Abstract
Purpose
Low vitamin D in COVID-19 have been related to worse outcomes. However, most of the studies conducted so far were not-controlled and retrospective, including biases potentially influencing this association. We evaluated 25(OH)vitamin D levels of patients with both severe and non-severe disease at hospital-admission, and in a cohort of control subjects. Moreover, we evaluated sACE-2 levels to investigate the mechanisms underlying the association between vitamin D and COVID-19.
Methods
COVID-19 patients were enrolled in a matched for age, sex and comorbidities 1:1-ratio based on the presence/or not of respiratory-distress/severe-disease at hospital-admission. Control matched subjects were enrolled from an outpatient-setting.
Results
Seventy-three COVID-19 patients (36 severe and 37 non-severe) and 30 control subjects were included. We observed a higher vitamin D deficiency (<20 ng/mL) prevalence in COVID-19 patients than control subjects (75% vs 43%). No differences were found regarding 25(OH)vitamin D and sACE-2 levels between patients with and without severe-disease at study entry. During the disease-course, in the severe group a life-threatening disease occurred in 17 patients (47.2%), and, in the non-severe group, a worsening disease occurred in 10 (27%). 25(OH)vitamin D levels, at admission, were negatively correlated with sACE-2 levels, and were lower in patients whose disease worsened as compared to those in whom it did not, independently from the disease severity at admission. In multivariate-analysis, lower 25(OH)vitamin D resulted as an independent risk factor for disease worsening.
Conclusions
25(OH)vitamin D levels at hospital-admission strongly predicted the occurrence of worsening outcomes in COVID-19 independently of the disease severity at presentation.
Keywords: COVID-19, Vitamin D, ACE-2, SARS-CoV-2, Hypovitaminosis D
Introduction
The negative role of vitamin D deficiency in patients affected by Coronavirus disease-19 (COVID-19) was hypothesized from the early pandemic spread due to the well-known influence of vitamin D on immune response and immunocompetence both regarding innate and adaptive immunity supporting antimicrobial and antiviral immune responses [1–4].
Low 25(OH) vitamin D levels were early reported as a possible risk factor for worse clinical outcomes in COVID-19 patients [1, 3] particularly in European Mediterranean Countries characterized by widespread vitamin D deficiency (and heavily impacted by the early pandemic spread) [5] in line with the associations between vitamin D deficiency and acute respiratory infections (ARIs) and immune-modulating role of vitamin D. Some observational studies have reported that higher 25(OH) vitamin D levels were independently associated with reduced risk for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severe COVID-19, which was confirmed by several systematic reviews and meta-analyses [6–12]. However, study designs were mainly retrospective without a control group and included patients with demographic differences and biases potentially influencing the correlations between lower 25(OH) vitamin D levels and severe COVID-19. In fact, a few prospective clinical studies regarding these associations reported contrasting results [13–17].
Membrane-bound Angiotensin-Converting Enzyme 2 (mACE-2) is part of the renin-angiotensin-aldosterone system (RAAS) and the main important host receptor for SARS-CoV-2 infection, and the internalization of the ACE-2-virus complex promotes its shedding increasing soluble ACE-2 (sACE-2) form in circulation [18–22]. Though, the role of sACE-2 in the overall SARS-CoV-2 infection process has not been clarified. Vitamin D is known to regulate and modulate RAAS negatively, promoting ACE-2/Ang‐(1‐7)/MasR axis activity by preventing mACE-2 shedding, stimulating their gene expressions [23] and inhibiting renin secretion and the ACE/Ang II/AT1R axis, leading to a potential protective role against acute lung injury (ALI) and acute respiratory-distress syndrome (ARDS) [24, 25].
Some authors proposed that low 25(OH) vitamin D levels found in upper and lower tract ARIs [26] as well as in other inflammatory diseases [2, 27, 28] could be part, of the so called “acute phase reaction” since acute inflammatory and immune responses are associated with an acute reduction of circulating vitamin D binding protein [29–33]. In fact, a systematic review showed that, in the presence of an acute inflammatory status, a reduction of about 30% in 25(OH) vitamin D levels occurred following the onset of the immune response [34].
Therefore, it is still a matter of debate if the low 25(OH) vitamin D levels observed in COVID-19 patients during acute illness are only a biochemical marker and epiphenomenon of the disease or a potential pre-existing risk factor for infection and worse outcomes [35–37]. Unfortunately, the data regarding the vitamin D role in COVID-19 patients are mostly based on retrospective collections and focused on severe patients in whom 25(OH) vitamin D levels were evaluated when the acute phase of the disease had already occurred. Therefore, at this time, the debate if severe COVID-19 could be the cause or the effect of a poor vitamin D status is of paramount interest and bears clear treatment implications [38].
In order to better understand the relationship between COVID-19 and vitamin D, we carried out this prospective study in which 25(OH) vitamin D levels were measured at first hospital evaluation of patients with both severe and non-severe disease matched for age, sex, and comorbidities. These patients were also matched with a cohort of control subjects enrolled in the same period in an outpatient-setting. Moreover, we evaluated the circulating sACE-2 levels of COVID-19 patients at the study entry to investigate the pathophysiological mechanisms underlying the associations between vitamin D deficiency and COVID-19.
Patients and methods
Study design
This was a prospective study performed at San Raffaele University Hospital, a tertiary healthcare center in Milan, Italy. The study protocol complies with the Declaration of Helsinki and was approved by the hospital ethics committee (protocol ABIO/NC/03 no. 367/2020). Signed informed consent was obtained from all patients participating in this study. Adult patients (age ≥18 years) admitted to San Raffaele University Hospital for COVID-19 from March to June 2021 were evaluated for the enrollment in the study. Confirmed COVID-19 was defined as positive real-time reverse-transcriptase polymerase chain reaction from a nasal and/or throat swab together with signs, symptoms, and/or radiological findings suggestive of COVID-19 pneumonia. Patients admitted for other reasons and subsequently diagnosed with superimposed SARS-CoV-2 infection were excluded.
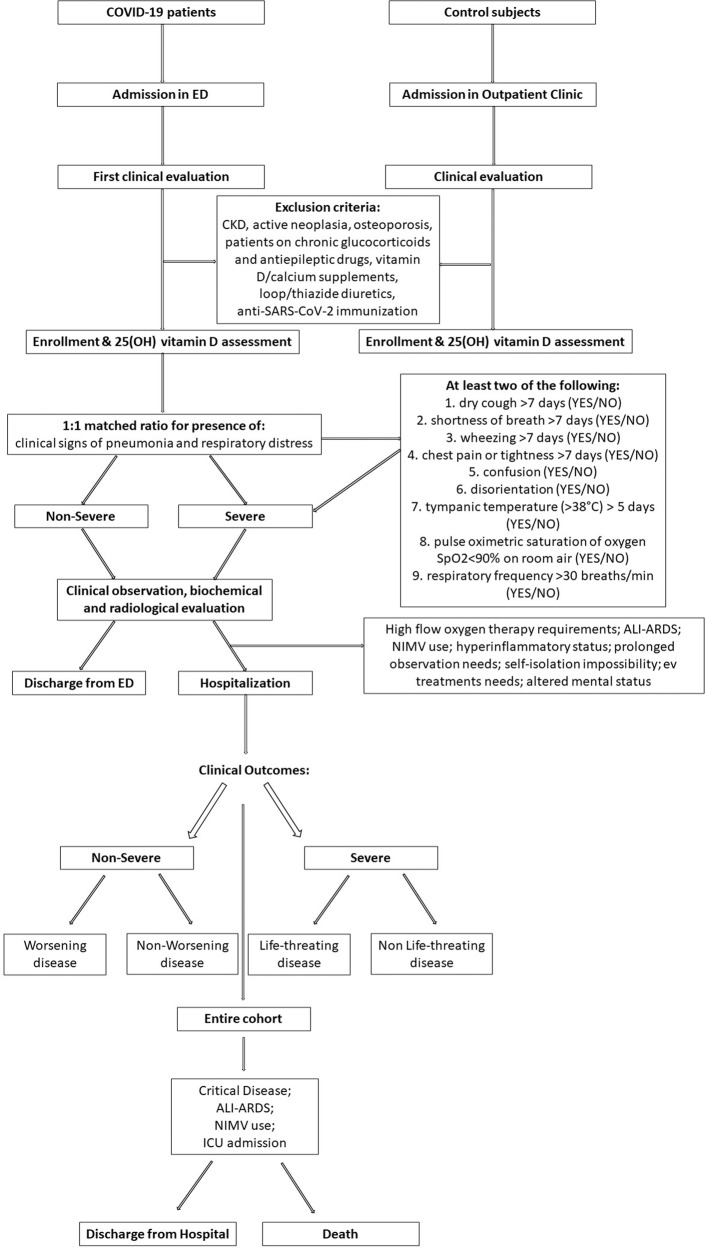
At first admission in-hospital emergency department (ED), before performing any biochemical or radiological evaluations, patients were consecutively enrolled in a matched for age, sex and comorbidities 1:1 ratio based on the presence or not of clinical signs of pneumonia and respiratory-distress (i.e., detection of at least two of the following signs and symptoms of lower respiratory disease longer than seven days [dry cough (YES/NO); shortness of breath (YES/NO); wheezing (YES/NO); chest pain or tightness (YES/NO)], general status impairment [confusion (YES/NO), disorientation (YES/NO)], fever (tympanic temperature >38 °C) longer than five days [YES/NO], pulse oximetric saturation of oxygen SpO2 < 90% on room air [YES, NO], respiratory frequency >30 breaths/min [YES, NO]) defining those with severe and non-severe disease [39]. This differentiation was confirmed by subsequent biochemical and radiological examinations (chest X-rays and/or CT scan) performed during the same day. Control matched subjects were enrolled from the outpatient Endocrinology Unit of the same hospital during the same time-period. The study design and the enrollment flow chart are summarized in Fig. 1.
