Abstract
Purpose of the Review:
This review addresses recent progress in our understanding of the role of Regulatory T (Treg) cells in enforcing immune tolerance and tissue homeostasis in the lung at steady state and in directing the immune response in asthmatic lung inflammation.
Recent findings:
Regulatory T cells regulate the innate and adaptive immune responses at steady state to enforce immune tolerance in lung tissues at steady state and their control of the allergic inflammatory responses induced by allergens. This regulatory function can break down in the context of chronic asthmatic airway inflammation such that the lung tissue Treg cells become skewed towards a pathogenic phenotype that aggravates and perpetuates disease. Subversion of lung tissue Treg cell function involves their upregulation of Notch4 expression, which in turn acts to amplify T helper type 2 and type 17 and innate lymphoid cell type 2 responses in the airways.
Summary:
A dual role for Treg cells has emerged both as immune regulators but also a potential disease effectors in asthma, with implications for disease therapy.
Keywords: Asthma, Regulatory T-cells, Treg cells, Pathogenesis, Homeostasis
Introduction
Asthma is an airway inflammatory disease associated with reversible airway obstruction (1). It is appreciated that the high prevalence of asthma over the last decades reflects the interaction of susceptibility genes in affected individuals with environmental and life-style changes ushered by the industrial revolution (2, 3). Communities that have diverged towards a modern, post-industrial life-style experience an increase in asthma prevalence compared to related ones that have maintained a pre-industrial life style (4, 5). Pathologically asthma is characterized by mucus production, Goblet cell hyperplasia, increased smooth muscle mass, and high infiltration of innate and adaptive immune cells most frequently associated with type 2 (allergic) immunity (6). Airway inflammation involves the activities of innate immune cells, including eosinophils, neutrophils, mast cells, innate lymphoid cells especially type 2 (ILC2), alveolar macrophages and dendritic cells, and adaptive immune cells including different T cell and B populations (6, 7). Tissue remodelling in the lung is the main hallmark of chronic asthma (8). It is manifested by structural changes and perturbations during inflammation (9, 10). Currently, the focus of therapeutic targets is on effector cellular and molecular mechanisms. Management and control of asthma include inhaled corticosteroids, leukotriene-receptor antagonists, leukotriene synthesis inhibitors, and various antibody biologics including anti-immunoglobulin E (IgE), anti-interleukin-5 (IL-5), anti-thymic stromal lymphopoietin (TSLP) and anti-IL4/IL13 antibodies (11-13). On the other hand, the role of immune regulatory mechanisms the pathogenesis of asthma has been relatively neglected until recently. Of particular interest is the role of regulatory T (Treg) cells in disease pathogenesis and their potential to be targeted in therapeutic approaches (14, 15). In this review, we cover recent progress in our understanding of the role of Treg cells at steady state in the lung as well as their pivotal role in asthma pathogenesis.
Role of Treg cells in Steady State and homeostasis
Treg cells constitute up to 10% of the CD4+ T-cell compartment (16, 17). They play a critical role in maintaining lung tissue homeostasis at steady state and in the context of acute and chronic lung inflammation (18). Treg cells can either develop naturally from the thymus (nTreg cells) or after induction in different effector organs (iTreg cells) (19). The main function of Treg cells is modulation of effector T-cell and other cells activity mainly by supressing their over-activity and restore tolerance (20). At steady state, Treg cell control the immune response by different mechanisms. They release perforin or granzyme A to initiate apoptosis in Antigen Presenting Cells (APCs) (21), or change their behaviour and phenotype using CTLA-4 and CD80/CD86 (22, 23). Moreover, IL-10, transforming growth factor beta (TGFβand/or IL-35 are cytokines produced by Treg cells to maintain tolerance in the lung (24-27). In addition to that Treg cells can exert their suppressive capacity using CD39, CD73 and T-cell immunoglobulin and ITIM domains (TIGIT), to suppress the immune response (28). On the other, a very recent role of miRNAs in Treg suppressive capacity have been described. King et al. have shown that Treg cells modulate the activity and functionality of Dendritic Cells (DCs) via transfer of micro RNA (miRNA), namely miR-150-5p and miR-142-3p. Both cause the upregulation of the anti-inflammatory cytokine IL-10 and down regulation of the proinflammatory cytokine IL-6 (29). In addition to DCs, lung Treg cells have the ability at steady state to supress IL-17-producing γδ T cells. Signalling via the IL-33 receptor (IL-33R, also known as ST2) found on a subpopulation of resident lung tissue Treg cells induces Ebi3, which together with IL-12 p35 forms the heterodimeric cytokine IL-35. In turn Treg cell-derived IL-35 suppresses lung innate γδ T cells and restrains allergic airway inflammation, thus integrating IL-33-dependent tissue repair function and suppression of the inflammatory response (30).
