Abstract
The human circadian system plays a vital role in many physiological processes, and circadian rhythms are found in virtually all tissues and organs. Disruption of circadian rhythms may lead to adverse health outcomes. Evidence from recent population-based studies was reviewed because they represent real-world behavior and can be useful in developing future studies to reduce risk of adverse health conditions, including cardiovascular diseases, obesity, and diabetes mellitus, which may occur due to circadian disruption. We performed an electronic search in PubMed and Web of Science (2012–2022). Selected articles were based on specific inclusion and exclusion criteria. We discuss five factors that may disrupt circadian rhythm alignment: shift work, late chronotype, late sleep timing, sleep irregularity, and late meal timing. Evidence from observational studies of these circadian disruptors suggests potential detrimental effects on cardiometabolic health, including higher BMI/obesity, higher blood pressure, greater dyslipidemia, greater inflammation, and diabetes. Future research should identify the specific underlying pathways in order to mitigate the health consequences of shift work. Further, optimal sleep and mealtimes for metabolic health can be explored in intervention studies. Lastly, it is important that the timing of external environmental cues, such as light, and behaviors that influence circadian rhythms are managed.
Keywords: circadian rhythm, cardiometabolic diseases
Introduction
Circadian rhythms or biological clocks are endogenous regulators in cells or organisms responsible for coordinating physiological and behavioral activities, allowing organisms to adapt to a changing environment within a 24-hour cycle (1, 2). The primary role of this circadian system is thought to be to organize physiological processes temporally in order to anticipate periods of activity and periods of rest (3). The rhythms of these processes are controlled by internal “clocks” with a central clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus in the brain that serves as the conductor of the clocks found in nearly all tissues of the body. The ubiquitous nature of these clocks throughout the body testifies to the importance of these rhythms for health.
