Abstract
During postnatal development, an emmetropization feedback mechanism uses visual cues to modulate the axial growth of eyes so that, with maturation, images of distant objects are in focus on the retina. If the visual cues indicate that the eye has become too long, it generates STOP signals that slow eye elongation. Myopia is a failure of this process where the eye becomes too long. The existing animal models of myopia have been essential in understanding the mechanics of emmetropization but use visual cues that lead to fast progressing myopia and don’t match the stimuli that lead to human myopia. Form deprivation removes esssentially all spatial contrast. Minus lens wear accurately guides axial elongation to restore sharp focus: technically it is not a model of myopia! In contrast, childhood myopia involves a slow drift into myopia, even with the presence of clear images. We hypothesize that, in the modern visual environment, STOP signals are present but not quite strong enough to halt myopic progression. Using tree shrews, small diurnal mammals closely related to primates, we have developed an animal model that we propose better represents this situation. We use limited bandwidth light to provide limited chromatic cues for emmetropization that are not quite enough to produce fully effective STOP signaling, resulting in a slow drift into myopia as seen in children. We hypothesize that this animal model of myopia may prove useful in evaluating anti-myopia therapies where form deprivation and minus lens wear would be too powerful.
Keywords: Emmetropization, Myopia, Animal Models, Refractive Error, Wavelength, Chromatic Aberration
1. Introduction
To achieve a maximum visual acuity of 30 cycles per degree (or more in some subjects), the axial length of a human eye must be matched to its optics (focal plane) with a precision of approximately 175 μm to produce clear images on the retina (Atchison et al., 2004; Mutti et al., 2018). The match of the location of the retina with that of the focal plane is achieved and maintained by an active feedback emmetropization mechanism that modulates the rate of axial growth of the eye to produce good focus as the optics mature with age (Norton, 1999; Troilo et al., 2019; Wildsoet, 1997). The inputs to this feedback mechanism are visual cues in the environment that allow it to determine the sign (retina in front or behind the focal plane) and the amount of mismatch between the position of the retina and the focal plane of the optics (Troilo et al., 2019). If the visual cues indicate that the eye is too short (retina in front of the focal plane), the emmetropization mechanism generates what have been described as GO signals that accelerate axial elongation. If the visual cues indicate the eye has become too long (retina behind the focal plane), it generates STOP signals that slow eye elongation (Rohrer & Stell, 1994).
We do not currently know the precise nature of GO and STOP, including whether these are single entities or multifactorial, or even if GO is simply the absence of STOP. However, the existence of a negative feedback system means that in some form these must exist. Children initially develop a non-Gaussian (leptokurtic and skewed) distribution of refractive errors, and the variance of the distribution of refractive errors is much smaller than the variance predicted by random selection of the distributions of the contributing refractive elements. (Flitcroft, 2014). This can only come about through a feedback system homing in on on a target, which in some form must have feedback signals derived from visual cues. Additionally, in one randomized clincial trial, children who had an over-minus prescription became on average more myopic than those who had a non over-minus prescription (Chen et al., 2021), even as children wearing multifocal lenses with additional plus power tend to become less myopic (Chamberlain et al., 2019). Therefore children, like the commonly used animal models, must have active emmetropization and STOP and GO signals.
Human juvenile-onset myopia is a failure of emmetropization in which the axial length of the eye becomes too long for that eye’s optics. This elongation does not just cause blurry distance vision, visual disability, and reduced quality of life (Rose et al., 2000), but also stresses posterior ocular tissues (retina and choroid), and is a major risk factor for potentially blinding ocular pathologies later in life (Bullimore et al., 2021; Flitcroft, 2012; Haarman et al., 2020). Public health concerns for myopia are increasing because the prevalence of myopia has increased dramatically in recent years (Sankaridurg et al., 2021), rising from approximately 25% in 1971–1972 to 42% in 1999–2004 in U.S. adults (Vitale et al., 2009) and affecting as many as 96% of the population in East Asia (Jung et al., 2012).
This epidemic of myopia must be largely environmental because human genetics cannot have changed so fast. However, the specific features of the modern visual environment that are responsible for this epidemic of myopia remain unclear. To study the visual cues used by the emmetropization mechanism and to learn the mechanisms that modulate axial growth, two methods — form deprivation and negative-lens wear — have been used in animal models in a variety of species (including primates, tree shrews, chicks, guinea pigs, and mice) over the past 45 years to produce axial myopia that is anatomically similar to juvenile-onset myopia in children (Troilo et al., 2019). Both alter the visual environment; form deprivation prevents clear images on the retina whereas negative-lens wear changes the focal plane of light (Norton, 1999; Troilo et al., 2019). In the form-deprivation model, a diffuser is placed in front of a postnatal developing eye, preventing the formation of sharp images on the retina. Deprived of clear visual images, the emmetropization mechanism presumably generates strong GO signals that rapidly increase the axial elongation rate of the eye so it becomes myopic. Because clear images remain absent, the emmetropization mechanism cannot evaluate focus. Apparently the emmetropization mechanism does not generate STOP signals during form deprivation, and axial elongation typically progresses to high levels of myopia (in six week old tree shrews, 11 days of form deprivation creates over −7D of myopia (El Hamdaoui et al., 2021)). In lens-induced myopia, a negative-power lens is placed over the eye, reducing the eye’s optical power, and shifting the focal plane behind the retina. The emmetropization mechanism then presumably generates GO signals to rapidly elongate the eye until it re-aligns the retina with the shifted focal plane so that images are again clearly focused, at which point STOP signals are generated to slow the axial elongation rate to maintain the retina at the new focal plane.
The use of these two approaches has led to fundamental advances in our understanding of how the eye regulates its growth and refractive state, revealing the existence of the emmetropization feedback mechanism that operates locally within the eye (demonstrated experimentally in rhesus monkeys, tree shrews, and chicks), and increasing our understanding of how it regulates eye growth and refractive development (Norton, 1999; Troilo et al., 2019; Wallman & Winawer, 2004). However, both methods fail to accurately mimic important aspects of the visual conditions typically experienced by myopic children. Unlike form-deprivation myopia, most human juvenile-onset myopia develops and progresses despite the presence of clear images on the retina (Bao et al., 2022; Chamberlain et al., 2019; Lam et al., 2020; Rappon et al., 2022; Walline et al., 2020). While pathological changes to the ocular adnexa or media can cause some degree of form deprivation in children (Gusek-Schneider & Martus, 2000; O’Leary & Millodot, 1979; von Noorden & Lewis, 1987), the vast majority of human school-age onset myopia does not involve gross elimination of form vision. Unlike lens-induced myopia, human juvenile myopia develops without any obvious target point for the emmetropization mechanism. It would be useful if a third way could be found to produce myopia that would avoid these deficiencies, allowing retinal image formation and without significantly moving the location of the focal plane. In addition, myopia in children generally develops slowly, in contrast to the rapid myopia development produced by form deprivation or lens wear. If a condition in an animal model could be found where the emmetropization mechanism would produce some STOP signals, albeit weaker ones and therefore a slower myopia progression, it would more closely match myopia progression in children.
Recent studies have found that when animals are exposed to ambient light in which the spectrum is limited to a narrow band of wavelengths (half-height ± 10 nm), eyes deviate from normal eye growth and develop refractive errors (Jiang et al., 2021; Khanal et al., 2021; Liu et al., 2011; Norton et al., 2021; Rucker, 2019; Rucker & Wallman, 2009; Smith et al., 2015). In tree shrews (small mammals closely related to primates), narrow-band long-wavelength (red) light exposure from 1 to 14 hours a day produces slowed axial growth and hyperopia (Gawne, Siegwart, et al., 2017; Gawne, Ward, et al., 2017), short-wavelength narrow-band blue light produces instability in refraction with a long-term trend towards myopia (Gawne et al., 2018), and short-wavelength narrow-band cyan light produces myopia (Norton et al., 2021). Like tree shrews, macaque monkeys exposed to red light for 12 hours a day for months develop hyperopia (Hung et al., 2018; Smith et al., 2015). Narrow-band lighting in other species also produces ametropia (Jiang et al., 2021; Liu et al., 2011; M. Wang et al., 2018). While the basis of these species-specific differences is not clear, the common denominator is that narrow-band light impairs the ability of the emmetropization mechanism to use defocus cues to guide the eye to achieve and maintain emmetropia (Gawne et al., 2018). It is possible that an important defocus-related visual cue that is missing in narrow-band light is normally provided by longitudinal chromatic aberration (LCA). All vertebrate eyes that have been tested for chromatic aberrations focus short-wavelength light closer to the front of the eye than long-wavelength light by approximately 1–3 diopters (Mandelman & Sivak, 1983). It has been proposed that, in broadband light, the differential focus of short-vs.-long wavelengths, along with other visual cues, is used as a signal by the emmetropization mechanism to guide and maintain eyes at emmetropia (Gawne, Siegwart, Ward & Norton, 2017)(Gawne & Norton, 2020)(Gawne, Grytz & Norton, 2021, Rucker & Wallman, 2009, Smith, Hung, Arumugam, Holden, Neitz & Neitz, 2015, Wildsoet, Howland, Falconer & Dick, 1993). If short wavelengths are in better focus than long, it indicates the retina is in front of the focal plane so GO signals are produced causing eyes to elongate. Conversely, if long wavelengths are better focused, the retina is behind the focal plane; STOP signals are generated to slow the elongation rate.
