Abstract
Background:
The long-term impact of prenatal alcohol exposure (PAE) on adult vascular health was assessed using a prospective cohort first identified while in utero.
Methods:
Participants with no PAE (n = 37, Mean Age = 36.7 (1.6) years) were compared to participants with PAE (n = 51, Mean Age = 36.3 (1.7) years). Their vascular health was assessed by arterial blood pressure (BP) as well as peripheral arterial tonometry, which yields an index of endothelial function (reactive hyperemia index) and a measure of arterial stiffness (augmentation index). Blood samples were collected to assess cholesterol levels and insulin resistance (glucose, hemoglobin A1C, and insulin). Path analysis was used to examine the direct and indirect effects of PAE on vascular health after adjusting for other known physical outcomes.
Results:
Participants with a history of PAE weighed less, trended towards being shorter, had smaller body mass, and had more alcohol-related dysmorphic features than those without PAE. Path analysis suggested that the impact of PAE on BP were through its indirect relationships with height, BMI, and dysmorphic features and resulted in protective effects relative to the Contrast group who were disproportionately overweight. In contrast, PAE was found to have a direct negative effect on endothelial function. An index of total alcohol-related dysmorphic features was negatively associated with both a direct effect on arterial stiffness and an indirect effect on endothelial function.
Conclusions:
PAE’s impact to vascular function is not independent of other common physical and environmental factors but endothelial function and arterial stiffness seemed most compromised after controlling for these other factors. Level of alcohol-related dysmorphic features seemed to be predictive of more adverse effects to endothelial function and vascular stiffness.
Keywords: prenatal alcohol exposure, Fetal alcohol spectrum disorders, vascular, adult health
INTRODUCTION
The vascular system is the oldest and largest organ in the body and may be particularly vulnerable to prenatal alcohol exposure (PAE) as often the highest levels of prenatal alcohol consumption occur before there is an awareness of the pregnancy (Bandoli et al., 2020). The physical dysmorphic features and functional deficits associated with alcohol-related impairment of the brain have been well-investigated since Fetal Alcohol Syndrome was first identified (Jones and Smith, 1973). The mechanisms by which alcohol effects these deficits is unfortunately less well understood. Alterations in angiogenesis and epigenetic changes to vascular development have been implicated as a probable mechanism (Jegou et al., 2012, Ramadoss and Magness, 2012, Chernoff, 1977, Randall et al., 1977, Solonskii et al., 2008). Preclinical models of PAE have found disruption in the reproductive vasculature of the mother’s placenta, including alterations to blood flow, abnormal resistance of vessels, and angiogenesis (Ramadoss and Magness, 2012, Lo et al., 2017, Parkington et al., 2014). PAE has also been found to alter the cortical vascular density and spatial orientation of microvessels and the receptor expression of vascular endothelial growth factors in preclinical models (Jegou et al., 2012). Parallel findings have been reported in the brains of deceased human fetuses where those with heavy PAE had alterations to the radial organizaton of the microvasculature, particularly among older fetuses (Jegou et al., 2012), and alterations in the overall amount of vessel area (Solonskii et al., 2008, Jegou et al., 2012).
Among living individuals exposed to PAE, functional assessments of the vascular system have been limited to clinical samples where such assessments are clinically indicated (Steeg and Woolf, 1979) but additional support is garnered for disruption of this system from assessments of blood pressure (BP) and arterial function in otherwise physically healthy individuals with PAE. In a cohort of 9 year-olds with a history of PAE, arterial BP was normal but pulse-wave velocity (Morley et al., 2010) was significantly increased, indicating increased vascular stiffness associated with PAE. Arterial vessels in the retinas of individuals impacted by PAE have also been found to be altered, such that there is greater tortuosity and reduced branching points (Hellstrom et al., 1997a, Hellstrom et al., 1997b). In another clinical sample of children diagnosed with FASD, increased rates of hypertension were found even when controlling for age, weight and race (Cook et al., 2019). The latter finding was supported by preclinical models of PAE where increased arterial pressure has been found (Turcotte et al., 2002, Gray et al., 2010). Skepticism remains, however, whether these associations are directly linked to their PAE status or whether the associations are the by-products of indirect relationships of PAE and other adverse postnatal factors, including lower socioeconomic status and increased trauma exposure, that seemed to account for mental health outcomes associated with PAE (Coles et al., 2022).
The following study takes advantage of an on-going prospective study of the long-term impact of PAE on adult health outcomes to explore the impact of prenatal alcohol on vascular function. The participants were initially identified while in utero and reconsented as adults for additional follow-up studies. Vascular function was evaluated by standard BP measurement and peripheral arterial tonometry (PAT), which provides estimates of endothelial function and arterial stiffness. Relationships between PAE and levels of alcohol-related dysmorphic features were examined as predictors of vascular function status using path analysis, a form of structural equation modeling, that allows for simultaneous evaluation of relationships between predictors, potential mediators, and the outcome. PAE was anticipated to adversely impact vascular function through both direct effects and indirect effects of dysmorphic features, growth, other health factors or demographic factors that may be linked to adverse outcome.
MATERIAL AND METHODS
Recruitment
The original recruitment of the participants in this study occurred between 1980 and 1986 at a large inner city hospital that provided care to a predominantly Black population. Mothers were interviewed in the first trimester about their pregnancy and prenatal use of alcohol. Participants were then followed prospectively over their lifespan. The PAE group (n = 51) included adults whose mothers self-reported drinking at least one ounce of absolute alcohol per week or more (≥ 2 or more drinks/week). Using estimates of their absolute ounces of alcohol per week derived from maternal interviews in pregnancy, the group had a mean level of 10.7 (SD = 11.9, 95% CI 8.2–13.2) absolute ounces of alcohol per week. The Contrast group consisted of adults who were either identified at birth by their mothers’ self-report of abstaining from alcohol during the pregnancy or adults who were identified in adolescences from the same community as in need of special education services but had no history of PAE (Contrast, n = 37). Of the 37, 27 mothers were recruited during pregnancy and 10 were recruited in adolescences based on their special education history.
Participant recruitment for this study was done in conjunction with a larger multi-site study of adult health outcomes that was funded by the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD). Additional exclusion criteria included having congenital heart disease, sickle cell disease, structural or valvular heart disease, cardiomyopathy, congestive heart failure, arrhythmias, significant lung, renal, liver, or hematologic disease, and life expectancy less than 6 months. Two eligible participants who were initially recruited were unable to complete the study. One was unable to carry out the study assessment procedures and one participant subsequently refused. The study was approved by the Institutional Review Board at Emory University. Consent was obtained from the adult participant or their legal guardian as needed. In such cases, assent was obtained from the participant. Data collection for this project took place from December 2019 to August 2021.
Procedures
Physical measurements (height, weight, head circumference, and BP) and blood samples were obtained upon arrival to our laboratory. Participants were required to have a minimum of a 4 hour fasting period with no food, beverages, or cigarettes. Blood samples were analyzed for total cholesterol, low-density lipoproteins (LDL), high-density lipoproteins (HDL), and triglycerides (Quest Diagnostics®). In addition to a basic metabolic panel and complete blood count, glucose, insulin, and hemoglobin A1C levels were also assessed.
Participants were also interviewed about their health status and completed a demographic questionnaire and a questionnaire regarding their physical activity, the Paffenbarger Physical Activity Questionnaire (Paffenbarger et al., 1978). The latter provides an estimate of energy expenditure over the course of a week that has been linked to heart disease (Paffenbarger et al., 2001). Body mass index (BMI) was computed from the physical measurements. Individuals were defined as being as Underweight if their BMI was below 18.5 kg/m2, Normal Weight if their BMI fell between 18.5 and 24.9 kg/m2, Overweight if their BMI fell between 25 and 29.9 kg/m2and Obese if there BMI was 30 kg/m2or greater (Center for Disease Control and Prevention, 2020).