Another subpopulation of Treg cells, T follicular helper regulatory (TFR) cells, mediates control of IgE responses by T follicular helper (TFH) cells (31). In allergic inflammatory responses, including airway inflammation and food allergy, TFH cells that express IL-13 and IL-4 (TFH13) play a critical role in the generation of high affinity IgE antibodies (32-34). Depletion of TFR cells, which are enriched in the airway draining lymph nodes, exacerbated the TFH13 response and potentiated IgE-dependent allergic airway inflammation (32).
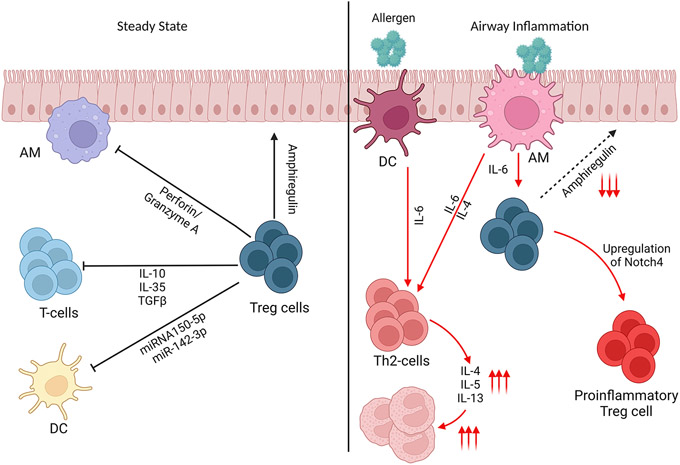
Treg cells have been shown to mediate dominant tolerance against particulate aeroallergens, such as pollen, house dust mites, and fungal spores (35). The function of Treg cell doesn’t stop at the level of immune cell suppression, it extends into an active role in tissue repair. Treg cells are recruited into the lung after tissue injury to induce repair via an Amphiregulin/IL-18 mechanism (36, 37). The inability to produce sufficient amphiregulin by Treg cells is associated with aggravated tissue damage during respiratory influenza virus infection (37, 38). The interplay of the different mechanisms by which Treg cells control tissue inflammation and repair are illustrated in Figure 1.
Figure 1.
Regulatory T-cell function at steady state and in airway inflammation. In Steady state, Treg cells contribute to tissue repair via Amphiregulin, suppression of innate Immunity via miRNA or via Perforin and Granzyme A. Treg cell regulation takes place through cell-cell contact and by means of immune modulatory cytokines including IL-10, IL-35 and TGFβ. In airway inflammation, the function of Treg cells is redirected in favour of promoting a potentially host-protective inflammatory response. In chronic inflammation and especially in severe cases, Treg cells turn into proinflammatory phenotype and act in a positive feedback loop to aggravate the inflammatory responses.
Role of Treg cells in Asthma
Regulatory T-cells play a pivotal role in the pathogenesis of Asthma. Treg cells maintain tolerance in the lung and supress untoward immune stimulation (39). Alveolar macrophages (AMs) and plasmacytoid dendritic cells (pDCs) are the main cells promoting Foxp3+ Treg cells in the lung (40-43). Treg cells have the capacity to induce tolerance against airway allergic inflammation in mice if the cells are exposed to allergen locally before systemic inflammation (44). This happens through antigen specific Treg cell responses after local exposure (45, 46).
