Significance
Tight junctions (TJs) are membrane structures formed by the polymerization of the palmitoylated membrane protein claudin. It has been canonically thought that claudin polymerization is driven by the protein–protein interaction with the scaffolding proteins, ZO. In this study, we reveal that the ZO proteins’ contribution to TJ formation is not limited to their scaffolding function; just as importantly, they function as an organizer of cholesterol-rich membrane domains in the vicinity of apical junctional complexes (AJCs). This enables claudins to accumulate through the affinity between their palmitoylated moiety and the cholesterol-enriched membrane.
Keywords: tight junction, cholesterol, membrane domain, claudin, epithelial cells
Abstract
Tight junctions (TJs) are cell-adhesion structures responsible for the epithelial barrier. We reported that accumulation of cholesterol at the apical junctions is required for TJ formation [K. Shigetomi, Y. Ono, T. Inai, J. Ikenouchi, J. Cell Biol. 217, 2373–2381 (2018)]. However, it is unclear how cholesterol accumulates and informs TJ formation—and whether cholesterol enrichment precedes or follows the assembly of claudins in the first place. Here, we established an epithelial cell line (claudin-null cells) that lacks TJs by knocking out claudins. Despite the lack of TJs, cholesterol normally accumulated in the vicinity of the apical junctions. Assembly of claudins at TJs is thought to require binding to zonula occludens (ZO) proteins; however, a claudin mutant that cannot bind to ZO proteins still formed TJ strands. ZO proteins were however necessary for cholesterol accumulation at the apical junctions through their effect on the junctional actomyosin cytoskeleton. We propose that ZO proteins not only function as scaffolds for claudins but also promote TJ formation of cholesterol-rich membrane domains at apical junctions.
Epithelial cells adhere to each other to form a continuous cell sheet, which covers the surface of organs. The epithelial cell sheet also functions as a barrier that separates the inside of the body from the outside and limits free diffusion of small substances such as ions. This function is assumed by tight junctions (TJs) that are located at the most apical side of the lateral membrane. Claudins, four transmembrane proteins, and zonula occludens (ZO) proteins, scaffolding proteins for claudins, are the major constituents of TJs (1). The widely accepted textbook model of TJ formation proposes that the incorporation of claudins into the characteristic sealing strands is regulated by the scaffolding function of ZO proteins. In other words, multimerization of ZO proteins at the apical junctional complexes (AJCs) serves as a platform to enable claudin polymerization (2, 3).
Confoundingly, our previous study revealed that the recruitment of ZO proteins to AJCs is insufficient to induce TJ formation. TJs are membrane domains rich in cholesterol and sphingomyelin (SM) with very long-chain fatty acids (4, 5). Clearly, to understand the mechanism of TJ formation, the contribution of plasma membrane lipids must be clarified (6). Removal of plasma membrane cholesterol by treatment with methyl-beta-cyclodextrin (MbCD) promptly disrupts TJs by inducing rapid removal of claudins from the plasma membrane without disturbing the localization of ZO proteins, which suggests that cholesterol-rich membrane domains, rather than ZO proteins themselves, informs TJ formation (4). However, it is unclear if the cholesterol-rich membrane domain is necessary and sufficient to induce TJs; it also remains possible that the presence of polymerized claudins enables the membrane domain to form. Furthermore, since the formation of adherens junctions (AJs) was not affected by MbCD treatment (4), the formation mechanisms of AJ and TJ clearly differ in terms of the requirement for cholesterol.
In the present study, in order to specifically address the mechanism of cholesterol accumulation at TJs and the reason why TJ formation depends on cholesterol, we established epithelial cells in which all claudin isoforms were knocked out by CRISPR. In these cells, cholesterol is enriched in the vicinity of apical junctions. We revealed that the accumulation of cholesterol depends on the actin cytoskeleton underlying apical junctions. Remarkably, we found that claudin that cannot bind to ZO proteins still accumulates at apical junctions, so long as a cholesterol-rich membrane domain is present. Finally, we showed that palmitoylation is required for claudin to interact with the said membrane domain. From these findings, we propose that the AJC-associated circumferential actin ring directs the formation of cholesterol-rich membrane domains at apical junctions, which promotes the TJ formation by facilitating the incorporation of palmitoylated claudins.
Results
Establishment of Epithelial Cells Lacking TJs by Knockout of All Expressed Claudin Isoforms.
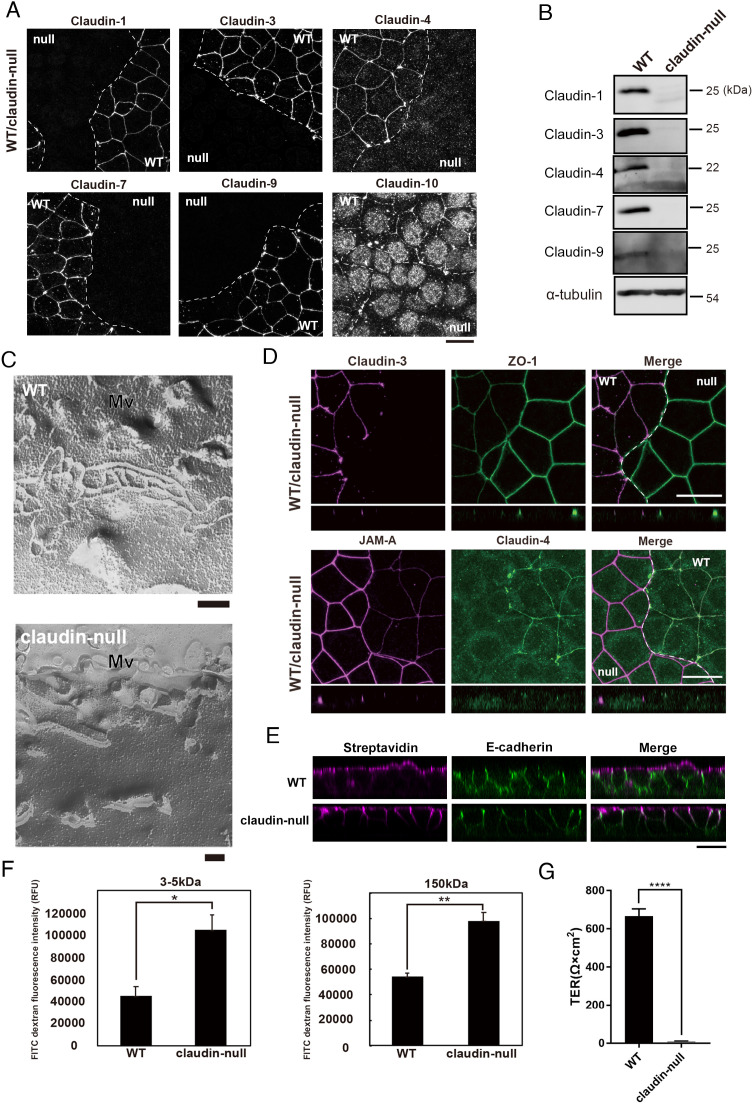
We generated claudin-null cells lacking all expressed claudin isoforms from the mouse-cultured mammary gland epithelial cell EpH4 (claudin-1, -3, -4, -7, -9, and -10) (7) by CRISPR-Cpf1-mediated gene knockout. The loss of claudin isoforms’ expression in claudin-null cells was confirmed by immunofluorescence microscopy and western blotting (Fig. 1 A and B). Freeze-fracture replica electron microscopy revealed that TJ strands, typically observed beneath microvilli in wild-type EpH4 cells, were never observed in claudin-null cells (Fig. 1C). We also examined the localization of other TJ proteins in claudin-null cells. One of the ZO proteins, ZO-1, was enriched at apical junctions in claudin-null cells but their amount increased relative to wildtype (Fig. 1D). In addition, the accumulation of JAM-A also increased in claudin-null cells (Fig. 1D).
Fig. 1.
