Summary
Background
Studies reporting on the impact of social determinants of health on childhood cancer are limited. The current study aimed to examine the relationship between health disparities, as measured by the social deprivation index, and mortality in paediatric oncology patients using a population-based national database.
Methods
In this cohort study of children across all paediatric cancers, survival rates were determined using the Surveillance, Epidemiology, and End Results (SEER) database from 1975 to 2016. The social deprivation index was used to measure and assess healthcare disparities and specifically the impact on both overall and cancer-specific survival. Hazard ratios were used to assess the association of area deprivation.
Findings
The study cohort was composed of 99,542 patients with paediatric cancer. Patients had a median age of 10 years old (IQR: 3–16) with 46,109 (46.3%) of female sex. Based on race, 79,984 (80.4%) of patients were identified as white while 10,801 (10.9%) were identified as Black. Patients from socially deprived areas had significantly higher hazard of death overall for both non-metastatic [1.27 (95% CI: 1.19–1.36)] and metastatic presentations [1.09 (95% CI: 1.05–1.15)] compared to in more socially affluent areas.
Interpretation
Patients from the most socially deprived areas had lower rates of overall and cancer-specific survival compared to patients from socially affluent areas. With an increase in childhood cancer survivors, implementation of social determinant indices, such as the social deprivation index, might aid improvement in healthcare outcomes for the most vulnerable patients.
Funding
There was no study sponsor or extramural funding.
Keywords: Social determinants of health, Survival outcomes, Social deprivation index, Socioeconomic status
Research in context.
Evidence before this study
Health disparities have been described in the United States for several paediatric cancers; however, the association between social determinants of health and paediatric cancer has been inconsistently reported. We searched PubMed and Google scholar for relevant articles written in English. There have been several studies reporting lower overall survival in paediatric cancer patients with acute lymphoblastic leukaemia and tumours of the central nervous system using the area deprivation index. Due to being derived from geographical data from the census tract, the area deprivation index has been unable to be applied beyond the state level. Limited studies have reported on the impact of health disparities and their outcomes in patients with paediatric cancer using national data.
Added value of this study
We used a national cancer registry to provide estimates of paediatric cancer mortality across the United States at the county-level for patients based on degree of social inequity. Using the social deprivation index, we show significantly worse overall and cancer-specific survival in paediatric cancer patients residing in areas of lower socioeconomic status. We found significantly worse outcomes for patients who reported being racially Black or who presented with nonmetastatic cancer.
Implications of all the available evidence
Among paediatric cancer patients, social inequities impact health outcomes. Social determinants of health screening tools would likely benefit the patient. While there are several available social determinants of health assessment tools, the social deprivation index can be uniquely applied to four geographical levels—county, census tract, aggregated Zip Code Tabulation Area, and Primary Care Service Area—therefore, the social deprivation index may be a valuable tool used in clinical practice to assess for health disparities at the patient, community, and national level.
Introduction
Childhood cancer is the leading cause for death by disease in the United States,1 despite 85% of children surviving past five years.2 The gap in childhood health care outcomes is greatest among racial and ethnic minorities particularly for cancers that are most amenable to treatment.3 These differences are thought to be due in large part to social determinants of health (SDoH),4 which are the environmental factors that impact well-being with four commonly cited examples being income, education, employment, and social support.5 Poverty has been found to have the single most profound effect on SDoH.6 In the United States (US) alone, 16% of children were determined to be living in poverty according to the US Census Bureau in 2020.7 While most of the published literature on SDoH focuses on poverty, inequalities in terms of educational attainment, food insecurity, health care access, housing, and transportation have been demonstrated to have an impact on healthcare outcomes.8, 9, 10, 11
Although SDoH have been reported to have a significant impact on a host of cancer outcomes in adults,5 studies reporting on the impact of SDoH in childhood cancer have been limited.12, 13, 14, 15 In a systematic review by Tran et al., in 2022, inconsistent findings have been found on the association of SDoH and paediatric cancer.16 In a prospective study on the topic, Bona et al. found that 20% of the 99 families with children receiving chemotherapy for a primary cancer had income ≤200% the federal poverty line and at least one episode of a food, energy, or housing insecurity.17 With an increasing incidence of paediatric cancer and rising minority population as described by Aristizabal et al., there is an increased need to address cancer health disparities in paediatric populations.12 Moreover, there is a need for practical interventions to improve outcomes in the paediatric population.18 Limited studies have assessed the impact of SDoH in paediatric cancer using the area deprivation index, using geographical data based on census tracts, in patients with acute lymphoblastic leukaemia19,20 and primary central nervous system tumours.21 Due to the lack of census tract data in large cancer databases, studies looking at national survival outcomes are unable to be performed using such data, however. The social deprivation index (SDI) is a similar measure of area level deprivation that has multiple levels of disaggregation including county.22 While the SDI has been defined and subsequently validated to assess healthcare outcomes in adults,22,23 the SDI has not been assessed in children. The SDI is based on a composite of weighted factors based on geographic data using income, education, employment, housing, household characteristics, and transportation. Importantly, the SDI is valuable in being able to quantify SDoH measures.22
Using the SDI, the purpose of this project is to stratify survival outcomes across all paediatric cancers and to describe differences in survival outcomes based on SDoH. The hypothesis is that patients from the most socially deprived areas will have worse oncological survival outcomes compared to patients from less socially deprived areas.
