Abstract
Background:
Nocardia is an environmental pathogen with a predilection for causing opportunistic infections in immunocompromised patients, including solid organ transplant (SOT) recipients. While risk factors have been identified for developing nocardiosis in this population, little is known regarding clinical factors resulting in poor outcomes. We evaluated a cohort of SOT recipients with nocardiosis for associations with 12-month mortality.
Methods:
We performed a multicenter retrospective cohort study of adult SOT recipients diagnosed with culture-confirmed nocardiosis from 2000-2020. Patients were followed for 12 months after diagnosis, unless abbreviated by mortality. Multivariable Cox regression was performed to analyze associations with 12-month mortality. A subgroup analysis of patients with disseminated nocardiosis was performed to analyze treatment variables.
Results:
125 SOT recipients met inclusion criteria. 12-month mortality was 16.8%. Liver transplantation (hazard ratio [HR] 3.52; 95% confidence interval [CI] 1.27-9.76) and time from symptom onset to presentation (HR 0.92 per day; 95% CI 0.86-0.99) were independently associated with 12-month mortality, while disseminated infection was not (HR 1.23; 95% CI 0.49-3.13). No treatment-specific factors were significantly associated with mortality in 33 patients with disseminated nocardiosis, though survivors had a higher rate of linezolid use.
Conclusions:
This study identified 2 independent associations with 12-month mortality, representing demographics and infection severity. Disseminated infection was not independently associated with poor outcomes, and specific sites of infection may be more important than dissemination itself. No treatment-specific factors were associated with mortality, though this analysis was likely underpowered. Further study of treatment strategies based on specific Nocardia syndromes is warranted.
Introduction
Nocardia is an environmental Gram-positive bacillus with a predilection for causing infection in immunocompromised individuals. Solid organ transplant (SOT) recipients have been reported to have rates of nocardiosis ranging from 0.6-2.65%, with variable incidence based on transplanted organ.1,2 Several studies have examined risk factors for development of nocardiosis, including augmented immunosuppression, cytomegalovirus (CMV) disease, supratherapeutic calcineurin inhibitor levels, and patient age.1,3 However, less is known regarding outcomes and risk factors for mortality in SOT recipients who develop Nocardia infection.
A recent multicenter European cohort of SOT recipients diagnosed with nocardiosis found a history of tumor within 5 years, invasive fungal infection (IFI) within 6 months, and increased donor age to be associated with 1-year mortality while acute rejection within the past year was protective.4 Outside of these factors, smaller studies have inconsistently identified potential predictors of outcomes including disseminated infection, heart transplant recipients, and use of trimethoprim-sulfamethoxazole (TMP-SMX) for treatment.2,5–7
There is even less data to guide treatment of nocardiosis, primarily due to the heterogeneity in disease presentation, organ involvement, species-specific characteristics, and underlying immunosuppression. Current guidelines from the American Society of Transplantation recommend 3-6 weeks of parenteral therapy followed by 9-12 total months of treatment for severe or disseminated infection in SOT recipients.8 However, few studies have compared length of therapy or different antibiotics in terms of outcomes,4,6,9 and optimal therapy remains unclear.
We sought to analyze our multicenter cohort of SOT recipients who developed nocardiosis and analyze risk factors for 12-month mortality. Furthermore, we aimed to compare therapeutic interventions in the subgroup of patients with disseminated infection.
Methods
Study design and setting
We performed a multicenter retrospective cohort study of SOT recipients diagnosed with nocardiosis at 3 Mayo Clinic sites in Arizona, Florida, and Minnesota between the years 2000 and 2020. All Mayo sites share common transplantation protocols, which include prophylaxis. Our centers do not routinely administer primary prophylaxis for Nocardia; however, the transplantation protocols include routine Pneumocystis prophylaxis. Prophylaxis is routinely administered for 6 months following kidney, kidney-pancreas, pancreas, and liver transplantation, 12 months following heart transplantation, and lifelong following lung and heart-lung transplantation. Standard prophylaxis is TMP-SMX 80-400 mg once daily, with dose adjustment for renal function. An exception to this protocol is for heart or heart-lung transplant recipients who are Toxoplasma seronegative and receive a transplant from a seropositive donor, as these patients receive lifelong TMP-SMX prophylaxis dosed 160-800 mg once daily. Our internal institutional review board approved the study protocol and granted it an exempt status and waived the need for informed consent (#21-002606).
Inclusion and exclusion criteria
SOT patients were identified through our internal transplant center registry. These patients were then searched in our microbiology database for either a diagnosis code of nocardiosis or a microbiology culture result with a Nocardia species. These patients were then manually screened through predetermined inclusion and exclusion criteria. Inclusion criteria were age ≥18 years at the date of nocardiosis diagnosis, receipt of SOT prior to diagnosis of nocardiosis, culture growth of Nocardia with accompanying signs, symptoms and/or radiographic findings consistent with nocardiosis, and receipt of initial Nocardia-directed therapy at 1 of the included sites. Exclusion criteria were age <18 years, diagnosis of nocardiosis prior to SOT, lack of culture confirmation of Nocardia, less than 12 months of postdiagnosis follow-up unless abbreviated by mortality, or lack of research authorization.
Once eligible patients were identified, data were manually extracted from the electronic medical record. Abstracted data included demographics, transplantation characteristics and complications such as acute rejection, maintenance immunosuppression, comorbid conditions, index Nocardia diagnosis, treatment details, and outcomes. Study data were collected and managed using REDCap electronic data capture tools hosted at Mayo Clinic.10,11
Identification and susceptibility testing
The clinical microbiology laboratory at Mayo Clinic in Rochester, Minnesota, received specimens for culture, identification, and susceptibility testing from Mayo Clinic sites. Clinical specimens were cultured in BD Bactec mycobacterial growth indicator tube (MGIT) 960 broth in mycobacterial growth indicator tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and on Middlebrook 7H11/7H11S agar biplates incubated at 35°C to 37°C for up to 6 weeks. Positive MGIT broth was subcultured to a Middlebrook 7H11 agar plate and isolated colony growth was identified using Sanger sequencing of a 500-bp region of the 16S rRNA gene. From August 2014, matrix-assisted laser desorption ionization time-of-flight mass spectrophotometry was added to supplement species identification using Sanger sequencing.12,13 Antimicrobial susceptibility testing was performed via broth microdilution using the Trek Sensititre Rapmyco plate and interpreted according to the Clinical and Laboratory Standards Institute guidelines during the respective period.14,15
Definitions
Nocardiosis was defined as culture growth of a Nocardia species with compatible signs, symptoms, and/or radiographic features consistent with infection. The index date was the date of initial culture ascertainment. Disseminated infection was defined as involvement of at least 2 noncontiguous organs, isolation of Nocardia in a blood culture, or isolated central nervous system (CNS) involvement without recent neurologic surgery or penetrating skull injury. Determination of nocardiosis as the attributable cause of death was determined by physician review of the medical record. CMV infection and IFI were defined according to published guidelines.16,17 Chronic kidney disease was defined as a baseline estimated glomerular filtration rate of less than 60 mL/min/1.73 m2 using the 2021 CKD-EPI equation.18 Charlson comorbidity index (CCI) was calculated with receipt of SOT credited as that specific transplanted organ’s comorbidity (i.e. liver transplantation credited as “moderate-to-severe liver disease”). When analyzing antimicrobial therapy, only antibiotics administered within the first 4 weeks of therapy for at least 7 days each were included.
