Abstract
Objective:
Eating disorders (EDs) are often stereotyped as disorders of adolescence and young adulthood; however, they can occur at any age. Prevalence of EDs at midlife are approximately 3.5% and specific symptoms at midlife can have prevalences’ as high as 29.3%. Studies also inconsistently suggest that EDs and related symptoms may be more prevalent in midlife aged women during perimenopause compared with midlife aged women at pre-menopause. To date few studies have examined the structure of and associations between ED symptoms in women specifically during perimenopause and early postmenopause. Thus, the purpose of the current study is to investigate the structure of ED symptoms specifically during perimenopause and early postmenopause.
Methods:
Participants included N=136 participants (45–61 years old) in a larger clinical trial who completed the Eating Disorder Examination Questionnaire (EDE-Q) at a baseline study visit. Network analysis statistical models were used to examine the structure of and associations between ED symptoms assessed via the EDE-Q.
Results:
Shape dissatisfaction and weight dissatisfaction were the top two central symptoms in the network.
Conclusions:
Results corroborate previous studies and indicate that, similar to young adult samples, dissatisfaction with body image is a core feature of ED pathology across the lifespan.
Keywords: perimenopause, midlife, eating disorder, binge eating, menopause
Introduction
Eating disorders (EDs) are serious mental health conditions characterized by disturbances in eating behavior and body image that occur in approximately 13.1% of women across the lifespan1. EDs are often stereotyped as a disease of adolescence and young adulthood. However, they can occur at any time during the lifespan, including at older ages such as midlife. The prevalence of any ED for women older than 40 is approximately 3.5%2 and specific ED symptoms, such as dissatisfaction with eating patterns, can be as high as 29.3% at midlife3. Serious complications are associated with EDs such as high mortality4, 5 and morbidity6, all of which can be exacerbated when present at older ages7. Research in the ED field is beginning to recognize the importance of including this typically underrepresented population in empirical studies, which is critical to a complete etiological understanding. However, such studies often fail to consider the developmental/reproductive stages of biological women at midlife (e.g., pre-menopause, perimenopause, menopause).
Indeed, although findings have been somewhat inconsistent, one study found that an ED diagnosis may be more prevalent in midlife aged women during the menopause transition (perimenopause) (9%) compared with midlife aged women at pre-menopause (2%)8. Women in perimenopause may also have the highest rates of dysregulated eating behaviors (e.g., weight control behaviors such as regular counting of calories or consumption of diet foods) of any reproductive stage at midlife and are significantly different from women at pre-menopause on assessments of body dissatisfaction (e.g., feelings of fatness)8. These findings have yet to be replicated9, 10; however, associations between EDs and symptoms of perimenopause (e.g., negative mood, depression, and fatigue)11 confirm that the perimenopause may be a particularly risky time for eating pathology.
Similarly, ED symptom prevalence across age groups appears to vary in middle and older adulthood. Significant differences between age groups in ED symptoms were found in one study that investigated differences in core ED (i.e., binge eating -- objective overeating associated with distress and a sense of loss of control over eating behavior, binge eating and purging, and purging in the absence of bingeing) and body image symptoms in women aged 50 and older. Thirty-percent of women aged 50–54 years reported current binge eating, which was higher than women aged 55–64, 65–74, and 75–84 who reported binge eating rates of 19%, 2%, and 1%, respectively12. In contrast, purging behaviors in the absence of bingeing within the past five years was significantly more prevalent in women aged 55–64 in comparison with women aged 50–54, 65–74, and 75–84, respectively12. Differences in symptom prevalence in these age groups demonstrate that ED symptom structure may vary between middle and older adulthood. Furthermore, 50–54 roughly maps onto the developmental/reproductive stages of midlife as perimenopause onset is 47.5 years and median age of menopause is between 50–52 years13–17, however timing of these reproductive stages trend younger in developing countries18–22. However, while such differences across age in symptom prevalence could suggest that the importance of specific ED symptomatology varies across age groups; to date, such studies have not accounted for the developmental/reproductive stages of biological women at midlife.
Network analysis is a statistical analysis technique which is uniquely suited for comparing the structure and importance of specific ED symptoms across developmental/reproductive stages. Network analysis allows for the identification of specific relationships among many symptoms simultaneously and provides opportunities to visualize illness pathways (i.e., relationships among individual symptoms) and identify central symptoms (i.e., symptoms that are highly connected with other symptoms in the network). A number of previous studies have applied network analysis to ED symptomatology and findings consistently identify aspects of body dissatisfaction as central to EDs across a range of age groups, including midlife24, 25. While body dissatisfaction is central in ED symptomology across the lifespan, specific items within the overarching umbrella of body dissatisfaction appear to vary in importance across different ages. In young adults, central body dissatisfaction items relate to preoccupation with body shape and weight and desire to lose weight24, 26, whereas body dissatisfaction items related to dissatisfaction with weight were found to be most central for women at midlife24, 27. Differing associations between symptoms may indicate differential pathways to ED development and maintenance across the lifespan such that risk factors to EDs and treatment targets may also change with age.
Taken together, previous research has investigated the network structure and central symptoms of EDs across the lifespan, yet studies to date have not considered the developmental/reproductive stages of women at midlife. Additionally, most prior research has relied on age categories to define reproductive stages at midlife. Here, we investigate the network structure and central symptoms of ED symptomology in midlife women using menstrual cycle changes to determine peri- and post-menopausal status. Given previous associations observed between ED symptoms and menopausal symptoms and inconsistent evidence that ED symptomatology may be increased in midlife women during perimenopause compared with other developmental/reproductive stages at midlife, perimenopause may be a window of risk for development or re-development of ED symptoms28, 29. More precisely identifying reproductive stage and associated hormonal milieu will advance understanding of hormonally relevant etiology. Identifying central ED symptoms within women in perimenopause has potential to further our understanding of the etiology of EDs in general as well as at midlife and specifically during perimenopause. Further understanding EDs in this typically underrepresented group lays the foundation for the development of better treatment strategies for women at midlife and, more specifically, for women across reproductive stages.
