Abstract
Echinacea purpurea modulates tumor progression, but the underlying mechanism is poorly defined. We isolated and purified a novel homogeneous polysaccharide from E. purpurea (EPPA), which was shown to be an arabinogalactan with a mean molecular mass (Mr) of 3.8 × 104 Da and with α- (1 → 5) -L-Arabinan as the backbone and α-L-Araf-(1→, →6)-β-D-Galp-(1→, and →4)-α-D-GalpA-(1→ as the side chains. Interestingly, oral administration of EPPA suppresses tumor progression in vivo and shapes the immune cell profile (e.g., facilitating M1 macrophages) in tumor microenvironment by single-cell RNA sequencing (scRNA-seq) analysis. More importantly, EPPA activates the inflammasome through a phagocytosis-dependent mechanism and rewires transcriptomic and metabolic profile, thereby potentiating M1 macrophage polarization. Collectively, we propose that EPPA supplementation could function as an adjuvant therapeutic strategy for tumor suppression.
Graphical abstract

Public summary
-
•
An Echinacea purpurea-derived homogeneous polysaccharide (EPPA) is found to have anti-tumor efficacy.
-
•
Single-cell RNA sequencing analysis demonstrates that oral EPPA administration targets immune cell functions in tumor microenvironment.
-
•
EPPA activates inflammasome in M1 macrophages via phagocytosis-mediated endocytosis mechanism.
-
•
EPPA potentiates M1 macrophage polarization by reprogramming transcriptomic and metabolic profiles.
Introduction
The composition and polarization of the cellular constituents (e.g., tumor cells, endothelial cells, and immune cells) of the tumor microenvironment (TME) determine tumorigenesis and affect the outcomes of cancer treatment.1,2 Thus, these cellular constituents (chiefly immune cells) could be considered as attractive targets for cancer therapy.1,3 Indeed, immunotherapy is a promising approach for cancer treatment. For example, cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) or programmed death-1 (PD-1)/programmed death ligand 1 (PD-L1)-based immune checkpoint inhibitors can inhibit tumor growth by affecting the TME and the functions of immune cells.4,5,6 However, most patients do not benefit from immunotherapy, as they develop immunotherapy resistance and/or suffer substantial side effects.7,8,9 Therefore, there is an urgent need to modulate the microenvironment (e.g., shaping the functions of immune cells) to avoid immune evasion, and consequently, promote the anti-tumor immunity.
The use of natural products as part of conventional cancer therapy has received growing attention as an important practical approach.10,11 Echinacea purpurea is a medicinal plant that is commonly used for the prevention and treatment of upper respiratory tract infections in Europe and North America.12 The pharmacological activities of Echinacea preparations (e.g., immunomodulatory activity, antifungal and antibacterial activities, and antioxidant property) depend on their bioactive constituents, including alkamides, caffeic acid derivatives, and polysaccharides. However, because of differences in the types of solvents used for extraction and/or variations in extraction procedures (all of which influence both the chemical profile and the biological activity of Echinacea preparations), the functions of different E. purpurea constituents and/or their derivatives remain largely unknown.
The current interest in the medicinal use of Echinacea is focused on its immunomodulatory effects.13 For example, Echinacea polysaccharides have strong adjuvant effects on the stimulation of T cell responses.14,15 However, delineation of specific constituents of Echinacea polysaccharides and their corresponding immune activities (especially for the anti-tumor immunity efficacy) is poorly defined.
Here, we first isolated and purified a homogeneous polysaccharide from E. purpurea (EPPA) and then used H22 ascites tumor-bearing and orthotopic colorectal cancer mouse models to evaluate the anti-tumor activity of EPPA. We found that oral administration of EPPA inhibits tumor growth in mice and promotes M1 macrophage polarization according to single-cell sequencing (scRNA-seq) analysis. Mechanistically, after phagocytosis, EPPA potently boosts the production of interleukin (IL)-1β by enhancing the activation of cellular inflammasome and reprogramming the transcriptomic and metabolic profile in M1 macrophages. Collectively, these findings highlight the potential application of EPPA supplementation as an adjuvant therapy in tumor by targeting M1 macrophages.
Results
Chemical structure of EPPA
A crude polysaccharide of E. purpurea was obtained from the roots of E. purpurea (L.) Moench and then eluted, dialyzed, and freeze-dried to prepare EPPA. The homogeneity and molecular weight of EPPA were then identified using high-performance gel permeation chromatography (HPGPC). The symmetrical peak on the HPGPC indicated that the isolated EPPA had an average molecular weight of 3.8 kDa (Figure 1A). The monosaccharide composition of EPPA was composed of L-Ara, D-Gal, and D-GalA in a ratio of 2.7:1:1, as determined by reduction, acid hydrolysis, aldononitrile acetate derivatization, and gas chromatography analysis. The corresponding H/C signals were assigned on the basis of one-dimensional (1D) and two-dimensional (2D) nuclear magnetic resonance (NMR) spectra and are listed in Table S1. The chemical shifts in the 1H NMR spectrum exhibited three strong signals in the anomeric region at 5.07, 5.10, and 5.14 ppm, which may be involved in the main chain structure of polysaccharides. The other three weaker signals were 4.47, 4.94, and 5.09 ppm. The 13C spectrum of CYP3 (Figure 1B) mainly showed six anomeric carbons at δ 108.2, 108.1, 103.9, 99.8, 100.9, and 107.8 ppm, which were designated as A, B, C, D, E, and F residues, respectively.
Figure 1.
Extraction, purification, and chemical characterization of homogeneous polysaccharide (EPPA)
(A and B) HPGPC (A) and 1H and 13C NMR spectra (B) of homogeneous polysaccharide.
(C) The putative structure of EPPA.
