Abstract
N6-Methyladenosine (m6A) is one of the most abundant modifications of the epitranscriptome and is found in cellular RNAs across all kingdoms of life. Advances in detection and mapping methods have improved our understanding of the effects of m6A on mRNA fate and ribosomal RNA function, and have uncovered novel functional roles in virtually every species of RNA. In this Review, we explore the latest studies revealing roles for m6A-modified RNAs in chromatin architecture, transcriptional regulation and genome stability. We also summarize m6A functions in biological processes such as stem-cell renewal and differentiation, brain function, immunity and cancer progression.
The RNA building block adenosine can be methylated at several distinct nitrogen atoms of the nucleobase (N1, N2 or N6) or on the 2′ oxygen of the ribose (BOX 1). RNA N6-methyladenosine (m6A) was identified in mammalian mRNA in the 1970s1,2, but more sensitive detection and mapping techniques (TABLE 1) have facilitated transcriptome-wide mapping of m6A methylation in mRNA and have enabled its detection in nearly all types of RNAs, including ribosomal RNAs (rRNAs), mRNAs, small nuclear RNAs (snRNAs) and several species of regulatory RNAs. The expansion of new techniques coupled with the characterization of enzymes that methylate, demethylate and bind to m6A (TABLE 2) has accelerated research in the past decade, establishing m6A as a major determinant of RNA fate. This epitranscriptomic modification affects most aspects of RNA metabolism, increasing the versatility of and information encoded in RNA, and has fundamental roles in development, organism homeostasis and disease.
Box 1 |. Functions of other adenosine methylation modifications.
Adenosines can be monomethylated in either the N1, N2 or N6 position of the nucleobase, resulting in N1-methyladenosine (m1A), N2-methyladenosine (m2A) or N6-methyladenosine (m6A), respectively, or can be 2′-O-methylated in the ribose yielding 2′-O-methyladenosine (Am). Additionally, adenosines can be dimethylated as is the case for N6,2′-O-dimethyladenosine (m6Am) or N6,N6-dimethyladenosine (m6,2A) (see the figure). m2A has thus far only been identified on adenosine 2503 of the 23S ribosomal RNA (rRNA) in prokaryotes where it helps confer a fitness advantage198, whereas Am (and other 2′-O-methylated bases) is prevalent on rRNAs56 and the first and second nucleosides of mRNAs directly after the N7-methylguanosine (m7G) cap1,199. The other modified adenosines are discussed in more detail below.
m1A in tRnAs, rRnA and mRnA
m1A is deposited by the TRM10 and TRM6/61A methyltransferases on conserved nucleosides 9, 14, 22, 57 and 58 in cytosolic and mitochondrial tRNAs to regulate structural stability and proper folding (reviewed elsewhere200). These methylation events are dynamic, increasing in response to glucose, and A58 can be demethylated by ALKBH1 to slow translation initiation and elongation191.
m1A has also been found to be methylated by RRP8 at a conserved adenosine in the 28S rRNA (A1322) from yeast to humans, which is required for appropriate translation initiation and translation of specific sets of transcripts201–204.
The presence and prevalence of m1A in mRNAs has been highly debated. Initial m1A-sequencing mapping studies reported m1A to be abundant in mammalian mRNAs and enriched near start codons, and it has been proposed to regulate translation149,150. However, subsequent single-nucleotide resolution mapping studies found limited m1A in mRNAs at low stoichiometry, and its presence leads to translation repression151,152,205. A study using multiple m1A mapping techniques confirmed the very low prevalence of m1A in mRNAs and suggested that conflicting reports were due to non-specific antibody binding153. It has been proposed151,152,205 that cells may tend to avoid mRNA m1A deposition because m1A disrupts base pairing, which blocks translation.
m6Am in mRnA and snRnA
m6Am is an abundant RNA modification, found on at least 25% of vertebrate mRNAs and certain viral RNAs, but is restricted to the first transcribed nucleotide following the m7G cap206,207. 2′-O-Methyladenosines are N6-methylated by PCIF1 (REFS.172–175). The phenotypic consequences and effects on RNA fate of PCIF1 deletion under basal conditions seem to be minimal, and the effects on RNA fate seem to be modest and often not in agreement in different studies172,174,175,208. m6Am has been reported to enhance translation126,172, inhibit translation175 or promote the stability of a subset of transcripts126,174. m6Am is a dynamic modification as FTO can remove m6Am both in vitro and in mammalian cells126. Recently, m6Am has been linked to obesity in mice because a high-fat diet stimulates FTO-mediated demethylation of m6Am in selected metabolic genes in the liver resulting in decreases in their translation209. Interestingly, viruses can hijack host PCIF1 to methylate viral RNAs to evade the immune system210.
Several small nuclear RNAs (snRNAs), including the U1 and U2 snRNAs, are also m6Am methylated on their caps and this methylation is removed by FTO211 (FIG. 2d). Cap m6Am modifications in U1 and U2 snRNAs cause altered alternative splicing211. In addition, U2 snRNAs are internally N6,2′-O-dimethylated at adenosine 30 by METTL4, which also increases splicing189,190 (FIG. 2d). Future experiments are needed to determine whether these snRNA modifications communicate with each other to create an epitranscriptomic code and how adenosine methylations help determine splicing specificity.
m6,2A in rRnA
m6,2A occurs at two conserved adjacent adenosines of eukaryotic 18S rRNAs (1850, 1851)12 and the consequences on rRNA function are just beginning to be deciphered. Although these methylation events have a role in the processing of 18S rRNA186,212, recent reports demonstrated that 18S rRNA N6,N6-dimethylation is induced in response to sulfur starvation in yeast and mammalian cells213 or starvation in Caenorhabditis elegans141, suggesting that rRNA m6,2A is not constitutive and could therefore contribute to ribosome heterogeneity to facilitate the translation of unique sets of stress resistance genes. DIMT1 is the 18S rRNA N6,N6-dimethyltransferase both in vitro and in vivo141,186–188. Recent work from our laboratory suggests that worms transmit elevated m6,2A 18S rRNA to their naive progeny in response to starvation, which helps confer an intergenerational hormesis phenotype (whereby low exposure to a stress induces the stress response pathway and allows a more favourable response to stress in the future)141.

Table 1 |.
Techniques for detecting adenine methylation
| Technique | Description | Sensitivity | Limitations | Refs. |
|---|---|---|---|---|
| 2D TLC | RNase T1 digestion and radioactive labelling followed by 2D TLC to separate different modified bases | High relative quantification | No qualitative info | 90,143 |
| UHPLC-MS/MS | Chemical separation by UHPLC followed by detection with tandem MS/MS | Highly quantitative | Need to ensure that methylated bases are not being contributed by contaminating mycoplasma or digestion enzymes | 144,145 |
| SCARLET | RNase H site-specific cleavage, splinted ligation, p32 labelling, digestion and TLC to separate and quantify specific methylation sites. | Highly quantitative and qualitative | Only assesses one specific site per assay; the assay is laborious | 146 |
| MazF and MAZTERseq | Site-specific fluorescent quantification of m6A found in specific context upon MazF cleavage or can be expanded to transcriptome-wide mapping by sequencing in MAZTERseq | High qualitative info, can provide stoichiometry information | Can only detect methylation in specific context of MazF cleavage sites (ACA triplet) | 147,148 |
| meRIP-seq and meRIP-qPCR | Immunoprecipitation followed by sequencing or qPCR | Medium | Depends on immunoprecipitation efficiency Not single-nucleotide resolution Can be prone to misreading other modifications (for example, m1A) as m6A |
3,4,51, 149–153 |
| RT-based detection methods | Several techniques detect signatures created by RT enzymes (such as 4SedTTP-RT, Tth polymerase and RT-KTQ polymerase) that terminate reverse transcription, or incorporate an incorrect base, when they encounter m6A | Medium to low | Higher false-positive rate than other methods | 154–156 |
| miCLIP and m6A-CLIP | Antibody crosslinking followed by sequencing to detect mutations | Qualitative but dependent on mutations being accurately detected | Large amount of starting material required | 8,157 |
| m6A-LAIC-seq | Immunoprecipitation of polyA selected transcripts with m6A antibody followed by sequencing of eluate and supernatant Calling a methylated site requires both enrichment in eluate and depletion of the same region in the supernatant |
Medium | Dependent on antibody specificity | 158 |
| m6A ELISA | ELISA-based method to detect the amount of m6A antibody-enriched RNA | Medium | Dependent on antibody specificity and lack of contaminations in normalization controls | 159 |
| Nm-seq | Harnesses chemical properties of 2′-O-methylation to expose base and then adaptors added and sequenced | Nucleoside resolution, more sensitive than RiboMeth-seq which takes advantage of 2′-O-methylation resistance to alkaline hydrolysis | – | 160 |
| m6A melting-qPCR | Harnesses capacity of different polymerases to retrotranscribe N6-adenosine methylated or unmethylated regions to do comparative RT-qPCR | Medium sensitivity | – | 161 |
| SMRTseq and nanopore | Transcriptome-wide mapping of modifications based on detecting change of kinetic incorporation of new bases or travelling through pores | High qualitative information if optimized for appropriate base detection | High error rates if not optimized | 162–165 |
| meClickseq | Click chemistry to attach small molecules to methylated substrates for targeted degradation Uses enzymes to introduce alkyne rather than methyl group |
Highly accurate | Measures depletion rather than enrichment | 166 |
| scDARTseq | Single-cell m6A profiling approach that can map methylomes of thousands of cells Reveals that most transcripts are methylated in a small fraction of cells and there is a high degree of methylation heterogeneity |
Highly sensitive for single-cell analyses | – | 5 |
| T3 DNA ligase-qPCR and SELECT | Harness changes in ligation efficiency of methylated bases T3 DNA ligase displays significantly higher selectivity for unmethylated adenosines than m6A, which can be used with qPCR to compare methylation levels in different samples In SELECT, m6A hinders Bst DNA polymerase elongation and ligation efficiency of nicks and uses qPCR for quantification |
Highly sensitive at specific sites | – | 167,168 |
Detection and high-throughput approaches for mapping of m6A in RNA offer the advantage that they can inform on both the presence of a modification in RNA and its precise location at single-nucleotide resolution. Several of the antibody-based techniques, however, have the caveat that antibodies may bind to non-specific sequences resulting in a high degree of false-positives sites. m6A, N6-methyladenosine; MazF, RNA endonuclease cleaves only non-methylated RNA in ACA sequence but not m6ACA; MAZTERseq, RNA digestion via m6A sensitive RNase; miCLIP, m6A individual nucleotide resolution crosslinking and immunoprecipitation; MS, mass spectrometry; qPCR, quantitative PCR; RT, reverse transcriptase; SCARLET, site-specific cleavage and radioactive labelling of the modified nucleotides followed by ligation-assisted extraction and thin-layer chromatography; scDARTseq, single-cell deamination adjacent to RNA modification targets; SELECT, single-base elongation-based and ligation-based qPCR amplification; SMRTseq, single-molecule real-time sequencing; TLC, thin-layer chromatography; UHPLC, ultra-high performance liquid chromatography.
Table 2 |.
Enzymes that regulate adenine methylation
| Enzymes | Substrates | Prevalence | Refs. |
|---|---|---|---|
| Methyltransferases | |||
| METTL3–METTL14 | m6A mRNAs | 25% of mRNAs3,4 | 6,169 |
| IME4 | m6A mRNAs | NA | 170 |
| METTL16 | m6A snRNA, mRNAs, and other ncRNAs | NA | 65,66,171 |
| PCIF1 | m6Am mRNA cap | 30% of mRNAs | 172–175 |
| CMTR1 | Am mRNA | NA | 176 |
| FBL | Am rRNA | 40–100%56 | 177–180 |
| TRMT61A | m1A58 tRNA | Most tRNAs181 | 182 |
| TRMT61B | m1A nuclear encoded tRNA | NA | 183 |
| TRMT10B | m1A nuclear encoded tRNA | NA | 184 |
| TRMT10C | m1A mitochondrial mRNA | NA | 185 |
| DIM1 | m6,2A 18S rRNA A1850, 1851 | NA | 186–188 |
| METTL4 | m6Am snRNA | NA | 189,190 |
| Demethylases | |||
| FTO | m6A, m6Am RNA | NA | 10,126 |
| ALKBH5 | m6A RNA | NA | 9 |
| ALKBH1 | m1A, m5C and hm5C tRNA | NA | 191,192 |
| ALKBH3 | m1A RNA and DNA | NA | 193 |
| Binding proteins | |||
| YTHDC1 | m6A mRNAs | NA | 194,195 |
| YTHDC2 | m6A mRNAs | NA | 100,101 |
| YTHDF1 | m6A mRNAs | NA | 38,196 |
| YTHDF2 | m6A mRNAs | NA | 4,41 |
| YTHDF3 | m6A mRNAs | NA | 4 |
| ELAVL1 | m6A mRNAs | NA | 4 |
| eIF3 | m6A mRNAs | NA | 37 |
| HNRNPC/G | m6A mRNAs | NA | 26,197 |
| HNRNPA2B1 | m6A mRNAs | NA | 44 |
| IGF2BP1–3 | m6A mRNAs | NA | 33 |
| FMRP | m6A mRNAs | NA | 34 |
Am, 2′-O-methyladenosine; eIF3, eukaryotic translation initiation factor 3; FMRP, fragile X mental retardation protein; hm5C, 5-hydroxymethylcytosine; HNRNP, heterogeneous nuclear ribonucleoprotein; IGF2BP, insulin-like growth factor 2 binding protein; m1A, N1-methyladenosine; m5C, C5-cytosine methylation; m6A, N6-methyladenosine; m6,2A, N6,N6-dimethyladenosine; m6Am, N 6,2′-O-dimethyladenosine; NA, not available; ncRNA, non-coding RNA; rRNA, ribosomal RNA; snRNA, small nuclear RNA.