Methods
Participants
Participants included women aged 45–60 that participated in the Perimenopausal Estrogen Replacement Therapy Study (PERT). The PERT Study was a clinical trial (NCT01308814) examining the effect of estrogen replacement therapy on depression30 and indices of cardiovascular risk31, 32 during the menopause transition. Stages of Reproductive Aging Workshop (STRAW) +1033 stages included in the current study were: early menopausal transition (Stage −2), late menopausual transition (Stage −1), and early postmenopause (Stage +1a, +1b), collectively referred to as perimenopause. Early and late perimenopause and early postmenopause were defined as outlined in STRAW+10 criteria33. Early perimenopause was defined as persistent menstrual cycle length change of at least 7 days longer than typical for that individual; late perimenopause was defined as an interval of amenorrhea of at least 60 days; early postmenopause was defined as within two years of their final menstrual period30. Within early postmenopause hormone variability is still present as are vasomotor menopause symptoms33. The baseline assessment occurred before participants were randomized and began the hormone therapy (HT) regimen. Further, current use of HT at enrollment was an exclusion criteria for the study. Exclusion criteria for the primary trial included: depressive, stress-related, and psychotic disorders, current use of psychotropic medications, herbal supplements for menopausal symptoms, or HT, and history of medical conditions which may put participants at risk while taking transdermal estradiol and oral micronized progesterone. An exhaustive description of inclusion/exclusion criteria can be found in Gordon et al., 2018. Enrolled participants were randomized in a double-blind, placebo-controlled fashion to either a 12-months of estrogen plus progesterone therapy, specifically transdermal estradiol plus intermittent micronized progesterone, or placebo. The current report includes women who completed an eating disorder symptom questionnaire at their baseline study visit, prior to randomization. The PERT study has been described in detail elsewhere30 and was approved by the University of North Carolina at Chapel Hill IRB.
Measures
Eating Disorder Examination Questionnaire
The Eating Disorder Examination Questionnaire (EDE-Q) was used to examine ED symptoms26. In order to compare our findings to previous studies, we used the twenty-three items assessing subscales of restraint (e.g., restraint and avoidance of eating, dietary rules, food avoidance), eating concern (e.g. preoccupation with food, fear of a loss of control over eating, social and secretive eating), shape concern (e.g. dissatisfaction or discomfort with shape, fear of weight gain, preoccupation with shape), and weight concern (e.g. dissatisfaction with weight desire to lose weight, preoccupation with weight) for analysis. These 23 items have been used frequently in previous studies24, 34–36 so will allow us to directly compare our findings in young adults and heterogenous older age samples. The EDE-Q has been extensively tested and demonstrates good reliability and validity37.
Network Analyses
Analysis were completed in R38 (Version 4.0.0) and based on a publicly available script35. Networks were estimated with EBICglasso function in the R package qgraph39 to eliminate spurious correlations and minimize network sizes39. Specifically, the Glasso function computes partial correlation networks such that each correlation accounts for all other items in the network40. Within the network, nodes represent EDE-Q items and are shown as circles in network graphs. Network edges represent partial correlations between EDE-Q items and are shown as lines connecting nodes in network graphs. In the graphs, blue lines represent a positive correlation whereas red lines represent a negative correlation. Edge thickness corresponds to correlation strength.
Network Centrality and Stability
We used the qgraph package39 to calculate network centralities. Network centrality represents the likelihood that specific symptom activation will subsequently be followed by activation of other symptoms within the network41. In other words, identifying the most central symptom(s) of the network. As has been done in prior studies24, 34, 42, we report strength centrality to assess the relative importance of network nodes, which has consistently reported higher stability in similar network analyses than other measures of centrality41, 43, 44. Strength is calculated as the sum of absolute weights for all edges connected to a target node. Using the R bootnet package45, we examined the stability of strength centrality, which represents the maximum proportion of data that can be dropped while still retaining a correlation of 0.7 between the network with partial data and the network with full data. It is recommended stability should be at least 0.25 to interpret networks46.