In monochromatic or narrow-band light, it is not possible to use chromatic cues to evaluate focus. In this case, a feedback system based on differential contrast between short and long wavelengths of light will not be able to poperate normally and can produce erratic results that are wavelength and species dependent (Gawne, Ward & Norton, 2018).
The Gawne & Norton model provides a method to calculate the strength of the spectral STOP drive that is produced to slow axial growth (Gawne & Norton, 2020). A spectral STOP drive of 1.0, which occurs in broadband lighting as used in our tree shrew colony, produces a strong STOP signal so an eye does not elongate past emmetropia. We calculated that if the bandwidth of a narrow-band short-wavelength (blue) light is widened slightly to include longer-wavelengths (a limited-bandwidth light), the widened bandwidth will allow emmetropization to use chromatic cues to evaluate defocus, so that STOP is produced but is weaker than is produced in broad-band light. Our hypothesis was that these STOP signals would allow myopia to develop, but at a slower rate than the classical animal models of myopia. We thus examined the effect of housing tree shrews in ambient light of limited bandwidth with two different spectral STOP drives intermediate in strength between broad-band colony lighting and narrow-band short-wavelength light. We followed their refractive development over a prolonged period, from juveniles to adolescence to better correspond with the developmental period in which children typically develop myopia.
2. Methods
Twelve northern tree shrews (Tupaia belangeri) were initially raised by their mothers in the University of Alabama at Birmingham (UAB) Tree Shrew Core. All animals were maternally raised in a temperature-controlled colony on a 14 hours/day light, 10 hours/day dark cycle, starting at 6.30 AM. Tree shrews are born with their eyes closed, so we use as our starting point the first day at which both eyes are open (typically around three weeks after birth) and record subsequent time as Days of Visual Experience (DVE). Limited-bandwidth treatment began at 24 ± 1 DVE. By this age, tree shrews typically complete their initial emmetropization process and achieve low hyperopia (approximately 1.4 D (Siegwart & Norton, 2010)), providing a stable baseline for evaluating the effect of wavelengths on emmetropization. All animal care adhered to the guidelines of the Association for Research in Vision and Ophthalmology for the use of animals in ophthalmic and visual research. The UAB Institutional Animal Care and Use Committee approved all experimental procedures in the study.
Tree shrews are small diurnal mammals that are very closely related to primates (Janecka et al., 2007). Like most mammals, tree shrews are dichromats, with short and long wavelength sensitive cone types (Petry & Hárosi, 1990). Most (not all) humans are trichromatic, with short, medium, and long wavelength sensitive cones - but there is evidence that, as regards emmetropization, humans are functionally dichromatic with the medium and long wavelength sensitive cone activities pooled to produce a single “longer” wavelength cone signal (Gawne et al., 2021). If this is the case, then tree shrew cone spectral tuning is virtually identical to that of the human. Unlike tree shrews, primates have a fovea, but experiments in rhesus monkeys have shown that the fovea is not necessary for emmetropization (Smith et al., 2007) and it appears that it is the peripheral retina that is important. Humans have a much higher visual acuity than tree shrews, nominally 30 cycles per degree (higher in some subjects) vs. about 2 cycles per degree (Petry et al., 1984), but emmetropization appears to use mid-range spatial frequencies and the differences as regards emmetropization between human and tree shrew may be modest (Gawne et al., 2021; Gawne & Norton, 2020). While accommodation and central perception need not use the same mechanisms as emmetropization, it has also been shown that the critical band of frequencies in humans for guiding accommodation and estimating defocus are much lower than maximal acuities, in the range of 4–8 cycles per degree. (Burge & Geisler, 2011, Owens, 1980). Tree shrews have approximately 55 horizontal degrees of binocular vision, but as emmetropization appears to be local to each retina, binocular vision and stereopsis are not relevant. One significant difference is that humans often engage in sustained accommodation, but tree shrews do not appear to even in the presence of sustained hyperopic defocus (Norton et al., 2006). Thus, for the purposes of this specific study, tree shrews are in many ways a good model for human.
2.1. Experimental groups
Animals were allocated into the Blue+Green group (n=6) or the Blue+Cyan group (n=6). Treatment began when animals were placed in cages where the only light was provided by limited–bandwidth blue+green light (n=6) or limited–bandwidth blue+cyan (n=6) and lasted until a maximum of 150 DVE. Refractive error and ocular component dimension measurements of these animals were compared with a previously studied Colony group of seven animals raised continuously in standard colony fluorescent broad-band lighting in identical cages and environmental conditions (Gawne, Siegwart, et al., 2017).
2.2. Lighting conditions
Figure 1 shows the spectra of the light sources as determined using an Ocean Optics STS Microspectrometer (Largo, FL). The colony lighting (Figure 1A) was provided by fluorescent bulbs (type F34CW RS WM ECO, General Electric Lighting), that contained a wide range of wavelengths spanning the visible spectrum. All animals experienced this lighting until the two treatment groups were moved to limited-bandwidth conditions. The Colony group remained in the broadband lighting. The blue+green and blue+cyan-illuminated cages were located in separate light-tight bays in a dedicated wavelength treatment room (see Supplementary Figure 1). Between the outer corridor and the inner bays was a dimly illuminated access corridor, so that animals in a bay were isolated from the light from both the outer corridor and other bays. Limited-bandwidth animals remained on the 14-hour on/10-hour off lighting schedule, starting at 6.30 AM. The light cycle on each cage was verified using individual light monitors that were part of a ClockLab (Actimetrics, Wilmette, IL) circadian rhythm monitoring system.
Figure 1.
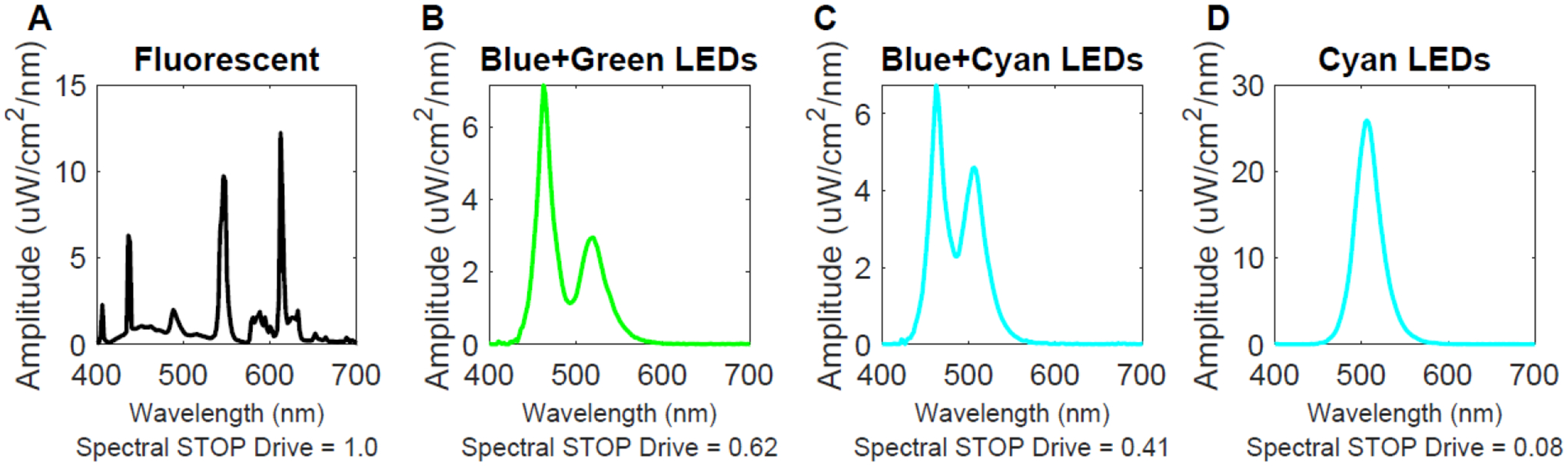
Ambient light spectra. Panel A illustrates the spectra of the colony fluorescent lights. This has a “spectral STOP drive” (normalized to 1.0) to oppose becoming myopic. Tree shrews emmetropize reliably and accurately under this illumination. Panels B and C show the spectra of ambient lights of limited bandwidth: Blue+Green, and Blue+Cyan. These lights have progressively lower “spectral STOP drive” numbers, and hence are potentially less effective in providing emmetropization system information to generate strong STOP signals that prevent myopia. Panel D shows the spectrum of cyan narrow-band light which has a spectral STOP drive of 0.08 and very little STOP signal so that myopia develops rapidly (Norton et al., 2021).
The illuminance was measured with a digital illuminance meter (LX1330B, Hisgadget Inc). For the colony group, illuminance on the floor of the cage typically ranged from 100–300 human lux. Illuminance for both the blue+green (Figure 1B) and blue+cyan (Figure 1C) lighting arrays was approximately 800 human lux. Along the back wall of the cages was an elevated white shelf with 40% higher illuminance than on the cage floor. These lighting levels are within a range that does not seem to affect emmetropization in this species (Norton & Siegwart, 2013).
