Abstract
[Purpose] We aimed to assess diffusion tensor fractional anisotropy to outline the brain regions associated with the long-term motor and cognitive functional outcomes of patients with stroke. [Participants and Methods] Eighty patients from our previous study were enrolled. Fractional anisotropy maps were acquired on days 14–21 after stroke onset, and tract-based spatial statistics were applied. Outcomes were scored using the Brunnstrom recovery stage and Functional Independence Measure motor and cognition components. Fractional anisotropy images were assessed in relation to outcome scores using the general linear model. [Results] For both the right (n=37) and left (n=43) hemisphere lesion groups, the corticospinal tract and the anterior thalamic radiation were most strongly associated with the Brunnstrom recovery stage. In contrast, the cognition component involved large regions encompassing the anterior thalamic radiation, superior longitudinal fasciculus, inferior longitudinal fasciculus, uncinate fasciculus, cingulum bundle, forceps major, and forceps minor. The results for the motor component were intermediate between those for the Brunnstrom recovery stage and those for the cognition component. [Conclusion] Motor-related outcomes were associated with fractional anisotropy decreases in the corticospinal tract, whereas cognitive outcomes were related to broad regions of association and commissural fibers. This knowledge will help scheduling appropriate rehabilitative treatments.
Keywords: Prediction, Prognosis, Stroke
INTRODUCTION
The brain imaging assessment of clinical severity is crucial for facilitating successful rehabilitation. Various brain imaging techniques have been developed, including computed tomography, magnetoencephalography, and magnetic resonance imaging1,2,3). Of these, magnetic resonance diffusion tensor imaging (DTI) is considered to be one of the most useful techniques for stroke rehabilitation1). This unique modality enables the assessment of neural fiber integrity in vivo. Of various parameters derived from DTI, fractional anisotropy (FA) is used as an index of neural fiber degeneration4). Indeed, a body of literature has been published focusing on the relationships between FA and stroke patient outcomes1).
Patients with stroke often develop neurological sequelae in motor and cognitive domains5, 6). The most typical clinical manifestation in motor function is hemiparesis, which often leads to decreased hand dexterity and lower locomotive ability. Cognitive dysfunctions such as aphasia, apraxia, and spatial neglect are also commonly observed, with subsequent decreased functional independence. As such, most moderate-to-severe stroke patients exhibit combined symptoms in both motor and cognitive functions.
Several DTI-FA studies have investigated the relationships between neural fiber degeneration and functional outcomes. However, few have touched upon the relationships of FA decreases with both motor and cognitive outcomes5, 6). Thus, the aim of this study was to outline the brain regions responsible for functional outcomes in motor and cognitive components in stroke patients.
PARTICIPANTS AND METHODS
This study is a continuation of our research on DTI and comprises a summary of reanalyses of our previously published data5,6,7,8,9,10). The study was conducted with approval from the Ethics Committee of Hyogo Medical University (No. 2454). Information about study inclusion was posted on the hospital’s website, and consent was obtained via the opt-out method. The study comprised 80 patients with first-ever stroke of supratentorial lesions. The patients’ profiles were detailed in our previous studies5, 6). Typically, the patients were transferred to our hospital soon after onset and were diagnosed with stroke by using computed tomography and/or magnetic resonance imaging. They were then managed conservatively with medications, and surgery was performed when necessary. Patients also received physical, occupational, and speech therapy for up to 180 min (combined daily total). The rehabilitative regimen was administered in accordance with the Japanese Guidelines for the Management of Stroke11).
DTI data were acquired on days 14–21 after admission by using a 3.0-T magnetic resonance imaging scanner (MAGNETOM Trio; Siemens AG, Erlangen, Germany) with a 32-channel head coil. The image acquisition protocol was detailed in our previous report12). Briefly, the DTI protocol acquired 12 images with non-collinear diffusion gradients (b=1,000 s/mm2) and one non-diffusion-weighted image (b=0 s/mm2) using a single-shot echo-planar imaging sequence. Sixty-four contiguous axial slices were obtained for each patient. The field of view was 230.4 mm × 230.4 mm (acquisition matrix, 128 × 128; slice thickness, 3 mm with no gap; echo time, 83 ms; repetition time, 7,000 ms).