Fig. 1.
Study design and enrollment flow chart
Data collection
Data were collected directly by patient interview or from medical chart review and entered in a dedicated electronic case record form specifically developed for the study. Before the analysis, data were cross-checked with medical charts and verified by data managers and clinicians for accuracy. For this study the following variables were collected: age, sex, body mass index (BMI) (calculated as the ratio of weight in kilograms divided by height in meters squared; overweight was defined as a BMI > 25 kg/m2 [40]), SpO2/FiO2 and PaO2/FiO2 ratios (calculated as the ratio between the pulse oximetric saturation and arterial partial pressure of oxygen, respectively, and the fraction of inspired oxygen), high-sensitivity C-reactive protein (CRP, mg/dL), ferritin (ng/mL) on admission to the ED, comorbidities (including history of hypertension, diabetes mellitus, cardiovascular disease, active and history of neoplasia, chronic kidney disease [CKD]), and clinical outcomes occurred during the entire disease course (hospitalization, need for non-invasive mechanical ventilation [NIMV], admission to intensive care unit [ICU], and mortality).
25(OH) vitamin D was measured, at study enrollment in ED, by Roche Cobas 8000 WKC/MET/036 using electrochemiluminescence immunoassays (ECLIA) (ng/mL) (coefficient of variation of 5%). Vitamin D deficiency was defined as 25(OH) vitamin D levels below 20 ng/mL, according to the cut-off values reported by Sempos et al. [41]. Ionized serum calcium (Ca2+) was measured on arterial blood gas test (RapidPoint 500 Analyzer, Siemens Healthcare, VA, USA, mmol/L) and hypocalcemia was defined as a Ca2+ level below 1.18 mmol/L.
Circulating sACE-2 levels (ng/mL) were measured by Human ACE-2 enzyme-linked immunosorbent assay (ELISA) Kit (no. MBS849243 MyBioSource) (detection range: 0.391 ng/mL–25 ng/mL).
Patients were classified as severe and non-severe according to the presence or absence of clinical signs of pneumonia and respiratory-distress at first admission [39]. Worsening disease was defined by the occurrence of critical clinical course (critical COVID-19) in patients who were initially characterized as non-severe in ED, during the entire clinical course of the disease. Critical COVID-19 was defined in the entire cohort by the requirement of high flow oxygen therapy and/or NIMV at admission in ED or during the hospitalization and/or ICU admission and/or those who died for COVID-19 related complications, evaluating the entire clinical course of the disease [42]. Life-threatening disease was defined in the severe group by the occurrence of serious and fatal outcomes including the requirement of NIMV and/or ICU admission and/or death for COVID-19 related complications, evaluating the entire clinical course of the disease. Hyperinflammation was defined in the entire cohort as an increase in either the serum inflammation marker CRP (≥100 mg/L) or ferritin (≥900 ng/mL), or both, evaluating the entire clinical course of the disease [43]. Based on PaO2/FiO2 ratio values and on degree of hypoxemia, ARDS and ALI were defined as PaO2/FiO2 ≤ 200 and ≤300 mmHg, respectively, evaluating the entire clinical course of the disease [44, 45]. High flow oxygen therapy, NIMV use, and ICU admission requirement were decided on the basis of severity of patients’ respiratory impairment [44, 45] and physicians experience. Needs of hospitalization was based on patients’ disease severity, patients’ social conditions (i.e., self-isolation impossibility) and physicians decision to prudently prolong or not the hospital observation.
Patients with the following comorbidities and concomitant active therapies influencing vitamin D metabolism were excluded: CKD, active neoplasia, osteoporosis, patients on chronic glucocorticoids and antiepileptic drugs, vitamin D/calcium, loop/thiazide diuretics, and patients with an estimated glomerular filtration rate of less than or equal to 30 mL/min/1.73 m2 using creatinine levels at initial evaluation. In addition, also patients with at least one anti-SARS-CoV-2 immunization dose were not enrolled.
Statistical analysis
Descriptive statistics were obtained for all study variables. Categorical variables were summarized as counts and percentages. Kolmogorov–Smirnov normality test was performed (p < 0.05) and continuous variables were expressed as medians and interquartile range [IQR] [25th–75th percentile]. Fisher exact test or χ2 test and the Wilcoxon signed-rank test or the Kruskal–Wallis test were used to determine the statistical significance of differences in proportions and medians, respectively. Correlations were analysed using the Spearman rank correlation analysis. Multiple logistic regression analyses were used to estimate the odds ratios (ORs) with 95% confidence interval (CI) of variables included in the study for the different outcomes evaluated. The performances of 25(OH) vitamin D levels in predicting the different outcomes were estimated using the area under the receiver operating characteristic curve (AUROC) with the 95% CI, and the optimal cut-off value was chosen to maximize the sum of the sensitivity and specificity on the Youden index. All statistical tests were two-sided. P value of <0.05 was considered statistically significant. Statistical analysis was conducted using IBM SPSS Statistics (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) and GraphPad Prism (GraphPad Software for Windows, version 9.0.0, San Diego, California USA).
Results
COVID-19 patients vs. control subjects
A total of 73 COVID-19 patients and 30 control subjects were enrolled in this study. Statistical differences regarding demographic characteristics and concomitant comorbidities prevalence between these groups are summarized in Table 1.
Table 1.
Demographic characteristics, concomitant comorbidities and vitamin D status in COVID-19 patients and control subjects
| COVID-19 patients (n.73) | Control subjects (n.30) | P value | |
|---|---|---|---|
| Age, years | 68 [54–73] | 65 [62–70] | 0.89 |
| Male gender, n. (%) | 43 (58.9%) | 18 (60%) | >0.99 |
| BMI, kg/m2 | 27.1 [25–31] | 26.6 [24–28.1] | 0.1 |
| Overweight, n. (%) | 55 (75.3%) | 21 (70%) | 0.4 |
| Hypertension, n. (%) | 42 (57.5%) | 20 (66.7%) | 0.5 |
| Cardiovascular disease, n. (%) | 10 (13.7%) | 5 (16.7%) | 0.76 |
| Diabetes mellitus, n. (%) | 20 (27.4%) | 5 (16.7%) | 0.31 |
| History of neoplasia, n. (%) | 8 (11%) | 5 (16.7%) | 0.52 |
| 25(OH) vitamin D levels, ng/mL | 13.8 [8.8–20.1] | 23.6 [16.3–28.2] | <0.001 |
| Vitamin D deficiency, n. (%) | 55 (75%) | 13 (43%) | 0.002 |
n. number, BMI body mass index
P values reported in bold are those statistically significant
Median [IQR] age of COVID-19 patients was 68 [54–73] years and 43 (58.9%) were male. The most frequent concomitant comorbidity in this cohort was history of hypertension (57.5%), and none of the patients was affected by active malignancy and/or CKD. There were no statistical differences regarding demographic characteristics and comorbidity prevalence between COVID-19 and control cohort (Table 1).
Median 25(OH) vitamin D level in the entire cohort was 16.2 [10.4–25.1] ng/mL and vitamin D deficiency was found in 68 patients (66%). Although there was no difference in demographic characteristics, we observed lower 25(OH) vitamin D levels and higher prevalence of vitamin D deficiency in COVID-19 patients than control subjects (13.8 [8.8–20.1] vs. 23.6 [16.3–28.2] ng/mL, p < 0.001) (75% vs. 43%, p = 0.002).
25(OH) Vitamin D in COVID-19 patients: descriptive and correlation analyses
Baseline data: the whole COVID-19 cohort
A total of 73 COVID-19 patients were prospectively enrolled in the study in a 1:1 ratio for the presence or not of severe disease matched for age, sex, and comorbidities. The cohort included 36 (49.3%) and 37 (50.7%) patients with and without severe disease, respectively.
In the whole COVID-19 cohort, we observed a higher prevalence of vitamin D deficiency in male patients than female (83.7% vs. 63.3%; p = 0.047), and in those overweight than normal weight (81.8% vs. 50%, p = 0.013). No other statistically significant differences were found according to vitamin D deficiency status.
Sixty-two patients (84.9%) were hospitalized, and 11 (15.1%) patients were discharged from the hospital, respectively, after the evaluation in ED. Those who were discharged did not require any other following in-hospital evaluations for the management of their acute illness. In those hospitalized, critical COVID-19 occurred in 46 patients (64%), an ALI-ARDS form in 43 (60%), hyperinflammation in 32 (45%), and 18 (24.7%) and 8 (11%) patients required NIMV and ICU admission, respectively. Three patients (4.1%) died for COVID-19 related complications.