Approximately 25% of mammalian mRNAs contain m6A, with an average of one to three modifications per transcript3,4. However, m6A-modified transcripts seem to be highly heterogeneous from cell to cell5. For mRNA and other RNA Polymerase II-synthesized transcripts (including most small RNAs and microRNAs (miRNAs)), the METTL3–METTL14–WTAP methyltransferase complex is mainly responsible for the deposition of m6A in newly synthesized transcripts in the cell nucleus6 (FIG. 1a and TABLE 2). m6A was found to be enriched in conserved locations in the 3′ untranslated regions (UTRs) and near stop codons of transcripts that regulate differentiation and development, whereas it is relatively depleted in housekeeping transcripts3,4. Mapping has refined the conserved sequence of m6A to the motif DRACH (where D = A, G or U; R = A or G; H = A, C or U)3,4,7,8. Importantly, m6A in mRNA is dynamic and reversible, as the methyl group can be removed by the action of the demethylases ALKBH5 (REF.9) and FTO10, restoring the methylated base back to the canonical adenine base (FIG. 1a). By contrast, m6A modification of rRNAs is thought to be constitutive. Unique highly conserved sites in the 28S and 18S rRNA of eukaryotes11,12 are N6-adenosine methylated by ZCCHC4 (REFS.13–16) and METTL5 (REFS.16–23), respectively.
Fig. 1 |. Molecular consequences of m6A modification of mRNA.

a | N6-Adenosine methylation is a dynamic modification that occurs in the nucleus and is regulated by both a methyltransferase complex (METTL3–METTL14–WTAP) and by demethylases (FTO and ALKBH5). N6-Methyladenosine (m6A) has been implicated in regulating various nuclear and cytoplasmic processes in the mRNA life cycle, including subcellular localization, splicing (part b), export (part c), stability or degradation (part d) and translation (part e). b | m6A regulates pre-mRNA splicing by both direct and indirect mechanisms. In a direct mechanism, YTHDC1 binding to m6A leads to exon inclusion by blocking binding of SRSF10 and recruitment of SRSF3. In the absence of N6-adenosine methylation, SRSF10 binds resulting in exon skipping. In an indirect mechanism, termed an m6A switch, m6A induces a conformational change in RNA folding, exposing a heterogeneous nuclear ribonucleoprotein C (HNRNPC) binding motif, which leads to HNRNPC recruitment and exon retention. c | N6-Adenosine methylation facilitates mRNA nuclear export. Binding of YTHDC1 to N6-adenosine methylated transcripts promotes export by mediating interactions with SRSF3 and nuclear export mediator NXF1. Alternatively, binding of fragile X mental retardation protein (FMRP) to m6A facilitates export by mediating a physical association with the nuclear export protein CMR1. d | m6A can promote or inhibit mRNA degradation. YTHDF2 promotes the degradation of m6A-modified transcripts by recruiting the CCR4–NOT1 deadenylase complex. By contrast, insulin-like growth factor 2 binding proteins (IGF2BPs) bind m6A-containing mRNAs and protect them from degradation. e | m6A either promotes or slows translation depending on its location within the mRNA. If the methylated adenosine is located within the 5′ untranslated region (UTR), binding of eukaryotic translation initiation factor 3 (eIF3) recruits the 43S translation initiation complex to promote cap-independent translation. If m6A occurs in the 3′ UTR, YTHDF1 or YTHDF3 will bind and stimulate translation. However, if m6A is localized in the coding region it can slow translation elongation rates by inhibiting efficient tRNA selection. m7G, N7-methylguanosine; TF, transcription factor.
m6A elicits a wide range of effects on RNA through changes in RNA stability, conformation and folding or by directly modulating the interactions of modified RNAs with binding proteins that affect RNA fate and function. Although m6A does not prevent Watson–Crick A:U base pairing, it does have a subtle destabilization effect on RNA24, which has been proposed to be important in regulating certain types of interactions, such as between miRNAs and mRNA25,26. m6A also destabilizes Hoogsteen base pairing27 and can inhibit folding of RNA in a sequence-dependent fashion28. The functional consequences of m6A are not restricted to the modified RNAs; once methylated RNAs are degraded, methylated adenosines can also function as signalling molecules by acting as ligands for the adenosine A3 receptor29. However, the majority of the functional consequences of m6A methylation in RNA fate and function are thought to be mediated by m6A-binding proteins (TABLE 2) (reviewed elsewhere30,31). In humans, the best characterized m6A-binding proteins belong to the YTH family3,4,30,32, but other binding proteins include insulin-like growth factor 2 binding proteins (IGF2BPs)33 and fragile X mental retardation protein (FMRP)34. By contrast, the presence of m6A has been shown to prevent binding of certain proteins, including the G3BP stress granule proteins34.
In this Review, we focus on the functions of RNA m6A methylation at the molecular, genomic and organismal level. We describe the functional consequences of m6A deposition on RNA substrates, including mRNA, rRNAs and regulatory RNAs. We review recent advances that clarify the mechanisms by which RNA methylation has an impact on chromatin architecture, epigenetic regulation of gene expression and genome stability. Finally, we summarize the key roles of m6A in fundamental biological processes during early development and in adult tissue homeostasis, including stem-cell differentiation, neurogenesis and brain function, and innate immune response, as well as m6A dysregulation in diseases, such as cancer.
Molecular consequences of m6A on RNA
At the molecular level, m6A participates in nearly every aspect of RNA metabolism, including mRNA expression3,4, splicing4,26,35, nuclear export9,36, translation efficiency37–40, RNA stability41,42 and miRNA processing43,44. Its effect on these processes seems to be largely regulated by binding proteins. However, the specific determinants that dictate whether an m6A-modified RNA is regulated at the level of splicing, export, stability or translation, or at multiple levels, are still unclear.
m6A mediates pre-mRNA splicing and nuclear export.
m6A affects the alternative splicing of mRNAs either through its direct interaction with nuclear reader YTHDC1 or through an ‘m6A switch’ mechanism involving indirect binding of heterogeneous nuclear ribonucleoproteins (HNRNPs), which are abundant nuclear proteins known to regulate pre-mRNA processing (FIG. 1b). Work in Drosophila melanogaster45,46 and human cell lines47 demonstrated that YTHDC1 binds directly to m6A-modified mRNAs and modulates alternative splicing by recruiting the splicing factor SRSF3, which promotes exon inclusion, and by inhibiting binding of splicing factor SRSF10 to drive exon skipping (FIG. 1b). In an example of the m6A-switch mechanism, m6A affects the conformation of local RNA structure by exposing the HNRNPC binding motif, which in turn promotes exon retention26 (FIG. 1b). Future work will need to decipher how specificity of splicing is determined for interactions with YTHDC1 or HNRNPs.
m6A-Binding proteins also facilitate interactions with nuclear export components to regulate the subcellular localization of modified mRNAs. YTHDC1 binds to the export proteins SRSF3 and NXF1, and FMRP physically associates with the export protein CRM1 to accelerate the export of N6-adenosine methylated transcripts from the nucleus48–50 (FIG. 1c). Thus, m6A seems to facilitate shuttling of mRNAs to the cytoplasm for translation by multiple mechanisms. It remains to be determined how nuclear export specificity is determined and whether these two pathways function independently or coordinate to facilitate nuclear export and subsequent processing in the cytoplasm.
m6A regulates mRNA degradation and stability.
Initial work in mouse embryonic stem (mES) cells demonstrated that m6A-modified mRNAs are less stable than unmodified transcripts, resulting in their reduced abundance and translation potential51. Subsequent studies showed that m6A-marked transcripts are actively targeted for degradation through direct binding to YTHDF2 (REFS.52,53), which recruits the CCR4–NOT deadenylase complex, a complex responsible for mRNA decay52 (FIG. 1d). Alternatively, YTHDF2 can physically interact with HRSP12 to recruit RNaseP/MRP endoribonucleases to cleave m6A transcripts53. However, the effects of m6A on stability seem to be complex, as binding of IGF2BPs to methylated mRNAs stabilizes them by protecting them from degradation33 (FIG. 1d). Furthermore, work in the parasite Trypanosoma brucei found that when methylation of the polyA tail of transcripts occurs in conjunction with variant surface glycoproteins, there is an increase in mRNA stability42. Taken together, these data suggest that additional factors determine whether m6A-containing transcripts are degraded or stabilized; additional work is needed to determine what these factors might be.
m6A promotes or inhibits mRNA translation in a context-dependent manner.
m6A promotes translation rates by multiple mechanisms depending on the location of m6A within the mRNA transcript. If m6A is located at the 5′ UTR, it can promote cap-independent translation in response to stress through binding of eukaryotic translation initiation factor 3 (eIF3), which directs the recruitment of the 43S translation complex to initiate translation37 (FIG. 1e). When m6A is located in the 3′ UTR, it can promote cap-dependent translation through direct interactions with YTHDF1 or YTHDF3, resulting in enhanced translation38,54 (FIG. 1e). YTHDF1 is postulated to affect translation initiation at the 5′ end of the transcript through a physical interaction with eIF3 that results in the looping of YTHDF1 to the transcriptional start site38 (FIG. 1e). However, m6A on mRNAs has also been shown to inhibit translation by affecting the interaction of transcripts with tRNAs, thereby slowing translation elongation55 (FIG. 1e).
m6A on rRNA refines ribosome binding.
rRNAs are modified at more than 200 nucleotides56 (Box 1). However, they are N6-adenosine methylated at only two conserved positions, corresponding to adenosine 1832 of human 18S rRNA and adenosine 4220 of human 28S rRNA. Recent discovery of the 28S rRNA methyltransferase ZCCHC4 in mammals13,14 and the 18S rRNA methyltransferase METTL5 in various Metazoans16–21 has begun to shed light on the functional role of these methylations in regulating translation (FIG. 2a). Genetic knockout of ZCCHC4 in mammalian cell lines resulted in a reduction of global translation and inhibition of cell proliferation of hepatocellular carcinoma cell lines13. Loss of 18S m6A methylation by genetic knockout or knockdown of Mettl5 resulted in a wide range of phenotypes, including loss of pluripotency and decreased differentiation potential in mES cells18,21, increased stress resistance in Caenorhabditis elegans17 and behavioural defects in D. melanogaster20. Together, these findings raise the possibility that m6A in rRNAs can expand ribosome heterogeneity, which could induce the translation of specific sets of transcripts under different contexts or in response to diverse stimuli to regulate specific cellular processes.
Fig. 2 |. Molecular consequences of m6A modification of non-coding RNAs.

a | N6-Adenosine methylation of ribosomal RNAs (rRNAs) can promote translation or specify mRNAs to be translated. ZCCHC4 N6-methylates adenosine 4220 in human 28S rRNA resulting in increased translation and inhibition of cell proliferation. METTL5-mediated N6-methylation of adenosine 1832 on human 18S rRNA seems to result in the selective increased translation of a unique sets of transcripts. b | N6-Adenosine methylation facilitates microRNA (miRNA) processing. N6-Methyladenosine (m6A) deposition on primary (pri)-miRNAs is recognized by the heterogeneous nuclear ribonucleoprotein HNRNPA2B1, which in turn facilitates their processing to precursor (pre)-miRNAs via the Drosha–DGCR8 microprocessor complex. c | m6A regulates circular RNA (circRNA) biogenesis and function. m6A induces back-splicing of circRNA through a YTHDC1-dependent mechanism. In addition, YTHDF3 binds to m6A-modified circRNAs to enhance cap-independent translation, resulting in protein synthesis from circRNA transcripts. d | m6A regulates small nuclear RNAs (snRNAs) to facilitate spliceosome assembly. METTL16-dependent m6A deposition on U6 snRNAs regulates spliceosome assembly or 5′ splice site recognition. N6,2′-O-Dimethyladenosine (m6Am) modification of U2 snRNAs by METTL4 increases splicing whereas FTO demethylation of U2 snRNAs inhibits splicing.
m6A mediates microRNA processing.
