Abstract
Background:
Release of neutrophil extracellular traps (NETosis) may mediate post-injury organ dysfunction, but mechanisms remain unclear. The intracellular serine protease inhibitor (serpin) B1 is vital to neutrophil function and has been shown to restrict NETosis in inflammatory settings. In this study, we utilized discovery proteomics to identify the proteomic signature of trauma-induced NETosis. We hypothesized that serpinB1 would be a major component of this NET protein profile and associated with adverse outcomes.
Methods:
This was a post hoc analysis of data collected as part of the COMBAT randomized clinical trial. Blood was collected from injured patients at a single Level I Trauma Center. Proteomic analyses were performed through targeted liquid chromatography coupled with mass spectrometry. Abundances of serpinB1 and known NETosis markers were analyzed with patient and injury characteristics, clinical data, and outcomes.
Results:
SerpinB1 levels on ED arrival were significantly correlated with proteomic markers of NETosis, including core histones, transketolase, and S100A8/A9 proteins. More severely injured patients had elevated serpinB1 and NETosis markers on ED arrival. Levels of serpinB1 and top NETosis markers were significantly elevated on ED arrival in non-survivors and patients with fewer ventilator- and ICU-free days. In proteome-wide ROC analysis, serpinB1 was consistently among the top proteins associated with adverse outcomes. Among NETosis markers, levels of serpinB1 early in the patient’s course exhibited the greatest separation between patients with fewer and greater ventilator- and ICU-free days. Gene Ontology analysis of top predictors of adverse outcomes further supports NETosis as a potential mediator of post-injury organ dysfunction.
Conclusions:
We have identified a proteomic signature of trauma-induced NETosis, and NETosis is an early process following severe injury that may mediate organ dysfunction. In addition, serpinB1 is a major component of this NET protein profile that may serve as an early marker of excessive NETosis after injury.
Evidence:
Level III, Prognostic/Epidemiological
Keywords: NETosis, SerpinB1, Proteomics, Trauma, Injury
Introduction
Trauma remains a leading cause of morbidity and mortality worldwide [1]. Many severely injured patients who survive the initial stabilization period experience a dysregulated innate immune response to injury that leads to subsequent organ dysfunction and adverse outcomes. Neutrophils are a major component of this innate immune response and believed to play a key role in the pathology of inflammatory complications in trauma [2–5]. One mechanism by which neutrophils have been proposed to mediate post-injury sterile inflammation is via release of neutrophil extracellular traps (NETs) [5–8]. This phenomenon, termed NETosis, involves an apoptosis-like process of nuclear envelope dissolution with extrusion of decondensed chromatin decorated with histones and other bactericidal proteins and proteases [9–12]. First described in 2004 as an antimicrobial effort, a growing body of evidence now suggests NETs may modulate the inflammatory and immune responses after injury, aggravating tissue damage and contributing to organ dysfunction [6,7,10]. Despite this body of work, the role of NETosis in trauma as well as the underlying mechanisms of NET formation in this setting remain poorly understood [13–14].
SerpinB1, also called “neutrophil (or leukocyte) elastase inhibitor”, is an intracellular serine protease inhibitor (serpin) that is vital to neutrophil function. Despite its importance in innate immunity, serpinB1 has been a subject of very limited investigation in trauma. Located primarily in the cytosol of neutrophils, serpinB1 protects the cell from enzymatic destruction by its own proteases [15]. Remold-O’Donnell et al. elegantly demonstrated this with neutrophils in serpinB1-knockout mice exhibiting significantly decreased viability and immune function in the setting of acute pulmonary injury [16]. Multiple studies have also shown that serpinB1 is protective in inflammatory lung and bowel diseases and suggested it plays a broader regulatory role within the neutrophil to limit inflammation and improve clinical outcomes [15,17–20]. While the specific mechanisms underlying serpinB1’s protective and immuno-regulatory effects remain elusive, there is evidence that NETosis is involved. Prior in vitro and in vivo findings suggest serpinB1 has a vital role in restricting NET production. SerpinB1-deficient neutrophils produce increased NETs, and serpinB1-knockout mice infected with bacterial and viral pathogens experience protracted inflammation and early mortality associated with increased NETosis [17,20–22]. The crucial, regulatory step appears to take place prior to NET formation and inside the nucleus where serpinB1 migrates and restricts chromatin decondensation [22–23]. The details of this process, however, are not clear.
Much remains unknown regarding the roles and interplay of serpinB1 and NETs in human disease; even less is known about how these innate immune mediators contribute specifically to trauma pathology and how this manifests in measures of serpinB1 and other NET-associated proteins in the plasma of severely injured patients. Levels of serpinB1 in the circulating compartment have been found elevated in chronic disease states, such as diabetes, but plasma serpinB1 has not been investigated as an acute marker and potential mediator of dysregulated innate immunity following traumatic injury [24–25]. Furthermore, a NET-focused proteomics analysis of trauma patients has not been reported prior. Therefore, the present study employed discovery proteomics to capture the proteomic signature of NETosis and measure association with plasma serpinB1 in severely injured patients. Given that NETosis has been observed immediately following injury, we hypothesized that the early proteomic signature of NETosis would define trauma patients by injury severity and clinical trajectory and that serpinB1 would be a major component of this trauma-associated NET profile [13].