For residue A, the anomeric proton signal at δ 5.07 ppm correlated with the signals at δ 108.2 ppm in the heteronuclear single quantum coherence (HSQC) spectrum. The signal values of 81.5, 77.2, and 82.9 were assigned to C2, C3, and C4 by the heteronuclear multiple bond connectivity (HMBC) and HSQC-total correlation spectroscopy (HSQC-TOCSY). The correlation between H-5 (3.88, 3.95) and C-5 (66.9) indicated that C-5 was involved in the connection of glycosidic linkage. Thus, the glycosidic linkage was identified to be →5)-α-L-Araf-(1→. Similarly, residues B–F were identified as 3,5) -α-L-Araf- (1 →, →6)-β-D-Galp-(1→, →4)-α-D-GalpA-(1→, →4)-α-D-GalpAMe- (1 →, and α-L-Araf- (1 →, respectively.16 For residue F, according to a previous report,17 C-2-C-5 was not involved in the glycosidic linkage. Thus, this glycosidic linkage was identified to be α-L-Araf- (1 → (residue D), which is the non-reducing end Ara. The molar ratio of these glycosidic linkages → 3,5) -α-L-Araf- (1 →, → 5) -α-L-Araf- (1 →, and α-L-Araf- (1 →) was about 1:1:1 by 1H NMR integration, indicating that α-L-Araf- (1 → participated in branch linkage formation. For residue D, the chemical shift of C-6 was 176.1 ppm, suggesting that it presented as carboxyl acid. For residue E, the chemical shift of C-6 at 171.3 ppm indicated that the carboxyl group was esterified by methyl (3.79/53.5) group. C-4 of residue E at 79.7 ppm showed that it is involved in glycosidic linkage formation.
Using the HMBC assay, the sequences of glycoside residues were determined as shown in Figure 1B. The cross peaks at δ 66.9/5.10 ppm (A [C5]/[H1] B) and δ 67.2/5.07 ppm (B [C5]/[H1] A) suggested that these two glycosidic linkages were linked to each other, and that the main chain comprised α- (1 → 5) -L-Arabinan. Also, it suggested that the C-3 of → 3,5) -α-L-Araf- (1 → was involved in the formation of the branch chain. Similarly, HMBC results indicated that H-1 of residue C was correlated with C-3 of residue B ( → 3,5) -α-L-Araf- (1), indicating that the two glycosidic residues were directly connected. There was also a correlation between H-1 of residue F and C-4 of residue D or E.
Taken together, these results indicate that EPPA, whose putative structure of EPPA is presented in Figure 1C, is an arabinogalactan that mainly consists of L-Ara, D-Gal, and D-GalA.
Oral EPPA administration suppresses tumor growth in mice
To decipher the bioactivity of EPPA, we assessed its effects on tumor growth. Results showed that EPPA had no effect on the proliferation of tumor cells in vitro (e.g., H22 cells) (Figures S1A–S1F). Subsequently, we investigated the effect of EPPA on tumor progression in mice that were initially transplanted with murine H22 hepatocarcinoma cells, with cyclophosphamide as positive control (Figures S2A and S2C). It was observed that oral EPPA administration, but not intratumoral EPPA administration, showed inhibitory effects on tumor growth in mice accompanied by a reduction in tumor weight and size (Figures S2B–S2N).
We also used the orthotopic colorectal cancer mouse model to further investigate the anti-tumor efficacy of EPPA in vivo (Figure 2A). It was shown that oral EPPA administration substantially reduced tumor burden in the colon of mice (Figures 2B and 2C), but it had little effect on the corresponding colon length (Figures 2B and 2D). Furthermore, oral EPPA administration increased the number of IFN-γ+ and F4/80+ cells (marked for T cells and macrophages, respectively, which are associated with anti-tumor immunity) (Figures 2E and 2F). Taken together, these findings show that oral EPPA administration inhibits tumor growth in mice and shapes host immune status.
Figure 2.
Effects of EPPA on tumor growth of colorectal cancer in mice
(A) Schematic image for the establishment of colorectal cancer mouse model with or without EPPA treatment.
(B–D) The tumor number (C) and colon length (D) of the mice in different groups at 15 weeks post-induction (n = 12).
(E) Representative IHC staining image for IFN-γ and F4/80. Scale bar, 100 μm.
(F) Positive staining area/total tissue area in colorectal tumor tissue sections (n = 6–9).
Data were analyzed using unpaired t tests (C, D, and F) and are represented as mean ± SD. ∗p < 0.05, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
Oral EPPA administration changes immune responses in the tumor microenvironment
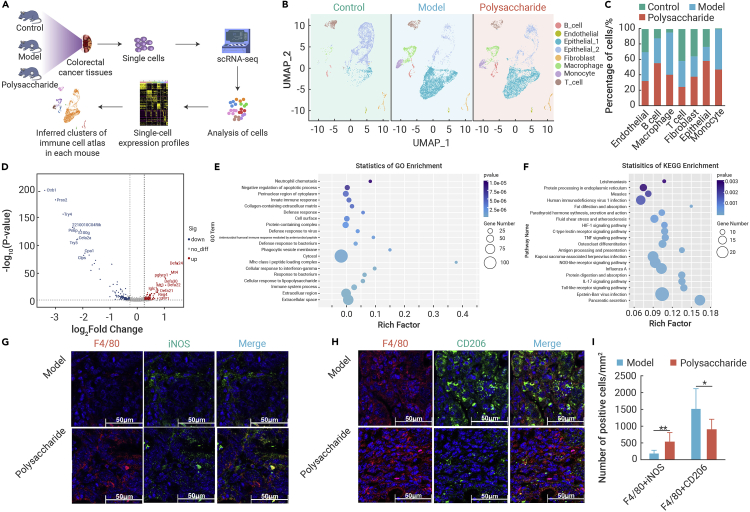
To further clarify the immunoregulatory effects of EPPA in vivo, we generated scRNA-seq data from the colorectal cancer tissues of control, model, and EPPA-treated mice (Figure 3A). We defined immune cell populations in murine colorectal cancer tissues, including monocytes, macrophages, T cells, and B cells (Figures 3B, S3A, and S3B). We also determined cell counts and examined major differences in transcriptional landscape of macrophages, T cells, and B cells (Figure 3C).