In this study, outcomes were assessed using the Brunnstrom recovery stage (BRS)13) and the Functional Independence Measure (FIM)14). BRS is typically used to evaluate the functionality of the proximal (shoulder/elbow/forearm) and distal (hand/finger) portions of the upper extremities and the entire lower extremities on the affected side (hemiparesis). In this study, we used the sum of the BRS score (BRS-total) as an index of the gross severity of the hemiparesis. FIM is a widely used tool for evaluating independence in terms of activities of daily living. FIM comprises two components, motor (13 items) and cognition (5 items). All items are scored on a 7-point scale (1=total assistance; 7=complete independence), and the total scores of both FIM-motor (scale range, 13–91) and FIM-cognition (scale range, 5–35) are commonly used in stroke rehabilitation5, 6). BRS and FIM scores were assessed monthly, and data were collected from our affiliated long-term rehabilitation facility at discharge. The total length of hospital stay was also documented for each patient. The aim of this study was to characterize DTI data in relation to long-term outcomes. To facilitate the interpretation of the results obtained from image analyses, correlations among BRS-total, FIM-motor, and FIM-cognition were assessed separately for right and left hemisphere lesion groups (Pearson test, p<0.05).
For this study, tract-based spatial statistics (TBSS)15), part of the FMRIB Software Library (FSL) software package16), was used to identify brain regions with neural fiber degeneration (a FA decrease) induced by stroke. TBSS enables evaluation of the integrity of the cerebral white matter based on intrinsic anisotropic properties; the FA of local tract structures is projected onto a virtual skeleton in the standard brain, thus providing an alignment-invariant depiction of the median aspects of the tract. The image processing procedures were detailed in our previous publications5, 6). The brain image analyses were performed separately for the right and left hemisphere lesion groups.
To assess the brain regions associated with outcome measures, we applied voxel-wise spatial statistics by permutation testing (N=5,000) using the “randomise” function implemented in FSL. It allows the use of the general linear model (GLM)17); the scores of outcome measures were entered as covariates in the GLM analysis. The analysis was performed separately for BRS-total, FIM-motor, and FIM-cognition data. To minimize potential confounds arising from a FA decline due to aging18,19,20), patients’ age data were also used as additional covariates for each analysis. Thresholding was achieved using the threshold-free cluster enhancement methodology21), and a p-value less than 0.05 was considered statistically significant.
To further evaluate the brain regions showing statistically significant relationships with outcome scores, we applied the region-of-interest (ROI) method5, 6). The numbers of voxels considered statistically significant were abstracted from the images from the GLM analyses. In reference to the standard white matter brain map (JHU-ICBM-tracts-maxprob-thr0) implemented in FSL22), ROIs were defined as follows: the corticospinal tract (CST), the anterior thalamic radiation (ATR), the superior longitudinal fasciculus (SLF), the inferior longitudinal fasciculus (ILF), the uncinate fasciculus (UNF), the cingulum bundle (CNG), the forceps major (FMJ), and the forceps minor (FMN). The inferior fronto-occipital fasciculus was excluded from the ROIs because it largely overlaps the UNF and ILF and the hippocampus was also excluded because its voxel size was markedly smaller than that of the other ROIs. For clarity, we limited our ROI analyses to the lesioned hemisphere, except for the commissural fibers (FMJ and FMN), which connect the right and left hemispheres.
RESULTS
Table 1 presents the patients’ profiles. The study samples comprised 37 patients with right hemisphere lesions and 43 patients with left hemisphere lesions. Table 2 shows the results of the correlation analyses. For both hemisphere lesion groups, the correlations between BRS-total and FIM-motor were significant. Although less evident, the correlations between FIM-motor and FIM-cognition were also significant. In contrast, the correlations between BRS-total and FIM-cognition were not significant.