Establishment of claudin-null cells. (A) Representative immunofluorescence images of a coculture of EpH4 WT and claudin-null cells stained for claudin-1, claudin-3, claudin-4, claudin-7, claudin-9, and claudin-10b. (Scale bar, 20 µm.) (B) Immunoblotting of whole-cell lysates of EpH4 WT and claudin-null cells. Claudin-1, claudin-3, claudin-4, claudin-7, and claudin-9 were not detected in claudin-null cells. Endogenous claudin-10b could not be detected by several commercially available antibodies due to its low expression level. (C) Freeze-fracture EM images of TJ strands in EpH4 WT and claudin-null cells. TJ strands were never observed in claudin-null cells. Mv: microvilli. (Scale bar, 200 nm.) (D) Representative immunofluorescence images of a coculture of EpH4 WT and claudin-null cells stained for claudin-3 and ZO-1 (Upper) and JAM-A and claudin-4 (Lower). (Scale bar, 20 µm.) (E) Sulfo-NHS-biotin was added to the apical compartment to visualize the paracellular tracer flux. The distribution of biotin was detected by staining with streptavidin. Lateral membranes are marked by E-cadherin. Note that in claudin-null cells, biotin freely passes through the paracellular space in the absence of the TJ barrier. (Scale bar, 20 µm.) (F) The paracellular permeability to macromolecules in EpH4 WT and claudin-null cells was examined by measuring the flux of membrane-impermeable tracers of various sizes (FITC-dextran, 3 to 5 and 150 kD). Apical-to-basal permeability of 3 to 5 kD and 150 kD FITC-dextran was significantly increased in claudin-null cells (n = 3, Student’s t test, *P < 0.05, **P < 0.01). (G) Transepithelial resistance (TER) was significantly decreased in claudin-null cells indicating loss of epithelial barrier in claudin-null cells (n = 3, Student’s t test, ****P < 0.0001).
Based on our observations so far, it should follow that the barrier function of claudin-null cells is impaired. Indeed, in the biotin tracer assay, only the apical membrane is biotinylated in wild-type cells, as visualized by FITC-conjugated streptavidin. In contrast, biotinylation is detected at the lateral membrane in claudin-null cells, indicating that the biotin tracer freely passed through the intercellular spaces (Fig. 1E). We also tested the barrier function against macromolecules in claudin-null cells using FITC-dextran with molecular weights of 3 to 5 kDa and 150 kDa (Fig. 1F). In claudin-null cells, the permeability of both molecular weight macromolecules was significantly increased compared to wild-type cells. Finally, we confirmed the loss of the TJ barrier in claudin-null cells by transepithelial electric resistance (TER) measurement (Fig. 1G). All told, we concluded that TJs are structurally and functionally absent in claudin-null cells.
Claudin Null Cells Are Normally Polarized.
As TJs are considered to function as a fence at the boundary between apical and basolateral membranes, which contributes to epithelial polarization, we next examined the apicobasal polarity of claudin-null cells. When the apical marker proteins GFP-tagged podocalyxin-1 and prominin-2 were expressed in claudin-null cells, both localized at the apical membrane as well as in wild-type cells (SI Appendix, Fig. S1A). Na-K ATPase α1, a lateral membrane marker protein, was correctly localized at the lateral membrane in claudin-null cells (SI Appendix, Fig. S1B). Furthermore, Par-3, an essential component of the polarity protein complex, was concentrated at apical junctions in claudin-null cells as in wild-type cells (SI Appendix, Fig. S1C). These results indicate that cell polarity is maintained even in the absence of TJs, consistent with previous studies (2, 8, 9).
Aberrant AJs Containing JAM-A Are Formed in Claudin-Null Cells.
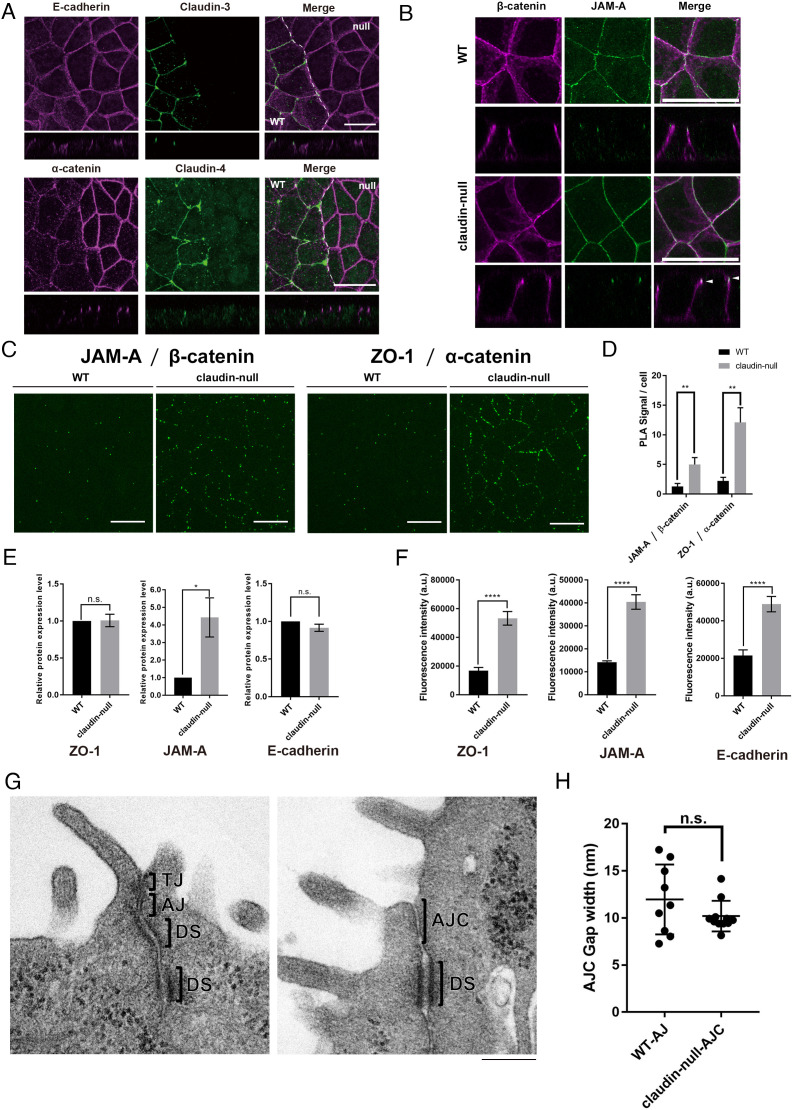
Next, we investigated the localization of proteins associated with AJs. In claudin-null cells, E-cadherin and α-catenin were concentrated more at apical junctions than in wild-type cells (Fig. 2A). As α-catenin is strongly enriched at the most apical region of the lateral membrane in claudin-null cells, we also examined the colocalization of β-catenin and the TJ component JAM-A (Fig. 2B). JAM-A partially colocalized with β-catenin in claudin-null cells (Fig. 2B). Since it is difficult to evaluate the quantitative degree of colocalization between β-catenin and JAM-A in the z-axis direction due to the limitations of light microscopy, we then performed a proximity ligation assay (PLA) to evaluate the degree of spatial distinction between TJ and AJ components. Both JAM-A and ZO-1 were significantly more likely to be in the vicinity of β-catenin and α-catenin, respectively, in claudin-null cells, which indicates that TJ and AJ proteins are spatially intermixed in the absence of functional TJs (Fig. 2 C and D). Therefore, we concluded that aberrant AJs containing JAM-A are formed in claudin-null cells. From these observations, it is clear that JAM-A and the AJ cadherin-catenin complex are unable to clearly segregate in claudin-null cells. Although AJ and TJ are collectively defined as AJC in normal epithelial cells, we define AJs containing JAM-A as AJCs in claudin-null cells in the following.
Fig. 2.
AJs containing JAM-A are formed in claudin-null cells. (A) Representative immunofluorescence images of a coculture of EpH4 WT and claudin-null cells stained for E-cadherin and claudin-3 or α-catenin and claudin-4. (Scale bar, 20 μm.) (B) Representative immunofluorescence images of EpH4 WT and claudin-null cells stained for JAM-A and β-catenin. Arrowhead indicates colocalization of β-catenin and JAM-A. (Scale bar, 20 μm.) (C) Representative fluorescence images of PLA signals between either JAM-A and β-catenin or ZO-1 and α-catenin in EpH4 WT and claudin-null cells. (Scale bar, 20 μm.) (D) Graph showing the average number of PLA signals detected per cell. Details of the experiment are described in Materials and Methods (n = 3, Student’s t test, **P < 0.01). (E) Graph showing the protein levels of ZO-1, JAM-A, and E-cadherin in EpH4 WT and claudin-null cells based on the western blotting analyses (n = 3, Student’s t test, *P < 0.05). (F) Graph showing the signal intensities of ZO-1, JAM-A, and E-cadherin at bicellular junctions in EpH4 WT and claudin-null cells based on the immunofluorescence images (n = 3, Student’s t test, ****P < 0.0001). (G) Representative transmission EM images of ultrathin sections of EpH4 WT and claudin-null cells. TJs with membrane appositions were observed in EpH4 WT. On the other hand, TJs were absent, and the intercellular space was widened in claudin-null cells. (Scale bar, 200 nm.) (H) Quantification of the intracellular gaps at AJs in EpH4 WT and at AJCs in claudin-null cells between adjacent cells based on the transmission EM images of ultrathin sections of EpH4 WT and claudin-null cells (n = 9, Student’s t test).