No statistical differences were found regarding demographic characteristics and comorbidity prevalence in patients with critical and/or ALI-ARDS COVID-19 form as compared to those without, as well between those with or without hyperinflammation. A higher BMI was observed in patients who required hospitalization as compared to those non-hospitalized (28.1 [25.6–32] vs. 26 [20.3–27] kg/m2, p = 0.005) (Table 2), and/or NIMV (31 [28–36] vs. 26.3 [25–29] kg/m2, p = 0.003) and/or ICU admission (33 [31–37] vs. 26.5 [25–29.7] kg/m2, p = 0.014), and no other statistical differences were found regarding demographic characteristics and comorbidity prevalence in these groups. Five patients presented at admission a glomerular filtration rate between 30 and 60 mL/min/1.73 m2; three were in severe and two in non-severe group (p = 0.67), and no statistical differences were observed regarding their outcomes compared to the other patients. Median Ca2+ level was 1.15 [1.11–1.17] mmol/L and hypocalcemia was observed in 55 patients (75%). We observed significantly lower Ca2+ levels (but not significantly higher prevalence of hypocalcemia) in patients with critical vs non critical COVID-19 (1.12 [1.1–1.17] vs. 1.15 [1.13–1.19] mmol/L, p = 0.028), and in those requiring hospitalization (1.13 [1.1–1.17] vs. 1.16 [1.15–1.21] mmol/L, p = 0.013), NIMV (1.12 [1.09–1.15] vs. 1.15 [1.11–1.18] mmol/L, p = 0.039), and ICU admission (1.1 [1.07–1.13] vs. 1.15 [1.11–1.17] mmol/L, p = 0.028), as compared to patients who did not require these measures.
Table 2.
Demographic characteristics and concomitant comorbidities prevalence differences in whole patients hospitalized and not hospitalized
| Hospitalized (n.62) | Non-Hospitalized (n.11) | P value | |
|---|---|---|---|
| Age, years | 68 [55–74] | 53 [39–70] | 0.1 |
| Male gender, n. (%) | 37 (59.6%) | 6 (54.5%) | 0.75 |
| BMI, kg/m2 | 28.1 [25.6–32] | 26 [20.3–27] | 0.005 |
| Overweight, n. (%) | 49 (79%) | 6 (54.5%) | 0.12 |
| Hypertension, n. (%) | 35 (56%) | 7 (63.6%) | 0.75 |
| Cardiovascular disease, n. (%) | 9 (14.5%) | 1 (9%) | >0.99 |
| Diabetes mellitus, n. (%) | 18 (29%) | 2 (18%) | 0.71 |
| History of neoplasia, n. (%) | 8 (13%) | 0 (0%) | 0.59 |
n. number, BMI body mass index
P values reported in bold are those statistically significant
We observed significantly lower 25(OH) vitamin D levels in patients with critical disease, with an ALI-ARDS form and those with hyperinflammation, as compared to those without (Table 3). Furthermore, we found significant lower 25(OH) vitamin D levels in patients who required NIMV and ICU admission, as compared to those who did not (Table 3). We showed more frequent vitamin D deficiency in patients with critical COVID-19, in those with ALI-ARDS forms, and who required NIMV and ICU admission (Table 3).
Table 3.
25(OH) vitamin D levels and vitamin D deficiency prevalence among the different disease outcomes and characteristics in whole cohort of COVID-19 patients
| 25(OH) vitamin D levels (ng/mL) | P value | ||
|---|---|---|---|
| Hospitalization, yes/no | Yes (n.62): 13.5 [8–20] | No (n.11): 13.2 [10–22] | 0.78 |
| NIMV, yes/no | Yes (n.18): 8.5 [6–16] | No (n.55): 14.4 [11–23] | 0.003 |
| ICU, yes/no | Yes (n.8): 6.9 [6–10.4] | No (n.65): 14 [9.7–21] | 0.01 |
| Death, yes/no | Yes (n.3): 13 [8–22] | No (n.70): 13.2 [9–20] | 0.55 |
| Critical disease, yes/no | Yes (n.46): 11.7 [7–17] | No (n.27): 16.7 [11–31] | 0.007 |
| ALI-ARDS, yes/no | Yes (n.43): 10.8 [7–11] | No (n.30): 17 [13–29] | <0.001 |
| Hyperinflammation, yes/no | Yes (n.32): 11.2 [7.8–17] | No (41): 16.2 [10.7–23.2] | 0.039 |
| Vitamin D deficiency, n. (%) | |||
|---|---|---|---|
| Hospitalization, yes/no | Yes: 47 (75.8%) | No: 8 (72.7%) | >0.99 |
| NIMV, yes/no | Yes: 17 (94%) | No: 38 (69%) | 0.032 |
| ICU, yes/no | Yes: 7 (87%) | No: 48 (74%) | 0.67 |
| Death, yes/no | Yes: 1 (33.3%) | No: 54 (77%) | 0.15 |
| Critical disease, yes/no | Yes: 39 (85%) | No: 16 (59%) | 0.015 |
| ALI-ARDS, yes/no | Yes: 39 (91%) | No: 16 (53%) | 0.001 |
| Hyperinflammation, yes/no | Yes: 28 (87%) | No: 27 (66%) | 0.054 |
n. number, NIMV non-invasive mechanical ventilation, ICU intensive care unit, ALI Acute Lung Injury, ARDS Acute Respiratory-Distress Syndrome
P values reported in bold are those statistically significant
Patients with vitamin D deficiency presented significant lower levels of SpO2/FiO2 and PaO2/FiO2 ratios (SpO2/FiO2: 433 [408–448] vs. 447 [438–458], p = 0.005) (PaO2/FiO2: 261 [303–230] vs. 311 [298–357], p < 0.001) and a non-significant trend toward lower Ca2+ levels (1.13 [1.1–1.15] vs. 1.15 [1.11–1.17] mmol/L, p = 0.081) as compared to those without vitamin D deficiency. In correlation analyses, there was a significantly positive correlation between 25(OH) vitamin D levels and SpO2/FiO2 and PaO2/FiO2 ratios (p = 0.021 r = 0.32; p < 0.001 r = 0.48; respectively).
Considering the whole cohort, higher sACE-2 levels were observed in patients with critical disease as compared to those without (1.47 [0.7–3.8] vs. 0.97 [0.5–1.65] ng/mL, p = 0.025). In correlation analyses, significant negative correlations were found between 25(OH) vitamin D and sACE-2 levels (p = 0.027 r = −0.3) and patients with vitamin D deficiency had significantly higher sACE-2 levels than those without vitamin D deficiency (1.37 [0.6–3.76] vs. 1 [0.47–1.65] ng/mL, p = 0.04).
Severe COVID-19 patients
In ED were prospectively enrolled 36 patients with severe disease, matched for age, sex, and comorbidities with non-severe patients. No statistical differences were found regarding demographic characteristics, comorbidity prevalence, 25(OH) vitamin D and sACE-2 levels between these two groups (Table 4).
Table 4.
Demographic characteristics and concomitant comorbidities prevalence differences in severe and non-severe COVID-19 groups
| Severe (n.36) | Non-Severe (n.37) | P value | |
|---|---|---|---|
| Age, years | 68 [56–74] | 64 [52–73] | 0.32 |
| Male gender, n. (%) | 21 (58.3%) | 22 (59%) | >0.99 |
| BMI, kg/m2 | 29.1 [25.2–32] | 27.7 [25–29.7] | 0.28 |
| Overweight, n. (%) | 27 (75%) | 28 (76%) | >0.99 |
| Hypertension, n. (%) | 20 (55.5%) | 22 (59.4%) | 0.81 |
| Cardiovascular disease, n. (%) | 6 (16.6%) | 4 (11%) | 0.51 |
| Diabetes mellitus, n. (%) | 11 (30.5%) | 9 (24.3%) | 0.6 |
| History of neoplasia, n. (%) | 5 (13.8%) | 3 (8.1%) | 0.48 |
| 25(OH) vitamin D levels, ng/mL | 12.3 [8–18.9] | 13.9 [9.9–22.6] | 0.33 |
| Vitamin D deficiency, n. (%) | 29 (80%) | 26 (70%) | 0.42 |
| sACE-2, ng/mL | 1.48 [0.56–3.47] | 1.01 [0.58–1.74] | 0.36 |
n. number, BMI body mass index, sACE-2 soluble angiotensin-converting enzyme 2
In the severe group (n.36), all patients (100%) were hospitalized after the evaluation in ED. Critical COVID-19 was observed in all severe patients (100%), an ALI-ARDS form in 29 (80.6%), hyperinflammation in 18 (50%). Moreover, 16 (44.4%) and 7 (19.4%) patients required NIMV and ICU admission, respectively. Three patients (8.3%) died for COVID-19 related complications. Life-threatening disease occurred in 17 patients (47.2%).
No statistical differences were found regarding demographic characteristics and comorbidity prevalence in patients with ALI-ARDS COVID-19 form as compared to those without, as well between those with or without hyperinflammation, and those admitted and not to ICU. BMI was higher in patients with life-threatening disease than those without (31 [26.4–35.5] vs. 26.2 [25–31] kg/m2, p = 0.033) and those who required NIMV than those did not need it (31 [26.4–35.5] vs. 26 [24.8–31] kg/m2, p = 0.02), and no other statistical differences were found regarding demographic characteristics and comorbidity prevalence between these groups.
In severe COVID-19 group, we observed significantly lower 25(OH) vitamin D levels in patients with an ALI-ARDS form as compared to those without, who required NIMV and ICU admission as compared to those who did not require, and we showed more frequent vitamin D deficiency in patients with ALI-ARDS form (Table 5). sACE-2 levels were found similar between these groups, particularly between those with or without life-threatening disease (1.64 [0.83–3.8] vs. 1.15 [0.48–2.5] ng/mL, p = 0.41).
Table 5.