Figure 3.
Transcriptional landscape of macrophages in tumor tissue of colorectal cancer in mice by scRNA-seq analysis
(A) Schematic diagram of scRNA-seq process in mice.
(B) Immune cell profiles of tumor tissues in different groups of mice.
(C) The percentage of cells in control, model, and polysaccharide group.
(D–F) Volcano map (D), GO analysis (E), and KEGG analysis (F) of differential gene obtained from macrophages between model group and polysaccharide group.
(G–I) Immunofluorescence analysis of F4/80+ iNOS+ macrophages (G and I) and F4/80+ CD206+ macrophages (H and I) in tumor tissue of colorectal cancer in mice (n = 5–7). Scale bar, 50 μm.
Data were analyzed using an unpaired t test (I) and are represented as mean ± SD. ∗p < 0.05 and ∗∗p < 0.01.
Considering macrophages are the most abundant immune cell type in TME,18,19 we primarily compared the differently expressed genes (DEGs) in macrophages from the model versus EPPA-treated mice (Figure 3D). Transcription of genes involved in innate immune response, cellular response to interferon-gamma/lipopolysaccharide, and immune system process were observed according to the Gene Ontology (GO) enrichment analysis (Figure 3E). The Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis demonstrated that the DEGs in macrophages were highly enriched in HIF-1 signaling pathway, TNF signaling pathway, NOD-like receptor signaling pathway, and Toll-like receptor signaling pathway (Figure 3F), which are related to M1 macrophage activation. These findings were consistent with our immunofluorescent staining analysis showing that EPPA treatment increased F4/80+iNOS+ cells (M1 macrophages, which inhibits tumor growth) and decreased F4/80+CD206+ cells (tumor-associated macrophages [M2-like], which favors tumor growth) in mouse colorectal cancer tissues (Figures 3G–3I).
Given that EPPA increased the number of IFN-γ+ cells (Figures 2F and 2G), we also explored the landscape of T cell subsets (Figures S4A and S4B). Notably, the KEGG enrichment showed that these DEGs were enriched in T cell receptor signaling pathway, IL-17 signaling pathway, and MAPK signaling pathway (Figure S4C). Furthermore, we found that EPPA increased the number of IFN-γ+CD8+ T cells, which exert cytotoxicity response to tumors, suggesting that oral EPPA treatment also affects T cell function in the context of our experimental settings. Overall, the scRNA-seq analysis indicates that oral EPPA administration targets immune cell functions (e.g., M1 macrophage polarization) in TME.
EPPA enhances M1 macrophage inflammation dependent on transcriptional reprogramming
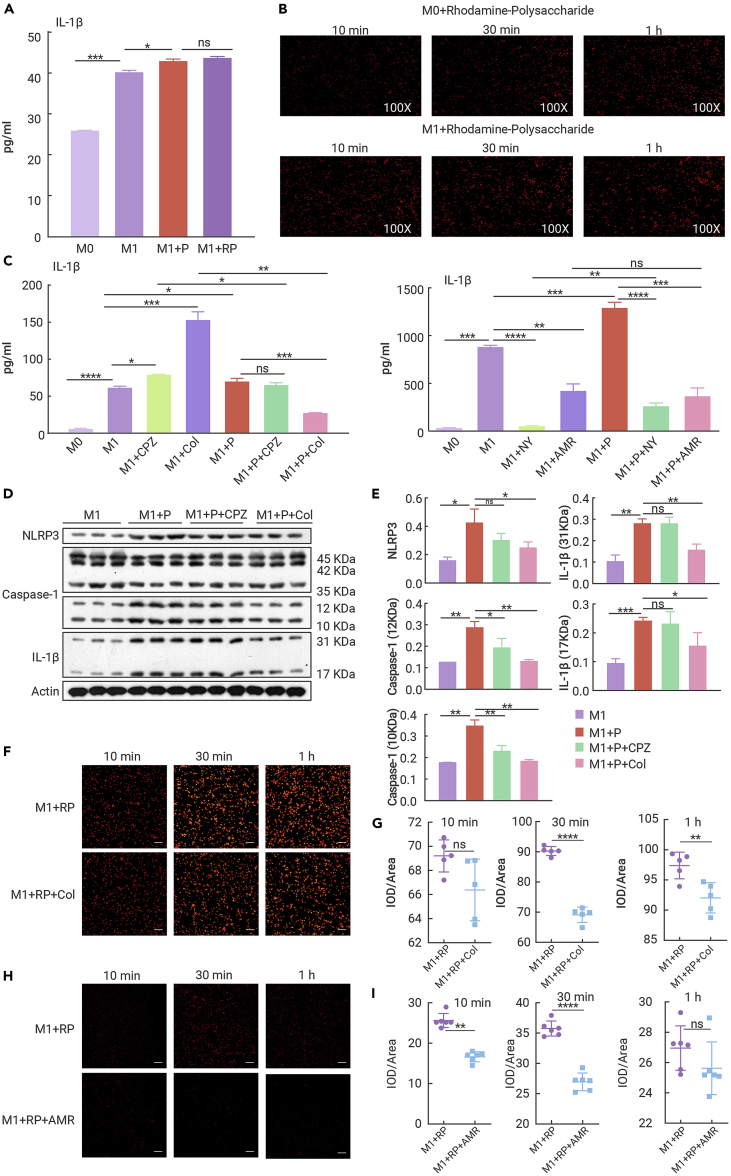
Considering the importance of macrophages against tumor cells, we further explored the direct effect of EPPA on cell viability of macrophages. EPPA did not exhibit significant toxicity against M0 macrophages (Figure S5A). As M1 macrophages determine the orchestration of the immune responses and exert inhibitory effect on tumor cells,20 we determined whether EPPA could influence the physiological state of M1 macrophages. EPPA promoted the proliferation of M1 macrophages, whereas it had little effect on the apoptosis of M1 macrophages (Figures S5B and S5C), especially at a dose of 500 μg/mL, which was used in subsequent experiments. M1 macrophages are characterized by secreting a large amount of mediators, including IL-1β and TNF-α.21,22 EPPA significantly enhanced the secretion of IL-1β (Figure 4A), while reducing the TNF-α concentration in M1 macrophages (Figure 4B).