Methods
This study was a post hoc analysis of data collected as part of the Control Of Major Bleeding After Trauma (COMBAT) trial, a single center, randomized clinical trial (NCT01838863). Institutional Review Board approval of our research protocol was obtained under exemption from informed consent requirements for emergency research (21 CFR 50.24) [26]. The STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE) checklist was used to ensure abidance to the Enhancing the QUAlity and Transparency Of Health Research (EQUATOR) guidelines for reporting observational studies (SDC 1: STROBE Checklist). Adherence to the reporting standards for clinical trials and a CONSORT flow diagram have been previously published for the COMBAT trial [27].
Patients and Sample Collection
The inclusion criteria for the COMBAT trial have been previously published [27–28]. Briefly, adult trauma patients with hypotension in the field due to hemorrhage were included, and blood samples were collected in the field and immediately upon emergency department (ED) arrival, followed by samples at 2, 4, 6, 12, and 24 hours. Samples collected at timepoints beyond the ED were only used in temporal trend analyses, and non-survivors were excluded in these analyses to avoid survival bias. Therefore, the number of patient samples at each timepoint were as follows: 107 at ED (including non-survivors), 94 at ED (survivors only), 68 at 2 hours, 82 at 4 hours, 83 at 6 hours, 73 at 12 hours, and 68 at 24 hours. An appropriately matched cohort of 100 prospectively collected healthy volunteers was used as a control group.
Sample Measurement (Proteomics and Metabolomics)
Whole blood samples were collected on ice, centrifuged to yield platelet-free plasma, and flash frozen in liquid nitrogen as previously described [27]. Banked plasma samples were digested with trypsin in the S-Trap 96-well plate (Protifi, Huntington, NY) after reduction and alkylation. Each sample was loaded onto individual Evotips for desalting and washing. The Evosep One system (Evosep, Odense, Denmark) was used to separate peptides on a Pepsep column. The system was coupled to the timsTOF Pro mass spectrometer (Bruker Daltonics, Bremen, Germany) via the nano-electrospray ion source (Captive Spray, Bruker Daltonics). Raw data files were converted to peak lists and queried for peptide identification against the human proteome (Uniprot database) using Spectronaut (Biognosys, Schlieren, Switzerland). Peptide identifications were summed (area under the curve) to generate protein level abundance quantities. Metabolites were extracted from plasma samples for mass spectrometric analysis using previously described methods [29]. Briefly, metabolites were extracted using a methanol, acetonitrile, and water (5:3:1) solution. Extracts were vortexed vigorously and centrifuged to pellet insoluble fractions. Supernatants were transferred to autosampler vials and loaded into a Vanquish UHLPC coupled to a Q-Exactive mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) with a Kinetex C18 column (Phenomenex). Raw data was processed using Maven (Princeton University, Princeton, NJ, USA) equipped with the KEGG database.
Outcome Measures
Patients were followed for 28 days. Outcome measures included mortality as well as 28-day ventilator- and ICU-free days (VFD and IFD, respectively). Massive transfusion (MT) was defined as >10 RBC units within six hours post-injury or death within six hours post-injury after receiving at least one RBC unit. TBI was defined as AIS head or neck ≥3.
Variables of Interest
Several investigators have contributed to the identification of the proteomic signature of NETosis, and this prior work has facilitated our identification of NETosis markers in our proteome-wide analyses [30–32]. We also mined our proteomics and metabolomics data for known protein markers of tissue damage as well as metabolites associated with hypoperfusion in the setting of hemorrhagic shock to investigate the individual contributions of tissue injury and hypoperfusion [33–34]. To further characterize patients by injury severity and degree of hemorrhagic shock, we included the new injury severity score (NISS) and the clinical laboratory value of the arterial base excess (BE) measured on ED arrival. For some of our analyses, patients were stratified into “shock/injury phenotype” groups defined by combinations of NISS and BE using the cutoffs NISS≥25 versus <25 and BE<−10 vs ≥−10 mEq/L; these cutoffs were based on previous studies, including the original description of the COMBAT randomized controlled trial [27].
Statistical Analysis
All statistical analyses were performed using R version 4.1.0 (2021-05-18). Spearman correlations, Kruskal-Wallis tests, Dunn’s tests with Bonferroni P-value adjustment, and Mann-Whitney tests were used as appropriate. For this data, absolute values of Spearman’s rho >0.70 were considered very strong correlations, 0.50 to 0.70 strong, 0.30 to 0.50 moderate, and 0.00 to 0.30 weak/negligible. Simple logistic regression with receiver operating characteristic (ROC) curve analyses were employed proteome-wide to evaluate the performance of individual protein measures on ED arrival as predictors of adverse outcomes. The 100 proteins with the highest areas under the curve (AUC) were considered “top predictors”. To categorize patients into low and high VFD and IFD, the median VFD and IFD were used as cutoffs. In addition, for each outcome, the top 100 proteins from ROC analysis were selected for Gene Ontology (GO) analysis to identify enriched biological processes [35–37].