A peripheral arterial tonometric (PAT) plethysmographic device (EndoPAT 2000, Itamar Medical®) was used to assess peripheral microvascular endothelial function. Digital volume changes accompanying pulse-waves were measured using a device consisting of a finger probe and a transducer. A pressure of 40–70mmHg was applied by the probe to the index fingers for venous occlusion of both hands to record arterial pulse amplitude. While participants were in supine position, a blood pressure cuff was placed on the test arm. Amplitudes were then recorded from both the test and contralateral arms. After a 10 minute period of equilibration, reactive hyperemia index (RHI) was obtained by analyzing the three distinct components: (1) baseline amplitude signal detection across 5 minutes, (2) arterial occlusion by suprasystolic blood pressure cuff inflation for 5 minutes, and (3) post-occlusion amplitude signal detection for a 6 minute duration. The resulting RHI value is a unit-less measure that is partly nitric-oxide dependent, is associated with atherosclerotic risk factors, and evaluates endothelial function. RHI is determined by EndoPAT® automated software which takes into account the baseline signal and adjusts it to the changes in the contralateral arm. RHI < 1.67 are prognostic (Bonetti et al., 2004). PAT-RHI has been used to assess microvascular function in various studies of cardiovascular disease, including the Framingham Heart Study (Nayor et al., 2021, Tanaka et al., 2018). Endothelial dysfunction is an early predictor of atherosclerosis and its associated cardiovascular disease (Motozato et al., 2020).
An augmentation index (AI) is also generated from the PAT procedure that reflects the arterial stiffness. AI is derived from the pulse waveform analysis of the signal generated from the PAT during the baseline period. The result is then normalized to a heart rate of 75 bpm and a percentage value relative to sex and age is generated. Lower AI values reflect better arterial elasticity and higher values reflect more arterial stiffness. Arterial stiffness is an important risk factor that is associated with conditions such as hypertension, diabetes, and hyperlipidemia, and an independent risk factor for cardiovascular disease as it is typically not correlated with endothelial function (Yang et al., 2011, O’Rourke et al., 2002).
Other lifestyle variables were also assessed to determine if they were potential confounders (i.e., educational level, other prenatal exposures, adverse childhood experiences (ACEs, (Reuben et al., 2016)) of the relationship between PAE status and the outcomes. In addition to assessments conducted as part of this study, archival data were available on the sample and were used to characterize the participants. An assessment of intellectual functioning was obtained when participants were adolescents (Wechsler Intelligence Scale for Children-III (Wechsler and Psychological Corporation., 1991)) and adults (Wechsler Abbreviated Scale of Intelligence, 2nd edition (Wechsler, 2011)) and a measure of alcohol-related dysmorphic features was collected using a common genetics dysmorphic features checklist that is modified to differentially weight PAE-related dysmorphic features (e.g., hypoplastic philtrum is a “3”) (Fernhoff et al., 1980). Individuals prenatally exposed to alcohol have historically received higher scores in comparison to the non-exposed Contrast group on this measure (Coles et al., 1997, Coles et al., 1991).
Participants were provided $50 for compensation of their time and transportation was provided to the laboratory as needed. Participant recruitment and the study’s procedures were carried out in accordance with the guidelines established by the Declaration of Helsinki. Of the 88 participants enrolled, all had their blood pressure assessed but only 80 completed the PAT assessments and 2 of the assessments were invalid. An additional 2 participants did not have valid AI data.
Data Analysis Procedures
Statistical analysis was carried out using SPSS 28.0 and IBM’s SPSS AMOS 28. Group differences were examined using descriptive statistics and frequency distributions. As multiple factors may be needed to understand the relationship between PAE and vascular outcome, path analysis (Sarwono, 2017) was used to examine the contribution of PAE on outcomes while allowing for the contribution of various potentially confounding and mediating factors. Factors were selected based on well-known relationships with outcomes of interest (i.e. relationships between height and BP (Bourgeois et al., 2017)) and any group differences in lifestyle or metabolic characteristics within the sample. Data reduction was done on the multiple indices of lipid metabolism provided to obtain an estimate of hyperlipemia status. A principal components factor analysis with a varimax rotation was done combining triglycerides, LDL cholesterol, HDL cholesterol, total cholesterol and the cholesterol to HDL cholesterol ratio. A two factor solution was generated that accounted for 87.2% percent of the variance. The first factor accounted for 65.7% of the variance and was positively loaded with total cholesterol (.872), triglycerides (.568), LDL cholesterol (.954), chol/hdl ratio (.912), and non HDL cholesterol (.983) and was negatively loaded on HDL cholesterol (−.384). The second factor accounted for 21.5% of the variance and was positively loaded on HDL (.884), total cholesterol (.487), LDL-cholesterol (.227), and non HDL cholesterol (.163) and was negatively loaded on triglycerides (−.305) and chol/hdl ratio (−.318). The first factor was used in modeling risk for hyperlidemia in subsequent analyses. Based on previous research with this cohort, insulin level was also included in the proposed models as PAE was associated with alterations in insulin level that varied as a function of BMI status (Kable et al., 2021)
In addition to PAE status, participant’s age, biological sex, insulin level, hyperlipidemia status, height, BMI, and dysmorphology were used in the path models (see Supplemental Figures 1–4). Each path analysis involved a series of multiple regression analyses to evaluate the direct and indirect (mediated) effects of PAE on the vascular outcome. In order to minimize the overall experiment-wise error, these equations are estimated simultaneously using structural equation modeling. Bootstrapping was done using 2000 samples of the data to obtain an estimate of bias-corrected results at a 90% confidence interval. Exogenous variables, which are those not dependent on other variables but may be correlated, were PAE status, age, and biological sex. Endogenous variables, which are those impacted by other factors in the model, were hyperlipidemia, insulin, BMI, height, and dysmorphic features. Vascular measures evaluated were systolic and diastolic BP, RHI, and AI. An alpha level of .05 was used for rejecting the null hypothesis for specific relationships within each model.
RESULTS
Mean Characteristics of Sample.
Data regarding the characteristics of the sample is provided in Table 1. There were no group differences in the sex distribution, chronological age, educational level, and adverse childhood events. The groups varied in racial identity in that the PAE group had more individuals who identified as being African American (98.0%) relative to the Contrast group (86.5%). From these variables with no pre-existing group differences, only age and sex were included in subsequent path models as a result of their known effects on the outcomes of interest. Group status was significant for weight with those with PAE weighing less. BMI and risk for being overweight were higher in the Contrast as compared to the individuals with PAE. There was a trend for those with a history of PAE being shorter than those in the Contrast group. The groups did not differ in weekly energy expenditure from physical activity or in the percentage who had physical activity levels above 2000 kcal/week. Higher levels of alcohol-related dysmorphic features and lower overall IQ were reported in the PAE group relative to the Contrast Group, which are well-known effects of PAE (Kable et al., 2016).
Table 1.
Descriptive information is provided regarding the demographic characteristics and FASD-related characteristics of the sample by group status.
