of cocaine-mediated changes in gut endothelial permeability as well as intestinal flora may open new opportunities for drug addiction research and promote innovative development of novel treatments for addiction.
Supplementary Material
Financial Disclosures and Funding Source.
This work was supported by grants from the National Institute of Drug Abuse R01 DA045596 (Fitting), R03DA057164 (Jiang), R21NS118393 (Haque), F32 DA044782 (Anna Kruyer), Merit Review Award Number (CX002422, Jiang) from the United States (U.S.) Department of Veterans Affairs Office of Research and Development (CSR&D) Service, the Veterans Administration (1IOBX001262, 1I01 BX004269), the Deutsche Forschungsgemeinschaft 488/1–1, 1–2, and the Brain & Behavior Research Foundation NARSAD Young Investigator Award 2018 (Amato).
Footnotes
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The human study was approved by the Institutional Review Board (IRB) for Human Research at the Medical University of South Carolina (PRO00062384).
All animal procedures were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina.
HUMAN AND ANIMAL RIGHTS
All human procedures followed were in accordance with the Helsinki Declaration of 1975.
All animal research procedures were followed in accordance with the standards set forth in the eighth edition of Guide for the Care and Use of Laboratory Animals (published by the National Academy of Sciences, The National Academies Press, Washington, D.C.).
CONSENT FOR PUBLICATION
All participants were adults over the age of 21 years and provided written consent
CONFLICT OF INTEREST
None.
Declarations. All authors have contributed to, seen, and approved the final, submitted version of the manuscript for publication. This manuscript has not been previously published and is not being submitted for publication elsewhere. None of the authors have a financial conflict with the studies presented in this manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
AVAILABILITY OF DATA AND MATERIALS
Data are available upon request.
Reference:
- 1.Yamada T, Yamanaka I. Microglial localization of alpha-interferon receptor in human brain tissues. Neurosci Lett. 1995;189(2):73–6. Epub 1995/04/14. doi: 10.1016/0304-3940(95)11452-3. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu F, Nishihara H, Sano Y, Takeshita Y, Takahashi S, Maeda T, et al. Markedly increased IP-10 production by blood-brain barrier in neuromyelitis optica. PLoS One. 2015;10(3):e0122000. Epub 2015/03/27. doi: 10.1371/journal.pone.0122000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmermann J, Emrich M, Krauthausen M, Saxe S, Nitsch L, Heneka MT, et al. IL-17A Promotes Granulocyte Infiltration, Myelin Loss, Microglia Activation, and Behavioral Deficits During Cuprizone-Induced Demyelination. Mol Neurobiol. 2018;55(2):946–57. Epub 2017/01/14. doi: 10.1007/s12035-016-0368-3. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed SH, Lutjens R, van der Stap LD, Lekic D, Romano-Spica V, Morales M, et al. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci U S A. 2005;102(32):11533–8. doi: 10.1073/pnas.0504438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piechota M, Korostynski M, Solecki W, Gieryk A, Slezak M, Bilecki W, et al. The dissection of transcriptional modules regulated by various drugs of abuse in the mouse striatum. Genome Biol. 2010;11(5):R48. Epub 2010/05/13. doi: 10.1186/gb-2010-11-5-r48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poon HF, Abdullah L, Mullan MA, Mullan MJ, Crawford FC. Cocaine-induced oxidative stress precedes cell death in human neuronal progenitor cells. Neurochem Int. 2007;50(1):69–73. Epub 2006/09/08. doi: 10.1016/j.neuint.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Dalvi P, Wang K, Mermis J, Zeng R, Sanderson M, Johnson S, et al. HIV-1/cocaine induced oxidative stress disrupts tight junction protein-1 in human pulmonary microvascular endothelial cells: role of Ras/ERK1/2 pathway. PLoS One. 2014;9(1):e85246. Epub 2014/01/11. doi: 10.1371/journal.pone.0085246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chivero ET, Ahmad R, Thangaraj A, Periyasamy P, Kumar B, Kroeger E, et al. Cocaine Induces Inflammatory Gut Milieu by Compromising the Mucosal Barrier Integrity and Altering the Gut Microbiota Colonization. Sci Rep. 2019;9(1):12187. doi: 10.1038/s41598-019-48428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng J, Sindberg GM, Roy S. Disruption of gut homeostasis by opioids accelerates HIV disease progression. Front Microbiol. 