Maintaining synchrony is key to optimal health, and this includes synchrony between our internal clocks and the external world, as well as synchrony between all the internal clocks (Figure 1). Our internal rhythms are synchronized with the external world primarily through light signals that reach the SCN through the eye via retinal ganglion cells (3). The central clock, in turn, regulates peripheral clocks through several mechanisms, including controlling rhythms in body temperature, autonomic nervous system activity, and various hormones, such as cortisol and melatonin (1, 4). The peripheral clocks, however, can also be synchronized through other signals, including feeding/fasting (5). Therefore, chronic disruption of the circadian rhythm resulting from factors such as night-shift work, late or irregular sleep timing, or meal timing may contribute to the deleterious effects related to chronic diseases such as cardiovascular disease (CVD), cancer, and diabetes (6).
Figure 1.
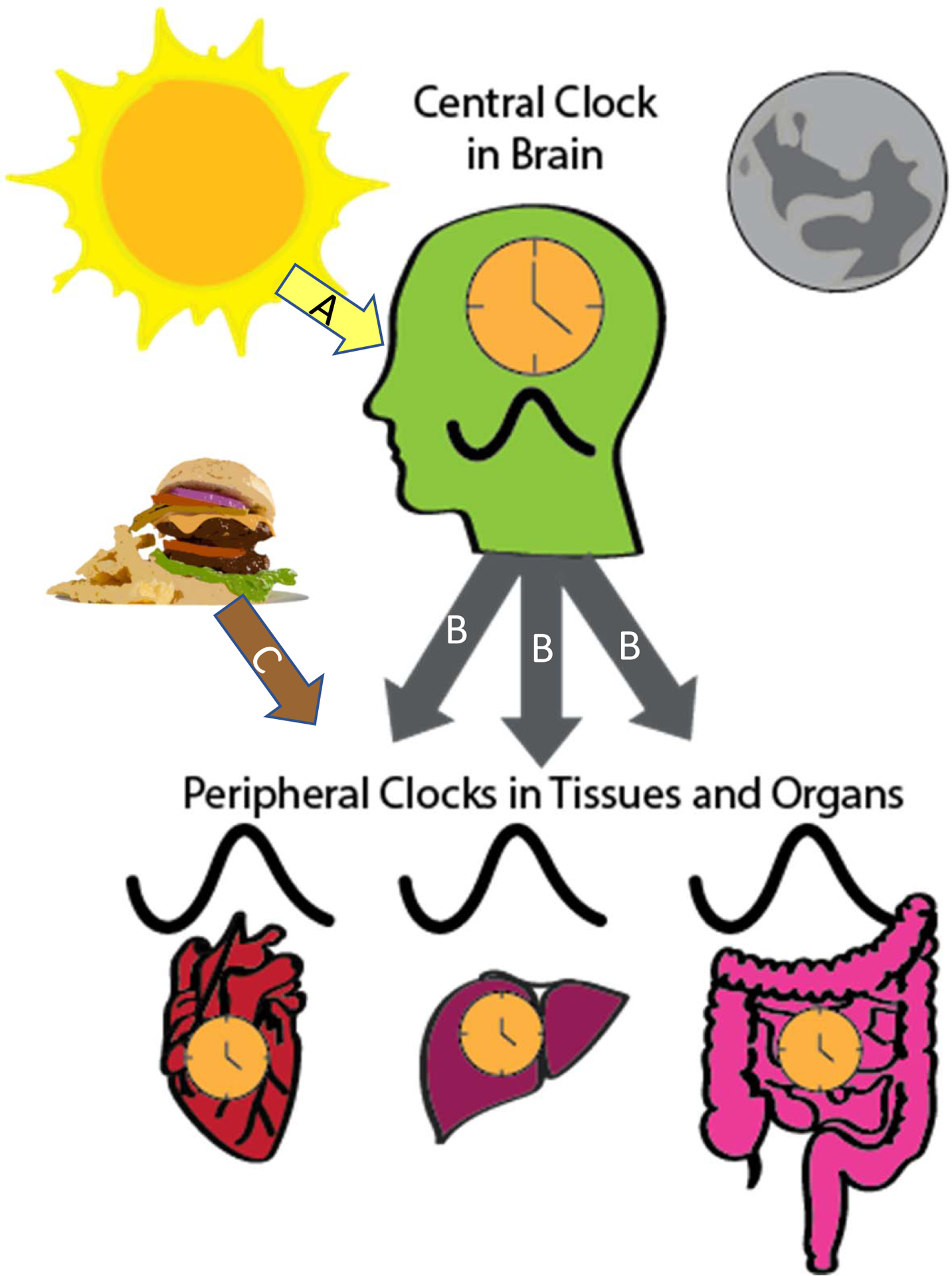
The circadian system is hierarchical with a central clock in the SCN of the hypothalamus and peripheral clocks in tissues and organs throughout the body. The primary timing signal that synchronizes the central clock is light (A arrow), while peripheral clocks are synchronized through several pathways. The central clock regulates circadian rhythms of peripheral tissues through several mechanisms (B arrows), such as control of body temperature, sympathetic nervous system activity, and hormones, like melatonin. Timing of peripheral clocks can also be synchronized through feeding (C arrow).
This review focuses on observational studies that examine health and behavior in real-world settings to determine if associations exist between habitual behaviors and health. We summarize the observational evidence for an association been markers of potential circadian disruption and cardiometabolic outcomes. Certain behaviors and environmental exposures, such as light, can influence our internal central and peripheral clocks and lead to circadian disruption (Figure 1). Shift work, which often involves shifting sleep and mealtimes between night and day, can be an extreme form of circadian disruption. Other factors that can impact circadian rhythm alignment include chronotype, meal timing, sleep regularity, and sleep timing. Observational studies have found that night or rotating shift workers, later chronotypes, sleep timing, sleep irregularity, and meal timing can have adverse effects on cardiometabolic outcomes (Figure 2). It is important to note that observational studies do not infer causality, however, an association between these factors and cardiometabolic diseases highlights their importance in future research and in public health.
Figure 2.