Table 1. Patient demographics and clinical characteristics.
| Rt hemisphere lesion (n=37) | Lt hemisphere lesion (n=43) | |
| Gender (male/female) | 18/19 | 29/14 |
| Age (years) | 65.5 ± 11.6 | 63.4 ± 13.2 |
| Type of stroke (hemorrhagic/ischemic) | 18/19 | 22/21 |
| Total length of hospital stay (days) | 138.3 ± 52.0 | 143.1 ± 56.5 |
| BRS Shoulder/Elbow/Forearm | 4.3 ± 1.4 | 4.0 ± 1.7 |
| BRS Hand/Finger | 4.2 ± 1.5 | 3.7 ± 1.7 |
| BRS Lower Extremity | 4.8 ± 1.1 | 4.4 ± 1.2 |
| BRS-total | 13.3 ± 3.7 | 12.1 ± 4.9 |
| FIM-motor | 75.9 ± 9.6 | 77.1 ± 6.9 |
| FIM-cognition | 30.9 ± 3.6 | 28.3 ± 5.7 |
BRS: Brunnstrom recovery stage; FIM: Functional Independence Measure; Lt: left; Rt: right.
Table 2. Results from correlation analyses.
| Rt hemisphere lesions (n=37) | FIM-motor | FIM-cognition |
| BRS-total | r=0.546*** | r=0.173 |
| FIM-motor | - | r=0.506** |
| Lt hemisphere lesions (n=43) | FIM-motor | FIM-cognition |
| BRS-total | r=0.504*** | r=0.127 |
| FIM-motor | - | r=0.334* |
BRS: Brunnstrom recovery stage; FIM: Functional Independence Measure; Lt: left; Rt: right. *p<0.05, **p<0.01, ***p<0.001.
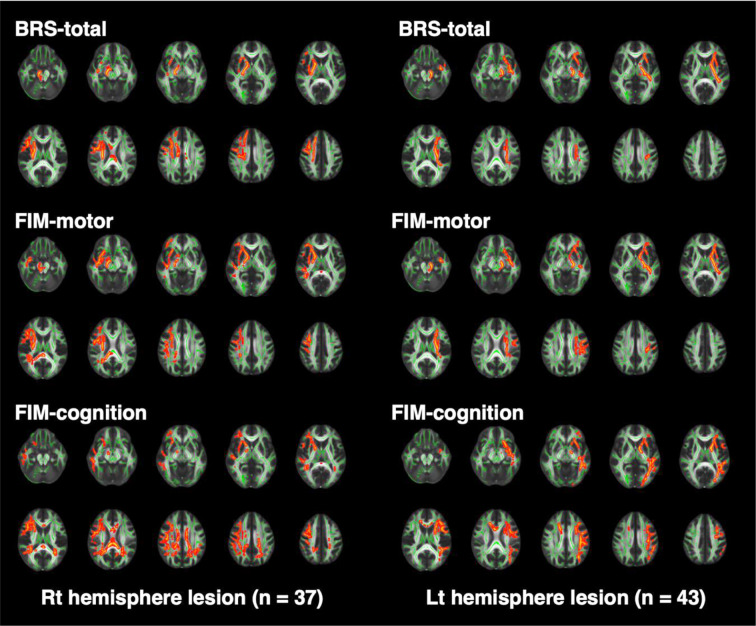
Figure 1 shows the brain regions exhibiting significant relationships with the outcome scores. Table 3 indicates the numbers of significant voxels in the ROIs. For both the right and left hemisphere lesion groups, the CST and ATR were the brain regions most strongly associated with BRS-total scores (Fig. 1, upper panels). In contrast, for FIM-cognition scores, numerous areas were involved, including the ATR, SLF, ILF, UNF, CNG, FMJ, and FMN (lower panels). Meanwhile, images for FIM-motor (middle panels) revealed significant findings mainly in the CST, ATR, and SLF, whose aspects were intermediate between the findings for BRS-total and those for FIM-cognition.
Fig. 1.
Brain images obtained from tract-based spatial statistics.
The FA skeleton representing tract centers is shown in green, whereas voxels with significantly smaller FA values are shown in red. To aid visualization, voxels showing statistical significance (p<0.05) were thickened using the “tbss_fill” command implemented in FSL.
FA: fractional anisotrophy; FSL: FMRIB Software Library; BRS: Brunnstrom recovery stage; FIM: Functional Independence Measure; Lt: left; Rt: right.