Methods
Data source and study population
The Surveillance of Epidemiology and End Results (SEER) database represents one of the largest datasets in describing outcomes in cancer, accounting for 30% of the population in the US.24 SEER importantly contains data that is valuable for an analysis on a population level to describe survival outcomes including for childhood cancer.25 The SEER 18 dataset is based on 18 population-based cancer registries from 13 states.24 After IRB approval (1871434-3), the SEER data was used to analyse paediatric patients (≤19 years old) diagnosed between 1975 and 2016. Patients were included using the International Classification for Oncology, third edition (ICD-O-3) to classify patients with a paediatric cancer.26 Primary cancer type was defined by the World Health Organization based on the International Classification of Childhood Cancer (ICCC).27 A total of 1223 cases were excluded due to missing census SDI demographic data from Honolulu county, Kauai county, Hawaii county, Maui county, or Alaska county. A total of 72 cases were excluded with missing county data. A total of 281 cases with survival months missing were excluded. A summary of patients included in the study can be found in Fig. 1. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Fig. 1.
Flow diagram for study participants.
Social deprivation index (SDI)
Social deprivation for each patient was determined using the 5-year American Community Survey (ACS) county level data from 2011 to 2015. The ACS provides population-level estimates of the US population annually based on random sampling of housing units. Further information on the collection of the data and survey methods can be found on the ACS website: https://www.census.gov/programs-surveys/acs. The latest SDI scores were quantified as originally described by Butler et al., in 2015.22 The SDI uses seven SDoH characteristics: percent living in poverty, percent with less than 12 years of education, percent single parent household, percent living in rented housing unit, percent living in overcrowded housing unit, percent of households without a car, and percent non-employed adults under 65 years of age.22 The SDI scores are weighted based on the SDoH characteristics with a range given of 1–100 from national county percentile rankings.22 Higher SDI scores are associated with worse social conditions. We defined SDI based in quartiles for analysis (Quartile 1 = 0–25, Quartile 2 = 26–50, Quartile 3 = 51–75, Quartile 4 = 76–100), as published previously.23
Variables
Patient demographic data included age (in years), gender, race, and ethnicity. As healthcare outcomes based on racial and ethnic status are recognized to have an impact on social determinants of health,28 these variables were included.
Endpoints
The primary endpoint in our study was overall survival with the secondary endpoint of cancer-specific survival among patients with a paediatric cancer diagnosis. Based on the SEER classification, cancer-specific survival was defined based on survival from the primary cancer with other causes of death censored.29 Survival time is based on the period from cancer diagnosis to either mortality or loss to follow-up. Survival time was evaluated up to a period of 10 years.
Statistical analysis
For continuous variables the median and interquartile range were recorded while for categorical variables the frequency and percentage were recorded. Patient demographics were assessed using the Chi-square test for categorical variables and Kruskal–Wallis test for continuous variables to assess overall differences in SDI quartiles. Kaplan–Meier curves were performed to assess both overall and cancer-specific survival based on SDI quartiles for up to 10 years. To assess statistical differences in the survival curves, the log-rank test was utilized. Separate sub analyses were performed using metastatic (M) stage and race/ethnicity. A cox proportional hazard regression model was performed of factors associated with overall survival. A backward stepwise technique was used with elimination of nonsignificant covariates to produce a multivariable model. Significance was defined for all tests using a two-tail p-value of <0.05. Basic analysis in this study was conducted using both the Social Sciences version 28.0 (IBM Corporation) and Stata version 17 (Stata Corporation). The RCommander package of R version 4.1.0 was used for statistical analysis.30
Results
Patient population and characteristics
A total of 99,542 paediatric oncology patients met inclusion criteria. In the entire cohort, patients had a median age of 10 years old (IQR: 3–16) with 46,109 (46.3%) identified as being female sex. Based on race, 79,984 (80.4%) patients were recorded as white while 10,801 (10.9%) patients were Black. According to metastatic (M) stage, 45,776 (46.0%) of patients had defined M0 stage disease while 38,551 (38.7%) of patients had defined M1 stage disease in the population.
In Table 1, a summary of the patient demographics can be found based on SDI quartiles. Significant differences (p < 0.001) in patients being recorded as Black was found across SDI quartiles. Black patients were most likely to be from the most socially deprived area (17.1% vs. 4.1%) compared to the least deprived area. There were similarly significant differences (p < 0.001) in patients having M1 stage disease at presentation across SDI quartiles. Patients with M1 stage disease at presentation were most often to be from the most socially deprived area (42.2% vs. 35.5%).
Table 1.
Baseline patient demographics among all paediatric cancer patients by Social Deprivation Index Quartiles.