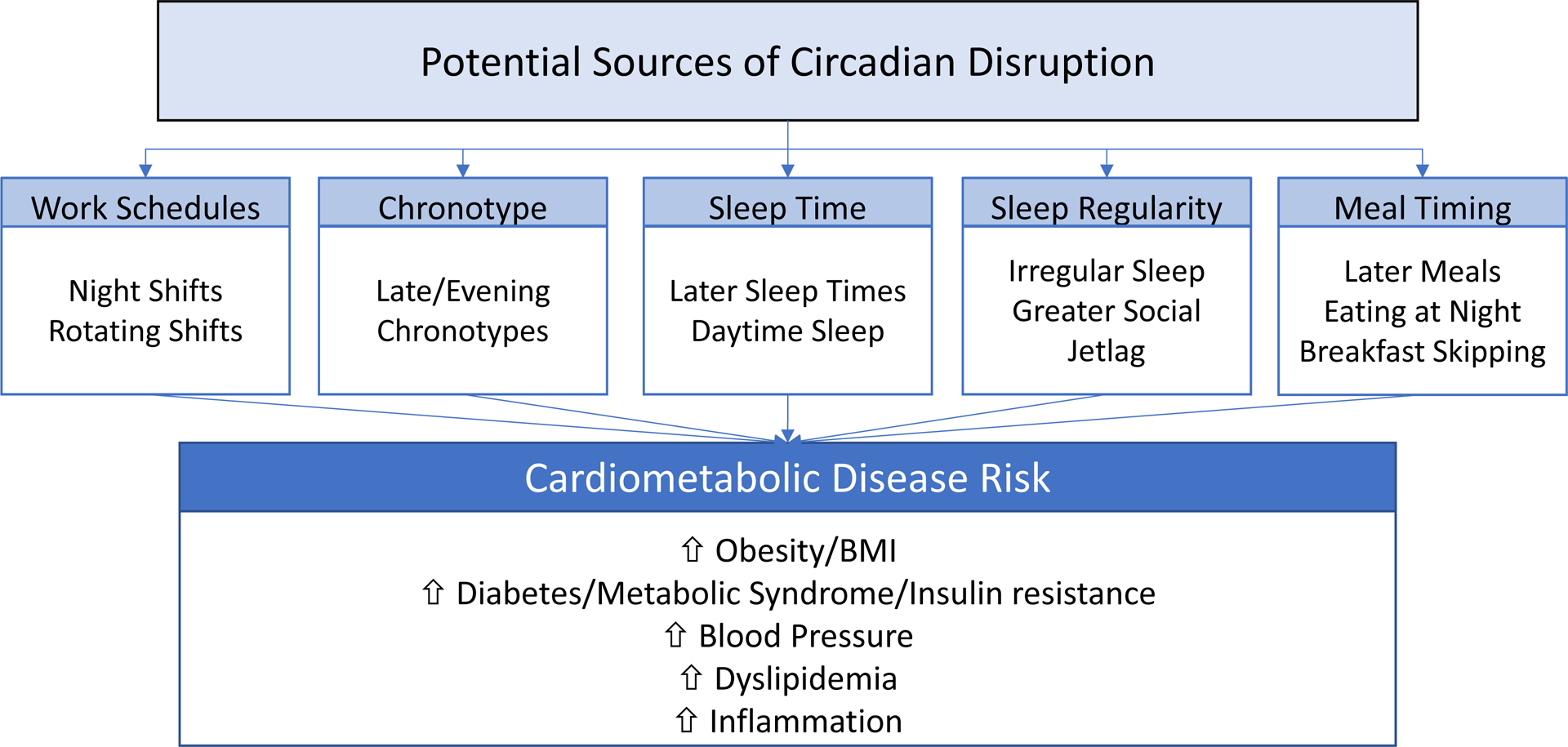
Sources of potential circadian disruption and their associations with specific cardiometabolic risk factors.
Methods
We searched PubMed and Web of Science using relevant keywords such as “circadian disruption, circadian rhythm, sleep irregularity, social jet lag, sleep timing, circadian misalignment, meal timing, time-restricted eating, chronotype, shift work, and cardiometabolic diseases”. We used “AND” and “OR” operators for these keywords. We included peer-reviewed studies in humans that were published in the last 10 years (i.e., 2012–2022). The reference list of selected articles was also screened for relevant studies. Outcomes of interest were cardiometabolic outcomes including obesity/body mass index (BMI), food intake, diabetes, blood pressure, inflammation, and cardiovascular diseases (CVD). This review focused on observational studies and included relevant reviews. There was no restriction on sample size and study location, however, only studies published in the English language and had adult participants were included in this review.