To distinguish whether the increased immunofluorescence of ZO-1, JAM-A, and E-cadherin at AJCs in claudin-null cells was due to the increased amounts of these proteins or to increased localization, we quantified and compared their expression levels along with their localization signals in wild-type and claudin-null cells (Fig. 2 E and F). The protein levels of ZO-1 and E-cadherin were not changed between wild-type cells and claudin-null cells, while JAM-A was significantly increased in claudin-null cells (Fig. 2E). On the other hand, signals of ZO-1, JAM-A, and E-cadherin staining were significantly increased in claudin-null cells (Fig. 2F). These findings suggest that the increased signal intensity of ZO-1, JAM-A, and E-cadherin at cell–cell contacts in claudin-null cells is more likely to be caused by altered localization than by increased transcription and translation of these proteins.
We next performed ultrathin section electron microscopic analyses to closely examine AJCs in claudin-null cells (Fig. 2G). We found that the plasma membrane contacts formed between adjacent cells (kissing points) were never observed and only AJ-like structures are formed in claudin-null cells. We measured the width of the gap between the adjoining cells based on the EM images and found that the gaps at the AJCs in claudin-null cells are comparable to that at the AJ in wild-type cells (Fig. 2H).
Cholesterol Is Highly Enriched at the Outer Leaflet of the Plasma Membrane at AJCs in Claudin-Null Cells.
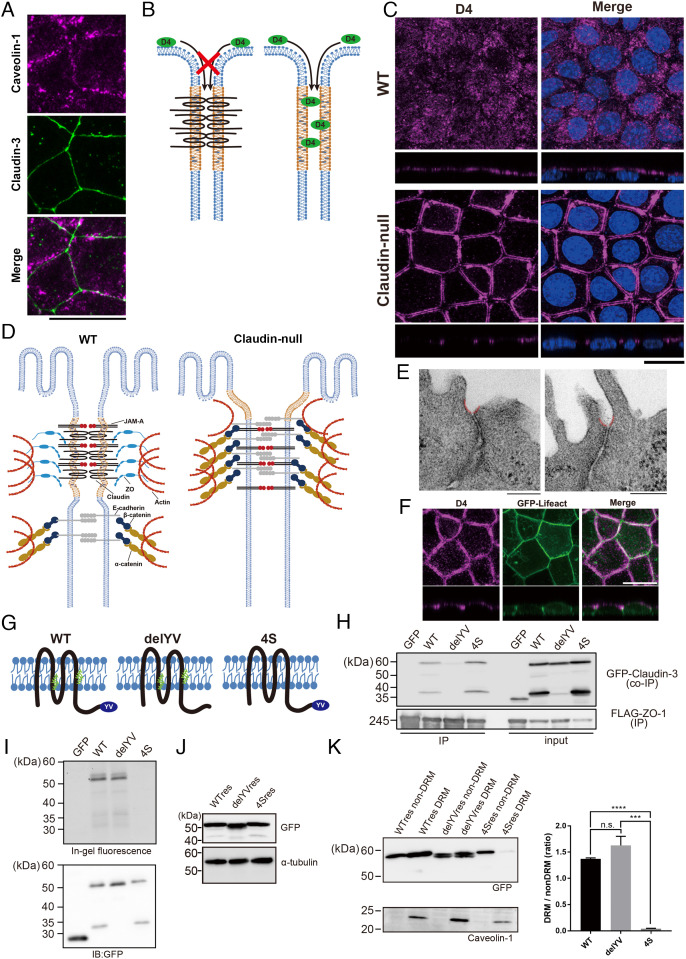
We recently reported that the plasma membrane of TJs is enriched in cholesterol and SM with very long-chain fatty acids (4). That claudin-3 partially colocalized with the membrane raft marker caveolin-1, as previously described (5), further support the notion that TJs are distinct raft-like membrane domains that are rich in cholesterol (5) (Fig. 3A). The question then is whether the formation of this cholesterol-rich membrane domain is induced by the accumulation and polymerization of claudins or is formed separately from claudin strand assembly.
Fig. 3.
Accumulation of cholesterol at AJCs is maintained without TJs in claudin-null cells. (A) Representative immunofluorescence images of EpH4 WT stained for caveolin-1 and claudin-3. (Scale bar, 20 μm.) (B) Schematic of the cholesterol localization analysis using the cholesterol-binding protein, RFP-D4. RFP-D4 cannot access the lateral membrane in WT cells because its bulk and hydrophilicity prohibit its passage across the TJ barrier. Under conditions where the barrier is absent, such as in claudin-null cells, RFP-D4 is able to access the lateral membrane, enabling direct verification of either the presence or the absence of cholesterol enrichment around AJCs. (C) Distribution of cholesterol at the outer leaflet of plasma membrane visualized by D4 staining in EpH4 WT and claudin-null cells. (Scale bar, 20 µm.) (D) In claudin-null cells, the intrinsic contractility of the actomyosin ring anchoring AJCs pulls apart the more apical membrane regions between adjoining cells since they are no longer tethered by TJs. The gap between parallel D4 staining in Fig. 2B reflects this disassociated state at the presumptive TJ region. (E) Representative transmission EM images of ultrathin sections of claudin-null cells. In claudin-null cells, the plasma membrane regions immediately apical to AJCs is caved inward (colored red). (Scale bars, 200 nm.) (F) Representative fluorescence images of GFP-Lifeact-expressing claudin-null cells stained with RFP-D4. (Scale bar, 20 μm.) (G) Molecular schematic of claudin mutants. The delYV mutant lacks the C-terminal PDZ-binding motif (YV) that is essential for binding to ZO proteins. In the 4S mutant, all four cysteine residues that undergo palmitoylation (green) were changed to serine. (H) Binding of GFP-fused claudin mutants to ZO-1 was assessed by immunoprecipitation of FLAG-tagged ZO-1. (I) Palmitoylation was detected using fluorescent palmitic acid. As expected, fluorescent palmitic acid is not incorporated by the 4S mutant. (J) Establishment of claudin-null cells stably expressing GFP-fused WT res, the delYV mutant (delYV res), or the 4S mutant (4S res). Whole-cell lysates were blotted with anti-GFP antibody and anti-alpha tubulin antibody. (K) Immunoblot analysis of the DRM and non-DRM fractions of claudin-null cells stably expressing WT claudin-3 (WT res), the delYV mutant (delYV res), or the 4S mutant (4S res). The DRM fraction is enriched with the DRM marker protein caveolin-1. The ratio of protein levels between DRM and non-DRM fractions were quantified (n = 3, Student’s t test, ***P < 0.001, ****P < 0.0001).
Therefore, we directly visualized cholesterol enrichment in claudin-null cells using a cholesterol probe. We opted to use the protein-based probe derived from domain 4 (D4) of perfringolysin O from among the available cholesterol probes since it most specifically and sharply visualizes cholesterol-enriched domains, rather than free cholesterol, in the membrane (10, 11). In wild-type cells, the D4 probe (molecular weight ∼40 kD) cannot pass across the TJ to access the lateral membranes due to the TJ barrier against macromolecules, whereas in claudin-null cells, it can access the entire lateral membrane since the TJ barrier is impaired (Fig. 3B). Accordingly, only the apical membrane was stained in wild-type cells; by contrast, D4 additionally stained the most apical region of the lateral membrane in claudin-null cells (Fig. 3C). That D4 stained such a limited region of the lateral membrane in claudin-null cells strongly indicates that cholesterol enrichment in the vicinity of AJCs occurs independently from TJ strand formation (Fig. 3D).