25(OH) vitamin D levels and vitamin D deficiency prevalence among the different disease outcomes and characteristics in severe COVID-19 patients (n.36)
| 25(OH) vitamin D levels (ng/mL) | P value | ||
|---|---|---|---|
|
Life-threatening disease, yes/no |
Yes (n.17): 9.3 [6.5–18.2] | No (n.19): 13.9 [10–23.4] | 0.06 |
| NIMV, yes/no | Yes (n.16): 9.1 [6.5–16.7] | No (n.20): 14.5 [10.2–23] | 0.026 |
| ICU, yes/no | Yes (n.7): 7.3 [6.5–10.8] | No (n.29): 13.9 [9–19.4] | 0.04 |
| Death, yes/no | Yes (n.3): 13 [8–22] | No (n.33): 12.2 [8–17.6] | 0.4 |
| ALI-ARDS, yes/no | Yes (n.29): 15.9 [11.4–29] | No (n.7): 23.6 [16.3–28] | <0.001 |
| Hyperinflammation, yes/no | Yes (n.18): 10.9 [7.4–17.5] | No (n.18): 15.4 [8.2–24.2] | 0.21 |
| Vitamin D deficiency, n. (%) | |||
|---|---|---|---|
|
Life-threatening disease, yes/no |
Yes: 15 (85%) | No: 14 (73%) | 0.41 |
| NIMV, yes/no | Yes: 15 (94%) | No: 14 (70%) | 0.1 |
| ICU, yes/no | Yes: 6 (86%) | No: 23 (79%) | 0.99 |
| Death, yes/no | Yes: 1 (33.3%) | No: 28 (84%) | 0.1 |
| ALI-ARDS, yes/no | Yes: 27 (93%) | No: 2 (28%) | 0.001 |
| Hyperinflammation, yes/no | Yes: 17 (94%) | No: 12 (67%) | 0.08 |
n. number, NIMV non-invasive mechanical ventilation, ICU intensive care unit, ALI Acute Lung Injury, ARDS Acute Respiratory-Distress Syndrome
P values reported in bold are those statistically significant
Non-severe COVID-19 patients
In ED we prospectively enrolled 37 patients with non-severe disease, matched for age, sex, and comorbidities with patients with severe disease. As reported above, no statistical differences were found regarding demographic characteristics, comorbidity prevalence and 25(OH) vitamin D levels between these two groups (Table 4).
In the non-severe group (n.37), 26 patients (70%) were hospitalized after the evaluation in ED. Worsening disease was observed in 10 patients (27%), an ALI-ARDS form in 14 (37.8%), hyperinflammation in 14 (37.8%). Two (5.4%) and one (2.7%) patient required NIMV and ICU admission, respectively. No patients died for COVID-19 related complications.
BMI was higher in hospitalized patients as compared to those discharged from the ED (28 [26–30.5] vs. 26 [20.3–27] kg/m2, p = 0.01). No statistical differences were found regarding demographic characteristics and comorbidities between patients with or without ALI-ARDS form, hyperinflammation, worsening disease, NIMV requirement, and ICU admission.
In non-severe COVID-19 group, we observed significantly lower 25(OH) vitamin D levels in patients with worsening disease, critical disease and an ALI-ARDS form as compared to those without, and who required NIMV and ICU admission as compared to those who did not require them, and we observed more frequent vitamin D deficiency in patients with worsening disease (Table 6). sACE-2 levels were similar between these groups, particularly between those with or without worsening disease (1.14 [0.83–4.26] vs. 0.97 [0.5–1.65] ng/mL, p = 0.19).
Table 6.
25(OH) vitamin D levels and vitamin D deficiency prevalence among the different disease outcomes and characteristics in non-severe COVID-19 patients (n.37)
| 25(OH) vitamin D levels (ng/mL) | P value | ||
|---|---|---|---|
| Hospitalization, yes/no | Yes (n.26): 14.1 [9.6–25] | No (n.11): 13.2 [9.8–22] | 0.78 |
|
Worsening, yes/no |
Yes (n.10): 8.6 [5.7–14] | No (n.27): 16.7 [11.4–31] | 0.004 |
| NIMV, yes/no | Yes (n.2): 6.2 [5.8–6.3] | No (n.35): 14.4 [10.6–23.1] | 0.04 |
| ICU, yes/no | Yes (n.1): 5.8 [5.8–5.8] | No (n.36): 14.1 [10.1–22.9] | 0.22 |
| ALI-ARDS, yes/no | Yes (n.14): 11 [5.7–18.9] | No (n.23): 15.9 [11.4–28.7] | 0.03 |
| Hyperinflammation, yes/no | Yes (n.14): 11.2 [7.9–19.1] | No (n.23): 16.7 [11.4–23.1] | 0.21 |
| Vitamin D deficiency, n. (%) | |||
|---|---|---|---|
| Hospitalization, yes/no | Yes: 18 (69%) | No: 8 (73%) | >0.99 |
|
Worsening, yes/no |
Yes: 10 (100%) | No: 16 (59%) | 0.018 |
| NIMV, yes/no | Yes: 2 (100%) | No: 24 (68%) | >0.99 |
| ICU, yes/no | Yes: 1 (100%) | No: 25 (69%) | >0.99 |
| ALI-ARDS, yes/no | Yes: 12 (86%) | No: 14 (61%) | 0.15 |
| Hyperinflammation, yes/no | Yes: 11 (78%) | No: 15 (65%) | 0.47 |
n. number, NIMV non-invasive mechanical ventilation, ICU intensive care unit, ALI Acute Lung Injury, ARDS Acute Respiratory-Distress Syndrome
P values reported in bold are those statistically significant
25(OH) vitamin D in COVID-19 patients: multiple logistic regression analyses and ROC curves
Multiple regression analyses including demographic characteristics, comorbidities, and 25(OH) vitamin D levels were performed for all outcomes of the study to evaluate the role of 25(OH) vitamin D levels as outcome predictor in COVID-19 patients.
In non-severe group, lower 25(OH) vitamin D level resulted as the only variable significantly and independently associated with the worsening disease (p = 0.026, OR 1.32 CI 1.03–1.68) (Table 7). As well, also in severe group, 25(OH) vitamin D level was the only variable with a trend although not statistically significant toward an independent association with the life-threatening disease (p = 0.081, OR 1.14 CI 0.96–1.35) (Table 8).
Table 7.
Multiple logistic regression analysis of possible risk factors to predict worsening disease in non-severe patients at admission
| OR | 95% CI | P value | |
|---|---|---|---|
| Sex | 0.22 | 0.014–3.53 | 0.29 |
| BMI | 0.82 | 0.64–1.05 | 0.12 |
| Age | 0.94 | 0.842–1.04 | 0.22 |
| Hypertension | 3.1 | 0.18–5.3 | 0.43 |
| Cardiovascular disease | 0.2 | 0.008–5.21 | 0.33 |
| Diabetes mellitus | 0.64 | 0.026–1.61 | 0.78 |
| History of neoplasia | 0.21 | 0.005–8.1 | 0.39 |
| 25(OH) vitamin D levels | 1.32 | 1.03–1.68 | 0.026 |
BMI body mass index, OR odds ratio, CI confidence interval
P values reported in bold are those statistically significant
Table 8.
Multiple logistic regression analysis of possible risk factors to predict life-threatening disease in severe patients at admission
| OR | 95% CI | P value | |
|---|---|---|---|
| Sex | 0.5 | 0.04–6.54 | 0.6 |
| BMI | 1.009 | 0.77–1.32 | 0.9 |
| Age | 1.08 | 0.96–1.21 | 0.19 |
| Hypertension | 0.1 | 0.003–3.61 | 0.21 |
| Cardiovascular disease | 0.8 | 0.09–2.72 | 0.75 |
| Diabetes mellitus | 0.24 | 0.01–4.46 | 0.34 |
| History of neoplasia | 0.81 | 0.03–2.15 | 0.9 |
| 25(OH) vitamin D levels | 1.14 | 0.96–1.35 | 0.081 |
BMI body mass index, OR odds ratio, CI confidence interval
P values reported in bold are those statistically significant
In the whole population, lower 25(OH) vitamin D level resulted together with age and sex independently associated with NIMV (p = 0.008, OR 1.17 CI 1.04–1.31), and with critical disease (p = 0.01, OR 1.12 CI 1.03–1.21), and, as the only variable independently associated with ALI-ARDS form (p = 0.013, OR 1.1 CI 1.02–1.15). No significant associations of 25(OH) vitamin D level were found for the other outcomes. Regarding worsening outcomes in whole COVID-19 population in combination with worsening disease in non-severe group and life-threatening disease in severe group, lower 25(OH) vitamin D levels (p = 0.002, OR 1.161 CI 1.057–1.275) were a significant risk factor whereas lower BMI was protective (p = 0.031, OR 0.865 CI 0.758–0.987) (Table 9).
Table 9.