Results and Discussion
Shift work
Shift workers have work schedules outside the traditional 9 AM to 5 PM work schedule. Individuals classified as shift workers have early morning, night, rotating, and/or evening schedules. Shift work alters the normal sleep-wake cycle as they often have to sleep during daytime hours, which disrupts circadian rhythms and thus increases the risk for chronic diseases (7), and shift workers often have light exposure outside of natural daytime. The circadian rhythm disruption associated with shift work is a risk factor for CVD, diabetes, obesity, hypertension, sleep disorders, and metabolic disorders (7, 8).
Observational studies have demonstrated an adverse effect of shift work on cardiometabolic diseases and risk factors. For example, meta-analyses have found that shift workers were approximately 23% more likely to be overweight or obese (9), had a 14% increased risk of incident diabetes (10), 11–35% increased odds of metabolic syndrome (11, 12), and had 10% increased odds of prevalent hypertension and 30% odds of incident hypertension (13). A systematic review of 45 epidemiological studies also found that night shift workers had significantly higher systolic and diastolic blood pressure (14).
Shift work has been associated with some risk factors for cardiometabolic disease. A cross-sectional study of female hospital employees found that women who were rotating shift workers had a higher cardiometabolic risk score, which was based on a composite of blood pressure, fasting blood glucose, triglycerides, and waist circumference measure (15). This study also demonstrated differences in the diurnal pattern of urinary cortisol levels; the shift workers had lower total 24-hour cortisol production and had a flatter pattern over 2 days of urine collection compared with day workers (15). Lipid levels are also associated with shift work. A systematic review of 66 articles showed that shift workers, particularly those who worked the night shift, had higher levels of total cholesterol, increased triglycerides, and lower high-density cholesterol levels (16), which are known risk factors for CVD.
Inflammation has also been associated with shift work. A study among male shift workers found that high-sensitivity C-reactive protein (hsCRP), a marker of inflammation, was significantly higher in former shift workers compared to daytime workers (17). Similar findings were observed in another study that showed higher levels of hsCRP in female shift workers compared to female daytime workers (18). Another study among female nurses found that those who worked rotating night shifts had a higher CRP level compared to those who never worked rotating shifts (19). One study (20), however, did not find a relationship between shift work and hsCRP, but a significant relationship was found between long work hours and higher hsCRP as well as an interaction effect between long work hours and shift work on low-grade inflammation. Inflammation is a risk factor for CVD, type 2 diabetes, and obesity, thus chronic inflammation among shift workers may play a role in the initiation and progression of these conditions.
Blood pressure levels and diurnal rhythms play an important role in cardiovascular health and disease risk and blood pressure have a circadian rhythm that can be disrupted by shift work. Blood pressure values are elevated during wake, particularly during work times compared to non-work times, and since the work periods occur at a biological time (night) when blood pressure levels should be lower, shift work, especially night shift work, may impair the normal diurnal blood pressure pattern leading to an increased risk of CVD (21). Indeed, blood pressure is often elevated among shift workers compared to day workers (22).
Health behaviors, such as diet and sleep, may partially explain elevated cardiometabolic disease risk among shift workers. Shift workers may consume less healthy foods such as saturated fats and soft drinks as compared to day workers and eat meals at night when the body is primed for sleep (23). Shift work may contribute to changes in meal timing, eating around the clock, and meal skipping that may affect appetite-regulating hormones and can lead to internal circadian misalignment, increasing the risk of cardiometabolic diseases (23). Results from a cross-sectional study found that shift workers had a higher energy intake (56 kcal/day higher) as compared to day workers possibly due to work hours (24). Poor sleep is another factor common among shift workers and may partially explain increased cardiometabolic disease risk. Results among nurses showed poor sleep quality among shift and non-shift work nurses with shift work as an independent risk factor for poor sleep (25). Poor sleep health is associated with increased cardiometabolic risk (26).
These highlighted studies demonstrated that shift work has an impact on cardiometabolic health, and potential mechanisms could be related to inflammation, impaired blood pressure regulation, or unhealthy behaviors. One limitation to note is that the definition of shift work is not consistent among studies and different work schedules may vary in the degree of circadian disruption or impairment of cardiometabolic health.