| Contrast n = 37 | Prenatal Alcohol n = 51 | Statistic | |
|---|---|---|---|
| Age (Mean, SD) | 36.7 (1.6) | 36.3 (1.7) | F (1,86) = 1.785, p < .185 |
| Gender (n, % Female) | 24, 64.9 | 32, 62.7 | χ = .042, p < .838 |
| Race (n, % African American) | 32, 86.5 | 50, 98.0 | χ = 4.505, p < .034 |
| In a Partnered Relationship (n, %) | 13, 35.1 | 13, 25.5 | χ = 0.958, p < .328 |
| Educational Level1 (Mean, SD) | 4.4 (1.4) | 4.2 (1.1) | F (1,86) = .398, p < .530 |
| Adverse Childhood Events Total (ACES)2 (Mean, SD) | 2.0 (1.9) | 2.5 (2.4) | F (1,86) = .856, p < .357 |
| Weight (kg) (Mean, SD) | 100.9 (31.7) | 78.9 (21.9) | F (1,86) = 14.825, p < .001 |
| Height (cm) (Mean, SD) | 171.3 (10.3) | 166.8 (11.4) | F (1,86) = 3.543, p < .063 |
| Body Mass Index ( kg/m2) (Mean, SD) | 34.5 (10.6) | 28.4 (7.8) | F (1,86) = 9.527, p < .003 |
| BMI Overweight or Obese (n, % Risk) | 34, 91.9 | 30, 58.8 | χ = 11.822, p < .001 |
| Total Dysmorphic Features Score3 (Mean, SD) | 1.91 (2.2) | 4.72 (6.4) | F (1,81) = 5.807, p < .018 |
| Small Palpebral Length (n, %) | 0, 0 | 6, 11.8 | χ = 4.671, p < .031 |
| Hypoplastic Philtrum (n, %) | 2, 5.4 | 12, 23.5 | χ = 5.265, p < .022 |
| Thinned Vermillion (n, %) | 0, 0 | 3, 5.9 | χ = 2.253, p < .133 |
| IQ4 (Mean, SD) | 84.2 (11.1) | 76.3 (15.9) | F (1,83) = 6.193, p < .015 |
| Physical Activity Index (kcal/wk) (Mean, SD) | 7853.0 (10,274.52) | 9249.3 (12,360.1) | F (1, 85) = 0.309, p < .580 |
| Log Physical Activity Index (Mean, SD) | 3.39 (1.2) | 3.67 (.54) | F (1, 85) = 2.064, p < .154 |
| Physical Activity >=2,000 kcal/wk (n, %) | 22, 61.1 | 36, 70.6 | χ = 0.853, p < .356 |
Educational level ranged from 1 to 7 with 1, Less than 7th grade; 2, Junior high/Middle school (9th grade); 3, Partial high school (10th or 11th); 4, High school graduate; 5, Partial college (At least one year) or specialized training (technical school); 6, College or University graduation; 7, Partial graduate.
Adverse Childhood Events Total score ranges from 0–11(Reuben et al., 2016).
Total Alcohol-Related Dysmorphic Features score was obtained from an assessment when participants were in their 20’s.
Obtained from the average of the Wechsler Intelligence Scale for Children-III (Wechsler and Psychological Corporation., 1991) administered at the teenage assessment and the Wechsler Abbreviated Scale of Intelligence (WASI) administered when participants were in their 20s.
Relative to their metabolic status, group differences were not found in levels of glucose, HbA1c, or insulin. There was a significant effect on triglycerides with those in the Contrast group having higher levels than did those in the PAE group and trends were found for higher LDL cholesterol and non HDL levels being higher in the Contrast group. No group differences were found in HDL cholesterol, total cholesterol or the cholesterol to HDL cholesterol ratio. See Table 2 for details. Simple mean group differences were also not found in the levels of systolic or diastolic BP, RHI, or AI and there were no group differences in those categorized as having elevated or hypertensive BP or as having a clinical risk for peripheral microvascular endothelial dysfunction based on their RHI values (see Table 3 for details).
Table 2.
Levels of lipids and diabetes-related indicators are reported relative to group status.
| Contrast n = 37 | Prenatal Alcohol n = 51 | Statistic | |
|---|---|---|---|
| Total Cholesterol mg/dL | 185.6 (43.9) | 171.1 (38.5) | F (1,86) = 2.696, p < .104 |
| LDL1 Cholesterol mg/dL | 111.9 (37.2) | 98.0 (38.5) | F (1,86) = 2.819, p < .097 |
| HDL2 Cholesterol mg/dL | 52.8 (10.9) | 55.5 (17.2) | F (1,86) = 0.742, p < .392 |
| Triglycerides mg/dL | 111.4 (82.7) | 83.8 (39.5) | F (1,86) = 4.337, p < .040 |
| Non HDL Cholesterol mg/dL | 132.8 (41.4) | 115.6 (42.0) | F (1,86) = 3.660, p < .059 |
| Cholesterol/HDL Ratio mg/dL | 3.6 (0.95) | 3.4 (1.43) | F (1,86) = 0.671, p < .451 |
| Glucose mg/dL | 99.8 (55.2) | 91.5 (24.7) | F (1,86) = 0.920, p < .340 |
| Hemoglobin A1C3 | 5.54 (1.2) | 5.49 (.94) | F (1,86) = 0.058, p < .811 |
| Insulin uIU/mL | 8.6 (6.2) | 8.4 (8.8) | F (1,86) = 0.016, p < .898 |
LDL refers to low density lipoprotein;
HDL refers to high density lipoproteins;
% Total hemoglobin
Table 3.
Descriptive information regarding the vascular outcome variables is provided by group status.1
| Contrast | Prenatal Alcohol | Statistic | |
|---|---|---|---|
| Systolic Blood Pressure (mm Hg) (Mean, SD) | 129.9 (15.7) | 125.6 (17.6) | F (1,86) = 1.444, p < .233 |
| Diastolic Blood Pressure (mm Hg) (Mean, SD) | 83.7 (11.7) | 82.3 (12.6) | F (1,86) = 0.28, p < .598 |
| Hypertension2 (n, %) | 26, 70.3 | 30, 58.8 | χ = 1.214, p < .380 |
| Reactive Hyperemia Index (RHI) (Mean, SD) | 2.03 (.59) | 1.85 (.52) | F (1,76) = 2.039, p < .157 |
| Clinical Risk Level of RHI (< 1.67) (n, %) | 9, 29 | 15, 31.9 | χ = 0.73, p < .787 |
| Augmentation Index (AI) Percentile3 (Mean, SD) | 58.4 (28.3) | 64.8 (28.5) | F (1,74) = 0.931, p < .338 |
Sample size for the Contrast group is 37 for BP measurements and 31 for RHI and AI. Sample size for the PAE group is 51 for the BP measurements and 47 for the RHI and AI.
Number and precentage of cases of hypertension using the American Heart Association criteria for elevated BP or hypertension (Kaneko et al., 2021).
AI is reported relative to age and gender norms
Path Analysis of Vascular Function:
Model Prediction of Specific Outcomes:
Table 4 contains the phi and Pearson correlations between the variables used in the path analysis. For systolic and diastolic BP, the default model was rejected and the proposed model had a good fit (χ2 (11, N = 88)= 9.791, p < .549, CFI= 1.00, TLI = 1.03, RMSEA = .000) with 29.4% of the variance accounted for in Systolic BP and 22.9% in Diastolic BP. For RHI, the default model was rejected and proposed model had a good fit (χ2 (11, N = 78) = 9.416, p < .584, CFI= 1.00, TLI = 1.043, RMSEA = .000) with 28.3% of the variance accounted for in RHI. For AI, the default model of no effect was also rejected χ2 (11, N = 76)= 9.344, p < .590, CFI= 1.00, TLI = 1.041, RMSEA = .000) with 36.1 % of the variance accounted for in AI.
Table 4.
Pearson and phi coefficients are provided to assess the simple linear relationships between variables used in the path model
| Effect | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. PAE Statusρ | 1.00 | −.022 | −.164 | −.014 | −.199 T | .316** | .276** | −.129 | −.057 | .104 | .119 |
| 2. Sexρ | 1.00 | .051 | .123 | −.605 *** | .156 | −.055 | −.016 | .023 | .013 | .292 * | |
| 3. Hyperlipidemia | 1.00 | .233 * | −.195 | .053 | .021 | .062 | .078 | −.234 * | −.087 | ||
| 4. Insulin | 1.00 | −.174 | .527 *** | .001 | .225 * | .241 * | −.348 ** | −.222 T | |||
| 5. Height | 1.00 | −.071 | −.358 *** | .176 | .170 | .092 | −.208 T | ||||
| 6. BMI | 1.00 | −.199 T | .335 *** | .302 ** | −.213 T | −.384 *** | |||||
| 7. Dysmorphology | 1.00 | −.050 | −.089 | .078 | .277 * | ||||||
|
| |||||||||||
| 8. BP-Systolic | 1.00 | .862 *** | −.071 | .057 | |||||||
| 9. BP-Diastolic | 1.00 | −.116 | .016 | ||||||||
| 10. PAT-RHI | 1.00 | .250 * | |||||||||
| 11. PAT-AI | 1.00 | ||||||||||
Notes: Pearson correlation coefficients are presented in the table in all cases but where variables are marked with a ρ, which indicates correlations are phi coefficients. PAE = prenatal alcohol exposure (coded 1 as yes and 0 as no); Sex coded as 1 is male and 2 is female). PAT = peripheral arterial tonometry; RHI = reactive hyperemia index; AI = augmentation index,
< .10;
< .05;
< .01;
< .001.
