Abstract
Background.
Rotator cuff disease is a common cause of shoulder pain. Comorbidities such as diabetes, hypertension, and hyperlipidemia may be associated with rotator cuff disease, likely because of mechanisms related to vascular insufficiency.
Objectives.
We performed a systematic review of the association of diabetes, hypertension, and hyperlipidemia with the diagnosis of rotator cuff disease.
Methods.
Following systematic queries of PubMed, Embase, Cochrane, CINAHL, and Science Direct, articles meeting eligibility criteria and reporting on the association of one or more risk factors (diabetes, hypertension, and hyperlipidemia) and rotator cuff disease were considered. Meta-analysis was performed to quantitatively summarize the associations between each risk factor and rotator cuff disease. We assessed study quality with the Newcastle-Ottawa Scale (NOS) and performed a qualitative assessment of risk of bias.
Results.
After a full-text review of 212 articles, 12 articles assessing diabetes, 5 assessing hypertension and 8 assessing hyperlipidemia were eligible. The odds of having rotator cuff disease was increased with diabetes (odds ratio [OR] 1.49, 95% confidence interval [CI] 1.43–1.55), hypertension (OR 1.40, 95% CI 1.19–1.65) and hyperlipidemia/dyslipidemia (OR 1.48, 95% CI 1.42–1.55). Diabetes was also specifically associated with rotator cuff tears (OR 1.28, 95% CI 1.07–1.52). Synthesizing assessment for risk of bias suggested that current epidemiologic evidence for an association was plausible for diabetes and hyperlipidemia but not hypertension.
Conclusions.
Diabetes, hypertension, and hyperlipidemia were associated with rotator cuff disease in our meta-analysis. However, the possibility of bias exists for all 3 co-morbidities evaluated and is likely highest for hypertension. High-quality studies with the ability to incorporate time since first diagnosis of co-morbidity are scarce and much needed.
Introduction
Rotator cuff disease is the most common cause of shoulder pain. The biology and factors associated with degeneration of the rotator cuff tendon are poorly understood. Degeneration of the tendon is likely propagated by a relatively avascular tendon tissue [1], leading to poor regenerative ability and recurrent symptomatology. Comorbid risk factors such as diabetes, hypertension, and hyperlipidemia can lead to vascular insufficiency and are therefore likely associated with degenerative rotator cuff disease. Previous literature has reported the association between these risk factors and rotator cuff disease [2–18]; however, to our knowledge, results from these studies have not been systematically synthesized.
The objective of this study was to qualitatively and quantitatively synthesize existing evidence from the literature on the association between diabetes, hypertension, and hyperlipidemia and risk of rotator cuff disease. We also performed a meta-analysis to provide estimates on the association of each risk factor with rotator cuff tears. Data on these risk factors can help identify patients at greatest risk.
Methods
This systematic review and meta-analysis adhered to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) criteria [19] (Supplementary Material 5). The review was not registered because only clinical trials are required to be registered.
Search strategy
We performed systematic queries of multiple databases (PubMed, Embase, Cochrane, CINAHL, and Science Direct) on July 10, 2019 to search for articles on the association between putative risk factors and rotator cuff disease. The review guidelines were established before performing this study and there were no deviations from the guidelines. Search terms were built and queried with the help of a librarian. Search terms implemented for each of the databases are provided in Supplementary Material 1. The database search and manual bibliography review of relevant systematic reviews identified a total of 7,332 articles, including duplicates, for screening as title and abstract review. Articles were screened (by DO’H) out for the following reasons: published in a language other than English, not related to rotator cuff disease, evaluated surgical procedures/outcomes or evaluated genetics as risk factors, a description of case report/series, not original research (opinions, editorials, systematic reviews and meta-analyses) or abstracts only. Only English-language articles were included because we did not have access to other language interpreters for non-English articles. A total of 212 articles underwent full-text review, when articles were searched for risk factors associated with rotator cuff disease. Only articles with a proper comparison group or control group were included. DO’H performed full-text review, and articles with uncertainty for inclusion or exclusion were adjudicated by AG and NJ.
We focused this meta-analysis on factors that potentially contribute to the etiology of rotator cuff disease through mechanisms relating to hypovascularity, namely, diabetes, hypertension and hyperlipidemia. We included analytic observational studies (case–control, cross-sectional, or cohort) in adults (≥ 18 years old) that reported appropriate effect estimates (odds ratios [ORs], risk ratios, or hazard ratios [HRs]) or provided sufficient information to compute these estimates. We included only studies that had at least 30 cases of rotator cuff disease and at least 30 controls to include reports with relatively stable estimates. When 2 or more articles reported estimates from the same population or included overlapping populations, only the article reporting effect estimates from the largest sample size was included. At least 2 studies meeting our eligibility criteria were required for a given risk factor to be considered in a meta-analysis.
Assessment of rotator cuff disease (outcome)
The main outcome for this meta-analysis was rotator cuff disease as a composite dichotomous outcome. This approach was used because studies did not consistently distinguish rotator cuff tears from rotator cuff disease. Hence, rotator cuff disease in our study included the terms rotator cuff tear, rotator cuff syndrome, rotator cuff tendinopathy, rotator cuff tendonitis, rotator cuff tendinosis, rotator cuff injury, and rotator cuff disease. We accepted the diagnosis of rotator cuff disease as presented in the included studies.
Assessment of risk factors
We considered diabetes, hypertension and hyperlipidemia as risk factors for rotator cuff disease.If studies reported comparison of mean/median laboratory values such as mean hemoglobulin A1c level, fasting glucose, systolic blood pressure, diastolic blood pressure, and levels of high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total cholesterol, or triglycerides, then they were not considered for quantitative assessment in this meta-analysis because of considerable heterogeneity. We limited the analyses to studies that reported the association between any one or more of these risk factors as a dichotomous (present/absent) variable and rotator cuff disease. For hyperlipidemia, we included studies that reported hyperlipidemia, hypercholesterolemia and dyslipidemia as a composite dichotomous exposure, which we termed hyperlipidemia for this meta-analysis.
Data abstraction
Data abstraction (performed by AG) involved using a standardized approach that included the following fields from each article (as applicable): study tittle, publication date, journal, first author, study design, rotator cuff disease specifics (tear, syndrome, disease, or tendonitis), definition for the diagnosis of rotator cuff disease (imaging with MRI, CT, ultrasonography, surgical repair codes, medical notes), diabetes status, hypertension status, hyperlipidemia status, number of cases and controls, number of cases and controls by exposure status (exposed cases, unexposed cases, exposed controls, unexposed controls) to compute the effect estimate when available, crude effect estimate if provided, and multivariable adjusted effect estimate if provided. When multiple effect estimates were presented for different definitions of rotator cuff disease, all estimates were abstracted and flagged to avoid double counting of correlated estimates in a given meta-analysis. When estimates were presented for 2 or more independent populations in a given study (e.g., men and women, and as a composite), then all 3 estimates were abstracted for completeness.