Incidentally, D4 staining in claudin-null cells shows a striking intercellular gap. Ultrastructural analysis of the apical membrane around AJCs revealed that the plasma membrane caves inward from the base of the microvilli toward the AJ (Fig. 3E). Since the plasma membrane here is constitutively subject to the contractile force of the circumferential actin ring, we can surmise that the presumptive TJ plasma membrane regions are pulled apart in the absence of claudin-mediated adhesions to bind them. In order to determine whether cholesterol-rich membrane domains are actually formed in the vicinity of AJCs in claudin-null cells, we established cells stably expressing GFP-Lifeact in claudin-null cells to visualize the circumferential actin ring associated with AJCs and stained them with D4. GFP-Lifeact colocalized with D4, supporting the conclusion that cholesterol-rich domains are formed in the vicinity of AJCs (Fig. 3F). Based on these observations, we concluded that the cholesterol-rich membrane domain is formed in the vicinity of AJCs independently of claudin polymerization.
The Nonpalmitoylated 4S Claudin Mutant Cannot Interact with Cholesterol-Rich Membrane Domains.
The above findings suggest that there are at least two mechanisms by which claudins could accumulate at AJCs, one directed by protein–protein interaction and the other by interaction with the cholesterol-enriched plasma membrane domain. The former is the well-characterized interaction between claudins and ZO proteins. The C-terminal PDZ binding motif of claudin is well conserved among claudin isoforms and binds to the PDZ domain of ZO proteins. ZO proteins themselves form higher-order multimeric complexes that may assist claudin polymerization and cells lacking ZO proteins do not form TJs (2, 12). Therefore, the assumption has been that the interaction between claudins and ZO proteins is essential for TJ formation but this has never been tested directly in previous studies.
Separately, we have shown that cholesterol-rich membrane domains are present at AJCs even in the absence of polymerized claudins. Intriguingly, four cysteine residues of claudin-14 reportedly undergo palmitoylation and these palmitoylation sites are well-conserved among claudin isoforms (13). In general, palmitoylation is an important posttranslational modification that regulates the localization of membrane proteins at the plasma membrane (14). Molecular dynamics simulation in a recent study predicts that claudin palmitoylation is important for its localization at the plasma membrane through the interaction with cholesterol and SM (15). Thus, this lipid modification may underlie the selective accumulation of claudins at AJCs by dictating the affinity of claudins for the cholesterol-rich membrane domains.
We therefore decided to test the contribution of each of these two possible mechanisms by rescuing claudin-null cells with mutants of claudin-3, one lacking the carboxyl-terminal PDZ-binding region required for interaction with ZO proteins (delYV) and the other lacking the palmitoylation modification sites (4S; Fig. 3G). Immunoprecipitation assay confirmed that wild-type and the 4S mutant, but not the delYV mutant, bind to ZO-1 (Fig. 3H). Likewise, the 4S mutant was not palmitoylated (Fig. 3I).
We then established clones of claudin-null cells expressing wild-type claudin-3 (WT res), the delYV mutant claudin-3 (delYV res), or the 4S mutant claudin-3 (4S res) at similar expression levels to characterize the affinity of these proteins for the cholesterol-rich membrane domain in epithelial cells (Fig. 3J). When we examined their segregation into Triton X-100-insoluble detergent-resistant membranes (DRMs), we found that the proportion of the 4S mutant in the DRM fraction was significantly reduced compared to both wild-type and the delYV mutant (Fig. 3K).
The delYV Claudin Mutant Deficient in Interaction with ZO Proteins Form TJ Strands.
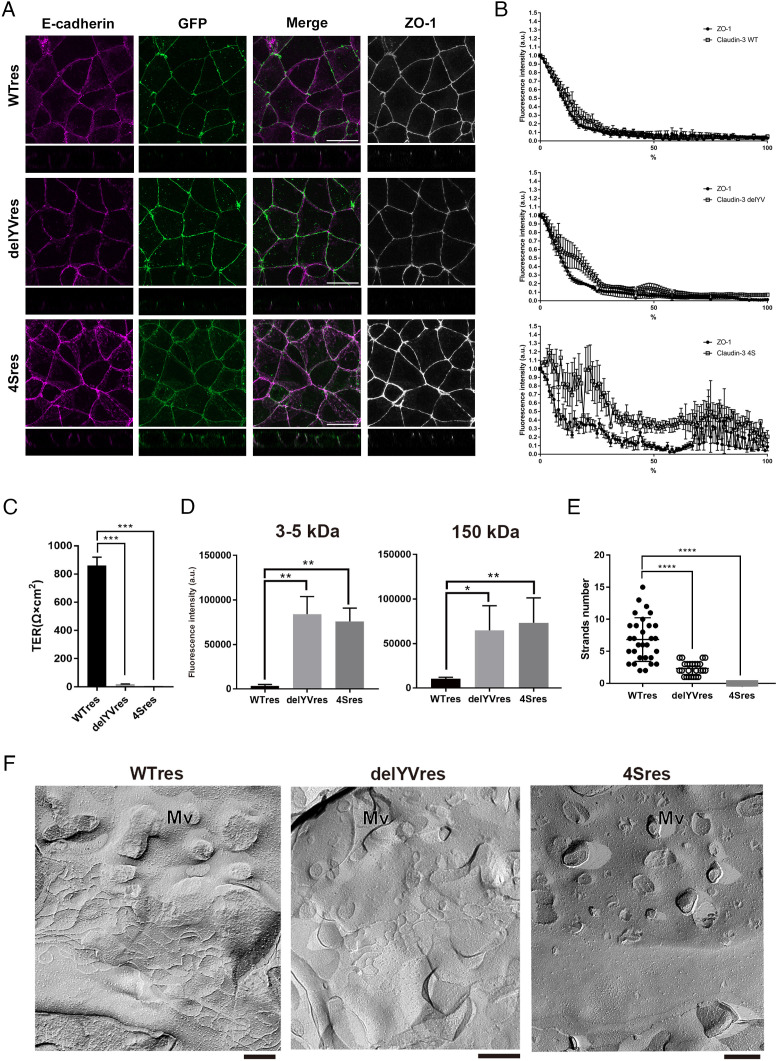
Since the affinity for the cholesterol-rich membrane domains is lost in the 4S mutant, these results suggest that palmitoylation could mediate claudin accumulation at AJCs. Therefore, we next examined the localization of the claudin mutants and their ability to polymerize into TJ strands. Surprisingly, the delYV mutant, like WT res, was correctly localized at AJCs, which suggests that binding to the ZO proteins is not required for claudins to accumulate at AJCs. By contrast, a large proportion of the 4S mutant remained in the lateral membrane relative to AJCs (Fig. 4A). We quantified the extent to which the localizations of WT res, the delYV mutant, and the 4S mutant are restricted to AJCs (Fig. 4B). The delYV mutant, but not the 4S mutant, was enriched at AJCs, indicating that the affinity to cholesterol-rich membrane domain is crucial for the accumulation of claudins at AJCs.
Fig. 4.
Claudin-3 delYV mutants induce TJ strand formation in claudin-null cells independently of ZO proteins. (A) Representative immunofluorescence images of WT res, delYV res, or 4S res cells stained for ZO-1 and E-cadherin. (Scale bar, 20 μm.) (B) Enrichment of claudin mutants shown in A relative to the AJC marker ZO-1 was analyzed by line scan analysis. The highest fluorescence of GFP-claudin-3 signal was set as the apical-most point of the lateral membrane, from which cell height was measured along the lateral membrane revealed by E-cadherin immunofluorescence. Total cell height was normalized to 100% to plot the percentage of the lateral membrane covered by the GFP signals. Error bars show SD calculated based on three independent experiments. (C) The TER values were significantly decreased in delYV res cells or 4S res cells as compared to WT res cells (n = 3, Student’s t test, ***P < 0.001). (D) Apical-to-basal permeability of 3 to 5 kD and 150 kD FITC-dextran was compromised in delYV res and 4S res cells as compared to WT res cells (n = 3, Student’s t test, *P < 0.05, **P < 0.01). (E) Number of TJ strands were quantified based on the freeze-fracture EM images (n = 25, Student’s t test, ****P < 0.001). (F) Representative freeze-fracture EM images of TJ strands in WT res, delYV res, and 4S res cells. Mv: microvilli. (Scale bar, 200 nm.)
Next, we examined whether functional TJs are formed in claudin-null cells expressing the delYV mutant or the 4S mutant. Although the delYV mutant superficially accumulated at AJCs, the TER value of these cells, as well as the 4S mutant, did not recover to the extent of claudin-null cells expressing WT res (Fig. 4C). Likewise, the barrier function against macromolecules was not meaningfully restored in either mutant-rescued cells, as indicated by the increased permeability of both low and high molecular weight FITC-dextran compared to wild-type-rescued cells (Fig. 4D).