The blue+green lighting was provided by 0.5-meter-long strips of 12-volt DC red+green+blue LEDs NFLS RGB 15X2-WHT-LC4 (superbrightleds.com, Earth City, MO) with only the blue (peak intensity, 462 ± 10 nm, half-max) and green (peak intensity, 518 ± 18 nm) LEDs activated. The strips were affixed to aluminum channels for secure mounting and heat dissipation and were placed on top of the cages. A custom pulse-width modulated controller adjusted the intensity of the strips to achieve 400 lux for the blue and green arrays, each measured separately.
The blue+cyan lights were of a similar design, but cyan LEDs are less commonly available. For each cage, we used four Rebel LEDs (Luxeon Star LEDs, Lethbridge, Alberta, Canada), with the Cyan peak intensity at 505 ± 17 nm, and each had three LEDs mounted in a TriStar cool base. A Carclo 44 degree frosted 20 mm circular beam optics was mounted on each one, with an additional white diffuser. These assemblies were put on a single aluminum T-bar facing down into the cage. Dimming of the cyan LEDs was achieved with layers of neutral gray fiberglass mesh. Figure S1 illustrates one of the cages illuminated with blue+cyan light. For both the blue+cyan and blue+green lighting the midpoint of the wavelength range was shifted slightly away from the ‘middle’ of the visible spectrum (nominally 550 nm) and more towards the short-wavelength end of the visible light spectrum. If emmetropization were optimizing retinal image sharpness regardless of wavelength, then because shorter wavelengths are focused in front of longer ones, this should have caused the eyes to become less than a diopter hyperopic compared to the colony animals (because of the LCA function for tree shrews (Gawne & Norton, 2020)), which did not happen. Therefore, we do not feel that the change in the midpoint of the lighting spectrum would be a major factor in our results.
We have previously proposed that chromatic signals provide the dominant cue for emmetropization. (Norton, Khanal & Gawne, 2021). In broad-spectrum ambient lighting, the differential image sharpness between the short and longer-wavelength cones provides a strong cue for the magnitude and polarity of defocus, and (at least under natural conditions) typically allows for accurate emmetropization. With monochromatic light (or sufficiently narrow-bandwidth light), emmetropization cannot function properly. If a monochromatic light has a wavelength that stimulates both short and longer-wavelength cones, then both cone types will sense identical image sharpness and there can be no feedback based on chromatic cues. Here we sought to generate limited bandwidth light to provide an intermediate condition: one where there are signals allowing for chromatic cues to guide eye growth, but where these signals have been limited in magnitude. We define a spectral STOP drive, which quantifies the strength of the chromatically-generated STOP signals under the condition of −2D of myopia, based on a previously published model (Gawne & Norton, 2020). In brief, we calculate the effective images as sensed by both the short and longer-wavelength cones integrated across the entire visual spectrum, taking into account both the continuous shift in focus with wavelength, and the sensitivities of the cones with wavelength. We then calculate an ‘edge spread function’: the response of both cone classes to a visual step-edge, which has been proposed as an especially useful method of characterizing optical blur (Westheimer, 2021). We take the integrated difference between the edge spread functions for the short and longer wavelength cones as a measure of the chromatic signal for optical defocus. We do not know if this calculation has any parallels to whatever the effective calculation used by the retina, but only propose it as one method of quantifying the ability of a given lighting spectrum to generate chromatic cues for defocus.
Broad-spectrum colony lighting would produce the maximum spectral STOP drive, and we have normalized this to a value of 1.0. Narrow-band cyan lighting (Figure 1D) would produce a very low STOP signal of 0.08, again normalized compared to colony lighting (perfect monochromatic light would produce a spectral STOP drive of 0.0). Blue+green lighting has a spectral STOP drive of 0.62, and the the blue+cyan lighting has a spectral STOP drive of 0.41. The differences in these two limited bandwidth spectra might seem small, but these spectra were selected to be right at the edge of where emmetropization would begin to fail, and so it is not surprising that apparently small changes in bandwidth could have large effects on emmetropization.
2.3. Pedestal Installation
One to three days before the onset of limited bandwidth light exposure, a dental acrylic pedestal was installed on the skull of the experimental animals to align animals accurately during refractive and axial component dimension measurements. These procedures have been described in extensive detail previously (Siegwart & Norton, 1994). In summary, animals were removed from their mother’s cage and anesthetized. The dental acrylic pedestal was rigidly attached to the skull with six stainless steel machine screws, allowing for precise control of head and eye position during refractive and axial measures. Following recovery from anesthesia, animals were weaned and housed in individual cages in the main colony under broad-band fluorescent lighting. At 24 ± 1 DVE, the animals were transferred to individual cages for exposure to limited bandwidth lighting in the wavelength treatment room, for a period lasting until 150 DVE.
2.4. Refractive Error and Ocular Axial Measurements
The refractive errors of each eye were measured in awake and gently restrained animals with a Nidek ARK-700A autorefractor (Marco Ophthalmic, Jacksonville, FL). During autorefraction, the eye of the animal was aligned with the instrument using the dental acrylic pedestal. In our experience tree shrews make infrequent and very limited eye movements so that accurate eye alignment is readily achieved by holding the head. We recorded ten measurements in each eye and obtained net refractive errors as the average of five measures of the highest quality rating. In the absence of at least five measures with quality metrics of “7” or greater for each eye, the measurements were repeated. The refractive error measurements were converted to spherical equivalent at the corneal plane and corrected for the “small eye artifact” (Glickstein & Millodot, 1970), previously reported to be approximately +4 D in tree shrews (Norton et al., 2003; Sajdak et al., 2019).
As with previous studies in this laboratory (Khanal et al., 2021; Norton et al., 2021), non-cycloplegic measures were recorded because cycloplegic drugs may interfere with emmetropization (McBrien et al., 1993). Moreover, non-cycloplegic measures have been shown to provide a consistent estimate of refractive errors in this species. When measured in the same animals, the difference between a non-cycloplegic and cycloplegic refractive error is a relatively consistent 0.8 D (Norton et al., 2003), indicating that tree shrews normally have a small and relatively constant accommodative tone.
The refractive errors were measured in a dimly lit room between approximately 10 AM and 11 AM approximately every four days, although scheduling issues often caused this timing to change. Animals were kept in darkness while being transported between the limited-bandwidth lit cage and the measurement room. The measurement room was very dim, with the ambient lighting generated by the display screens of the instruments and a small strip of blue and green LEDs on one wall. Animals were kept in their nest tubes except for the brief periods when measurements were taken, to minimize any exposure to different lighting. During measurements, the internal target light of the autorefractor was disabled to avoid exposing the animal to another source of relatively bright broad-band light.
Immediately after refractive measurements, axial component dimensions were measured with a LenStar LS-900 optical biometer. The raw data were analyzed using a custom MATLAB program using tree shrew-specific refractive indices (El Hamdaoui et al., 2019). This system gives comparable axial length values to A-scan ultrasonography, but with better precision and repeatability (Penha et al., 2012). The axial measures allow us to determine whether changes in refractive errors are due to changes in axial eye size and not merely changes in the power of the cornea and lens.
2.5. Data Analysis
In this study, we followed the refractive development of the animals over a considerable period (from 24 to 150 DVE, or 127 days). We were unable to record all outcome measures at the same time points for each animal due to weekends and holidays. Therefore, a standard repeated-measures ANOVA was not readily applicable. Hence, for statistical analysis, for each animal, we fit an exponential function to the refractive error as a function of time:
Where D is the mean refractive error of the left and right eyes in diopters, a and b are scaling factors, DVE is time in Days of Visual Experience, and c is the time constant (in days). We then performed a Kruskal-Wallis Chi-squared test on the refractive errors at 24 DVE (the start of the study), the projected value at 150 DVE, and the time constant “c”. Data are expressed as mean ± standard errors of the mean (SEM), unless stated otherwise.
3. Results
Figure 2A illustrates the refractive error (average of left and right eyes) as a function of time for each of the six animals in the Blue+Green limited-bandwidth group. Mean data for the colony animals is also included in this, and the other three panels of Figure 2. The group mean is shown in Figure 3A. Within a few days after treatment began, all animals showed a myopic shift in refractive error. The average slope over the first four days was −0.30 ± 0.13 D/day. This initial rate of myopia development in blue+green limited-bandwidth light was faster than in colony fluorescent light (−0.15 ± 0.08 D/day) but slower than in −5D lens wear (−0.67 ± 0.15 D/day) reported previously (Norton, Amedo, et al., 2010). For one animal, data collection stopped after 54 DVE because the animal died. This animal had exhibited normal behavior during measurements, normal behavior and appetite between measures while in the home cage, and the data trended with the initial data from the animals in the same group. Necropsy showed no obvious pathology. The remaining five animals showed a prolonged period (from 107 to 150 DVE) during which refractions gradually became more myopic. Figure 2B shows the corresponding vitreous chamber depths for the Blue+Green animals. Soon after the onset of limited-bandwidth treatment, the vitreous chamber depths of the Blue+Green animals became longer than that of the Colony group and remained longer over time. The vitreous chamber depth of both the Colony and the Blue+Green animals decreased over time. The decrease occurred because the crystalline lens increased in thickness more rapidly than the increase in axial length (Figure 3C).
Figure 2.