In mRNAs, m6A and miRNA binding sites are both enriched in the 3′ UTR and near stop codons, leading to the proposal that crosstalk might occur between them in miRNA-dependent control of gene expression3. Indeed, m6A is enriched at miRNA binding sites in mouse embryonic fibroblasts and human cancer cell lines57–59, and depletion of Dicer, a regulator of biogenesis of mature miRNA, results in reduced m6A deposition in these cells. Whether m6A on mRNAs can have an impact on the targeting of miRNAs is still unclear. However, m6A directly affects miRNA processing and biogenesis of mature miRNAs. The mirNa microprocessor complex subunit DGCR8 is recruited to m6A-modified primary (pri)-miRNAs, usually via the HNRNPA2B1 m6A-binding protein, and pri-miRNAs are then processed to mature miRNAs; depletion of m6A from pri-miRNAs disrupts this process and leads to lower levels of mature miRNAs43,44 (FIG. 2b). These findings begin to illustrate how m6A could affect the biogenesis of specific miRNAs, and thereby add another level of regulation to miRNA-mediated gene silencing.
m6A promotes circular RNA biogenesis and function.
Circular RNAs (circRNAs) are frequently produced by back-splicing of exons, whereby a 5′ splice donor site is fused to an upstream 3′ splice acceptor site60. The functions of circRNAs are just beginning to be characterized, but some circRNAs suppress specific miRNAs by functioning as miRNA ‘sponges’61,62. Growing in vitro and in vivo evidence suggests that N6-adenosine methylation of circRNAs regulates their biogenesis and translation potential63,64. A single m6A is sufficient to initiate cap-independent translation of an artificial circRNA reporter construct63 and can increase circRNA biogenesis and stimulate cap-independent translation from an open reading frame in circ-ZNF609 via binding of YTHDF3 and eIF4G2 (REF.64) (FIG. 2c). Although the functions of polypeptides derived from circRNA-encoded open reading frames are still unknown, the fact that circRNA biogenesis and translation are modulated by m6A implies that these by-products of alternative splicing of exons have important regulatory functions.
m6A modulates spliceosome assembly and function.
The deposition of m6A in snRNAs, such as the spliceosome component U6 snRNA, is mediated by the METTL16 methyltransferase65,66. METTL16 specifically modifies adenosine 43 (A43) in U6 snRNA65,66, but it is unclear whether this modification is constitutive or reversible (FIG. 2d). Interestingly, A43 of U6 snRNAs interacts with the 5′ splice site of pre-mRNAs and it has been proposed that A43 methylation might fine-tune snRNA–pre-mRNA interactions, thereby regulating spliceosome assembly at, or recognition of, the 5′ splice site66. These findings suggest that m6A can have a dual role in pre-mRNA splicing: in addition to regulating alternative splicing in subsets of m6A-modified transcripts as discussed above, it can also directly modulate spliceosome function.
m6A, chromatin and genome integrity
In the past decade, it has become increasingly clear that N6-adenosine methylation is not only instrumental in regulating RNA fate and metabolism but also has broader roles in epigenetic regulation of gene expression, in shaping genomic architecture and maintaining genomic stability. These effects of m6A on genome stability and function were found to be mediated by various types of RNAs, including nascent pre-mRNA transcripts, long non-coding RNAs (lncRNAs), chromosome-associated regulatory RNAs (carRNAs), endogenous retrovirus RNAs (ERVs) and R-loops.
m6A in pre-mRNA mediates crosstalk with chromatin modifications.
Several lines of evidence indicate extensive crosstalk between m6A in pre-mRNAs and chromatin modifications, providing an additional layer of regulation in the control of gene expression and genome stability. Mapping experiments show that m6A on mRNAs overlaps significantly with high levels of histone H3 trimethylated at lysine 36 (H3K36me3) at the corresponding genomic location, and deletion of the H3K36 trimethyltransferase SETD2 results in global reduction of m6A levels and reduced levels of METTL3 complexes binding to target mRNAs67. Interestingly, METTL14 binds to H3K36me3-modified histones and recruits METTL3 to sites that are actively transcribed by RNA polymerase II so that m6A is deposited co-transcriptionally67. Reciprocally, co-transcriptional N6-adenosine methylation of nascent mRNA transcripts facilitates the removal of H3 dimethylated at lysine 9 (H3K9me2), a traditionally repressive histone modification68, via recruitment of the H3K9me2 demethylase KDM3B, which physically interacts directly with the m6A-binding protein YTHDC1 (REF.68) (FIG. 3a). The N6-adenosine methyltransferase complex also directly regulates transcription by inducing the release of RNA polymerase II from a paused state69, and the presence of m6A inhibits binding of the transcriptional termination integrator complex70. This coordinated communication between m6A and the chromatin modifying machinery further highlights how epitranscriptomic and epigenetic modifications form a complex regulatory network with multiple modules and switches that increase the flexibility of gene expression control.
Fig. 3 |. Genomic consequences of m6A.
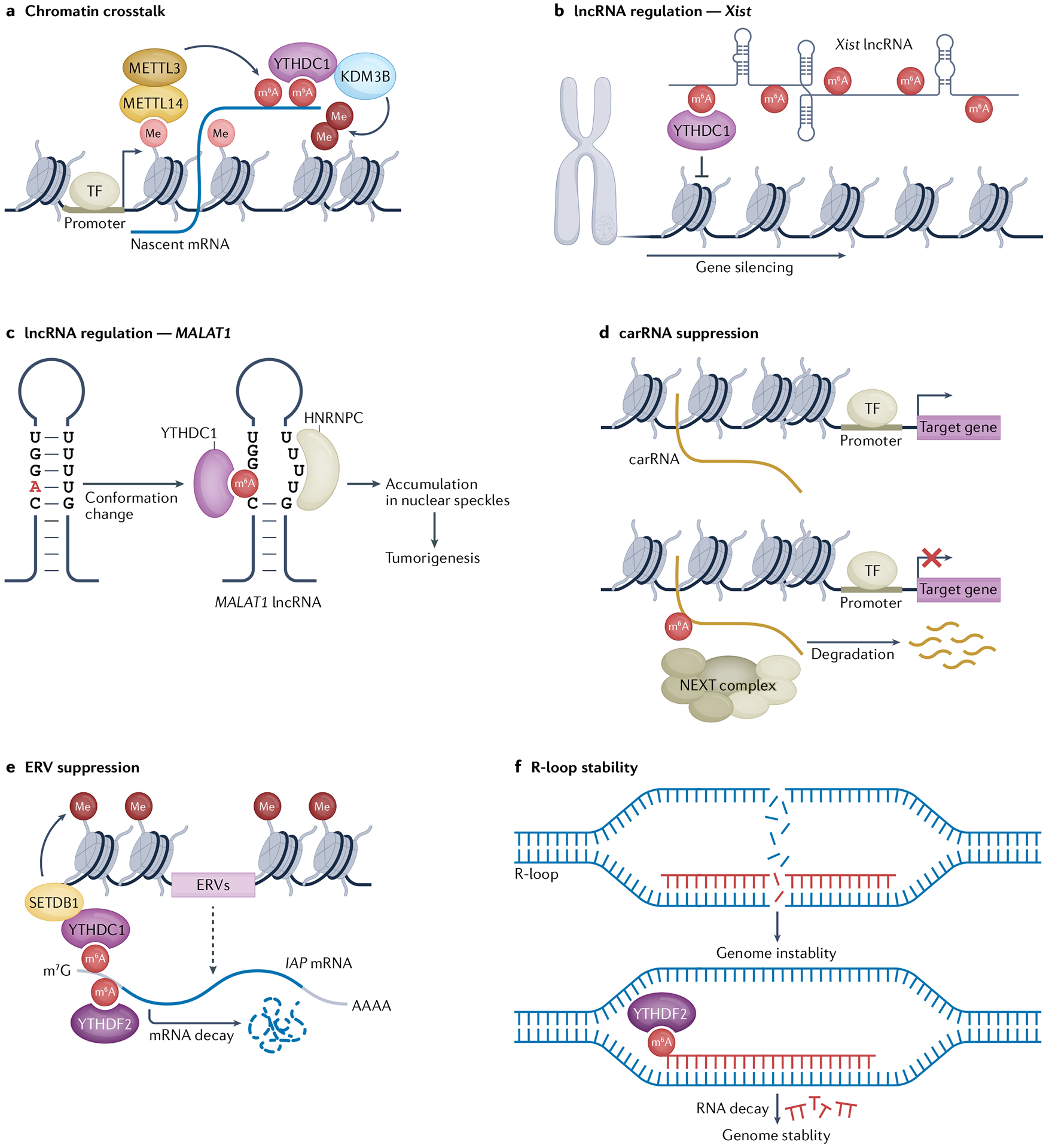
a | Crosstalk between N6-methyladenosine (m6A) and chromatin modifications reinforces epigenetic signatures. YTHDC1 can bind to both m6A-modified transcripts and to the histone H3 lysine 9 (H3K9) demethylase KDM3B to induce histone demethylation to reinforce chromatin accessibility in regions being actively transcribed. Moreover, methylation of H3 lysine 36 (H3K36) can recruit the METTL3–METTL14 N6-adenosine methylation complex to m6A-methylate nascent transcripts in regions of active chromatin. b | m6A regulates activity and folding of long non-coding RNAs (lncRNAs). Xist lncRNA is N6-adenosine methylated at many locations and m6A promotes Xist-mediated gene silencing and X chromosome inactivation in a YTHDC1-dependent manner. c | N6-Adenosine methylation of MALAT1 lncRNA induces a conformational change, which leads to heterogeneous nuclear ribonucleoprotein C (HNRNPC) binding and alterations in nuclear organization and tumorigenesis. d | N6-Adenosine methylation of chromosome-associated regulatory RNAs (carRNAs) causes their degradation via the nuclear exosome targeting complex (NEXT) complex, resulting in gene repression. e | m6A facilitates endogenous retrovirus RNA (ERV) suppression by multiple mechanisms to maintain genome stability. m6A-modified intracisternal A-particle (IAP) mRNA is bound by YTHDC1, which recruits the histone H3K9 methyltransferase SETDB1, resulting in the deposition of repressive chromatin marks. In addition, YTHDF2 binds to m6A-modified IAP transcripts to induce degradation of IAP mRNAs. f | m6A prevents genome instability caused by RNA:DNA hybrids (R-loops). N6-Adenosine methylation of RNA in R-loops results in YTHDF2-dependent RNA degradation, which suppresses the formation of R-loops and prevents genomic instability. m7G, N7-methylguanosine; TF, transcription factor.
m6A can also indirectly affect chromatin modifications, and presumably other methylated substrates, by regulating levels of the predominant methyl donor, S-adenosyl methionine (SAM). METTL16, whose homologue is essential for mouse embryonic development71, mediates m6A methylation of a hairpin in the 3′ UTR of MAT2A pre-mRNA (encoding the SAM synthetase protein). When unmodified, this hairpin inhibits splicing of MAT2A transcripts, causing intron retention and RNA decay, thereby limiting SAM levels65. However, when the levels of SAM are too low, METTL16 is recruited to and methylates the 3′ UTR hairpin, which facilitates efficient splicing of MAT2A pre-mRNA and stimulates SAM synthesis65. By contrast, in C. elegans, METT-10 (the worm ort-hologue of METTL16) inhibits endogenous SAM bio-synthesis via a negative feedback loop in response to changes in diet that increase intracellular SAM levels72; METT-10-mediated N6-adenosine methylation of sams3 transcripts (which encode SAM synthetase) at their 3′ splice sites inhibits their splicing, leading to lower levels of SAM72. Maintaining appropriate SAM levels via METT-10/METTL16 seems to be a conserved process, albeit through inhibition or promotion of SAM synthesis depending on the organism. This work exemplifies some of the crosstalk that mRNA methylation can have with other epigenetic cues including histone methylation.
m6A in lncRNAs facilitates oncogenesis and X chromosome gene silencing.
Initial mapping in mouse tissue and human cell line studies revealed that m6A is prevalent in lncRNAs3,4, and subsequent studies have revealed important biological consequences of m6A for the functions of the lncRNAs Xist (which has a pivotal role in X chromosome inactivation)73 and Malat1 (an abundant nuclear lncRNA that is often upregulated in metastatic cancers).
Directed by the lncRNA Rmb15, METTL3 methylates 78 sites in lncRNA Xist74, which causes YTHDC1 to bind to Xist, an interaction that is required for X chromosome inactivation (FIG. 3b). In an elegant experiment, the artificial tethering of YTHDC1 to Xist bypasses the requirement for m6A for transcriptional repression of the X chromosome, indicating that the primary function of m6A is to recruit YTHDC1 to Xist74 (FIG. 3b). However, the extent of the involvement of m6A in X chromosome inactivation and how YTHDC1 influences Xist-mediated gene silencing remain unclear, although it has been proposed that YTHDC1 facilitates the interaction of Xist with gene silencing proteins74,75.