| Characteristics |
No. (%) |
Total |
p-valueb | |||
|---|---|---|---|---|---|---|
| Quartile 1a |
Quartile 2 |
Quartile 3 |
Quartile 4 |
|||
| No. | 23,239 | 24,906 | 24,343 | 27,054 | 99,542 | |
| Age, Median (IQR), y | 9.8 [3–16] | 9.7 [3–16] | 9.7 [3–16] | 9.6 [3–16] | 10.0 [3–16] | 0.001 |
| Sex | ||||||
| Female | 10,941 (47.1) | 11,467 (46.0) | 11,151 (45.8) | 12,550 (46.4) | 46,109 (46.3) | 0.032 |
| Male | 12,298 (52.9) | 13,439 (54.0) | 13,192 (54.2) | 14,504 (53.6) | 53,433 (53.7) | |
| Race | ||||||
| White | 21,245 (91.4) | 19,854 (79.7) | 18,561 (76.2) | 20,324 (75.1) | 79,984 (80.4) | <0.001 |
| Black | 946 (4.1) | 2036 (8.2) | 3204 (13.2) | 4615 (17.1) | 10,801 (10.9) | |
| Otherc | 1048 (4.5) | 3016 (12.1) | 2578 (10.6) | 2115 (7.8) | 8757 (8.8) | |
| Ethnicity | ||||||
| Hispanic | 1715 (7.4) | 4001 (16.1) | 6787 (27.9) | 11,692 (43.2) | 24,195 (24.3) | |
| M Stage | ||||||
| M0 | 10,729 (46.2) | 11,304 (45.4) | 11,544 (47.4) | 12,199 (45.1) | 45,776 (46.0) | <0.001 |
| M1 | 8257 (35.5) | 9197 (36.9) | 9677 (39.8) | 11,420 (42.2) | 38,551 (38.7) | |
| Unknown | 4253 (18.3) | 4405 (17.7) | 3122 (12.8) | 3435 (12.7) | 15,215 (15.3) | |
| Primary Site | ||||||
| Leukaemia | 5537 (23.8) | 6133 (24.6) | 6474 (26.6) | 7811 (28.9) | 25,955 (26.1) | <0.001 |
| CNS | 4278 (18.4) | 4273 (17.2) | 3942 (16.2) | 4214 (15.6) | 16,707 (16.8) | |
| Lymphoma | 3407 (14.7) | 3754 (15.1) | 3358 (13.8) | 3594 (13.3) | 14,113 (14.2) | |
| Miscellaneousd | 2900 (12.5) | 3267 (13.1) | 3078 (12.6) | 3471 (12.8) | 12,716 (12.8) | |
| Carcinoma | 1949 (8.4) | 2144 (8.6) | 2120 (8.7) | 2117 (7.8) | 8330 (8.4) | |
| Germ Cell | 1395 (6.0) | 1511 (6.1) | 1602 (6.6) | 1875 (6.9) | 6383 (6.4) | |
| Soft Tissue | 1431 (6.2) | 1604 (6.4) | 1537 (6.3) | 1803 (6.7) | 6375 (6.4) | |
| Bone | 1401 (6.0) | 1369 (5.5) | 1448 (5.9) | 1594 (5.9) | 5812 (5.8) | |
| Skin | 892 (3.8) | 807 (3.2) | 714 (2.9) | 520 (1.9) | 2933 (2.9) | |
| Unspecified | 49 (0.2) | 44 (0.2) | 70 (0.3) | 55 (0.2) | 218 (0.2) | |
| Follow-up time, Median (IQR), y | 8.3 (2.4–17.8) | 8.0 (2.3–16.8) | 6.1 (1.9–12.3) | 6.6 (1.8–13.8) | 7.1 (2.1–14.8) | <0.001 |
Increasing quartiles indicate increasing levels of social deprivation.
p values detected overall differences using ANOVA for continuous variables and Pearson Chi-Square for categorical variables.
Includes American Indian/Alaska Native, Asian/Pacific Islander.
Includes Wilms, neuroblastoma, embryonal, paragangliomas, myeloma, mast cell or other lymphoreticular tumours.
SDI-related risk of death
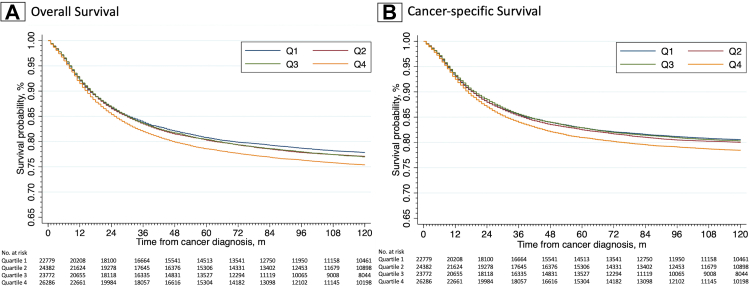
Survival differences were determined based on quartiles. 10-year overall and cancer-specific survival hazard ratios are described based on quartiles in Table 2. Based on overall survival, patients had significantly (p < 0.001) higher hazard of death [1.15 (95% CI: 1.11–1.19)] in the most socially deprived area compared to the least deprived area. A linear trend was found based on overall survival with worst survival in the most deprived areas. Based on cancer-specific survival, patients had significantly (p < 0.001) higher hazard of death [1.15 (95% CI: 1.10–1.19)] in Q4. Patients from Q4 similarly had worse cancer-specific survival than in each of the other quartiles. Kaplan–Meier curves for overall and cancer-specific survival based on quartiles for all paediatric cancer patients can be found in Fig. 2. Patients in Table 2 from Q4 had a 10-year overall survival of 73.8% (95% CI: 73.3–74.4%) and cancer-specific survival of 77.1% (95% CI: 76.5–77.7%).
Table 2.
10 year overall and cancer-specific survival with hazard ratios stratified by Social Deprivation Index quartiles.
| SDI Quartile | Overall survival |
Cancer-specific survival |
||||
|---|---|---|---|---|---|---|
| Survival % (95% CI) | Hazard ratio | p value | Survival % (95% CI) | Hazard ratio | p value | |
| Quartile 1 | 76.7 (76.1–77.3) | 1 [referent] | NA | 79.6 (79.1–80.2) | 1 [referent] | NA |
| Quartile 2 | 75.9 (75.3–76.4) | 1.04 (1.00–1.07) | 0.07 | 79.0 (78.4–79.5) | 1.04 (0.99–1.08) | 0.086 |
| Quartile 3 | 75.8 (75.2–76.4) | 1.05 (1.01–1.09) | 0.014 | 79.3 (78.7–79.8) | 1.02 (0.97–1.06) | 0.45 |
| Quartile 4 | 73.8 (73.3–74.4) | 1.15 (1.11–1.19) | <0.001 | 77.1 (76.5–77.7) | 1.15 (1.10–1.19) | <0.001 |
SDI, Social deprivation index; NA, Not applicable.