Statistical analysis
Our primary outcome was mortality within 12 months after nocardiosis diagnosis. Secondary outcomes were graft loss within 12 months and a composite of graft loss and mortality within 12 months. Continuous variables are presented as mean (standard deviation) or median (interquartile range [IQR]), as appropriate. Categorical variables are presented as number (percentage). Chi-square test or Fisher’s exact test were used to analyze categorical variables and 2-sample t-test or Mann-Whitney test were used to analyze continuous variables. Kaplan-Meier curves were constructed and compared utilizing a log-rank test for survival between groups. A multivariable Cox regression analysis was performed to analyze independent associations with 12-month mortality. Variables with P < 0.20 in univariable testing were eligible for inclusion in the final Cox model. A subgroup analysis of SOT recipients with disseminated nocardiosis was performed to analyze treatment variables. All analyses were performed using BlueSky Statistics version 7.40 software (BlueSky Statistics LLC, Chicago, Illinois).
Results
A total of 125 unique patients met the inclusion and exclusion criteria, all with complete follow-up. Mean age was 58.3 ± 11.5 years, the majority were male (66.4%), and nocardiosis was diagnosed a median of 400 days after transplantation (IQR: 155, 1487). Kidney was the most common transplant type (43.2%), followed by heart (18.4%) and lung (11.2%). Most patients were receiving tacrolimus, mycophenolate, and prednisone as maintenance immunosuppression with a median prednisone dose of 7.5 mg (IQR: 5, 10). Thirty-four (27.2%) were receiving TMP-SMX prophylaxis at the time of Nocardia diagnosis. Within 6 months prior to Nocardia diagnosis, 27 (21.6%) and 14 (11.2%) had acute rejection or CMV infection, respectively. The most common Nocardia species were N. farcinica (22.4%), N. cyriacigeorgica (15.5%), and N. nova (15.5%). Nine isolates were not identified to the species level (Figure 1). Thirty-three (26.4%) had disseminated infection. The most common sites of involvement were lung (88.8%), skin (20.8%), and CNS (14.4%).
Figure 1:
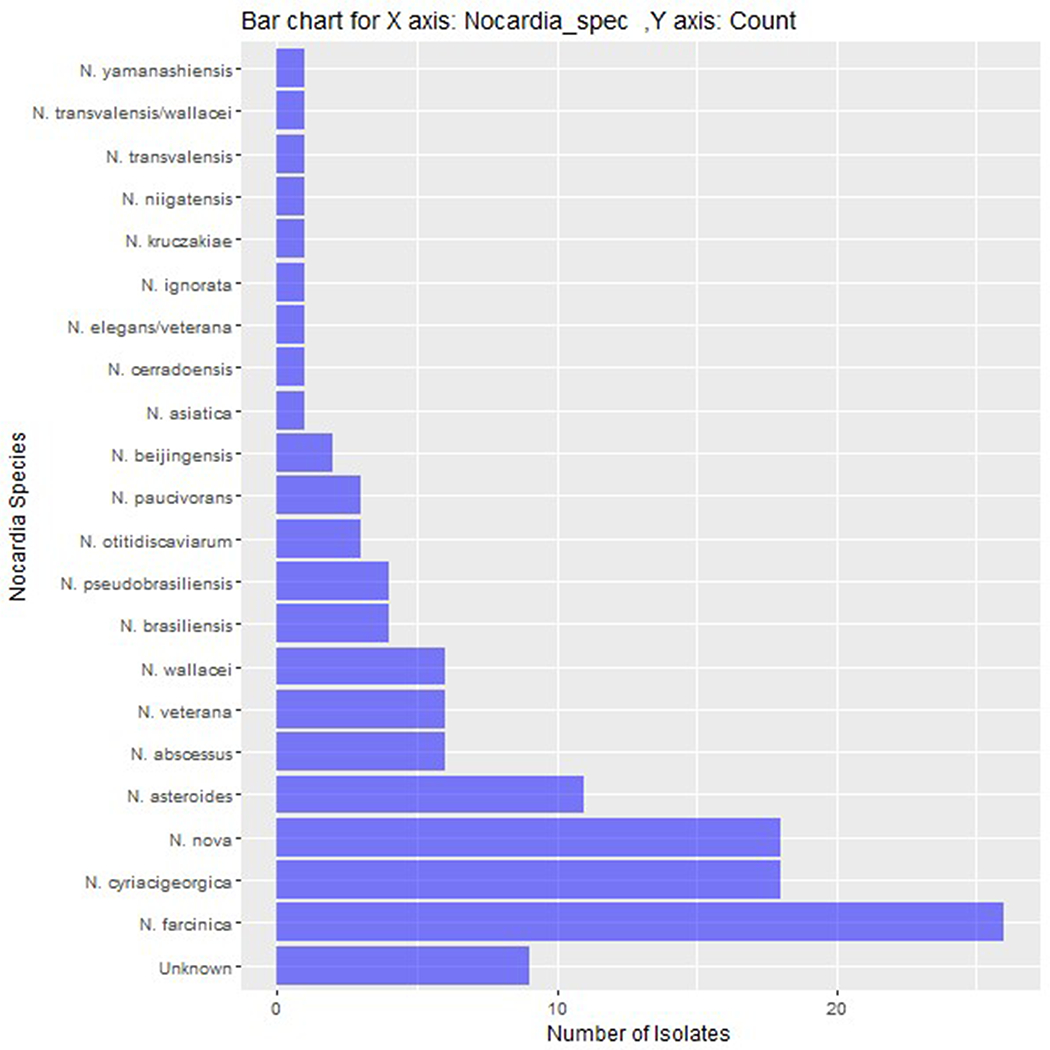
Bar chart of the number of each identified Nocardia species.