Malat1 is known to localize to nuclear speckles, associate with splicing factors and affect the expression of oncogenes through interactions with transcription factors and chromatin modifying enzymes76. It has recently been shown that m6A modifications in Malat1 create a scaffold for the recruitment of YTHDC1 in nuclear speckles. Both the m6A modifications in Malat1 and the YTHDC1 localization to nuclear speckles seem to be necessary for the oncogenic effects of Malat1 (REF.77). N6-Adenosine methylation of Malat1 has also been proposed to destabilize an RNA hairpin, thereby exposing an RNA-binding motif and promoting interaction with HNRNPC26 (FIG. 3c); although the functional consequences of this interaction remain unclear, binding presumably facilitates the localization of HNRNPC to nuclear speckles.
As lncRNAs have been shown to play roles in various processes, ranging from regulating the immune system, to development, to regulating genome stability and ageing78, the prevalence of m6A on lncRNAs could, in turn, affect each of these processes by altering binding of specific lncRNAs to proteins, RNAs or DNA or through modulation of local RNA structure.
m6A in carRNAs and ERVs suppresses expression of repetitive elements.
m6A on regulatory RNA species could facilitate communication between RNAs and chromatin to help reinforce the functional consequences of m6A. Deletion of the m6A methyltransferase Mettl3 or binding protein Ythdc1 in mES cells resulted in reduced m6A levels on several classes of carRNAs, such as promoter-associated RNAs, enhancer RNAs and repeat RNAs, and in broad increases in chromatin accessibility and transcriptional activation79,80. m6A was shown to facilitate the degradation of certain carRNAs, such as long interspersed element 1 (LINE-1; also known as L1) repeat RNAs, by recruitment of the nuclear exosome targeting complex (NEXT)79 (FIG. 3d). As L1 repeat RNAs promote chromatin accessibility during transcriptional activation, the m6A-mediated decay of specific carRNAs might regulate the chromatin state during mES cell homeostasis.
Suppression of expression from large swaths of repetitive DNA is essential for cell viability and the regulated expression of appropriate transcripts. Repetitive elements and retrotransposons can cause genomic instability by inducing recombination-mediated chromosomal rearrangements, such as deletions, insertions and trans-locations, that affect the expression of nearby genes or by causing mutations through transposition events in the genome81. In a genome-wide CRISPR–Cas9 screen in mES cells, several components of the Mettl3 complex were shown to be required for the repression of the intracisternal A-particle (IAP)-type family of ERVs82. The suppression of these IAPs is mediated by N6-adenosine methylation of the 5′ UTRs of IAP mRNAs, which promotes their decay through an YTHDF2-dependent mechanism82 (FIG. 3e). METTL3 also facilitates heterochromatin formation and subsequent transcriptional repression by binding and recruiting the H3K9me3 methyltransferase SETDB1 to IAP-encoding genomic regions83 (FIG. 3e).
Taken together, these studies reveal that N6-adenosine methylation can facilitate chromatin reorganization to regulate the appropriate suppression of retrotransposons to safeguard genome stability.
m6A promotes genome stability at R-loops.
R-loops are frequently formed during transcription and have roles in transcription initiation and elongation, replication and DNA repair84. Interestingly, the RNA strand of R-loops is often N6-adenosine methylated by METTL3 in human pluripotent stem cells85. Depletion of the m6A-binding protein YTHDF2 results in accumulation of m6A-methylated R-loops, leading to cell growth retardation and increased accumulation of histone γH2AX, a marker of DNA double-strand breaks85. These results suggest that m6A in R-loops mediates R-loop removal, thereby safeguarding genome stability, but the underlying mechanism of YTHDF2-mediated R-loop degradation remains unclear (FIG. 3f). There may be a dual or context-dependent role for m6A in R-loop formation and maintenance, as an earlier study performed in HeLa cells suggested that m6A promotes R-loop formation to facilitate transcription termination86. Interestingly, DNA damage signals induce the ataxia-telangiectasia mutated (ATM) protein to phosphorylate METTL3, which is then recruited to sites of double-strand breaks in R-loops where it methylates the RNA strand at N6-adenosines87. Subsequent recruitment of YTHDC1 causes an increased accumulation of RNA:DNA hybrids, which results in the recruitment of RAD51 and BRCA1 to drive homologous recombination-mediated repair87. Therefore, the N6-adenosine methylation of RNA in R-loops seems to induce a multipronged, context-specific response that can regulate numerous biological consequences of R-loop formation.
m6A in development and differentiation
Given the emerging roles of m6A as a versatile modification that regulates RNA metabolism, protein synthesis, gene expression and genome stability, it is unsurprising that m6A regulates diverse biological processes during development, differentiation and adult tissue homeostasis. The importance of m6A in early developmental decisions is underscored by the fact that METTL3 and METTL14 are essential for mouse and plant embryonic development88–90. In this section, we explore the roles of m6A in stem-cell renewal and differentiation, and in developmental transitions.
m6A facilitates ES cell self-renewal and differentiation.
The role of m6A in regulating the stem-cell state is highlighted by the observation that genetic knockdown of components of the METTL3–METTL14 complex in mES cells causes a loss of self-renewal capacity owing to the increased stability of N6-adenosine methylated pluripotency factors such as Nanog, SOX2 and KLF4 (REFS.51,88,91). Furthermore, genetic knockout of METTL3 or METTL14 in mES cells and human ES cells disrupts their exit from the self-renewal pathway and progression towards differentiation91, resulting in a failure to undergo lineage priming and transition to the post-implantation primed stage leading to embryonic lethality88,89. METTL3 complex localization is regulated by two opposing zinc-finger proteins. ZFP217, a transcriptional activator of core pluripotency genes, binds to and sequesters METTL3, thereby limiting m6A deposition on transcripts required for pluripotency, resulting in impaired stem-cell renewal92. Conversely, ZC3H13 anchors the m6A methyltransferase complex in the nucleus of mES cells, facilitating m6A deposition and promoting stem-cell renewal93 (FIG. 4a). Importantly, the deposition of m6A on transcripts encoding pluripotency factors is responsive to extracellular signalling cues. Activation of TGFβ–activin signalling in human pluripotent stem cells leads to the physical association of transcription factors SMAD2/3 with the METTL3–METTL14–WTAP methyltransferase complex, promoting m6A deposition on a subset of mRNAs encoding pluripotency factors, including Nanog94. Conversely, inhibition of TGFβ–activin signalling destabilizes these mRNAs and causes their rapid degradation, allowing a timely exit from pluripotency and the induction of neuroectoderm differentiation94. Precisely how the changes in N6-adenosine methylation are interpreted is still unclear. The YTHDF2 binding protein is a good candidate for interpreting changes in m6A in stem cells as it has recently been implicated in destabilizing neural-specific RNA transcripts during induced pluripotent stem cell differentiation95. However, owing to the functional redundancy of YTHDF proteins, it is plausible that other members of the family might also contribute to the degradation of core pluripotency factor transcripts96. Taken together, these findings underscore the importance of m6A in regulating ES cell renewal, differentiation and pluripotency through marking key transcripts that maintain cell state or facilitate differentiation.
Fig. 4 |. m6A is a critical modification during early development and embryogenesis.
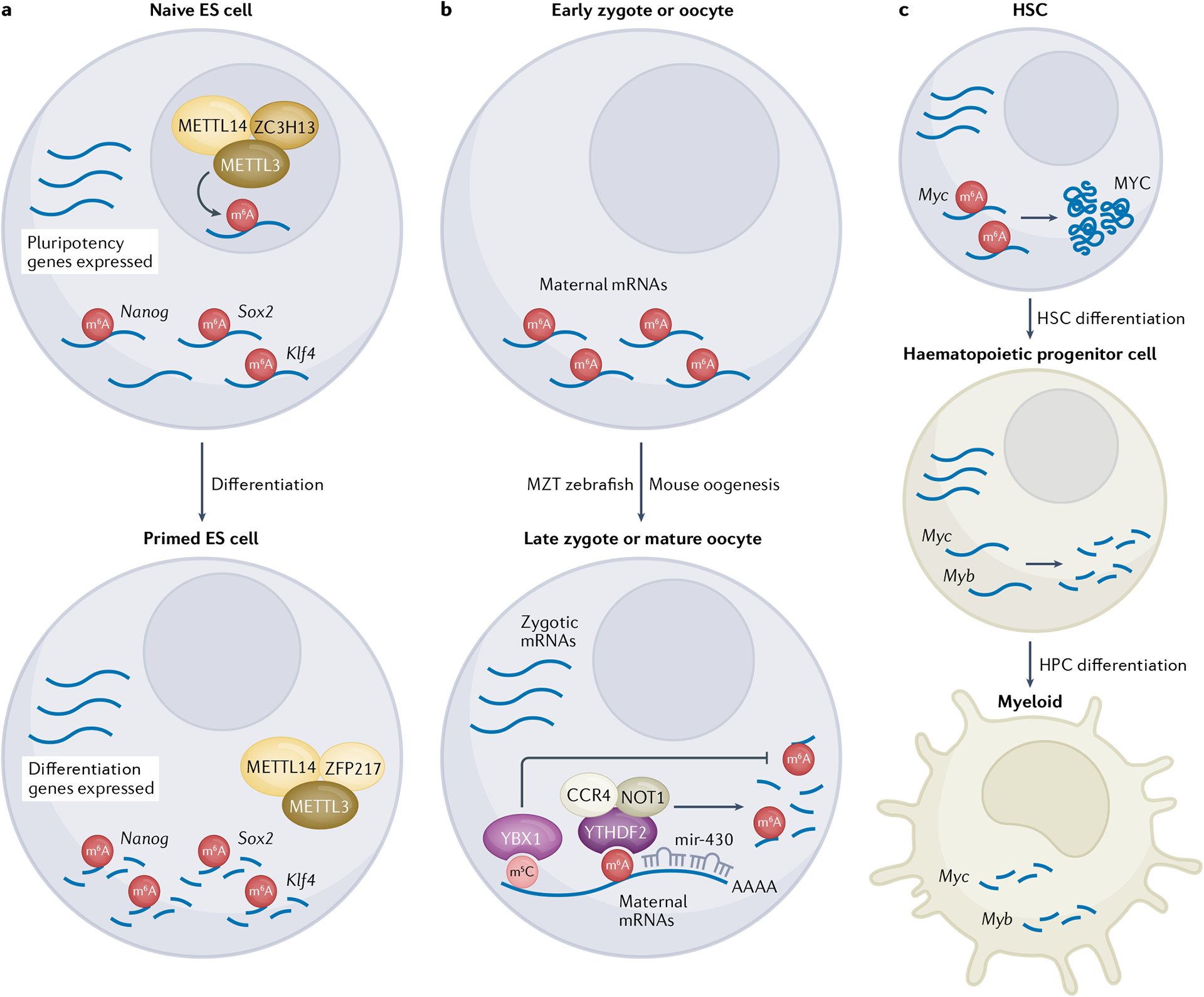
a | N6-Methyladenosine (m6A) modulates embryonic stem (ES) cell renewal and differentiation. Transcripts encoding ES cell pluripotency factors, such as SOX2 and NANOG, are selectively m6A-modified in the nucleus of naive ES cells by the METTL3–METTL14 methyltransferase complex in conjunction with the zinc-finger protein ZC3H13. m6A-dependent degradation of pluripotency factor mRNAs drives ES cell differentiation to the primed state, whereas new m6A deposition is inhibited by sequestration of the METTL3–METTL14 complex to the cytoplasm by the zinc-finger protein ZFP217. b | m6A facilitates clearance of maternal mRNAs during the earliest developmental stages of zebrafish and mice. During zebrafish maternal-to-zygotic transition (MZT), YTHDF2 binds to m6A-modified transcripts to direct degradation via the CCR4–NOT deadenylase complex. Binding of the microRNA mir-430 to maternal transcripts further promotes their clearance. By contrast, C5-cytosine methylation (m5C) of maternal transcripts promotes stabilization. c | m6A promotes haematopoietic stem cell (HSC) differentiation at several points in the differentiation pathway. m6A deposition on Myc mRNA stimulates MYC protein synthesis, which promotes HSC differentiation to haematopoietic progenitor cells. Subsequently, m6A-dependent degradation of Myc and Myb transcripts drives haematopoietic progenitor cell (HPC) differentiation to the myeloid cell lineage.
m6A facilitates developmental transitions.