Statistical analysis
Overall, we performed meta-analyses on the association of the 3 risk factors (diabetes status, hypertension status, and hyperlipidemia status) with rotator cuff disease. The following analytic plan was designed a priori and applied to each of the questions uniformly. We first performed inverse-variance weighted fixed-effects and random-effects meta-analyses for each risk factor. The OR was the most used effect estimate across studies, so it was chosen as the effect estimate for the meta-analysis and is reported with corresponding 95% confidence intervals (95% CIs). Studies reporting estimates from 2 or more independent populations (e.g., men and women) were allowed to contribute 2 or more of these independent estimates to a given meta-analysis. Preference was given to multivariable adjusted estimates over crude estimates, followed by manual calculation of the OR by meta-analysis when multivariable-adjusted or crude estimates were not provided. Because very few studies reported risk ratios and HRs, these were treated as ORs to maximize the number of studies in a meta-analysis and for ease of synthesis. If studies reported more than one effect estimate based on the rotator cuff disease definition, then the primary analysis included estimates from the larger of the 2 or more sample sizes. When a sufficient number of studies reported the association between risk factors and rotator cuff tears specifically, we performed separate meta-analyses on this subset of effect estimates. In sensitivity analyses, the smallest and largest effect estimates were also substituted instead of estimates from the largest sample to provide a range of meta-analysis estimates. When there were 10 or more effect estimates available for a given meta-analysis, we constructed funnel plots for visual inspection of publication bias and performed the Egger’s test [20]. Heterogeneity was assessed and reported as the I2 statistic. All meta-analyses involved using the metan package in STATA/MP 16.0 (StataCorp, College Station, TX, USA).
Assessment of study quality
We used the Newcastle-Ottawa Scale (NOS)[21] to assess the quality of the observational studies that were eligible for meta-analysis. Briefly, the NOS is a composite assessment strategy that provides one score for each study based on questions related to 3 key domains: selection bias, comparability for assessment of confounding, and outcome/exposure definition. Standard questionnaires are available for cohort and case–control studies. Because of lack of availability of questionnaires specific to cross-sectional studies, the case–control study form was used for this study design instead. We also performed a qualitative risk of bias (RoB) assessment for each study for a given exposure-outcome assessment and rated RoB as low, low-to-moderate, moderate, moderate-to-high, or high. A study received an NOS score and RoB assessment for each exposure–outcome association assessed. For example, if a study was used in the meta-analysis evaluating diabetes and rotator cuff disease and also for hypertension and rotator cuff disease, the study received 2 NOS scores and 2 RoB assessments because each exposure– outcome association may have its own set of relevant confounding factors. NOS scores and RoB assessments are summarized in Table 1, and detailed assessments for each exposure–outcome assessment are provided (Supplementary Material 2, 3, 4). In addition to qualitatively assessing the likelihood of bias, we also provide the potential direction of bias (toward or away from the null) and note the most important source of bias for each exposure–outcome assessment relevant to each study included in meta-analyses. When we had a sufficient number of studies, we performed ad-hoc sensitivity analyses by RoB assessments: low or low-to-moderate studies only, low-to-moderate studies only, and moderate or higher-risk studies only.
Table 1.
Summary of studies evaluating diabetes, hypertension and dyslipidemia in relation to rotator cuff disease.
| Study* | Age (years) (range) and country | Eligibility criteria | N cases/controls | Risk factor values in cases; controls (Presented as n of participants (%) or mean (SD) unless otherwise noted); Confounders adjusted | Results | NOS Assessment scale/Risk of Bias (RoB) assessment |
|---|---|---|---|---|---|---|
| Abboud et al (2009) [15] | Cases: 66.1 (21–93) Control: 67.2 USA | Patients with confirmed full-thickness rotator cuff tear upon surgery, MRI, and examination; Exclusion criteria included smoking, previous shoulder infections, surgery, younger than 21 years, frozen shoulder, calcific tendinitis, chronic steroid or floxacin use Controls presented with shoulder complaints and normal rotator cuff | 74 rotator cuff tear Cases 73 Controls | Total Cholesterol: 237mg/dL; 194 mg/dl Total Cholesterol>2 40 mg/dL: 63%; 28% Confounders considered: NA; Comparison of means | Patients with rotator cuff tears had a higher incidence of hypercholestere mia (p=0.02) | NOS: NA – Study was not included in meta-analysis due to difference in metric. RoB (Dyslipidemia): likely moderate to high. Compared cholesterol concentrations between cases and controls. Likely confounded. |
| Bodin et al. 2012(1) [3] | 38.7 France | 96 cases were diagnosed with RCS based on intermittent pain for more than 4 of the past 7 days worsened by elevation and positive shoulder tests. 1,360 workers without RCS as controls | 96 Cases 1,360 Controls | Please see paper for incidence of RCS broken down by work and personal characteristic as too detailed to present Confounders: NA; study not in meta-analysis | Men: Age 45–49 OR: 4.7 (2.210.0); (ref<40 yrs) Above shoulder/high exertion: 3.3 (1.3–8.4) low coworker support: 2.0 (1.1–3.9) Women: Age: 50–59 OR: 5.4 (2.3–13.2); (ref. <40 yrs) Working with colleagues in temp. employment:2.2 (1.2–4.2) Repeated Arm abduction (60–90°): 2.6 (1.4–5.0) | NOS: NA-Study not included in any meta-analysis due to overlapping study population with Roquelaure et al. RoB: Not assessed |
| Chung et al (2016)* [4] | 60.1, 46–76 Korea | Patients with chronic, symptomatic full thickness rotator cuff tear surgically treated at institution; Controls are age and sex matched patients who visited hospital for regular health examination with no shoulder symptoms or disease | 48 Cases 48 Controls | Diabetes: 5 (10%); 4 (8%) Hypertension: 14 (29%); 11 (22%) Thyroid disease: 5 (10%); 3 (6%) Heart disease: 3 (6%); 3 (6%) Smoking: 6 (12%); 7 (14%) Work level (low:med: High): 13:18:17; 12:21:15 BMI: 23.5 (2.6); 22.6 (2.6) Additional characteristics available in full paper Confounders: NA; no regressions performed; only raw numbers provided | Diabetes: P=0.726 Hypertension: P=0.485 Thyroid disease: P=0.46 Heart disease: P=1.000 Smoking: P=0.765 Work level: P=0.820 BMI: P=0.211 Grip strength (kg): P=0.041 Palmar Pinch(kg): P=0.007 Key Pinch(kg): P=0.050 | NOS (Diabetes): 3 NOS (Hypertension): 3 NOS (Dyslipidemia): NA RoB (Diabetes): Moderate to High RoB (Hypertension): Moderate to High RoB (Dyslipidemia): NA |