Antimicrobial susceptibility testing was attempted in all isolates, 121 (96.8%) of which had valid results (Table S1). Initial combination antibiotic therapy was utilized in 97 (77.6%) patients, with 37 (29.6%) initially receiving at least 3 antibiotics. Most patients received a sulfa antibiotic (73.6%), primarily TMP-SMX. Other commonly used antibiotics were carbapenems (36.8%), which included both imipenem (20.8%) and meropenem (16.0%), and fluoroquinolones (22.4%). 102 patients who did not die within 12 months were treated for a median of 296 days (IQR: 191.25, 389.25), with 2 patients not having completed antibiotic therapy at last follow-up. Eight (7.8%) were treated for less than 120 days. Thirteen patients with CNS infection who completed therapy were treated for a median of 499 days (IQR: 364-528). Details regarding initial antimicrobial therapy are outlined in Table S2. Fifty-nine (47.2%) of SOT recipients underwent a reduction in 1 or more of their maintenance immunosuppressive agents. This was most commonly mycophenolate (88.1%) or tacrolimus (20.3%). Twenty-two (17.6%) patients underwent therapeutic surgical procedures for nocardiosis. These included 11 surgeries for soft tissue or osteoarticular infection, 7 thoracic surgeries, and 4 neurosurgical procedures.
12-month mortality
From our initial cohort, 21 (16.8%) died within 12 months of diagnosis. Nine of these deaths were directly attributed to nocardiosis. Furthermore, 10 (8.0%) suffered graft loss within 12 months and 28 (22.4%) experienced either graft loss or mortality within 12 months. Cohort characteristics stratified by 12-month mortality are presented in Table 1. Statistically significant differences between groups in univariable analyses included dialysis dependency, pleural space involvement, bloodstream infection, time from symptom onset to presentation, intensive care unit admission, number of antibiotics used, and use of a carbapenem for nocardiosis therapy. CCI, infection with Nocardia farcinica, disseminated Nocardia infection, and coinfection were numerically higher or more prevalent in SOT recipients who died within 12 months, though none were statistically significant in univariable analyses. Tumor within 5 years, acute rejection, and recent IFI were not associated with 12-month mortality. Kaplan-Meier curves comparing transplant types, disseminated infection, dialysis dependency, and Nocardia bloodstream infection are presented in Figure 2.
Table 1:
Baseline characteristics and univariable analyses of 12-month mortality in 125 solid organ transplant recipients with nocardiosis
| Alive at 12 months (N = 104) | Died within 12 months (N = 21) | Total (N = 125) | P value | |
|---|---|---|---|---|
| Age, y, mean (SD) | 58.1 (11.6) | 59.3 (11.0) | 58.3 (11.5) | 0.647 |
|
| ||||
| Male sex | 71 (68.3) | 12 (57.1) | 83 (66.4) | 0.325 |
|
| ||||
| Race | 0.730 | |||
| - American Indian or Alaska Native | 5 (4.8) | 0 (0.0) | 5 (4.0) | |
| - Asian | 5 (4.8) | 2 (9.5) | 7 (5.6) | |
| - Black or African American | 9 (8.7) | 3 (14.3) | 12 (9.6) | |
| - Hispanic White | 12 (11.5) | 2 (9.5) | 14 (11.2) | |
| - Non-Hispanic White | 72 (69.2) | 14 (66.7) | 86 (68.8) | |
| - Unknown | 1 (1.0) | 0 (0.0) | 1 (0.8) | |
|
| ||||
| Type of transplant | 0.009 | |||
| - Kidney | 48 (46.2) | 6 (28.6) | 54 (43.2) | |
| - Liver | 5 (4.8) | 6 (28.6) | 11 (8.8) | |
| - Heart | 20 (19.2) | 3 (14.3) | 23 (18.4) | |
| - Lung | 10 (9.6) | 4 (19.0) | 14 (11.2) | |
| - Pancreas | 3 (2.9) | 1 (4.8) | 4 (3.2) | |
| - Multiorgana | 18 (17.3) | 1 (4.8) | 19 (15.2) | |
|
| ||||
| Charlson comorbidity index, median (IQR) | 3.0 (2.0, 4.0) | 4.0 (3.0, 5.0) | 3.0 (2.0, 4.0) | 0.061 |
|
| ||||
| Diabetes mellitus | 53 (51.0) | 9 (42.9) | 62 (49.6) | 0.498 |
|
| ||||
| Chronic kidney disease | 66 (63.5) | 13 (61.9) | 79 (63.2) | 0.893 |
|
| ||||
| Dialysis | 3 (2.9) | 5 (23.8) | 8 (6.4) | 0.003 |
|
| ||||
| History of tumor | 10 (9.6) | 4 (19.0) | 14 (11.2) | 0.252 |
|
| ||||
| eGFR, mL/min/1.73 m2, mean (SD)b | 53.3 (24.0) | 50.4 (26.9) | 52.9 (24.3) | 0.515 |
|
| ||||
| Leukocyte count, x109, mean (SD) | 8.7 (5.2) | 9.9 (9.0) | 8.9 (6.0) | 0.550 |
|
| ||||
| Neutrophil count, x109, mean (SD)c | 7.1 (4.8) | 8.4 (8.4) | 7.3 (5.6) | 0.749 |
|
| ||||
| Lymphocyte count, x109, mean (SD)c | 0.7 (0.6) | 0.7 (0.6) | 0.7 (0.6) | 0.729 |
|
| ||||
| Maintenance immunosuppression | ||||