During oocyte maturation and early embryogenesis, development is driven by the inherited pool of maternal mRNA transcripts. The activation of zygotic transcription occurs during the tightly regulated maternal-to-zygotic transition (MZT)97. In zebrafish, N6-adenosine methylation of mRNAs plays a critical role in the clearance of maternal mRNAs, as approximately one third of maternal transcripts are m6A-modified and elimination of YTHDF2 by genetic knockout limits the decay of the maternal mRNAs and delays development98. Interestingly, YTHDF2 may not function on its own to recognize m6A-modified maternal transcripts, as all three YTHDF binding proteins act redundantly to control MZT and oogenesis99 (FIG. 4b). Similarly, m6A is important for mouse oocyte maturation, which is also characterized by a wave of degradation of maternal mRNAs when oocytes proceed through meiosis (M-decay) and another wave in the early zygote during MZT (Z-decay). The effects of METTL3-dependent m6A deposition during mouse oocyte differentiation are mediated by the coordinated activity of YTHDF2, YTHDC1 and YTHDC2 (REFS.100–104). Oocyte-specific inactivation of YTHDF2 causes infertility owing to impaired degradation of a subset of maternal transcripts at or prior to the two-cell stage103. Moreover, knockdown of Mettl3 in developing oocytes impairs maturation and MZT due to decreased mRNA translation and inefficient maternal mRNA clearance105. m6A coordinates with other regulatory pathways to rapidly clear maternal mRNAs during MZT. In zebrafish, mir-430 is induced during MZT and facilitates the deadenylation and clearance of hundreds of maternal transcripts through binding to their 3′ UTRs106. The co-occurrence of m6A modifications and the miR-430 seed in the 3′ UTR seems to accelerate the destabilization of maternal mRNAs99 (FIG. 4b). Interestingly, other RNA modifications have also been implicated in maternal mRNA clearance during MZT in zebrafish. In contrast to m6A, binding of YBX1 to a pool of C5-cytosine methylation (m5C)-modified maternal mRNA was shown to increase their stability86 (FIG. 4b). Moreover, addition of a uracil tail to maternal mRNAs by the terminal uridylyltransferases TUT4 and TUT7 facilitates their clearance during MZT107. It will be important in future studies to decipher how m6A on maternal mRNAs communicates with miRNA-mediated regulation and to examine whether an epitranscriptomic code exists — a mechanistic crosstalk between m6A and the other RNA modifications — which facilitates the read-out of multiple mRNA modifications to elicit a stronger biological consequence.
N6-Adenosine methylation also seems to be critical for the endothelial-to-haematopoietic transition during zebrafish embryogenesis, a process governed by numerous interconnected signalling pathways, including NOTCH1 signalling, which drives haematopoietic stem and progenitor cell specification108. Genetic knockout of mettl3 in zebrafish embryos blocks the production of haematopoietic stem/progenitor cells during endothelial to haematopoietic transition109,110, and these effects were shown to be mediated by YTHDF2-directed degradation of Notch1a mRNA, resulting in reduced NOTCH1 signalling. This m6A-mediated regulation of endothelial-to-haematopoietic transition through NOTCH signalling is conserved in mice109.
m6A regulates adult stem-cell differentiation.
N6-Adenosine methylation of mRNA is also important for the homeostasis and differentiation of adult stem-cell lineages, including haematopoietic stem cells (HSCs) and neuronal stem cells (NSCs), which is underscored by the emerging roles of m6A in cancer stem-cell biology especially in acute myeloid leukaemia (AML) and glioblastoma (see below). Deletion of Mettl3 in adult mouse HSCs resulted in their impaired differentiation in vivo111. The role of m6A in regulating HSCs seems to be mediated by the N6-adenosine methylation of Myc mRNAs, which enhances their translation, with the resulting increase in MYC protein driving HSC differentiation111 (FIG. 4c). Interestingly, the results observed for a Mettl3-knockout mouse contradict earlier studies in human HSC cells, which reported that knockdown of METTL3 and METTL14 impairs human progenitor haematopoietic stem and progenitor cell self-renewal and leads to myeloid differentiation by limiting expression of the MYB and MYC transcription factor112,113. These discrepancies might arise from differential effects of m6A methylation in progenitor cells versus adult HSC cells or in differences between knocking out and knocking down the mRNA m6A regulatory machinery. It will be important for future studies to resolve the role of m6A in adult HSC differentiation and to identify which binding proteins mediate the effects of m6A on MYC and other developmental regulators.
m6A also affects NSC renewal and differentiation in the cortex of the mouse brain. m6A depletion by Mettl14 knockout or Mettl3 knockdown prolongs the cell cycle and causes delayed differentiation of NSCs owing to increased stability of transcripts encoding neural developmental regulators that are normally kept at low levels114. Mouse NSCs lacking Mettl14 show decreased proliferation and premature differentiation, suggesting a defect in their self-renewal potential115. Interestingly, this study demonstrated that m6A destabilizes the mRNAs of histone modifying enzymes, suggesting a model by which m6A influences NSC self-renewal and differentiation through regulation of chromatin structure115. The importance of m6A in neural lineage differentiation is further highlighted by the revelation that Ythdf2-knockout mice exhibit delayed cortical neurogenesis owing to impaired degradation of m6A-modified mRNAs associated with neuronal development and differentiation116. Moreover, knockout of the gene encoding the FTO demethylase causes defects in neurogenesis, which lead to impaired learning and memory owing to dysregulated gene expression in the hippocampus117. Taken together, these data suggest that m6A can regulate NSC renewal and differentiation by multiple mechanisms that involve the destabilization of neural development regulators and alterations in chromatin structure.
Organism-level effects of m6A
m6A is dynamically regulated in response to numerous physiological stimuli, and has been implicated in the control of numerous homeostatic mechanisms in the cell, including the UV-induced and acute restraint stress responses, circadian rhythm regulation, neurobehaviour and the innate immune system responses. Given the wide-ranging role of m6A in maintaining homeostasis, when m6A regulation goes awry, disease such as cancer develops118,119. In the following section, we focus on three emerging roles of m6A: neuronal synaptic function and neurobehaviour; T cell homeostasis and immune responses; and cancer stem-cell establishment.
m6A in neural synaptic function and behaviour.
In addition to the roles of m6A in regulation of neurogenesis, and consistent with the elevated levels of m6A in mRNAs in adult brains3, several lines of evidence suggest a role for m6A in synapse activity and plasticity during learning and memory. Learning and the formation and maintenance of memory depend on activity-dependent changes in the strength of synapses in the hippocampus, which is achieved through changes in local protein synthesis120. Several regulatory mechanisms involving polyA tail length and the activity of miRNAs and RNA-binding proteins have been implicated, but recent evidence suggests that m6A on mRNA can impact activity-induced local translation at the synapse. Low-input sequencing-based analysis of m6A in synaptosomal RNA in the forebrain revealed that ~3,000 synaptic N6-adenosine methylated transcripts are enriched for functions in synapse synthesis and modulation of synaptic transmission121. Interestingly, the N6-adenosine methyltransferase and demethylase and m6A-binding proteins are located in dendrites and adjacent to synapses, raising the possibility that subcellular modification or recruitment of modified mRNAs could have a role in synaptic activity121 (FIG. 5a). Excitingly, knockdown of the genes encoding the m6A-binding proteins YTHDF1 or YTHDF3 in cultured hippocampal pyramidal neurons reduces translation of the dendritically localized Arc mRNAs, which encode a synaptic protein that promotes internalization of specific glutamate receptors important for fast excitatory neurotransmission; the knockout cells also showed synaptic dysfunction and impaired synaptic transmission121 (FIG. 5a). Conversely, axon-specific knockdown or chemical inhibition of FTO promotes local translation of mRNAs in axons of cultured neurons122.
Fig. 5 |. m6A function in adult cell homeostasis in the nervous system and immune reactions.

a | In neurons, N6-methyladenosine (m6A) is proposed to regulate local translation of selected dendritic transcripts encoding proteins that facilitating memory consolidation during learning and fear conditioning, such as ARC, a synaptic protein important for fast excitatory neurotransmission. The N6-adenosine methyltransferase METTL3, demethylase FTO and binding proteins YTHDF1–YTHDF3, as well as m6A-modified transcripts, are enriched near synapses, raising the possibility of localized regulation of these transcripts. b | m6A facilitates degradation of specific transcripts in immune cells to allow a rapid response to viral infection and a return to basal states once the virus has been inhibited. Viral infection induces type I interferon activation to inhibit additional viral infection and, subsequently, N6-adenosine methylation of mRNA encoding interferon-β (IFNβ) elicits its decay, facilitating a return to basal IFNβ levels. In macrophages, following vesicular stomatitis virus (VSV) infection, mRNA encoding 2-oxyglutarate dehydrogenase (OGDH) are N6-adenosine methylated, which causes their degradation and blocks synthesis of the naturally produced itaconate, which is an activator of VSV replication.
Numerous recent studies report that dynamic regulation of m6A in response to experience or acute stress is required for regulation of complex behaviours such as fear conditioning123–125. Fear conditioning results in a rapid decrease in expression of FTO demethylase in the mouse medial prefrontal cortex and near synapses in the hippocampal CA1 neurons, with a concomitant increase in m6A levels123,124. Targeted depletion of FTO or METTL3 in the medial prefrontal cortex or hippocampus results in enhanced fear memory consolidation following behavioural training123–125. Given the opposing activities of FTO and METTL3 with respect to m6A deposition, and the fact that FTO can also demethylate the cap-adjacent dimethyladenosine (m6Am) modification126 (Box 1), it is possible that some of the FTO-dependent behavioural effects are mediated by m6Am. These results raise the possibility that, following experience, changes in m6A deposition near synapses could result in enhanced local translation of synaptic transcripts that facilitate memory consolidation. In support of this hypothesis, conditional inactivation of Mettl3 specifically in the hippocampus results in long-term memory defects owing to impaired translation of early response synaptic transcripts, whereas Mettl3 overexpression enhances learning efficacy and long-term memory formation127. Similarly, Ythdf1-knockout mice display defects in translation of m6A-methylated transcripts following neuronal stimulation which correlate with defects in hippocampal synaptic transmission and learning and memory128. Taken together, these studies support a model whereby m6A facilitates local translation of synaptic transcripts to facilitate synaptic signalling and confer memory consolidation.
m6A in T cell homeostasis and innate immune response.
m6A on mRNA has been shown to have critical roles in the immune response, including modifying T cell proliferation and differentiation, regulating the turnover of essential host transcripts and methylating viral transcripts to facilitate evasion of the host immune system. Deletion of Mettl3 or Mettl14 in naive T cells blocks proliferation and differentiation to effector T cells129. Loss of N6-adenosine methylation on transcripts encoding inhibitors of STAT5 increased their stability, causing inhibition of STAT5 and subsequent disruption of T cell homeostasis129. Additionally, the mRNA encoding interferon-β (IFNβ) (the main cytokine in the type I interferon response) is N6-adenosine methylated, enabling its fast turnover following activation of the immune response130 (FIG. 5b). CRISPR-induced depletion of m6A (through deletion of METTL3) or reducing the ability of cell to detect and respond to m6A (through depletion of the m6A-binding protein YTHDF2) in both human and mouse cells results in interferon stabilization and activation of interferon-stimulated genes, thereby facilitating protection against viral proliferation130.
Host immune systems have used m6A methylation to effectively fight viral infections. In response to infection with vesicular stomatitis virus (VSV), macrophages inhibit the enzymatic activity of ALKBH5 (an m6A demethylase) resulting in a concomitant increase in m6A levels on RNA131. Mechanistically, it was shown that increased m6A methylation on mRNA encoding 2-oxyglutarate dehydrogenase (OGDH) reduces its stability and OGDH protein expression, which leads to reduced levels of a metabolite (itaconate) required for viral replication131 (FIG. 5b). Therefore, m6A-mediated degradation of OGDH transcripts in macrophages prevents viral proliferation.
Unsurprisingly, viruses have also evolved mechanisms that take advantage of m6A mRNA methylation to evade the host immune system. The RIG-I and MDA5 RNA receptor sensing pathways, which are normally activated upon recognition of unmethylated viral RNA, trigger the expression of type I interferons132. However, human metapneumovirus (HMPV), a negative-sense single-strand RNA virus, possesses m6A on its viral RNAs, which promotes viral replication and facilitates evasion of the innate immune response in human cells. Interestingly, depletion of m6A from HMPV RNA enhances binding and activation of RIG-I and the interferon response pathway133. In a separate example, VSV infection causes the translocation of METTL3 to the cytoplasm and m6A deposition on viral RNAs, which facilitates its evasion of the RIG-I and MDA5 sensing pathways. Depletion of m6A from VSV viral RNAs enhances the formation of double-stranded RNA domains that are recognized by RIG-I and MDA5 to trigger the activation of the interferon response pathway134. Double-strand DNA viruses and certain single-strand RNA viruses can also generate viral circRNAs, which similarly to viral RNAs trigger the RIG-I innate immune response pathway when unmethylated135. Taken together, these findings suggest that diverse viruses use m6A to abrogate the host cell immune response.
m6A in cancer stem cells in acute myeloid lymphoma and glioblastoma.
m6A is central to the regulation of RNA metabolism and fate in numerous developmental and homeostatic cellular processes, and thus disruption of the genetic networks that establish, modulate and bind to m6A ultimately leads to pathologies such as cancer, psychiatric disorders and metabolic disorders118,119. m6A dysregulation has been implicated in various human cancers including breast cancer, hepatic cancers, brain cancer, cervical cancer, lung cancer and leukaemias (for a review of m6A role in cancer, see REF.118). Here, we briefly highlight some of the roles of m6A in cancer stem cells, using AML and glioblastoma as examples.