Multiple logistic regression analysis of possible risk factors to predict worsening disease in non-severe and life-threatening disease in severe patients at admission
| OR | 95% CI | P value | |
|---|---|---|---|
| Sex | 0.347 | 0.088–1.365 | 0.13 |
| BMI | 0.865 | 0.758–0.987 | 0.03 |
| Age | 0.996 | 0.942–1.053 | 0.88 |
| Hypertension | 0.649 | 0.146–2.894 | 0.57 |
| Cardiovascular disease | 3.124 | 0.442–22.092 | 0.25 |
| Diabetes mellitus | 0.576 | 0.125–2.652 | 0.47 |
| History of neoplasia | 0.349 | 0.036–3.346 | 0.36 |
| 25(OH) vitamin D levels | 1.161 | 1.057–1.275 | 0.002 |
BMI body mass index, OR odds ratio, CI confidence interval
P values reported in bold are those statistically significant
ROC analyses showed that the global performances of 25(OH) vitamin D level to predict worsening disease in non-severe patients, and critical disease, NIMV requirement, ICU admission and ALI-ARDS in the whole cohort forms were significant. The best Youden Index J for the 25(OH) vitamin D value in predicting worsening disease was 11.55 ng/mL (74% sensitivity, 70% specificity and AUROC 80% (p = 0.005, CI 0.65–0.96)); in predicting NIMV requirement was 9.4 ng/mL (84% sensitivity, 61% specificity and AUROC 73% (p = 0.003, CI 0.6–0.87)); in predicting ICU admission was 9.4 ng/mL (78% sensitivity, 75% specificity and AUROC 78% (p = 0.01, CI 0.6–0.96)); in predicting critical disease was 12.85 ng/mL (67% sensitivity, 57% specificity and AUROC 69% (p = 0.007, CI 0.56–0.81)); in predicting ALI-ARDS forms was 12.85 ng/mL (77% sensitivity, 65% specificity and AUROC 76% (p < 0.001, CI 0.64–0.86)). Regarding worsening outcomes in whole COVID-19 population in combination with worsening disease in non-severe group and life-threatening disease in severe group, the best Youden Index J for the 25(OH) vitamin D value in predicting worsening outcomes was 11.55 ng/mL (74% sensitivity, 69% specificity and AUROC 75% (p = 0.001, CI 0.62–0.86)) (Fig. 2).
Fig. 2.
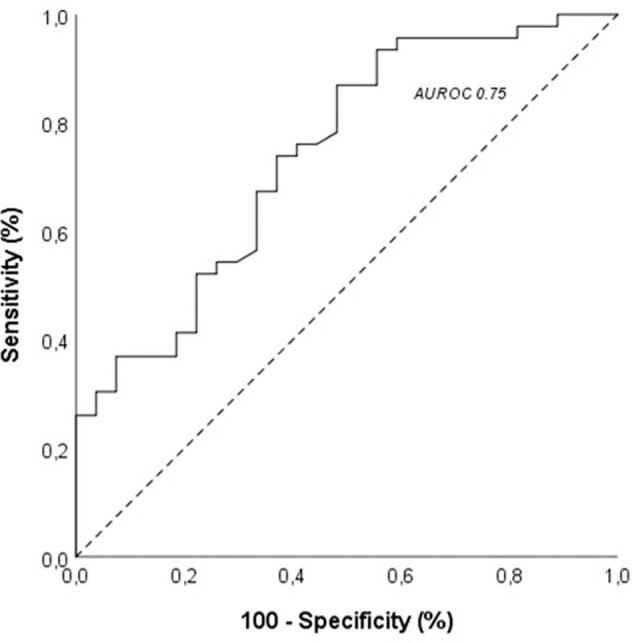
Correlation and receiver operating characteristic (ROC) analysis and area under the receiver operating characteristic curve (AUROC) of 25(OH) vitamin D levels to predict worsening disease in non-severe and life-threatening disease in severe patients at admission
Discussion
The main finding of our prospective controlled study was that 25(OH) vitamin D levels at hospital admission strongly predicted the occurrence of critical disease and worse outcomes independently of the severity of the disease at presentation and of all other clinical and biochemical factors so far associated with negative prognosis in COVID-19 patients.
Vitamin D metabolites are known to support the antimicrobial and antiviral immune responses through several mechanisms of action and regulate the adaptive immune response. They promote a shift from pro-inflammatory to tolerogenic state by downregulating the immune responses mediated by T-helper-1 lymphocytes cells and inhibiting the production of pro-inflammatory cytokines [3, 4]. Interestingly, causality between poor vitamin D status and severity of COVID-19 was hypothesized. In fact, in vitro data suggest that vitamin D is among the molecules that might be able to attenuate the effects of COVID-19 through its effects on gene expression [46, 47]. Many retrospective case–control studies, cohort studies, as well as meta-analyses of observational studies, revealed inverse associations between serum 25(OH) vitamin D level and the risk of developing severe COVID-19, including an increased risk of mortality, ICU admission, length of ICU stay, and need for mechanical ventilation. However, despite several observational studies and recently published meta-analyses [48–51] reported significant benefits of vitamin D supplementation in terms of fewer rates of ICU admission and mortality, a recent randomized trial was not able to show a positive impact of fixed supplementation doses of vitamin D in COVID-19 patients [52].
A retrospective observational study found that low 25(OH) vitamin D levels associated with severity of lung involvement as assessed by CT in male patients with COVID-19 [53]. Also, a negative impact of vitamin D deficiency, usually at hospital admission, on mortality besides other clinical endpoints of severe COVID-19 independently of other clinical risk factors was also reported [54–58]. However, available studies are not concordant since a recent large Mendelian randomization study based on the UK Biobank, suggested no association between pre-existing hypovitaminosis D, as resulting from serum 25(OH) vitamin D levels assayed on samples obtained several years before, and with development of severe COVID-19 [59]. In fact, the two major causes for these discrepant evidences and hence of uncertainty in the field were suggested to be the patients selection bias and the difficulty to demonstrate causality. In reference to the former aspect, enrollment of heterogeneous patient groups with largely different degrees of disease severity in the emergency setting which characterized the first wave of the pandemic, lack of control population and of adjustment for already ascertained predictive factors such as diabetes and obesity with which vitamin D deficiency is known to associate [10] appear to be the major issues. Concerning the latter aspect several authors have hypothesized that 25(OH) vitamin D levels may be just decreased by the acute illness per se [60] representing not the (con)cause of severe COVID-19 but the effect implying therefore a possible reverse causality [35, 38].
Interestingly, the design of our study allowed us to overcome the limitations of previous studies since also thanks to a decreased hospital emergency pressure it was possible to subdivide patients at first hospital visit in those with severe and non-severe conditions based on clinical judgment which was subsequently confirmed by biochemical and radiologic data. This gave us the chance to verify if severity of disease per se was able to affect 25(OH) vitamin D levels and exclude that the negative outcomes could only have been just the result of a combination of clinical factors already conditioning the presentation of the disease to which vitamin D was just associated. Moreover, the careful selection of a control population allowed us to correct our COVID-19 patients data for the large prevalence of hypovitaminosis D in our Country. In fact, in our whole study population vitamin D deficiency, as defined by stringent criteria [41], was frequent in line with Italian epidemiological data [61]. Noteworthy, in our COVID-19 cohort, vitamin D deficiency appeared a strikingly prevalent feature observed in three-quarters of them at hospital evaluation being significantly more prevalent (with lower absolute levels) than in control subjects matched for age, sex and comorbidities and enrolled during the same period time. These data were consistent with the results of different systematic reviews and meta-analyses reporting association of higher risk of SARS-CoV-2 infection with lower 25(OH) vitamin D levels with the odds ratios ranging from 1.43 to 2.71 [6, 8, 9]. Our data reinforced and improved these findings since most of the studies included in these meta-analyses were affected by several biases with control subjects and COVID-19 patients often different in age, concomitant comorbidities, and timing of 25(OH) vitamin D evaluation, performed often only during the hospitalization and therefore possibly influenced by the disease progression, institutionalization, and concomitant systemic therapeutic treatment. In our study, no differences were found between control and COVID-19 cohorts regarding demographics and past medical history. 25(OH) vitamin D levels, in agreement with the previous literature, were 40% lower in the COVID-19 cohort than controls with a higher prevalence of vitamin D deficiency of 75%, despite the high prevalence of hypovitaminosis D (43%) found also in the control population. However, if these comparative findings vs controls clearly demonstrate that poor vitamin D status is a distinctive feature of COVID-19 they could not clarify the issue of possible reverse causality due to the nature of the control population.
In fact, the higher susceptibility to SARS-CoV-2 infection observed in subjects with vitamin D deficiency might be related to the immunomodulatory role of vitamin D, previously associated with COVID-19 severity and worse outcome regarding ICU admission and mortality [6, 8, 9]. Also, these studies had several biases in terms of inclusion criteria and study design, and a few conducted prospectively reported contrasting results [13–17]. Instead, we enrolled patients in 1:1 ratio for the presence of severe disease at early evaluation in ED and matched for age, sex, and comorbidities, and the patients’ groups with and without severe outcomes were homogeneous.
Interestingly, we were unable to find significant differences in 25(OH) vitamin D levels between patients with different severity at first hospital evaluation. This observation is consistent with the hypothesis that while low vitamin D may predispose to SARS-CoV-2 infection it does not condition the initial clinical presentation of COVID-19 which is likely influenced by many other clinical factors. Moreover, it suggested that the disease per se is not able to influence 25(OH) vitamin D levels, the decrease of which seems based on our data unlikely to be an effect, i.e., a biochemical marker, of severity of the disease.
Importantly instead, the prospective evaluation particularly of patients who were firstly identified as affected by a not-severe COVID-19 disclosed that those who developed a worsening disease, presented lower 25(OH) vitamin D levels and higher prevalence of vitamin D deficiency at study enrollment as compared to those who did not worsen. In line with these findings, also those patients identified as severe at presentation in whom worse (life-threatening) outcomes occurred, presented lower 25(OH) vitamin D levels and higher prevalence of vitamin D deficiency at study enrollment as compared to those without a serious disease progression. These findings were confirmed in multiple regression analyses showing lower 25(OH) vitamin D level as an independent risk factor for different outcomes evaluated in this study. In particular, we observed that lower 25(OH) vitamin D level was the only variable statistically associated with the occurrence of a worsening disease in those firstly admitted in not-severe conditions. Additionally, 25(OH) vitamin D level remained a strong predictor of poor outcomes also combining severe patients at presentation who developed a life-threatening disease with non-severe patients who underwent worsening disease. Only in this specific analysis, and not in the separate analyses of severe and non-severe patients, also a higher BMI resulted as an independent risk factor for this worse outcome occurrence. On one hand, these findings suggest that BMI does not seem to play a crucial role in worsening of disease particularly in non-severe patients. On the other hand, this result is in line with the previous published data that early reported overweight, obesity and highly visceral adiposity as some of the main important clinical factors strongly and negatively associated with a more severe disease course of patients affected by SARS-CoV-2 infection [62–65]. In multiple regression analyses we did not observe an independent effect of male sex, although male patients in previous studies were consistently characterized by a worse COVID-19 and lower 25(OH) vitamin D levels [66].