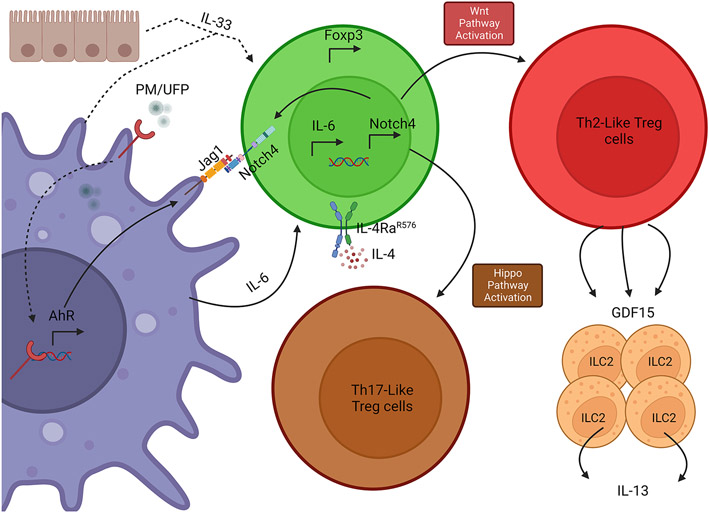
In contrast to the role of Treg cells in maintaining lung tissue homeostasis, the function of these cells may become subverted in the context of chronic asthmatic inflammation (15, 47, 48). In severe Asthma, the number of Foxp3+ Treg cells is significantly decreased compared to healthy control (49). Furthermore, their suppressive capacity depicted by their CCR5 expression was as well dramatically decreased (49). Multiple studies have shown a balance between Treg cells and Th17 cells that regulates tolerance in the lung (50, 51). Recently, there have been multiple studies showing that different compounds have the ability to reduce different hallmarks of asthma by shifting the balance of Treg/Th17 cells toward more Treg cells in the lungs (50, 52-54). A major factor disturbing this balance is particulate matter (PM), which act to amplify the Th2/ Th17 cell responses and reduce Treg cell numbers (55, 56). A key mechanism by which PM amplify the asthmatic inflammatory response involves the destabilization of the Treg cells toward Th2 and Th17 cell phenotypes (57). This regulation has been shown to be epigenetically mediated (58, 59). Furthermore, Treg cell DNA methylation contributes fully to the pathogenesis of allergic airway inflammation (60). DNA demethylation of the CNS2 Locus of the Foxp3 gene is important for the suppressive capacity of Treg cells and their cell identity and stability (61-63). In PM-augmented allergic airway inflammation, AM cells internalize PM, allowing the polycyclic aromatic hydrocarbon components of PM to activate Aryl Hydrocarbon Receptor (AhR), which results in the upregulation of Notch ligand Jagged 1 (Jag1) (42). Moreover, and whereas AM cells promote the formation of iTreg cells at steady state (41), the function of those cells is altered in the context of allergic inflammation. Under the latter conditions, AM cells promote the formation of iTreg cells that express Notch4 in an IL-6-dependent manner, and the interaction of Notch4 with its ligand Jag1 on AM leads alters the functionality of the Treg cells to paradoxically promote a proinflammatory response in the lung (41, 57, 64). Interestingly, blockade of IL-6 by an IL-6R antibody led to an increase in the immunosuppressive capacity of Treg cells in association with suppression of Notch4 expression (57), consistent with previously described inhibitory function of IL-6 in suppressing lung Treg cell response (65).
The mechanisms by which Notch4 subverts Treg cell function have been recently elucidated. Expression of Notch4 on Treg cells acts as a molecular switch to activate downstream pathways that destabilize the Treg cells and impair their regulatory/suppressive capacity (58, 57). Treg cells and due to upregulation of Notch4 shift their toleragenic profile into more proinflammatory profile by upregulating both Th2 and Th17 phenotypes. Notch4-dependent activation of the Hippo pathway in Treg cells promotes the translocation to the nucleus of the downstream effectors Yap1 and Taz, which in turn drive Th17 destabilization of Treg cells by interacting with the transcription factor RORγt (57, 66). Critically, the Notch4-activated Hippo pathway acts to epigenetically destabilize Treg cells by increasing the methylation of conserved non-coding sequence 2 (CNS2) in the Foxp3 locus (57). On the other hand, Notch4-dependent Wnt pathway was found to underlie the Th2 cell-like phenotype of dysregulated Treg cells (57). Wnt signalling has previously been shown to limit the suppressive capacity of Treg cells by modulating TCF-1 resulting in the inhibiting Foxp3 expression and the promotion of T effector cell phenotypes (67, 68). These results provided a putative mechanism by which Notch4-dependent Wnt signalling in Treg cells promoted their T effector-like skewing towards a Th2 cell-like phenotype.
A novel mechanism by which Notch4-mediated Wnt signalling in Treg cells promoted lung tissue allergic responses involved the production by the Notch4+ Treg cells of the cytokine Growth and Differentiation Factor 15 (GDF15). This cytokine is a member of the TGFβ superfamily that has emerged as a prominent regulator of organismal metabolism (69). GDF15 has been implicated in protecting against Inflammation-Induced tissue damage by promoting hepatic triglyceride metabolism (70). GDF15 transcripts are highly enriched in Notch4+ lung TR cells in a β-catenin-dependent manner (57). In vitro studies indicated that GDF activates ILC2 to produce more IL-13 and consequently augment the allergic inflammation in the lung (57). Furthermore, bacterially-derived recombinant GDF heightened AHR and other parameters of airway inflammation in mice lacking Notch4 in their Treg cells. While GDF15 can be derived from several cellular sources (69), our studies suggest that Notch4+ TR cells may play a particularly privileged source of GDF15 in allergic airway inflammation. The full role of GDF15 in allergic airway inflammation and in asthma remains to be established.