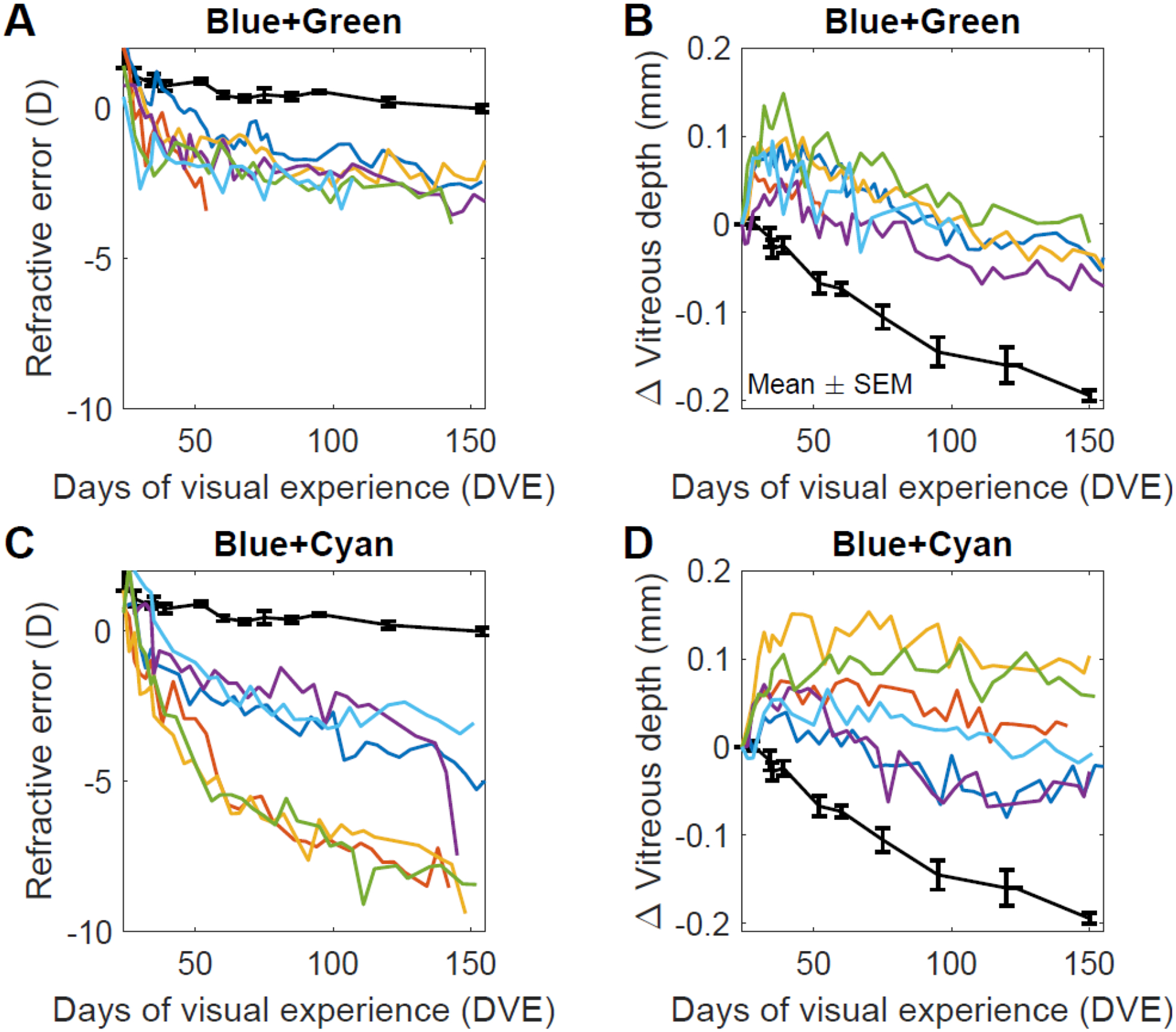
Refraction and vitreous chamber depth as a function of time. Black line in each panel: mean ± standard errors of the mean, (SEM) of the Colony group. A. Refractive error (average of left and right eyes) of animals in the Blue+Green limited-bandwidth group. B. Change in vitreous chamber depth of the Blue+Green animals relative to the starting measurement at 24 DVE. C. Refractive error (average of left and right eyes) of animals in the Blue+Cyan limited-bandwidth group. D) Change in vitreous chamber depth of the Blue+Cyan animals relative to the starting measurement at 24 DVE.
Figure 3.
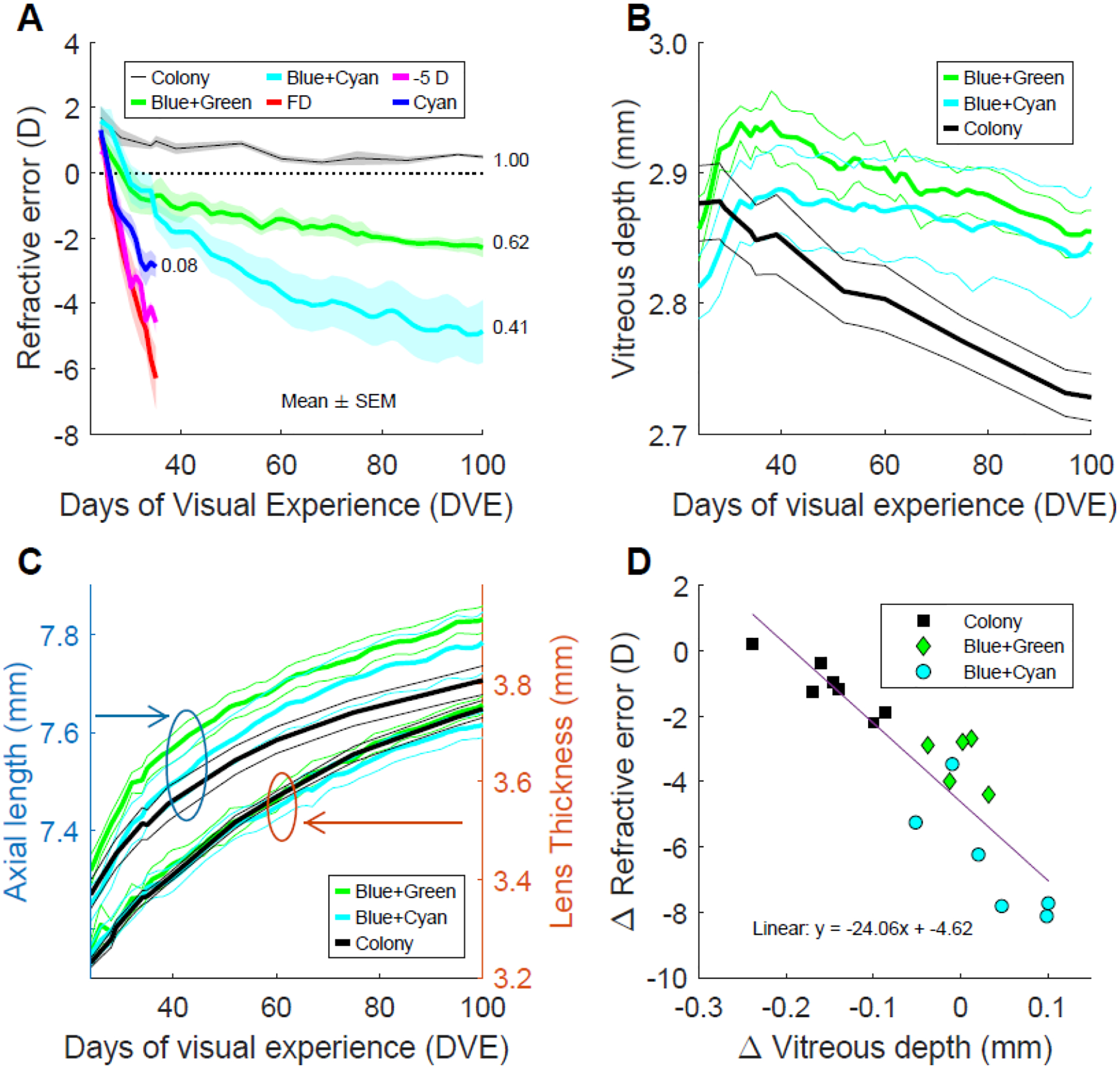
A. Myopia progression in limited-bandwidth light compared with progression in form deprivation (FD), negative-lens (−5D) wear, and cyan narrow-band light. Black line: mean refractive errors over time of the Colony group (n=7). Green line: mean interpolated refractive errors of the Blue+Green group (n=5). Cyan line: mean interpolated refractive errors the Blue+Cyan group (n=6). Red line: mean refractive errors of eyes with a diffuser (n=6)(Norton et al. 2010). Magenta line: mean refractive errors of eyes with a −5 D lens (n=5)(Norton, et al. 2010). Blue line: mean refractive errors of eyes in cyan light. Shaded regions show ± SEM. Numbers beside the plots indicate the spectral STOP drive to resist myopia progression. B. Change in vitreous chamber depth in Colony, Blue+Green and Blue+Cyan lighting. C. Change in axial length and lens thickness in Colony, Blue+Green and Blue+Cyan lighting showing that lens growth is faster than axial length increase. The vertical scales are the same for both components, but the lens (right scale) is considerably thinner than the axial length. D. Change in refraction vs. change in vitreous chamber depth from 24 to 100 DVE in Colony, Blue+Green and Blue+Cyan lighting. The solid purple line indicates the linear regression fit.