| Davis et al (2017) [16] | Cases: 57.5 (41–76) Controls: 53.7 (18.1–42.8) USA | Patients undergoing shoulder surgery and had an MRI in past year confirming intact RC or a full thickness rotator cuff tear of at least one tendon. Exclusion criteria included history of shoulder infection, surgery, partial tear, inflammatory arthritis, smoking, alcoholism, meds for hypercholestere mia Controls were patients meeting criteria with intact rotator cuff | 40 Cases 37 Controls | Age: 57.5; 53.7 Cholesterol: 69.5 mg/dl; 84 mg/dl HDL: 21.05 mg/dl; 22 mg/dl Non-HDL: 51.5 mg/dl; 48 mg/dl Triglycerides: 18 mg/dl; 21 mg/dl Serum Median Levels: Cholesterol: 186.5 mg/dl; 189 mg/dl HDL: 44 mg/dl; 48 mg/dl Non-HDL: 134.95 mg/dl; 130 mg/dl Triglycerides: 95 mg/dl; 101 mg/dl Confounders: NA; study not in meta-analysis | Synovial Fluid levels P-Values: Cholesterol: P=0.172 HDL: P=0.419 Non-HDL: P=0.832 TG: P=0.184 Serum Levels P-Values: Cholesterol: P=0.807 HDL: P=0.101 Non-HDL: P=0.625 Triglycerides :P=0.937 | NOS (Diabetes): NA NOS (Hypertension): NA NOS (Dyslipidemia): Did not assess RoB (Diabetes): NA RoB (Hypertension): NA RoB (Dyslipidemia): Did not assess Study was not included in meta-analysis since authors only compared mean lipid levels across cases and controls and this outcome did not meet meta-analysis end point determined a priori |
| Djerbi et al (2015)* [5] | Cases: 57.8 Controls: 59.4 France | Patients undergoing arthroscopic RC repair Controls are patients with asymptomatic shoulders in same orthopedic unit | 206 Cases 100 Controls | Age: 57.8; 59.4 BMI: 27.34; 26.35 Smoker: 54%; 10% Alcoholism: 13%; 8% High BP: 13%; 23% Dyslipidemia: 35%; 7% CV history: 21%; 4% Diabetes: 14%; 9% # of Cardiovascular risk factors: 2.09; 0.74 Confounders adjusted (for diabetes): None Confounders adjusted (for hypertension): None Confounders adjusted (for hyperlipidemia): BMI, smoking, cardiovascular history | Multivariate analysis OR(CI): BMI: 1.686 (0.842–3.377); P=0.1407 Smoker: 8.715 (4.192–18.118) P<0.0001 Dyslipidemia: 4.920 (2.04611.834) P=0.004 Cardiovascular history: 2.390 (0.731–7.813) P=0.1495 Univariate analysis rotator cuff tear Occurrence OR(CI): Age: 1.546 (0.96–2.5) P=0.3441 Alcoholism: 1.735 (0.76–3.97) P=0.192 HBP: 2.040 (1.18–3.52) P=0.0102 Diabetes: 1.420 (0.64–3.17) P=0.3919 | NOS (Diabetes): 5 NOS (Hypertension): 5 NOS (Dyslipidemia): 6 RoB (Diabetes): Moderate to High RoB (Hypertension): Moderate to High RoB (Dyslipidemia): Low |
| Huang et al (2016)* [6] | Participan ts >60 years of age: 47.2% Taiwan | Retrospective nationwide population-based study based on Taiwan Longitudinal Health Insurance Database 2005. Identified 58,652 diabetics and age-and sex-matched (1:2) 175,956 non diabetics and followed records for rotator cuff tear surgery. | Cases: 342 had surgical procedures for rotator cuff tear during follow-up Controls: 117,956 individuals did not have codes for rotator cuff tear surgery. | Incidence of rotator cuff tear in DM patients: 41 per 100,000 person-years; Incidence of rotator cuff tear in non-DM patients: 26 per 100,000 person-years 50.9% females Confounders: controls for age and sex through matching; Includes diabetes, hypertension, hyperlipidemia in model No adjustment for obesity | Diabetes OR (95% CI): 1.33 (1.05,1.68) Hypertension OR (95% CI): 1.01 (0.79, 1.27) Hyperlipidemia OR (95% CI): 1.31 (1.03, 1.68) | NOS (Diabetes): 6.5 NOS (Hypertension): 5 NOS (Dyslipidemia): NA RoB (Diabetes): Low RoB (Hypertension): Low to Moderate RoB (Dyslipidemia): NA |
| Gumina et al (2013)* [14] | Cases: 59 (47–68) Controls: 62 (47–75) Italy | Patients who underwent arthroscopic repair of rotator cuff. Exclusion criteria included primary osteoarthritis, previous shoulder operation, os acromiale, inflammatory joint disease Controls are patients undergoing orthopedic examination for non-shoulder pathologies | 408 Cases 201 Controls | Mean age: Small tear: 56.5 (8.4) Large tear: 64.8 (7.9) Massive tear: 70.4 (4.0) Control: 63.9 (8.9) Hypertension Present: Small Tear: 16 (17%) Large tear: 109 (51%) Massive tear: 73 (75%) Control: 66 (33%) Mean months of antihypertension therapy: Small Tear: 13 (33) Large tear: 38 (44) Massive tear: 75 (54) Control: 21 (37) Confounders adjusted: none; authors only present univariate statistics for hypertension | Hypertension and tear occurrence: OR: 2.05 (1.41–2.98) Hypertension and small tear OR: 0.63 (0.33–1.19) Hypertension and large tear OR: 2.09 (1.39–3.16) Hypertension and Massive tear OR: 4.30 (2.44–7.58) | NOS (Diabetes): NA NOS (Hypertension): 5.5 NOS (Dyslipidemia): NA RoB (Diabetes): NA RoB (Hypertension): Moderate to High RoB (Dyslipidemia): NA |
| Lin et al. (2015)* [8] | Overall age mean: 48.8 Taiwan | Subset of National Health Insurance Research Database diagnosed with Diabetes or hyperlipidemia. Cases were diagnosed with rotator cuff disease Exclusion criteria includes less than 30 years old, died before 2001, rheumatoid arthritis, previous rotator cuff disease diagnosis, shoulder fractures Controls were in database without rotator cuff disease | 26,664 Cases 472,014 Controls | Diabetes: 2,436 (9%); 24,228 (5%) Hyperlipidemi a: 2,475 (9%); 24,189 (5%) Confounders adjusted (for diabetes): age, sex and hyperlipidemia; provides estimates of insulin vs. noninsulin use Confounders adjusted (for dyslipidemia): age, sex and diabetes; provides estimates with and without statin. No adjustment for BMI in both analyses. | Multivariate analysis HR Diabetes: 1.47 (1.41–1.54) -insulin: 1.64 (1.53–1.75) + insulin: 1.43 (1.35–1.51) Hyperlipidemia:1. 48 (1.42–1.55) statin: 2.01 (1.892.13) +statin: 1.16 (1.10–1.23) | NOS (Diabetes): 8 NOS (Hypertensio n): NA NOS (Dyslipidemia): 8 RoB (Diabetes): Low RoB (Hypertension): NA RoB (Dyslipidemia): Low |
| Longo et al. (2008) [9] | Cases: 62.9 (37–82) Controls: 61.6 (3680) UK | Patients undergoing arthroscopic repair surgery for rotator cuff. Exclusion criteria included osteoarthritis, previous shoulder operations, inflammatory joint disease, hypertension, diabetes, hypercholestere mia Control patients undergoing arthroscopic meniscectomy for meniscal tear with no evidence of shoulder pathology | 120 Cases 120 Controls | Mean plasma glucose: 99.17 (9.04); 95.45 (9.87) mg/dl BMI M: 27.90; 26.97 BMI F: 27.81; 26.65 Age M: 59.8; 58.9 Age F: 64.75; 63.2 Confounders adjusted for: NA | Patients with rotator cuff tear higher fasting Plasma glucose: P=0.007 | NOS (Diabetes): did not assess NOS (Hypertensio n): NA NOS (Dyslipidemia ): NA RoB (Diabetes): did not assess RoB (Hypertension): NA RoB (Dyslipidemia): NA Study compared fasting plasma glucose in cases and controls. This outcome was not eligible for meta-analysis a priori by design. |