Interestingly, ROC analyses confirmed the role of 25(OH) vitamin D levels in predicting the disease clinical course, reporting excellent AUROCs for different outcomes. In particular, the best cut-off 25(OH) vitamin D value for the occurrence of worse outcomes was around 12 ng/mL, the level recognized as the cut-off value to define the severe vitamin D deficiency status [41]. Currently, this threshold is mainly associated with an increased risk of rickets and osteomalacia, but our data support its use also to identify patients and subjects at higher risk to develop the more severe inflammatory and infectious complications.
Mechanistically, vitamin D deficient patients presented with higher levels of inflammatory parameters and a higher prevalence of hyperinflammation, associated with worse COVID-19 outcomes [43]. In patients with hyperinflammation, who had similar demographic, anthropometric and past medical history characteristics, we observed lower 25(OH) vitamin D levels and a higher prevalence of vitamin D deficiency.
This may be explained by vitamin D opposing the effect of RAAS promoting the expression of mACE-2, MasR, and Ang‐(1‐7) and inhibited the ACE/Ang II/AT1R axis favoring the conversion of angiotensin I (Ang I) to Ang 1–9 and Ang II into Ang 1–7 peptides which trigger vasodilation and have anti-inflammatory and antiproliferative properties protecting against organ injury in many human diseases, including cardiovascular disease, CKD, liver diseases and lung injury [18, 24, 25].
Vitamin D supplementation decreased lipopolysaccharide (LPS)‐induced ALI by inducing ACE-2/Ang‐(1‐7) axis and suppressing renin and the ACE/Ang II/AT1R axis [67] and enhancing the mRNA expression of ACE-2 receptor in rat models of LPS‐induced ALI [68]. Also, vitamin D supplementation decreased ACE concentration and ACE/ACE-2 ratio and enhanced ACE-2 concentration in diabetic rats [69]. In cell cultures, 1,25(OH)2 vitamin D directly suppressed renin gene transcription by a VDR-dependent mechanism [70, 71] and, in a large cohort of patients, 25(OH) and 1,25(OH)2 vitamin D were related to an upregulated RAAS activity and emerged as independent predictors of plasma renin and Ang II concentrations [72].
Consistently, we reported for the first time to our knowledge, a negative correlation between 25(OH) vitamin D and sACE-2 levels in COVID-19 patients, and patients with vitamin D deficiency were characterized by higher levels of sACE-2 as compared to those not deficient. These data could reflect the previously mentioned negative effects of vitamin D deficiency on RAAS activity with increased levels of sACE-2.
Soluble ACE-2 binds circulating SARS-CoV-2 viral S protein, with a similar pathway to mACE-2, forming sACE-2-virus complexes and then mediates viral entry into cells spreading the infection to other organs [73–75]. Supporting this potentially detrimental role, several studies have reported higher levels of sACE-2 in patients with a more severe COVID-19 and worse disease outcomes, proposing its clinical utility as a possible novel biochemical marker for disease severity, inflammation, and tissue damage in patients with SARS-CoV-2 infection [76–84]. Confirming the possible role as useful biomarker of disease severity of sACE-2 in COVID-19 patients, as previously reported in literature, we observed higher sACE-2 levels in patients with a critical vs non critical disease. However, interestingly, sACE-2 levels at presentation were not able to predict at odds with respect to 25(OH) vitamin D the worsening of disease either separately in severe or non-severe patients at presentation or in our global population. Therefore, it can be concluded that sACE-2 and vitamin D may have different roles in COVID-19. In fact, sACE-2 can represent a marker of the disease (and of vitamin D deficiency) whereas vitamin D may be a (con)cause of the poor outcome of COVID-19. In fact, the higher levels of sACE-2 in more severe patients may reflect an increased viral shedding and inactivation of mACE-2 reducing its anti-inflammatory effects and altering RAAS activity in favor of Ang II, which is seen in more severe COVID-19 patients [85–88].
Finally, we confirmed that the effects of 25(OH) vitamin D occur as part of a distinct and emerging osteo-metabolic COVID-19 phenotype typically observed in these patients [89, 90], characterized, in addition to the highly reported rates of vitamin D deficiency, by also a marked acute reduction of calcium levels in patients with a more severe disease, as reported by consistent previous published data [91, 92] and also confirmed in this study. In fact, although no statistical differences in prevalence of hypocalcemia between severe and non-severe patients, likely due at least in part to this quite widespread finding in our cohort, it cannot be excluded based on our data that hypocalcemia may be one other possible mechanism underlying negative prognostic role of low vitamin D levels in COVID-19 [93].
Our study has limitations. First of all, the number of enrolled patients was relatively limited but as previously mentioned this was due to the use of stringent entry criteria excluding those with comorbidities possibly affecting vitamin D metabolism, additionally, this, combined with decreased hospital emergency pressure, allowed us to carefully and accurately carry out a 1:1 enrollment in order to include the patients in two severity groups matched for age, sex and comorbidities. Therefore, the highly controlled nature of the study made it candidate to recognize at best the possible role of vitamin D deficiency in COVID-19 including possible reverse causality. Moreover, this study was conducted in a region heavily affected by vitamin D deficiency and potentially reducing the statistical ability to highlight this negative role in our patients. However, the enrollment of a matched control population was able to minimize this effect. Finally, the study was not designed specifically to evaluate the other biologically active forms of serum vitamin D or other parameters of bone metabolism such as serum phosphate and the whole biochemical ACE-2/RAAS axis and all its components. This latter aspect should be considered for further larger studies to clarify the underlying mechanisms of the relationship between vitamin D and sACE-2.
In conclusion, we reported a higher prevalence of vitamin D deficiency in patients affected by COVID-19 as compared to control subjects matched for age, sex and comorbidities; our study suggests that lower 25(OH) vitamin D levels are unlikely to be an effect of the severity of the underlying disease therefore not supporting key role for reverse causality between COVID-19 and vitamin D. In fact, we were able to provide evidence that levels below 12 ng/mL of 25(OH) vitamin D were independent predictors of worsening of the disease particularly in hospitalized non-severe patients. The clinical implications of these findings are extensive suggesting that repletion of vitamin D in deficient subjects, particularly in areas in which vitamin D deficiency is endemic, reaching levels above that threshold could reduce the susceptibility to the SARS-CoV-2 infection but even more importantly reduce the risk of a worsening of the disease also in patients with non-severe presentation at hospital admission thereby acting clinically in a similar way with respect to available vaccines [94, 95] and giving a sound pathophysiological basis for interventional studies in patients with non-severe disease and eventually for a possible synergistic effect of vitamin D repletion and vaccination in improving COVID-19 outcomes.
Acknowledgements
The authors wish to thank Abiogen Pharma S.p.A, Pisa, Italy, for supporting this study.
Data availability
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Compliance with ethical standards
Conflict of interest
F.N. is an employee of Abiogen Pharma S.p.A. A.G. is consultant for Abiogen and Takeda and received research grant to Institution from Takeda. All other authors have no competing interests to declare.