Fig. 2.
Kaplan–Meier estimates by social deprivation quartile based on (A) overall and (B) cancer-specific survival.
Metastatic stage of presentation-related risk of death
Survival differences were further defined based on metastatic disease for the cohort. In Table 3, 10-year overall and cancer-specific survival hazard ratios are described for quartiles based on the presence or absence of metastatic disease. Patients from Q4 with non-metastatic cancer were found to have a significantly (p < 0.001) higher risk of overall [1.27 (95% CI: 1.19–1.36)] and cancer-specific [1.28 (95% CI: 1.19–1.38)] hazard of death. Patients from Q4 with metastatic cancer were similarly found to have a higher risk of both overall [1.09 (95% CI: 1.05–1.15)] and cancer-specific hazard of death [1.08 (95% CI: 1.02–1.14)] with significant differences (p = 0.001 overall and p = 0.007 cancer specific). Kaplan–Meier curves for overall and cancer-specific survival based on quartiles for both non-metastatic and metastatic disease can be found in Fig. 3. In metastatic presentations, there is no statistical difference noted between Q1, Q2 and Q3. The only difference is seen in the most deprived Q4 population. Non-metastatic patients in Table 3 had a 10-year overall survival from Q4 of 81.3% (95% CI: 80.6–82.1%) and cancer-specific survival of 83.8% (95% CI: 83.1–84.5%). Metastatic patients in Table 3 had a 10-year overall survival from Q4 of 66.9% (95% CI: 66.0–67.9%) and cancer-specific survival of 70.7% (95% CI: 69.8–71.6%).
Table 3.
10 year overall and cancer-specific survival stratified by metastatic and non-metastatic disease.
| SDI Quartile | No. (%) | Overall survival |
Cancer-specific survival |
||||
|---|---|---|---|---|---|---|---|
| Survival % (95% CI) | Hazard ratio | p value | Survival % (95% CI) | Hazard ratio | p value | ||
| M0 Disease | |||||||
| Quartile 1 | 10,729 (23.4) | 85.0 (84.2–85.7) | 1 [referent] | NA | 87.1 (86.4–87.8) | 1 [referent] | NA |
| Quartile 2 | 11,304 (24.7) | 84.1 (83.3–84.5) | 1.06 (0.99–1.13) | 0.1 | 86.4 (85.7–87.0) | 1.05 (0.98–1.14) | 0.17 |
| Quartile 3 | 11,544 (25.2) | 82.4 (81.6–83.2) | 1.19 (1.11–1.27) | <0.001 | 85.5 (84.8–86.1) | 1.13 (1.05–1.22) | <0.001 |
| Quartile 4 | 12,199 (26.6) | 81.3 (80.6–82.1) | 1.27 (1.19–1.36) | <0.001 | 83.8 (83.1–84.5) | 1.28 (1.19–1.38) | <0.001 |
| M1 Disease | |||||||
| Quartile 1 | 8257 (21.4) | 69.1 (68.1–70.2) | 1 [referent] | NA | 72.5 (71.5–73.5) | 1 [referent] | NA |
| Quartile 2 | 9197 (23.9) | 67.7 (66.7–68.7) | 1.05 (0.99–1.10) | 0.1 | 71.5 (70.5–72.5) | 1.04 (0.98–1.10) | 0.24 |
| Quartile 3 | 9677 (25.1) | 69.8 (68.8–70.8) | 0.97 (0.92–1.03) | 0.29 | 73.7 (72.7–74.6) | 0.95 (0.89–1.00) | 0.06 |
| Quartile 4 | 11,420 (29.6) | 66.9 (66.0–67.9) | 1.09 (1.04–1.15) | 0.001 | 70.7 (69.8–71.6) | 1.08 (1.02–1.14) | 0.007 |
M, Metastases; SDI, Social deprivation index; NA, Not applicable.
Fig. 3.
Kaplan–Meier estimates based on metastatic disease and social deprivation quartile. Denoted is (A) overall and (B) cancer-specific survival for localized disease along with (C) overall and (D) cancer-specific survival for metastatic disease.
Race/ethnicity-related risk of death
Patients were separately subdivided by race and ethnicity in Table 4. Black patients had lower 10-year overall [67.6% (95% CI: 66.1–69.0%) vs. 75.2% (95% CI: 74.5–75.8%)] and cancer-specific [71.5% (95% CI: 70.1–72.9%) vs. 78.2% (95% CI: 77.6–78.8%)] survival in Q4 compared to non-Black patients. In contrast, patients of Hispanic ethnicity had similar 10-year overall [74.3% (95% CI: 73.4–75.2%) vs. 73.5% (95% CI: 72.7–74.3%)] and cancer-specific [77.5% (95% CI: 76.7–78.4%) vs. 76.7% (95% CI: 76.0–77.5%)] survival in Q4 compared to non-Hispanic patients.
Table 4.