Genetic factors may also contribute to the failure of Treg cells to control inflammation in asthmatics. A case in point is the interleukin (IL)-4 receptor alpha chain variant arginine 576 (IL-4Rα-R756), which is associated with asthma and asthma severity and exacerbated airway inflammation in transgenic mice (71-75). This variant promotes conversion of induced Treg (iTreg) cells toward a T helper 17 (Th17) cell fate (48, 64). This effect is mediated by recruitment to the IL-4Rα-R756 of growth-factor-receptor-bound protein 2 (GRB2) adaptor protein to the Il4ra which then induces IL-6 production by activating a microtubule-associated protein kinase (MAPK) cascade. In turn, IL-6 production acts in an autocrine fashion to skew the nascent lung iTreg cells towards IL-17 expression (48, 64). Mice whose IL-4Rα chain carries the human R576 substitution exhibit exaggerated airway Th2/Th17 cell inflammation which can be suppressed with a neutralizing anti-IL-6 antibody therapy which prevented iTreg cell reprogramming into Th17-like cells (48). Furthermore, the IL-4Rα-R756-activated GRB2/IL-6 axis leads to the upregulation of Notch4 on Treg cells and thus their further corruption (64). These results established the centrality of the Treg cell IL- 6- Notch4 circuit in promoting and amplifying airway inflammation by common variants such as the IL-4Rα-R756.
Patients with Asthma have decreased Treg cells and further lower suppressive capacity in these cells (76-78) (Figure 2). On the other hand, restoring Treg cell functionality and activity has recently been the focus of multiple therapeutic interventions. Allergen-specific immunotherapy has shown the capacity to promote Treg cell number and functionality (79, 80). Other therapeutic approaches have suggested that reduction of TNF-alpha levels would cause a significant increase in Treg numbers and suppressive capacity (81). Targeting pathways that impair lung Treg cell function such as the IL-6R may provide benefit in patients with severe asthma (82). Similarly, targeting Notch4 with precision therapies could also provide innovative therapeutic approach in restoring Treg function and tolerance in asthma (57) and viral lung infection (38). Thus, approaches that focus on restoring tolerance in the lung, may complement existing ones that aim to restrain pathogenic effector mechanisms to provide innovative therapies for asthma.
Figure 2.
Role of Particulate Matter in augmentation of inflammatory responses in the lung. PM/UFP inhalation leads to activation of the aryl hydrocarbon receptor (AhR) in alveolar macrophages (AM) which in turn upregulates the expression of Jagged1 (Jag1) in AM (41). Jag1 along with IL-6 (and to a lesser extent IL-33) leads to the upregulation of Notch4 on Treg cells and the activation of downstream Hippo and Wnt pathway. Hippo pathway activation destabilizes the Treg cells towards a Th17 cell-like phenotype, while the Wnt pathway causes the Treg to acquire a Th2 cell-like phenotype (57). Production of Growth and Differentiation Factor 15 (GDF15) by Th2-like Treg cell leads in turn to upregulation of Innate Lymphoid Cells type 2 (ILC2) activity and higher production of IL-13. The IL-6-Notch4 circuit is amplified by the pro-asthmatic IL-4Rα-R576 variant to exacerbate allergic airway inflammation.
Conclusion
Accumulated evidence points to Treg cells playing a pivotal role in the pathogenesis of Asthma. At steady state, lung Treg cells maintain tissue homeostasis and restrain untoward activation of the local innate immune cells thus inhibiting the aberrant activation of inflammatory conditions. In contrast, in asthmatic inflammation, lung tissue Treg cells actively participate in disease pathophysiology: they lose their suppressive capacity and function to direct the asthmatic inflammatory response. In particular, we were able to identify a molecular switch involving Notch signalling in lung Treg cells that promoted immune tolerance breakdown and the upregulation of proinflammatory cell responses. The pro-inflammatory function of Notch4 was related to both the promotion of adaptive Th2/Th17 cell responses and the mobilization of innate immune responses including ILC2 activation by Treg cell-derived and Notch4-induced GDF15. It can be envisioned that while pro-inflammatory function of Treg cells in the context of a parasitic lung infection could prove highly beneficial to the host by loosening the immune regulatory response in favour of an inflammatory one, in could be highly deleterious in the context of a chronic allergen-driven disease process such as asthma. In conclusion, the dual nature of Treg cells in suppressing or promoting lung tissue inflammation in a context-dependent manner provides novel opportunity for disease therapy that targets the immune regulatory response either alone or in conjunction with effector immune pathways.