Why is the delYV mutant nonfunctional despite its correct localization? To address this question, we performed ultrastructural analysis of claudin-null rescue cells. Consistent with its lateral localization, TJ strands were never observed in the 4S mutant rescue cells (Fig. 4 E and F). By contrast, bona fide TJ strands were observed in the delYV mutant rescue cells although their number was significantly reduced relative to wild-type rescue cells, which explains why the TER was underdeveloped and the paracellular barrier remained compromised in delYV rescue cells (Fig. 4 E and F). Nevertheless, delYV claudin-3 irrefutably formed the hallmark TJ ultrastructure, indicating that claudins can not only accumulate at AJCs but also polymerize, albeit minimally, without interacting with ZO proteins.
To determine whether these claudin-3 mutants have TJ-forming activity in cells other than EpH4 cells, we established L fibroblasts, which lack endogenous claudins, that stably express these mutants. The delYV mutant is accumulated at intercellular adhesion sites and formed a TJ network, similar to WT (SI Appendix, Fig. S2). On the other hand, the 4S mutant was uniformly distributed at the plasma membrane and did not accumulate at cell–cell contacts (SI Appendix, Fig. S2). These results suggest that affinity for cholesterol-rich membrane domains via palmitoylation modification, rather than binding to ZO proteins, is essential for claudins to polymerize into TJ strands, even in such a heterologous cell model as L fibroblasts.
Given that TJ strands form independently of claudin binding to ZO proteins and that cholesterol is still enriched near AJCs in the absence of claudin assembly, we supposed that claudins accumulate at AJCs through their affinity for cholesterol-rich membrane domains and thereafter self-assemble into TJ strands. If this were true, the claudin-3 delYV mutant would be more sensitive to cholesterol removal than WT since the former lacks the anchor to the circumferential actin ring through ZO proteins. Accumulation of WT at AJCs was unaffected by treatment with up to 50 mM MbCD. By contrast, 25 mM MbCD was enough to weaken the accumulation of the delYV mutant at AJCs (SI Appendix, Fig. S3). These results clearly support an active role for cholesterol-rich membrane domains in concentrating claudin at AJCs.
As the delYV mutant undergoes palmitoylation (Fig. 3I), while the 4S mutant does not, it is evident that claudin accumulation at AJCs requires the interaction between its palmitoylated moiety and the cholesterol-rich membrane domain. Moreover, this lipid-directed concentration is sufficient to polymerize claudins into TJ strands. However, the density of TJ strands in claudin-null cells expressing the delYV mutant was inadequate to restore a functional barrier, indicating that protein–protein interaction with ZO proteins is required to form enough number of TJ strands to achieve complete epithelial barrier function.
In claudin-null cells expressing claudin-3 WT and delYV, the enhanced accumulation of E-cadherin at AJ decreased with the restoration of TJ strand formation. In contrast, E-cadherin remained elevated at AJ in claudin-null cells expressing the 4S mutant. These findings indicate that TJ strand formation suppresses the formation of excessive AJs (SI Appendix, Fig. S4).
Roles of ZO Protein as Membrane Organizer.
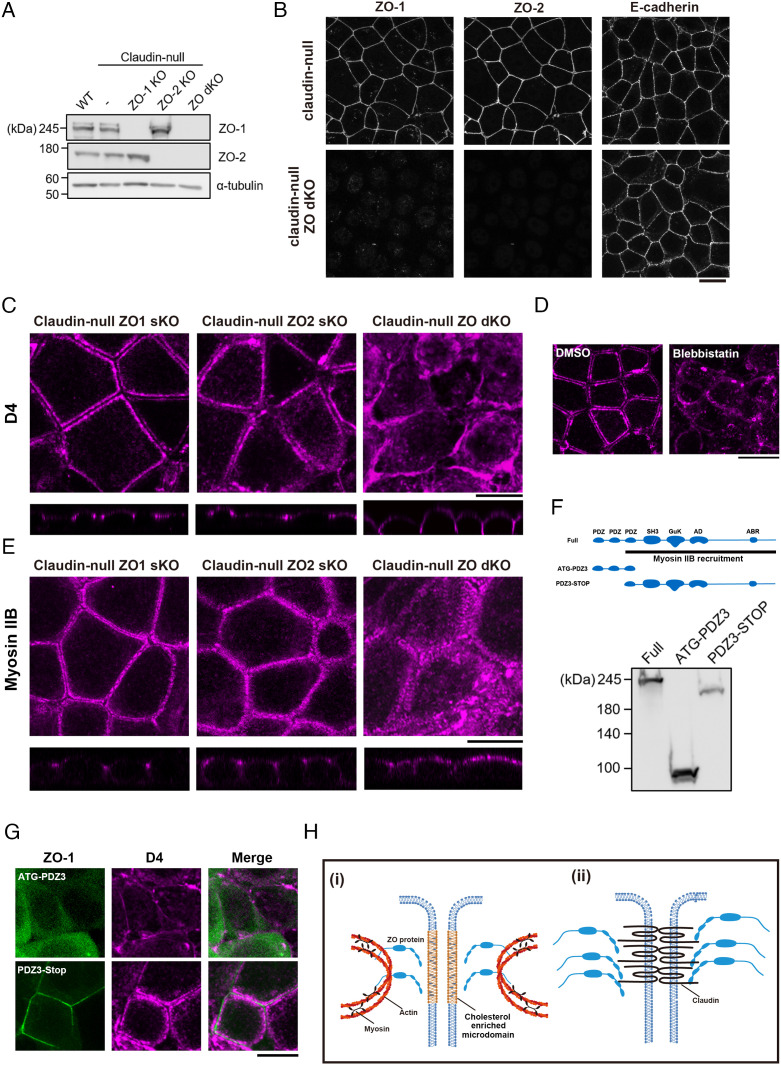
When we previously reported that epithelial cells lacking ZO-1 and ZO-2 (ZO dKO cells) fail to form TJs, our interpretation of this result was that ZO proteins, as claudin scaffolds, determine where TJ strands are polymerized (2, 16). However, the delYV mutant lacking the association with ZO proteins still accumulates at AJCs and forms TJ strands, implying that binding to scaffolding proteins is dispensable for claudin accumulation. Therefore, we reexamined the role of ZO proteins in TJ formation by regarding them as organizers of membrane domains instead. To elucidate the role of ZO proteins in cholesterol accumulation, we established claudin-null cells lacking ZO proteins [claudin-null ZO-1 single KO (claudin-null ZO-1 sKO), claudlin-null ZO-2 single KO (claudin-null ZO-2 sKO), and claudin-null ZO-1/ZO-2 double KO cells (claudin-null ZO dKO)] (Fig. 5 A and B).
Fig. 5.
ZO proteins enable the formation of cholesterol-rich membrane domains in the vicinity of AJCs via contractile actomyosin cable formation. (A and B) Establishment of claudin-null ZO-1 sKO, claudin-null ZO-2 sKO, and claudin-null dKO cells. Immunoblots of whole-cell lysates prepared from each cell lines are shown in A. Representative immunofluorescence images of claudin-null cells and claudin-null ZO dKO cells stained for ZO-1, ZO-2, and E-cadherin are shown in B. (Scale bar, 20 µm.) (C) Distribution of cholesterol at the outer leaflet of plasma membrane visualized by D4 staining in claudin-null ZO-1 sKO, claudin-null ZO-2 sKO, and claudin-null ZO dKO cells. Note that the accumulation of cholesterol at AJCs is lost and that cholesterol is redistributed uniformly across the lateral membranes in claudin-null ZO dKO cells. (Scale bar, 20 µm.) (D) Distribution of cholesterol at the outer leaflet of plasma membrane visualized by D4 staining in claudin-null cells treated with 100 µM blebbistatin. (Scale bar, 20 µm.) (E) Distribution of Myosin IIB in claudin-null ZO-1 sKO, claudin-null ZO-2 sKO, and claudin-null ZO dKO cells. (Scale bar, 20 µm.) (F) Schematics of the ZO-1 deletion mutants used in this study and immunoblots of whole-cell lysates prepared from HEK293 cells expressing each construct. (G) Distribution of cholesterol at the outer leaflet of plasma membrane visualized by D4 staining in claudin-null ZO dKO cells expressing GFP-tagged ATG-PDZ3 or GFP-tagged PDZ3-STOP. (Scale bar, 20 µm.) (H) Working model of how ZO proteins accumulate claudins at AJCs by two different molecular mechanisms. (i) Contractile actin ring directs accumulation of cholesterol in the vicinity of AJCs. ZO proteins possibly mediate the enrichment of cholesterol directly or indirectly. Palmitoylated claudins are recruited to the cholesterol-rich membrane domains, resulting in the polymerization of claudins into TJ strands. (ii) The polymerization of ZO proteins further increases the density of claudins at the plasma membrane and enables the development of the functional TJ network.