2015;6:643. Epub 2015/07/15. doi: 10.3389/fmicb.2015.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sindberg GM, Sharma U, Banerjee S, Anand V, Dutta R, Gu CJ, et al. An infectious murine model for studying the systemic effects of opioids on early HIV pathogenesis in the gut. J Neuroimmune Pharmacol. 2015;10(1):74–87. Epub 2014/12/17. doi: 10.1007/s11481-014-9574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee S, Sindberg G, Wang F, Meng J, Sharma U, Zhang L, et al. Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol. 2016;9(6):1418–28. Epub 2016/02/26. doi: 10.1038/mi.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao K, Guo M, Niu F, Yang L, Callen SE, Buch S. Cocaine-mediated induction of microglial activation involves the ER stress-TLR2 axis. J Neuroinflammation. 2016;13:33. Epub 2016/02/11. doi: 10.1186/s12974-016-0501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.da Fonseca AC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, et al. The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci. 2014;8:362. Epub 2014/11/19. doi: 10.3389/fncel.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1–12. Epub 2014/12/03. doi: 10.1016/j.bbi.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halpern JH, Sholar MB, Glowacki J, Mello NK, Mendelson JH, Siegel AJ. Diminished interleukin-6 response to proinflammatory challenge in men and women after intravenous cocaine administration. J Clin Endocrinol Metab. 2003;88(3):1188–93. Epub 2003/03/12. doi: 10.1210/jc.2002-020804. [DOI] [PubMed] [Google Scholar]
- 16.Kubera M, Filip M, Basta-Kaim A, Nowak E, Siwanowicz J, Zajicova A, et al. The effect of cocaine sensitization on mouse immunoreactivity. Eur J Pharmacol. 2004;483(2–3):309–15. Epub 2004/01/20. doi: 10.1016/j.ejphar.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Yao H, Duan M, Buch S. Cocaine-mediated induction of platelet-derived growth factor: implication for increased vascular permeability. Blood. 2011;117(8):2538–47. doi: 10.1182/blood-2010-10-313593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17(9):564–80. Epub 2016/06/30. doi: 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]
- 19.Scorza C, Piccini C, Martinez Busi M, Abin Carriquiry JA, Zunino P. Alterations in the Gut Microbiota of Rats Chronically Exposed to Volatilized Cocaine and Its Active Adulterants Caffeine and Phenacetin. Neurotox Res. 2019;35(1):111–21. doi: 10.1007/s12640-018-9936-9. [DOI] [PubMed] [Google Scholar]
- 20.Volpe GE, Ward H, Mwamburi M, Dinh D, Bhalchandra S, Wanke C, et al. Associations of cocaine use and HIV infection with the intestinal microbiota, microbial translocation, and inflammation. J Stud Alcohol Drugs. 2014;75(2):347–57. Epub 2014/03/22. doi: 10.15288/jsad.2014.75.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chabot S, Wagner JS, Farrant S, Neutra MR. TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J Immunol. 2006;176(7):4275–83. Epub 2006/03/21. doi: 10.4049/jimmunol.176.7.4275. [DOI] [PubMed] [Google Scholar]
- 22.Kruyer A, Ball LE, Townsend DM, Kalivas PW, Uys JD. Post-translational S-glutathionylation of cofilin increases actin cycling during cocaine seeking. PLoS One. 2019;14(9):e0223037. Epub 2019/09/25. doi: 10.1371/journal.pone.0223037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67(1):81–4. Epub 2009/09/01. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu X, Cheng D, Luo Z, Wagner A, Fitting S, Cong X, et al. Oral Enrichment of Streptococcus and its Role in Systemic Inflammation Related to Monocyte Activation in Humans with Cocaine Use Disorder. J Neuroimmune Pharmacol. 