Ethics approval
The study protocol complies with the Declaration of Helsinki, was approved by the Hospital Ethics Committee (protocol ABIO/NC/03 no. 367/2020).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Luigi di Filippo, Melin Uygur, Massimo Locatelli, Fabrizio Nannipieri, Stefano Frara, Andrea Giustina
References
- 1.A. Giustina, A.M. Formenti, Does hypovitaminosis D play a role in the high impact of COVID infection in Italy? Brit. Med. J. (2020). https://www.bmj.com/content/368/bmj.m810/rr-36
- 2.Bouillon R, Marcocci C, Carmeliet G, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr. Rev. 2019;40(4):1109–1151. doi: 10.1210/er.2018-00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilezikian JP, Bikle D, Hewison M, et al. MECHANISMS IN ENDOCRINOLOGY: Vitamin D and COVID-19. Eur. J. Endocrinol. 2020;183(5):R133–R147. doi: 10.1530/EJE-20-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charoenngam N, Holick MF. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients. 2020;12(7):2097. doi: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO COVID-19 Dashboard. Geneva: World Health Organization, 2020. https://covid19.who.int/ (last cited: [09/20/2022])
- 6.Dissanayake HA, de Silva NL, Sumanatilleke M, et al. Prognostic and Therapeutic Role of Vitamin D in COVID-19: Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2022;107(5):1484–1502. doi: 10.1210/clinem/dgab892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hopefl R, Ben-Eltriki M, Deb S. Association Between Vitamin D Levels and Inflammatory Markers in COVID-19 Patients: A Meta-Analysis of Observational Studies. J. Pharm. Pharm. Sci. 2022;25:124–136. doi: 10.18433/jpps32518. [DOI] [PubMed] [Google Scholar]
- 8.Chiodini I, Gatti D, Soranna D, et al. Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes. Front. Public Health. 2021;9:736665. doi: 10.3389/fpubh.2021.736665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akbar MR, Wibowo A, Pranata R, Setiabudiawan B. Low Serum 25-hydroxyvitamin D (Vitamin D) Level Is Associated With Susceptibility to COVID-19, Severity, and Mortality: A Systematic Review and Meta-Analysis [published correction appears in Front Nutr. 2021 Sep 27;8:754539] Front. Nutr. 2021;8:660420. doi: 10.3389/fnut.2021.660420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.di Filippo L, Allora A, Doga M, et al. Vitamin D Levels Are Associated With Blood Glucose and BMI in COVID-19 Patients, Predicting Disease Severity. J. Clin. Endocrinol. Metab. 2022;107(1):e348–e360. doi: 10.1210/clinem/dgab599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman HW, Niles JK, Kroll MH, Bi C, Holick MF. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE. 2020;15(9):e0239252. doi: 10.1371/journal.pone.0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charoenngam N, Shirvani A, Reddy N, Vodopivec DM, Apovian CM, Holick MF. Association of Vitamin D Status With Hospital Morbidity and Mortality in Adult Hospitalized Patients With COVID-19. Endocr. Pr. 2021;27(4):271–278. doi: 10.1016/j.eprac.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogliolo L, Cereda E, Klersy C, et al. Vitamin D 25OH deficiency and mortality in moderate to severe COVID-19: a multi-center prospective observational study. Front. Nutr. 2022;9:934258. doi: 10.3389/fnut.2022.934258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalichuran S, van Blydenstein SA, Venter M, Omar S. Vitamin D status and COVID-19 severity. S Afr. J. Infect. Dis. 2022;37(1):359. doi: 10.4102/sajid.v37i1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parra-Ortega I, Alcara-Ramírez DG, Ronzon-Ronzon AA, et al. 25-Hydroxyvitamin D level is associated with mortality in patients with critical COVID-19: a prospective observational study in Mexico City. Nutr. Res Pr. 2021;15(Suppl 1):S32–S40. doi: 10.4162/nrp.2021.15.S1.S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bianconi V, Mannarino MR, Figorilli F, et al. Prevalence of vitamin D deficiency and its prognostic impact on patients hospitalized with COVID-19. Nutrition. 2021;91-92:111408. doi: 10.1016/j.nut.2021.111408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campi I, Gennari L, Merlotti D, et al. Vitamin D and COVID-19 severity and related mortality: a prospective study in Italy. BMC Infect. Dis. 2021;21(1):566. doi: 10.1186/s12879-021-06281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amraei R, Rahimi N. COVID-19, renin-angiotensin system and endothelial dysfunction. Cells. 2020;9:E1652. doi: 10.3390/cells9071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE-2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frara S, Allora A, Castellino L, di Filippo L, Loli P, Giustina A. COVID-19 and the pituitary. Pituitary. 2021;24(3):465–481. doi: 10.1007/s11102-021-01148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Zhao H, An Y. ACE-2 Shedding and the Role in COVID-19. Front Cell Infect. Microbiol. 2022;11:789180. doi: 10.3389/fcimb.2021.789180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dusso AS, Bauerle KT, Bernal-Mizrachi C. Non-classical Vitamin D Actions for Renal Protection. Front Med (Lausanne) 2021;8:790513. doi: 10.3389/fmed.2021.790513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Getachew B, Tizabi Y. Vitamin D and COVID-19: Role of ACE-2, age, gender, and ethnicity. J. Med Virol. 2021;93(9):5285–5294. doi: 10.1002/jmv.27075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malek Mahdavi A. A brief review of interplay between vitamin D and angiotensin-converting enzyme 2: Implications for a potential treatment for COVID-19. Rev. Med Virol. 2020;30(5):e2119. doi: 10.1002/rmv.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jolliffe DA, Griffiths CJ, Martineau AR. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J. Steroid Biochem Mol. Biol. 2013;136:321–329. doi: 10.1016/j.jsbmb.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Bilezikian JP, Formenti AM, Adler RA, et al. Vitamin D: Dosing, levels, form, and route of administration: Does one approach fit all. Rev. Endocr. Metab. Disord. 2021;22(4):1201–1218. doi: 10.1007/s11154-021-09693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giustina A, Bouillon R, Binkley N, et al. Controversies in Vitamin D: A Statement From the Third International Conference. JBMR Plus. 2020;4(12):e10417. doi: 10.1002/jbm4.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waldron JL, Ashby HL, Cornes MP, et al. Vitamin D: a negative acute phase reactant. J. Clin. Pathol. 2013;66(7):620–622. doi: 10.1136/jclinpath-2012-201301. [DOI] [PubMed] [Google Scholar]
- 30.Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2(1):76–89. doi: 10.1016/S2213-8587(13)70165-7. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan A, Ochola J, Mundy J, et al. Acute fluid shifts influence the assessment of serum vitamin D status in critically ill patients. Crit. Care. 2010;14(6):R216. doi: 10.1186/cc9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louw JA, Werbeck A, Louw ME, Kotze TJ, Cooper R, Labadarios D. Blood vitamin concentrations during the acute-phase response. Crit. Care Med. 1992;20(7):934–941. doi: 10.1097/00003246-199207000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Bang UC, Novovic S, Andersen AM, Fenger M, Hansen MB, Jensen JE. Variations in serum 25-hydroxyvitamin D during acute pancreatitis: an exploratory longitudinal study. Endocr. Res. 2011;36(4):135–141. doi: 10.3109/07435800.2011.554937. [DOI] [PubMed] [Google Scholar]
- 34.Silva MC, Furlanetto TW. Does serum 25-hydroxyvitamin D decrease during acute-phase response? A systematic review. Nutr. Res. 2015;35(2):91–96. doi: 10.1016/j.nutres.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68(1):2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smolders J, van den Ouweland J, Geven C, Pickkers P, Kox M. Letter to the Editor: Vitamin D deficiency in COVID-19: Mixing up cause and consequence. Metabolism. 2021;115:154434. doi: 10.1016/j.metabol.2020.154434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta D, Menon S, Criqui MH, Sun BK. Temporal Association of Reduced Serum Vitamin D with COVID-19 Infection: Two Single-Institution Case-Control Studies. Nutrients. 2022;14(13):2757. doi: 10.3390/nu14132757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.J.P. Bilezikian, N. Binkley, H.F. De Luca et al. Consensus and Controversial Aspects of Vitamin D and COVID-19. J. Clin. Endocrinol. Metab. dgac719 (2022) 10.1210/clinem/dgac719 [DOI] [PubMed]
- 39.Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for covid-19 [published correction appears in BMJ] BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 40.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 854, 1–452 (1995) [PubMed]
- 41.Sempos CT, Heijboer AC, Bikle DD, et al. Vitamin D assays and the definition of hypovitaminosis D: results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharm. 2018;84(10):2194–2207. doi: 10.1111/bcp.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.WHO World Health Organization. COVID-19 Clinical management Living guidance, (2021). WHO reference number: WHO/2019-nCoV/clinical/2021.1
- 43.Cavalli G, Larcher A, Tomelleri A, et al. Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: a cohort study. Lancet Rheumatol. 2021;3(4):e253–e261. doi: 10.1016/S2665-9913(21)00012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 45.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 46.Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr. Rev. 2008;29(6):726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah Alam M, Czajkowsky DM, Aminul Islam M, Ataur Rahman M. The role of vitamin D in reducing SARS-CoV-2 infection: An update. Int. Immunopharmacol. 2021;97:107686. doi: 10.1016/j.intimp.2021.107686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varikasuvu SR, Thangappazham B, Vykunta A, et al. COVID-19 and vitamin D (Co-VIVID study): a systematic review and meta-analysis of randomized controlled trials. Expert Rev. Anti Infect. Ther. 2022;20(6):907–913. doi: 10.1080/14787210.2022.2035217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hosseini B, El Abd A, Ducharme FM. Effects of Vitamin D Supplementation on COVID-19 Related Outcomes: A Systematic Review and Meta-Analysis. Nutrients. 2022;14(10):2134. doi: 10.3390/nu14102134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study”. J. Steroid Biochem Mol. Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maghbooli Z, Sahraian MA, Jamalimoghadamsiahkali S, et al. Treatment With 25-Hydroxyvitamin D3 (Calcifediol) Is Associated With a Reduction in the Blood Neutrophil-to-Lymphocyte Ratio Marker of Disease Severity in Hospitalized Patients With COVID-19: A Pilot Multicenter, Randomized, Placebo-Controlled, Double-Blinded Clinical Trial. Endocr. Pract. 