Table 3. Numbers of voxels reaching statistical significance.
| BRS-total | FIM-motor | FIM-cognition | ||||
| Rt | Lt | Rt | Lt | Rt | Lt | |
| CST (5,537/5,485) | 2,694 | 1,746 | 967 | 1,724 | 173 | 16 |
| ATR (6,614/7,717) | 1,176 | 885 | 1,383 | 1,217 | 1,136 | 1,324 |
| SLF (9,570/9,978) | 2,225 | 584 | 2,597 | 1,376 | 3,801 | 3,862 |
| ILF (5,069/5,320) | 4 | 534 | 672 | 323 | 635 | 857 |
| UNF (1,103/1,357) | 35 | 658 | 394 | 597 | 128 | 539 |
| CNG (1,535/2,429) | 26 | 0 | 35 | 0 | 323 | 12 |
| FMJ (5,603) | 0 | 0 | 242 | 0 | 211 | 186 |
| FMN (6,057) | 123 | 0 | 4 | 0 | 49 | 184 |
Numbers in parentheses indicate the total number of voxels in the ROIs (Rt/Lt). FMJ and FMJ have no laterality (Rt/Lt) because they are commissural fibers.
ATR: anterior thalamic radiation; BRS: Brunnstrom recovery stage; CNG: cingulum bundle; CST: corticospinal tract; FIM: Functional Independence Measure; FMJ: forceps major; FMN: forceps minor; ILF: inferior longitudinal fasciculus; Lt: left; Rt: right; SLF: superior longitudinal fascicles; UNF: uncinate fasciculus; ROI: region of interest.
DISCUSSION
This study outlined the brain regions with neural fiber degeneration in relation to different dimensions of stroke outcomes. Hemiparesis severity indexed by BRS was related to the CST and ATR. Cognitive decline indexed by FIM-cognition was associated with wider regions in association fibers, such as the SLF, ILF, UNF, and CNG, and in commissural fibers, such as the FMJ and FMN. These findings were compatible with the results obtained from the correlation analyses in which BRS-total and FIM-cognition scores were orthogonalized. Meanwhile, the findings for FIM-motor were intermediate between the results for BRS and those for FIM-cognition.
In our previous studies, we first focused on the relationships between FA decreases in the CST and motor-related outcomes7, 8, 10). However, stroke lesions affect not only the CST but also other brain areas such as the SLF. In an attempt to cover broader brain areas, we then employed TBSS which allows whole brain analyses5, 6, 9). This study further extended TBSS using the GLM in relation to various outcome scores including BRS, FIM-motor and cognition. Owing to such an extended methodology, the results of the present study revealed the brain areas responsible for these different components of the long-term outcomes.
Using the BRS index that evaluates hemiparesis severity, upper and lower extremity functions were found to be associated with the CST and ATR (Table 3). Considering the nature of the motor control of and the sensory input from the extremities, this finding is reasonable. In contrast, the SFL was most evidently involved in FIM-cognition, with over 3,800 voxels for both hemisphere groups considered statistically significant (Table 3). Recent imaging studies revealed that the functional role of the SLF includes working memory, motor control, language function, and visual recognition23,24,25,26,27). Previous neuroimaging studies of stroke patients reported that neural degeneration in the left SLF was associated with aphasia28, 29) while the right SLF was associated with unilateral neglect30, 31). As far as we know, our study is the first DTI-FA study to use GLM for both motor and cognition domains. The findings are still in line with the established knowledge in neurology, indicating the appropriateness of our methodology.
In this study, the results from correlation analyses (Table 2) indicated that BRS and FIM-cognition scores were not significantly correlated with each other. Meanwhile, FIM-motor scores were significantly correlated with both BRS and FIM-cognition scores. FIM-motor comprises 13 items: eating, grooming, bathing, dressing the upper body, dressing the lower body, toileting, bladder control, bowel control, transfer to bed, chair, or wheelchair, transfer to toilet, transfer to tub or shower, walking or wheelchair propulsion, and stair climbing. The ability to perform these items cannot be attributed to simple motor outputs. For example, transfer to wheelchair requires visual recognition and motor controls for the body trunk and extremities. Furthermore, dressing apraxia is one of the higher brain dysfunctions often observed in patients with right hemisphere lesions. As shown here, FIM-motor scores reflect abilities involving both motor and cognitive functions. The present brain imaging findings confirm such a specificity of the FIM-motor scores from a neurobiological point of view.