Figure 4.
EPPA suppresses M1 macrophage inflammation
(A and B) The secretion of IL-1β (A) and TNF-α (B) from M1 macrophages treated with or without EPPA (500 μg/mL) (n = 3). Results represent three independent experiments.
(C) Heatmap analysis of DEGs in M1 macrophages treated with or without EPPA (500 μg/mL) (n = 4).
(D) Lists of signaling pathways (count ≥ 3) in M1 macrophages treated with or without EPPA (500 μg/mL) according to KEGG analysis (n = 4).
(E–G) Heatmap analysis of DEGs related to cytokine-cytokine receptor interaction (E), NF-κB signaling pathway (F), and NOD-like receptor signaling pathway (G) in M1 macrophages treated with or without EPPA (500 μg/mL) (n = 4).
(H) Protein abundance of p65, p-p65, NALP1, NLRP3, NLRC4, AIM2, caspase-1, and IL-1β in M1 macrophages treated with or without EPPA (500 μg/mL) (n = 4).
Data were analyzed using one-way ANOVA with Bonferroni correction (A, B, and H, right) and are represented as mean ± SD.∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
We then conducted bulk RNA-seq to determine how EPPA influences M1 macrophage function. To this end, cluster analysis revealed that EPPA significantly affected the transcriptomic profile of M1 macrophages (Figures 4C and S5D–S5F), in which 591 genes were up-regulated, while 575 genes were down-regulated (fold change > 2, p < 0.05) (Figure S5D). Functional enrichment analysis showed that the DEGs were mostly enriched in biological processes (Figure S5G), including “positive regulation of defense response,” “positive regulation of immune response,” “response to lipopolysaccharide,” and “cellular response to lipopolysaccharide” (Figure S5H). Moreover, in agreement with the previous scRNA-seq results, KEGG pathway analysis revealed that the DEGs (count > 3) were also enriched in signaling pathways (36 items) (e.g., NOD-like receptor signaling pathway, TNF signaling pathway, HIF-1 signaling pathway, and Toll-like receptor signaling pathway) (Figures 4D and S5I), and metabolic pathways (32 items) (Figure S5I), that are crucial for the activation of M1 macrophages. Besides, RNA-seq data showed that EPPA significantly promoted the expression of M1 polarization-related genes enriched in cytokine-cytokine receptor interaction, NF-κB signaling pathway, and NOD-like receptor signaling pathway (Figures 4E–4G). These findings suggest that EPPA treatment transcriptionally enhances M1 macrophage inflammation.
Western blotting results also showed that EPPA substantially increased the protein abundance of inflammasome components, such as NALP1, NLRP3, and cleaved caspase-1 (Figure 4H), which are the vital platforms for the key inflammatory mediator (IL-1β) production.23 Furthermore, EPPA highly increased the protein expression of pro-IL-1β and matured IL-1β (Figure 4H). However, EPPA had little effect on the activation p65 signaling (a proxy for NF-κB activation, which is the main signaling pathway for TNF-α expression) (Figure 4H), despite the fact that EPPA could promote expression of several genes enriched in NF-κB signaling pathway according to RNA-seq analysis (Figure 4E). In summary, EPPA is critical for modulating macrophage function through transcriptional reprogramming.
EPPA activates the inflammasome in M1 macrophages through phagocytosis
To investigate how EPPA affects macrophage function and given that endocytosis is the main cellular entry mode of macromolecules,24 we used rhodamine B-labeled EPPA and found that the secretion of IL-1β was comparable between EPPA-treated M1 macrophages and rhodamine B-EPPA-treated M1 macrophages (Figure 5A). Furthermore, using fluorescent inverted microscopy we observed that the uptake of EPPA by macrophages was obvious and evenly distributed in the cytoplasm (Figure 4B).
Figure 5.
EPPA activates inflammasome in M1 macrophages via endocytosis
(A) The secretion of IL-1β from M1 macrophages treated with EPPA (500 μg/mL) or rhodamine B-labeled EPPA (500 μg/mL) (n = 3). Results represent two independent experiments.
(B) Representative images of the uptake of EPPA (500 μg/mL) by M0 and M1 macrophages at different time points (10 min, 30 min, and 1 h) (×100, n = 3).
(C) The secretion of IL-1β from CPZ (10 μM), Col (10 μM), NY (50 μg/mL), or AMR (50 μM)-pretreated M1 macrophages with or without EPPA (500 μg/mL) supplementation (n = 3). Results represent two independent experiments.
(D and E) Protein abundance of NLRP3, caspase-1, and IL-1β in CPZ (10 μM) or Col (10 μM)-pretreated M1 macrophages with EPPA (500 μg/mL) supplementation (n = 3).
(F and G) Representative images (F) and statistical analysis (G) of the uptake of EPPA (500 μg/mL) by Col (10 μM)-pretreated M1 macrophages at different time points (10 min, 30 min, and 1 h) (n = 5). Results represent two independent experiments. Scale bar, 10 μm.
(H and I) Representative images (H) and statistical analysis (I) of the uptake of EPPA (500 μg/mL) by AMR (50 μM)-pretreated M1 macrophages at different time points (10 min, 30 min, and 1 h) (n = 6). Results represent two independent experiments. Scale bar, 10 μm.