Finally, centrality difference tests were conducted to determine if central symptoms were significantly more central than other symptoms. Using the R package bootnet47, we performed node centrality difference tests to determine whether the nodes that have higher centrality statistics are in fact significantly different from other nodes with lower values48. In other words, we were able to determine not only if symptoms were highly central, but if they were significantly more central than other symptoms in the network. We report only the top central nodes before any sharp observable drop in network strength centrality similar to previous studies24, 34 (Figure 1). Subsequently, in our results, we report only the central nodes that were statistically different from other nodes (approximately 85%–92% of other nodes). EDE-Q item abbreviations (Table 1) in the networks followed previously published studies for ease of comparability24, 34.
Figure 1. Strength Differences of EDE-Q Network.
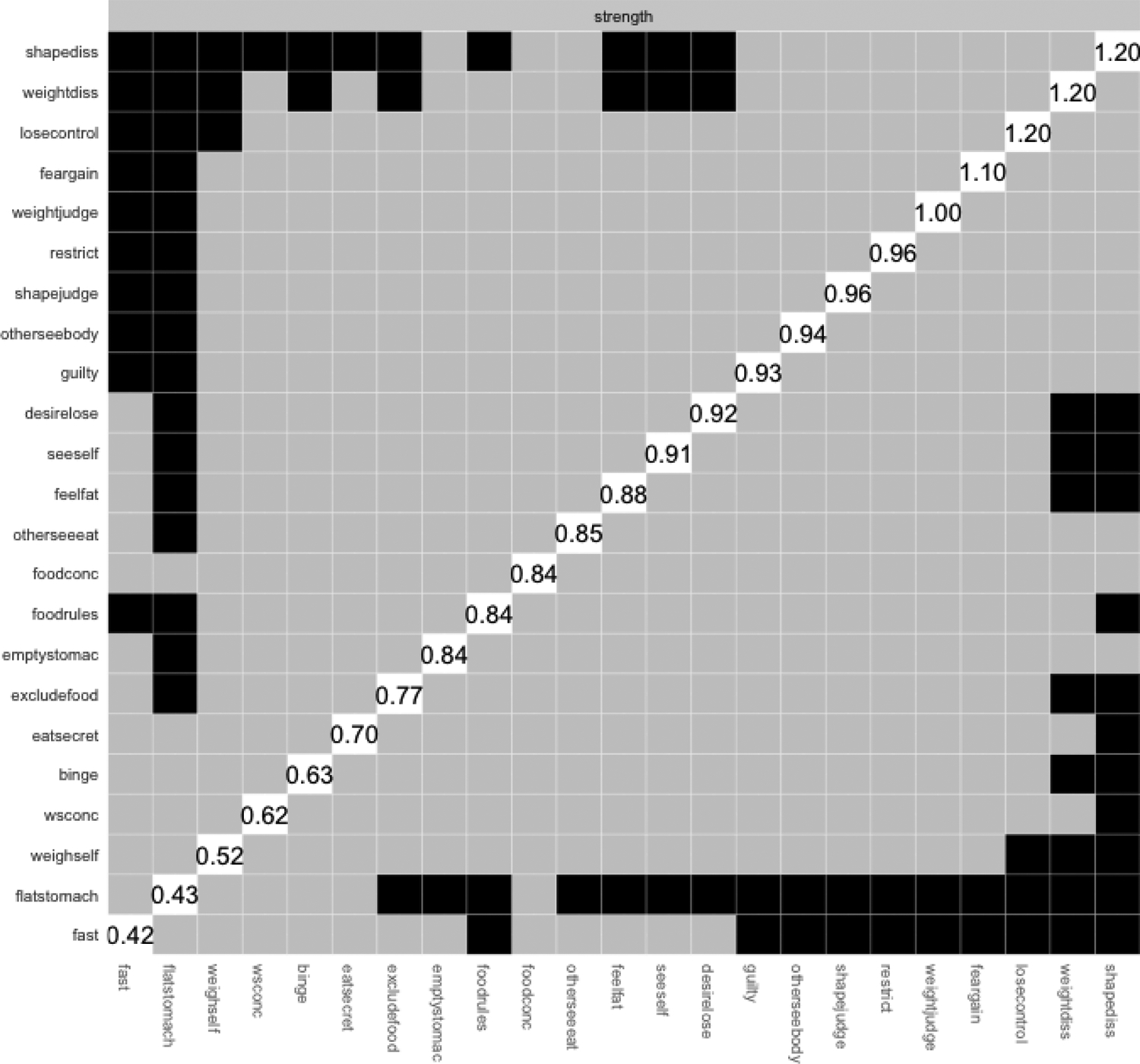
The strength differences graph visualizes if central symptoms are significantly more central than other symptoms. The R package bootnet was used to perform node centrality difference tests. We report only the top central nodes before any sharp observable drop in network strength centrality (approximately statistically different from 85%−92% of other nodes).
Table 1.
EDE-Q Items Abbreviations as Network Nodes
| Abbreviation | Item |
|---|---|
| restrict | Have you been deliberately trying to limit the amount of food you eat to influence your shape or weight? |
| fast | Have you gone for long periods of time without eating anything in order to influence your shape or weight? |
| excludefood | Have you tried to avoid eating any foods which you like in order to influence your shape or weight? |
| foodrules | Have you tried to follow definite rules regarding your eating in order to influence your shape or weight? |
| emptystomac | Have you wanted your stomach to be empty? |
| foodconc | Has thinking about food or its calorie content made it much more difficult to concentrate on things you are interested in? |
| losecontrol | Have you been afraid of losing control over eating? |
| binge | Have you had episodes of binge-eating? |
| eatsecret | Have you eaten in secret? |
| flatstomach | Have you definitely wanted your stomach to be flat? |
| wsconc | Has thinking about shape or weight made it more difficult to concentrate on things you are interested in…? |
| feargain | Have you had a definite fear that you might gain weight or become fat? |
| feelfat | Have you felt fat? |
| desirelose | Have you had a strong desire to lose weight? |
| guilty | On what proportion of times that you have eaten have you felt guilty because of the effect on your shape or weight? |
| weightjudge | Has your weight influenced how you think about (judge) yourself as a person? |
| shapejudge | Has your shape influenced how you think about (judge) yourself as a person? |
| weighself | How much would it upset you if you had to weigh yourself once a week for the next four weeks? |
| weightdiss | How dissatisfied have you felt about your weight? |
| shapediss | How dissatisfied have you felt about your shape? |
| otherseeeat | How concerned have you been about other people seeing you eat? |
| otherseebody | How uncomfortable have you felt seeing your body? |
| seeself | How uncomfortable have you felt about others seeing your body? |
Results
Participant Characteristics
The final analysis sample included N=136 women (Mage = 51.54 ± 3.5). Racial composition was primarily White (75%) or African American (21.3%), but also included Native American (1.5%), Hispanic (0.7%), and Asian (0.7%) women. See Table 2 for participant demographics. Roughly half (50.7%) of women in the study were in late perimenopause, 19.1% in early postmenopause, and 16.2% in early perimenopause (Table 3).
Table 2.
Participant Demographics
| Mean | SD | |
|---|---|---|
| Age | 51.54 | 3.5 |
| Number | % | |
| Race | ||
| White | 102 | 75 |
| African American | 29 | 21.3 |
| Native American | 2 | 1.5 |
| Hispanic | 1 | 0.7 |
| Asian | 1 | 0.7 |
| Menopausal Stage | ||
| Early Perimenopause | 22 | 16.2 |
| Late Perimenopause | 69 | 50.7 |
| Early Postmenopause | 26 | 19.1 |
N = 136 women.
SD = standard deviation.
Early and late perimenopause and early postmenopause were defined as outlined in the Stages of Reproductive Aging Workshop +10 criteria33. Early perimenopause was defined as persistent menstrual cycle length change of at least 7 days longer than typical for that individual; late perimenopause was defined as an interval of amenorrhea of at least 60 days; early postmenopause was defined as within two years of their final menstrual period30. Within early postmenopause hormone variability is still present as are vasomotor menopause symptoms33.
Table 3.
Participant Demographics by Menopause Transition Stage
| Menopause Stage | Age | White | African American | Native American | Hispanic | Asian |
|---|---|---|---|---|---|---|
| Early perimenopause (n=22) | 49.05 ± 2.63 | 15 (68.2%) | 5 (22.7%) | 1 (4.5%) | 0 | 1 (4.5%) |
| Late perimenopause (n=69) | 51.41 ± 3.35 | 56 (81.2%) | 12 (17.4%) | 1 (1.4%) | 0 | 0 |
| Early postmenopause (n=26) | 54.5 ± 2.44 | 21 (80.8%) | 4 (15.4%) | 0 | 1 (3.8%) | 0 |
n=19 missing.
Early and ate perimenopause and early postmenopause were defined as outlined in the Stages of Reproductive Aging Workshop +10 criteria33. Early perimenopause was defined as persistent menstrual cycle length change of at least 7 days longer than typical for that individual; late perimenopause was defined as an interval of amenorrhea of at least 60 days; early postmenopause was defined as within two years of their final menstrual period30. Within early postmenopause hormone variability is still present as are vasomotor menopause symptoms33.