| Longo et al. (2009) [18] | Cases: 64.86 (40–83) Controls: 63.91 (3878) | Patients undergoing arthroscopic repair surgery for rotator cuff. Exclusion criteria included osteoarthritis, previous shoulder operations, inflammatory joint disease, hypertension, diabetes, hypercholestere mia Control patients are undergoing arthroscopic meniscectomy for meniscal tear with no history of rotator cuff symptoms | 120 Cases 120 Controls | M BMI: 27.36; 27.81 F BMI: 27.88; 26.82 M Age: 63.43; 63.31 F Age: 65.73; 64.28 M Serum triglycerides: 158.42 (122.29) mg/dl;139.87 (75.56) mg/Dl F Serum triglycerides: 131.81 (55.9) mg/dl; 120.48 (53.75) mg/dL Confounders adjusted for: NA | Triglycerides: p=0.6 Cholesterol: P=0.1 | NOS (Diabetes): NA NOS (Hypertension): NA NOS (Dyslipidemia): did not assess RoB (Diabetes): NA RoB (Hypertensio n): NA RoB (Dyslipidemia): did not assess Same study population as above. Study compared lipid levels in cases and controls. This outcome was not eligible for meta-analysis a priori by design. |
| Passaretti et al. (2013)* [10] | Cases: 64 (54–78) Controls: 66 (58–72) Italy | Patients treated arthroscopically for full-thickness rotator cuff tear and controls with no history of shoulder pathologies. Exclusion criteria included previous shoulder operation, inflammatory or rheumatologic joint disease, primary osteoarthritis, BMI>25, hypertension, diabetes, hypercholestere mia Controls included consecutive patients with no history of shoulder pathology seen at outpatient hospital clinic | 249 full thickness rotator cuff tear 428 Controls | Age: 64; 66 Alcohol intake: 23; 19 g/day Smoking: 102 (41%); 176 (41%) Diabetes: 93 (37.3%); 122 (29%) Confounders adjusted for: Unclear; insufficient description to determine if and what was controlled for. | Age OR: 0.96 (0.94–0.98) P<0.001 Alcohol OR: 1.02 (1.01–1.04) P<0.001 Smoking OR: 0.71 (0.51–0.98) P=0.004 Diabetes OR: 3.2 (2.3–4.5) P<0.001 | NOS (Diabetes): 4 NOS (Hypertension): NA NOS (Dyslipidemia): NA RoB (Diabetes): High RoB (Hypertension): NA RoB (Dyslipidemia): NA |
| Rechardt et al. (2010)* [11] | 6,237 Men and women residing in Finland over the age of 30 taking Health 2000 survey. Exclusion criteria included missing information on shoulder disorders, rheumatoid arthritis, positive rheumatoid factor Cases had chronic rotator tendinopathy, defined as history of rotator cuff pain | Former smoker: M 4% F: 3% Current smokers > 20 pack years: M 3% F 1% BMI 25.0–29.9: M 3% F 2% BMI >30: M: 3% F: 4% Type I diabetes: M: 5% Type II diabetes: M: 6% F: 4% Data for additional characteristics available in paper Confounders adjusted: aging, education, physical work | Former smoker OR: M: 1.3 (0.7–2.3) F: 1.0 (0.5–1.8) Current Smoker > 20 pack years OR: M: 1.3 (0.5–3.3) F: 0.4 (0.1–1.7) BMI 252–9.9 OR (ref 18.5–24.9): M: 1.6 (0.9–2.7) F: 1.0 (0.6–1.7) BMI >30.0 OR (ref 18.5–24.9) M: 1.7 (0.8–3.6) F: 1.2 (0.6–2.3) Type I Diabetes OR: M: 4.7 (1.1–20.3) Type II Diabetes OR: M: 1.6 (0.7–3.5) F: 0.7 (0.3–1.8) | NOS (Diabetes): 7 NOS (Hypertension): NA NOS (Dyslipidemia): NA RoB (Diabetes): Low to moderate RoB (Hypertension): NA RoB (Dyslipidemia): NA | ||
| Roquelau re et al. (2011)* [12] | 38.7 France | 3710 French workers, distribution similar to regional workforce, completing questionnaire regarding personal factors and work exposures | 3710 workers in total Rotator cuff syndrome in 6.6% of total | BMI 25–29.9: 55; 700 BMI >30: 18; 157 Diabetes: 4; 36 Thyroid disorder: 2; 31 Confounders adjusted for: aging, BMI, workload, work type, and working class. Multivariable estimates only provided for females, and presented unadjusted estimates for males (reporting bias) | Univariate analysis Age OR (I year increment) M: 1.07 (1.05–1.09) F: 1.08 (1.06–1.10) BMI 25–29.9 OR (ref 18.5–24.9) M: 1.0 (0.7–1.5) F: 1.3 (0.8–2.0) BMI >30 OR (ref 18.5–24.90 M: 1.4 (0.8–2.5) F: 2.8 (1.0–8.1) Diabetes M: 1.0 (0.3–2.8) F: 2.8 (1.0–8.1) Thyroid disorders OR: M: 0.7 (0.2–3.2) F: 0.9 (0.4–1.7) *please see paper for work related factors | NOS (Diabetes): 5.5 NOS (Hypertension): NA NOS (Dyslipidemia): NA RoB (Diabetes): Low to Moderate RoB (Hypertension): NA RoB (Dyslipidemia): NA |
| Titchener et al. (2014)* [13] | 55 UK | Cases were randomly selected patients with recorded diagnosis of rotator cuff disease within THIN database of UK patient records. Controls were matched by age, sex, and general practice without rotator cuff disease | 5000 Cases 5000 controls | BMI 25.1–30: 1281 (26%); 1063 (21%) BMI 30.1–40: 751 (15%);614 (12%) Current smoker: 925 (19%);826 Ex-smoker: 981 (19%); 763 (17%) Heavy alcohol use: 280 (6%);247 (5%) Insulin: 105 (2.1%);43 (1%) Oral antidiabetics: 1234 (25%);671 (13%) Rheumatoid arthritis: 39 (1%); 37 (1%) Confounders adjusted: 1:1 age, sex and general practice matched | Multivariate analysis BMI 25.1–30: 1.15 (1.02–1.31) BMI 30.1–40: 1.1 (0.95–1.27) Current smoker: 0.94(.83–1.06) Ex-smoker: 1.09 (0.96–1.23) Heavy alcohol use: 0.91 (0.65–1.28) Insulin: 1.77 (1.20–2.61) Oral antidiabetics: 1.66 (1.48–1.87) Rheumatoid Arthritis: 0.85 (0.53–1.36) | NOS (Diabetes): 4.5 NOS (Hypertension): NA NOS (Dyslipidemia): NA RoB (Diabetes): Low to moderate RoB (Hypertension): NA RoB (Dyslipidemia): NA |
| Kang et al. (2010)* [7] | DM: 62.6 Non-DM: 56.9 Taiwan | Patients undergoing physical and ultrasound examination, older than 40yrs, chronic shoulder pain for longer than 1 month, no rheumatologic disease, malignancy, major trauma, recent steroid injection, shoulder fracture with cases being diabetic shoulders Controls being non-diabetic shoulders | 91 cases 362 controls | SS tears: 33 (36%); 127 (35%) IS Tears: 4(4%); 11 (3%) SC Tears: 2 (2.2%); 3 (1%) Confounders adjusted for: age, sex and handedness No adjustment for BMI, or other comorbidities | Adjusted ORs for RC lesions on ultrasound of diabetics compared to non-diabetics: SC tears: 1.32 (0.79–2.2) P=0.288 IS Tears: 1.13 (0.46–1.26) P=0.288 SC tears: 2.44 (0.37–16.25) P=0.356 | NOS (Diabetes): 3.5 NOS (Hypertension): NA NOS (Dyslipidemia): NA RoB (Diabetes): Moderate RoB (Hypertension): NA RoB (Dyslipidemia): NA |
| Kim et al. (2016) [17] | Cases: 55.6 Controls: 58.1 Korea | Patients treated for supraspinatus tendinopathy with or without tear with no prior shoulder surgery, prior steroid injection, capsular pattern, neurological diseases leading to shoulder pain, or lipid lowering medication. Cases including patients with hyperlipidemia Controls being patients with non–hyperlipidemia | 50 Cases 49 Controls | Supraspinatus tear: 37 (76%); 29 (58%) | Rotator cuff tears in hyperlipidemia group not statistically significant: P=0.06 | NOS (Diabetes): NA NOS (Hypertensio n): NA NOS (Dyslipidemia): did not assess RoB (Diabetes): NA RoB (Hypertension): NA RoB (Dyslipidemia ): did not assess Authors posed a question asking whether hyperlipidemia affects treatment of supraspinatus tendinopathy with and without tear. Not relevant for our question of interest specifically. |
Study included in one or more meta-analysis; BMI, body mass index; DM, diabetes mellitus; HBP, high blood pressure; HDL, high-density lipoprotein; HTN, hypertension; IS, infraspinatus; NOS, Newcastle-Ottawa Scale; NA, not applicable; OR, odds ratio; RCS, rotator cuff syndrome; RC, rotator cuff; SC, subscapularis; SS, supraspinatus
The rationale for the 30-case threshold is based on our efforts to have enough statistical power to make meaningful conclusions.