Data were analyzed using one-way ANOVA with Bonferroni correction (A, C, and E) or unpaired t tests (G and I) and are represented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Endocytosis occurs through a variety of mechanisms, specifically clathrin-mediated endocytosis and caveolae-mediated endocytosis.25,26 We treated M1 macrophages with chlorpromazine (CPZ; a clathrin-mediated endocytosis inhibitor), colchicine (Col; a phagocytosis inhibitor), nystatin (NY; a caveolae-mediated endocytosis inhibitor), or amiloride (AMR; a macropinocytosis inhibitor) before EPPA supplementation. We found that both CPZ and Col increased IL-1β secretion from M1 macrophages and EPPA failed to increase IL-1β secretion from CPZ- or Col-pretreated M1 macrophages, while only Col inhibited IL-1β secretion from M1 macrophages with EPPA treatment (Figure 5C, left). Moreover, we also demonstrated that both NY and AMR decreased IL-1β secretion from M1 macrophages and EPPA-treated M1 macrophages, respectively, whereas EPPA failed to promote IL-1β secretion from AMR-pretreated M1 macrophages (Figure 5C, right). Notably, the activation of inflammasome (NLRP3 and cleaved caspase-1) in EPPA-treated M1 macrophages was extremely dampened by Col (Figures 5D and 5E). Likewise, only Col reduced the abundance of IL-1β in EPPA-treated M1 macrophages (Figures 5D and 5E). We confirmed that the endocytosis of EPPA by M1 macrophages was significantly inhibited by Col (Figures 5F and 5G) and AMR (Figures 5H and 5I). Collectively, these data demonstrate that EPPA activates inflammasome in M1 macrophages through a phagocytosis-dependent mechanism.
EPPA shapes the metabolic profile of M1 macrophages
As cellular metabolism is highly associated with the functional output in immune system,27 our bulk RNA-seq data showing that most DEGs enriched in metabolic pathways (including pentose phosphate pathway, pyruvate metabolism, glycolysis, citrate cycle, and oxidative phosphorylation [OXPHOS]) were down-regulated (Figures S6A and S6B) suggest that EPPA may affect cellular metabolism in M1 macrophages. Therefore, we performed metabolomics to identify the metabolites in M1 macrophages with or without EPPA treatment. The intracellular metabolites in M1 macrophages were clearly from those in M1 macrophages with EPPA treatment (Figures 6A, S6C and S6D). The differential metabolites could be enriched in pyruvate metabolism and glycolysis (Figure S6E), which are in line with the results of RNA-seq (Figures S5A and S5B). Furthermore, several significant differential metabolites enriched in cellular metabolism were down-regulated, including glucose, glucose-6P, pyruvate, and lactate (Figure 6B). These findings indicated that EPPA restricts glycolysis of M1 macrophages. Assessment of cellular bioenergetics showed that M1 macrophages with EPPA treatment had lower ECAR (extracellular acidification rate; a measure of glycolysis) (Figures 6C and 6D). Taken together, these data show that EPPA shapes the metabolic profile and restricts glycolysis of M1 macrophages.
Figure 6.
EPPA shapes the metabolic profile of M1 macrophages
(A) Heatmap analysis of different metabolites in M1 macrophages treated with or without EPPA (500 μg/mL) (n = 6).
(B) Fold change of the levels of different metabolites in glucose metabolism of M1 macrophages treated with or without EPPA (500 μg/mL) (n = 6). Blue, decreased; gray, undetermined; black, unchanged.
(C and D) The glycolysis, glycolic capacity, and glycolic reserve of M1 macrophages treated with or without EPPA (500 μg/mL) (n = 10). Results represent two independent experiments.
Data were analyzed using unpaired t tests (B and D) and are represented as mean ± SD.∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
EPPA impairs mitochondrial morphology and inhibits OXPHOS in M1 macrophages
It is well documented that mitochondria serves as a signaling platform via the generation of reactive oxygen species (ROS) and metabolites of the TCA cycle in regulation of macrophage biology.28 Thus, we investigated whether EPPA treatment could influence mitochondria biology, thereby promoting M1 macrophage polarization. The results show that EPPA did not have a direct effect on the biological functions of mitochondria (as shown by the levels of biomass, MMP, and mtROS) in M1 macrophages (Figures S7A–S7D). Furthermore, EPPA altered mitochondrial dynamics (especially inhibiting fusion-related OPA1 expression) (Figures S7E and S7F) and significantly reduced the mean length of mitochondria in M1 macrophages (Figures S7G and S7H), which is consistent with reports that the functional dynamics of metabolism is intertwined with changes in mitochondrial morphology.29
Most DEGs enriched in OXPHOS were down-regulated (Figure S6B), implying that EPPA blocks OXPHOS in M1 macrophages. We found that EPPA significantly reduced electron transport chain (ETC)-related protein abundance (Figures S7I and S7J). On the basis of this finding, assessment of cellular bioenergetics confirmed that M1 macrophages with EPPA treatment became less “oxidative” compared with M1 macrophages, on the basis of the lower OCR (oxygen consumption rate; a measure of OXPHOS) in ATP production, maximal respiration, and spare capacity (Figures S7K and S7L). Collectively, the aforementioned findings indicate that EPPA alters mitochondrial morphology and inhibits OXPHOS capacity of M1 macrophages.
Discussion
A fundamental role of E. purpurea is to stimulate activation of the immune system.30,31 In the present study, we also find that EPPA inhibits tumor growth in vivo, possibly through the regulation of immune responses in the TME, especially for M1 macrophages and IFN-γ+CD8+ T cells. Specifically, EPPA potentiates IL-1β production in M1 macrophages by promoting the activation of cellular inflammasome, and rewiring the transcriptomic and metabolic profile in M1 macrophages involved in phagocytosis-associated endocytosis. This study, thus supports EPPA supplementation as an adjuvant therapy in macrophage-associated inflammatory diseases (e.g., cancer).