Network Analyses
See Figures 2 and 3 for the EDE-Q network. Table 1 includes descriptions of EDE-Q items. Stability strength for the network was low (strength = 0.36), likely due to our smaller sample size, but still acceptable for interpretation45.
Figure 2. EDE-Q Network.
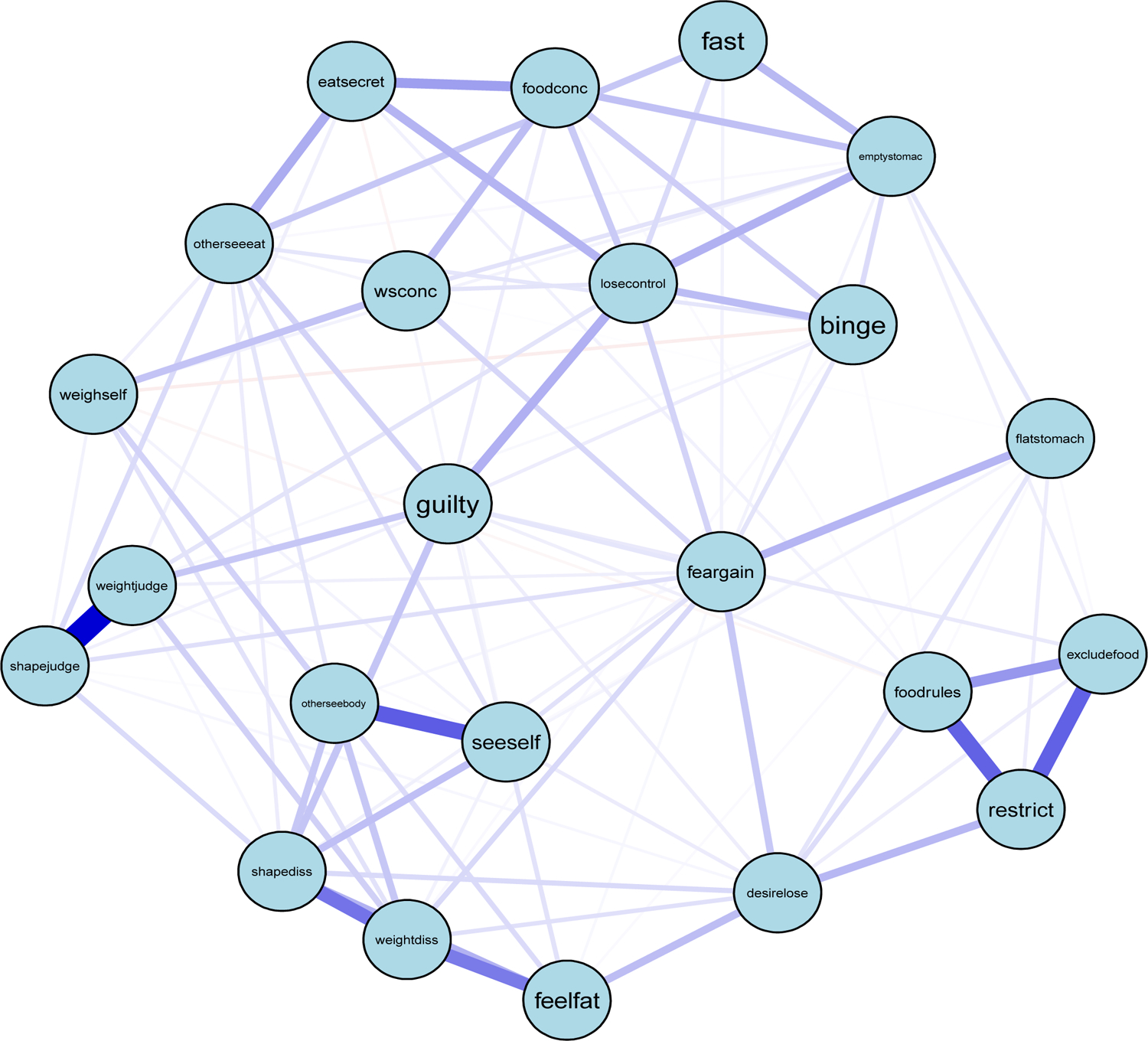
The network graph visualizes associations between EDE-Q items. Networks were estimated with EBICglasso function in the R package qgraph to eliminate spurious correlations and minimize network sizes. Within the network, nodes represent EDE-Q items and are shown as circles in network graphs. Network edges represent partial correlations between EDE-Q items and are shown as lines connecting nodes in network graphs. In the graphs, blue lines represent a positive correlation whereas red lines represent a negative correlation. Edge thickness corresponds to correlation strength.
Figure 3. EDE-Q Network Strength Centrality.