| - Cyclosporine | 3 (2.9) | 1 (4.8) | 4 (3.2) | 0.526 |
| - Tacrolimus | 97 (93.3) | 19 (90.5) | 116 (92.8) | 0.646 |
| - Sirolimus | 1 (1.0) | 0 (0.0) | 1 (0.8) | 1 |
| - Mycophenolate | 90 (86.5) | 16 (76.2) | 106 (84.8) | 0.313 |
| - Azathioprine | 4 (3.8) | 2 (9.5) | 6 (4.8) | 0.265 |
| - Belatacept | 1 (1.0) | 0 (0.0) | 1 (0.8) | 1 |
| - Prednisone | 98 (94.2) | 20 (95.2) | 118 (94.4) | 1 |
|
| ||||
| Acute rejection within 6 months | 20 (19.2) | 7 (33.3) | 27 (21.6) | 0.158 |
|
| ||||
| CMV infection within 6 months | 10 (9.6) | 4 (19.0) | 14 (11.2) | 0.252 |
|
| ||||
| TMP-SMX prophylaxis at diagnosis | 26 (25.0) | 8 (38.1) | 34 (27.2) | 0.219 |
|
| ||||
| IFI within 6 months | 13 (12.5) | 0 (0.0) | 13 (10.4) | 0.123 |
|
| ||||
| Time from transplantation, days, median (IQR) | 457.5 (149.3, 1607.5) | 341 (192.0, 1179.0) | 400.0 (155.0, 1487.0) | 0.604 |
|
| ||||
| Nocardia farcinica d | 18 (18.8) | 8 (40.0) | 26 (20.8) | 0.072 |
|
| ||||
| Disseminated Infection | 25 (24.0) | 8 (38.1) | 33 (26.4) | 0.183 |
|
| ||||
| Brain MRI performed | 72 (69.2) | 12 (57.1) | 84 (67.2) | 0.282 |
|
| ||||
| Site of infection | ||||
| - Lung | 91 (87.5) | 20 (95.2) | 111 (88.8) | 0.461 |
| - Pleural space | 4 (3.8) | 4 (19.0) | 8 (6.4) | 0.027 |
| - CNS | 14 (13.5) | 4 (19.0) | 18 (14.4) | 0.503 |
| - Bloodstream | 8 (7.7) | 5 (23.8) | 13 (10.4) | 0.043 |
| - Endocarditis | 2 (1.9) | 0 (0.0) | 2 (1.6) | 1 |
| - Skin/soft tissue | 22 (21.2) | 4 (19.0) | 26 (20.8) | 1 |
| - Osteoarticular | 2 (1.9) | 1 (4.8) | 3 (2.4) | 0.427 |
| - Othere | 6 (5.8) | 3 (14.3) | 9 (7.2) | 0.175 |
|
| ||||
| Coinfectionf | 26 (25.0) | 9 (42.9) | 35 (28.0) | 0.096 |
|
| ||||
| Time from symptom onset to presentation, days, median (IQR) | 11.0 (6.0, 21.0) | 7.0 (4.0, 9.0) | 10.0 (5.0, 18.0) | 0.005 |
|
| ||||
| Time from presentation to treatment initiation, days, mean (SD) | 3.0 (1.0, 7.0) | 5.0 (1.0, 11.0) | 3.0 (1.0, 8.0) | 0.347 |
|
| ||||
| ICU admission | 11 (10.6) | 8 (38.1) | 19 (15.2) | 0.004 |
|
| ||||
| Surgical intervention | 19 (18.3) | 3 (14.3) | 22 (17.6) | 1 |
|
| ||||
| Decrease in immunosuppression | 48 (47.1) | 11 (52.4) | 59 (48.0) | 0.657 |
|
| ||||
| Number of antibiotics used, mean (SD) | 2.0 (0.7) | 2.5 (0.7) | 2.1 (0.8) | 0.011 |
|
| ||||
| Number of active antibiotics, mean (SD)g | 1.7 (0.7) | 2.0 (0.8) | 1.8 (0.8) | 0.157 |
|
| ||||
| ≥ 2 antibiotics within first 4 weeks | 77 (74.0) | 20 (95.2) | 97 (77.6) | 0.042 |
|
| ||||
| Initial treatment | ||||
| - Sulfa antibiotic | 78 (75.0) | 14 (66.7) | 92 (73.6) | 0.429 |
| - Carbapenem | 32 (30.8) | 14 (66.7) | 46 (36.8) | 0.002 |
| - Fluoroquinolone | 23 (22.1) | 5 (23.8) | 28 (22.4) | 1 |
| - Macrolide | 9 (8.7) | 2 (9.5) | 11 (8.8) | 1 |
Data are n (%) unless otherwise specified. Bold values indicate P < 0.05.
Abbreviations: CMV, cytomegalovirus; CNS, central nervous system; eGFR, estimated glomerular filtration rate; IFI, invasive fungal infection; IQR, interquartile range; MRI, magnetic resonance imaging; SD, standard deviation; y, years
Multiorgan transplant recipients included kidney-pancreas (11), kidney-liver (4), heart-kidney (3), and heart-lung (1). From this group, only 1 kidney-pancreas recipient died within 12 months.
n = 117, excluding those requiring dialysis.
n = 124
n = 116, excluding 9 patients whose Nocardia isolate was not identified to a species level.
Other sites of infection included pelvic abscess (3), lymphadenitis (3), muscle abscess (2), and peritonitis (1).
Coinfections included 40 organisms from 35 patients comprised of 12 fungi, 9 Gram-negative bacteria, 8 CMV, 4 Gram-positive bacteria, 4 respiratory viruses, and 3 Clostridioides difficile infection.
n = 121, excluding those without valid antibiotic susceptibility test results.
Figure 2:
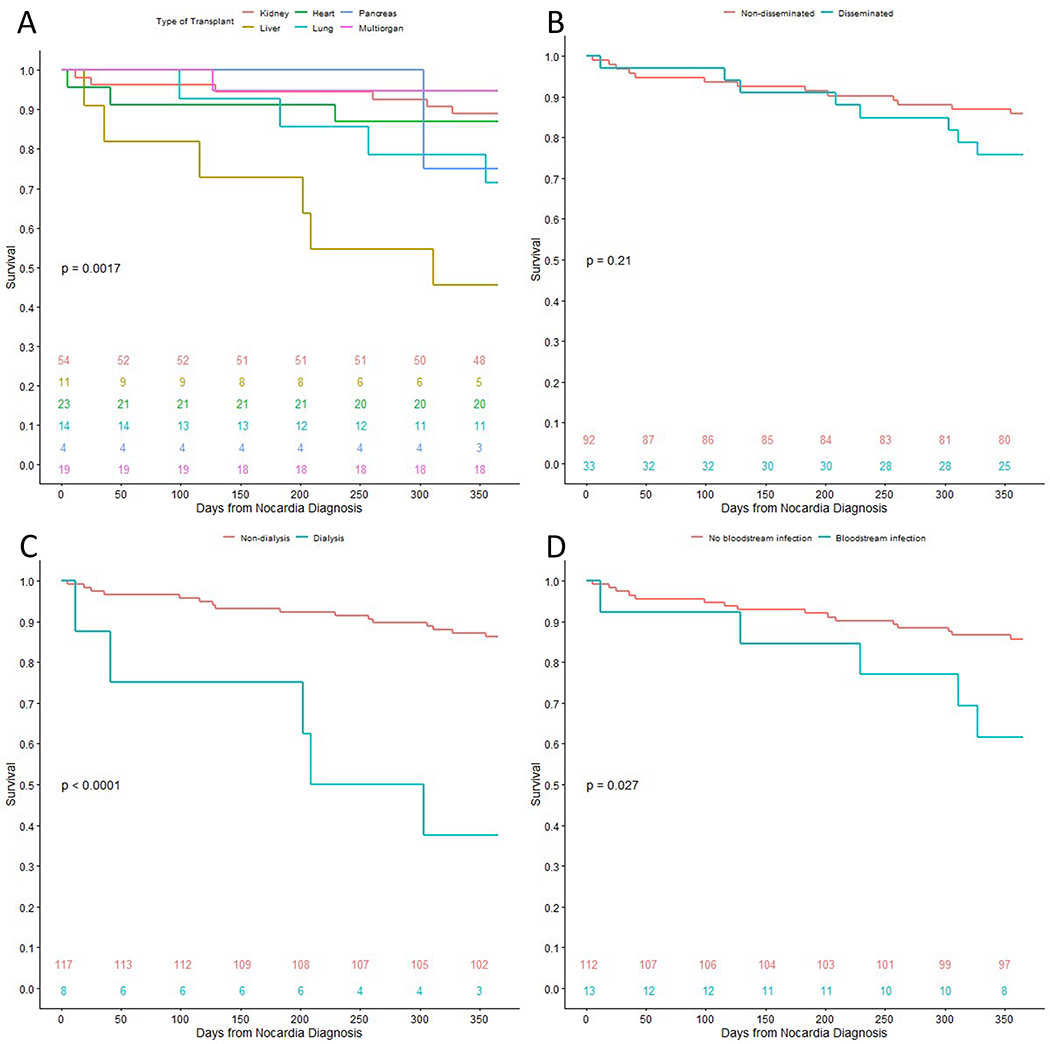
Kaplan-Meier curves comparing 12-month survival after diagnosis of nocardiosis in solid organ transplant recipients, stratified by variables of interest. The numbers near the bottom of the graph indicate number of patients alive at the respective time-point by group. The p-values are calculated through log-rank tests. A, stratified by transplanted organ type. B, stratified by presence of disseminated infection at presentation. C, stratified by chronic dialysis dependency at time of Nocardia infection diagnosis. D, stratified by presence of Nocardia bloodstream infection at presentation.