The putative active compounds in E. purpurea include polysaccharides, cichoric acid, alkamides, and glycoproteins. Unfortunately, the understanding of the precise chemical structure of these active compounds is still limited. For example, only 4 different polysaccharides with immune stimulating properties have been isolated from E. purpurea and characterized, including two neutral fucogalactoxyloglucans with a mean molecular mass (Mr) of 1×104 and 2.5 × 104, an acidic arabinogalactan with a mean Mr of 7.5×104, and a 4-O-methylation of glucuronic acid xylan with a mean Mr of 3.5 × 104.32,33,34 In the present study, we isolated and purified an EPPA from E. purpurea that that was shown to be an arabinogalactan with a mean Mr of 3.8 × 104 Da, and with α- (1 → 5)-L-Arabinan as backbone and α-L-Araf-(1→, →6)-β-D-Galp-(1→ and →4)-α-D-GalpA-(1→ as the side chains.
Echinacea polysaccharides show highly immunomodulatory effects13; however, the immunological and pharmacological potentials of Echinacea polysaccharides, especially for the anti-tumor immunity efficacy, are poorly understood. Results of the present study demonstrate that oral EPPA administration inhibits tumor growth in vivo by affecting the immune cell profile in TME, especially macrophages and IFN-γ+CD8+ T cells, by conducting scRNA-seq analysis. This finding is consistent with the fact that macrophages are the key inflammatory cells in the TME and pro-inflammatory macrophages always have anti-tumor and immune-enhancing effects.35 As scRNA-seq data demonstrate that oral EPPA administration affects the landscape of T cell subsets in tumor tissues, it will be interesting to further explore the effects of EPPA on T cells (e.g., IFN-γ+CD8+ T cells). Also, considering that intestinal microbiota is closely related to tumor pathogenesis and only oral EPPA administration represses tumor growth, it will be meaningful for future studies to investigate whether EPPA supplementation inhibits tumor growth by shaping intestinal microbiota composition and metabolism.
It has been well demonstrated using cell culture assays that high-purity polysaccharides from Echinacea effectively activate macrophages36,37,38,39,40,41 by promoting the production of inflammatory mediators (e.g., IL-10 and IL-1β) from macrophages. We also find that EPPA enhances IL-1β production in activated macrophages. However, similar to a previous study demonstrating that Echinacea polysaccharides inhibit the production of inflammatory cytokines in macrophages, including TNF-α,42 we notice that EPPA reduces TNF-α production in macrophages. The possible explanation for the discrepancy in the production of inflammatory mediators (e.g., IL-1β and TNF-α) may due to the diverse varieties and origins of Echinacea and the different types of solvent and extraction procedures used. These factors influence both the chemical profile and the biological activity of Echinacea preparation.
The production of IL-1β in pro-inflammatory macrophages (M1) mainly depends on canonical inflammasome-mediated processing.43 The canonical inflammasomes include cytosolic sensor molecules (NOD-like receptor [NLR] [e.g., NALP1, NLRP3, NLRC4] and absent in melanoma [AIM] 2-like receptor [ALR] families), caspase-1, and adaptor molecule ASC.44 The present study provides evidence that EPPA provokes the inflammasome-mediated IL-1β production, especially via NALP1 and NLRP3 activation. However, whether and how EPPA regulates the assembly of NLRP3 and activation of NLAP1 into functional inflammation still needs to be investigated. Additionally, ASC is an adapter protein that is required for the formation of the inflammasomes.45,46 Therefore, it is interesting to investigate whether EPPA influences NLRP3 inflammasome activation by modulating their physical interaction with several components (e.g., ASC).
Innate immune cells, such as macrophages, internalize biomacromolecules and the invading pathogens by endocytosis mediated by various mechanisms, including clathrin- and caveolae-mediated endocytosis, macropinocytosis, and phagocytosis.47 Our results show that EPPA activates phagocytosis to promote inflammasome activation in M1 macrophages. Endocytosis broadly participates in the signaling of inflammation. For example, macrophages stimulated by LPS activate TLR4, which is translocated to endosomes where it activates the downstream pathways (e.g., TRAM/TRIF) and consequently amplifies inflammatory responses.47,48 Thus, research on the function of EPPA in the modulation of phagocytosis-associated endocytosis involved in the signaling transduction of TLR4 requires further clarification.
Macrophage metabolism is highly associated with the corresponding functions49 and the metabolic conversion of macrophages from OXPHOS to glycolysis alters macrophage gene expression and ultimately affects tumor growth.50 On the basis of our RNA-seq and metabonomics data, we clearly demonstrated that EPPA affects glycolysis and OXPHOS of M1 macrophages to boost IL-1β production. Likewise, there were considerable alterations of intracellular metabolites in EPPA-treated M1 macrophages, but how EPPA influences the levels of those metabolites and whether EPPA potentiates IL-1β production in M1 macrophages via those metabolite-mediated mechanisms remains to be investigated.
In conclusion, we identified and purified a new EPPA derived from Echinacea and showed that oral EPPA administration could be a strategy to tackle tumors by tailoring M1 macrophage function. Nevertheless, further research using the mouse model to assess the “clinic significance” (e.g., the pharmacologic mechanism, pharmacokinetics, clinical application, and adverse reaction) of EPPA will be warranted before conducting any human experiments with this product.
Materials and methods
Antibody information, preparation, and structural characterization of homogeneous polysaccharide (EPPA), establishment of tumor model, isolation of peritoneal macrophages, cell proliferation and cytotoxicity analysis, cell apoptosis analysis, analysis of EPPA uptake, immunohistochemistry, immunofluorescence, western blotting, ELISA, detection of mitochondria function, RNA-seq, metabolite profiling analysis, seahorse assay, transmission electron microscopy (TEM), Chromium 10× Genomics library and sequencing, and statistical analysis are described in the supplemental information.
Acknowledgments
We thank Prof. Charles Martin Nyachoti (Department of Animal Science, University of Manitoba) and Dr. Leli Wang (Institute of Subtropical Agriculture, Chinese Academy of Sciences) for reviewing the full manuscript. This study was financially supported by Key Realm R&D Program of Guangdong Province (2020B020221001), the Open Competition Program of Ten Major Directions of Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province (2022SDZG07), Chinese Academy of Science Key Project (QYZDY-SSW-SMC008) and National Natural Science Foundation of China grants (32225047, 31922079, and 32130099).