Systolic BP.
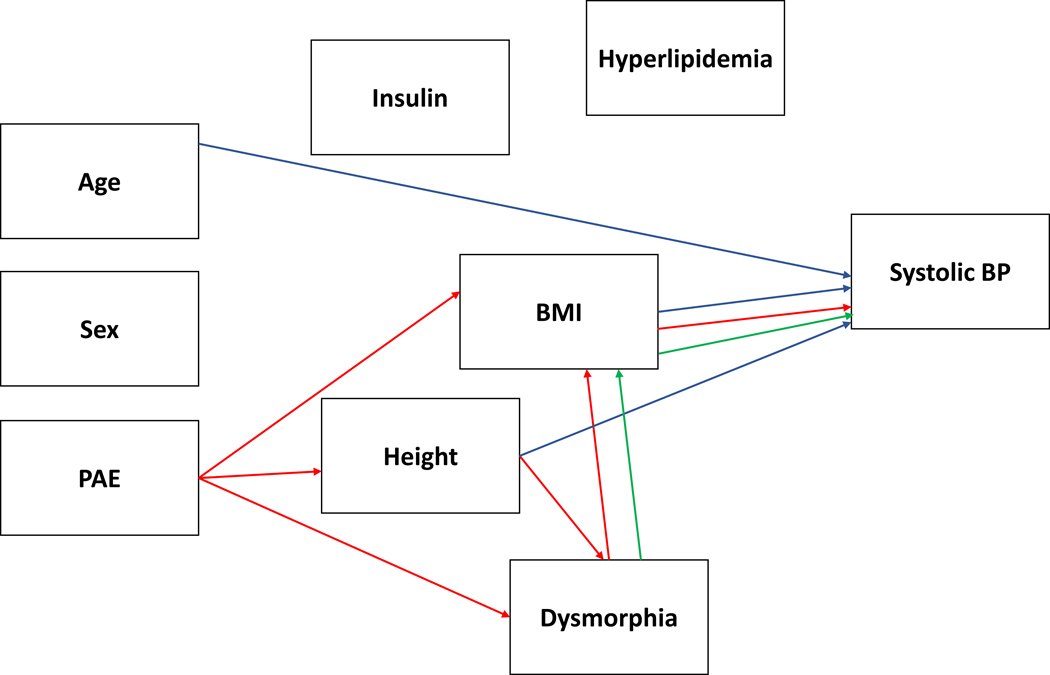
In predicting systolic BP, direct effects were found for height (β = .346, p < .008), BMI (β = .337, p < .003) and age (β = .297, p < .003). Indirect effects were found for PAE (β = −.154, p < .008) and dysmorphic features (β = −.072, p < .027). The indirect effect of PAE was via its effect on height (p < .008), BMI (p < .005), height to dysmorphic features to BMI (p < .015) and dysmorphic features to BMI (p < .029). The indirect effect of dysmorphic features was via its effect on BMI (p < .027). The impact of PAE on lowering stature and BMI was protective relative to the Contrast group in this study. Supplementary Figure 1 displays the model with the standardized beta values for each of the relationships and a graphical depiction of the simplified model is in Figure 1.
Figure 1.
displays the simplified model for systolic blood pressure (BP) with the significant PAE-related effects (direct and indirect) plotted in red and dysmorphia effects independent of PAE in green. A blue line reflects direct or indirect relationships with the outcome variable that are independent of PAE or dysmorphia.
Diastolic BP.
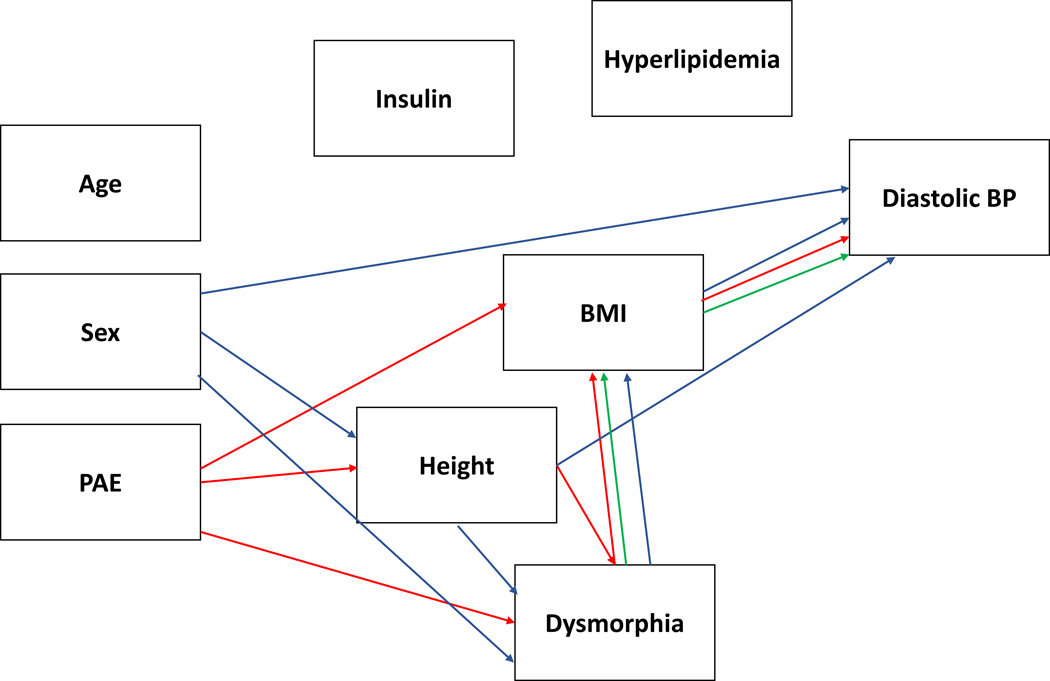
For prediction of diastolic BP, direct effects were found for height (β = .403, p < .004), sex (β = .226, p < .029), and BMI (β = .294, p < .007) and indirect effects were found for PAE (β = −.172, p < .005), dysmorphic features (β = −.067, p < .024) and sex (β = −.181, p < .040). Both PAE (p < .005) and dysmorphic features (p < .028) had an indirect effect through BMI and PAE had an indirect effect via the dysmorphic features to BMI path (p < .030). PAE also had an indirect effect via its effect on height (p < .006) and via height to dysmorphic features to BMI (p < .015). Each of these pathways points to protective effect of PAE resulting from the effect PAE has on reducing stature and BMI relative to the Contrast group who are disproportionately overweight relative to national norms. The indirect effect of sex was via two paths, including height to dysmorphic features to BMI (p < .014) and dysmorphic features to BMI (p < .019). Supplementary Figure 2 displays the model with the standardized beta values for each of the relationships and a graphical depiction of the simplified model is in Figure 2.
Figure 2.
displays the simplified model for diastolic blood pressure (BP) with the significant PAE-related effects (direct and indirect) plotted in red and dysmorphia effects independent of PAE in green. A blue line reflects direct or indirect relationships with the outcome variable that are independent of PAE or dysmorphia.
RHI.