Given the importance of N6-adenosine methylation for the homeostasis of the HSC and NSC lineages, it is unsurprising that m6A plays a critical role in AML, an aggressive clonal disorder of HSCs, and in the tumorigenesis of glioblastoma stem-like cells (GSCs). METTL3 and METTL14 promote the m6A-dependent translation of MYC and MYB transcription factors, which are both activated and causal for the development of AML, indicating that m6A can enable the maintenance and self-renewal of leukaemia stem/initiation cells and the survival of AML cell lines112,113. However, (R)-2-hydroxyglutarate (R-2HG), which inhibits FTO activity and increases m6A levels, inhibits the proliferation of leukaemia cells by inducing the degradation of m6A-modified transcripts, including those encoding MYC136. YTHDF2 has been implicated in mediating some of the effects of m6A in the progression of AML; inactivation of YTHDF2 increases the half-life of m6A-modified transcripts and, specifically, compromises cancer stem-cell initiation and propagation137. YTHDC1 has also been shown in ex vitro studies to be required for AML tumorigenesis by sequestering N6-adenosine methylated transcripts to specific regions of the cell to prevent their degradation138.
Similarly, knockdown or overexpression of the genes encoding METTL3/METTL14 or FTO, respectively, alters the self-renewal and tumorigenesis of GSCs139. In addition, the ALKBH5 demethylase is required for the proliferation and tumorigenesis of GSCs, as inactivation of ALKBH5 inhibits the proliferation of GSCs. ALKBH5 demethylates transcripts encoding the FOXM1 transcription factor, leading to increased FOXM1 expression and stimulation of cell proliferation140. Taken together, these findings underscore the critical roles of m6A in tumorigenesis of cancer stem cells and provide a rationale for targeting components of the m6A machinery for the development of cancer therapeutics.
Conclusions and future perspectives
In this Review, we have delineated the chemical consequences of adenine methylations on RNA, described the functional effects of methylation of various different substrates and provided illustrative examples of how these methylation events affect various biological processes, including stem-cell pluripotency, memory formation, immune responses and tumorigenesis. It has become clear that the addition of this small chemical moiety to RNA (which can physically alter the RNA or signal to binding proteins to treat the RNA molecule differently) plays an outsized role in regulating virtually every aspect of biology.
With so many diverse functions assigned to m6A, the next step for the field will be to distinguish direct consequences of this modification from secondary or tertiary consequences that have been inappropriately ascribed to m6A. Independent validation of m6A functions using orthogonal approaches will be necessary, and we believe that analysing the functional consequences of directed RNA methylation or demethylation of specific residues79 will play an important part in confirming the biological importance of m6A. Moreover, complementing directed editing with directed chemical labelling of specific m6A modifications141, for accurate visualization and tracking, will further bolster confidence in specific biological consequences of m6A.
Moving forwards, it will also be important to determine how different epitranscriptomic modifications communicate with each other and with chromatin modifications, and whether an epitranscriptomic code exists that can help integrate m6A modifications with other epitranscriptomic or epigenetic signalling pathways. In addition, questions remain about how this abundant modification, which has been implicated in regulating so many diverse processes, can achieve specificity: do methyl-binding proteins alone provide the required specificity or are other systems involved? Precision editing and an improved understanding of m6A stoichiometry should help provide answers. Finally, RNA methylation has already been demonstrated to have a role in subcellular localization48–50, so it is exciting to speculate that m6A could also mark specific RNAs for inheritance through cell divisions or even across generations, similar to what our recent preprint reports m6,2A to do on 18S rRNA141.
These are exciting times for the field. With the rapid improvement of existing methods and development of new technology, the study of m6A is poised to uncover new biological regulatory systems and m6A may even find use as a therapeutic target142.
Acknowledgements
Work from the Greer laboratory is supported by grants from the US National Institutes of Health (NIH) (DP2AG055947 and R01AI151215). The authors apologize for literature omitted owing to space limitations.
Glossary
- Hoogsteen base pairing
An alternative base pairing in which the purine is flipped and form different hydrogen bonds with partner bases. For adenines, the second hydrogen bond with the pyrimidine base is formed with N6 rather than N1. These alternative base pairs allow for additional structures beyond double helix including triplexes and quadruplexes.
- miRNA microprocessor complex
A protein complex involved in the early stages of processing microRNA (miRNA) and RNA interference in animal cells.
- Spliceosome
A large RNA–protein complex that catalyses the removal of introns from nuclearpre-mRNA.
- Long non-coding RNAs
(lncRNAs). Non-coding RNAs longer than 200 nucleotides.
- Chromosome-associated regulatory RNAs
(carRNAs). regulatory RNAs associated with the chromatin.
- Endogenous retrovirus RNAs
(ERVs). The prevalent endogenous viral elements that are derived from retroviruses that have become integrated into the genome.
- R-loops
These RNA:DNA hybrids form three-stranded structures when nascent RNA transcripts hybridize with one strand of the DNA template.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Wei CM, Gershowitz A & Moss B Methylated nucleotides block 5′ terminus of HeLa cell messenger RNA. Cell 4, 379–386 (1975). [DOI] [PubMed] [Google Scholar]
- 2.Sommer S, Lavi U & Darnell JE Jr. The absolute frequency of labeled N-6-methyladenosine in HeLa cell messenger RNA decreases with label time. J. Mol. Biol 124, 487–499 (1978). [DOI] [PubMed] [Google Scholar]
- 3.Meyer KD et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149, 1635–1646 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominissini D et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206 (2012). [DOI] [PubMed] [Google Scholar]; Together with Meyer et al. (2012), this paper reports the mapping of m6A in the human and mouse transcriptome.
- 5.Tegowski M, Flamand MN & Meyer KD scDART-seq reveals distinct m6A signatures and mRNA methylation heterogeneity in single cells. Mol. Cell 82, 868–878.e10 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J et al. A METTL3–METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol 10, 93–95 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports that the METTL3–METTL14–WTAP complex mediates N6-adenosine methylation.
- 7.Wei CM & Moss B Nucleotide sequences at the N6-methyladenosine sites of HeLa cell messenger ribonucleic acid. Biochemistry 16, 1672–1676 (1977). [DOI] [PubMed] [Google Scholar]
- 8.Linder B et al. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat. Methods 12, 767–772 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng G et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 49, 18–29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies and characterizes ALKBH5 as an m6A demethylase, demonstrating that m6A is a dynamic reversible modification in mRNA.
- 10.Jia G et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol 7, 885–887 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies and characterizes FTO as an m6A demethylase, suggesting that m6A is a dynamic reversible modification in mRNA; it should be read in conjunction with Mauer et al. (2017), which suggests that FTO is an m6Am demethylase.
- 11.Piekna-Przybylska D, Decatur WA & Fournier MJ The 3D rRNA modification maps database: with interactive tools for ribosome analysis. Nucleic Acids Res. 36, D178–D183 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sergiev PV, Aleksashin NA, Chugunova AA, Polikanov YS & Dontsova OA Structural and evolutionary insights into ribosomal RNA methylation. Nat. Chem. Biol 14, 226–235 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Ma H et al. N6-methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation. Nat. Chem. Biol 15, 88–94 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren W et al. Structure and regulation of ZCCHC4 in m6A-methylation of 28S rRNA. Nat. Commun 10, 5042 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto R et al. The human methyltransferase ZCCHC4 catalyses N6-methyladenosine modification of 28S ribosomal RNA. Nucleic Acids Res. 48, 830–846 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Tran N et al. The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res. 47, 7719–7733 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberman N et al. N6-Adenosine methylation of ribosomal RNA affects lipid oxidation and stress resistance. Sci. Adv 6, eaaz4370 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xing M et al. The 18S rRNA m6A methyltransferase METTL5 promotes mouse embryonic stem cell differentiation. EMBO Rep. 21, e49863 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rong B et al. Ribosome 18S m6A methyltransferase METTL5 promotes translation initiation and breast cancer cell growth. Cell Rep. 33, 108544 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Leismann J et al. The 18S ribosomal RNA m6A methyltransferase Mettl5 is required for normal walking behavior in Drosophila. EMBO Rep. 21, e49443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ignatova VV et al. The rRNA m6A methyltransferase METTL5 is involved in pluripotency and developmental programs. Genes Dev. 34, 715–729 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sendinc E, Valle-Garcia D, Jiao A & Shi Y Analysis of m6A RNA methylation in Caenorhabditis elegans. Cell Discov. 6, 47 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sepich-Poore C et al. The METTL5–TRMT112 N6-methyladenosine methyltransferase complex regulates mRNA translation via 18S rRNA methylation. J. Biol. Chem 298, 101590 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kierzek E & Kierzek R The thermodynamic stability of RNA duplexes and hairpins containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines. Nucleic Acids Res. 31, 4472–4480 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer KD & Jaffrey SR The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat. Rev 15, 313–326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu N et al. N6-methyladenosine-dependent RNA structural switches regulate RNA–protein interactions. Nature 518, 560–564 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes a mechanism by which m6A affects RNA folding to indirectly have an impact on the binding of proteins to RNA.
- 27.Wang S et al. The m6A methylation perturbs the Hoogsteen pairing-guided incorporation of an oxidized nucleotide. Chem. Sci 8, 6380–6388 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashraf S, Huang L & Lilley DMJ Effect of methylation of adenine N6 on kink turn structure depends on location. RNA Biol. 16, 1377–1385 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogawa A et al. N6-methyladenosine (m6A) is an endogenous A3 adenosine receptor ligand. Mol. Cell 81, 659–674.e7 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Patil DP, Pickering BF & Jaffrey SR Reading m6A in the transcriptome: m6A-binding proteins. Trends Cell Biol. 28, 113–127 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi H, Wei J & He C Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol. Cell 74, 640–650 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaccara S, Ries RJ & Jaffrey SR Reading, writing and erasing mRNA methylation. Nat. Rev 20, 608–624 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Huang H et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol 20, 285–295 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edupuganti RR et al. N6-methyladenosine (m6A) recruits and repels proteins to regulate mRNA homeostasis. Nat. Struct. Mol. Biol 24, 870–878 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ping XL et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 24, 177–189 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fustin JM et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell 155, 793–806 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Meyer KD et al. 5′ UTR m6A promotes cap-independent translation. Cell 163, 999–1010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X et al. N6-methyladenosine modulates messenger RNA translation efficiency. Cell 161, 1388–1399 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Meyer et al. (2015), this paper establishes a role for m6A in regulating mRNA translation.
- 39.Zhou J et al. Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature 526, 591–594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li A et al. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 27, 444–447 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper establishes a role for m6A in modulating mRNA stability.
- 42.Viegas IJ et al. N6-methyladenosine in poly(A) tails stabilize VSG transcripts. Nature 604, 362–370 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alarcon CR, Lee H, Goodarzi H, Halberg N & Tavazoie SF N6-methyladenosine marks primary microRNAs for processing. Nature 519, 482–485 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper establishes a role for m6A in modulating miRNA processing.
- 44.Alarcon CR et al. HNRNPA2B1 is a mediator of m6A-dependent nuclear RNA processing events. Cell 162, 1299–1308 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lence T et al. m6A modulates neuronal functions and sex determination in Drosophila. Nature 540, 242–247 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Haussmann IU et al. m6A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature 540, 301–304 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Xiao W et al. Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol. Cell 61, 507–519 (2016). [DOI] [PubMed] [Google Scholar]; This study establishes a role for m6A in modulating splicing.
- 48.Roundtree IA et al. YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. eLife 6, e31311 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edens BM et al. FMRP modulates neural differentiation through m6A-dependent mRNA nuclear export. Cell Rep. 28, 845–854.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsu PJ et al. The RNA-binding protein FMRP facilitates the nuclear export of N6-methyladenosine-containing mRNAs. J. Biol. Chem 294, 19889–19895 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y et al. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol 16, 191–198 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study establishes a role for METTL3 and METTL14 in stemcell renewal via reduced stability of m6A-modified transcripts.
- 52.Du H et al. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4–NOT deadenylase complex. Nat. Commun 7, 12626 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park OH et al. Endoribonucleolytic cleavage of m6A-containing RNAs by RNase P/MRP complex. Mol. Cell 74, 494–507.e8 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Shi H et al. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 27, 315–328 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi J et al. N6-methyladenosine in mRNA disrupts tRNA selection and translation-elongation dynamics. Nat. Struct. Mol. Biol 23, 110–115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taoka M et al. Landscape of the complete RNA chemical modifications in the human 80S ribosome. Nucleic Acids Res. 46, 9289–9298 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen T et al. m6A RNA methylation is regulated by microRNAs and promotes reprogramming to pluripotency. Cell Stem Cell 16, 289–301 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Das Mandal S & Ray PS Transcriptome-wide analysis reveals spatial correlation between N6-methyladenosine and binding sites of microRNAs and RNA-binding proteins. Genomics 113, 205–216 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Kanoria S, Rennie WA, Carmack CS, Lu J & Ding Y N6-methyladenosine enhances post-transcriptional gene regulation by microRNAs. Bioinform Adv. 2, vbab046 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen LL The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev 21, 475–490 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Memczak S et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Hansen TB et al. Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Yang Y et al. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 27, 626–641 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Timoteo G et al. Modulation of circRNA metabolism by m6A modification. Cell Rep. 31, 107641 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Pendleton KE et al. The U6 snRNA m6A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell 169, 824–835.e14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that METTL16 m6A methyltransferase regulates SAM synthetase mRNA splicing, modulating SAM levels and, thereby, indirectly regulating the methylation of proteins and nucleic acids.