Author contributions
All authors have made substantial, direct, and intellectual contributions to the work discussed in this publication. W.R. and H.W. conceived and designed the project. J.B., Y.X., F.Z., C.Y., H.J., H.H., M.J., M.L., and Y. Yuan performed the experiments. J.B., Y.X., and F.Z. analyzed the data and wrote the draft. W.R., Z.L., Y. Yin, and H.W. discussed the data and revised the manuscript. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published Online: February 9, 2023
Footnotes
It can be found online at https://doi.org/10.1016/j.xinn.2023.100391.
Contributor Information
Yulong Yin, Email: yinyulong@isa.ac.cn.
Hong Wu, Email: wh@scau.edu.cn.
Lead contact website
Supplemental information
References
- 1.Greten F.R., Grivennikov S.I. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y., Wang Y., Ren Y., et al. Metabolic modulation of immune checkpoints and novel therapeutic strategies in cancer. Semin. Cancer Biol. 2022;86:542–565. doi: 10.1016/j.semcancer.2022.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Nicolas A.M., Pesic M., Engel E., et al. Inflammatory fibroblasts mediate resistance to neoadjuvant therapy in rectal cancer. Cancer Cell. 2022;40:168–184.e13. doi: 10.1016/j.ccell.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borghaei H., Paz-Ares L., Horn L., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer J.R., Tykodi S.S., Chow L.Q.M., et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren D., Hua Y., Yu B., et al. Predictive biomarkers and mechanisms underlying resistance to PD1/PD-L1 blockade cancer immunotherapy. Mol. Cancer. 2020;19:19. doi: 10.1186/s12943-020-1144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binnewies M., Roberts E.W., Kersten K., et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldominos P., Barbera-Mourelle A., Barreiro O., et al. Quiescent cancer cells resist T cell attack by forming an immunosuppressive niche. Cell. 2022;185:1694–1708.e19. doi: 10.1016/j.cell.2022.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirza B., Croley C.R., Ahmad M., et al. Mango (Mangifera indica L.): a magnificent plant with cancer preventive and anticancer therapeutic potential. Crit. Rev. Food Sci. Nutr. 2021;61:2125–2151. doi: 10.1080/10408398.2020.1771678. [DOI] [PubMed] [Google Scholar]
- 11.Yuan X., Duan Y., Xiao Y., et al. Vitamin E enhances cancer immunotherapy by reinvigorating dendritic cells via targeting checkpoint SHP1. Cancer Discov. 2022;12:1742–1759. doi: 10.1158/2159-8290.CD-21-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aucoin M., Cooley K., Saunders P.R., et al. The effect of Echinacea spp. on the prevention or treatment of COVID-19 and other respiratory tract infections in humans: a rapid review. Adv. Integr. Med. 2020;7:203–217. doi: 10.1016/j.aimed.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobrange E., Peshev D., Loedolff B., Van den Ende W. Fructans as immunomodulatory and antiviral agents: the case of Echinacea. Biomolecules. 2019;9:615. doi: 10.3390/biom9100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park S.J., Lee M., Kim D., et al. Echinacea purpurea extract enhances natural killer cell activity in vivo by upregulating MHC II and Th1-type CD4(+) T cell responses. J. Med. Food. 2021;24:1039–1049. doi: 10.1089/jmf.2021.K.0064. [DOI] [PubMed] [Google Scholar]
- 15.Fonseca F.N., Papanicolaou G., Lin H., et al. Echinacea purpurea (L.) Moench modulates human T-cell cytokine response. Int. Immunopharmacol. 2014;19:94–102. doi: 10.1016/j.intimp.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan J., Dong Q., Ding K., Fang J. Characterization of a pectic polysaccharide from the leaves of Diospyros kaki and its modulating activity on lymphocyte proliferation. Biopolymers. 2010;93:649–656. doi: 10.1002/bip.21430. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y., Dong Q., Qiu H., et al. Structural characterization of an arabinogalactan from Platycodon grandiflorum roots and antiangiogenic activity of its sulfated derivative. Biomacromolecules. 2010;11:2558–2566. doi: 10.1021/bm100402n. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y., Yang D., Yang Q., et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat. Commun. 2020;11:6322. doi: 10.1038/s41467-020-20059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z., Li B., Li S., et al. Metabolic control of CD47 expression through LAT2-mediated amino acid uptake promotes tumor immune evasion. Nat. Commun. 2022;13:6308. doi: 10.1038/s41467-022-34064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehla K., Singh P.K. Metabolic regulation of macrophage polarization in cancer. Trends Cancer. 2019;5:822–834. doi: 10.1016/j.trecan.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia Y., He F., Wu X., et al. GABA transporter sustains IL-1β production in macrophages. Sci. Adv. 2021;7:eabe9274. doi: 10.1126/sciadv.abe9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia Y., Zhang Q., Ye Y., et al. Melatonergic signalling instructs transcriptional inhibition of IFNGR2 to lessen interleukin-1β-dependent inflammation. Clin. Transl. Med. 2022;12:e716. doi: 10.1002/ctm2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Huang H., Liu B., et al. Inflammasomes as therapeutic targets in human diseases. Signal. Transduct. Target. Ther. 2021;6:247. doi: 10.1038/s41392-021-00650-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinze C., Boucrot E. Local actin polymerization during endocytic carrier formation. Biochem. Soc. Trans. 2018;46:565–576. doi: 10.1042/BST20170355. [DOI] [PubMed] [Google Scholar]