Multivariable Cox regression analysis is shown in Table 2. Liver transplantation was associated with higher risk of 12-month mortality while more days from symptom onset to presentation was associated with lower risk of mortality. Disseminated infection was not significantly associated with mortality. A secondary multivariable analysis was performed where disseminated infection was replaced by CNS infection, which yielded similar results (hazard ratio 1.29, 95% confidence interval 0.43-3.84; p = .652).
Table 2:
Multivariable Cox regression analyzing risk of 12-month mortality for 125 solid organ transplant recipients with nocardiosis
| Variable | Hazard Ratio (95% Confidence Interval) | P value |
|---|---|---|
| Liver transplant | 3.52 (1.27-9.76) | 0.02 |
| Disseminated infection | 1.23 (0.49-3.13) | 0.66 |
| Time from symptom onset to presentation (per day) | 0.92 (0.86-0.99) | 0.03 |
Bold values indicate p < 0.05.
Disseminated infection
From the 33 SOT recipients with disseminated nocardiosis, kidney transplant (57.6%) was the most common SOT type. All patients had pulmonary involvement and a majority had CNS involvement. Characteristics and treatment of SOT recipients with disseminated nocardiosis are detailed in Table 3. Overall, 8 (24.2%) patients with disseminated infection died within 12 months of diagnosis compared to 13 (14.1%) of patients without disseminated infection. All 3 patients requiring chronic dialysis at diagnosis died within 12 months (p = .01). Those who died within 12 months also had higher rates of prenocardiosis TMP-SMX prophylaxis (50%), N. farcinica infection (50%), and Nocardia bloodstream infection (62.5%), though none of these were statistically significant. There were similar rates of surgical intervention and decrease in immunosuppression.
Table 3:
Baseline characteristics of 33 solid organ transplant recipients with disseminated nocardiosis
| Overall | Alive at 12 months (N = 25) | Died within 12 months (N = 8) | Total (N=33) | P value |
|---|---|---|---|---|
| Age, y, mean (SD) | 57.8 (12.7) | 57.4 (12.4) | 57.7 (12.4) | 0.966 |
|
| ||||
| Male sex | 17 (68.0) | 4 (50.0) | 21 (63.6) | 0.420 |
|
| ||||
| Type of Transplant | 0.189 | |||
| - Kidney | 16 (64.0) | 3 (37.5) | 19 (57.6) | |
| - Liver | 3 (12.0) | 3 (37.5) | 6 (18.2) | |
| - Heart | 2 (8.0) | 1 (12.5) | 3 (9.1) | |
| - Lung | 1 (4.0) | 0 (0.0) | 1 (3.0) | |
| - Pancreas | 0 (0.0) | 1 (12.5) | 1 (3.0) | |
| - Kidney-pancreas | 3 (12.0) | 0 (0.0) | 3 (9.1) | |
|
| ||||
| Charlson comorbidity index, median (IQR) | 4.0 (2.0, 4.0) | 4.0 (3.0, 5.0) | 4.0 (2.0, 4.0) | 0.260 |
|
| ||||
| Dialysis | 0 (0.0) | 3 (37.5) | 3 (9.1) | 0.010 |
|
| ||||
| Tumor | 4 (16.0) | 2 (25.0) | 6 (18.2) | 0.616 |
|
| ||||
| Leukocyte count, x109, mean (SD) | 9.3 (6.0) | 12.7 (10.1) | 10.1 (7.1) | 0.501 |
|
| ||||
| Neutrophil count, x109, mean (SD) | 7.7 (5.7) | 11.2 (9.5) | 8.5 (6.8) | 0.324 |
|
| ||||
| Lymphocyte count, x109, mean (SD) | 0.5 (0.4) | 0.8 (0.7) | 0.6 (0.5) | 0.344 |
|
| ||||
| Maintenance immunosuppression | ||||
| - Cyclosporine | 1 (4.0) | 0 (0.0) | 1 (3.0) | 1 |
| - Tacrolimus | 24 (96.0) | 7 (87.5) | 31 (93.9) | 0.432 |
| - Mycophenolate | 23 (92.0) | 7 (87.5) | 30 (90.9) | 1 |
| - Azathioprine | 0 (0.0) | 1 (12.5) | 1 (3.0) | 0.242 |
| - Prednisone | 23 (92.0) | 8 (100) | 31 (93.9) | 1 |
|
| ||||
| Acute rejection within 6 months | 4 (16.0) | 1 (12.5) | 5 (15.2) | 1 |
|
| ||||
| CMV within 6 months | 4 (16.0) | 0 (0.0) | 4 (12.1) | 0.550 |
|
| ||||
| TMP-SMX prophylaxis at diagnosis | 4 (16.0) | 4 (50.0) | 8 (24.2) | 0.074 |
|
| ||||
| IFI within 6 months | 3 (12.0) | 0 (0.0) | 3 (9.1) | 0.560 |
|
| ||||
| Time from transplantation, days, median (IQR) | 387.0 (199.0, 1463.0) | 660.5 (324.8, 1925.0) | 398 (204.0, 1463.0) | 0.501 |
|
| ||||
| Nocardia farcinica a | 9 (37.5) | 4 (50.0) | 13 (39.4) | 0.684 |
|
| ||||
| Site of infection | ||||
| - Lung | 25 (100) | 8 (100) | 33 (100) | 1 |
| - Pleural space | 0 (0.0) | 1 (12.5) | 1 (3.0) | 0.242 |
| - CNS | 14 (56.0) | 4 (50.0) | 18 (54.5) | 1 |
| - Bloodstream | 8 (32.0) | 5 (62.5) | 13 (39.4) | 0.213 |
| - Endocarditis | 2 (8.0) | 0 (0.0) | 2 (6.1) | 1 |
| - Skin/soft tissue | 11 (44.0) | 3 (37.5) | 14 (42.4) | 1 |
| - Osteoarticular | 1 (4.0) | 1 (12.5) | 2 (6.1) | 0.432 |
| - Otherb | 4 (16.0) | 3 (37.5) | 7 (21.2) | 0.320 |
|
| ||||
| Coinfectionc | 6 (24.0) | 2 (25.0) | 8 (24.2) | 1 |
|
| ||||
| Time from symptom onset to presentation, days, median (IQR) | 9.0 (7.0, 18.0) | 9.0 (7.3, 11.8) | 9.0 (7.0, 16.0) | 0.512 |
|
| ||||
| Time from presentation to treatment initiation, days, median (IQR) | 3.0 (1.0, 5.0) | 4.5 (1.8, 8.3) | 3.0 (1.0, 7.0) | 0.582 |
|
| ||||
| Surgical intervention | 8 (32.0) | 3 (37.5) | 11 (33.3) | 1 |
|
| ||||
| Decrease in immunosuppression | 16 (64.0) | 5 (62.5) | 21 (65.6) | 1 |