Key Points.
Aberrant lung tissue Treg cell response plays a key role in asthma pathogenesis
Targeting pathways that subvert the lung tissue Treg cell response may provide precision therapy in moderate to severe asthma.
Financial support and Sponsorship
This work was supported by a National Institutes of Health grants R01 AI115699 and R01 AI065617 to T.A.C., U01 AI160087 and U01 AI143514.
Footnotes
Conflict of Interest
T.A.C., H.H., and A.M. are inventors on published US patent application No. WO2019178488A1 submitted by The Children's Medical Center Corporation, titled “Method for treating asthma or allergic disease”. T.A.C. and H.H. are scientific co-founders of and hold equity in Alcea Therapeutics.
References
- 1.National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. [DOI] [PubMed] [Google Scholar]
- 2.Platts-Mills TA. The allergy epidemics: 1870-2010. J Allergy Clin Immunol. 2015;136(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355(21):2226–35. [DOI] [PubMed] [Google Scholar]
- 4.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N Engl J Med. 2016;375(5):411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatila TA. Innate Immunity in Asthma. N Engl J Med. 2016;375(5):477–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45–56. [DOI] [PubMed] [Google Scholar]
- 7.**. Hammad H, Lambrecht BN. The basic immunology of asthma. Cell. 2021;184(6):1469–85. This review provides a comprehensive overview of the immunology of asthma.
- 8.Bergeron C, Tulic MK, Hamid Q. Airway remodelling in asthma: from benchside to clinical practice. Can Respir J. 2010;17(4):e85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bos IS, Gosens R, Zuidhof AB, Schaafsma D, Halayko AJ, Meurs H, et al. Inhibition of allergen-induced airway remodelling by tiotropium and budesonide: a comparison. Eur Respir J. 2007;30(4):653–61. [DOI] [PubMed] [Google Scholar]
- 10.Hough KP, Curtiss ML, Blain TJ, Liu RM, Trevor J, Deshane JS, et al. Airway Remodeling in Asthma. Front Med (Lausanne). 2020;7:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harb H, Chatila TA. Mechanisms of Dupilumab. Clin Exp Allergy. 2020;50(1):5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calhoun WJ, Chupp GL. The new era of add-on asthma treatments: where do we stand? Allergy Asthma Clin Immunol. 2022;18(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.**. Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N Engl J Med. 2021;384(19):1800–9. This study reports on the efficacy of Tezepelumab, an anti-TSLP human monoclonal antibody, in the treatment of severe uncontrolled asthma.
- 14.Khan MA. Regulatory T cells mediated immunomodulation during asthma: a therapeutic standpoint. J Transl Med. 2020;18(1):456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noval Rivas M, Chatila TA. Regulatory T cells in allergic diseases. J Allergy Clin Immunol. 2016;138(3):639–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Georgiev P, Charbonnier LM, Chatila TA. Regulatory T Cells: the Many Faces of Foxp3. J Clin Immunol. 2019;39(7):623–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alroqi FJ, Chatila TA. T Regulatory Cell Biology in Health and Disease. Curr Allergy Asthma Rep. 2016;16(4):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity. 2009;31(3):438–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30(5):626–35. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13(6):461–7. [DOI] [PubMed] [Google Scholar]
- 21.Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104(9):2840–8. [DOI] [PubMed] [Google Scholar]
- 22.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30(6):1538–43. [DOI] [PubMed] [Google Scholar]
- 23.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4(12):1206–12. [DOI] [PubMed] [Google Scholar]
- 24.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566–9. [DOI] [PubMed] [Google Scholar]
- 25.Paterson AM, Lovitch SB, Sage PT, Juneja VR, Lee Y, Trombley JD, et al. Deletion of CTLA-4 on regulatory T cells during adulthood leads to resistance to autoimmunity. J Exp Med. 2015;212(10):1603–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9(3):239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner JA, Stephen-Victor E, Wang S, Rivas MN, Abdel-Gadir A, Harb H, et al. Regulatory T Cell-Derived TGF-beta1 Controls Multiple Checkpoints Governing Allergy and Autoimmunity. Immunity. 2020;53(6):1202–14 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non-inflammatory conditions. Semin Immunol. 2011;23(6):424–30. [DOI] [PubMed] [Google Scholar]