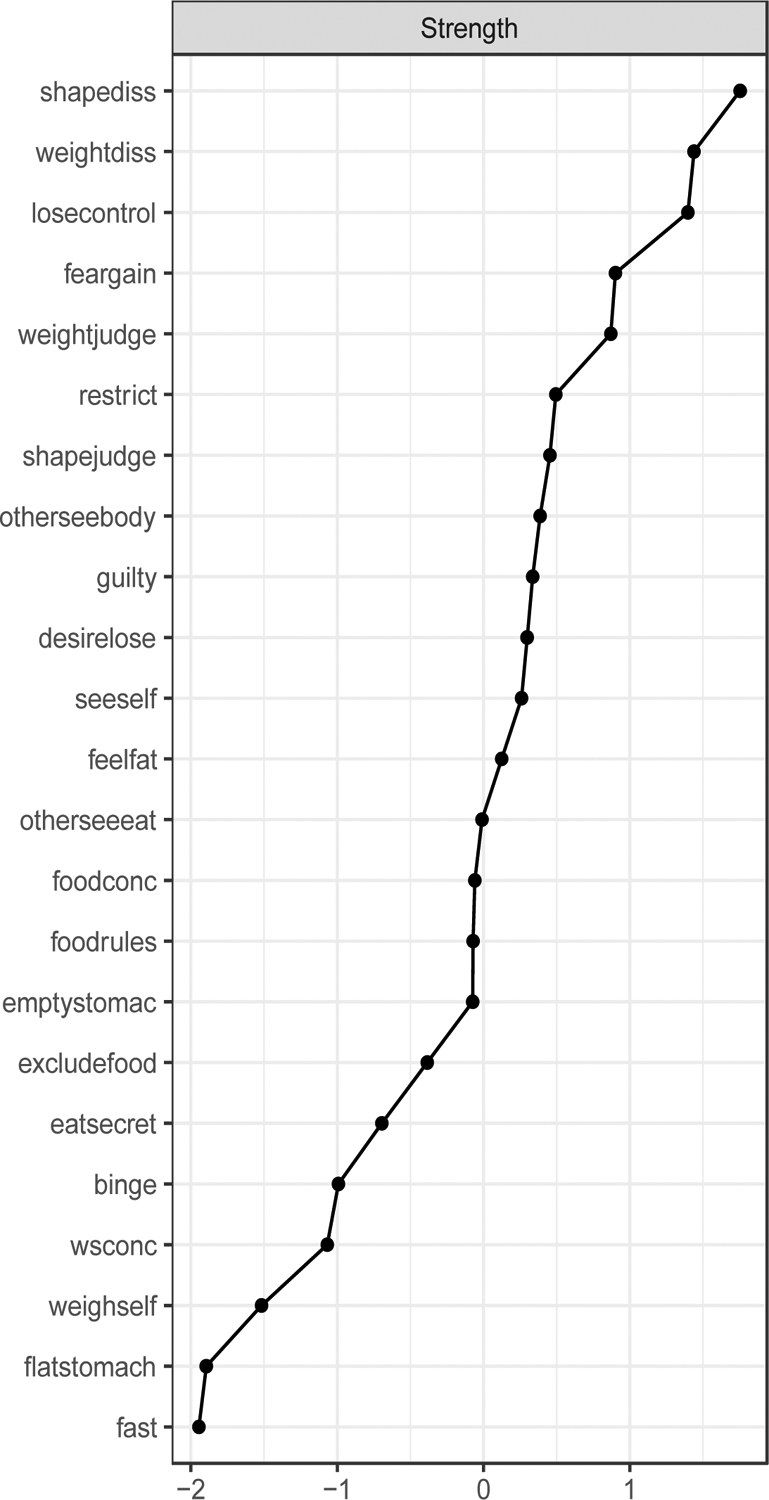
The strength centrality graph visualizes the likelihood that specific symptom activation will subsequently be followed by activation of other symptoms in the network. Strength is calculated as the sum of absolute weights for all edges connected to a target node and was determined using the R bootnet package.
Network
Visually, some shape and weight concern subscale items (node seeself, otherseebody, feelfat, weightdiss, and shapediss) formed a cluster, and some restraint subscale items (node foodrules, excludefood, and restrict) formed a cluster. Judgments influenced by shape and judgements influenced by weight (node shapejudge and weight judge) had a strong and positive partial correlation with each other. As defined by strength centrality (Table 4; Figure 3), central symptoms for the network were: shapediss (S=1.75), weightdiss (S=1.75), losecontrol (S=1.40), feargain (S=0.90), and weightjudge (S=0.87). The top two central symptoms created a cluster (i.e., shapediss and weightdiss) as did the next three (i.e., losecontrol, feargain, weightjudge), albeit at a lower strength centrality than the top two nodes, but still observably higher than all remaining subsequent nodes (Figure 3). The top two central symptoms were significantly different from 48% (p<0.05) and 35% (p<0.05) of all nodes in the network, respectively, and the next three top nodes were significantly different from 13% and 9% of all other nodes in the network (Figure 1).
Table 4.
Top 5 Central EDE-Q Nodes by Strength
| Strength | |
|---|---|
| Shapediss | 1.75 |
| Weightdiss | 1.44 |
| Losecontrol | 1.40 |
| Feargain | 0.90 |
| Weightjudge | 0.87 |
Abbreviations of nodes can be found in Table 1.
Discussion
The current study was the first to examine central symptoms of ED symptomology in midlife women, specifically during the menopause transition (perimenopause) as confirmed by gold standard reproductive staging criteria. Our study found fear of gaining weight and fear of losing control over eating are central ED symptoms in women during perimenopause and early postmenopause. In studies evaluating women at midlife, regardless of reproductive stage, these have not been previously observed as central symptoms. This may suggest that fear of gaining weight and fear of losing control over eating are of specific importance during perimenopause. In general, our results support those from the young adult literature indicating body dissatisfaction is a central component of ED symptomatology.
Network analysis of ED symptoms to date has mostly focused on central symptoms in adolescence and young adulthood. Current findings corroborate the importance of body dissatisfaction yet also suggest that specific central symptom items, apart from weight dissatisfaction, may differ in perimenopause from those as seen in adolescence and young adulthood. ED central symptoms in early and late adolescence were preoccupation with weight, overeating (i.e., eating an unusually large amount), desire to lose weight, desire to have an empty stomach, and shape influenced by self-judgement24. However, these specific items were not found to be highly central in the present study. Results also differed from those seen in young adulthoods where central symptoms were desire to have an empty stomach, preoccupation with weight, overeating, and binge eating24. This suggests that relationships between specific symptoms change in midlife women and specifically in perimenopause as compared with adolescence and young adulthood. While speculative, the possibility exists that these differences may be influenced by different reproductive stages and associated hormonal profiles that occur across the lifespan in women.
Indeed, our findings confirm the importance of the overarching symptom cluster of body dissatisfaction to ED symptomatology during midlife but suggest differences in central symptoms may be more nuanced and change across reproductive stages. In a heterogenous sample of women 46 and older, Christian et al.24 found weight and shape dissatisfaction, desire to have an empty stomach, and overeating to be central symptoms whereas we observed fear of losing control over eating, fear of gaining weight, and weight influenced by self-judgement to be top central symptoms, in addition to weight and shape dissatisfaction. Differences between findings of the current study and the study by Christian and colleagues (2020) may be due to differences in study populations as the current study included only women in perimenopause or early postmenopause to control for potential variation in the structure of ED symptoms, namely core symptoms, that may change as women age. As such, central symptoms found in this study may be related to the physical and body changes that occur with perimenopause, which could subsequently manifest as fear and self-judgement tied to changes in weight and shape. Together, this suggests that central symptoms and network structure of EDs may vary across reproductive stages.