- 66.Warda AS et al. Human METTL16 is a N6-methyladenosine (m6A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 18, 2004–2014 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang H et al. Histone H3 trimethylation at lysine 36 guides m6A RNA modification co-transcriptionally. Nature 567, 414–419 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y et al. N6-methyladenosine co-transcriptionally directs the demethylation of histone H3K9me2. Nat. Genet 52, 870–877 (2020). [DOI] [PubMed] [Google Scholar]
- 69.Akhtar J et al. m6A RNA methylation regulates promoter-proximal pausing of RNA polymerase II. Mol. Cell 81, 3356–3367.e6 (2021). [DOI] [PubMed] [Google Scholar]
- 70.Xu W et al. Dynamic control of chromatin-associated m6A methylation regulates nascent RNA synthesis. Mol. Cell 82, 1156–1168.e7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mendel M et al. Methylation of structured RNA by the m6A writer METTL16 is essential for mouse embryonic development. Mol. Cell 71, 986–1000.e11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mendel M et al. Splice site m6A methylation prevents binding of U2AF35 to inhibit RNA splicing. Cell 184, 3125–3142.e25 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang L, Froberg JE & Lee JT Long noncoding RNAs: fresh perspectives into the RNA world. Trends Biochem. Sci 39, 35–43 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patil DP et al. m6A RNA methylation promotes XIST-mediated transcriptional repression. Nature 537, 369–373 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper establishes a role for m6A in Xist-mediated silencing.
- 75.Nesterova TB et al. Systematic allelic analysis defines the interplay of key pathways in X chromosome inactivation. Nat. Commun 10, 3129 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kopp F & Mendell JT Functional classification and experimental dissection of long noncoding RNAs. Cell 172, 393–407 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X et al. N6-methyladenosine modification of MALAT1 promotes metastasis via reshaping nuclear speckles. Dev. Cell 56, 702–715.e8 (2021). [DOI] [PubMed] [Google Scholar]
- 78.Andergassen D & Rinn JL From genotype to phenotype: genetics of mammalian long non-coding RNAs in vivo. Nat. Rev. Genet 23, 229–243 (2022). [DOI] [PubMed] [Google Scholar]
- 79.Liu J et al. N6-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science 367, 580–586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study performs targeted epigenomic editing to test the direct role of m6A on specific RNAs.
- 80.Lee JH et al. Enhancer RNA m6A methylation facilitates transcriptional condensate formation and gene activation. Mol. Cell 81, 3368–3385.e9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Romanish MT, Cohen CJ & Mager DL Potential mechanisms of endogenous retroviral-mediated genomic instability in human cancer. Semin. Cancer Biol 20, 246–253 (2010). [DOI] [PubMed] [Google Scholar]
- 82.Chelmicki T et al. m6A RNA methylation regulates the fate of endogenous retroviruses. Nature 591, 312–316 (2021). [DOI] [PubMed] [Google Scholar]; This study establishes a role for m6A in suppressing ERVs to help maintain genome stability.
- 83.Xu W et al. METTL3 regulates heterochromatin in mouse embryonic stem cells. Nature 591, 317–321 (2021). [DOI] [PubMed] [Google Scholar]
- 84.Niehrs C & Luke B Regulatory R-loops as facilitators of gene expression and genome stability. Nat. Rev 21, 167–178 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abakir A et al. N6-methyladenosine regulates the stability of RNA:DNA hybrids in human cells. Nat. Genet 52, 48–55 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper establishes a role for m6A in regulating R-loop formation.
- 86.Yang X et al. m6A promotes R-loop formation to facilitate transcription termination. Cell Res. 29, 1035–1038 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang C et al. METTL3 and N6-methyladenosine promote homologous recombination-mediated repair of DSBs by modulating DNA–RNA hybrid accumulation. Mol. Cell 79, 425–442.e7 (2020). [DOI] [PubMed] [Google Scholar]
- 88.Geula S et al. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science 347, 1002–1006 (2015). [DOI] [PubMed] [Google Scholar]; This paper establishes a role for METTL3 in mES cell renewal and differentiation and shows that m6A reduces pluripotency factor transcript stability to facilitate ES cell differentiation.
- 89.Meng TG et al. Mettl14 is required for mouse postimplantation development by facilitating epiblast maturation. FASEB J. 33, 1179–1187 (2019). [DOI] [PubMed] [Google Scholar]
- 90.Zhong S et al. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant. Cell 20, 1278–1288 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Batista PJ et al. m6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell 15, 707–719 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aguilo F et al. Coordination of m6A mRNA methylation and gene transcription by ZFP217 regulates pluripotency and reprogramming. Cell Stem Cell 17, 689–704 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wen J et al. Zc3h13 regulates nuclear RNA m6A methylation and mouse embryonic stem cell self-renewal. Mol. Cell 69, 1028–1038.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bertero A et al. The SMAD2/3 interactome reveals that TGFβ controls m6A mRNA methylation in pluripotency. Nature 555, 256–259 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heck AM, Russo J, Wilusz J, Nishimura EO & Wilusz CJ YTHDF2 destabilizes m6A-modified neural-specific RNAs to restrain differentiation in induced pluripotent stem cells. RNA 26, 739–755 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zaccara S & Jaffrey SR A unified model for the function of YTHDF proteins in regulating m6A-modified mRNA. Cell 181, 1582–1595.e18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yartseva V & Giraldez AJ The maternal-to-zygotic transition during vertebrate development: a model for reprogramming. Curr. Top. Dev. Biol 113, 191–232 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao BS et al. m6A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature 542, 475–478 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study establishes a role for m6A in maternal transcript clearance during zebrafish MZT.
- 99.Kontur C, Jeong M, Cifuentes D & Giraldez AJ Ythdf m6A readers function redundantly during zebrafish development. Cell Rep. 33, 108598 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wojtas MN et al. Regulation of m6A transcripts by the 3′ → 5′ RNA helicase YTHDC2 is essential for a successful meiotic program in the mammalian germline. Mol. Cell 68, 374–387.e12 (2017). [DOI] [PubMed] [Google Scholar]
- 101.Hsu PJ et al. Ythdc2 is an N6-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 27, 1115–1127 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bailey AS et al. The conserved RNA helicase YTHDC2 regulates the transition from proliferation to differentiation in the germline. eLife 6, e26116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ivanova I et al. The RNA m6A reader YTHDF2 is essential for the post-transcriptional regulation of the maternal transcriptome and oocyte competence. Mol. Cell 67, 1059–1067.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kasowitz SD et al. Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLoS Genet. 14, e1007412 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sui X et al. METTL3-mediated m6A is required for murine oocyte maturation and maternal-to-zygotic transition. Cell Cycle 19, 391–404 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Giraldez AJ et al. Zebrafish miR-430 promotes deadenylation and clearance of maternal mRNAs. Science 312, 75–79 (2006). [DOI] [PubMed] [Google Scholar]
- 107.Chang H et al. Terminal uridylyltransferases execute programmed clearance of maternal transcriptome in vertebrate embryos. Mol. Cell 70, 72–82.e7 (2018). [DOI] [PubMed] [Google Scholar]
- 108.Wu Y & Hirschi KK Regulation of hemogenic endothelial cell development and function. Annu. Rev. Physiol 83, 17–37 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang C et al. m6A modulates haematopoietic stem and progenitor cell specification. Nature 549, 273–276 (2017). [DOI] [PubMed] [Google Scholar]
- 110.Lv J et al. Endothelial-specific m6A modulates mouse hematopoietic stem and progenitor cell development via Notch signaling. Cell Res. 28, 249–252 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee H et al. Stage-specific requirement for Mettl3-dependent m6A mRNA methylation during haematopoietic stem cell differentiation. Nat. Cell Biol 21, 700–709 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vu LP et al. The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat. Med 23, 1369–1376 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weng H et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m6A modification. Cell Stem Cell 22, 191–205.e9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yoon KJ et al. Temporal control of mammalian cortical neurogenesis by m6A methylation. Cell 171, 877–889.e17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y et al. N6-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nat. Neurosci 21, 195–206 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li M et al. Ythdf2-mediated m6A mRNA clearance modulates neural development in mice. Genome Biol. 19, 69 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li L et al. Fat mass and obesity-associated (FTO) protein regulates adult neurogenesis. Hum. Mol. Genet 26, 2398–2411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu J, Harada BT & He C Regulation of gene expression by N6-methyladenosine in cancer. Trends Cell Biol. 29, 487–499 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang C et al. The role of m6A modification in physiology and disease. Cell Death Dis. 11, 960 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Buffington SA, Huang W & Costa-Mattioli M Translational control in synaptic plasticity and cognitive dysfunction. Annu. Rev. Neurosci 37, 17–38 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Merkurjev D et al. Synaptic N6-methyladenosine (m6A) epitranscriptome reveals functional partitioning of localized transcripts. Nat. Neurosci 21, 1004–1014 (2018). [DOI] [PubMed] [Google Scholar]; This paper reports the identification of subcellular synapse-specific m6A-methylated transcripts.
- 122.Yu J et al. Dynamic m6A modification regulates local translation of mRNA in axons. Nucleic Acids Res. 46, 1412–1423 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Widagdo J et al. Experience-dependent accumulation of N6-methyladenosine in the prefrontal cortex is associated with memory processes in mice. J. Neurosci 36, 6771–6777 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Walters BJ et al. The role of the RNA demethylase FTO (fat mass and obesity-associated) and mRNA methylation in hippocampal memory formation. Neuropsychopharmacology 42, 1502–1510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Engel M et al. The role of m6A/m-RNA methylation in stress response regulation. Neuron 99, 389–403.e9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mauer J et al. Reversible methylation of m6Am in the 5′ cap controls mRNA stability. Nature 541, 371–375 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study characterizes FTO as an m6Am demethylase that regulates mRNA stability, suggesting that m6Am is a dynamic reversable modification; it should be read in conjunction with Jia et al. (2011), which suggests that FTO is a m6A demethylase.
- 127.Zhang Z et al. METTL3-mediated N6-methyladenosine mRNA modification enhances long-term memory consolidation. Cell Res. 28, 1050–1061 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shi H et al. m6A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature 563, 249–253 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals a regulatory role for m6A in neurobehavioural phenotypes by promoting translation of selected transcripts.
- 129.Li HB et al. m6A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature 548, 338–342 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Winkler R et al. m6A modification controls the innate immune response to infection by targeting type I interferons. Nat. Immunol 20, 173–182 (2019). [DOI] [PubMed] [Google Scholar]
- 131.Liu Y et al. N6-methyladenosine RNA modification-mediated cellular metabolism rewiring inhibits viral replication. Science 365, 1171–1176 (2019). [DOI] [PubMed] [Google Scholar]; This paper shows that m6A-marked transcripts allow a levelled response to viral infection.
- 132.Chow KT, Gale M Jr & Loo YM RIG-I and other RNA sensors in antiviral immunity. Annu. Rev. Immunol 36, 667–694 (2018). [DOI] [PubMed] [Google Scholar]
- 133.Lu M et al. N6-methyladenosine modification enables viral RNA to escape recognition by RNA sensor RIG-I. Nat. Microbiol 5, 584–598 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Qiu W et al. N6-methyladenosine RNA modification suppresses antiviral innate sensing pathways via reshaping double-stranded RNA. Nat. Commun 12, 1582 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen YG et al. N6-methyladenosine modification controls circular RNA immunity. Mol. Cell 76, 96–109.e9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Su R et al. R-2HG exhibits anti-tumor activity by targeting FTO/m6A/MYC/CEBPA signaling. Cell 172, 90–105.e23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Paris J et al. Targeting the RNA m6A reader YTHDF2 selectively compromises cancer stem cells in acute myeloid leukemia. Cell Stem Cell 25, 137–148.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cheng Y et al. N6-methyladenosine on mRNA facilitates a phase-separated nuclear body that suppresses myeloid leukemic differentiation. Cancer Cell 39, 958–972.e8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cui Q et al. m6A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 18, 2622–2634 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang S et al. m6A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell 31, 591–606.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liberman N et al. Intergenerational hormesis is regulated by heritable 18S rRNA methylation. Preprint at bioRxiv 10.1101/2021/1109.1127.461965 (2021). [DOI] [Google Scholar]
- 142.Yankova E et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature 593, 597–601 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies small molecules that can effectively inhibit METTL3, raising the possibility of therapeutic options for myeloid leukaemia and other m6A-related pathologies.