- 29.King BC, Esguerra JL, Golec E, Eliasson L, Kemper C, Blom AM. CD46 Activation Regulates miR-150-Mediated Control of GLUT1 Expression and Cytokine Secretion in Human CD4+ T Cells. J Immunol. 2016;196(4):1636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faustino LD, Griffith JW, Rahimi RA, Nepal K, Hamilos DL, Cho JL, et al. Interleukin-33 activates regulatory T cells to suppress innate gammadelta T cell responses in the lung. Nat Immunol. 2020;21(11):1371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Q, Dent AL. Regulation of the IgE response by T follicular regulatory cells. J Allergy Clin Immunol. 2022;150(5):1048–9. [DOI] [PubMed] [Google Scholar]
- 32.Clement RL, Daccache J, Mohammed MT, Diallo A, Blazar BR, Kuchroo VK, et al. Follicular regulatory T cells control humoral and allergic immunity by restraining early B cell responses. Nat Immunol. 2019;20(10):1360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gowthaman U, Chen JS, Zhang B, Flynn WF, Lu Y, Song W, et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science. 2019;365(6456):eaaw6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gowthaman U, Chen JS, Eisenbarth SC. Regulation of IgE by T follicular helper cells. J Leukoc Biol. 2020;107(3):409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bacher P, Heinrich F, Stervbo U, Nienen M, Vahldieck M, Iwert C, et al. Regulatory T Cell Specificity Directs Tolerance versus Allergy against Aeroantigens in Humans. Cell. 2016;167(4):1067–78 e16. [DOI] [PubMed] [Google Scholar]
- 36.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155(6):1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, et al. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162(5):1078–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.**. Harb H, Benamar M, Lai PS, Contini P, Griffith JW, Crestani E, et al. Notch4 signaling limits regulatory T-cell-mediated tissue repair and promotes severe lung inflammation in viral infections. Immunity. 2021;54(6):1186–99 e7. This study reports on the role of Notch4 pathway on lung tissue Treg cells in promoting severe respiratory viral infections.
- 39.Mansouri S, Katikaneni DS, Gogoi H, Pipkin M, Machuca TN, Emtiazjoo AM, et al. Lung IFNAR1(hi) TNFR2(+) cDC2 promotes lung regulatory T cells induction and maintains lung mucosal tolerance at steady state. Mucosal Immunol. 2020;13(4):595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lippitsch A, Baal N, Chukovetskyi Y, Cunningham S, Michel G, Dietert K, et al. Plasmacytoid dendritic cell depletion modifies FoxP3+ T cell homeostasis and the clinical course of bacterial pneumonia in mice. J Leukoc Biol. 2019;106(4):977–85. [DOI] [PubMed] [Google Scholar]
- 41.Xia M, Harb H, Saffari A, Sioutas C, Chatila TA. A Jagged 1-Notch 4 molecular switch mediates airway inflammation induced by ultrafine particles. J Allergy Clin Immunol. 2018;142(4):1243–56 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia M, Viera-Hutchins L, Garcia-Lloret M, Noval Rivas M, Wise P, McGhee SA, et al. Vehicular exhaust particles promote allergic airway inflammation through an aryl hydrocarbon receptor-notch signaling cascade. J Allergy Clin Immunol. 2015;136(2):441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, et al. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med. 2013;210(4):775–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8(9):1024–32. [DOI] [PubMed] [Google Scholar]
- 45.Lee SM, Batzer G, Ng N, Lam D, Pattar SS, Patel ND, et al. Regulatory T cells contribute to allergen tolerance induced by daily airway immunostimulant exposures. Am J Respir Cell Mol Biol. 2011;44(3):341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marazuela EG, Rodriguez R, Fernandez-Garcia H, Garcia MS, Villalba M, Batanero E. Intranasal immunization with a dominant T-cell epitope peptide of a major allergen of olive pollen prevents mice from sensitization to the whole allergen. Mol Immunol. 2008;45(2):438–45. [DOI] [PubMed] [Google Scholar]