Results
There were 118 (94%) patients of the 125 patients included in the COMBAT trial with proteomics data for analysis, and of those 118, there were 107 (91%) with plasma samples at the ED time-point. Based on eligibility criteria for the study, this was a severely injured patient cohort with median NISS 27 and BE −9.0 mEq/L. While roughly half of patients were randomized to receive two units of pre-hospital fresh frozen plasma (FFP) as part of the COMBAT trial, omics analyses demonstrated no differences between COMBAT study groups for the NETosis markers, tissue damage proteins, and shock-associated analytes included in this study. Therefore, the two COMBAT study groups were combined into a single entity for the analyses detailed subsequently. Additional patient characteristics and outcomes can be viewed in Table 1.
Table 1.
Baseline characteristics and outcomes for patients with plasma samples at the ED timepoint. TBI (Traumatic Brain Injury), NISS (New Injury Severity Score), IQR (Interquartile Range), ED (Emergency Department), BE (Base Excess), RBC (Red Blood Cell), FFP (Fresh Frozen Plasma), VFD (Ventilator-free Days), IFD (ICU-free Days).
| VARIABLE | Total (N=107) |
|---|---|
| Received pre-hospital plasma * | 56 (52%) |
| Median Age in years (IQR) | 33 (26-49) |
| Male Sex | 86 (80%) |
| Penetrating Mechanism | 52 (49%) |
| TBI Present | 29 (27%) |
| Median NISS (IQR) | 27 (11-41) |
| Median ED BE in mEq/L (IQR) | −9.0 (−13.0 to −5.75) |
| Missing ED BE | 14 (13%) |
| Shock/Injury Phenotypes | |
| Group 1 (NISS<25, BE≥−10) | 24 (22%) |
| Group 2 (NISS<25, BE<−10) | 14 (13%) |
| Group 3 (NISS≥25, BE≥−10) | 29 (27%) |
| Group 4 (NISS≥25, BE<−10) | 26 (24%) |
| Required Massive Transfusion | 25 (23%) |
| Median RBC units in 6hr (IQR) | 2 (0-8) |
| Median FFP units in 6hr (IQR) | 2 (1-6) |
| Median VFD (IQR) | 27 (14-28) |
| Median IFD (IQR) | 25 (10-26) |
| Mortality | 13 (12%) |
Randomized to receive two units of pre-hospital fresh frozen plasma as part of COMBAT trial.
Proteome-wide Spearman’s correlations with serpinB1 demonstrated significant associations between ED levels of serpinB1 and known proteomic markers of NETosis (Figure 1). Top protein correlates included core histones, transketolase, and S100A8/A9 proteins. Less strong but still significant correlations at this early timepoint included additional, classic NETosis markers, neutrophil defensin and myeloperoxidase. To further support serpinB1 as a specific marker of NETosis in these injured patients, fibrinogen alpha chain, a biomarker used in trauma but not associated with NETosis, demonstrated zero significant associations with NETosis proteins. In addition to NETosis proteins, serpinB1 on ED arrival was significantly correlated with plasma malate dehydrogenase, actin, and myoglobin, which are markers of tissue damage. SerpinB1 and the NET protein correlates were also all strongly correlated with each other at the ED timepoint and subsequent timepoints throughout the first 24 hours, though most strongly in the first 6 hours, further supporting their involvement in a common post-injury process (SDC 2: Protein and metabolite correlograms). This latter correlation analysis over 24 hours also demonstrated that, overall, NETosis markers, including serpinB1, correlated more strongly with NISS and tissue damage proteins than BE or markers of hypoperfusion/hemorrhagic shock, lactate and succinate. A complete list of NETosis and tissue damage protein correlates with serpinB1 can be viewed in Panel B of Figure 1.
Figure 1.

Proteome-wide Spearman’s correlations with serpinB1 for trauma patient plasma samples collected on ED arrival demonstrating significant correlations with NETosis and tissue damage markers. A: U-plot of proteome-wide Spearman’s correlations with serpinB1 with the X-axis displaying the correlation coefficient and the Y-axis displaying the the -log10 transformed P-value for each correlation. Significant (P<0.05) positive correlates are displayed in blue color while insignificant correlates are colored grey. The top significant positive protein correlates were previously identified NETosis and tissue damage markers, which are labeled on the U-plot. B: Table of all included NETosis and tissue damage markers included in this study with most identified on the U-plot in panel A. The table includes the gene name, description, and Uniprot accession number.
Stratifying patients into groups (“phenotypes”) by injury severity and hemorrhagic shock on arrival, more severely injured patients had higher levels of serpinB1 and NETosis markers on ED arrival (Figure 2). This same pattern was also observed for the tissue damage protein myoglobin. Among those severely injured (NISS≥25), there was no statistical difference between patients with and without hemorrhagic shock, further suggesting a weaker influence of hypoperfusion on post-injury NETosis than tissue damage. Analyses of additional NETosis markers and tissue damage proteins by shock/injury phenotypes are consistent with those exhibited in Figure 2 (SDC 3: Extended NETosis and tissue damage markers by shock/injury phenotype).
Figure 2.