- 143.Kruse S et al. A novel synthesis and detection method for cap-associated adenosine modifications in mouse mRNA. Sci. Rep 1, 126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yuki H, Kawasaki H, Imayuki A & Yajima T Determination of 6-methyladenine in DNA by high-performance liquid chromatography. J. Chromatogr 168, 489–494 (1979). [DOI] [PubMed] [Google Scholar]
- 145.Thuring K, Schmid K, Keller P & Helm M LC–MS analysis of methylated RNA. Methods Mol. Biol 1562, 3–18 (2017). [DOI] [PubMed] [Google Scholar]
- 146.Liu N et al. Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA 19, 1848–1856 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Imanishi M, Tsuji S, Suda A & Futaki S Detection of N6-methyladenosine based on the methyl-sensitivity of MazF RNA endonuclease. Chem. Commun 53, 12930–12933 (2017). [DOI] [PubMed] [Google Scholar]
- 148.Garcia-Campos MA et al. Deciphering the “m6A code” via antibody-independent quantitative profiling. Cell 178, 731–747.e16 (2019). [DOI] [PubMed] [Google Scholar]
- 149.Dominissini D et al. The dynamic N1-methyladenosine methylome in eukaryotic messenger RNA. Nature 530, 441–446 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li X et al. Transcriptome-wide mapping reveals reversible and dynamic N1-methyladenosine methylome. Nat. Chem. Biol 12, 311–316 (2016). [DOI] [PubMed] [Google Scholar]
- 151.Safra M et al. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature 551, 251–255 (2017). [DOI] [PubMed] [Google Scholar]
- 152.Li X et al. Base-resolution mapping reveals distinct m1A methylome in nuclear- and mitochondrial-encoded transcripts. Mol. Cell 68, 993–1005.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Grozhik AV et al. Antibody cross-reactivity accounts for widespread appearance of m1A in 5′ UTRs. Nat. Commun 10, 5126 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Harcourt EM, Ehrenschwender T, Batista PJ, Chang HY & Kool ET Identification of a selective polymerase enables detection of N6-methyladenosine in RNA. J. Am. Chem. Soc 135, 19079–19082 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hong T et al. Precise antibody-independent m6A identification via 4SedTTP-involved and FTO-assisted strategy at single-nucleotide resolution. J. Am. Chem. Soc 140, 5886–5889 (2018). [DOI] [PubMed] [Google Scholar]
- 156.Aschenbrenner J et al. Engineering of a DNA polymerase for direct m6A sequencing. Angew. Chem. Int. Ed 57, 417–421 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ke S et al. A majority of m6A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes Dev. 29, 2037–2053 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Molinie B et al. m6A-LAIC-seq reveals the census and complexity of the m6A epitranscriptome. Nat. Methods 13, 692–698 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bringmann P & Luhrmann R Antibodies specific for N6-methyladenosine react with intact snRNPs U2 and U4/U6. FEBS Lett. 213, 309–315 (1987). [DOI] [PubMed] [Google Scholar]
- 160.Dai Q et al. NM-seq maps 2′-O-methylation sites in human mRNA with base precision. Nat. Methods 14, 695–698 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Castellanos-Rubio A et al. A novel RT-QPCR-based assay for the relative quantification of residue specific m6A RNA methylation. Sci. Rep 9, 4220 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Flusberg BA et al. Direct detection of DNA methylation during single-molecule, real-time sequencing. Nat. Methods 7, 461–465 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Garalde DR et al. Highly parallel direct RNA sequencing on an array of nanopores. Nat. Methods 15, 201–206 (2018). [DOI] [PubMed] [Google Scholar]
- 164.Chen Y et al. Study of the whole genome, methylome and transcriptome of Cordyceps militaris. Sci. Rep 9, 898 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pratanwanich PN et al. Identification of differential RNA modifications from nanopore direct RNA sequencing with xPore. Nat. Biotechnol 39, 1394–1402 (2021). [DOI] [PubMed] [Google Scholar]
- 166.Mikutis S et al. meCLICK-Seq, a substrate-hijacking and RNA degradation strategy for the study of RNA microsoftethylation. ACS Cent. Sci 6, 2196–2208 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu W et al. Identification of a selective DNA ligase for accurate recognition and ultrasensitive quantification of N6-methyladenosine in RNA at one-nucleotide resolution. Chem. Sci 9, 3354–3359 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xiao Y et al. An elongation- and ligation-based qPCR amplification method for the radiolabeling-free detection of locus-specific N6-methyladenosine modification. Angew. Chem. Int. Ed 57, 15995–16000 (2018). [DOI] [PubMed] [Google Scholar]
- 169.Bokar JA, Shambaugh ME, Polayes D, Matera AG & Rottman FM Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA 3, 1233–1247 (1997). [PMC free article] [PubMed] [Google Scholar]; This paper identifies METTL3 as the catalytic subunit of the complex that mediates N6-adenosine methylation.
- 170.Clancy MJ, Shambaugh ME, Timpte CS & Bokar JA Induction of sporulation in Saccharomyces cerevisiae leads to the formation of N6-methyladenosine in mRNA: a potential mechanism for the activity of the IME4 gene. Nucleic Acids Res. 30, 4509–4518 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shima H et al. S-Adenosylmethionine synthesis is regulated by selective N6-adenosine methylation and mRNA degradation involving METTL16 and YTHDC1. Cell Rep. 21, 3354–3363 (2017). [DOI] [PubMed] [Google Scholar]
- 172.Akichika S et al. Cap-specific terminal N6-methylation of RNA by an RNA polymerase II-associated methyltransferase. Science 363, eaav0080 (2019). [DOI] [PubMed] [Google Scholar]
- 173.Sun H, Zhang M, Li K, Bai D & Yi C Cap-specific, terminal N6-methylation by a mammalian m6Am methyltransferase. Cell Res. 29, 80–82 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Boulias K et al. Identification of the m6Am methyltransferase PCIF1 reveals the location and functions of m6Am in the transcriptome. Mol. Cell 75, 631–643.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sendinc E et al. PCIF1 catalyzes m6Am mRNA methylation to regulate gene expression. Mol. Cell 75, 620–630.e9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Belanger F, Stepinski J, Darzynkiewicz E & Pelletier J Characterization of hMTr1, a human Cap1 2′-O-ribose methyltransferase. J. Biol. Chem 285, 33037–33044 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tollervey D, Lehtonen H, Jansen R, Kern H & Hurt EC Temperature-sensitive mutations demonstrate roles for yeast fibrillarin in pre-rRNA processing, pre-rRNA methylation, and ribosome assembly. Cell 72, 443–457 (1993). [DOI] [PubMed] [Google Scholar]
- 178.Kiss-Laszlo Z, Henry Y, Bachellerie JP, Caizergues-Ferrer M & Kiss T Site-specific ribose methylation of preribosomal RNA: a novel function for small nucleolar RNAs. Cell 85, 1077–1088 (1996). [DOI] [PubMed] [Google Scholar]
- 179.Nicoloso M, Qu LH, Michot B & Bachellerie JP Intron-encoded, antisense small nucleolar RNAs: the characterization of nine novel species points to their direct role as guides for the 2′-O-ribose methylation of rRNAs. J. Mol. Biol 260, 178–195 (1996). [DOI] [PubMed] [Google Scholar]
- 180.Tycowski KT, Smith CM, Shu MD & Steitz JA A small nucleolar RNA requirement for site-specific ribose methylation of rRNA in Xenopus. Proc. Natl Acad. Sci. USA 93, 14480–14485 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Juhling F et al. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res 37, D159–D162 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ozanick S, Krecic A, Andersland J & Anderson JT The bipartite structure of the tRNA m1A58 methyltransferase from S. cerevisiae is conserved in humans. RNA 11, 1281–1290 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chujo T & Suzuki T Trmt61B is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial tRNAs. RNA 18, 2269–2276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vilardo E et al. Functional characterization of the human tRNA methyltransferases TRMT10A and TRMT10B. Nucleic Acids Res 48, 6157–6169 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vilardo E et al. A subcomplex of human mitochondrial RNase P is a bifunctional methyltransferase — extensive moonlighting in mitochondrial tRNA biogenesis. Nucleic Acids Res 40, 11583–11593 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lafontaine D, Delcour J, Glasser AL, Desgres J & Vandenhaute J The DIM1 gene responsible for the conserved m62Am62A dimethylation in the 3′-terminal loop of 18S rRNA is essential in yeast. J. Mol. Biol 241, 492–497 (1994). [DOI] [PubMed] [Google Scholar]
- 187.Suvorov AN, van Gemen B & van Knippenberg PH Increased kasugamycin sensitivity in Escherichia coli caused by the presence of an inducible erythromycin resistance (erm) gene of Streptococcus pyogenes. Mol. Gen. Genet 215, 152–155 (1988). [DOI] [PubMed] [Google Scholar]
- 188.Shen H, Stoute J & Liu KF Structural and catalytic roles of the human 18S rRNA methyltransferases DIMT1 in ribosome assembly and translation. J. Biol. Chem 295, 12058–12070 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chen H et al. METTL4 is an snRNA m6Am methyltransferase that regulates RNA splicing. Cell Res 30, 544–547 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Goh YT, Koh CWQ, Sim DY, Roca X & Goh WSS METTL4 catalyzes m6Am methylation in U2 snRNA to regulate pre-mRNA splicing. Nucleic Acids Res 48, 9250–9261 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liu F et al. ALKBH1-mediated tRNA demethylation regulates translation. Cell 167, 816–828.e16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kawarada L et al. ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nucleic Acids Res 45, 7401–7415 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Aas PA et al. Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature 421, 859–863 (2003). [DOI] [PubMed] [Google Scholar]
- 194.Xu C et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat. Chem. Biol 10, 927–929 (2014). [DOI] [PubMed] [Google Scholar]
- 195.Theler D, Dominguez C, Blatter M, Boudet J & Allain FH Solution structure of the YTH domain in complex with N6-methyladenosine RNA: a reader of methylated RNA. Nucleic Acids Res 42, 13911–13919 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xu C et al. Structural basis for the discriminative recognition of N6-methyladenosine RNA by the human YT521-B homology domain family of proteins. J. Biol. Chem 290, 24902–24913 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liu N et al. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res 45, 6051–6063 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Toh SM, Xiong L, Bae T & Mankin AS The methyltransferase YfgB/RlmN is responsible for modification of adenosine 2503 in 23S rRNA. RNA 14, 98–106 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Furuichi Y et al. Methylated, blocked 5 termini in HeLa cell mRNA. Proc. Natl Acad. Sci. USA 72, 1904–1908 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Oerum S, Degut C, Barraud P & Tisne C m1A post-transcriptional modification in tRNAs. Biomolecules 7, 20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Peifer C et al. Yeast Rrp8p, a novel methyltransferase responsible for m1A 645 base modification of 25S rRNA. Nucleic Acids Res 41, 1151–1163 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Waku T et al. NML-mediated rRNA base methylation links ribosomal subunit formation to cell proliferation in a p53-dependent manner. J. Cell Sci 129, 2382–2393 (2016). [DOI] [PubMed] [Google Scholar]
- 203.Yokoyama W et al. rRNA adenine methylation requires T07A9.8 gene as rram-1 in Caenorhabditis elegans. J. Biochem 163, 465–474 (2018). [DOI] [PubMed] [Google Scholar]
- 204.Sharma S et al. A single N1-methyladenosine on the large ribosomal subunit rRNA impacts locally its structure and the translation of key metabolic enzymes. Sci. Rep 8, 11904 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schwartz S m1A within cytoplasmic mRNAs at single nucleotide resolution: a reconciled transcriptome-wide map. RNA 24, 1427–1436 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wei C, Gershowitz A & Moss B N6,O2′-Dimethyladenosine a novel methylated ribonucleoside next to the 5′ terminal of animal cell and virus mRNAs. Nature 257, 251–253 (1975). [DOI] [PubMed] [Google Scholar]
- 207.Keith JM, Muthukrishnan S & Moss B Effect of methylation of the N6 position of the penultimate adenosine of capped mRNA on ribosome binding. J. Biol. Chem 253, 5039–5041 (1978). [PubMed] [Google Scholar]
- 208.Pandey RR et al. The mammalian Cap-specific m6Am RNA methyltransferase PCIF1 regulates transcript levels in mouse tissues. Cell Rep 32, 108038 (2020). [DOI] [PubMed] [Google Scholar]
- 209.Ben-Haim MS et al. Dynamic regulation of N6,2′-O-dimethyladenosine (m6Am) in obesity. Nat. Commun 12, 7185 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tartell MA et al. Methylation of viral mRNA cap structures by PCIF1 attenuates the antiviral activity of interferon-β. Proc. Natl Acad. Sci. USA 118, e2025769118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mauer J et al. FTO controls reversible m6Am RNA methylation during snRNA biogenesis. Nat. Chem. Biol 15, 340–347 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zorbas C et al. The human 18S rRNA base methyltransferases DIMT1L and WBSCR22-TRMT112 but not rRNA modification are required for ribosome biogenesis. Mol. Biol. Cell 26, 2080–2095 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liu K et al. Regulation of translation by methylation multiplicity of 18S rRNA. Cell Rep 34, 108825 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