- 47.Krishnamoorthy N, Khare A, Oriss TB, Raundhal M, Morse C, Yarlagadda M, et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med. 2012;18(10):1525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Massoud AH, Charbonnier LM, Lopez D, Pellegrini M, Phipatanakul W, Chatila TA. An asthma-associated IL4R variant exacerbates airway inflammation by promoting conversion of regulatory T cells to TH17-like cells. Nat Med. 2016;22(9):1013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kraszula L, Eusebio MO, Kuna P, Pietruczuk M. Relationship between CCR5(+)FoxP3(+) Treg cells and forced expiratory volume in 1 s, peak expiratory flow in patients with severe asthma. Postepy Dermatol Alergol. 2021;38(2):262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li P, Tsang MS, Kan LL, Hou T, Hon SS, Chan BC, et al. The Immuno-Modulatory Activities of Pentaherbs Formula on Ovalbumin-Induced Allergic Rhinitis Mice via the Activation of Th1 and Treg Cells and Inhibition of Th2 and Th17 Cells. Molecules. 2021. Dec 31;27(1):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Zhao H, Wang T, Zhao X, Wang J, Wang Q. Anti-Inflammatory and Anti-asthmatic Effects of TMDCT Decoction in Eosinophilic Asthma Through Treg/Th17 Balance. Front Pharmacol. 2022;13:819728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiang CY, Chang JH, Chuang HC, Fan CK, Hou TY, Lin CL, et al. Schisandrin B promotes Foxp3(+) regulatory T cell expansion by activating heme oxygenase-1 in dendritic cells and exhibits immunomodulatory effects in Th2-mediated allergic asthma. Eur J Pharmacol. 2022;918:174775. [DOI] [PubMed] [Google Scholar]
- 53.Wang C, Huang CF, Li M. Sodium houttuynia alleviates airway inflammation in asthmatic mice by regulating FoxP3/RORgammaT expression and reversing Treg/Th17 cell imbalance. Int Immunopharmacol. 2022;103:108487. [DOI] [PubMed] [Google Scholar]
- 54.Shen X, Zhang H, Xie H, Chen L, Li S, Zheng J, et al. Reduced CCR6(+)IL-17A(+)Treg Cells in Blood and CCR6-Dependent Accumulation of IL-17A(+)Treg Cells in Lungs of Patients With Allergic Asthma. Front Immunol. 2021;12:710750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang C, Wang D, Zhao H, Wang J, Liu N, Shi H, et al. Traffic-related PM2.5 and diverse constituents disturb the balance of Th17/Treg cells by STAT3/RORgammat-STAT5/Foxp3 signaling pathway in a rat model of asthma. Int Immunopharmacol. 2021;96:107788. [DOI] [PubMed] [Google Scholar]
- 56.Brandt EB, Myers JM, Ryan PH, Hershey GK. Air pollution and allergic diseases. Curr Opin Pediatr. 2015;27(6):724–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harb H, Stephen-Victor E, Crestani E, Benamar M, Massoud A, Cui Y, et al. A regulatory T cell Notch4-GDF15 axis licenses tissue inflammation in asthma. Nat Immunol. 2020;21(11):1359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126(4):845–52 e10. [DOI] [PubMed] [Google Scholar]
- 59.Hew KM, Walker AI, Kohli A, Garcia M, Syed A, McDonald-Hyman C, et al. Childhood exposure to ambient polycyclic aromatic hydrocarbons is linked to epigenetic modifications and impaired systemic immunity in T cells. Clin Exp Allergy. 2015;45(1):238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J, Sha J, Sun L, Zhu D, Meng C. Contribution of Regulatory T Cell Methylation Modifications to the Pathogenesis of Allergic Airway Diseases. J Immunol Res. 2021;2021:5590217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feng Y, Arvey A, Chinen T, van der Veeken J, Gasteiger G, Rudensky AY. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell. 2014;158(4):749–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463(7282):808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li X, Liang Y, LeBlanc M, Benner C, Zheng Y. Function of a Foxp3 cis-element in protecting regulatory T cell identity. Cell. 2014;158(4):734–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.**. Benamar M, Harb H, Chen Q, Wang M, Chan TMF, Fong J, et al. A common IL-4 receptor variant promotes asthma severity via a T(reg) cell GRB2-IL-6-Notch4 circuit. Allergy. 2022;77(11):3377–87. This study demonstrates that a common IL-4 receptor alpha chain variant associated with asthma and asthma severity acts by upregulating an IL-6-Notch4 circuit on lung tissue Treg cells.