Accordingly, fluctuations in reproductive hormones that occur during perimenopause may serve as a potential risk factor to ED symptomology28. Changes in estrogen and progesterone levels across the menstrual cycle are associated with change in ED symptoms, which suggests that estrogen and progesterone may play a role in risk for ED development and/or symptom exacerbation28. Specifically, we have previously examined the association between estrogen and progesterone change across the menstrual cycle in women during early perimenopause and observed that estrogen and progesterone interact to influence ED symptoms. When progesterone is high, estrogen was positively associated with body dissatisfaction; however, when progesterone was low, estrogen was negatively associated with body dissatisfaction49. Hypotheses regarding the underlying mechanisms of these associations draw from previous literature investigating the association between reproductive hormones in postpartum depression and associations between estrogen and feeding behavior in animal models. Research investigating the role of reproductive hormones in postpartum depression, another time of fluctuation in ovarian hormones like the menopause transition, hypothesizes that postpartum depression is a result of abnormal neural response to fluctuations in reproductive hormones during the perinatal period50–52. Furthermore, a recent study found that estradiol and progesterone administration triggered depression symptoms in women who had a history of postpartum depression and decreased activity in brain areas which respond to reward53. Given previous studies have also shown an association between ED symptoms and response to reward54–57, the associations between ED symptoms and the menopause transition may therefore be a result of a similar mechanism. Fluctuations in ovarian hormones during perimenopause and early postmenopause may pose as a risk factor to ED symptomology via changes in brain activity and aberrant neural responses in areas of the brain related to response to reward thereby altering salience of cues related to food and body image. Simultaneously, animal literature findings predominately demonstrate that estrogen decreases homeostatic feeding behavior, meal size, and meal frequency while increasing hedonic feeding58. Fluctuations in ovarian hormones may thus elevate risk for development/redevelopment or exacerbation of ED symptomology during perimenopause and early postmenopause by directly impacting eating behavior in women during this time. Investigation into the proposed mechanistic ideas should be investigated in future research. Therefore, research with women at perimenopause is of particular interest in the development or redevelopment of EDs because estrogen levels are characteristically erratic during this time 59, similar to puberty, a known risk period for the development of EDs.
This study must be considered within the context of limitations. First, in comparison to previous network analysis studies, our sample size is smaller. Despite the smaller sample size, our stability, although on the lower end, was still acceptable for interpretation. Our results also generally align with previous studies (i.e., importance of body dissatisfaction to the network) suggesting that our results are interpretable. Secondly, we chose a prioi to include items from the EDE-Q that were used in previous studies in the current network analysis. This decision was made because one of the purposes of this study was to address whether the structure and association of ED symptomatology are similar across the lifespan. In order for our results to be comparable to published studies in other age groups24, 34, it was important to replicate previous measures/items used. Otherwise, any differences in results obtained could simply be attributable to the differences in items included in the network. Third, our study was limited to participants who met inclusion/exclusion criteria for the primary clinical trial, which may lend to inclusion bias. Furthermore, as we performed secondary level analysis, our study is hypothesis generating. Future research should examine core symptoms of ED pathology within women at perimenopause and early postmenopause to validate if the current findings are specific to perimenopause and early postmenopause. Despite limitations, a strength of the current study was the focus on a specific developmental window during midlife, perimenopause, which has yet to be investigated via network analysis until now. Previous studies have been extremely heterogenous as they have included a wide range of women at many different developmental stages with large variations in reproductive hormones where the present study indirectly controlled for such variations by including only women in perimenopause and early postmenopause.
Conclusions
Taken together, the results of our study support body dissatisfaction as a key symptom for ED symptomatology across development and proposes specific symptoms that may be particularly relevant for women in perimenopause. Results on central symptoms specific to perimenopause have potential to provide more targeted/individualized treatments for women at midlife. For example, within the cognitive behavioral therapy framework different treatment targets may have more clinical utility at differing stages of development. Consequently, treatment priorities for women in perimenopause may marginally differ from those in adolescence, young adulthood, or in older, postmenopausal women. However, overarching constructs such as body dissatisfaction remain relevant across all ages. In other words, our results suggest that the treatment of body dissatisfaction may be nuanced across the lifespan such that specific aspects of body dissatisfaction used as treatment targets may need to change with age. Future research should replicate our findings within larger samples and examine bridge symptoms of EDs and menopausal symptoms throughout midlife as fluctuations in reproductive hormones across the perimenopause have been hypothesized to be a risk mechanism to EDs at midlife.
Sources of funding:
Time to analyze data and draft the manuscript was partially funded by the National Institute of Health R21MH121726 (PI: Baker).
Footnotes
Financial disclosures/Conflict of interest: None reported.