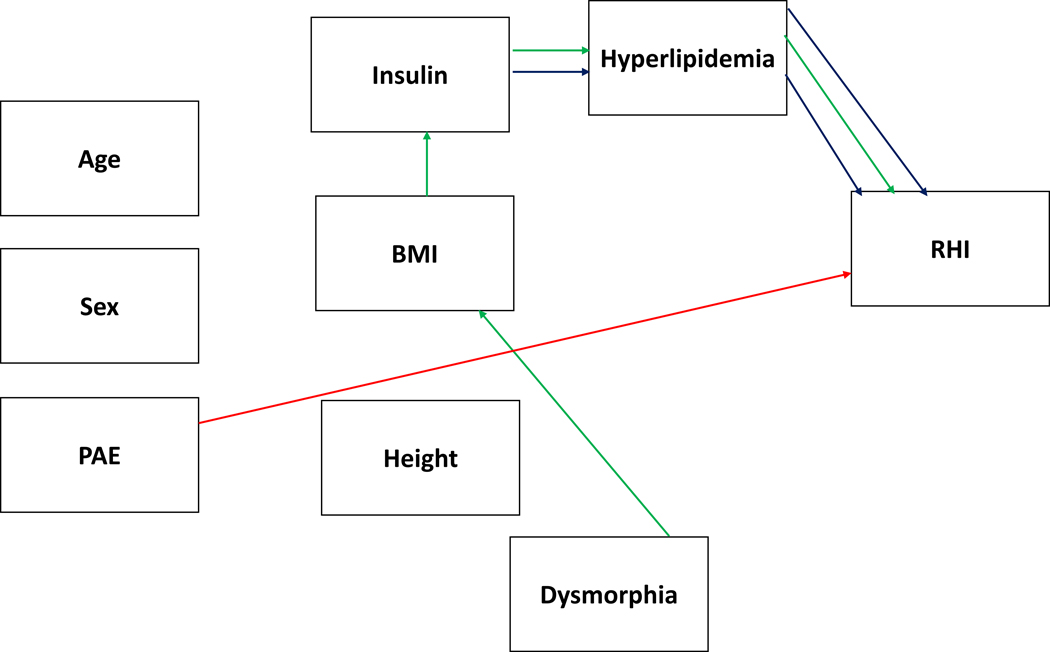
Direct effects were found for PAE (β = −.312, p < .024) and hyperlipidemia ( β = −.195, p < .050) and indirect effects were found for dysmorphic features (β = .060, p < .026) and insulin (β = −.041, p < .046). The direct effects of PAE and hyperlipidemia on RHI were negative in nature for both PAE and hyperlipidemia as both resulted in lower RHI scores, reflecting greater endothelial dysfunction. Dysmorphic features also negatively impacted RHI indirectly via its effect on BMI to insulin to hyperlipidemia (p < .042) and insulin indirectly had a negative impact on RHI via its effect on hyperlipidemia (p < .047). Supplementary Figure 3 displays the model with the standardized beta values for each of the relationships and Figure 3 displays a graphical depiction of the simplified model.
Figure 3.
displays the simplified model for reactive hyperemia index (RHI) with the significant PAE-related effects (direct and indirect) plotted in red and dysmorphia effects independent of PAE in green. A blue line reflects direct or indirect relationships with the outcome variable that are independent of PAE or dysmorphia.
AI.
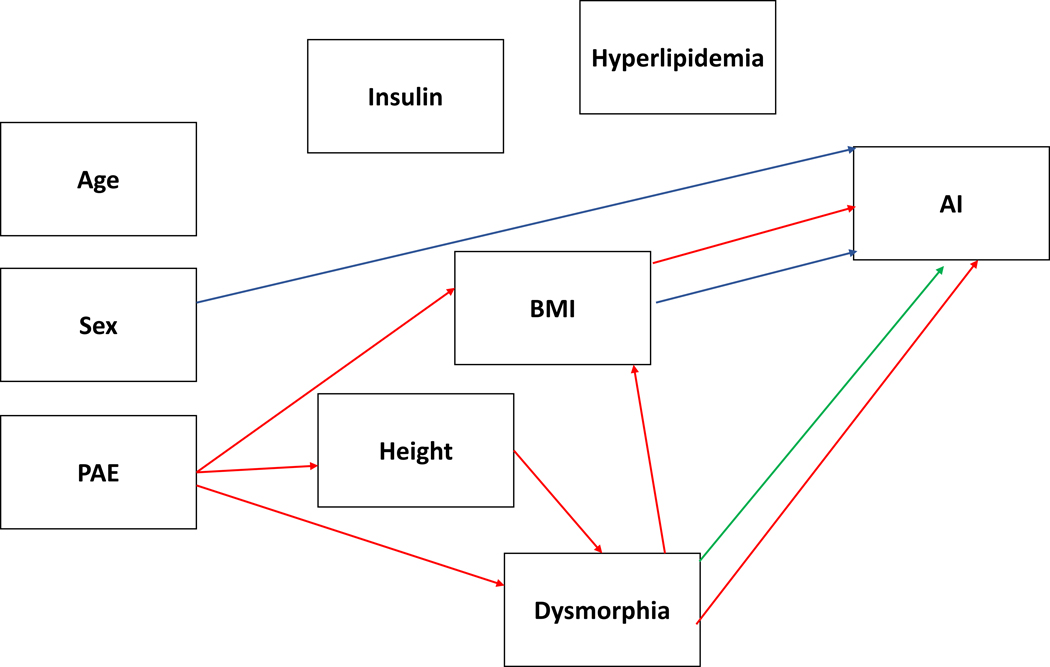
Direct effects were found on biological sex (β = .459, p < .003), BMI (β = −.408, p < .009) and dysmorphic features (β = .279, p < .020). Being female and level of alcohol-related dysmorphic features were associated with increased arterial stiffness as indicated by higher scores but increased levels of BMI were associated with greater arterial elasticity. PAE had an indirect negative effect (β = .237, p < .005) via its effects on dysmorphic features, height, and BMI that also resulted in increased arterial stiffness. The pathways involved (1) PAE through dysmorphic features (p < .029), (2) PAE through dysmorphic features to BMI (p < .038), (3) PAE, height, and dysmorphic features (p < .013), (4) PAE, height, dysmorphic features, and BMI (p < .033), and (5) BMI alone (p < .005). Supplementary Figure 4 displays the standardized beta values for each of the relationships and Figure 4 displays the associated simplified model.
Figure 4.
displays the simplified model for the augmentation index (AI) with the significant PAE-related effects (direct and indirect) plotted in red and dysmorphia effects independent of PAE in green. A blue line reflects direct or indirect relationships with the outcome variable that are independent of PAE or dysmorphia.
DISCUSSION
Linking prenatal and early life exposures to later health outcomes has been a primary goal of those exploring the developmental origins of health and disease (DOHaD) hypothesis (Barker, 1990). The evidence in this study links PAE to mid-life health outcomes on vascular function using a prospective cohort initially identified while in utero. When contrasted to a cohort of individuals of similar age, ethnicity, social economic status, and educational background, PAE was associated with negative impacts on endothelial function and arterial elasticity but not to arterial BP. These findings suggest a long-term impact of PAE on particular aspects of vascular function and extends previous findings of disruption to cerebral vascular development while in utero (see review, (Bukiya and Dopico, 2018)) and in later childhood (Cook et al., 2019, Morley et al., 2010) after a history of PAE.
In the case of BP, PAE was found to be protective relative to the Contrast group used in this study who had elevated risk for vascular impairment based on the high frequency of being categorized as overweight and obese (91.9%). The protection provided by PAE was through its effects on lowering physical stature and body mass. Caution should be used in interpreting that PAE is protective against elevated arterial BP relative to a general population as both groups in this study had increased risk for hypertension when compared to large-scale population databases. The National Health and Nutrition Examination Survey (NHANES) data collected between 2007–2010 indicated that 24.4% of males and 17.6% of females between 35–44 years have hypertension (Go et al., 2013) as compared to 70.3% of the Contrast group and 58.8% of the PAE group in this study, suggesting both groups have increased risk for vascular disease associated with their lower socioeconomic status and history of social adversity (Coles et al., 2022) when compared to the overall population of the United States. Even when the NHANES data are stratified by race (39.9% Black men and 42.7% Black women had hypertension after adjusting for age), participants in this study sample appear to carry increased risk for vascular disease. Previous results on the effect of PAE on BP have been mixed (Morley et al., 2010, Cook et al., 2019). The findings from this study are more supportive of an increased risk relative to the general population but not an increased risk when compared to groups who are matched in sociodemographic characteristics and social adversity. Discrepancies in the previous literature may simply be an artifact of these types of group differences in other factors that may also influence arterial BP, suggesting the relative importance of other biological and environmental influences on arterial BP outcomes that may co-occur with PAE histories. The Cook et al study involved clinical patients diagnosed with an FASD rather than an exposure cohort as was used in this sample where a continuum of PAE effects would be anticipated. In contrast, support for an adverse effect of PAE on arterial pressure has been reported in preclinical models of PAE (Turcotte et al., 2002, Gray et al., 2010) where environmental factors are typically comparable between exposed and non-exposed groups.