- 65.Doganci A, Eigenbrod T, Krug N, De Sanctis GT, Hausding M, Erpenbeck VJ, et al. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005;115(2):313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geng J, Yu S, Zhao H, Sun X, Li X, Wang P, et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of TH17 cells and Treg cells. Nat Immunol. 2017;18(7):800–12. [DOI] [PubMed] [Google Scholar]
- 67.van Loosdregt J, Fleskens V, Tiemessen MM, Mokry M, van Boxtel R, Meerding J, et al. Canonical Wnt signaling negatively modulates regulatory T cell function. Immunity. 2013;39(2):298–310. [DOI] [PubMed] [Google Scholar]
- 68.van der Veeken J, Glasner A, Zhong Y, Hu W, Wang ZM, Bou-Puerto R, et al. The Transcription Factor Foxp3 Shapes Regulatory T Cell Identity by Tuning the Activity of trans-Acting Intermediaries. Immunity. 2020;53(5):971–84 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Breit SN, Brown DA, Tsai VW. The GDF15-GFRAL Pathway in Health and Metabolic Disease: Friend or Foe? Annu Rev Physiol. 2021;83:127–51. [DOI] [PubMed] [Google Scholar]
- 70.Luan HH, Wang A, Hilliard BK, Carvalho F, Rosen CE, Ahasic AM, et al. GDF15 Is an Inflammation-Induced Central Mediator of Tissue Tolerance. Cell. 2019;178(5):1231–44 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hershey GK, Friedrich MF, Esswein LA, Thomas ML, Chatila TA. The association of atopy with a gain-of-function mutation in the alpha subunit of the interleukin-4 receptor. N Engl J Med. 1997;337(24):1720–5. [DOI] [PubMed] [Google Scholar]
- 72.Rosa-Rosa L, Zimmermann N, Bernstein JA, Rothenberg ME, Khurana Hershey GK. The R576 IL-4 receptor alpha allele correlates with asthma severity. J Allergy Clin Immunol. 1999;104(5):1008–14. [DOI] [PubMed] [Google Scholar]
- 73.Wenzel SE, Balzar S, Ampleford E, Hawkins GA, Busse WW, Calhoun WJ, et al. IL4R alpha mutations are associated with asthma exacerbations and mast cell/IgE expression. Am J Respir Crit Care Med. 2007;175(6):570–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al-Muhsen S, Vazquez-Tello A, Alzaabi A, Al-Hajjaj MS, Al-Jahdali HH, Halwani R. IL-4 receptor alpha single-nucleotide polymorphisms rs1805010 and rs1801275 are associated with increased risk of asthma in a Saudi Arabian population. Annals of thoracic medicine. 2014;9(2):81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tachdjian R, Mathias C, Al Khatib S, Bryce PJ, Kim HS, Blaeser F, et al. Pathogenicity of a disease-associated human IL-4 receptor allele in experimental asthma. J Exp Med. 2009;206(10):2191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ, et al. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol. 2007;119(5):1258–66. [DOI] [PubMed] [Google Scholar]
- 77.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202(11):1539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sjaheim TB, Bjortuft O, Drablos PA, Kongerud J, Halstensen TS. Increased bronchial density of CD25+Foxp3+ regulatory T cells in occupational asthma: relationship to current smoking. Scand J Immunol. 2013;77(5):398–404. [DOI] [PubMed] [Google Scholar]
- 79.Bohm L, Maxeiner J, Meyer-Martin H, Reuter S, Finotto S, Klein M, et al. IL-10 and regulatory T cells cooperate in allergen-specific immunotherapy to ameliorate allergic asthma. J Immunol. 2015;194(3):887–97. [DOI] [PubMed] [Google Scholar]
- 80.Tian M, Wang Y, Lu Y, Jiang YH, Zhao DY. Effects of sublingual immunotherapy for Dermatophagoides farinae on Th17 cells and CD4(+) CD25(+) regulatory T cells in peripheral blood of children with allergic asthma. Int Forum Allergy Rhinol. 2014;4(5):371–5. [DOI] [PubMed] [Google Scholar]
- 81.Lin YL, Shieh CC, Wang JY. The functional insufficiency of human CD4+CD25 high T-regulatory cells in allergic asthma is subjected to TNF-alpha modulation. Allergy. 2008;63(1):67–74. [DOI] [PubMed] [Google Scholar]
- 82.Esty B, Harb H, Bartnikas LM, Charbonnier LM, Massoud AH, Leon-Astudillo C, et al. Treatment of severe persistent asthma with IL-6 receptor blockade. J Allergy Clin Immunol Pract. 2019;7(5):1639–42 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]