Results
Of the 212 articles eligible for full-text review, 17 reported associations between one or more risk factors (diabetes, hypertension, hyperlipidemia) and rotator cuff disease [2–18] (Table 1 and Fig. 1); 12 of these articles reported on the association between diabetes and rotator cuff disease [2–13], 5 hypertension and rotator cuff disease [2,4–6,14], and 8 hyperlipidemia and rotator cuff disease [2,5,6,8,15–18].
Figure 1.
PRISMA flow chart of the selection of articles.
Diabetes and rotator cuff disease
Of the 12 articles reporting on diabetes status and rotator cuff disease, 2 were not eligible for inclusion in the meta-analysis [3,12]. The study by Bodin et al. [3] was excluded because of an overlapping population with Roquelaure et al. [12]. The latter was chosen for inclusion because of its larger sample size. Longo et al. was not included in the meta-analysis because the authors reported on only mean glucose levels by case–control status [9]. The articles by Lin et al. [8] and Huang et al. [6] were both based on the National Health Insurance random sample in Taiwan, and were retained to be analyzed in separate meta-analysis because one evaluated rotator cuff disease and the other rotator cuff tear. Five[2,4,5,7,10] of 10 studies had RoB assessments of moderate or higher, and only 2 [6,8] was rated as low RoB. Lack of adjustment for confounding was the primary concern for studies rated as moderate or higher RoB, in addition to lack of temporality and selection bias due to improper sampling of controls. Five of 10 studies were case-control studies, 3 were cross-sectional studies and 2 were retrospective cohort studies. Five studies specifically reported on rotator cuff tear, 2 reported on tendinopathy/tendonitis, another 2 reported on rotator cuff disease and 1 reported on rotator cuff syndrome. Roquelaure et al. [12] and Rechardt et al. [11] provided separate estimates for men and women. These studies together provided 12 independent effect estimates for the meta-analysis (32,912 cases and 488,186 controls).
Overall, having diabetes was associated with rotator cuff disease (fixed effects OR 1.49, 95% CI 1.43–1.55) (Fig. 2a). The choice of model as random-effects analysis yielded similar estimates. Models showed low evidence of heterogeneity across studies (I2 = 11%) (Table 2; Fig. 2a). The funnel plot (Fig. 2b) and Egger’s test did not show evidence for small study bias (bias coefficient: −0.17; p = 0.68). We performed an a priori sensitivity analysis to assess the association between diabetes status and rotator cuff tears [4–7,10]. Meta-analysis of 5 studies revealed an attenuated but positive association (OR 1.28, 95% CI 1.07–1.52). Performing sub-group analyses according to RoB showed that studies with low or low-to-moderate risk had similar estimates as the main analyses (fixed-effects OR 1.49, 95% CI 1.43–1.55; n studies = 5) and studies with a moderate or higher likelihood of bias had lower magnitude in effect size (fixed effects OR 1.22, 95% CI 0.94–1.58). Only one study had low bias risk in the meta-analysis of rotator cuff disease. Removing this study to only include studies with low-to-moderate risk increased the effect estimate (fixed-effects OR 1.64, 95% CI 1.47–1.83).
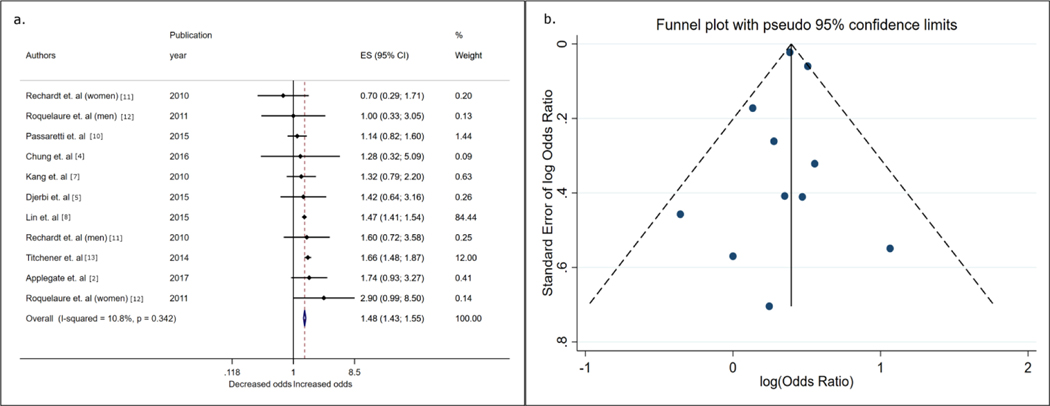
Figure 2. Forest plot and funnel plot from inverse variance fixed-effects meta-analysis showing associations between diabetes status and rotator cuff disease.
a) Shows effect estimates (odds ratios) and 95% confidence intervals and the assumed weights of contributing studies evaluating the association between diabetes and rotator cuff disease. The meta-analysis odds ratio was computed using the individual effect estimates weighted by the inverse of the variance for each study. b) Funnel plot of the estimates included in the meta-analysis depicted in 2a. The funnel plot shows the distribution of effect estimates as a function of the standard error of each reporting study. Each point represents a contributing effect estimate, and symmetrical distribution of these points around the central vertical line (the meta-analysis log[odds]) suggests a lack of evidence of small-study bias, whereas a lack of symmetry is suggestive of small-study bias. ES, effect estimate
Table 2.
Fixed effects and random effects meta-analysis results for diabetes, hypertension and hyperlipidemia in relation to rotator cuff disease and rotator cuff tear.
| Co-morbidity | N-estimates | OR | 95% CI | I2 |
|---|---|---|---|---|
| Diabetes - RCD | ||||
| FE | 11 | 1.49 | (1.43; 1.55) | 11% |
| RE | 11 | 1.50 | (1.39; 1.61) | 11% |
| Diabetes - RCT | ||||
| FE | 5 | 1.28 | (1.07; 1.52) | 0% |
| Hypertension - RCD | ||||
| FE | 5 | 1.40 | (1.19; 1.65) | 73% |
| RE | 5 | 1.58 | (1.10; 2.26) | 73% |
| Hypertension - RCT | ||||
| FE | 4 | 1.31 | (1.09; 1.57) | 75% |
| Hyperlipidemia - RCD | ||||
| FE | 3 | 1.48 | (1.42; 1.55) | 73% |
| RE | 3 | 1.74 | (1.12; 2.69) | 73% |
95% CI, 95% confidence interval; FE, fixed effects; I2, percent variation due to heterogeneity; OR, odds ratio; RCD, rotator cuff disease; RCT, rotator cuff tear; RE, random effects Present comorbidities were treated as dichotomous outcomes (Present/Absent).