2021;27(12):1242–1251. doi: 10.1016/j.eprac.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jolliffe DA, Holt H, Greenig M, et al. Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and covid-19: phase 3 randomised controlled trial (CORONAVIT) BMJ. 2022;378:e071230. doi: 10.1136/bmj-2022-071230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Smet D, De Smet K, Herroelen P, Gryspeerdt S, Martens GA. Serum 25(OH)D Level on Hospital Admission Associated With COVID-19 Stage and Mortality. Am. J. Clin. Pathol. 2021;155(3):381–388. doi: 10.1093/ajcp/aqaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seal KH, Bertenthal D, Carey E, Grunfeld C, Bikle DD, Lu CM. Association of Vitamin D Status and COVID-19-Related Hospitalization and Mortality. J. Gen. Intern Med. 2022;37(4):853–861. doi: 10.1007/s11606-021-07170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vanegas-Cedillo PE, Bello-Chavolla OY, Ramírez-Pedraza N, et al. Serum Vitamin D Levels Are Associated With Increased COVID-19 Severity and Mortality Independent of Whole-Body and Visceral Adiposity. Front Nutr. 2022;9:813485. doi: 10.3389/fnut.2022.813485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neves FF, Pott-Junior H, de Sousa Santos S, et al. Vitamin D deficiency predicts 30-day hospital mortality of adults with COVID-19. Clin. Nutr. ESPEN. 2022;50:322–325. doi: 10.1016/j.clnesp.2022.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z, Joshi A, Leopold K, et al. Association of vitamin D deficiency with COVID-19 infection severity: Systematic review and meta-analysis. Clin. Endocrinol. 2022;96(3):281–287. doi: 10.1111/cen.14540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pereira M, Dantas Damascena A, Galvão Azevedo LM, de Almeida Oliveira T, da Mota Santana J. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022;62(5):1308–1316. doi: 10.1080/10408398.2020.1841090. [DOI] [PubMed] [Google Scholar]
- 59.Butler-Laporte G, Nakanishi T, Mooser V, et al. Vitamin D and COVID-19 susceptibility and severity in the COVID-19 Host Genetics Initiative: A Mendelian randomization study. PLoS Med. 2021;18(6):e1003605. doi: 10.1371/journal.pmed.1003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bassatne A, Basbous M, Chakhtoura M, El Zein O, Rahme M, El-Hajj Fuleihan G. The link between COVID-19 and VItamin D (VIVID): A systematic review and meta-analysis. Metabolism. 2021;119:154753. doi: 10.1016/j.metabol.2021.154753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Minisola S, Colangelo L, Pepe J, Diacinti D, Cipriani C, Rao SD. Osteomalacia and Vitamin D Status: A Clinical Update 2020. JBMR . 2020;5(1):e10447. doi: 10.1002/jbm4.10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Puig-Domingo M, Marazuela M, Yildiz BO, Giustina A. COVID-19 and endocrine and metabolic diseases. An updated statement from the European Society of Endocrinology. Endocrine. 2021;72(2):301–316. doi: 10.1007/s12020-021-02734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marazuela M, Giustina A, Puig-Domingo M. Endocrine and metabolic aspects of the COVID-19 pandemic [published correction appears in Rev Endocr Metab Disord. 2021 Mar;22(1):145] Rev. Endocr. Metab. Disord. 2020;21(4):495–507. doi: 10.1007/s11154-020-09569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puig-Domingo M, Marazuela M, Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68(1):2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pranata R, Lim MA, Huang I, et al. Visceral adiposity, subcutaneous adiposity, and severe coronavirus disease-2019 (COVID-19): Systematic review and meta-analysis. Clin. Nutr. ESPEN. 2021;43:163–168. doi: 10.1016/j.clnesp.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brandi ML, Giustina A. Sexual Dimorphism of Coronavirus 19 Morbidity and Lethality. Trends Endocrinol. Metab. 2020;31(12):918–927. doi: 10.1016/j.tem.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharide‐induced acute lung injury via regulation of the renin‐angiotensin system. Mol. Med. Rep. 2017;16:7432–7438. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang J, Zhang H, Xu Z. Effect of vitamin D on ACE-2 and vitamin d receptor expression in rats with LPS‐induced acute lung. Injury. 2016;47:2816–2821. doi: 10.1016/j.injury.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 69.Riera M, Anguiano L, Clotet S, et al. Paricalcitol modulates ACE-2 shedding and renal ADAM17 in NOD mice beyond proteinuria. Am. J. Physiol. Ren. Physiol. 2016;310:F534–F546. doi: 10.1152/ajprenal.00082.2015. [DOI] [PubMed] [Google Scholar]
- 70.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J. Steroid Biochem. Mol. Biol. 2004;89-90(1-5):387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 71.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tomaschitz A, Pilz S, Ritz E, et al. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin. Chim. Acta. 2010;411(17-18):1354–1360. doi: 10.1016/j.cca.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 73.Rahman MM, Hasan M, Ahmed A. Potential detrimental role of soluble ACE-2 in severe COVID-19 comorbid patients. Rev. Med. Virol. 2021;31(5):1–12. doi: 10.1002/rmv.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karthika T, Joseph J, Das VRA, Nair N, Charulekha P, Roji MD, et al. SARS-CoV-2 cellular entry is independent of the ACE-2 cytoplasmic domain signaling. Cells. 2021;10(7):1814. doi: 10.3390/cells10071814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yeung ML, Teng JLL, Jia L, Zhang C, Huang C, Cai J-P, et al. Soluble ACE-2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin-angiotensin system. Cell. 2021;184(8):2212–2228.e12. doi: 10.1016/j.cell.2021.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Díaz-Troyano N, Gabriel-Medina P, Weber S, et al. Soluble angiotensin-converting enzyme 2 as a prognostic biomarker for disease progression in patients infected with SARS-CoV-2. Diagnostics. 2022;12(4):886. doi: 10.3390/diagnostics12040886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maza MDC, Úbeda M, Delgado P, et al. ACE-2 Serum Levels as Predictor of Infectability and Outcome in COVID-19. Front Immunol. 2022;13:836516. doi: 10.3389/fimmu.2022.836516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.V. Mariappan, P. Ranganadin, L. Shanmugam, S.R. Rao, A. Balakrishna Pillai, Early shedding of membrane-bounded ACE-2 could be an indicator for disease severity in SARS-CoV-2. Biochimie S0300-9084(22)00167-5 (2022). 10.1016/j.biochi.2022.06.005 [DOI] [PMC free article] [PubMed]
- 79.Vassiliou AG, Zacharis A, Keskinidou C, et al. Soluble Angiotensin Converting Enzyme 2 (ACE-2) Is Upregulated and Soluble Endothelial Nitric Oxide Synthase (eNOS) Is Downregulated in COVID-19-induced Acute Respiratory Distress Syndrome (ARDS) Pharmaceuticals. 2021;14(7):695. doi: 10.3390/ph14070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kornilov SA, Lucas I, Jade K, Dai CL, Lovejoy JC, Magis AT. Plasma levels of soluble ACE-2are associated with sex, Metabolic Syndrome, and its biomarkers in a large cohort, pointing to a possible mechanism for increased severity in COVID-19. Crit. Care. 2020;24(1):452. doi: 10.1186/s13054-020-03141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wallentin L, Lindbäck J, Eriksson N, et al. Angiotensin-converting enzyme 2 (ACE-2) levels in relation to risk factors for COVID-19 in two large cohorts of patients with atrial fibrillation. Eur. Heart J. 2020;41(41):4037–4046. doi: 10.1093/eurheartj/ehaa697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reindl-Schwaighofer R, Hödlmoser S, Eskandary F, et al. ACE-2 Elevation in Severe COVID-19. Am. J. Respir. Crit. Care Med. 2021;203(9):1191–1196. doi: 10.1164/rccm.202101-0142LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akin S, Schriek P, van Nieuwkoop C, et al. A low aldosterone/renin ratio and high soluble ACE-2 associate with COVID-19 severity. J. Hypertens. 2022;40(3):606–614. doi: 10.1097/HJH.0000000000003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kragstrup TW, Singh HS, Grundberg I, et al. Plasma ACE-2 predicts outcome of COVID-19 in hospitalized patients. PLoS ONE. 2021;16(6):e0252799. doi: 10.1371/journal.pone.0252799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lundström A, Ziegler L, Havervall S, et al. Soluble angiotensin-converting enzyme 2 is transiently elevated in COVID-19 and correlates with specific inflammatory and endothelial markers. J. Med. Virol. 2021;93(10):5908–5916. doi: 10.1002/jmv.27144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Narula S, Yusuf S, Chong M, Ramasundarahettige C, Rangarajan S, Bangdiwala SI, van Eikels M, Leineweber K, Wu A, Pigeyre M, Paré G. Plasma ACE-2 and risk of death or cardiometabolic diseases: a case-cohort analysis. Lancet. 2020;396:968–976. doi: 10.1016/S0140-6736(20)31964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jia H. Pulmonary angiotensin-converting enzyme 2 (ACE-2) and inflammatory lung disease. Shock. 2016;46:239–248. doi: 10.1097/SHK.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 88.Ramchand J, Patel SK, Kearney LG, Matalanis G, Farouque O, Srivastava PM, Burrell LM. Plasma ACE-2 activity predicts mortality in aortic stenosis and is associated with severe myocardial fibrosis. JACC Cardiovase Image. 2020;13:655–664. doi: 10.1016/j.jcmg.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 89.di Filippo L, Frara S, Giustina A. The emerging osteo-metabolic phenotype of COVID-19: clinical and pathophysiological aspects. Nat. Rev. Endocrinol. 2021;17(8):445–446. doi: 10.1038/s41574-021-00516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.di Filippo L, Frara S, Doga M, Giustina A. The osteo-metabolic phenotype of COVID-19: an update. Endocrine. 2022;78(2):247–254. doi: 10.1007/s12020-022-03135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.di Filippo L, Formenti AM, Giustina A. Hypocalcemia: the quest for the cause of a major biochemical feature of COVID-19. Endocrine. 2020;70(3):463–464. doi: 10.1007/s12020-020-02525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.di Filippo L, Doga M, Frara S, Giustina A. Hypocalcemia in COVID-19: Prevalence, clinical significance and therapeutic implications. Rev. Endocr. Metab. Disord. 2022;23(2):299–308. doi: 10.1007/s11154-021-09655-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.di Filippo L, Allora A, Locatelli M, et al. Hypocalcemia in COVID-19 is associated with low vitamin D levels and impaired compensatory PTH response. Endocrine. 2021;74(2):219–225. doi: 10.1007/s12020-021-02882-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data [published correction appears in Lancet. 2021 Jul 17;398(10296):212] Lancet. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.