Figures 2C and 2D show the average refractive error and vitreous chamber depth as a function of time for each of the six animals in the Blue+Cyan group. Due to scheduling issues, we followed these animals up to 142 to 150 DVE. The group mean is shown in Figure 3A. As with the Blue+Green group, all animals became myopic within a few days after the onset of limited-bandwidth blue+cyan light treatment with a similar rate over the first four days (−0.27 ± 0.11 D/day, unpaired t-test, p = 0.83). Subsequently, the Blue+Cyan animals developed myopia more rapidly than the Blue+Green animals and showed greater variability in the rate of myopia progression. We note that in Figure 2C the data appear to be split into two groups. We speculate that, as we limit the power of the available chromatic cues, some animals will “fall off the myopic cliff”, while others will barely hang on. As expected, there was a commensurately larger increase in the vitreous chamber depth relative to the Colony group (Figure 2D). The Blue+Cyan animals showed a continuing slow development of myopia over time but did so at a somewhat faster rate than did the Blue+Green group.
Exponential functions fit to the refractive error data as a function of time (DVE) for the individual animals in each group (supplemental Figure S2) accurately reflected the data and accounted for a mean of 82 percent of the variance (range between individual animals: 57% to 98%). Table 1 provides summary statistics of these fits. The model-fit refractive errors at 150 DVE for both Blue+Green (−2.3 ± 0.1 D) and Blue+Cyan (−6.0 ± 0.9 D) animals were significantly more myopic than for the Colony animals (0.4 ± 0.1 D), and those for the Blue+Cyan animals are significantly more myopic than the Blue+Green animals. The time constant was significantly different Blue+Cyan vs Colony animals, but not between Blue+Green and Colony animals, confirming that the myopia progression was faster in the Blue+Cyan group.
Table 1.
Comparison of refraction and model statistics among the three study groups.
| Colony (A) | Blue+Green (B) | Blue+Cyan (C) | Kruskal Wallis | Wilcoxon rank-sum p-values | |||
|---|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | p-value | A vs. B | A vs. C | B vs. C | |
| Refraction at 24 DVE (Diopters) | 1.7 ± 0.3 | 1.4 ± 0.4 | 1.6 ± 0.5 | 0.87 | |||
| Refraction at 150 ± 8 DVE (Diopters)* | −0.02 ± 0.1 | −2.9 ± 0.2 | −7.0 ± 0.4 | 0.001 | |||
| Predicted refraction at 150 DVE (Diopters) | 0.4 ± 0.1 | −2.3 ± 0.1 | −6.0 ± 0.9 | 0.0005 | 0.003 | 0.001 | 0.004 |
| Time constant (c) | 9.8 ± 2.4 | 21.6 ± 7.2 | 61.1 ± 26.3 | 0.02 | 0.34 | 0.002 | 0.25 |
Refraction data at 150 ± 8 DVE were available for only 4 animals in the Blue+Green group.
Figure 3A, shows the mean refractive errors of the Blue+Green, Blue+Cyan, and Colony groups over time. Because the measurement days were not always identical for Blue+Green and Blue+Cyan animals, we interpolated the data and then took the mean of the interpolated values. We plotted the data to 100 DVE, omitting the one Blue+Green animal in which data collection ended at 54 DVE. These results are compared with data from previous studies using short-term exposure from 24 to 35 DVE with a monocular −5D lens (Norton, Amedo, et al., 2010), with a monocular form diffuser (Norton, Hernan, et al., 2010) and in narrow-band cyan light. Compared to the rapid onset and progression of myopia in animals wearing diffuser, negative lens, or narrow-band cyan lighting, the onset and progression of myopia in limited-bandwidth lighting were considerably slower. The numbers at the end of the plots are the calculated spectral STOP drive values indicating the strength of the resistance to myopia progression and show a monotonic increase in myopia progression with decreasing spectral STOP drive.
Figure 3B plots the progression of the vitreous chamber depth in the Colony group, the Blue+Green and the Blue+Cyan limited bandwidth groups. Note that the initial vitreous chamber depth in the Blue+Cyan group was smaller than that of the Blue+Green group, yet the refractions (Figure 3A) were the same (even as with humans, males have on average longer eyes than females, but similar refractions, see (Hou, Norton, Hyman, Gwiazda & Group, 2018). As has been established in previous studies in tree shrews and other species (McBrien & Norton, 1992, Wallman, Gottlieb, Rajaram & Fugate-Wentzek, 1987, Wiesel & Raviola, 1977), the myopia shown in Figure 3A is produced almost entirely by an increase in the depth of the vitreous chamber relative to its initial value. During the first 10 days after the onset of limited-bandwidth treatment, the vitreous chamber depth in both the Blue+Green and Blue+Cyan groups increased roughly in parallel as did the myopia in both groups (Figure 3A). Between 40 and 100 DVE, the vitreous chamber depth in the Colony group decreased because (Figure 3C) the thickness of the crystalline lens increased throughout development at a faster rate than the increase in axial length. After around 40 DVE, the vitreous chamber depth in the two limited-bandwidth groups also decreased, but not as steeply as in the Colony group, producing a slow increase in the myopia. The decrease in vitreous chamber depth of the Blue+Cyan group was slower than that of the Blue+Green group, so that the myopia in the Blue+Cyan group became greater than that of the Blue+Green group.
Figure 3D shows the change in refractive error from 24 to 100 DVE as a function of the change in vitreous chamber depth from 24 to 100 DVE. The normal Colony animals showed on average approximately a −1.5 D shift in refraction and a 0.15 mm decrease in the vitreous chamber depth. All animals in the Blue+Green and Blue+Cyan groups showed a larger myopic change and either a smaller decrease or an increase in vitreous chamber depth over time. Other ocular components were also measured (supplementary Figure S3) but only the vitreous chamber and, consequently, the overall axial length (the sum of all other components), changed in keeping with the progression of the myopia in the Blue+Green and Blue+Cyan groups. The range of values for central corneal thickness, anterior chamber depth, and retinal thickness is relatively small, and these measurements are variable across animals with no obvious trends. The mean lens thickness as a function of age, with the removal of the one Blue+Cyan animal with an unusually thin lens, was remarkably precise and independent of the lighting condition.
4. Discussion
The primary finding of this study is that housing tree shrews in limited-bandwidth blue+cyan or blue+green lighting produces a gradually progressing myopia. This finding has two main implications. One is that exposure to limited-bandwidth light is a new way to produce myopia in tree shrews that does not deprive the eye of clear images, as occurs in form deprivation, and does not substantially change the focal plane (the target for the emmetropization mechanism) as does a negative-power lens (at least not initially). In this respect, the myopia produced by limited-bandwidth lighting appears to more closely resemble juvenile-onset myopia in children. A second important implication is that the rate of myopia progression in limited-bandwidth lighting is much slower than occurs with form deprivation or negative-lens wear, both of which induce myopia at a rapid pace relative to the normal axial growth of the eye. Although the rate of myopia progression in limited-bandwidth lighting, relative to overall eye growth, is proportionally more rapid in tree shrews than is myopia progression in most children, it is a much closer match than is the rapid myopia produced by either form deprivation or negative-lens wear. It is likely not possible to precisely define corresponding developmental ages in these two species. However, the period we used in tree shrews goes up to sexual maturity, comparable to adolescent humans when myopia development typically stabilizes. As illustrated in Figure 4, during this period in tree shrews axial elongation slows but is still present (Siegwart & Norton, 1998) even as it is in humans up to at least age 15 (Fledelius & Christensen, 1996, McCullough, Adamson, Breslin, McClelland, Doyle & Saunders, 2020). It has also been shown that tree shrews can develop myopia in response to form deprivation during this period (Siegwart & Norton, 1998). Therefore, in many aspects, the age range for the tree shrews in this study had many parallels with the age range in humans when they typically develop myopia.
Figure 4.