The analytical database for this study included both types of strokes: hemorrhagic and ischemic. The reduction in FA with symptoms might differ between these two types. For this reason, we previously compared the relationships between motor deficit and the FA decrease between ischemic and hemorrhagic stroke groups8). In terms of motor deficit, the hemorrhagic stroke patients showed more severe hemiparesis, as indexed by BRS. In parallel, the FA decreases were more evident in the hemorrhagic cases than in the ischemic cases. Consequently, the regression lines between FA values in the CST and BRS were very similar in the two groups8). For this reason, we treated the hemorrhagic and ischemic stroke patients as being in the same category in our analyses.
This study has several limitations. First, the outcome measure for the cognitive domain (FIM-cognition) is not specific to stroke symptoms. Patients with stroke have a variety of cognitive symptoms, including aphasia, apraxia, neglect, and memory disturbance. The FIM-cognition scale used in the analyses is not sufficient to describe such a variety of cognitive symptoms. Second, the sample size was not large. We had 37 right hemisphere lesion patients and 43 left hemisphere lesion patients. However, a recent systematic review showed a median sample size of 28 (interquartile range, 15–50) in the 71 studies included1). Our sample size was thus comparable to that of previous studies in the field. Third, in this study, we did not include patients with subarachnoid hemorrhage or those with infratentorial lesions because their symptoms (e.g., consciousness disturbance, ataxia, and nystagmus) are different from those in patients with supratentorial intramedullary lesions. Therefore, caution is needed when the present findings are being generalized to the entire stroke population.
In conclusion, the present DTI-FA study found that motor dysfunction is associated with FA decreases in the corticospinal tract, whereas cognitive dysfunction is related to broad regions of association and commissural fibers.
Funding
This work was partially supported by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (JSPS KAKENHI [JP16H03209]).
Conflict of interest
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Kim B, Winstein C: Can neurological biomarkers of brain impairment be used to predict poststroke motor recovery? A systematic review. Neurorehabil Neural Repair, 2017, 31: 3–24. [DOI] [PubMed] [Google Scholar]
- 2.Guggisberg AG, Koch PJ, Hummel FC, et al. : Brain networks and their relevance for stroke rehabilitation. Clin Neurophysiol, 2019, 130: 1098–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pellegrino G, Arcara G, Cortese AM, et al. : Cortical gamma-synchrony measured with magnetoencephalography is a marker of clinical status and predicts clinical outcome in stroke survivors. Neuroimage Clin, 2019, 24: 102092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu C, Zhu C, Zhang Y, et al. : A longitudinal diffusion tensor imaging study on Wallerian degeneration of corticospinal tract after motor pathway stroke. Neuroimage, 2009, 47: 451–458. [DOI] [PubMed] [Google Scholar]
- 5.Koyama T, Domen K: Diffusion tensor fractional anisotropy in the superior longitudinal fasciculus correlates with Functional Independence Measure cognition scores in patients with cerebral infarction. J Stroke Cerebrovasc Dis, 2017, 26: 1704–1711. [DOI] [PubMed] [Google Scholar]
- 6.Koyama T, Uchiyama Y, Domen K: Associations of diffusion-tensor fractional anisotropy and FIM outcome assessments after intracerebral hemorrhage. J Stroke Cerebrovasc Dis, 2018, 27: 2869–2876. [DOI] [PubMed] [Google Scholar]
- 7.Koyama T, Marumoto K, Uchiyama Y, et al. : Outcome assessment of hemiparesis due to intracerebral hemorrhage using diffusion tensor fractional anisotropy. J Stroke Cerebrovasc Dis, 2015, 24: 881–889. [DOI] [PubMed] [Google Scholar]
- 8.Koyama T, Koumo M, Uchiyama Y, et al. : Utility of fractional anisotropy in cerebral peduncle for stroke outcome prediction: comparison of hemorrhagic and ischemic strokes. J Stroke Cerebrovasc Dis, 2018, 27: 878–885. [DOI] [PubMed] [Google Scholar]
- 9.Koyama T, Uchiyama Y, Domen K: Comparison of fractional anisotropy from tract-based spatial statistics with and without lesion masking in patients with intracerebral hemorrhage: a technical note. J Stroke Cerebrovasc Dis, 2019, 28: 104376. [DOI] [PubMed] [Google Scholar]