In contrast, PAE was found to adversely impact endothelial function. PAE had a direct negative effect on endothelial function and total alcohol-related dysmorphic features had an indirect negative effect on endothelial function that was statistically independent or unique relative to the effect of PAE. This suggests that the extent of alcohol-related dysmorphic features is linked to the extent of the vascular damage and provides unique information over and above documentation of PAE alone. The extent of total alcohol-related dysmorphic features may be flagging PAE exposure early in pregnancy where it could have more impact on the early developing vascular system or simply flagging a nonlinear relationship between higher dosage levels of PAE and vascular outcome. Endothelial dysfunction is an early predictor of atherosclerosis and its associated cardiovascular disease (Motozato et al., 2020). Besides PAE, the other big contributor to impaired microvascular function was hyperlipidemia, which had a direct effect of its own and served as the final point along the path for the indirect effects of dysmorphic features and insulin. Hyperlipidemia is well-known for producing atherosclerosis, which involves the accumulation of lipids, cholesterol, and plaques within the walls of arteries, that reduce blood flow and the delivery of oxygen throughout the body (Mszar et al., 2022). Dietary intake was not assessed in this study so it is not possible to differentiate whether individual differences in lipid metabolism or intake may have contributed to the indirect negative effect of alcohol-related dysmorphic features had on endothelial function. Future research should include dietary intake measures to explore whether disruption to lipid metabolism is associated with PAE or simply impaired cognitive function may lead to less optimal dietary choices relative to dietary lipid intake.
The increased arterial stiffness found in this study is supportive of the previous studies of PAE related to arterial functioning, including findings of less contractile force in the umbilical cord arteries (Iveli et al., 2007) and increased stiffness using pulse wave analysis in 9 year-olds (Morley et al., 2010), suggesting these alterations to vascular elasticity last well into adulthood. Arterial stiffness is seen as a symptom of peripheral vascular disease and an independent risk factor for cardiovascular disease. The combination of reduced renal and vascular capacity seen after PAE was proposed by Parkington and colleagues (2010) as being key to predicting increased cardiovascular disease with ageing in those with PAE but others (Saha et al., 2021) have proposed that the on-going impact of arterial stiffness to everyday neural functioning resulting from reductions in cerebral blood flow may be problematic under conditions of high demand, leading to emotional and behavioral dysfunction. Understanding the biological mechanisms of arterial stiffening related to PAE may help with identifying potential therapeutic interventions that could have impact on both the long-term cardiovascular risk but also may help to improve everyday functioning of individuals with an FASD who struggle frequently when put in situations where they are over-stimulated.
Our study has several limitations. Both the Contrast and PAE group were from high-risk social backgrounds which may adversely impacted our ability to detect PAE-related effects. This would be the case if there were ceiling effects on the outcomes such that PAE-related group differences would be obscured by other factors that influence the outcomes. This concern is most pertinent to our results regarding the arterial BP and the findings that PAE was protective of hypertension in our sample. Both our Contrast and PAE groups were from backgrounds that were associated with elevated cardiovascular risk and a contrast group that was more representative of the typical U.S. sample may have provided aid in our analysis. Our sample size was also relatively small, making the findings vulnerable to spurious findings. Future replications in larger samples would help reduce these concerns. The sample size is also vulnerable to potential over-fitting of the data when using the path analysis chosen for this study. A minimum of 100 subjects are often recommended (Sarwono, 2017). Tanaka (1987) recommends 20 to 1 subject to variable ratios but others have argued that a 5 to 1 can be used (Bentler and Chou, 1987). Finally, the measure of arterial stiffness derived from Endo-PAT finger-tip tonometry in our study is not the most commonly used measure of arterial stiffness, which is typically carotid to femoral pulse wave analysis. PAT-derived AI, however, is still a reliable measure with strong repeatability and reasonable correlations have been reported between this technique and radial artery tonometry-derived arterial stiffness (Haller et al., 2007, McCrea et al., 2012, Heffernan et al., 2010).
Given that acute alcohol intoxication has effects on maternal vascular function (Cook et al., 2001), it is not surprising that there appears to be a cascade of impact on placental and fetal vascular development (Ramadoss and Magness, 2012, Lo et al., 2017, Parkington et al., 2014), including reductions in umbilical cord blood flow (Tseng et al., 2019, Pinson et al., 2022), reductions in fetal cerebral blood flow (Raghunathan et al., 2020, Tobiasz et al., 2018), increased vasoconstriction (Raghunathan et al., 2018, Shan et al., 2020), alterations in expression of genes linked to angiogenesis and vascular development (Xu et al., 2005), impaired cortical vascular network formation (Siqueira et al., 2021), decreased oxygen perfusion and saturation in fetal vasculature (Shan et al., 2020), and altered cerebral artery mitochondria (Bukiya, 2019). Our study provides support for the persistence of these early effects on peripheral vascular and microvascular function into adulthood.
The impact of PAE to the vascular system may also serve as a potential biochemical signature of PAE, leading to a much needed biomarker of PAE effect. Placental protein profiles linked to angiogenesis and early vascular development (i.e., VEGFR2 and annexin-A4) had moderate to high levels of diagnostic accuracy in predicting later neurodevelopmental impairment associated with PAE (Holbrook et al., 2019). Other promising candidates for biomarkers of PAE’s impact on vascular and brain development include PAE-related reductions in placental growth factor, which is critical for brain angiogenesis (Lecuyer et al., 2017), and changes in proteomics of cerebral arteries associated with binge drinking models in primates (Bisen et al., 2019).
PAE appears to have a long-term impact on vascular function that can increase an individual’s risk for cardiovascular disease. The severity of alcohol-related dysmorphia was predictive of more adverse effects on endothelial function and vascular stiffness.
Supplementary Material
Acknowledgements:
Research was funded by the NIH Research Grant R21AA027345 (Julie Kable, PI), funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
Data collection was conducted in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Additional information about CIFASD can be found at www.cifasd.org.
Footnotes
Conflict of Interest: The authors have no conflict of interest to report.
REFERENCES
- BANDOLI G, JONES K, WERTELECKI W, YEVTUSHOK L, ZYMAK-ZAKUTNYA N, GRANOVSKA I, PLOTKA L, CHAMBERS C & CIFASD 2020. Patterns of Prenatal Alcohol Exposure and Alcohol-Related Dysmorphic Features. Alcohol Clin Exp Res, 44, 2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKER DJ 1990. The fetal and infant origins of adult disease. BMJ, 301, 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENTLER P & CHOU C 1987. Practical issues in structural modeling. Sociological Methods & Research, 16, 78–117. [Google Scholar]
- BISEN S, KAKHNIASHVILI D, JOHNSON DL & BUKIYA AN 2019. Proteomic Analysis of Baboon Cerebral Artery Reveals Potential Pathways of Damage by Prenatal Alcohol Exposure. Mol Cell Proteomics, 18, 294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONETTI PO, PUMPER GM, HIGANO ST, HOLMES DR JR., KUVIN JT & LERMAN A 2004. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol, 44, 2137–41. [DOI] [PubMed] [Google Scholar]
- BOURGEOIS B, WATTS K, THOMAS DM, CARMICHAEL O, HU FB, HEO M, HALL JE & HEYMSFIELD SB 2017. Associations between height and blood pressure in the United States population. Medicine (Baltimore), 96, e9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUKIYA AN 2019. Fetal Cerebral Artery Mitochondrion as Target of Prenatal Alcohol Exposure. Int J Environ Res Public Health, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUKIYA AN & DOPICO AM 2018. Fetal Cerebral Circulation as Target of Maternal Alcohol Consumption. Alcohol Clin Exp Res, 42, 1006–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CENTER FOR DISEASE CONTROL AND PREVENTION. 2020. Defining Adult Overweight and Obesity [Online]. Available: https://www.cdc.gov/obesity/adult/defining.html [Accessed 8/20 2020].