SerpinB1, NETosis markers, and the tissue damage protein myoglobin (MB) are elevated on ED arrival in severely injured patients. Stratification by shock/injury (S/I) phenotypes as defined in Table 1 (Groups 1-4) as well as comparisons with levels in healthy volunteers demonstrate that patients with severe injury (NISS≥25, Groups 3 and 4) have higher serpinB1 and evidence of increased NETosis on ED arrival. In parallel with this pattern of NETosis, MB as a marker of tissue damage was also elevated in more severely injured patients. Lack of significant difference between Groups 3 and 4 may suggest severity of tissue damage is more a driver of post-injury NETosis than hypoperfusion. Displayed proteins in this figure were selected based on having the greatest statistical significance in comparisons between S/I phenotypes and healthy volunteers in Kruskal-Wallis tests; see Panel B of Figure 1 for protein identification. Abundance quantity refers to the label-free quantification of protein level. Plots contain boxes displaying the median and interquartile range (IQR) with whiskers denoting the highest and lowest values within 1.5xIQR; values outside this whisker range were considered outliers and indicated by individual points.
****P<0.0001, ***P<0.001, **P<0.01, *P<0.05
Plasma levels of serpinB1 and top NETosis markers were significantly elevated on ED arrival in non-survivors, patients with fewer than 27 VFD, and patients with fewer than 25 IFD (Figures 3–5, Panel A). Additionally, patients meeting MT criteria had significantly higher serpinB1 and NET proteins on ED arrival (SDC 4: Elevated NETosis markers are associated with massive transfusion). In proteome-wide receiver operating characteristic (ROC) curve analysis, NETosis proteins were top predictors of mortality, having fewer than 27 VFD, and having fewer than 25 IFD (Figure 3–5, Panel B). NETosis markers were also top predictors for meeting MT criteria (SDC 4: Elevated NETosis markers are associated with massive transfusion). SerpinB1 was consistently among these top proteins associated with adverse outcomes. In multiple logistic regression, elevated serpinB1 on ED arrival remained significantly associated with death, <27 VFD, and <25 IFD after adjustment for BE (all P<0.01) but not after adjustment for NISS, consistent with our other findings suggesting a stronger contribution of tissue injury to NETosis than hemorrhagic shock.
Figure 3.
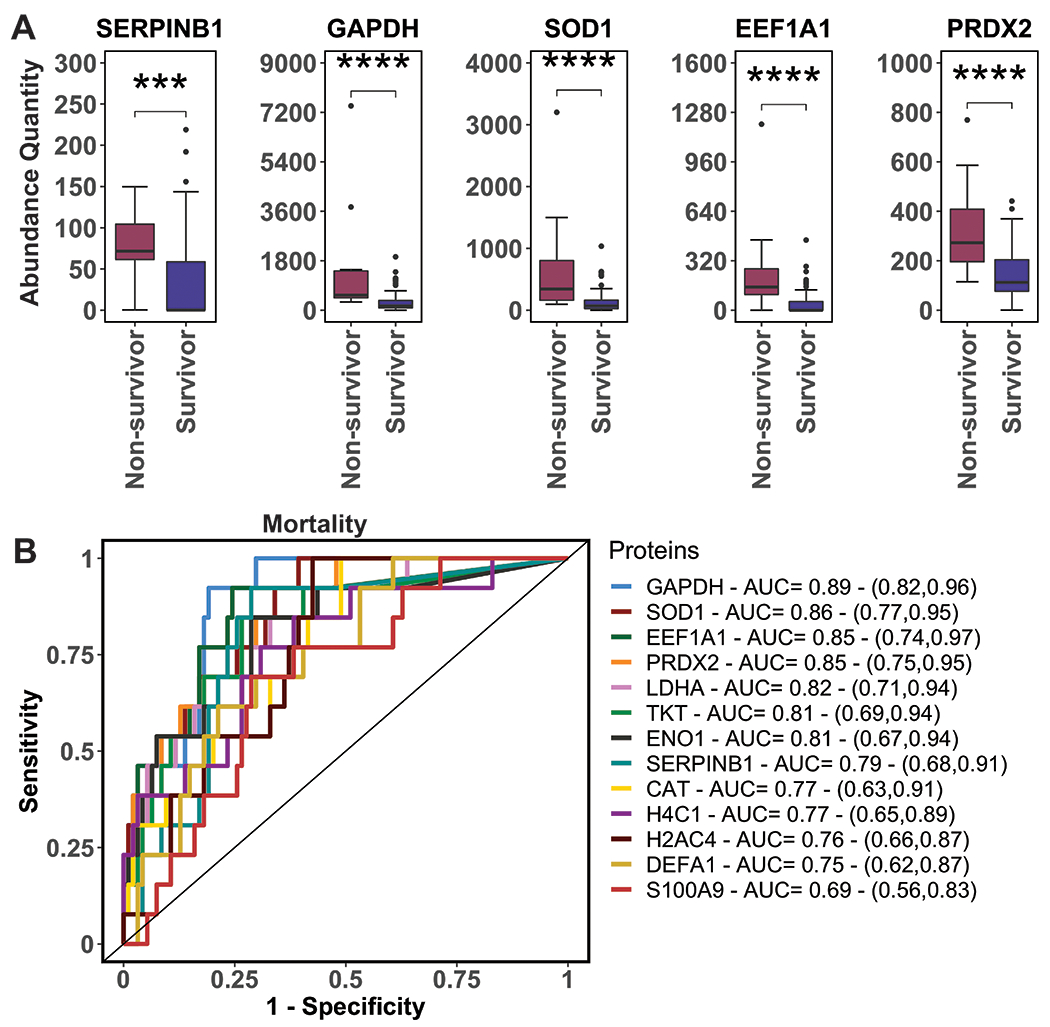
Elevated SerpinB1 and NETosis markers on ED arrival are associated with mortality. A: SerpinB1 and NETosis markers are elevated on ED arrival in non-survivors versus survivors. Displayed proteins were those with the greatest statistical significance in Mann-Whitney tests between non-survivors and survivors; see Panel B of Figure 1 for protein identification. Abundance quantity refers to the label-free quantification of protein level. Plots contain boxes displaying the median and interquartile range (IQR) with whiskers denoting the highest and lowest values within 1.5xIQR; values outside this whisker range were considered outliers and indicated by individual points. B: Receiver operating characteristic (ROC) curves from proteome-wide ROC analysis of top predictors of mortality based on levels measured on ED arrival. The legend shows corresponding areas under the curves (AUC) with 95% confidence intervals. Displayed proteins were NETosis markers with the highest AUC’s among top predictors of mortality. ***P<0.001, ****P<0.0001
Figure 5.