The accumulation of cholesterol at AJCs is unaffected in claudin-null ZO-1 sKO cells or claudin-null ZO-2 sKO cells, while cholesterol was no longer concentrated at AJCs but broadly distributed to the lateral membrane in claudin-null ZO dKO cells (Fig. 5C).
Then, how do ZO-1 and ZO-2 cooperatively contribute to cholesterol accumulation at AJCs? Although not a precise parallel, the circumferential actin ring at AJCs is similar to the contractile ring formed during cytokinesis in that both are contractile actomyosin ring structures associated with the plasma membrane. Intriguingly, cholesterol accumulation at the cleavage furrow during cytokinesis is dependent on actomyosin contractility, as disruption of the contractile actin ring by treatment with blebbistatin impairs it (17, 18). Therefore, we examined the effect of blebbistatin treatment on cholesterol distribution in claudin-null cells. We found that cholesterol accumulation at AJCs was abolished by blebbistatin treatment, suggesting that an analogous mechanism to the cleavage furrow is at work here (Fig. 5D).
It has been shown that ZO proteins are required for the formation of the circumferential actin ring and recruitment of the associated Myosin II, suggesting that ZO proteins could direct cholesterol accumulation by ensuring contractility at AJCs (19). In line with this supposition, Myosin IIB was absent from AJCs in claudin-null ZO dKO cells (Fig. 5E). Integration of Myosin II into the circumferential actin ring at AJCs requires the region from PDZ3 to the carboxyl terminus of ZO-1 (Fig. 5F, PDZ3-STOP fragment) (19). Accordingly, expression of the PDZ3-STOP fragment restored the accumulation of both Myosin II and cholesterol at AJCs in claudin-null ZO dKO cells (Fig. 5G and SI Appendix, Fig. S5A). Therefore, we concluded that the formation of the contractile circumferential actin ring, which requires ZO proteins, is a prerequisite for the accumulation of cholesterol at AJCs.
Previous studies suggest that the formation of the contractile ring and the enrichment of cholesterol at the cleavage furrow are interdependent (17, 18). Inhibiting cholesterol accumulation at the cleavage furrow causes cytokinesis failure by impairing contractile ring formation. Therefore, we tested whether the formation of the circumferential actin ring is similarly dependent on cholesterol accumulation at AJCs. Cholesterol removal by MbCD treatment had no effect on circumferential actin ring formation in claudin-null cells, leading us to conclude that the circumferential actin ring preceded cholesterol accumulation at AJCs and not vice versa (SI Appendix, Fig. S5B). We also examined the effect of cholesterol depletion on localizations of ZO-1 and Myosin IIB with respect to TJ strands by MbCD treatment in claudin-null cells expressing WT res, the delYV mutant claudin-3, or the 4S mutant claudin-3 (SI Appendix, Fig. S5C). The localization of ZO-1 and Myosin IIB in these rescue cells was unaffected by MbCD treatment, clearly indicating that the formation of AJCs containing Myosin IIB induces the formation of cholesterol-rich domains and not vice versa.
In summary, we concluded that ZO proteins play two distinct roles in TJ formation, not only through protein–protein interactions as previously thought but also through the induction of the cholesterol-rich membrane domain via the formation of the circumferential actin ring associated with AJCs, which enables claudin accumulation.
Discussion
The crux of this study is the observation that the circumferential actin ring underlying AJCs causes the formation of a cholesterol-rich membrane domain, which is essential for the formation of TJs. Altogether, our results strongly argue for a reconsideration of TJ formation, whereby not only protein–protein (claudin–ZO proteins) interaction as canonically believed but also protein–lipid (claudin–cholesterol) interaction supports polymerization of claudins into TJ strands. (Fig. 5H). Elucidating how the formation of cholesterol-rich domains at AJCs is induced by ZO proteins and the contractile circumferential actin rings is an important research topic for the future. Relevant to this question, it was recently reported that cholesterol-rich liquid order domains are increased in T cells by artificially stabilizing the actin cytoskeleton (20).
There are several possibilities as to how nonlabile actin structures could cause the accumulation of cholesterol and SM to the outer leaflet of the overlaying plasma membrane. It has been suggested that the actin cytoskeleton immobilizes phosphatidylserine (PS) with long-chain saturated fatty acids, resulting in the formation of membrane domains containing phospholipids with saturated fatty acids, including SM, GPI-anchored protein, and cholesterol on the outer leaflet by transbilayer coupling (21). Such transbilayer coupling of inner and outer leaflets may be caused not only by PS but also by phosphatidylinositol 4,5-bisphosphate (PIP2), which is abundant in the inner leaflet of the plasma membrane of the cleavage furrow and epithelial AJCs. PIP2 promotes polymerization of the actin cytoskeleton and activation of membrane-anchored proteins such as ERMs, which may promote further cholesterol accumulation at the outer leaflet via stabilization of the actin cytoskeleton; it is also conceivable that the accumulation of cholesterol in the outer layer of the plasma membrane is promoted by proteins recruited by ERM.
In addition to ERM family proteins, several cross-linking proteins such as Annexin A2 (22) and IRSp53 (23), which bind to both the actin filament and membrane lipids, are known to accumulate at AJCs. These molecules may be involved in the formation of circumferential actin ring-dependent cholesterol-rich domains in the vicinity of AJCs. More interestingly, it has been reported that contractile actomyosin filaments can regulate the size and dynamics of liquid-ordered phase (Lo) and liquid-disordered phase (Ld) domains of supported artificial lipid membranes (24, 25). Actomyosin fibers bind unequally to these lipid phases of different viscosity. The traction of the membrane domains by actomyosin contraction is counteracted by friction, but the difference in viscosity triggers reorientation of the Lo domain. Thus, actomyosin contractility itself may contribute to the rearrangement of cholesterol-rich Lo domains at AJCs. Indeed, it has been reported that cholesterol and raft-associated proteins such as flotillin accumulate in the cleavage furrow formed during cytokinesis, in a manner dependent on the contractile force of the actomyosin ring structure (26). Interestingly, the cleavage furrow has been reported to act as a diffusion barrier against membrane proteins during cell division (27). In claudin-null cells, the localization of apical and basolateral membrane proteins is not affected (SI Appendix, Fig. S1 A and B), indicating that the diffusion barrier at the boundary between apical and basolateral membranes is maintained even in the absence of TJ strands. This implies that the TJ structure in itself, i.e., the functional sum of its protein components, is not the actuator of the diffusion barrier. Astonishingly, that role may be assumed by the cholesterol-rich membrane domain formed on the basis of contractile actin rings, which was visualized here through the analysis of claudin-null cells. It is also notable that ZO proteins were essential for cholesterol accumulation at AJCs since it was reported that ZO-1 also accumulates at the cleavage furrow during cytokinesis (28). Therefore, it is possible that ZO proteins directly or indirectly regulate cholesterol accumulation to these membrane domains.
It has been shown that the preceding formation of AJs is required for the formation of TJs during the maturation of cell–cell contacts. In the present study, we demonstrated that AJ formation is excessively enhanced in claudin-null cells lacking TJs, indicating that TJ formation suppresses AJ formation. The existence of such mutual regulation between TJ and AJ has been observed in various experimental systems (29–32). At present, the molecular mechanisms by which TJ formation suppresses AJ formation are unclear; however, there are several possibilities. One possibility is that TJ strand formation suppresses AJ formation by physically eliminating the intercellular space, which prevents adhesion mediated by E-cadherins with large extracellular regions. Another possibility is that the relocalization of ZO proteins and cell polarity proteins, such as the Par complex, from AJs to TJs in conjunction with TJ formation suppresses AJ formation. The detailed elucidation of the mechanisms behind the mutual regulation of TJs and AJs is an important topic for future research, particularly in the context of the differentiation of membrane structures at the plasma membrane.