10 year overall and cancer-specific survival stratified by race/ethnicity.
| SDI Quartile | No. (%) | Overall survival |
Cancer-specific survival |
||||
|---|---|---|---|---|---|---|---|
| Survival % (95% CI) | Hazard ratio | p value | Survival % (95% CI) | Hazard ratio | p value | ||
| Race | |||||||
| non-Black | |||||||
| Q1 | 22,293 (25.1) | 76.9 (76.3–77.5) | 1 [referent] | NA | 79.8 (79.2–80.3) | 1 [referent] | NA |
| Q2 | 22,870 (25.8) | 76.3 (75.7–76.9) | 1.02 (0.98–1.06) | 0.24 | 79.4 (78.8–79.9) | 1.02 (0.98–1.07) | 0.29 |
| Q3 | 21,139 (23.8) | 76.8 (76.2–77.5) | 1.00 (0.96–1.04) | 0.87 | 80.2 (79.5–80.8) | 0.97 (0.93–1.02) | 0.24 |
| Q4 | 22,439 (25.3) | 75.2 (74.5–75.8) | 1.09 (1.05–1.13) | <0.001 | 78.2 (77.6–78.8) | 1.08 (1.04–1.13) | <0.001 |
| Black | |||||||
| Q1 | 946 (8.8) | 72.1 (68.8–75.1) | 1 [referent] | NA | 76.6 (73.5–79.5) | 1 [referent] | NA |
| Q2 | 2036 (18.9) | 70.5 (68.3–72.6) | 1.01 (0.87–1.17) | 0.91 | 74.3 (72.2–76.3) | 1.06 (0.89–1.25) | 0.52 |
| Q3 | 3204 (29.7) | 68.9 (67.1–70.7) | 1.09 (0.95–1.25) | 0.24 | 73.6 (71.9–75.3) | 1.11 (0.95–1.29) | 0.21 |
| Q4 | 4615 (42.7) | 67.6 (66.1–69.0) | 1.15 (1.01–1.31) | 0.04 | 71.5 (70.1–72.9) | 1.21 (1.05–1.41) | 0.01 |
| Ethnicity | |||||||
| non-Hispanic | |||||||
| Q1 | 21,524 (28.6) | 76.7 (76.1–77.3) | 1 [referent] | NA | 79.6 (79.0–80.2) | 1 [referent] | NA |
| Q2 | 20,905 (27.7) | 76.0 (75.4–76.6) | 1.03 (0.99–1.07) | 0.18 | 79.0 (78.4–79.6) | 1.04 (0.99–1.08) | 0.11 |
| Q3 | 17,556 (23.3) | 76.2 (75.5–76.9) | 1.03 (0.98–1.07) | 0.22 | 79.5 (78.9–80.2) | 1.00 (0.95–1.05) | 0.99 |
| Q4 | 15,362 (20.4) | 73.5 (72.7–74.3) | 1.17 (1.12–1.22) | <0.001 | 76.7 (76.0–77.5) | 1.16 (1.11–1.22) | <0.001 |
| Hispanic | |||||||
| Q1 | 1715 (7.1) | 76.7 (74.3–78.9) | 1 [referent] | NA | 79.9 (77.6–82.0) | 1 [referent] | NA |
| Q2 | 4001 (16.5) | 74.9 (73.4–76.4) | 1.08 (0.95–1.22) | 0.23 | 78.9 (77.5–80.3) | 1.05 (0.92–1.21) | 0.46 |
| Q3 | 6787 (28.1) | 74.5 (73.3–75.7) | 1.10 (0.98–1.24) | 0.11 | 78.6 (77.4–79.7) | 1.08 (0.95–1.22) | 0.26 |
| Q4 | 11,692 (48.3) | 74.3 (73.4–75.2) | 1.12 (1.01–1.25) | 0.04 | 77.5 (76.7–78.4) | 1.14 (1.01–1.29) | 0.04 |
Q, Quartile; SDI, Social deprivation index; NA, Not applicable.
Univariate and multivariate cox proportional hazards regression analysis
To identify predictors of overall survival, a univariate analysis was performed with significant factors included into the multivariate analysis in Table 5. SDI was significantly associated with overall survival (p < 0.001). Q4 was significantly associated with a higher hazard of death [1.08 (95% CI: 1.04–1.12)]. Patients of Black race [1.36 (95% CI: 1.31–1.41)] as well as Hispanic ethnicity [1.07 (95% CI: 1.04–1.11)] had a higher hazard of death. As SDI is based on ACS data from 2011 to 2015, a separate cox proportional hazards regression was performed with patients exclusively diagnosed during this period. This data shows similar findings and is provided in the Supplementary Table S1.
Table 5.
Multivariate cox proportional hazard model for overall mortality.
| Factor | HR (95% CI) | p value |
|---|---|---|
| Age | ||
| Adolescent (>11 years old) | 1 [referent] | <0.001 |
| Children (2–10 years old) | 0.71 (0.69–0.73) | |
| Infant (<2 years old) | 0.94 (0.90–0.99) | |
| Sex | ||
| Male | 1 [referent] | <0.001 |
| Female | 0.86 (0.84–0.88) | |
| Race | ||
| White | 1 [referent] | <0.001 |
| Black | 1.36 (1.31–1.41) | |
| Other | 1.06 (1.01–1.12) | |
| Ethnicity | ||
| Non-Hispanic | 1 [referent] | <0.001 |
| Hispanic | 1.07 (1.04–1.11) | |
| Metastasis | ||
| M0 | 1 [referent] | <0.001 |
| M1 | 3.31 (3.19–3.43) | |
| Unknown | 2.07 (2.0–2.16) | |
| Primary site | ||
| Leukaemia | 1 [referent] | <0.001 |
| CNS | 2.96 (2.82–3.11) | |
| Lymphoma | 0.88 (0.83–0.93) | |
| Miscellaneous | 1.49 (1.41–1.56) | |
| Carcinoma | 1.18 (1.10–1.26) | |
| Germ cell | 0.79 (0.73–0.85) | |
| Soft tissue | 2.42 (2.29–2.56) | |
| Bone | 2.90 (2.82–3.11) | |
| Skin | 0.92 (0.82–1.04) | |
| Unspecified | 2.23 (1.77–2.82) | |
| SDI Quartile | ||
| Q1 | 1 [referent] | <0.001 |
| Q2 | 1.02 (0.98–1.06) | |
| Q3 | 1.01 (0.97–1.05) | |
| Q4 | 1.08 (1.04–1.12) |
Q, Quartile; SDI, Social deprivation index.