|
| ||||
| Number of antibiotics, mean (SD) | 2.4 (0.6) | 2.4 (0.9) | 2.4 (0.7) | 0.962 |
|
| ||||
| Number of active antibiotics, mean (SD) | 2.1 (0.7) | 1.9 (0.8) | 2.0 (0.7) | 0.495 |
|
| ||||
| ≥ 2 initial antibiotics | 24 (96.0) | 7 (87.5) | 31 (93.9) | 0.962 |
|
| ||||
| ≥ 2 initial active antibiotics | 20 (80.0) | 5 (62.5) | 25 (75.8) | 0.495 |
|
| ||||
| Sulfa antibiotics | 23 (92.0) | 7 (87.5) | 30 (90.9) | 1 |
| - TMP-SMX | 19 (76.0) | 7 (87.5) | 26 (78.8) | 0.652 |
| - Sulfadiazine | 4 (16.0) | 0 (0.0) | 4 (12.1) | 0.550 |
|
| ||||
| Carbapenem | 14 (56.0) | 5 (62.5) | 19 (57.6) | 1 |
| - Imipenem | 8 (32.0) | 4 (50.0) | 12 (36.4) | 0.420 |
| - Meropenem | 6 (24.0) | 1 (12.5) | 7 (21.2) | 0.652 |
|
| ||||
| Fluoroquinolone | 4 (16.0) | 2 (25.0) | 6 (18.2) | 0.616 |
| - Ciprofloxacin | 1 (4.0) | 1 (12.5) | 2 (6.1) | 0.432 |
| - Moxifloxacin | 3 (12.0) | 1 (12.5) | 4 (12.1) | 1 |
|
| ||||
| Amikacin | 1 (4.0) | 0 (0.0) | 1 (3.0) | 1 |
|
| ||||
| Azithromycin | 1 (4.0) | 1 (12.5) | 2 (6.1) | 0.432 |
|
| ||||
| Ceftriaxone | 2 (8.0) | 1 (12.5) | 3 (9.1) | 1 |
|
| ||||
| Linezolid | 10 (40.0) | 1 (12.5) | 11 (33.3) | 0.218 |
|
| ||||
| Minocycline | 4 (16.0) | 2 (25.0) | 6 (18.2) | 0.616 |
Data are n (%) unless otherwise specified. Bold value indicates p < 0.05.
Abbreviations: CMV, cytomegalovirus; CNS, central nervous system; IFI, invasive fungal infection; IQR, interquartile range; SD, standard deviation; TMP-SMX, trimethoprim-sulfamethoxazole; y, years.
n = 32, excluding 1 patient whose Nocardia isolate was not identified to a species level.
Other sites of infection included pelvic abscess (3), lymphadenitis (2), muscle abscess (1), and peritonitis (1).
Coinfections included 9 organisms from 8 patients comprised of 2 fungi, 2 CMV, 2 Gram-positive bacteria, 2 respiratory viruses, and 1 Gram-negative bacteria.
In terms of antibiotic treatment, most patients (93.9%) with disseminated infection received initial combination therapy. Twelve (36.4%) received at least 3 initial agents, which was similar between those who did and did not die within 12 months. Likewise, use of at least 2 (80% vs 62.5%) or 3 (28% vs 25%) active agents based on in vitro susceptibility testing was similar between groups. Thirty patients (90.9%) received treatment including a sulfa antibiotic, 26 of which were TMP-SMX. All patients with disseminated infection had available susceptibility testing results. There were no statistically significant associations in agent selection with 12-month mortality, though those who died had a numerically lower rate of linezolid use. From those who did not die within 12 months, 23 received antibiotic therapy for a median of 402 days (IQR: 271.5, 536.0) and 2 were still receiving therapy at last follow-up.
Discussion
In this cohort, we identified 2 factors associated with 12-month mortality. Disseminated infection did not show a significant association after adjustment for liver transplantation and time from symptom onset to presentation. No treatment variables were significantly associated with 12-month mortality after our group was restricted to only those with disseminated infection.
We found a 12-month mortality rate of 16.8%, similar to recent reports of nocardiosis in SOT recipients with rates ranging from 11-16.2%.2,4 Prior publications have suggested differences in mortality based on type of transplanted organ. Goodlet et al. found a cohort of lung transplant recipients to have a 6-month mortality rate of 25%y.19 Other cohorts have found similarly high rates of all-cause or Nocardia-specific mortality in lung transplant recipients.20,21 Another study suggested heart transplant recipients may have improved survival compared to recipients of other organ transplants.5 Our data also show a difference in 12-month mortality based on SOT type, though with liver transplant recipients having the highest mortality rate. Liver transplant recipients have been shown to be at elevated risk for more severe or disseminated presentations of other opportunistic infections including cryptococcosis and aspergillosis.22,23 Liver transplant recipients have also been shown to have higher rates of mortality from invasive mold infections, compared to other types of SOT.24 Nocardia appears to follow a similar pattern, despite liver transplant recipients often receiving lower maintenance immunosuppression compared to other SOT types. This may be related to underlying hepatic dysfunction and alterations in physiologic processes such as iron homeostasis,22 though the present study does not specifically address this. However, these data do suggest liver transplant recipients are at highest risk of poor outcomes and efforts should be made to mitigate risks for development of nocardiosis in this population.