Finally, Otani et al. recently reported that JAM-A forms a functional barrier against 150 kDa macromolecules in the absence of TJs, using the canine kidney-derived epithelial cell line MDCKII, similarly lacking claudin expression (9). By contrast, the barrier against 150 kDa macromolecules was completely lost in our claudin-null EpH4 cells even though JAM-A was present at AJCs. The disagreement here could be due to the differences between MDCKII and EpH4 cells, but our result challenges the notion that JAM-A invariably forms a barrier against macromolecules independent of claudin-based TJs.
Concluding Remarks
It was long thought that ZO proteins bound to the actin cytoskeleton determine where claudins are assembled into functional TJs based on the analogy to other cell adhesion complexes such as AJs and desmosomes. It is increasingly clear, however, that TJs are fundamentally different structures. We previously demonstrated that the removal of cholesterol from the plasma membrane selectively impairs TJ formation without affecting AJ formation (4). Now, the present study shows that the scaffolding proteins and cytoskeleton associated with TJs promote the assembly of a functional adhesion structure by a mechanism completely unique from those of other cell adhesion structures.
Materials and Methods
Cells and Reagents.
EpH4 cells were grown in DMEM supplemented with 10% FBS. Claudin-null EpH4 cells were established using the CRISPR-Cpf1 system. The primary and secondary antibodies used for immunofluorescence microscopy and immunoblotting are listed in Supplementary Table 1. Cytochalasin D was added to the cells at a final concentration of 1 µM.
Expression Vectors.
pLenti CMV GFP Neo (Addgene Plasmid #17447) was used for the construction of expression vectors. DNA fragments encoding mouse claudin-3, claudin-3 lacking the COOH terminus KDYV (delYV), and claudin-3 carrying C113S, C116S, C192S, and C194S mutations (4S) were subcloned into the pLenti CMV GFP Neo vector. Claudin-null cells were generated by using the CRSPR-Cpf1 system. The target sequences were as follows:
claudin-1 (mouse), 5′-ATCCTGGCTTCTCTGGGATGGAT-3′; claudin-3 (mouse), 5′-GGCCTTCATCGGCAGCAGCATCA-3′; claudin-4 (mouse), 5′-CCTCTTCTGCCCGGAAGCCACCA-3′; claudin-7 (mouse), 5′-GCAGCTCATCATGCCGGTGCTCT-3′; claudin-9 (mouse), 5′-GTGTCCTGTGCCCTGCCACTGTG-3′; and claudin-10b (mouse), 5′-GCCAACCTGTGGAAGATCTGCGT-3′.
Electron Microscopy.
EpH4 cells and claudin-null cells were fixed with 2% paraformaldehyde and 2% glutaraldehyde in 0.1 M phosphate buffer (PB) pH 7.4 at 4 °C overnight. After fixation, the samples were washed three times with 0.1 M PB for 30 min each and were postfixed with 2% osmium tetroxide in 0.1 M PB at 4 °C for 90 min. The samples were dehydrated in graded ethanol solutions. The samples were infiltrated with propylene oxide (PO) twice for 20 min each and were put into a 70:30 mixture of PO and resin (Quetol-812: Nisshin EM) for 1 h, then the tube was left open overnight to allow PO to be volatilized. The samples were transferred to a fresh 100% resin and were polymerized at 60 °C for 48 h. The polymerized resins were ultrathin sectioned at 70 nm with a diamond knife using an ultramicrotome (Ultracut UCT; Leica), mounted on copper grids, then stained with 2% uranyl acetate at room temperature (RT) for 15 min. Stained samples were washed with distilled water followed then secondary-stained with lead stain solution (Sigma-Aldrich) at RT for 3 min. The grids were observed by a transmission electron microscope (JEM-l400Plus; JEOL) at an acceleration voltage of 100 kV.
Fluorescence Microscopy.
Immunofluorescence microscopy was performed as described previously (33). In brief, cells cultured on coverslips were fixed with 3% formalin in PBS for 10 min at RT, treated with 0.2% Triton X-100 in PBS for 5 min, and then washed with PBS. Blocking was done by incubating the fixed cells with 5% BSA in PBS for 30 min at RT. After the antibodies were diluted with the blocking solution, the cells were incubated at RT for 1 h with the primary antibody and for 1 h with the secondary antibody. For actin staining, Alexa Fluor 488 phalloidin (Life Technologies) was added to the secondary antibody. For imaging of cholesterol-rich domains, cells were cultured on a glass-bottom dish and incubated with recombinant RFP-tagged D4 for 30 min at 37 °C. After washing with HBSS thrice, cells were observed at 37 °C. Both immunofluorescence and live imaging samples were observed with a confocal microscope (LSM700; Carl Zeiss MicroImaging) equipped with a Plan-APO (63/1.40 NA oil-immersion) objective with appropriate binning of pixels and exposure time. The images were analyzed with ZEN 2012 (Carl Zeiss MicroImaging).
FLAG Immunoprecipitation.
HEK293 cells were transfected with FLAG-tagged ZO-1 and GFP-tagged claudin-3. Cells were washed with ice-cold PBS and lysed with FLAG IP buffer [20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, and protease inhibitors]. Lysates were incubated with 10 μL of anti-DYKDDDDK mAb beads (Wako Pure Chemical Industries) for 2 h. The beads were washed with FLAG IP buffer, and bound proteins were eluted in a SDS sample buffer. Aliquots of the lysates and eluates were immunoblotted with anti-DYKDDDDK and anti-GFP antibodies.
Detection of 17-Octadecynoic Acid-Labeled Proteins Using Click Chemistry.
HEK293 cells transiently expressing GFP-tagged claudin were labeled with 25 µM 17-octadecynoic acid (Sigma-Aldrich, O8382) in a normal culture medium for 24 h. The cells were washed with PBS and lysed in RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and protease inhibitors). Lysates were incubated with anti-GFP antibody overnight at 4 °C. Protein G Sepharose (GE Healthcare) was added to lysate and incubated for 3 h. Immunoprecipitated GFP-tagged proteins were eluted from the beads with 1% SDS, 50 mM HEPES pH −7.4 and adjusted to 1 mM CuSO4 (Wako, 030-04442), 1 mM Tris(2-carboxyethyl)phosphine hydrochloride (Sigma-Aldrich Co., C4706), 100 µM Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (Tokyo Chemical Industry, T2993), and 100 µM TAMRA-Azide-Biotin (Click Chemistry Tools, #1048). The reaction mixture was gently shaken at RT in darkness for 1 h. The click reactions were stopped by adding 10 volumes of ice-cold methanol, and proteins were precipitated overnight at −80 °C. The precipitated samples were spun down at 15,000 rpm at 4 °C for 30 min, rinsed in ice-cold methanol, and eluted in SDS sample buffer for analysis by SDS-PAGE. Gels were scanned with an Amersham Typhoon scanner (GE Healthcare).
Freeze-Fracture EM.
Freeze-fracture electron microscopy was performed as described previously (4). Confluent cells were fixed with 2.5% glutaraldehyde in PB, rinsed in PB, cryoprotected in 30% glycerol in PB, and then frozen in liquid propane. Frozen samples were fractured at −180 °C and platinum-shadowed unidirectionally at an angle of 45° in a JFD-7000 apparatus (JEOL). Replicas with cells were immersed in household bleach and then mounted on copper grids after the cells were removed. Processed samples were examined using a JEM-2100HCKM electron microscope (JEOL).
Measurement of TER and Analysis of Paracellular Tracer Flux.
Cells were cultured for 6 d on transwell filters, and TER was measured directly in culture media using a cellZscopeE (CellSeed). For the paracellular tracer flux assay, after 6 d of culture, FITC-dextran with molecular weights of 3 to 5 kDa and 150 kDa at concentrations of 200 µM and 50 µM was added to the medium in the inner compartment. After 90 min of incubation, a 100-µL aliquot of the medium was collected from the outer compartment, and the paracellular tracer flux was measured as the amount of FITC-dextran in the medium using a fluorometer.
Biotin Tracer Assay.