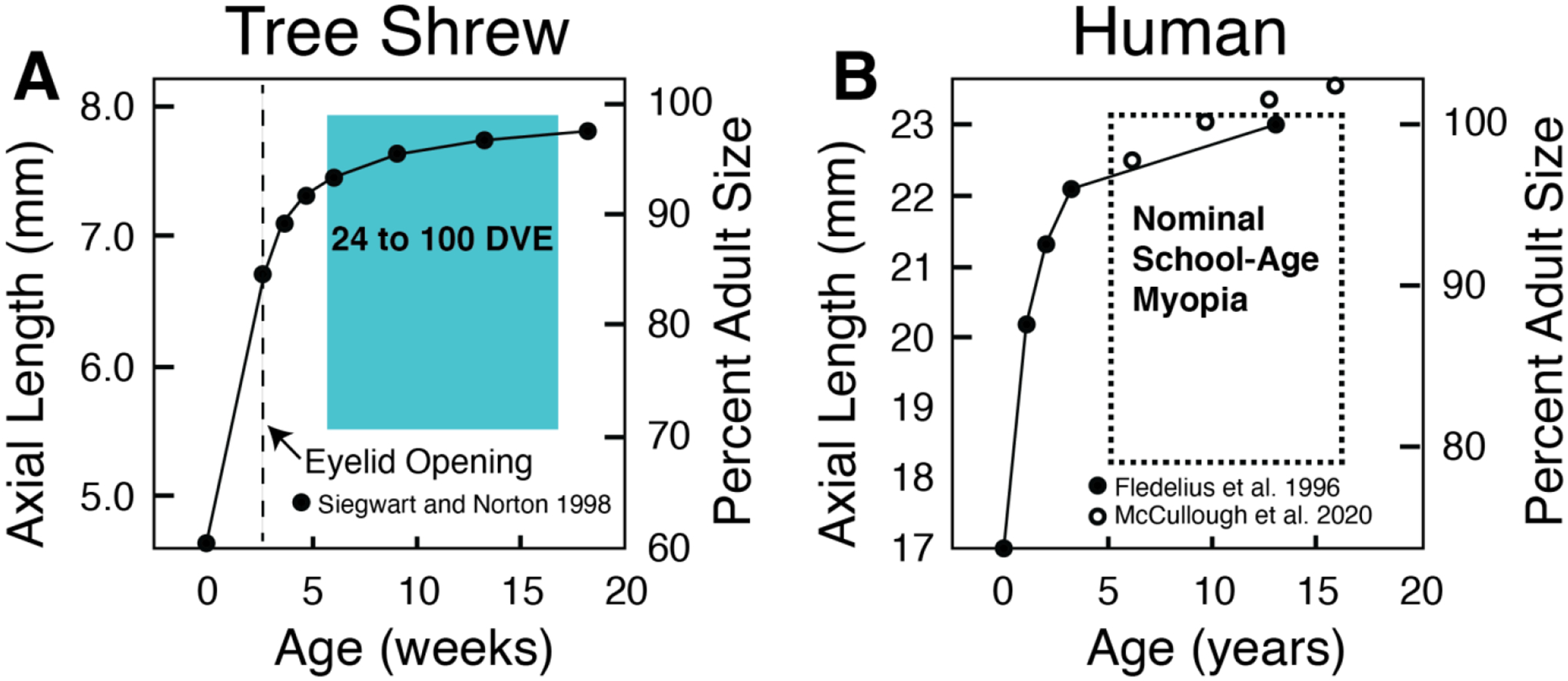
Redrawn from Siegwart and Norton 1998. A. Ocular axial length as a function of age for tree shrews. B. Ocular axial length for humans as a function of age. Both tree shrews and humans have an initial infantile phase where there is a rapid increase in axial length and the refractive state converges to emmetropia/low hyperopia. There is also a later juvenile phase where the rate of axial elongation has slowed but not stopped, and active emmetropization is requried to maintain emmetorpia. During this juvenile period tree shrews are still susceptible to myopia, and human school-age myopia typically develops.
Although genetics clearly play a role in myopia development in children (Hammond et al., 2001; Kurtz et al., 2007; Tedja et al., 2019; Zadnik et al., 1994), the success of optical treatments in slowing myopia progression (Chamberlain et al., 2022) distinctly shows that the visual environment plays a significant role in children. In animals, form deprivation and negative-lens wear provide such a strong stimulus for myopia progression that the role of genetics seems negligible. We suggest that these conditions prevent the emmetropization mechanism from producing the STOP signals needed to restrain axial elongation, throughout the duration of form deprivation and to the point of full compensation for a negative lens. A reason that limited-bandwidth lighting produces a slow myopia progression may be that this lighting only reduces (does not eliminate) the ability of the emmetropization mechanism to use environmental cues to produce STOP signals.
It is possible that there may be nothing wrong with the emmetropization mechanism in many human myopes (Siegwart & Norton, 2011). Most children who become myopic achieve near-emmetropia before they later develop myopia (Gwiazda et al., 1993), suggesting the presence of a functioning emmetropization mechanism. It may be that our modern visual environment contains relatively less of the visual cues that generate strong STOP signals to counteract intrinsic eye growth, thus increasing the risk of developing myopia. At present we don’t know what aspect of the modern visual environment could be responsible for this. We do point out that children initially have relatively strong LCA (i.e. a greater dioptric shift with change in wavelength), but the LCA function substantially declines with age - paralleling the period when initially-emmetropic children start to develop myopia (J. Wang et al., 2008). Of course, a weaker LCA function by itself will not unfailingly cause myopia, but if this reduces the ability of emmetropization to utilize chromatic cues for defocus, then in combination with our altered man-made visual environment, and individual genetic susceptibility, it could be a major contributor. Regardless, we hypothesize that limited-bandwidth lighting in tree shrews is a better functional model of human juvenile-onset myopia because it reduces (but does not eliminate) the production of STOP signals by the emmetropization mechanism in response to myopic defocus.
If, to control myopia progression in children, the emmetropization mechanism needs only to strengthen the STOP signals, it may be necessary to modulate the application of some optical treatments designed to slow myopia progression. For example, red light and amber light, provided throughout the day, actually cause hyperopia in tree shrews and macaque monkeys (Gawne, Ward, et al., 2017; Hung et al., 2018; Khanal et al., 2021) apparently because they cause the emmetropization mechanism to produce very strong STOP signals. Indeed, full-time red light in these species greatly reduces myopia produced by form deprivation and negative-lens wear (Hung et al., 2018; She et al., 2022) conditions that seem to block the production of STOP signals. If, as we have suggested, juvenile-onset myopia usually is due to conditions that only reduce the production of STOP signals, it may be possible to use these strong STOP-generating conditions for only brief treatment periods each day in order to control myopia progression utilizing the well-known ability of brief exposure to STOP-signal producing conditions to slow myopia development. Indeed, there have been reports that very brief treatment with bright long-wavelength light can control myopia progression in children (Zhou et al., 2022). We hypothesize that, in animal models, anti-myopia treatments that can either cause hyperopia, or significantly reduce form deprivation or minus lens myopia, would have to be extremely powerful. And yet, to reduce a slow drift to myopia in defiance of optical defocus, perhaps a small ‘boost’ towards hyperopia every day - one too small to be effective against lens-induced or form deprivation myopia - could be enough to maintain emmetropia?
Thus, another way in which using limited-bandwidth lighting may be useful is in testing putative myopia control treatment parameters in tree shrews. For instance, it could be used to test how often and for how long a strong STOP condition (e.g., narrow-band red, amber lights (Gawne, Ward, et al., 2017; Khanal et al., 2021)) needs to be presented to “boost” the STOP signaling enough to block the progression of a slowly-progressing myopia. In this case, one would raise tree shrews in constant limited-bandwidth light, but periodically expose them to (for example) red or amber light for brief periods during the day. A positive result using this paradigm in tree shrews could provide the justification for clinical trials in children.
Will limited bandwidth lighting also work to produce a slow myopia in other animal species? Studies have found inter-species variability in the response to narrow-band light. Species relatively distant to humans like chickens (Rucker & Wallman, 2009; Seidemann & Schaeffel, 2002; Wildsoet et al., 1993), guinea pigs (Liu et al., 2011; Long et al., 2009; Qian et al., 2013; Zou et al., 2018), and fish (Kröger & Fernald, 1994; Kröger & Wagner, 1996) appear to show contrasting responses (developing myopia in long-wavelength light) compared with tree shrews and monkeys that are more closely related to humans. Regardless of the species-specific variation in which wavelengths produce myopia, wavelength-induced myopia in every species is compatible with the notion that limited bandwidths of light produce myopia because they do not cause the emmetropization mechanism to produce strong enough STOP signals to maintain emmetropia. It may be that a range of limited bandwidths of light exists for each species that will produce a slowly progressing myopia. To the extent that this proves useful as a tool to understand how myopia is produced and possible treatments to control myopia progression, limited-bandwidth light-induced myopia may join form deprivation and negative-lens-wear as a tool to study myopia in animal models.
Supplementary Material
Acknowledgments
Supported by NEI grants RO1 EY028578 (TJG) and P30 EY003039 (core). The authors acknowledge the technical assistance of Russell Veale, Johanna Henry, and Eric Worthington.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Safal Khanal, Thomas T. Norton, and Timothy J. Gawne, have either patents or provisional patents on optical methods of preventing myopic progression related to the ideas presented here.
Safal Khanal: Writing- Reviewing and Editing, Visualization, Formal Analysis.
Thomas T. Norton: Writing- Reviewing and Editing, Visualization, Formal Analysis.
Timothy J. Gawne: Conceptualization, Writing - Original Draft, Review and Editing, Investigation, Visualization, Formal Analaysis.