2021. Epub 2021/08/28. doi: 10.1007/s11481-021-10007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo Z, Fitting S, Robinson C, Benitez A, Li M, Wu Y, et al. Chronic cannabis smoking-enriched oral pathobiont drives behavioral changes, macrophage infiltration, and increases beta-amyloid protein production in the brain. EBioMedicine. 2021;74:103701. Epub 2021/11/27. doi: 10.1016/j.ebiom.2021.103701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28(1):154–62. Epub 2013/01/02. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Z, Bian C, Luo Z, Guille C, Ogunrinde E, Wu J, et al. Progesterone decreases gut permeability through upregulating occludin expression in primary human gut tissues and Caco-2 cells. Sci Rep. 2019;9(1):8367. Epub 2019/06/12. doi: 10.1038/s41598-019-44448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee K, Vuong HE, Nusbaum DJ, Hsiao EY, Evans CJ, Taylor AMW. The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology. 2018;43(13):2606–14. Epub 2018/09/28. doi: 10.1038/s41386-018-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang M, Mischel RA, Bhave S, Komla E, Cho A, Huang C, et al. The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Sci Rep. 2017;7:42658. Epub 2017/02/18. doi: 10.1038/srep42658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauman BD, Meng J, Zhang L, Louiselle A, Zheng E, Banerjee S, et al. Enteric glial-mediated enhancement of intestinal barrier integrity is compromised by morphine. J Surg Res. 2017;219:214–21. Epub 2017/10/29. doi: 10.1016/j.jss.2017.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barlass U, Dutta R, Cheema H, George J, Sareen A, Dixit A, et al. Morphine worsens the severity and prevents pancreatic regeneration in mouse models of acute pancreatitis. Gut. 2018;67(4):600–2. Epub 2017/06/24. doi: 10.1136/gutjnl-2017-313717. [DOI] [PubMed] [Google Scholar]
- 32.Kiraly DD, Walker DM, Calipari ES, Labonte B, Issler O, Pena CJ, et al. Alterations of the Host Microbiome Affect Behavioral Responses to Cocaine. Sci Rep. 2016;6:35455. doi: 10.1038/srep35455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anda T, Yamashita H, Khalid H, Tsutsumi K, Fujita H, Tokunaga Y, et al. Effect of tumor necrosis factor-alpha on the permeability of bovine brain microvessel endothelial cell monolayers. Neurol Res. 1997;19(4):369–76. Epub 1997/08/01. doi: 10.1080/01616412.1997.11758599. [DOI] [PubMed] [Google Scholar]
- 34.Al-Sadi R, Guo S, Ye D, Rawat M, Ma TY. TNF-alpha Modulation of Intestinal Tight Junction Permeability Is Mediated by NIK/IKK-alpha Axis Activation of the Canonical NF-kappaB Pathway. Am J Pathol. 2016;186(5):1151–65. Epub 2016/03/08. doi: 10.1016/j.ajpath.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR 2nd, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189(1):111–26. Epub 2010/03/31. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pastorelli L, De Salvo C, Mercado JR, Vecchi M, Pizarro TT. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front Immunol. 2013;4:280. Epub 2013/09/26. doi: 10.3389/fimmu.2013.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulzke JD, Ploeger S, Amasheh M, Fromm A, Zeissig S, Troeger H, et al. Epithelial tight junctions in intestinal inflammation. Annals of the New York Academy of Sciences. 2009;1165:294–300. Epub 2009/06/23. doi: 10.1111/j.1749-6632.2009.04062.x. [DOI] [PubMed] [Google Scholar]
- 38.Yao H, Yang Y, Kim KJ, Bethel-Brown C, Gong N, Funa K, et al. Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration. Blood. 2010;115(23):4951–62. doi: 10.1182/blood-2010-01-266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson TL, Hargrave SL, Kearns DN, Clasen MM, Jones S, Wakeford AGP, et al. Cocaine impairs serial-feature negative learning and blood-brain barrier integrity. Pharmacol Biochem Behav. 2018;170:56–63. Epub 2018/05/14. doi: 10.1016/j.pbb.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Meckel KR, Kiraly DD. A potential role for the gut microbiome in substance use disorders. Psychopharmacology (Berl). 2019;236(5):1513–30. Epub 2019/04/15. doi: 10.1007/s00213-019-05232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158. Epub 2014/11/21. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request.