Elevated SerpinB1 and NETosis markers on ED arrival are associated with longer ICU stay. A: SerpinB1 and NETosis markers are elevated on ED arrival in patients with fewer than 25 (median) ICU-free days (IFD) versus those with 25 or more IFD. Displayed proteins were those with the greatest statistical significance in Mann-Whitney tests between groups; see Panel B of Figure 1 for protein identification. Abundance quantity refers to the label-free quantification of protein level. Plots contain boxes displaying the median and interquartile range (IQR) with whiskers denoting the highest and lowest values within 1.5xIQR; values outside this whisker range were considered outliers and indicated by individual points. B: Receiver operating characteristic (ROC) curves from proteome-wide ROC analysis of top predictors of having <25 IFD based on levels measured on ED arrival. The legend shows corresponding areas under the curves (AUC) with 95% confidence intervals. Displayed proteins were NETosis markers with the highest AUC’s among top predictors of having <25 IFD.
****P<0.0001
Temporal trend analysis revealed that, while all NETosis markers were elevated early, among them, levels of serpinB1 early in the patient’s course exhibited the greatest separation between patients with fewer and greater VFD and IFD (SDC 5: NET protein temporal trends by ≥27 vs <27 ventilator-free days and SDC 6: NET protein temporal trends by ≥25 vs <25 ICU-free days). Specifically, serpinB1 levels in patients with ≥27 VFD and ≥25 IFD were nearly undetectable and no different than healthy volunteer levels at all timepoints over 24 hours, yet levels in patients with fewer VFD and IFD were highest at the ED timepoint and dropped to healthy volunteer level by four hours. This trajectory of serpinB1 differed from that of the other NETosis markers in that the other markers remained elevated longer in patients with fewer VFD and IFD, and several were persistently elevated above healthy control level even among patients with ≥27 VFD and ≥25 IFD. Similar patterns were also observed for markers of tissue destruction. These temporal trends can be viewed in the context of the acute phase response as indicated by the rise in C-reactive protein from 6-24 hours.
In Gene Ontology (GO) analysis of the top predictors from the proteome-wide ROC curve analyses for the above clinical outcomes, enriched biological processes in patients with adverse outcomes included those linked to NETosis (SDC 7: Gene Ontology (GO) Analysis for top predictors of adverse outcomes). Enriched processes involved neutrophil activation, hydrogen peroxide metabolism, cellular processing of superoxide radical, and responses to pro-inflammatory cytokines, all further supporting an important role of NETosis in mediating post-injury organ dysfunction.
Discussion
In this study, we have identified a proteomic signature of trauma-induced NETosis, of which the neutrophil intracellular serine protease inhibitor serpinB1 is a major component. These data suggest that NETosis is an early process after injury, likely driven more by tissue damage than hemorrhagic shock, and injury has a dose-dependent effect on NETosis. Furthermore, NETosis was associated with mortality, longer ventilator requirement, and greater ICU utilization in injured patients, providing evidence for an influential role of this early post-injury process in mediating organ dysfunction after trauma. From this study, serpinB1 emerges as an important NETosis biomarker; its contribution to the early proteomic signature of NETosis, early elevations in more critical patients, and ability to differentiate clinical trajectory early in the injured patient’s course are consistent with a vital function upstream of NET formation and suggest it may be a specific, early plasma marker of excessive post-injury NETosis.
The best method for quantifying NETosis in plasma samples has yet to be established. Standardized assays for citrullinated histones are lacking, which has contributed to variable results in studying NETosis. Likewise, circulating cell-free DNA is not specific to NET formation, which adds to the challenge of investigating this process [13,38]. More recently, proteomic methods have been employed as a more global, integrative approach to studying NETosis in various inflammatory and infectious disease processes [9,31,39–40]. However, until now, no prior study has utilized modern mass spectrometry-based proteomics to analyze NETosis in severely injured trauma patients. Prior work has demonstrated that different insults and inflammatory milieus can produce different NET protein profiles, which can affect NET functions and the host response [9,32]. For example, Chapman et. al. found that, in a comparison of two autoimmune disorders, serpinB1 was significantly higher in the NET protein profiles in lupus patients versus rheumatoid arthritis patients [9]. With a characterization of the proteome of trauma-induced NETosis, we may better understand the drivers of this process and be able to translate knowledge gained from studies of non-trauma inflammatory disease states with similar NET protein profiles to studies of post-injury inflammation.