References
- 1.Stice E, Marti CN, Rohde P. Prevalence, incidence, impairment, and course of the proposed DSM-5 eating disorder diagnoses in an 8-year prospective community study of young women. J. Abnorm. Psychol 2013;122(2):445–57. doi: 10.1037/a0030679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangweth-Matzek B, Hoek HW. Epidemiology and treatment of eating disorders in men and women of middle and older age. Curr. Opin. Psychiatry 2017;30(6):446–51. doi: 10.1097/YCO.0000000000000356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus MD, Bromberger JT, Wei H-L, Brown C, Kravitz HM. Prevalence and selected correlates of eating disorder symptoms among a multiethnic community sample of midlife women. Ann. Behav. Med 2007;33:269–77. doi: 10.1007/BF02879909 [DOI] [PubMed] [Google Scholar]
- 4.Keshaviah A, Edkins K, Hastings ER, et al. Re-examining premature mortality in anorexia nervosa: a meta-analysis redux. Compr. Psychiatry 2014;55(8):1773–83. doi: 10.1016/j.comppsych.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 5.Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry. 2014;13(2):153–60. doi: 10.1002/wps.20128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler RC, Berglund PA, Chiu WT, et al. The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol. Psychiatry 2013;73(9):904–14. doi: 10.1016/j.biopsych.2012.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Podfigurna-Stopa A, Czyzyk A, Katulski K, et al. Eating disorders in older women. Maturitas. 2015;82(2):146–52. doi: 10.1016/j.maturitas.2015.06.036 [DOI] [PubMed] [Google Scholar]
- 8.Mangweth-Matzek B, Hoek HW, Rupp CI, Kemmler G, Pope HG Jr., Kinzl J. The menopausal transition-a possible window of vulnerability for eating pathology. Int J Eat Disord. 2013;46:609–16. doi: 10.1002/eat.22157 [DOI] [PubMed] [Google Scholar]
- 9.Slevec JH, Tiggemann M. Predictors of body dissatisfaction and disordered eating in middle-aged women. Clin. Psychol. Rev 2011;31(4):515–24. doi: 10.1016/j.cpr.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 10.Pearce G, Thøgersen-Ntoumani C, Duda J. Body image during the menopause transition: a systematic scoping review. Health Psychol. Rev 2014;8(4):473–89. doi: 10.1080/17437199.2013.848408 [DOI] [PubMed] [Google Scholar]
- 11.Schreiber DR. A Microlongitudinal Study of Menopause Symptoms and Problematic Eating in Midlife Women. Psychology. Richmond, Virginia: Virginia Commonwealth University; 2020. p. 146. [Google Scholar]
- 12.Gagne DA, Von Holle A, Brownley KA, et al. Eating disorder symptoms and weight and shape concerns in a large web-based convenience sample of women ages 50 and above: Results of the gender and body image (GABI) study. Int. J. Eat. Disord 2012;45(7):832–44. doi: 10.1002/eat.22030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14(2):103–15. doi: 10.1016/0378-5122(92)90003-m [DOI] [PubMed] [Google Scholar]
- 14.Greendale GA, Hogan P, Kritz-Silverstein D, Langer R, Johnson SR, Bush T. Age at Menopause in Women Participating in the Postmenopausal Estrogen/Progestins Interventions (PEPI) Trial: An Example of Bias Introduced by Selection Criteria. Menopause. 1995;2(1):27–34. https://journals.lww.com/menopausejournal/Abstract/1995/02010/Age_at_Menopause_in_Women_Participating_in_the.5.aspx. Accessed November, 10, 2022. [Google Scholar]
- 15.Luoto R, Kaprio J, Uutela A. Age at natural menopause and sociodemographic status in Finland. Am J Epidemiol. 1994;139(1):64–76. doi: 10.1093/oxfordjournals.aje.a116936 [DOI] [PubMed] [Google Scholar]
- 16.Stanford JL, Hartge P, Brinton LA, Hoover RN, Brookmeyer R. Factors influencing the age at natural menopause. J Chronic Dis. 1987;40(11):995–1002. doi: 10.1016/0021-9681(87)90113-5 [DOI] [PubMed] [Google Scholar]
- 17.Magurský V, Mesko M, Sokolík L. Age at the menopause and onset of the climacteric in women of Martin District, Czechoslovkia. Statistical survey and some biological and social correlations. Int J Fertil. 1975;20(1):17–23. https://europepmc.org/article/med/4380. Accessed November, 10, 2022. [PubMed] [Google Scholar]
- 18.Castelo-Branco C, Blümel JE, Chedraui P, et al. Age at menopause in Latin America. Menopause. 2006;13(4):706–12. doi: 10.1097/01.gme.0000227338.73738.2d [DOI] [PubMed] [Google Scholar]
- 19.Gonzales GF, Villena A, De La Cruz D. Age of natural menopause among women in Lima City, Peru. Int J Gynaecol Obstet. 1997;57(1):69–72. doi: 10.1016/s0020-7292(96)02823-8 [DOI] [PubMed] [Google Scholar]
- 20.McCarthy T. The prevalence of symptoms in menopausal women in the Far East: Singapore segment. Maturitas. 1994;19(3):199–204. doi: 10.1016/0378-5122(94)90072-8 [DOI] [PubMed] [Google Scholar]
- 21.Samil RS, Wishnuwardhani SD. Health of Indonesian women city-dwellers of perimenopausal age. Maturitas. 1994;19(3):191–7. doi: 10.1016/0378-5122(94)90071-x [DOI] [PubMed] [Google Scholar]
- 22.Wasti S, Robinson SC, Akhtar Y, Khan S, Badaruddin N. Characteristics of menopause in three socioeconomic urban groups in Karachi, Pakistan. Maturitas. 1993;16(1):61–9. doi: 10.1016/0378-5122(93)90134-4 [DOI] [PubMed] [Google Scholar]
- 23.Christian C, Perko VL, Vanzhula I, Tregarthen JP, Forbush KT, Levinson CA. Eating disorder core symptoms and symptom pathways across developmental stages: A network analysis. J. Abnorm. Psychol 2020;129(2):177–90. doi: 10.1037/abn0000477 [DOI] [PubMed] [Google Scholar]
- 24.Monteleone AM, Cascino G. A systematic review of network analysis studies in eating disorders: Is time to broaden the core psychopathology to non specific symptoms. Eur. Eat. Disord. Rev 2021;29(4):531–47. doi: 10.1002/erv.2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? International J. Eat. Disord 1994;16(4):363–70. https://pubmed.ncbi.nlm.nih.gov/7866415/. Accessed August, 23, 2022. [PubMed] [Google Scholar]
- 26.Wang SB, Jones PJ, Dreier M, Elliott H, Grilo CM. Core psychopathology of treatment-seeking patients with binge-eating disorder: a network analysis investigation. Psychol. Med 2019;49(11):1923–8. doi: 10.1017/S0033291718002702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker JH, Runfola CD. Eating disorders in midlife women: A perimenopausal eating disorder? Maturitas. 2016;85:112–6. doi: 10.1016/j.maturitas.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 28.Bird JL, Oinonen KA. Elevated eating disorder symptoms in women with a history of oral contraceptive side effects. Arch. Womens Ment. Health 2011;14(4):345–53. doi: 10.1007/s00737-011-0229-z [DOI] [PubMed] [Google Scholar]