References
- Atchison DA, Jones CE, Schmid KL, Pritchard N, Pope JM, Strugnell WE, & Riley RA (2004). Eye shape in emmetropia and myopia. Investigative Ophthalmology & Visual Science, 45(10), 3380–3386. [DOI] [PubMed] [Google Scholar]
- Bao J, Huang Y, Li X, Yang A, Zhou F, Wu J, Wang C, Li Y, Lim EW, Spiegel DP, Drobe B, & Chen H (2022). Spectacle lenses with aspherical lenslets for myopia control vs single-vision spectacle lenses: A randomized clinical trial. JAMA Ophthalmology. 10.1001/jamaophthalmol.2022.0401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullimore MA, Ritchey ER, Shah S, Leveziel N, Bourne RRA, & Flitcroft DI (2021). The risks and benefits of myopia control. Ophthalmology, 128(11), 1561–1579. [DOI] [PubMed] [Google Scholar]
- Burge J, & Geisler WS (2011). Optimal defocus estimation in individual natural images. Proc Natl Acad Sci U S A, 108 (40), 16849–16854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain P, Bradley A, Arumugam B, Hammond D, McNally J, Logan NS, Jones D, Ngo C, Peixoto-de-Matos SC, Hunt C, & Young G (2022). Long-term effect of dual-focus contact lenses on myopia progression in children. Optometry and Vision Science: Official Publication of the American Academy of Optometry, Publish Ahead of Print(3), 204–212. [DOI] [PubMed] [Google Scholar]
- Chamberlain P, Peixoto-de-Matos SC, Logan NS, Ngo C, Jones D, & Young G (2019). A 3-year Randomized Clinical Trial of MiSight Lenses for Myopia Control. Optometry and Vision Science: Official Publication of the American Academy of Optometry, 96(8), 556–567. [DOI] [PubMed] [Google Scholar]
- Chen AM, Erzurum SA, Chandler DL, Hercinovic A, Melia BM, Bhatt AR, Suh DW, Vricella M, Erickson JW, Miller AM, Marsh JD, Bodack MI, Martinson SR, Titelbaum JR, Gray ME, Holtorf HL, Kong L, Kraker RT, Rahmani B, … Pediatric Eye Disease Investigator Group. (2021). Overminus lens therapy for children 3 to 10 years of age with intermittent exotropia: A randomized clinical trial. JAMA Ophthalmology, 139(4), 464–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hamdaoui M, Gann DW, Norton TT, & Grytz R (2019). Matching the LenStar optical biometer to A-Scan ultrasonography for use in small animal eyes with application to tree shrews. Experimental Eye Research, 180, 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hamdaoui M, Levy AM, Gaonkar M, Gawne TJ, Girkin CA, Samuels BC, & Grytz R (2021). Effect of scleral crosslinking using multiple doses of genipin on experimental progressive myopia in tree shrews. Translational Vision Science & Technology, 10(5), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fledelius HC, & Christensen AC (1996). Reappraisal of the human ocular growth curve in fetal life, infancy, and early childhood. Br J Ophthalmol, 80 (10), 918–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitcroft DI (2012). The complex interactions of retinal, optical and environmental factors in myopia aetiology. Progress in Retinal and Eye Research, 31(6), 622–660. [DOI] [PubMed] [Google Scholar]
- Flitcroft DI (2014). Emmetropisation and the aetiology of refractive errors. Eye, 28(2), 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne TJ, Grytz R, & Norton TT (2021). How chromatic cues can guide human eye growth to achieve good focus. Journal of Vision, 21(5), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne TJ, & Norton TT (2020). An opponent dual-detector spectral drive model of emmetropization. Vision Research, 173, 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne TJ, Siegwart JT Jr, Ward AH, & Norton TT (2017). The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Experimental Eye Research, 155, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne TJ, Ward AH, & Norton TT (2017). Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Research, 140, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne TJ, Ward AH, & Norton TT (2018). Juvenile Tree Shrews Do Not Maintain Emmetropia in Narrow-band Blue Light. Optometry and Vision Science: Official Publication of the American Academy of Optometry, 95(10), 911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein M, & Millodot M (1970). Retinoscopy and eye size. Science, 168(3931), 605–606. [DOI] [PubMed] [Google Scholar]
- Gusek-Schneider G-C, & Martus P (2000). Stimulus deprivation myopia in human congenital ptosis: a preliminary report of 50 unilateral cases. Strabismus, 8(3), 169–177. [PubMed] [Google Scholar]
- Gwiazda JE, Thorn F, Bauer J, & Held R (1993). Myopic children show insufficient accommodative response to blur. Investigative Ophthalmology & Visual Science, 34(3), 690–694. [PubMed] [Google Scholar]
- Haarman AEG, Enthoven CA, Tideman JWL, Tedja MS, Verhoeven VJM, & Klaver CCW (2020). The Complications of Myopia: A Review and Meta-Analysis. Investigative Ophthalmology & Visual Science, 61(4), 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond CJ, Snieder H, Gilbert CE, & Spector TD (2001). Genes and environment in refractive error: the twin eye study. Investigative Ophthalmology & Visual Science, 42(6), 1232–1236. [PubMed] [Google Scholar]
- Hou W, Norton TT, Hyman L, Gwiazda J, & Group C (2018). Axial Elongation in Myopic Children and its Association With Myopia Progression in the Correction of Myopia Evaluation Trial. Eye Contact Lens, 44 (4), 248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung L-F, Arumugam B, She Z, Ostrin L, & Smith EL III. (2018). Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Experimental Eye Research, 176, 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecka JE, Miller W, Pringle TH, Wiens F, Zitzmann A, Helgen KM, Springer MS, & Murphy WJ (2007). Molecular and genomic data identify the closest living relative of primates. Science (New York, N.Y.), 318(5851), 792–794. [DOI] [PubMed] [Google Scholar]
- Jiang X, Pardue MT, Mori K, Ikeda S-I, Torii H, D’Souza S, Lang RA, Kurihara T, & Tsubota K (2021). Violet light suppresses lens-induced myopia via neuropsin (OPN5) in mice. Proceedings of the National Academy of Sciences of the United States of America, 118(22), e2018840118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SK, Lee JH, Kakizaki H, & Jee D (2012). Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in Seoul, South Korea. Investigative Ophthalmology & Visual Science, 53(9), 5579–5583. [DOI] [PubMed] [Google Scholar]
- Khanal S, Norton TT, & Gawne TJ (2021). Amber light treatment produces hyperopia in tree shrews. Ophthalmic & Physiological Optics: The Journal of the British College of Ophthalmic Opticians (Optometrists), 41(5), 1076–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger RH, & Fernald RD (1994). Regulation of eye growth in the African cichlid fish Haplochromis burtoni. Vision Research, 34(14), 1807–1814. [DOI] [PubMed] [Google Scholar]
- Kröger RH, & Wagner HJ (1996). The eye of the blue acara (Aequidens pulcher, Cichlidae) grows to compensate for defocus due to chromatic aberration. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology, 179(6), 837–842. [DOI] [PubMed] [Google Scholar]
- Kurtz D, Hyman L, Gwiazda JE, Manny R, Dong LM, Wang Y, Scheiman M, & COMET Group. (2007). Role of parental myopia in the progression of myopia and its interaction with treatment in COMET children. Investigative Ophthalmology & Visual Science, 48(2), 562–570. [DOI] [PubMed] [Google Scholar]
- Lam CSY, Tang WC, Tse DY-Y, Lee RPK, Chun RKM, Hasegawa K, Qi H, Hatanaka T, & To CH (2020). Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. The British Journal of Ophthalmology, 104(3), 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Qian Y-F, He JC, Hu M, Zhou X-T, Dai J-H, Qu X-M, & Chu R-Y (2011). Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Experimental Eye Research, 92(6), 447–453. [DOI] [PubMed] [Google Scholar]
- Long Q, Chen D, & Chu R (2009). Illumination with monochromatic long-wavelength light promotes myopic shift and ocular elongation in newborn pigmented guinea pigs. Cutaneous and Ocular Toxicology, 00(00), 090825080943088–090825080943085. [DOI] [PubMed] [Google Scholar]
- Mandelman T, & Sivak JG (1983). Longitudinal chromatic aberration of the vertebrate eye. Vision Research, 23(12), 1555–1559. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Moghaddam HO, & Reeder AP (1993). Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Investigative Ophthalmology & Visual Science, 34(1), 205–215. [PubMed] [Google Scholar]
- McBrien NA, & Norton TT (1992). The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri). Vision Research, 32(5), 843–852. [DOI] [PubMed] [Google Scholar]
- McCullough S, Adamson G, Breslin KMM, McClelland JF, Doyle L, & Saunders KJ (2020). Axial growth and refractive change in white European children and young adults: predictive factors for myopia. Sci Rep, 10 (1), 15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti DO, Sinnott LT, Lynn Mitchell G, Jordan LA, Friedman NE, Frane SL, & Lin WK (2018). Ocular component development during infancy and early childhood. Optometry and Vision Science: Official Publication of the American Academy of Optometry, 95(11), 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT (1999). Animal Models of Myopia: Learning How Vision Controls the Size of the Eye. ILAR Journal / National Research Council, Institute of Laboratory Animal Resources, 40(2), 59–77. [DOI] [PubMed] [Google Scholar]
- Norton TT, Amedo AO, & Siegwart JT Jr. (2010). The effect of age on compensation for a negative lens and recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri). Vision Research, 50(6), 564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Hernan CK, & Siegwart JT (2010). Initial Rates of Myopia Progression in Juvenile Tree Shrews with Monocular –5 D, –10D Lenses, and Form Deprivation. American Academy of Optometry Annual Meeting, San Francisco. [Google Scholar]