- 10.Koyama T, Uchiyama Y, Domen K: Outcome in stroke patients is associated with age and fractional anisotropy in the cerebral peduncles: a multivariate regression study. Prog Rehabil Med, 2020, 5: 20200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinohara Y, Yanagihara T, Abe K, et al. : VII. Rehabilitation. J Stroke Cerebrovasc Dis, 2011, 20: S145–S180. [DOI] [PubMed] [Google Scholar]
- 12.Koyama T, Marumoto K, Domen K, et al. : White matter characteristics of idiopathic normal pressure hydrocephalus: a diffusion tensor tract-based spatial statistic study. Neurol Med Chir (Tokyo), 2013, 53: 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunnstrom S: Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther, 1966, 46: 357–375. [DOI] [PubMed] [Google Scholar]
- 14.Heinemann AW, Linacre JM, Wright BD, et al. : Relationships between impairment and physical disability as measured by the functional independence measure. Arch Phys Med Rehabil, 1993, 74: 566–573. [DOI] [PubMed] [Google Scholar]
- 15.Smith SM, Jenkinson M, Johansen-Berg H, et al. : Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage, 2006, 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- 16.Jenkinson M, Beckmann CF, Behrens TE, et al. : FSL. Neuroimage, 2012, 62: 782–790. [DOI] [PubMed] [Google Scholar]
- 17.Winkler AM, Ridgway GR, Webster MA, et al. : Permutation inference for the general linear model. Neuroimage, 2014, 92: 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madden DJ, Bennett IJ, Song AW: Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev, 2009, 19: 415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett IJ, Madden DJ, Vaidya CJ, et al. : Age-related differences in multiple measures of white matter integrity: a diffusion tensor imaging study of healthy aging. Hum Brain Mapp, 2010, 31: 378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madden DJ, Bennett IJ, Burzynska A, et al. : Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta, 2012, 1822: 386–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SM, Nichols TE: Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage, 2009, 44: 83–98. [DOI] [PubMed] [Google Scholar]
- 22.Wakana S, Jiang H, Nagae-Poetscher LM, et al. : Fiber tract-based atlas of human white matter anatomy. Radiology, 2004, 230: 77–87. [DOI] [PubMed] [Google Scholar]
- 23.Bernal B, Altman N: The connectivity of the superior longitudinal fasciculus: a tractography DTI study. Magn Reson Imaging, 2010, 28: 217–225. [DOI] [PubMed] [Google Scholar]
- 24.Shinoura N, Midorikawa A, Onodera T, et al. : Damage to the left ventral, arcuate fasciculus and superior longitudinal fasciculus-related pathways induces deficits in object naming, phonological language function and writing, respectively. Int J Neurosci, 2013, 123: 494–502. [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez-Herreros B, Amengual JL, Gurtubay-Antolín A, et al. : Microstructure of the superior longitudinal fasciculus predicts stimulation-induced interference with on-line motor control. Neuroimage, 2015, 120: 254–265. [DOI] [PubMed] [Google Scholar]
- 26.Rizio AA, Diaz MT: Language, aging, and cognition: frontal aslant tract and superior longitudinal fasciculus contribute toward working memory performance in older adults. Neuroreport, 2016, 27: 689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita T, Saito DN, Ban M, et al. : Self-face recognition shares brain regions active during proprioceptive illusion in the right inferior fronto-parietal superior longitudinal fasciculus III network. Neuroscience, 2017, 348: 288–301. [DOI] [PubMed] [Google Scholar]
- 28.Breier JI, Hasan KM, Zhang W, et al. : Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. AJNR Am J Neuroradiol, 2008, 29: 483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyama T, Domen K: Reduced diffusion tensor fractional anisotropy in the left arcuate fasciculus of patients with aphasia caused by acute cerebral infarct. Prog Rehabil Med, 2016, 1: 20160008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thiebaut de Schotten M, Tomaiuolo F, Aiello M, et al. : Damage to white matter pathways in subacute and chronic spatial neglect: a group study and 2 single-case studies with complete virtual “in vivo” tractography dissection. Cereb Cortex, 2014, 24: 691–706. [DOI] [PubMed] [Google Scholar]
- 31.Lunven M, Bartolomeo P: Attention and spatial cognition: neural and anatomical substrates of visual neglect. Ann Phys Rehabil Med, 2017, 60: 124–129. [DOI] [PubMed] [Google Scholar]