- 29.Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Xia K, Schmidt PJ, Girdler SS. Efficacy of Transdermal Estradiol and Micronized Progesterone in the Prevention of Depressive Symptoms in the Menopause Transition: A Randomized Clinical Trial. JAMA Psychiatry. 2018;75(2):149–57. doi: 10.1001/jamapsychiatry.2017.3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon JL, Rubinow DR, Watkins L, Hinderliter AL, Caughey MC, Girdler SS. The Effect of Perimenopausal Transdermal Estradiol and Micronized Progesterone on Markers of Risk for Arterial Disease. J. Clin. Endocrinol. Metab 2019;105(5):e2050–e60. doi: 10.1210/clinem/dgz262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zannas AS, Gordon JL, Hinderliter AL, Girdler SS, Rubinow DR. IL-6 Response to Psychosocial Stress Predicts 12-month Changes in Cardiometabolic Biomarkers in Perimenopausal Women. J. Clin. Endocrinol. Metab 2020;105(10):e3757–e65. doi: 10.1210/clinem/dgaa476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop +10: addressing the unfinished agenda of staging reproductive aging. Climacteric. 2012;15(2):105–14. doi: 10.1097/gme.0b013e31824d8f40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christian C, Williams BM, Hunt RA, et al. A network investigation of core symptoms and pathways across duration of illness using a comprehensive cognitive-behavioral model of eating-disorder symptoms. Psychol. Med 2020:1–10. doi: 10.1017/S0033291719003817 [DOI] [PubMed] [Google Scholar]
- 34.Levinson CA, Brosof LC, Vanzhula I, et al. Social anxiety and eating disorder comorbidity and underlying vulnerabilities: Using network analysis to conceptualize comorbidity. Int. J. Eat. Disord 2018;51(7):693–709. doi: 10.1002/eat.22890 [DOI] [PubMed] [Google Scholar]
- 35.Calugi S, Sartirana M, Misconel A, Boglioli C, Grave RD. Eating disorder psychopathology in adults and adolescents with anorexia nervosa: A network approach. Int. J. Eat. Disord 2020;53(5):420–31. doi: 10.1002/eat.23270 [DOI] [PubMed] [Google Scholar]
- 36.Berg KC, Peterson CB, Frazier P, Crow SJ. Psychometric Evaluation of the Eating Disorder Examination and Eating Disorder Examination-Questionnaire: A Systematic Review of the Literature. Int. J. Eat. Disord 2012;45(3):428–38. doi: 10.1002/eat.20931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.R Core Team. R: A language and environment for statistical computing. 4.0.0 ed. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 38.Epskamp S, Cramer AO, Waldorp LJ, Schmittmann VD, Borsboom D. qgraph: Network visualizations of relationships in psychometric data. J. Stat. Softw 2012;48(4):1–18. doi: 10.18637/jss.v048.i04 [DOI] [Google Scholar]
- 39.Friedman J, Hastie T, Tibshirani R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics. 2008;9(3):432–41. doi: 10.1093/biostatistics/kxm045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNally RJ. Can network analysis transform psychopathology?. Behav. Res. Ther 2016;86:95–104. doi: 10.1016/j.brat.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 41.Vanzhula IA, Calebs B, Fewell L, Levinson CA. Illness pathways between eating disorder and post‐traumatic stress disorder symptoms: Understanding comorbidity with network analysis. Eur. Eat. Disord. Rev 2019;27(2):147–60. doi: 10.1002/erv.2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Opsahl T, Agneessens F, Skvoretz J. Node centrality in weighted networks: Generalizing degree and shortest paths. Soc. Networks 2010;32(3):245–51. doi: 10.1016/j.socnet.2010.03.006 [DOI] [Google Scholar]
- 43.Newman M. Networks: An introduction. Oxford, England: Oxford University Press; 2010. [Google Scholar]
- 44.Epskamp S, Borsboom D, Fried EI. Estimating psychological networks and their accuracy: A tutorial paper. Behav. Res. Methods 2018;50(1):195–212. doi: 10.3758/s13428-017-0862-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Epskamp S, Fried EI. A tutorial on regularized partial correlation networks. Psychol. Methods 2018;23(4):617–34. doi: 10.1037/met0000167 [DOI] [PubMed] [Google Scholar]
- 46.Epskamp S, Maris GK, Waldorp LJ, Borsboom D. Network psychometrics. arXiv preprint. 2016; arXiv: 1609.02818. doi: 10.48550/arXiv.1609.02818 [DOI] [Google Scholar]
- 47.Epskamp S, Kruis J, Marsman M. Estimating psychopathological networks: Be careful what you wish for. PLOS One. 2017;12(6):e0179891. doi: 10.1371/journal.pone.0179891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker JH, Eisenlohr-Moul T, Wu Y, Schiller CE, Bulik CM, Girdler SS. Ovarian hormones influence eating disorder symptom variability during the menopause transition: A pilot study. Eat. Behav 2019;35:101337. doi: 10.1016/j.eatbeh.2019.101337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moses-Kolko EL, Fraser D, Wisner KL, et al. Rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biol Psychiatry. 2011;70(4):395–9. doi: 10.1016/j.biopsych.2011.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry. 2010;167(11):1373–80. doi: 10.1176/appi.ajp.2010.09081235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silverman ME, Loudon H, Safier M, et al. Neural dysfunction in postpartum depression: an fMRI pilot study. CNS Spectr. 2007;12(11):853–62. doi: 10.1017/s1092852900015595 [DOI] [PubMed] [Google Scholar]
- 52.Schiller CE, Walsh E, Eisenlohr-Moul TA, et al. Effects of gonadal steroids on reward circuitry function and anhedonia in women with a history of postpartum depression. J. Affect. Disord 2022;314:176–84. doi: 10.1016/j.jad.2022.06.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avena NM, Bocarsly ME. Dysregulation of brain reward systems in eating disorders: neurochemical information from animal models of binge eating, bulimia nervosa, and anorexia nervosa. Neuropharmacol. 2012;63(1):87–96. doi: 10.1016/j.neuropharm.2011.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monteleone AM, Castellini G, Volpe U, et al. Neuroendocrinology and brain imaging of reward in eating disorders: A possible key to the treatment of anorexia nervosa and bulimia nervosa. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80(Pt B):132–42. doi: 10.1016/j.pnpbp.2017.02.020 [DOI] [PubMed] [Google Scholar]
- 55.Berner LA, Brown TA, Lavender JM, Lopez E, Wierenga CE, Kaye WH. Neuroendocrinology of reward in anorexia nervosa and bulimia nervosa: Beyond leptin and ghrelin. Mol Cell Endocrinol. 2019;497:110320. doi: 10.1016/j.mce.2018.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frank GK, Shott ME, Hagman JO, Mittal VA. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry. 2013;170(10):1152–60. doi: 10.1176/appi.ajp.2013.12101294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivera HM, Stincic TL. Estradiol and the control of feeding behavior. Steroids. 2018;133:44–52. doi: 10.1016/j.steroids.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J. Clin. Endocrinol. Metab 1996;81(4):1495–501. doi: 10.1210/jcem.81.4.8636357 [DOI] [PubMed] [Google Scholar]