The presented data are consistent with serpinB1 serving a critical function early in the NET formation process, and elevations in the plasma on ED arrival suggest already rampant NETosis in the severely injured patient. SerpinB1 normally functions intracellularly to restrict chromatin decondensation within the nucleus, thereby inhibiting an initial step in NET formation [22–23]. Detectably high levels in the plasma, therefore, may indicate a massive NETosis stimulus that has overwhelmed regulatory mechanisms. The fact that more severely injured patients and those who develop organ dysfunction have higher serpinB1 plasma levels early after injury may support the use of serpinB1 as an early biomarker of this potentially maladaptive, innate inflammatory process in the injured patient. Additionally, we show that serpinB1 and markers of NETosis are more associated with tissue damage proteins and injury severity than measures of hemorrhagic shock or metabolic markers of hypoperfusion. The influence of tissue damage is not surprising as damage-associated molecular patterns (DAMPs) are known, potent stimulators of NET release via activation of pattern recognition receptors on neutrophils [4,14]. While hemorrhagic shock has been shown to be a primary force in multiple pathologic processes after injury, our data suggest it may have less of an impact on NET formation. Further study is needed to elucidate the individual contribution of tissue injury and DAMPs, but our work here sheds light on potential mediators of trauma-induced NETosis.
As NETosis is already underway upon ED arrival and this early process is associated with adverse outcomes, intervention upstream of NET formation and release is not a practical therapeutic strategy in trauma. However, there are multiple promising countermeasures to NETosis that may have utility in the post-injury setting. Deoxyribonucleases (DNAses) break down NET structure, and recombinant DNAse infusion has been shown to reduce NET burden, improve microvascular perfusion, and decrease end-organ damage in mouse models of sepsis [41]. DNAses have also been suggested as potential counter-regulators of aberrant NETosis in trauma patients [42]. Additionally, extracellular histones, major components of NETs and released in high quantities during massive NETosis, are extremely cytotoxic; neutralization of histones has been shown to attenuate inflammation and limit organ dysfunction in models of sepsis and both trauma-associated and non-trauma lung injury [43–45]. Targeting other toxic granule proteins attached to the NET scaffold may be less desirable for risk of dismantling essential antimicrobial defenses and predisposing patients to infectious complications. In general, the harm/benefit ratio of NET inhibition remains unclear and requires further investigation.
While advancing our understanding of the pathomechanisms of trauma, augmenting our granularity for clinical outcome prediction, and illuminating potential therapeutic targets, this study has generated additional hypotheses to direct future research in the area of innate immune responses to injury. Future analyses may unveil specific DAMPs and soluble mediators responsible for the massive NETosis induced by traumatic injury. Additionally, induction of NETosis in the local environment of an endothelial cell monolayer in our in vitro permeability assays may further characterize the mechanism of NET-driven post-injury organ dysfunction. Animal models may be necessary to determine the individual contributions of tissue injury and hemorrhagic shock on NETosis as well as explore the impact on the NET proteome and the therapeutic potential of targeting NET-derived extracellular histones and removing NETs with DNAse.
This study has several limitations. Broadly, we are observing correlative human data analyzed retrospectively and therefore are unable to control for all variables contributing to illness severity and medical decision-making. Additionally, while we found no differences in NETosis markers, tissue damage proteins, or hemorrhagic shock-associated metabolites between patients who received pre-hospital FFP and those who did not, we did not analyze the impact of plasma resuscitation on NET proteomics, and our inclusion of both COMBAT study groups as a single entity is a potential limitation of this study. MT can also influence outcomes, such as mortality and parameters of lung injury, and we did not account for any independent contribution of MT on outcomes, which presents another limitation of this analysis. This study and others analyzing NETosis are also limited by the overlap of NET markers and tissue damage proteins. To further complicate this overlap, post-injury NETosis may cause further tissue damage, blurring the distinction between mediators and consequences of NETs in trauma. For these reasons, well-designed in vitro and in vivo experiments, as described above, are needed. Nonetheless, our global, proteome-wide analyses provide compelling evidence that tissue damage and NETosis are integrally linked processes after injury. We also did not investigate post-translational modifications (PTMs) of NET proteins, which have been observed and may impact NET function [9]. Future analyses using peptide-level proteomics will help identify these PTMs and determine their role in NET biology. Lastly, we did not use any of the previously published, non-proteomics methods for measuring NETosis in patient samples, including assays for cell-free DNA and citrullinated histone H3. These parallel analyses to compare with our proteomic signature, while lacking here and thus limiting this study, will be included in future investigations.
In summary, we have identified a proteomic signature of trauma-induced NETosis, and NETosis is an early process following severe injury that may be an important mediator of organ dysfunction. We further describe serpinB1 as a major component of this NET protein profile that may serve as an early biomarker of excessive NETosis after injury. Release of DAMPs from tissue damage may be more influential than hemorrhagic shock and hypoperfusion in provoking this innate immune response to trauma. Finally, our findings shed light on potential therapeutic targets and will guide future studies on the inflammo-pathology of trauma.
Supplementary Material
Figure 4.