- CHERNOFF GF 1977. The fetal alcohol syndrome in mice: an animal model. Teratology, 15, 223–9. [DOI] [PubMed] [Google Scholar]
- COLES CD, BROWN RT, SMITH IE, PLATZMAN KA, ERICKSON S & FALEK A 1991. Effects of prenatal alcohol exposure at school age. I. Physical and cognitive development. Neurotoxicol Teratol, 13, 357–67. [DOI] [PubMed] [Google Scholar]
- COLES CD, GRANT TM, KABLE JA, STONER SA, PEREZ A & COLLABORATIVE INITIATIVE ON FETAL ALCOHOL SPECTRUM, D. 2022. Prenatal alcohol exposure and mental health at midlife: A preliminary report on two longitudinal cohorts. Alcohol Clin Exp Res, 46, 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLES CD, PLATZMAN KA, RASKIND-HOOD CL, BROWN RT, FALEK A & SMITH IE 1997. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res, 21, 150–61. [PubMed] [Google Scholar]
- COOK JC, LYNCH ME & COLES CD 2019. Association Analysis: Fetal Alcohol Spectrum Disorder and Hypertension Status in Children and Adolescents. Alcohol Clin Exp Res, 43, 1727–1733. [DOI] [PubMed] [Google Scholar]
- COOK JL, ZHANG Y & DAVIDGE ST 2001. Vascular function in alcohol-treated pregnant and nonpregnant mice. Am J Physiol Regul Integr Comp Physiol, 281, R1449–55. [DOI] [PubMed] [Google Scholar]
- FERNHOFF PM, SMITH IE & FALEK A 1980. Dysmorphia Checklist. Document available through the Maternal Substance Abuse and Child Development Project, Division of Psychiatry, Emory University School of Medicine, Atlanta, GA. [Google Scholar]
- GO A, MOZAFFARIAN D, ROGER V, BENJAMIN E, BERRY J, BORDEN W, BRAVATA D, DAI S, FORD E, FOX C, FRANCO S, FULLERTON H, GILLESPIE C, HAILPERN S, HEIT J, HOWARD V, HUFFMAN M, KISSELA B, KITTNER S, LACKLAND D, LICHTMAN J, LISABETH L, MAGID D, MARCUS G, MARELLI A, MATCHAR D, MCGUIRE D, MOHLER E, MOY C, MUSSOLINO M, NICHOL G, PAYNTER N, SCHREINER P, SORLIE P, STEIN J, TURAN T, VIRANI S, WONG N, WOO D & TURNER M 2013. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation, 127, e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY SP, DENTON KM, CULLEN-MCEWEN L, BERTRAM JF & MORITZ KM 2010. Prenatal exposure to alcohol reduces nephron number and raises blood pressure in progeny. J Am Soc Nephrol, 21, 1891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLER MJ, SILVERSTEIN JH & SHUSTER JJ 2007. Correlation between radial artery tonometry- and fingertip tonometry-derived augmentation index in children with type 1 diabetes. Diab Vasc Dis Res, 4, 66. [DOI] [PubMed] [Google Scholar]
- HEFFERNAN KS, PATVARDHAN EA, HESSION M, RUAN J, KARAS RH & KUVIN JT 2010. Elevated augmentation index derived from peripheral arterial tonometry is associated with abnormal ventricular-vascular coupling. Clin Physiol Funct Imaging, 30, 313–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLSTROM A, CHEN Y & STROMLAND K 1997a. Fundus morphology assessed by digital image analysis in children with fetal alcohol syndrome. J Pediatr Ophthalmol Strabismus, 34, 17–23. [DOI] [PubMed] [Google Scholar]
- HELLSTROM A, SVENSSON E & STROMLAND K 1997b. Eye size in healthy Swedish children and in children with fetal alcohol syndrome. Acta Ophthalmol Scand, 75, 423–8. [DOI] [PubMed] [Google Scholar]
- HOLBROOK BD, DAVIES S, CANO S, SHRESTHA S, JANTZIE LL, RAYBURN WF, BAKHIREVA LN & SAVAGE DD 2019. The association between prenatal alcohol exposure and protein expression in human placenta. Birth Defects Res, 111, 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVELI MF, MORALES S, REBOLLEDO A, SAVIETTO V, SALEMME S, APEZTEGUIA M, CECOTTI N, DRUT R & MILESI V 2007. Effects of light ethanol consumption during pregnancy: increased frequency of minor anomalies in the newborn and altered contractility of umbilical cord artery. Pediatr Res, 61, 456–61. [DOI] [PubMed] [Google Scholar]
- JEGOU S, EL GHAZI F, DE LENDEU PK, MARRET S, LAUDENBACH V, UGUEN A, MARCORELLES P, ROY V, LAQUERRIERE A & GONZALEZ BJ 2012. Prenatal alcohol exposure affects vasculature development in the neonatal brain. Ann Neurol, 72, 952–60. [DOI] [PubMed] [Google Scholar]
- JONES KL & SMITH DW 1973. Recognition of the fetal alcohol syndrome in early infancy. Lancet, 302, 999–1001. [DOI] [PubMed] [Google Scholar]
- KABLE JA, MEHTA PK & COLES CD 2021. Alterations in Insulin Levels in Adults with Prenatal Alcohol Exposure. Alcohol Clin Exp Res, 45, 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KABLE JA, O’CONNOR MJ, OLSON HC, PALEY B, MATTSON SN, ANDERSON SM & RILEY EP 2016. Neurobehavioral Disorder Associated with Prenatal Alcohol Exposure (ND-PAE): Proposed DSM-5 Diagnosis. Child Psychiatry Hum Dev, 47, 335–46. [DOI] [PubMed] [Google Scholar]
- KANEKO H, YANO Y, ITOH H, MORITA K, KIRIYAMA H, KAMON T, FUJIU K, MICHIHATA N, JO T, TAKEDA N, MORITA H, NODE K, CAREY RM, LIMA JAC, OPARIL S, YASUNAGA H & KOMURO I 2021. Association of Blood Pressure Classification Using the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline With Risk of Heart Failure and Atrial Fibrillation. Circulation, 143, 2244–2253. [DOI] [PubMed] [Google Scholar]
- LECUYER M, LAQUERRIERE A, BEKRI S, LESUEUR C, RAMDANI Y, JEGOU S, UGUEN A, MARCORELLES P, MARRET S & GONZALEZ BJ 2017. PLGF, a placental marker of fetal brain defects after in utero alcohol exposure. Acta Neuropathol Commun, 5, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LO JO, SCHABEL MC, ROBERTS VH, WANG X, LEWANDOWSKI KS, GRANT KA, FRIAS AE & KROENKE CD 2017. First trimester alcohol exposure alters placental perfusion and fetal oxygen availability affecting fetal growth and development in a non-human primate model. Am J Obstet Gynecol, 216, 302 e1–302 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCREA CE, SKULAS-RAY AC, CHOW M & WEST SG 2012. Test-retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for clinical trial design. Vasc Med, 17, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORLEY R, DWYER T, HYNES KL, COCHRANE J, PONSONBY AL, PARKINGTON HC & CARLIN JB 2010. Maternal alcohol intake and offspring pulse wave velocity. Neonatology, 97, 204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOTOZATO K, SUEMATSU Y, NORIMATSU K, KUSUMOTO T & MIURA S-I 2020. Reactive Hyperemia Index Associated With Atherosclerotic Cardiovascular Disease Under Treatment for Lifestyle Diseases. Journal of clinical medicine research, 12, 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MSZAR R, WEBB GB, KULKARNI VT, AHMAD Z & SOFFER D 2022. Genetic Lipid Disorders Associated with Atherosclerotic Cardiovascular Disease: Molecular Basis to Clinical Diagnosis and Epidemiologic Burden. Med Clin North Am, 106, 325–348. [DOI] [PubMed] [Google Scholar]