Hypertension and rotator cuff disease
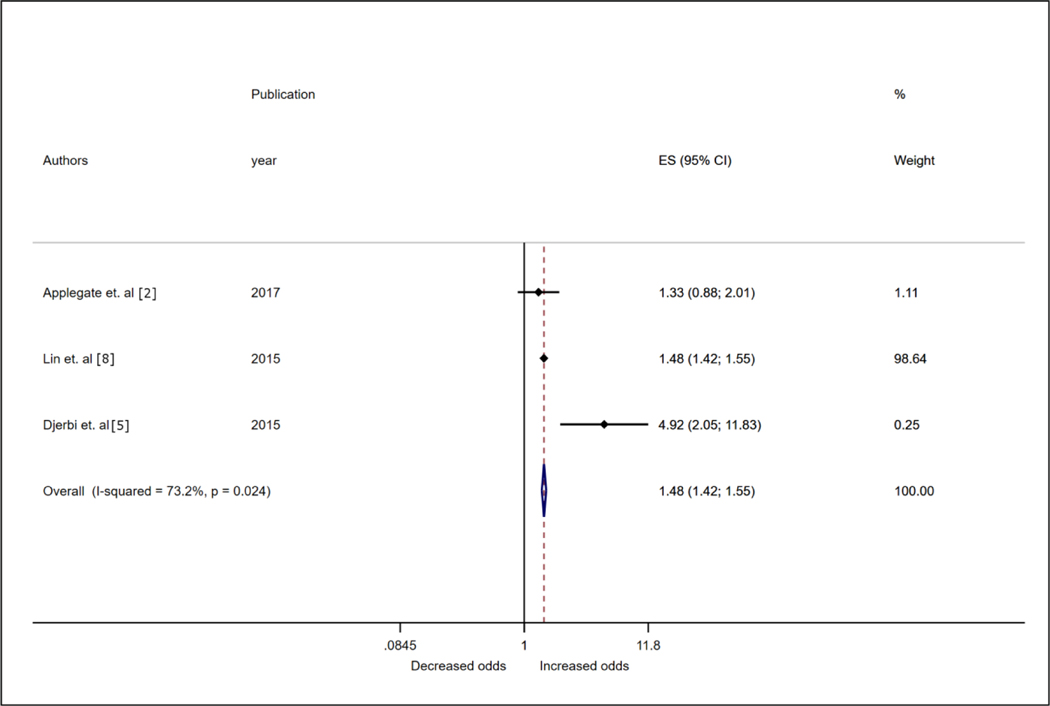
Five studies in total, 3 case–control, 1 cross-sectional study and one cohort study (1,160 cases and 177,033 controls) reported on the association between hypertension and rotator cuff disease. RoB assessments were moderate to high for 3 studies[4,5,14], moderate for 1 study[2] and low to moderate for 1 study[6]. Uncontrolled confounding was the primary reason for bias. Overall, the odds of rotator cuff disease was increased with hypertension (OR 1.40, 95% CI 1.19–1.65; Fig. 3). We found evidence for heterogeneity in effect estimates (I2 = 75%). Gumina et al. [22] reported more than one effect estimate, and substituting the largest effect estimate in the sensitivity analyses minimally changed the OR from 1.40 to 1.44 (95% CI 1.21–1.72). With the exception of Applegate et al. [2], all other studies specifically reported on the association between hypertension and rotator cuff tears. Limiting the analyses to studies focused on rotator cuff tears [4–6,22] resulted in an OR of 1.31 (95% CI 1.09–1.57). The study with the lowest risk of bias (Huang et al.) reported a null effect estimate on the association between hypertension status and rotator cuff disease (OR 1.01, 95% CI 0.80–1.18)[6].
Figure 3. Forest plot from inverse variance fixed-effects meta-analysis showing associations between hypertension status and rotator cuff disease.
Shows effect estimates (odds ratios) and 95% confidence intervals, and the assumed weights of contributing studies evaluating the association between hypertension and rotator cuff disease. The meta-analysis odds ratio was computed using the individual effect estimates weighted by the inverse of the variance for each study. ES, effect estimate
Hyperlipidemia and rotator cuff disease
Of the 8 studies that met eligibility, only 4 [2,5,6,8] reported effect estimates appropriate for this meta-analysis. Three of the excluded studies compared means or medians of high-density lipoprotein, low-density lipoprotein, or total cholesterol levels between cases and controls. One reported a positive association [15], another reported no evidence of association [18] and the third reported higher levels of lipids in controls versus cases [16]. Among studies eligible for meta-analyses, 2 were cohort studies, 1 a case–control study and the other a cross-sectional study. Both cohort studies were derived from the same base population; however, one analyzed rotator cuff disease [8] and the other included patients with rotator cuff tears [6]. Because of overlapping populations, only 1 of the 2 studies was used in a given meta-analysis. The study with the larger sample size was included as the primary study. The cohort study had low RoB[8] and the other 2 studies were assessed as low-to-moderate[5] and moderate RoB [2]. Overall, in the meta-analysis sample of 27,940 cases and 472,230 controls, the odds of rotator cuff disease was increased with hyperlipidemia/dyslipidemia (OR 1.48, 95% CI 1.42–1.55; Fig. 4).
Figure 4. Forest plot from inverse variance fixed-effects meta-analysis showing associations between hyperlipidemia/dyslipidemia status and rotator cuff disease.
Shows effect estimates (odds ratios) and 95% confidence intervals, and the assumed weights of contributing studies evaluating the association between hyperlipidemia/dyslipidemia and rotator cuff disease. The meta-analysis odds ratio was computed using the individual effect estimates weighted by the inverse of the variance for each study. ES, effect estimate
Substituting the effect estimate from Huang et al. with that of Lin et al. did not change the point estimate markedly (OR 1.41, 95% CI 1.15–1.73; I2 = 76%). The study with the lowest RoB reported a statistically significant finding between hyperlipidemia status at baseline and rotator cuff disease (HR 1.48, 95% CI 1.42–1.55).
Discussion
In this systematic review and meta-analysis, we synthesized evidence on the association of chronic cardiometabolic comorbidities such as diabetes, hypertension and hyperlipidemia with rotator cuff disease. Overall meta-analysis results suggested that diabetes, hypertension, and hyperlipidemia were associated with the disease. When we restricted the meta-analysis to studies that assessed patients with rotator cuff tears, we found an attenuated but positive association of diabetes and hypertension with rotator cuff tear. A qualitative synthesis of evidence incorporating meta-analysis estimates, study quality metrics and RoB assessments suggests that epidemiologic evidence is most robust for the association between diabetes and rotator cuff disease, is limited but plausible for hyperlipidemia and rotator cuff disease, and is most unreliable and insufficient for hypertension and rotator cuff disease.