Elevated SerpinB1 and NETosis markers on ED arrival are associated with longer ventilator requirement. A: SerpinB1 and NETosis markers are elevated on ED arrival in patients with fewer than 27 (median) ventilator-free days (VFD) versus those with 27 or more VFD. Displayed proteins were those with the greatest statistical significance in Mann-Whitney tests between groups; see Panel B of Figure 1 for protein identification. Abundance quantity refers to the label-free quantification of protein level. Plots contain boxes displaying the median and interquartile range (IQR) with whiskers denoting the highest and lowest values within 1.5xIQR; values outside this whisker range were considered outliers and indicated by individual points. B: Receiver operating characteristic (ROC) curves from proteome-wide ROC analysis of top predictors of having <27 VFD based on levels measured on ED arrival. The legend shows corresponding areas under the curves (AUC) with 95% confidence intervals. Displayed proteins were NETosis markers with the highest AUC’s among top predictors of having <27 VFD. ****P<0.0001
Funding Disclosure and Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health (T32 GM008315 and P50 GM049222) and the Department of Defense (USAMRAA, W81XWH-12-2-0028). The Control of Major Bleeding after Trauma (COMBAT W81XWH-12-2-2008) was funded by the Department of Defense. Other important funding sources include a series of grants through the Trans-agency Research Consortium for Trauma Induced Coagulopathy (TACTIC UM1-HL120877) from the National Heart, Lung and Blood Institute, NIH. The current major funding source is an RM-1 grant (1RM1GM131968-01) that runs through May of 2024. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other sponsors of the project.
Footnotes
Supplemental Digital Content (SDC):
SDC for this manuscript is comprised of seven image files. In addition to the STROBE reporting guidelines checklist, these graphics include correlograms of NETosis markers over the first 24 hours, extended NETosis marker analysis by shock/injury phenotypes and massive transfusion criteria, temporal trend comparisons between patients with fewer and greater ventilator- and ICU-free days, and Gene Ontology analyses for outcomes. The figures supplement descriptions of data in the Results section. Complete SDC figure legends can be viewed at the end of this document.
Conflict of Interest Statement: The authors report no conflicts of interest.
References
- 1.Injuries and violence [Internet]. Who.int. World Health Organization; 2021. [cited 2022 Jul 17]. Available from: https://www.who.int/news-room/fact-sheets/detail/injuries-and-violence [Google Scholar]
- 2.Schenck EJ, Ma KC, Murthy SB, Choi AMK. Danger signals in the ICU. Crit Care Med. 2018;46(5):791–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hesselink L, Spijkerman R, van Wessem KJP, Koenderman L, Leenen LPH, Huber-Lang M, et al. Neutrophil heterogeneity and its role in infectious complications after severe trauma. World J Emerg Surg. 2019;14(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finlay LDB, Conway Morris A, Deane AM, Wood AJT. Neutrophil kinetics and function after major trauma: A systematic review. World J Crit Care Med. 2021;10(5):260–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hazeldine J, Hampson P, Lord JM. The impact of trauma on neutrophil function. Injury. 2014;45(12):1824–33 [DOI] [PubMed] [Google Scholar]
- 6.Kovtun A, Messerer DAC, Scharffetter-Kochanek K, Huber-Lang M, Ignatius A. Neutrophils in tissue trauma of the skin, bone, and lung: Two sides of the same coin. J Immunol Res. 2018;2018:8173983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu F-C, Chuang Y-H, Tsai Y-F, Yu H-P. Role of neutrophil extracellular traps following injury. Shock. 2014;41(6):491–8 [DOI] [PubMed] [Google Scholar]
- 8.Margraf S, Lögters T, Reipen J, Altrichter J, Scholz M, Windolf J. Neutrophil-derived circulating free DNA (cf-DNA/NETs): a potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock. 2008;30(4):352–8 [DOI] [PubMed] [Google Scholar]
- 9.Chapman EA, Lyon M, Simpson D, Mason D, Beynon RJ, Moots RJ, et al. Caught in a trap? Proteomic analysis of neutrophil Extracellular Traps in rheumatoid arthritis and systemic lupus erythematosus. Front Immunol. 2019;10:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5 [DOI] [PubMed] [Google Scholar]
- 11.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18(4):581–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goswami J, MacArthur T, Bailey K, Spears G, Kozar RA, Auton M, et al. Neutrophil extracellular trap formation and syndecan-1 shedding are increased after trauma. Shock. 2021;56(3):433–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuboly E, Briggs GD, Balogh ZJ. The role of neutrophil extracellular traps in post-injury inflammation. In: Khajah MA, editor. Role of Neutrophils in Disease Pathogenesis. London: InTech; 2017 [Google Scholar]
- 15.Torriglia A, Martin E, Jaadane I. The hidden side of SERPINB1/Leukocyte Elastase Inhibitor. Semin Cell Dev Biol. 2017;62:178–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benarafa C, LeCuyer TE, Baumann M, Stolley JM, Cremona TP, Remold-O’Donnell E. SerpinB1 protects the mature neutrophil reserve in the bone marrow. J Leukoc Biol. 2011;90(1):21–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benarafa C, Priebe GP, Remold-O’Donnell E. The neutrophil serine protease inhibitor serpinb1 preserves lung defense functions in Pseudomonas aeruginosa infection. J Exp Med. 2007;204(8):1901–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi YJ, Kim S, Choi Y, Nielsen TB, Yan J, Lu A, et al. SERPINB1-mediated checkpoint of inflammatory caspase activation. Nat Immunol. 2019;20(3):276–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooley J, Takayama TK, Shapiro SD, Schechter NM, Remold-O’Donnell E. The serpin MNEI inhibits elastase-like and chymotrypsin-like Serine proteases through efficient reactions at two active sites. Biochemistry. 2001;40(51):15762–70 [DOI] [PubMed] [Google Scholar]