- NAYOR M, CHERNOFSKY A, SPARTANO NL, TANGUAY M, BLODGETT JB, MURTHY VL, MALHOTRA R, HOUSTIS NE, VELAGALETI RS, MURABITO JM, LARSON MG, VASAN RS, SHAH RV & LEWIS GD 2021. Physical activity and fitness in the community: the Framingham Heart Study. Eur Heart J, 42, 4565–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’ROURKE MF, STAESSEN JA, VLACHOPOULOS C, DUPREZ D & PLANTE GE 2002. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens, 15, 426–44. [DOI] [PubMed] [Google Scholar]
- PAFFENBARGER RS JR., BLAIR SN & LEE IM 2001. A history of physical activity, cardiovascular health and longevity: the scientific contributions of Jeremy N Morris, DSc, DPH, FRCP. Int J Epidemiol, 30, 1184–92. [DOI] [PubMed] [Google Scholar]
- PAFFENBARGER RS JR., WING AL & HYDE RT 1978. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol, 108, 161–75. [DOI] [PubMed] [Google Scholar]
- PARKINGTON HC, COLEMAN HA, WINTOUR EM & TARE M 2010. Prenatal alcohol exposure: implications for cardiovascular function in the fetus and beyond. Clin Exp Pharmacol Physiol, 37, e91–8. [DOI] [PubMed] [Google Scholar]
- PARKINGTON HC, KENNA KR, SOZO F, COLEMAN HA, BOCKING A, BRIEN JF, HARDING R, WALKER DW, MORLEY R & TARE M 2014. Maternal alcohol consumption in pregnancy enhances arterial stiffness and alters vasodilator function that varies between vascular beds in fetal sheep. J Physiol, 592, 2591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINSON MR, TSENG AM, ADAMS A, LEHMAN TE, CHUNG K, GUTIERREZ J, LARIN KV, CHAMBERS C, MIRANDA RC & COLLABORATIVE INITIATIVE ON FETAL ALCOHOL SPECTRUM, D. 2022. Prenatal alcohol exposure contributes to negative pregnancy outcomes by altering fetal vascular dynamics and the placental transcriptome. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed]
- RAGHUNATHAN R, LIU CH, KOUKA A, SINGH M, MIRANDA RC & LARIN KV 2020. Dose-response analysis of microvasculature changes in the murine fetal brain and the maternal extremities due to prenatal ethanol exposure. J Biomed Opt, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAGHUNATHAN R, WU C, SINGH M, LIU CH, MIRANDA RC & LARIN KV 2018. Evaluating the effects of maternal alcohol consumption on murine fetal brain vasculature using optical coherence tomography. J Biophotonics, 11, e201700238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMADOSS J & MAGNESS RR 2012. Vascular effects of maternal alcohol consumption. Am J Physiol Heart Circ Physiol, 303, H414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDALL CL, TAYLOR J & WALKER DW 1977. Ethanol-induced malformations in mice. Alcohol Clin Exp Res, 1, 219–24. [DOI] [PubMed] [Google Scholar]
- REUBEN A, MOFFITT TE, CASPI A, BELSKY DW, HARRINGTON H, SCHROEDER F, HOGAN S, RAMRAKHA S, POULTON R & DANESE A 2016. Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. J Child Psychol Psychiatry, 57, 1103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAHA PS, KNECHT TM, ARRICK DM, WATT MJ, SCHOLL JL & MAYHAN WG 2021. Constrictor responses of cerebral resistance arterioles in male and female rats exposed to prenatal alcohol. Physiol Rep, 9, e15079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARWONO J 2017. Path Analysis: Data Analysis Applicaiton, Using IBM SPSS and Stata, Columbia, S.C., Jonathan Sarwono. [Google Scholar]
- SHAN T, ZHAO Y, JIANG S & JIANG H 2020. In-vivo hemodynamic imaging of acute prenatal ethanol exposure in fetal brain by photoacoustic tomography. J Biophotonics, 13, e201960161. [DOI] [PubMed] [Google Scholar]
- SIQUEIRA M, ARAUJO APB, GOMES FCA & STIPURSKY J 2021. Ethanol Gestational Exposure Impairs Vascular Development and Endothelial Potential to Control BBB-Associated Astrocyte Function in the Developing Cerebral Cortex. Mol Neurobiol, 58, 1755–1768. [DOI] [PubMed] [Google Scholar]
- SOLONSKII AV, LOGVINOV SV & KUTEPOVA NA 2008. Development of brain vessels in human embryos and fetuses in conditions of prenatal exposure to alcohol. Neurosci Behav Physiol, 38, 373–6. [DOI] [PubMed] [Google Scholar]
- STEEG CN & WOOLF P 1979. Cardiovascular malformations in the fetal alcohol syndrome. Am Heart J, 98, 635–7. [DOI] [PubMed] [Google Scholar]
- TANAKA A, TOMIYAMA H, MARUHASHI T, MATSUZAWA Y, MIYOSHI T, KABUTOYA T, KARIO K, SUGIYAMA S, MUNAKATA M, ITO H, UEDA S, VLACHOPOULOS C, HIGASHI Y, INOUE T, NODE K & PHYSIOLOGICAL DIAGNOSIS CRITERIA FOR VASCULAR FAILURE, C. 2018. Physiological Diagnostic Criteria for Vascular Failure. Hypertension, 72, 1060–1071. [DOI] [PubMed] [Google Scholar]
- TANAKA J 1987. “How big is big enough?”: Sample size and goodness of fit in structural equation models with latent variables. Child Development, 58, 134–146. [Google Scholar]
- TOBIASZ AM, DUNCAN JR, BURSAC Z, SULLIVAN RD, TATE DL, DOPICO AM, BUKIYA AN & MARI G 2018. The Effect of Prenatal Alcohol Exposure on Fetal Growth and Cardiovascular Parameters in a Baboon Model of Pregnancy. Reprod Sci, 25, 1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSENG AM, CHUNG DD, PINSON MR, SALEM NA, EAVES SE & MIRANDA RC 2019. Ethanol Exposure Increases miR-140 in Extracellular Vesicles: Implications for Fetal Neural Stem Cell Proliferation and Maturation. Alcohol Clin Exp Res, 43, 1414–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURCOTTE LA, ABERLE NS, NORBY FL, WANG GJ & REN J 2002. Influence of prenatal ethanol exposure on vascular contractile response in rat thoracic aorta. Alcohol, 26, 75–81. [DOI] [PubMed] [Google Scholar]
- WECHSLER D 2011. Wechsler Abbreviated Scale of Intelligence–Second Edition (WASI-II), San Antonio, TX, NCS Pearson. [Google Scholar]
- WECHSLER D & PSYCHOLOGICAL CORPORATION. 1991. WISC-III : Wechsler Intelligence Scale for Children : manual, San Antonio, Psychological Corp. [Google Scholar]
- XU Y, XIAO R & LI Y 2005. Effect of ethanol on the development of visceral yolk sac. Hum Reprod, 20, 2509–16. [DOI] [PubMed] [Google Scholar]
- YANG W-I, PARK S, YOUN J-C, SON NH, LEE S-H, KANG S-M & JANG Y 2011. Augmentation Index Association With Reactive Hyperemia as Assessed by Peripheral Arterial Tonometry in Hypertension. American Journal of Hypertension, 24, 1234–1238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.