The biotin tracer assay was performed using the cell-surface biotinylation method as described by Chen et al. (1997) with some modifications (34). Cells cultured on transwell filters were washed with HEPES-buffered saline [HBS; 25 mM HEPES-NaOH (pH 7.2), 137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, 6 mM dextrose, and 1.8 mM CaCl2] to which 1 mg/mL EZ-Link sulfo-NHS-LC-biotin (Pierce) prepared in HBS was added to the upper chamber only. After 10 min of incubation, the cells were washed with DMEM and fixed with 100% methanol for 3 min at −20 °C. The cells were then washed with PBS, blocked with 1% BSA in PBS for 30 min, and incubated with rat anti-E-cadherin antibody for 1 h. After a wash with PBS, they were incubated for 30 min with streptavidin and anti-rat antibody conjugated with Texas Red and Cy2, respectively.
PLA.
PLA was performed on EpH4 wild-type and claudin-null cells using the Duolink PLA kit (Sigma-Aldrich). The cells cultured on coverslips were fixed with 100% methanol at −20 °C and then blocked with 1% BSA prepared in PBS for 30 min at RT. Blocked cells were incubated with primary antibodies for 1 h at RT. The combination of primary antibodies was either rabbit anti-JAM-A pAb and mouse β-catenin mAb or rabbit anti–α-catenin and mouse anti-ZO-1. After washing, the samples were incubated with PLA probe solution containing the secondary antibodies for 1 h at 37 °C. Then, the samples were washed and incubated with the ligation solution containing the DNA ligase for 30 min at 37 °C. After ligation, the samples were incubated with the amplification solution containing DNA polymerase and fluorescently labeled nucleotides for 100 min at 37 °C. Finally, the samples were washed, counterstained with DAPI in mounting medium, and observed with a confocal microscope. Images from three fields of view were acquired for each sample, with each field of view containing ≥20 nuclei as identified by DAPI staining. The number of PLA signals was divided by the number of nuclei to calculate the number of PLA signals per cell. Experiments were performed independently three times.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank all members of the Ikenouchi laboratory (Department of Biology, Faculty of Sciences, Kyushu University) for helpful discussions. We also thank the Ultramicroscopy Research Center of Kyushu University, Dr. T. Inai (Fukuoka Dental College), and Dr. Y. Fukazawa (Fukui university) for their invaluable guidance and assistance of EM studies. This work was supported by JSPS KAKENHI [JP22H02618 (J.I.), JP21K19231 (J.I.), JP21K15089 (K.S.), JP20K22632 (K.S.), and JP16H06280 (Grant-in-Aid for Scientific Research on Innovative Areas, Platforms for Advanced Technologies and Research Resources “Advanced Bioimaging Support”)], AMED-FORCE (21444781) (J.I.), JST-FOREST (JPMJFR204L) (J.I.), and grants from the Mitsubishi Foundation (J.I.) and the Cell Science Research Foundation (J.I.).
Author contributions
J.I. designed research; K.S., Y.O., and K.M. performed research; K.S., Y.O., and K.M. analyzed data; and K.S. and J.I. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. S.L.V. is a guest editor invited by the Editorial Board.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Tsukita S., Furuse M., Itoh M., Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2, 285–293 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Umeda K., et al. , ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 126, 741–754 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Beutel O., Maraspini R., Pombo-García K., Martin-Lemaitre C., Honigmann A., Phase separation of zonula occludens proteins drives formation of tight junctions. Cell 179, 923–936.e911 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Shigetomi K., Ono Y., Inai T., Ikenouchi J., Adherens junctions influence tight junction formation via changes in membrane lipid composition. J. Cell Biol. 217, 2373–2381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nusrat A., et al. , Tight junctions are membrane microdomains. J. Cell Sci. 113, 1771–1781 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Lingaraju A., Long T. M., Wang Y., Austin J. R., Turner J. R., Conceptual barriers to understanding physical barriers. Semin. Cell Dev. Biol. 42, 13–21 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamazaki Y., Tokumasu R., Kimura H., Tsukita S., Role of claudin species-specific dynamics in reconstitution and remodeling of the zonula occludens. Mol. Biol. Cell 22, 1495–1504 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikenouchi J., et al. , Lipid polarity is maintained in absence of tight junctions. J. Biol. Chem. 287, 9525–9533 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otani T., et al. , Claudins and JAM-A coordinately regulate tight junction formation and epithelial polarity. J. Cell Biol. 218, 3372–3396 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimada Y., Nakamura M., Naito Y., Nomura K., Ohno-Iwashita Y., C-terminal amino acid residues are required for the folding and cholesterol binding property of perfringolysin O, a pore-forming cytolysin. J. Biol. Chem. 274, 18536–18542 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Wilhelm L. P., Voilquin L., Kobayashi T., Tomasetto C., Alpy F., Intracellular and plasma membrane cholesterol labeling and quantification using filipin and GFP-D4. Methods Mol. Biol. 1949, 137–152 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Fanning A. S., Van Itallie C. M., Anderson J. M., Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol. Biol. Cell 23, 577–590 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Itallie C. M., Gambling T. M., Carson J. L., Anderson J. M., Palmitoylation of claudins is required for efficient tight-junction localization. J. Cell Sci. 118, 1427–1436 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Diaz-Rohrer B. B., Levental K. R., Simons K., Levental I., Membrane raft association is a determinant of plasma membrane localization. Proc. Natl. Acad. Sci. U.S.A. 111, 8500–8505 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajagopal N., Irudayanathan F. J., Nangia S., Palmitoylation of claudin-5 proteins influences their lipid domain affinity and tight junction assembly at the blood-brain barrier interface. J. Phys. Chem. B 123, 983–993 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Ikenouchi J., Umeda K., Tsukita S., Furuse M., Tsukita S., Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J. Cell Biol. 176, 779–786 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng M. M., Chang F., Burgess D. R., Movement of membrane domains and requirement of membrane signaling molecules for cytokinesis. Dev. Cell 9, 781–790 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Abe M., et al. , A role for sphingomyelin-rich lipid domains in the accumulation of phosphatidylinositol-4,5-bisphosphate to the cleavage furrow during cytokinesis. Mol. Cell Biol. 32, 1396–1407 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamazaki Y., et al. , ZO-1- and ZO-2-dependent integration of myosin-2 to epithelial zonula adherens. Mol. Biol. Cell 19, 3801–3811 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinic J., Ashrafzadeh P., Parmryd I., Actin filaments attachment at the plasma membrane in live cells cause the formation of ordered lipid domains. Biochim. Biophys. Acta 1828, 1102–1111 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Raghupathy R., et al. , Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell 161, 581–594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee D. B., Jamgotchian N., Allen S. G., Kan F. W., Hale I. L., Annexin A2 heterotetramer: Role in tight junction assembly. Am. J. Physiol. Renal Physiol. 287, F481–F491 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Massari S., et al. , LIN7 mediates the recruitment of IRSp53 to tight junctions. Traffic 10, 246–257 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Köster D. V., et al. , Actomyosin dynamics drive local membrane component organization in an in vitro active composite layer. Proc. Natl. Acad. Sci. U.S.A. 113, E1645–E1654 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogel S. K., Greiss F., Khmelinskaia A., Schwille P., Control of lipid domain organization by a biomimetic contractile actomyosin cortex. Elife 6, e24350 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon S., et al. , The lipid raft microdomain-associated protein reggie-1/flotillin-2 is expressed in human B cells and localized at the plasma membrane and centrosome in PBMCs. Immunobiology 205, 108–119 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Schmidt K., Nichols B. J., A barrier to lateral diffusion in the cleavage furrow of dividing mammalian cells. Curr. Biol. 14, 1002–1006 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Wang T., Yanger K., Stanger B. Z., Cassio D., Bi E., Cytokinesis defines a spatial landmark for hepatocyte polarization and apical lumen formation. J. Cell Sci. 127, 2483–2492 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Troxell M. L., et al. , Inhibiting cadherin function by dominant mutant E-cadherin expression increases the extent of tight junction assembly. J. Cell Sci. 113, 985–996 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Shigetomi K., Ikenouchi J., Cell adhesion structures in epithelial cells are formed in dynamic and cooperative ways. BioEssays 41, e1800227 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Campbell H. K., Maiers J. L., DeMali K. A., Interplay between tight junctions & adherens junctions. Exp. Cell Res. 358, 39–44 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatte G., Prigent C., Tassan J. P., Tight junctions negatively regulate mechanical forces applied to adherens junctions in vertebrate epithelial tissue. J. Cell Sci. 131, 208736 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Ikenouchi J., et al. , Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 171, 939–945 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y. -H., Merzdorf C., Paul D. L., Goodenough D. A., COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos. J. Cell Biol., 138, 891–899 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.