A longer time from symptom onset to presentation was shown to be protective from 12-month mortality. Longer time from symptom onset (an approximation of infection onset) to seeking healthcare evaluation may indicate less severe infection or infection with a less virulent Nocardia species. This may serve as a marker of disease severity to account for in future studies and could be applied in a clinical setting as one component to help judge urgency of evaluation or therapy initiation. It should be noted that this analysis was restricted to this interval as we were unable to appropriately evaluate impact of early versus delayed therapy and does not imply that later initiation of treatment is protective. This is due to confounding factors that may affect time to treatment initiation, primarily those related to site of infection and severity of disease.
This cohort did find a rate of disseminated infection of 26.4% in SOT recipients, similar to historical reports ranging from 12.5-42.7%.2–4,6,25 Our analysis did not find disseminated infection to be independently associated with 12-month mortality. This is consistent with a smaller study of 54 SOT recipients with nocardiosis, where disseminated infection was not associated with mortality in unadjusted analysis.2 One possible explanation is the traditional definition of disseminated infection is comprised of a heterogenous group of disease manifestations that likely have different rates of mortality and other undesirable outcomes.26 In univariable analysis, patients with Nocardia bloodstream infection or pleural space involvement appeared to have higher rates of mortality. Interestingly, CNS infection was not significantly associated with mortality despite rates of mortality in this subgroup being similar to past reports of CNS nocardiosis.4,27 However, it should be noted that about one-third of our cohort did not undergo advanced CNS imaging, and it is possible there were unrecognized cases of CNS infection. Another notable difference is that patients with disseminated infection received significantly more antibiotics during the initial 4 weeks of therapy compared to those with nondisseminated disease, and this may have influenced outcomes as well.
A recent study of nocardiosis that was not limited to SOT recipients found disseminated infection to be associated with 12-month mortality after adjusting for potential confounders.28 This different result may be related to differences in study population, as only 3 of 317 patients were SOT recipients. There was also a much lower rate of ICU admission as compared to the present study, which may reflect differences in disease acuity at presentation between SOT recipients and other populations. Our cohort also had a higher rate of skin involvement, a common manifestation of disseminated disease, which may represent a less severe form of dissemination. These aspects highlight potential differences in the progression of disseminated nocardiosis in these populations, and localized infection, specifically, may be more severe in SOT recipients.
Current guidelines for nocardiosis in SOT recipients suggest treating disseminated infection with combination therapy for several weeks until susceptibility testing results are available and clinical improvement has been achieved.8 However, in our disseminated infection subgroup, we did not identify specific treatment variables to be significantly associated with 12-month mortality in the first 4 weeks of therapy. Specifically, there was no difference in outcome based on the use of more than 1 active antibiotic based on in vitro susceptibility testing. However, there was a nonsignificant, higher rate of linezolid use in those without 12-month mortality. Linezolid was active in vitro against all Nocardia isolates in this cohort, consistent with a large study of Nocardia susceptibility testing results in a reference laboratory.29 While our group of 33 patients is underpowered to fully assess these interventions, this may suggest that utilizing at least 1 active antibiotic is sufficient, with linezolid being a reliable agent. Furthermore, a recent study of TMP-SMX monotherapy for nocardiosis in SOT recipients found low rates of mortality,30 noting TMP-SMX is active in >90% of isolates.29 While our cohort had a low rate of monotherapy, there were high rates of TMP-SMX use and susceptibility, consistent with these prior reports. It is important to note that linezolid has significant drug-drug interactions and duration-limiting adverse effects such as myelosuppression, and this difference in usage may simply be due to patients with more severe infection or other high-risk features receiving interacting medications or having other attributes that make linezolid an unattractive option.
This cohort had a relatively long length of therapy among patients who completed treatment. This is consistent with the current American Society of Transplantation guidelines, which recommend 6-12 months for all patients, with longer courses for those with disseminated or CNS infection.8 Those with CNS infection did receive longer courses, which may have been influenced by follow-up CNS imaging. However, since therapy is typically abbreviated by death in those with 12-month mortality, we were unable to adequately assess the influence of treatment length on this outcome.
This study has several limitations of note. This study was retrospective and observational and is thus subject to intrinsic sources of bias. While this was a large study relative to other published reports, we had a low absolute number of events which limited our primary analysis to 3 variables. There are likely other impactful factors that require further study. It is also possible that this study underestimates the effect of highly impactful factors on mortality if those patients were more likely to die prior to culture-confirmation of nocardiosis. The analysis on treatment variables was limited to the disseminated infection subgroup to reduce confounding. However, this limited the analysis to univariable comparisons, and the results should be interpreted with caution. Furthermore, most patients received more than 1 active agent and the hypothesis that monotherapy with an active agent results in equivalent outcomes compared to combination therapy will require further study. Also, our therapy definition was limited to the regimen that was used the longest during 4 weeks of treatment. However, these treatments were not necessary used for that entire duration. Finally, we were unable to evaluate the effect of donor age on mortality, which has been previously associated with 1-year mortality.4
In conclusion, liver transplantation and time from symptom onset to presentation were independently associated with 12-month mortality, while disseminated infection was not significantly associated in multivariable analysis. Specific sites of infection within those with disseminated disease may be more predictive of outcomes. We also did not see a difference in outcomes with the use of multiple active antibiotic agents. This suggests multiple agents may only be necessary prior to availability of susceptibility testing results, challenging the dogma of combination therapy for disseminated disease. Further prospective studies are needed to further delineate optimal therapeutic strategies for nocardiosis in SOT recipients.
Supplementary Material
Funding:
This work was supported by the National Institutes of Health [UL1TR002377]. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- CCI
Charlson comorbidity index
- CMV
Cytomegalovirus
- CNS
Central nervous system
- IFI
Invasive fungal infection
- IQR
Interquartile range
- MGIT
Mycobacterial growth indicator tube
- SOT
Solid organ transplant
- TMP-SMX
Trimethoprim-sulfamethoxazole
Footnotes
Disclosure: The authors declare no conflicts of interest.