Discussion
This is one of the first studies to quantify SDoH to assess a national cohort of paediatric oncology patients.31 The results of this study show that patients from the most socially deprived areas consistently have worse overall and cancer-specific survival compared to patients from more socially affluent areas. These data were evaluated in numerous ways and across different timelines to try and make the series more contemporary. Ultimately, the findings remained consistent whether the evaluation focused on a specific subset of malignancy, a more limited timeframe, or a more contemporary series. Most striking, paediatric oncology patients from Q4, the most socially deprived with non-metastatic disease had roughly 30% higher risk of death for both overall and cancer-specific survival compared to patients from Q1, the most socially affluent quarter of the cohort. These findings emphasize that health outcomes are impacted by social and community contexts.
Inequities based on race were found among patients from the most socially deprived areas, with patients more likely to be Black. In a SEER study by Tehranifar et al., racial and ethnic minorities were found to have worse survival in cancers that were more amenable to medical interventions.32 When Q4 was subdivided in our study, both non-Black and Black populations still were found to have lower survival rates than Q1, but the decrease in survival was more profound in the Black population. While Butler et al. excluded percent Black from the constructed SDI model, the disproportionately higher percentage of Black patients in Q4 and associated worse survival outcomes in this population cannot be completely accounted for currently.22 Although patients of Hispanic ethnicity were found to be disproportionately higher in Q4, this did not translate to worse survival outcomes. The question of how to connect both race and ethnicity in a disparity index will need to be evaluated with future research endeavours.
This study utilized SDI as a method to evaluate degree of social deprivation of a geographic area based on county data and followed patients over a 10-year period. The SDI is currently available at four geographical levels: county, census tract, aggregated Zip Code Tabulation Area (ZCTA), and Primary Care Service Area.33 Indexes that have previously been applied to the paediatric oncology population are available at only a single geographical level such as the area deprivation index which is based on census block groups or the composite index of socioeconomic status which is based on census tract groups.34,35 Data generated exclusively from smaller geographical regions offer more precise information, but increasingly raise concerns of patient information being reidentifiable; this data requires protective methods to maintain compliance.36 Moreover, the unavailability of census block group data in national cancer databases for the paediatric population has precluded its application beyond the state level. As socioeconomic data for census tracts in SEER have only been collected since 2000, follow-up time is limited.29
Overall, the findings in this manuscript can help push for improvements in patient access and screening, the delivery of health care, future research, and policy changes. In the literature, social needs screening and referrals in clinical workflows are the most cited tools for addressing SDoH issues.37,38 Community-level SDoH assessments can be used to inform health care policies based on lack of facilities or even provide detailed information on medical professional shortages that may affect care in a geographic area.39 Similarly, community-level SDoH assessments can be incorporated into the medical health record to stratify patient risk in a multitude of health care settings such as inpatient or outpatient.40,41 With most children surviving a primary malignancy, assessments that focus on childhood cancer survivorship is increasingly more relevant due to a higher incidence of not only secondary malignant neoplasms42 but also cardiovascular complications43 in this population. Both general and subspecialty medical health care providers have a valuable role in both risk-based surveillance and preventative medicine.44
This study has several limitations of note. While SDI is a composite measure to assess factors related to SDoH, this is not comprehensive by any means in measuring patient level healthcare inequities. Having more local or even block level metrics to measure SDoH would provide greater specificity to individual inequities, however, oftentimes at the risk of becoming too granular and only describing community pockets. Outcomes in this study are limited to county-level data but as there are varying levels of affluence and access across neighbourhoods the available data are further removed from the experience of an individual patient. Cancer treatments and outcomes have changed over the 40 years of the SEER database and some geographic areas 40 years earlier may have been different from a socioeconomic standpoint than they are today. Patient factors including comorbidity are unavailable in SEER, and therefore could not be accounted for in our study. While the data in SEER is robust in accounting for a large patient sample of oncology patients, it is by no means inclusive of the entire United States and as such data are primarily collected from academic hospitals and metropolitan areas. As such, the study is limited to patients recorded in the SEER registry. Future prospective studies will be needed to apply and use SDI scores as an intervention in practice.
Conclusions
Social determinants of health are a contributor to disparities in healthcare outcomes among paediatric oncology patients. Patients from the most socially deprived areas had significantly worse 10-year overall and cancer-specific survival rates across all paediatric cancers. After separating out patients with metastatic disease, the differences in survival were present but not as disparate as for non-metastatic disease. Of important note, a higher proportion of patients from the most socially deprived quartile had metastatic disease. This is in alignment with other studies that found the greatest differences in outcomes in patients with disease were most amenable to medical interventions. With a lack of any standard SDoH metric in clinical practice, patients would likely benefit from implementation and screening using electronic medical records. The social deprivation index may be a useful tool to stratify individual patients and communities who should be the target of more focused medical attention and support. This could be an especially valuable tool in healthcare models that are driven by value-based care. More focus needs to be diverted towards paediatric patients who are vulnerable, particularly those from socially deprived areas, to create more equitable outcomes in the field.