- 20.Gong D, Farley K, White M, Hartshorn KL, Benarafa C, Remold-O’Donnell E. Critical role of serpinB1 in regulating inflammatory responses in pulmonary influenza infection. J Infect Dis. 2011;204(4):592–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farley K, Stolley J, Cooley J, Remold-O’Donnell E. SerpinB1 functions in generating neutrophil extracellular traps. J Immunol. 2011. Apr 1;186(1 Supplement) [Google Scholar]
- 22.Farley K, Stolley JM, Zhao P, Cooley J, Remold-O’Donnell E. A serpinB1 regulatory mechanism is essential for restricting neutrophil extracellular trap generation. J Immunol. 2012;189(9):4574–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majewski P, Majchrzak-Gorecka M, Grygier B, Skrzeczynska-Moncznik J, Osiecka O, Cichy J. Inhibitors of Serine proteases in regulating the production and function of neutrophil extracellular traps. Front Immunol. 2016;7:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takebayashi K, Hara K, Terasawa T, Naruse R, Suetsugu M, Tsuchiya T, et al. Circulating SerpinB1 levels and clinical features in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2016;4(1):e000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glicksman M, Asthana A, Abel BS, Walter MF, Skarulis MC, Muniyappa R. Plasma serpinB1 is related to insulin sensitivity but not pancreatic β-Cell function in non-diabetic adults. Physiol Rep. 2017;5(5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chin TL, Moore EE, Coors ME, Chandler JG, Ghasabyan A, Harr JN, et al. Exploring ethical conflicts in emergency trauma research: the COMBAT (Control of Major Bleeding after Trauma) study experience. Surgery. 2015;157(1):10–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore HB, Moore EE, Chapman MP, McVaney K, Bryskiewicz G, Blechar R, et al. Plasma-first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: a randomised trial. Lancet. 2018;392(10144):283–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapman MP, Moore EE, Chin TL, Ghasabyan A, Chandler J, Stringham J, et al. Combat: Initial experience with a randomized clinical trial of plasma-based resuscitation in the field for traumatic hemorrhagic shock. Shock. 2015;44 Suppl 1:63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nemkov T, Reisz JA, Gehrke S, Hansen KC, D’Alessandro A. High-throughput metabolomics: Isocratic and gradient mass spectrometry-based methods. Methods Mol Biol. 2019;1978:13–26 [DOI] [PubMed] [Google Scholar]
- 30.Bruschi M, Petretto A, Santucci L, Vaglio A, Pratesi F, Migliorini P, et al. Neutrophil Extracellular Traps protein composition is specific for patients with Lupus nephritis and includes methyl-oxidized αenolase (methionine sulfoxide 93). Sci Rep. 2019;9(1):7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against candida albicans. PLoS Pathog. 2009;5(10):e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim CH, Adav SS, Sze SK, Choong YK, Saravanan R, Schmidtchen A. Thrombin and plasmin alter the proteome of neutrophil extracellular traps. Front Immunol. 2018;9:1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dzieciatkowska M, Wohlauer MV, Moore EE, Damle S, Peltz E, Campsen J, et al. Proteomic analysis of human mesenteric lymph. Shock. 2011;35(4):331–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clendenen N, Nunns GR, Moore EE, Reisz JA, Gonzalez E, Peltz E, et al. Hemorrhagic shock and tissue injury drive distinct plasma metabolome derangements in swine. J Trauma Acute Care Surg. 2017;83(4):635–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gene Ontology Consortium. The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 2021;49(D1):D325–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mi H, Muruganujan A, Ebert D, Huang X, Thomas PD. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019;47(D1):D419–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thålin C, Aguilera K, Hall NW, Marunde MR, Burg JM, Rosell A, et al. Quantification of citrullinated histones: Development of an improved assay to reliably quantify nucleosomal H3Cit in human plasma. J Thromb Haemost. 2020;18(10):2732–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Donoghue AJ, Jin Y, Knudsen GM, Perera NC, Jenne DE, Murphy JE, et al. Global substrate profiling of proteases in human neutrophil extracellular traps reveals consensus motif predominantly contributed by elastase. PLoS One. 2013;8(9):e75141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barr FD, Ochsenbauer C, Wira CR, Rodriguez-Garcia M. Neutrophil extracellular traps prevent HIV infection in the female genital tract. Mucosal Immunol. 2018;11(5):1420–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDonald B, Davis RP, Kim S-J, Tse M, Esmon CT, Kolaczkowska E, et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129(10):1357–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng W, Paunel-Görgülü A, Flohé S, Witte I, Schädel-Höpfner M, Windolf J, et al. Deoxyribonuclease is a potential counter regulator of aberrant neutrophil extracellular traps formation after major trauma. Mediators Inflamm. 2012;2012:149560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng Q, Pan B, Alam HB, Liang Y, Wu Z, Liu B, et al. Citrullinated histone H3 as a therapeutic target for endotoxic shock in mice. Front Immunol. 2019;10:2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med. 2013;187(2):160–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122(7):2661–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