In our synthesis, the evidence of association was strongest for diabetes and rotator cuff disease. This meta-analysis had the largest number of studies available including contributions from 2 large retrospective cohort studies and 1 large electronic health record-based case–control study. We assessed for small study/publication bias and did not find sufficient evidence to suggest that the positive association between diabetes and rotator cuff disease was driven by this phenomenon. When we restricted the meta-analysis to studies evaluating rotator cuff tears, the association with diabetes was slightly attenuated but remained positive, which adds further confidence in this association. A sub-group analysis of studies with less than moderate RoB showed larger effect estimates than studies rated as moderate or higher bias. Lack of control for confounding was the greatest source of bias in these studies, and the fact that investigators chose not to report multivariable adjusted estimates in these studies represents well-known and problematic practices of not reporting or under-reporting results that are not statistically significant. However, 2 studies (Lin et al. and Titchener et al.), which were incidentally also 2 studies with lower RoB scores, account for more than 96% of the weight in the meta-analysis (Fig. 2a).
The mechanism behind the association of diabetes and rotator cuff disease is an active area of research. Hyperglycemia may increase the susceptibility to tendon tearing due to excessive accumulation of advanced glycation end products in connective tissue and non-enzymatic glycosylation of collagen [23–26]. Hyperglycemia may contribute to tendonitis by upregulating proinflammatory mediators [23,27–29]. Also, hyperglycemia may lead to microvascular dysfunction at various tissue sites including regions of the rotator cuff. This may cause eventual weakening of the rotator cuff. Evidence for the role of vascularity in rotator cuff disease is inconsistent. Studies report regions of the supraspinatus tendon on the articular side that may be hypervascular in individuals with ruptured tendons [30–32]. Individuals with tendinopathy in the acute stages may have a lower degree of vascularity than those without tendinopathy [33]. However, in chronic tendinopathy, tears may be associated with hypervascularity due to neovascularization caused as a healing response to degeneration and ischemia [33]. Consequently, crowding of blood vessels due to neovascularization in the tendon matrix may in some cases further weaken the integrity of the tendon, for more susceptibility to tears [30,34].
Evidence of an association for hyperlipidemia and rotator cuff disease is based on a limited number of studies available for synthesis. The study by Lin et al. showed that hyperlipidemia at baseline is associated with rotator cuff disease and also provided data on the stronger association in hyperlipidemic patients who do not use statins as compared with those who use statins. In a mouse model, hypercholesterolemia increased the likelihood of tendon tears [35]. Excess circulating low-density-lipoprotein level is known to accumulate in the Achilles tendon [36], and a similar mechanism leading to accumulation and infiltration of fat into the rotator cuff tendon may increase the risk of rupture or cause delayed healing. Despite the biologic plausibility, our meta-analysis is limited by the relatively few number studies in this area and even fewer with sound design and analytic approaches. Most of the evidence for this association relies on 1 study, Lin et al., and there is a dire need for more well-designed studies for sound inferences.
Our meta-analysis showed a positive association of hypertension and rotator cuff disease; however, synthesis of the quality of evidence and RoB suggests that evidence for this apparent association is likely severely biased. The only cohort study with a temporal assessment of hypertension and rotator cuff tear reported a lack of association (HR 1.01, 95% CI 0.79–1.27). Although we could not use analytic methods to assess small-study publication bias here because of the few studies, the possibility of publication bias due to selective reporting of statistically significant studies is high in this meta-analysis. The possible mechanism of the association of hypertension and rotator cuff disease may be due to damage to the microvascular network [37], a mechanism akin to that postulated for diabetes. However, from an epidemiologic perspective, we are not confident in suggesting an association between hypertension and rotator cuff disease.
The limitations of our meta-analysis are inherent to the studies that contributed to it. The definition of cases and controls of rotator cuff disease/tears was not standardized across studies. The number of studies assessing these risk factors was relatively small, which can lead to a bias, including publication bias of statistically significant results. We cannot establish temporality, and hence cannot infer cause from our analysis. We also cannot ascertain whether uncontrolled (versus controlled) diabetes, hypertension, and hyperlipidemia increases the risk of rotator cuff disease. The inability to adjust for confounding bias by occupation is also a limitation.
Conclusions
This systematic review and meta-analyses suggest that co-morbidities such as diabetes and hyperlipidemia may be associated with the diagnosis of rotator cuff disease; however, the evidence for hypertension as a causal factor is least convincing. High-quality studies with the ability to incorporate time since first diagnosis of co-morbidity with granular information on treatment patterns to incorporate parameters involving control of co-morbid conditions are needed to determine causality between these co-morbidities and rotator cuff disease.
Supplementary Material
Highlights.
The underlying mechanisms of tendinopathy leading to rotator cuff tear are poorly understood.
Diabetes, hypertension, and hyperlipidemia were associated with cuff tears.
The current approach is based on addressing the anatomic defect rather than vascularity.
In addition to the current approach, we suggest more focus on vascularity in management of cuff tears.
Funding.
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (award no. R01AR074989). Dr. Giri was funded by the Building Interdisciplinary Research Careers in Women’s Health program as a scholar (grant no.: 5K12HD043483-18; PI: K E. Hartmann) and the National Institute of Diabetes and Digestive and Kidney Diseases (grant no.: 1K01DK120631-01A1; PI: A Giri) when this work was performed. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest.
The institution of one or more of the authors (NBJ and AG) received funding from National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant no. R01AR074989).
Footnotes
Location: This work was performed at Vanderbilt University Medical Center
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dean BJF, Franklin SL, Carr AJ. A systematic review of the histological and molecular changes in rotator cuff disease. Bone Joint Res 2012;1:158–66. 10.1302/2046-3758.17.2000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Applegate KA, Thiese MS, Merryweather AS, Kapellusch J, Drury DL, Wood E, et al. Association Between Cardiovascular Disease Risk Factors and Rotator Cuff Tendinopathy: A Cross-Sectional Study. J Occup Environ Med 2017;59:154–60. 10.1097/JOM.0000000000000929. [DOI] [PubMed] [Google Scholar]
- [3].Bodin J, Ha C, Chastang J-F, Descatha A, Leclerc A, Goldberg M, et al. Comparison of risk factors for shoulder pain and rotator cuff syndrome in the working population. Am J Ind Med 2012;55:605–15. 10.1002/ajim.22002. [DOI] [PubMed] [Google Scholar]