Contributors
VC and AAS were responsible for project conceptualization. VC, CR, and AAR were responsible for data curation and data analysis. AAR and SMB provided project administration, supervision, and resources. VC and AAR wrote the original draft. All authors edited the final version. VC and CR had access to all the data and had final responsibility for the decision to submit for publication.
Data sharing statement
The SEER 18 data is publicly available at https://seer.cancer.gov/data/. The dataset creation plan for this study is available from the corresponding author upon request.
Role of the funding source
There was no study sponsor or extramural funding.
Declaration of interests
The authors declare that they have no conflicts of interest.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100454.
Appendix A. Supplementary data
References
- 1.Raab C.P., Gartner J.C., Jr. Diagnosis of childhood cancer. Prim Care. 2009;36(4):671–684. doi: 10.1016/j.pop.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Zavala V.A., Bracci P.M., Carethers J.M., et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021;124(2):315–332. doi: 10.1038/s41416-020-01038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alcaraz K.I., Wiedt T.L., Daniels E.C., Yabroff K.R., Guerra C.E., Wender R.C. Understanding and addressing social determinants to advance cancer health equity in the United States: a blueprint for practice, research, and policy. CA Cancer J Clin. 2020;70(1):31–46. doi: 10.3322/caac.21586. [DOI] [PubMed] [Google Scholar]
- 5.Braveman P., Gottlieb L. The social determinants of health: it's time to consider the causes of the causes. Public Health Rep. 2014;129 Suppl 2(Suppl 2):19–31. doi: 10.1177/00333549141291S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh G.K., Daus G.P., Allender M., et al. Social determinants of health in the United States: addressing major health inequality trends for the nation, 1935–2016. Int J MCH AIDS. 2017;6(2):139–164. doi: 10.21106/ijma.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox L. 2018. The Supplemental Poverty Measure: 2017. (Current Population Reports P60265) [Google Scholar]
- 8.Dean E.B., French M.T., Mortensen K. Food insecurity, health care utilization, and health care expenditures. Health Serv Res. 2020;55(Suppl 2):883–893. doi: 10.1111/1475-6773.13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahn R.A., Truman B.I. Education improves public health and promotes health equity. Int J Health Serv. 2015;45(4):657–678. doi: 10.1177/0020731415585986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolfe S., Garnham L., Godwin J., Anderson I., Seaman P., Donaldson C. Housing as a social determinant of health and wellbeing: developing an empirically-informed realist theoretical framework. BMC Public Health. 2020;20(1):1138. doi: 10.1186/s12889-020-09224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Syed S.T., Gerber B.S., Sharp L.K. Traveling towards disease: transportation barriers to health care access. J Community Health. 2013;38(5):976–993. doi: 10.1007/s10900-013-9681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aristizabal P., Winestone L.E., Umaretiya P., Bona K. Disparities in pediatric oncology: the 21st century opportunity to improve outcomes for children and adolescents with cancer. Am Soc Clin Oncol Educ Book. 2021;41:e315–e326. doi: 10.1200/EDBK_320499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berkman J.M., Dallas J., Lim J., et al. Social determinants of health affecting treatment of pediatric brain tumors. J Neurosurg Pediatr. 2019;24(2):159–165. doi: 10.3171/2019.4.PEDS18594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frederiksen L.E., Mader L., Feychting M., et al. Surviving childhood cancer: a systematic review of studies on risk and determinants of adverse socioeconomic outcomes. Int J Cancer. 2019;144(8):1796–1823. doi: 10.1002/ijc.31789. [DOI] [PubMed] [Google Scholar]
- 15.Gruszczynski N.R., Low C.M., Choby G., Meister K.D., Smith B.H., Balakrishnan K. Effects of social determinants of health care on pediatric thyroid cancer outcomes in the United States. Otolaryngol Head Neck Surg. 2021;166:1045. doi: 10.1177/01945998211032901. [DOI] [PubMed] [Google Scholar]
- 16.Tran Y.H., Coven S.L., Park S., Mendonca E.A. Social determinants of health and pediatric cancer survival: a systematic review. Pediatr Blood Cancer. 2022;69(5) doi: 10.1002/pbc.29546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bona K., London W.B., Guo D., Frank D.A., Wolfe J. Trajectory of material hardship and income poverty in families of children undergoing chemotherapy: a prospective cohort study. Pediatr Blood Cancer. 2016;63(1):105–111. doi: 10.1002/pbc.25762. [DOI] [PubMed] [Google Scholar]
- 18.Chung E.K., Siegel B.S., Garg A., et al. Screening for social determinants of health among children and families living in poverty: a guide for clinicians. Curr Probl Pediatr Adolesc Health Care. 2016;46(5):135–153. doi: 10.1016/j.cppeds.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oluyomi A., Aldrich K.D., Foster K.L., et al. Neighborhood deprivation index is associated with weight status among long-term survivors of childhood acute lymphoblastic leukemia. J Cancer Surviv. 2021;15(5):767–775. doi: 10.1007/s11764-020-00968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schraw J.M., Peckham-Gregory E.C., Rabin K.R., Scheurer M.E., Lupo P.J., Oluyomi A. Area deprivation is associated with poorer overall survival in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2020;67(9) doi: 10.1002/pbc.28525. [DOI] [PubMed] [Google Scholar]