- Norton TT, Khanal S, & Gawne TJ (2021). Tree shrews do not maintain emmetropia in initially-focused narrow-band cyan light. In Experimental Eye Research (Vol. 206, p. 108525). 10.1016/j.exer.2021.108525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, & Siegwart JT Jr. (2013). Light levels, refractive development, and myopia--a speculative review. Experimental Eye Research, 114, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Siegwart JT Jr, & Amedo AO (2006). Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Investigative Ophthalmology & Visual Science, 47(11), 4687–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Wu WW, & Siegwart JT Jr. (2003). Refractive state of tree shrew eyes measured with cortical visual evoked potentials. Optometry and Vision Science: Official Publication of the American Academy of Optometry, 80(9), 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DJ, & Millodot M (1979). Eyelid closure causes myopia in humans. Experientia, 35(11), 1478–1479. [DOI] [PubMed] [Google Scholar]
- Owens DA (1980). A comparison of accommodative responsiveness and contrast sensitivity for sinusoidal gratings. Vision Res, 20 (2), 159–167. [DOI] [PubMed] [Google Scholar]
- Penha AM, Burkhardt E, Schaeffel F, & Feldkaemper MP (2012). Ultrasonography and optical low-coherence interferometry compared in the chicken eye. Optometry and Vision Science: Official Publication of the American Academy of Optometry, 89(6), 916–921. [DOI] [PubMed] [Google Scholar]
- Petry HM, Fox R, & Casagrande VA (1984). Spatial contrast sensitivity of the tree shrew. Vision Research, 24(9), 1037–1042. [DOI] [PubMed] [Google Scholar]
- Petry HM, & Hárosi FI (1990). Visual pigments of the tree shrew (Tupaia belangeri) and greater galago (Galago crassicaudatus): a microspectrophotometric investigation. Vision Research, 30(6), 839–851. [DOI] [PubMed] [Google Scholar]
- Qian Y-F, Liu R, Dai J-H, Chen M-J, Zhou X-T, & Chu R-Y (2013). Transfer from blue light or green light to white light partially reverses changes in ocular refraction and anatomy of developing guinea pigs. Journal of Vision, 13(11). 10.1167/13.11.16 [DOI] [PubMed] [Google Scholar]
- Rappon J, Chung C, Young G, Hunt C, Neitz J, Neitz M, & Chalberg T (2022). Control of myopia using diffusion optics spectacle lenses: 12-month results of a randomised controlled, efficacy and safety study (CYPRESS). The British Journal of Ophthalmology, bjophthalmol-2021–321005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, & Stell WK (1994). Basic fibroblast growth factor (bFGF) and transforming growth factor beta (TGF-beta) act as stop and go signals to modulate postnatal ocular growth in the chick. Experimental Eye Research, 58(5), 553–561. [DOI] [PubMed] [Google Scholar]
- Rose KA, Harper R, Tromans C, Waterman C, Goldberg D, Haggerty C, & Tullo A (2000). Quality of life in myopia. The British Journal of Ophthalmology, 84(9), 1031–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker FJ (2019). Monochromatic and white light and the regulation of eye growth. Experimental Eye Research, 184, 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker FJ, & Wallman J (2009). Chick eyes compensate for chromatic simulations of hyperopic and myopic defocus: evidence that the eye uses longitudinal chromatic aberration to guide eye-growth. Vision Research, 49(14), 1775–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdak BS, Salmon AE, Cava JA, Allen KP, Freling S, Ramamirtham R, Norton TT, Roorda A, & Carroll J (2019). Noninvasive imaging of the tree shrew eye: Wavefront analysis and retinal imaging with correlative histology. Experimental Eye Research, 185(107683), 107683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaridurg P, Tahhan N, Kandel H, Naduvilath T, Zou H, Frick KD, Marmamula S, Friedman DS, Lamoureux E, Keeffe J, Walline JJ, Fricke TR, Kovai V, & Resnikoff S (2021). IMI impact of myopia. Investigative Ophthalmology & Visual Science, 62(5), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidemann A, & Schaeffel F (2002). Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Research, 42(21), 2409–2417. [DOI] [PubMed] [Google Scholar]
- She Z, Norton TT, & Gawne T (2022). Narrow-band, long-wavelength lighting caused hyperopia in normal eyes and retarded minus lens-induced myopia and form deprivation myopia for juvenile tree shrews. Investigative Ophthalmology & Visual Science, 63(7), 1889–A0018. [Google Scholar]
- Siegwart JT Jr, & Norton TT (1994). Goggles for controlling the visual environment of small animals. Laboratory Animal Science, 44(3), 292–294. [PubMed] [Google Scholar]
- Siegwart JT Jr, & Norton TT (1998). The susceptible period for deprivation-induced myopia in tree shrew. Vision Research, 38(22), 3505–3515. [DOI] [PubMed] [Google Scholar]
- Siegwart JT Jr, & Norton TT (2010). Binocular lens treatment in tree shrews: Effect of age and comparison of plus lens wear with recovery from minus lens-induced myopia. Experimental Eye Research, 91(5), 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegwart JT Jr, & Norton TT (2011). Perspective: how might emmetropization and genetic factors produce myopia in normal eyes? Optometry and Vision Science: Official Publication of the American Academy of Optometry, 88(3), E365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL 3rd, Hung L-F, Arumugam B, Holden BA, Neitz M, & Neitz J (2015). Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Investigative Ophthalmology & Visual Science, 56(11), 6490–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Ramamirtham R, Qiao-Grider Y, Hung LF, Huang J, Kee C-SS, Coats D, & Paysse E (2007). Effects of foveal ablation on emmetropization and form-deprivation myopia. Investigative Ophthalmology & Visual Science, 48(9), 3914–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedja MS, Haarman AEG, Meester-Smoor MA, Kaprio J, Mackey DA, Guggenheim JA, Hammond CJ, Verhoeven VJM, Klaver CCW, & CREAM Consortium. (2019). IMI - myopia genetics report. Investigative Ophthalmology & Visual Science, 60(3), M89–M105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Smith EL 3rd, Nickla DL, Ashby R, Tkatchenko AV, Ostrin LA, Gawne TJ, Pardue MT, Summers JA, Kee C-S, Schroedl F, Wahl S, & Jones L (2019). IMI - report on experimental models of emmetropization and myopia. Investigative Ophthalmology & Visual Science, 60(3), M31–M88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale S, Sperduto RD, & Ferris FL 3rd. (2009). Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Archives of Ophthalmology, 127(12), 1632–1639. [DOI] [PubMed] [Google Scholar]
- von Noorden GK, & Lewis RA (1987). Ocular axial length in unilateral congenital cataracts and blepharoptosis. Investigative Ophthalmology & Visual Science, 28(4), 750–752. [PubMed] [Google Scholar]
- Walline JJ, Walker MK, Mutti DO, Jones-Jordan LA, Sinnott LT, Giannoni AG, Bickle KM, Schulle KL, Nixon A, Pierce GE, Berntsen DA, & BLINK Study Group. (2020). Effect of high add power, medium add power, or single-vision contact lenses on myopia progression in children: The BLINK randomized clinical trial. JAMA: The Journal of the American Medical Association, 324(6), 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman J, & Adams JI (1987). Developmental aspects of experimental myopia in chicks: Susceptibility, recovery and relation to emmetropization. Vision Research, 27(7), 1139–1163. [DOI] [PubMed] [Google Scholar]
- Wallman J, Gottlieb MD, Rajaram V, & Fugate-Wentzek LA (1987). Local retinal regions control local eye growth and myopia. Science, 237 (4810), 73–77. [DOI] [PubMed] [Google Scholar]
- Wallman J, & Winawer J (2004). Homeostasis of eye growth and the question of myopia. Neuron, 43(4), 447–468. [DOI] [PubMed] [Google Scholar]
- Wang J, Candy TR, Teel DFW, & Jacobs RJ (2008). Longitudinal chromatic aberration of the human infant eye. Journal of the Optical Society of America. A, Optics, Image Science, and Vision, 25(9), 2263–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Schaeffel F, Jiang B, & Feldkaemper M (2018). Effects of light of different spectral composition on refractive development and retinal dopamine in chicks. Investigative Ophthalmology & Visual Science, 59(11), 4413–4424. [DOI] [PubMed] [Google Scholar]
- Westheimer G (2021). Objective measures of retinal image degradation due to refractive corrections. Optometry and Vision Science: Official Publication of the American Academy of Optometry, 98(6), 654–664. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, & Raviola E (1977). Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature, 266(5597), 66–68. [DOI] [PubMed] [Google Scholar]
- Wildsoet CF (1997). Active emmetropization--evidence for its existence and ramifications for clinical practice. In Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists) (Vol. 17, Issue 4, pp. 279–290). https://doi.org/S0275-5408(97)00003-3 [pii] [PubMed] [Google Scholar]
- Wildsoet CF, Howland HC, Falconer S, & Dick K (1993). Chromatic aberration and accommodation: their role in emmetropization in the chick. Vision Research, 33(12), 1593–1603. [DOI] [PubMed] [Google Scholar]
- Zadnik K, Satariano WA, Mutti DO, Sholtz RI, & Adams AJ (1994). The effect of parental history of myopia on children’s eye size. JAMA: The Journal of the American Medical Association, 271(17), 1323–1327. [PubMed] [Google Scholar]
- Zhou L, Xing C, Qiang W, Hua C, & Tong L (2022). Low-intensity, long-wavelength red light slows the progression of myopia in children: an Eastern China-based cohort. Ophthalmic & Physiological Optics: The Journal of the British College of Ophthalmic Opticians (Optometrists), 42(2), 335–344. [DOI] [PubMed] [Google Scholar]
- Zou L, Zhu X, Liu R, Ma F, Yu M, Liu H, & Dai J (2018). Effect of altered retinal cones/opsins on refractive development under monochromatic lights in guinea pigs. Journal of Ophthalmology, 2018, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