References
- 1.Peleg AV, Husain S, Qureshi ZA, et al. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: a matched case-control study. Clin Infect Dis. 2007;44(10):1307–1314. doi: 10.1086/514340 [DOI] [PubMed] [Google Scholar]
- 2.Majeed A, Beatty N, Iftikhar A, et al. A 20-year experience with nocardiosis in solid organ transplant (SOT) recipients in the Southwestern United States: a single-center study. Transpl Infect Dis. 2018;20(4):e12904. doi: 10.1111/tid.12904 [DOI] [PubMed] [Google Scholar]
- 3.Coussement J, Lebeaux D, Van Delden C, et al. Nocardia infection in solid organ transplant recipients: a multicenter European case-control study. Clin Infect Dis. 2016;63(3):338–345. doi: 10.1093/cid/ciw241 [DOI] [PubMed] [Google Scholar]
- 4.Lebeaux D, Freund R, Van Delden C, et al. Outcome and treatment of nocardiosis after solid organ transplantation: new insights from a European study. Clin Infect Dis. 2017;64(10):1396–1405. doi: 10.1093/cid/cix124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemmersbach-Miller M, Stout JE, Woodworth MH, Cox GM, Saullo JL. Nocardia infections in the transplanted host. Transpl Infect Dis. 2018;20(4):e12902. doi: 10.1111/tid.12902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tashiro H, Takahashi K, Kusaba K, et al. Relationship between the duration of trimethoprim/sulfamethoxazole treatment and the clinical outcome of pulmonary nocardiosis. Respir Investig. 2018;56(2):166–172. doi: 10.1016/j.resinv.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 7.Martinez Tomas R, Menendez Villanueva R, Reyes Calzada S, et al. Pulmonary nocardiosis: risk factors and outcomes. Respirology. 2007;12(3):394–400. doi: 10.1111/j.1440-1843.2007.01078.x [DOI] [PubMed] [Google Scholar]
- 8.Restrepo A, Clark NM. Nocardia infections in solid organ transplantation: guidelines from the infectious diseases community of practice of the American Society of Transplantation. Clin Transplant. 2019;33(9):1–12. doi: 10.1111/ctr.13509 [DOI] [PubMed] [Google Scholar]
- 9.Yetmar ZA, Wilson JW, Beam E. Recurrent nocardiosis in solid organ transplant recipients: An evaluation of secondary prophylaxis. Transpl Infect Dis. 2021;23(6). doi: 10.1111/tid.13753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel R MALDI-TOF MS for the diagnosis of infectious diseases. Clin Chem. 2015;61(1). doi: 10.1373/clinchem.2014.221770 [DOI] [PubMed] [Google Scholar]
- 13.Buckwalter SP, Olson SL, Connelly BJ, et al. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of Mycobacterium species, Nocardia species, and other Aerobic Actinomycetes. J Clin Microbiol. 2016;54(2). doi: 10.1128/JCM.02128-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woods GL, Wengenack NL, Lin G, et al. Susceptibility Testing of Mycobacteria, Nocardia Spp., and Other Aerobic Actinomycetes. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [PubMed] [Google Scholar]
- 15.Woods GL, Wengenack NL, Lin G, et al. Performance Standards for Susceptibility Testing of Mycobacteria, Nocardia Spp., and Other Aerobic Actinomycetes. 1st ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [PubMed] [Google Scholar]
- 16.Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64(1):87–91. doi: 10.1093/cid/ciw668 [DOI] [PubMed] [Google Scholar]
- 17.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367–1376. doi: 10.1093/cid/ciz1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inker LA, Eneanya ND, Coresh J, et al. New Creatinine- and Cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodlet KJ, Tokman S, Nasar A, et al. Nocardia prophylaxis, treatment, and outcomes of infection in lung transplant recipients: a matched case‐control study. Transpl Infect Dis. 2021;23(2). doi: 10.1111/tid.13478 [DOI] [PubMed] [Google Scholar]
- 20.Husain S, McCurry K, Dauber J, et al. Nocardia infection in lung transplant recipients. J Hear Lung Transplant. 2002;21(3):354–359. doi: 10.1016/S1053-2498(01)00394-1 [DOI] [PubMed] [Google Scholar]
- 21.Poonyagariyagorn HK, Gershman A, Avery R, et al. Challenges in the diagnosis and management of Nocardia infections in lung transplant recipients. Transpl Infect Dis. 2008;10(6):403–408. doi: 10.1111/j.1399-3062.2008.00338.x [DOI] [PubMed] [Google Scholar]
- 22.Singh N, Sun H-Y. Iron overload and unique susceptibility of liver transplant recipients to disseminated disease due to opportunistic pathogens. Liver Transplant. 2008;14(9):1249–1255. doi: 10.1002/lt.21587 [DOI] [PubMed] [Google Scholar]
- 23.Baddley JW, Forrest GN. Cryptococcosis in solid organ transplantation—Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9). doi: 10.1111/ctr.13543 [DOI] [PubMed] [Google Scholar]
- 24.Husain S, Silveira FP, Azie N, et al. Epidemiological features of invasive mold infections among solid organ transplant recipients: PATH Alliance ® registry analysis. Med Mycol. 2017;55(3):269–277. doi: 10.1093/mmy/myw086 [DOI] [PubMed] [Google Scholar]
- 25.Santos M, Gil-Brusola A, Morales P. Infection by nocardia in solid organ transplantation: thirty years of experience. Transplant Proc. 2011;43(6):2141–2144. doi: 10.1016/j.transproceed.2011.06.065 [DOI] [PubMed] [Google Scholar]
- 26.Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87(4):403–407. doi: 10.1016/j.mayocp.2011.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corsini Campioli C, Castillo Almeida NE, O’Horo JC, et al. Clinical presentation, management, and outcomes of patients with brain abscess due to nocardia species. Open Forum Infect Dis. 2021;8(4). doi: 10.1093/ofid/ofab067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takamatsu A, Yaguchi T, Tagashira Y, et al. Nocardiosis in Japan: a multicentric retrospective cohort study. Antimicrob Agents Chemother. 2022;66(2). doi: 10.1128/aac.01890-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamdi AM, Fida M, Deml SM, Abu Saleh OM, Wengenack NL. Retrospective analysis of antimicrobial susceptibility profiles of nocardia species from a tertiary hospital and reference laboratory, 2011 to 2017. Antimicrob Agents Chemother. 2020;64(3). doi: 10.1128/AAC.01868-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conan P, Matignon M, Bleibtreu A, et al. Trimethoprim/sulfamethoxazole for nocardiosis in solid organ transplant recipients: real‐life data from a multicentre retrospective study. Transpl Infect Dis. 2021;23(4). doi: 10.1111/tid.13669 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