- 21.Adel Fahmideh M., Schraw J.M., Chintagumpala M., Lupo P.J., Oluyomi A.O., Scheurer M.E. Neighborhood socioeconomic deprivation and mortality in children with central nervous system tumors. Cancer Epidemiol Biomarkers Prev. 2021;30(12):2278–2285. doi: 10.1158/1055-9965.EPI-21-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler D.C., Petterson S., Phillips R.L., Bazemore A.W. Measures of social deprivation that predict health care access and need within a rational area of primary care Service delivery. Health Serv Res. 2013;48(2pt1):539–559. doi: 10.1111/j.1475-6773.2012.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bevan G.H., Freedman D.A., Lee E.K., Rajagopalan S., Al-Kindi S.G. Association between ambient air pollution and county-level cardiovascular mortality in the United States by social deprivation index. Am Heart J. 2021;235:125–131. doi: 10.1016/j.ahj.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Duggan M.A., Anderson W.F., Altekruse S., Penberthy L., Sherman M.E. The surveillance, Epidemiology, and End results (SEER) program and pathology: toward strengthening the critical relationship. Am J Surg Pathol. 2016;40(12):e94–e102. doi: 10.1097/PAS.0000000000000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallicchio L., Elena J.W., Fagan S., et al. Utilizing SEER cancer registries for population-based cancer survivor epidemiologic studies: a feasibility study. Cancer Epidemiol Biomarkers Prev. 2020;29(9):1699–1709. doi: 10.1158/1055-9965.EPI-20-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization . 3rd ed. World Health Organization; Geneva: 2013. International classification of diseases for oncology (ICD-O) 1st revision ed. [Google Scholar]
- 27.Steliarova-Foucher E., Stiller C., Lacour B., Kaatsch P. International classification of childhood cancer, third edition. Cancer. 2005;103(7):1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 28.Fiscella K., Sanders M.R. Racial and ethnic disparities in the quality of health care. Annu Rev Public Health. 2016;37:375–394. doi: 10.1146/annurev-publhealth-032315-021439. [DOI] [PubMed] [Google Scholar]
- 29.Surveillance, Epidemiology, and End results (SEER) program. https://seer.cancer.gov Updated January 17, 2023. Available from:
- 30.Kanda Y. Statistical analysis using freely-available "EZR (Easy R)" software. Rinsho ketsueki. 2015;56(10):2258–2266. doi: 10.11406/rinketsu.56.2258. [DOI] [PubMed] [Google Scholar]
- 31.Kehm R.D., Spector L.G., Poynter J.N., Vock D.M., Altekruse S.F., Osypuk T.L. Does socioeconomic status account for racial and ethnic disparities in childhood cancer survival? Cancer. 2018;124(20):4090–4097. doi: 10.1002/cncr.31560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tehranifar P., Neugut A.I., Phelan J.C., et al. Medical advances and racial/ethnic disparities in cancer survival. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2701–2708. doi: 10.1158/1055-9965.EPI-09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Academy of Family Physicians . 2023. Social Deprivation Index (SDI)https://www.graham-center.org/rgc/maps-data-tools/social-deprivation-index.html Available from: [Google Scholar]
- 34.Hu J., Kind A.J.H., Nerenz D. Area deprivation index predicts readmission risk at an urban teaching hospital. Am J Med Qual. 2018;33(5):493–501. doi: 10.1177/1062860617753063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu M., Tatalovich Z., Gibson J.T., Cronin K.A. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control. 2014;25(1):81–92. doi: 10.1007/s10552-013-0310-1. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y., Conway D., Wan Z., Kantarcioglu M., Vorobeychik Y., Malin B.A. De-identifying socioeconomic data at the census tract level for medical research through constraint-based clustering. AMIA Annu Symp Proc. 2021;2021:793–802. [PMC free article] [PubMed] [Google Scholar]
- 37.Berry C., Paul M., Massar R., Marcello R.K., Krauskopf M. Social needs screening and referral program at a large US public hospital system, 2017. Am J Public Health. 2020;110(S2):S211–S214. doi: 10.2105/AJPH.2020.305642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiori K.P., Rehm C.D., Sanderson D., et al. Integrating social needs screening and community health workers in primary care: the community linkage to care program. Clin Pediatr (Phila) 2020;59(6):547–556. doi: 10.1177/0009922820908589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Streeter R.A., Snyder J.E., Kepley H., Stahl A.L., Li T., Washko M.M. The geographic alignment of primary care Health Professional Shortage Areas with markers for social determinants of health. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0231443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kostelanetz S., Di Gravio C., Schildcrout J.S., Roumie C.L., Conway D., Kripalani S. Should we implement geographic or patient-reported social determinants of health measures in cardiovascular patients. Ethn Dis. 2021;31(1):9–22. doi: 10.18865/ed.31.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen C., Popescu F., Sommer E.C., Adams L.B., Barkin S. The utility of the brokamp area deprivation index as a prescreen for social risk in primary care. J Pediatr. 2022;249:43–49. doi: 10.1016/j.jpeds.2022.06.028. [DOI] [PubMed] [Google Scholar]
- 42.Brown A.L., Arroyo V.M., Agrusa J.E., Scheurer M.E., Gramatges M.M., Lupo P.J. Survival disparities for second primary malignancies diagnosed among childhood cancer survivors: a population-based assessment. Cancer. 2019;125(20):3623–3630. doi: 10.1002/cncr.32356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bansal N., Blanco J.G., Sharma U.C., Pokharel S., Shisler S., Lipshultz S.E. Cardiovascular diseases in survivors of childhood cancer. Cancer Metastasis Rev. 2020;39(1):55–68. doi: 10.1007/s10555-020-09859-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long-term follow-up care for pediatric cancer survivors. Pediatrics. 2009;123(3):906–915. doi: 10.1542/peds.2008-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.