- 25.Doherty G.J., McMahon H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 26.El-Sayed A., Harashima H. Endocytosis of gene delivery vectors: from clathrin-dependent to lipid raft-mediated endocytosis. Mol. Ther. 2013;21:1118–1130. doi: 10.1038/mt.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lercher A., Baazim H., Bergthaler A. Systemic immunometabolism: challenges and opportunities. Immunity. 2020;53:496–509. doi: 10.1016/j.immuni.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Li N., Zhang X., Horng T. Mitochondrial metabolism regulates macrophage biology. J. Biol. Chem. 2021;297:100904. doi: 10.1016/j.jbc.2021.100904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia Y., Chen S., Zeng S., et al. Melatonin in macrophage biology: current understanding and future perspectives. J. Pineal Res. 2019;66:e12547. doi: 10.1111/jpi.12547. [DOI] [PubMed] [Google Scholar]
- 30.Nagoor Meeran M.F., Javed H., Sharma C., et al. Can Echinacea be a potential candidate to target immunity, inflammation, and infection - the trinity of coronavirus disease 2019. Heliyon. 2021;7:e05990. doi: 10.1016/j.heliyon.2021.e05990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cadiz M.P., Schara M.R., Kemp B.H., Gibbons Johnson R.M. Echinacea purpurea root extract increases tumor necrosis factor production by concanavalin A-activated murine splenocytes. J. Med. Food. 2019;22:1146–1150. doi: 10.1089/jmf.2019.0065. [DOI] [PubMed] [Google Scholar]
- 32.Wagner H., Proksch A., Riess-Maurer I., et al. Immunostimulating action of polysaccharides (heteroglycans) from higher plants. Arzneimittelforschung. 1985;35:1069–1075. [PubMed] [Google Scholar]
- 33.Wagner H., Stuppner H., Schäfer W., Zenk M. Immunologically active polysaccharides of Echinacea purpurea cell cultures. Phytochemistry. 1988;27:119–126. [Google Scholar]
- 34.Proksch A., Wagner H. Structural analysis of a 4-O-methyl-glucuronoarabinoxylan with immuno-stimulating activity from Echinacea purpurea. Phytochemistry. 1987;26:1989–1993. [Google Scholar]
- 35.Allavena P., Sica A., Garlanda C., Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol. Rev. 2008;222:155–161. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 36.Roesler J., Steinmüller C., Kiderlen A., et al. Application of purified polysaccharides from cell cultures of the plant Echinacea purpurea to mice mediates protection against systemic infections with Listeria monocytogenes and Candida albicans. Int. J. Immunopharmacol. 1991;13:27–37. doi: 10.1016/0192-0561(91)90022-y. [DOI] [PubMed] [Google Scholar]
- 37.Steinmüller C., Roesler J., Gröttrup E., et al. Polysaccharides isolated from plant cell cultures of Echinacea purpurea enhance the resistance of immunosuppressed mice against systemic infections with Candida albicans and Listeria monocytogenes. Int. J. Immunopharmacol. 1993;15:605–614. doi: 10.1016/0192-0561(93)90078-d. [DOI] [PubMed] [Google Scholar]
- 38.Luettig B., Steinmüller C., Gifford G.E., et al. Macrophage activation by the polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea. J. Natl. Cancer Inst. 1989;81:669–675. doi: 10.1093/jnci/81.9.669. [DOI] [PubMed] [Google Scholar]
- 39.Lee T.T., Huang C.C., Shieh X.H., et al. Flavonoid, phenol and polysaccharide contents of Echinacea purpurea L. And its immunostimulant capacity in vitro. Int. J. Environ. Science & Development. 2010;1:5–9. [Google Scholar]
- 40.Li Q., Yang F., Hou R., et al. Post-screening characterization of an acidic polysaccharide from Echinacea purpurea with potent anti-inflammatory properties in vivo. Food Funct. 2020;11:7576–7583. doi: 10.1039/d0fo01367f. [DOI] [PubMed] [Google Scholar]
- 41.Fu A., Wang Y., Wu Y., et al. Echinacea purpurea extract polarizes M1 macrophages in murine bone marrow-derived macrophages through the activation of JNK. J. Cell. Biochem. 2017;118:2664–2671. doi: 10.1002/jcb.25875. [DOI] [PubMed] [Google Scholar]
- 42.Fast D.J., Balles J.A., Scholten J.D., et al. Echinacea purpurea root extract inhibits TNF release in response to Pam3Csk4 in a phosphatidylinositol-3-kinase dependent manner. Cell. Immunol. 2015;297:94–99. doi: 10.1016/j.cellimm.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Lamkanfi M., Dixit V.M. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Rathinam V.A.K., Zhao Y., Shao F. Innate immunity to intracellular LPS. Nat. Immunol. 2019;20:527–533. doi: 10.1038/s41590-019-0368-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ainscough J.S., Frank Gerberick G., Zahedi-Nejad M., et al. Dendritic cell IL-1α and IL-1β are polyubiquitinated and degraded by the proteasome. J. Biol. Chem. 2014;289:35582–35592. doi: 10.1074/jbc.M114.595686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janeway C.A., Jr., Janeway J. How the immune system works to protect the host from infection: a personal view. Proc. Natl. Acad. Sci. USA. 2001;98:7461–7468. doi: 10.1073/pnas.131202998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li T., Qin K., Li N., et al. An endosomal LAPF is required for macrophage endocytosis and elimination of bacteria. Proc. Natl. Acad. Sci. USA. 2019;116:12958–12963. doi: 10.1073/pnas.1903896116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marongiu L., Gornati L., Artuso I., et al. Below the surface: the inner lives of TLR4 and TLR9. J. Leukoc. Biol. 2019;106:147–160. doi: 10.1002/JLB.3MIR1218-483RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryan D.G., O'Neill L.A.J. Krebs cycle reborn in macrophage immunometabolism. Annu. Rev. Immunol. 2020;38:289–313. doi: 10.1146/annurev-immunol-081619-104850. [DOI] [PubMed] [Google Scholar]
- 50.Xu H., Li D., Ma J., et al. The IL-33/ST2 axis affects tumor growth by regulating mitophagy in macrophages and reprogramming their polarization. Cancer Biol. Med. 2021;18:172–183. doi: 10.20892/j.issn.2095-3941.2020.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.