- [4].Chung SW, Yoon JP, Oh K-S, Kim HS, Kim YG, Lee H-J, et al. Rotator cuff tear and sarcopenia: are these related? J Shoulder Elbow Surg 2016;25:e249–255. 10.1016/j.jse.2016.02.008. [DOI] [PubMed] [Google Scholar]
- [5].Djerbi I, Chammas M, Mirous M-P, Lazerges C, Coulet B, French Society For Shoulder and Elbow (SOFEC). Impact of cardiovascular risk factor on the prevalence and severity of symptomatic full-thickness rotator cuff tears. Orthop Traumatol Surg Res 2015;101:S269–273. 10.1016/j.otsr.2015.06.011. [DOI] [PubMed] [Google Scholar]
- [6].Huang S-W, Wang W-T, Chou L-C, Liou T-H, Chen Y-W, Lin H-W. Diabetes mellitus increases the risk of rotator cuff tear repair surgery: A population-based cohort study. J Diabetes Complicat 2016;30:1473–7. 10.1016/j.jdiacomp.2016.07.015. [DOI] [PubMed] [Google Scholar]
- [7].Kang J-H, Tseng S-H, Jaw F-S, Lai C-H, Chen H-C, Chen S-C. Comparison of ultrasonographic findings of the rotator cuff between diabetic and nondiabetic patients with chronic shoulder pain: a retrospective study. Ultrasound Med Biol 2010;36:1792–6. 10.1016/j.ultrasmedbio.2010.08.006. [DOI] [PubMed] [Google Scholar]
- [8].Lin TT-L, Lin C-H, Chang C-L, Chi C-H, Chang S-T, Sheu WH-H. The effect of diabetes, hyperlipidemia, and statins on the development of rotator cuff disease: a nationwide, 11-year, longitudinal, population-based follow-up study. Am J Sports Med 2015;43:2126–32. 10.1177/0363546515588173. [DOI] [PubMed] [Google Scholar]
- [9].Longo UG, Franceschi F, Ruzzini L, Spiezia F, Maffulli N, Denaro V. Higher fasting plasma glucose levels within the normoglycaemic range and rotator cuff tears. Br J Sports Med 2009;43:284–7. 10.1136/bjsm.2008.049320. [DOI] [PubMed] [Google Scholar]
- [10].Passaretti D, Candela V, Venditto T, Giannicola G, Gumina S. Association between alcohol consumption and rotator cuff tear. Acta Orthop 2016;87:165–8. 10.3109/17453674.2015.1119599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rechardt M, Shiri R, Karppinen J, Jula A, Heliövaara M, Viikari-Juntura E. Lifestyle and metabolic factors in relation to shoulder pain and rotator cuff tendinitis: a population-based study. BMC Musculoskelet Disord 2010;11:165. 10.1186/1471-2474-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Roquelaure Y, Bodin J, Ha C, Petit Le Manac’h A, Descatha A, Chastang J-F, et al. Personal, biomechanical, and psychosocial risk factors for rotator cuff syndrome in a working population. Scand J Work Environ Health 2011;37:502–11. 10.5271/sjweh.3179. [DOI] [PubMed] [Google Scholar]
- [13].Titchener AG, White JJE, Hinchliffe SR, Tambe AA, Hubbard RB, Clark DI. Comorbidities in rotator cuff disease: a case-control study. J Shoulder Elbow Surg 2014;23:1282–8. 10.1016/j.jse.2013.12.019. [DOI] [PubMed] [Google Scholar]
- [14].Gumina S, Arceri V, Carbone S, Albino P, Passaretti D, Campagna V, et al. The association between arterial hypertension and rotator cuff tear: the influence on rotator cuff tear sizes. Journal of Shoulder and Elbow Surgery 2013;22:229–32. 10.1016/j.jse.2012.05.023. [DOI] [PubMed] [Google Scholar]
- [15].Abboud JA, Kim JS. The effect of hypercholesterolemia on rotator cuff disease. Clin Orthop Relat Res 2010;468:1493–7. 10.1007/s11999-009-1151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Davis DE, Narzikul A, Sholder D, Lazarus M, Namdari S, Abboud J. Shoulder Synovial Fluid Lipoprotein Levels and Their Relationship to the Rotator Cuff. Med Sci Sports Exerc 2017;49:396–402. 10.1249/MSS.0000000000001120. [DOI] [PubMed] [Google Scholar]
- [17].Kim J-M, Kim M-W, Do H-J. Influence of Hyperlipidemia on the Treatment of Supraspinatus Tendinopathy With or Without Tear. Ann Rehabil Med 2016;40:463–9. 10.5535/arm.2016.40.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Longo UG, Franceschi F, Spiezia F, Forriol F, Maffulli N, Denaro V. Triglycerides and total serum cholesterol in rotator cuff tears: do they matter? Br J Sports Med 2010;44:948–51. 10.1136/bjsm.2008.056440. [DOI] [PubMed] [Google Scholar]
- [19].Brooke BS, Schwartz TA, Pawlik TM. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg 2021. 10.1001/jamasurg.2021.0522. [DOI] [PubMed] [Google Scholar]
- [20].Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wells G, Shea B, O’Connell D, Robertson J, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis n.d.:21. [Google Scholar]
- [22].Gumina S, Candela V, Passaretti D, Latino G, Venditto T, Mariani L, et al. The association between body fat and rotator cuff tear: the influence on rotator cuff tear sizes. J Shoulder Elbow Surg 2014;23:1669–74. 10.1016/j.jse.2014.03.016. [DOI] [PubMed] [Google Scholar]
- [23].Abate M, Schiavone C, Salini V. Sonographic evaluation of the shoulder in asymptomatic elderly subjects with diabetes. BMC Musculoskelet Disord 2010;11:278. 10.1186/1471-2474-11-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].DeGroot J. The AGE of the matrix: chemistry, consequence and cure. Curr Opin Pharmacol 2004;4:301–5. 10.1016/j.coph.2004.01.007. [DOI] [PubMed] [Google Scholar]
- [25].Dutta U, Cohenford MA, Guha M, Dain JA. Non-enzymatic interactions of glyoxylate with lysine, arginine, and glucosamine: a study of advanced non-enzymatic glycation like compounds. Bioorg Chem 2007;35:11–24. 10.1016/j.bioorg.2006.07.005. [DOI] [PubMed] [Google Scholar]
- [26].Reiser KM. Nonenzymatic glycation of collagen in aging and diabetes. Proc Soc Exp Biol Med 1998;218:23–37. 10.3181/00379727-218-44264. [DOI] [PubMed] [Google Scholar]
- [27].Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, et al. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation 2002;105:816–22. 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- [28].Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 2006;114:597–605. 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- [29].Steenvoorden MMC, Toes REM, Ronday HK, Huizinga TWJ, Degroot J. RAGE activation induces invasiveness of RA fibroblast-like synoviocytes in vitro. Clin Exp Rheumatol 2007;25:740–2. [PubMed] [Google Scholar]
- [30].Factor D, Dale B. Current concepts of rotator cuff tendinopathy. Int J Sports Phys Ther 2014;9:274–88. [PMC free article] [PubMed] [Google Scholar]
- [31].Rudzki JR, Adler RS, Warren RF, Kadrmas WR, Verma N, Pearle AD, et al. Contrast-enhanced ultrasound characterization of the vascularity of the rotator cuff tendon: age- and activity-related changes in the intact asymptomatic rotator cuff. J Shoulder Elbow Surg 2008;17:96S–100S. 10.1016/j.jse.2007.07.004. [DOI] [PubMed] [Google Scholar]
- [32].Lohr JF, Uhthoff HK. The microvascular pattern of the supraspinatus tendon. Clin Orthop Relat Res 1990:35–8. [PubMed] [Google Scholar]
- [33].Levy O, Relwani J, Zaman T, Even T, Venkateswaran B, Copeland S. Measurement of blood flow in the rotator cuff using laser Doppler flowmetry. J Bone Joint Surg Br 2008;90:893–8. 10.1302/0301-620X.90B7.19918. [DOI] [PubMed] [Google Scholar]
- [34].Abate M, Gravare Silbernagel K, Siljeholm C, Di Iorio A, De Amicis D, Salini V, et al. Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Research & Therapy 2009;11:235. 10.1186/ar2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Beason DP, Abboud JA, Kuntz AF, Bassora R, Soslowsky LJ. Cumulative effects of hypercholesterolemia on tendon biomechanics in a mouse model. J Orthop Res 2011;29:380–3. 10.1002/jor.21255. [DOI] [PubMed] [Google Scholar]
- [36].Tsouli SG, Kiortsis DN, Argyropoulou MI, Mikhailidis DP, Elisaf MS. Pathogenesis, detection and treatment of Achilles tendon xanthomas. Eur J Clin Invest 2005;35:236–44. 10.1111/j.1365-2362.2005.01484.x. [DOI] [PubMed] [Google Scholar]
- [37].Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HAJ Microcirculation in Hypertension. Circulation 2001;104:735–40. 10.1161/hc3101.091158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.