Chronotype
Chronotype is a construct designed to identify the preferred or actual timing of activities, such as sleep (27, 28). Chronotype can be assessed using standard validated self-assessment questionnaires and two commonly used ones are the Morningness-Eveningness Questionnaire (MEQ) (29), which assesses the preferred timing of behaviors, and the Munich Chronotype Questionnaire (MCTQ) (30), which assesses the actual timing of behaviors such as sleep. Objective estimates of sleep timing, such as wrist actigraphy, can also be used to assess the chronotype of individuals (31). Chronotype, particularly using the MEQ, is often grouped into morning-types, evening-types, and intermediate types. Individuals who are morning-types prefer earlier clock times and tend to wake up and go to bed early while evening-types have later wakeup and bedtimes and peak performance later in the day (28).
Observational studies have linked evening chronotypes to increased prevalence of several cardiovascular and metabolic diseases, including higher prevalence of diabetes, metabolic syndrome, and cardiovascular diseases (32, 33, 34, 35, 36). A meta-analysis of cross-sectional studies reported that evening chronotypes were more likely to have diabetes (OR:1.30; 95% CI: 1.20, 1.41; n=7 studies) than morning types, but no association was observed for hypertension (OR 0.99, 95% CI 0.77, 1.27; n=5 studies) (37). In the Nurses’ Health Study 2 of more than 64,000 women, evening chronotype was not associated with prevalent diabetes compared to intermediate types, however, early types had a slightly reduced odds of prevalent diabetes (OR 0.87, 95% CI 0.77–0.98) compared to intermediate types, but in prospective analysis in 319 participants with a follow-up period of ~2 years, chronotype was not associated with incident diabetes in fully adjusted models (38). Analysis of the UK Biobank with almost 400,000 participants, found that early chronotype was associated with reduced risk of incident CVD (HR 0.93, 95% CI 0.89, 0.97) and reduced risk of incident coronary heart disease (HR 0.92, 0.87, 0.98) over a median of 8.5 years of follow-up (39).
Risk factors or subclinical predictors of cardiometabolic disease have also been associated with chronotype. In the meta-analyses of cross-sectional studies previously mentioned, compared to morning types, evening chronotype had significantly higher fasting blood glucose (mean difference [MD]: 7.82; 95% CI: 3.18, 12.45; n=8 studies) higher hemoglobin A1c (MD: 7.6; 95% CI: 3.1, 12.2; n=8 studies), higher LDL cholesterol (MD: 13.69; 95% CI: 6.8, 20.5; n=6 studies) and higher triglycerides (MD: 12.62; 95% CI: 0.90, 24.4; n=8 studies), but no significant differences were observed for BMI (n=33 studies), energy intake (n=16 studies) or BP (n=9 studies) (37). One study found that evening chronotype was associated with higher levels of circulating proteins, including plasminogen activator inhibitor 1 (PAI-1) (34), and these circulating proteins have been linked to insulin resistance (40).
The association between evening chronotypes and the risk of cardiometabolic diseases may be due to environmental and behavioral factors. For example, individuals with evening chronotype may have poorer quality diets and higher dietary energy density as compared to morning or intermediate chronotypes (36, 41) and lower physical activity (36, 42), which could lead to poor cardiovascular health. Evening chronotypes also are exposed to light at inopportune times, which may lead to circadian disruption (6). Chronotype influences sleep timing (43) and meal timing (41), which are also associated with cardiometabolic health as discussed below. Individuals with a later chronotype had a later sleep timing and a later mealtime as compared to those with an earlier chronotype in a study among (n=872) middle to older-aged adults (43). Two studies examined the combination of chronotype and shift work but did not find significant associations. One study of 527 nurses did not find increased cardiometabolic risk associated with match/mismatched (i.e., aligning work time with sleep preference or not) chronotype-work shift (44), and another of 596 healthcare workers observed no difference in cardiometabolic risk factors among shift workers with different chronotypes (45).
Overall, findings from these observational studies suggest that later/evening chronotypes are associated with adverse health outcomes compared to early/morning chronotypes, however more prospective studies are needed. Further, the underlying mechanisms need to be identified. It may be that health risks associated with evening chronotype are due to the mismatch in one’s preferred chronotype and one’s social obligations that lead to unhealthy or inappropriately timed behaviors.
Sleep timing and variability
The timing of sleep, i.e., the time of day that sleep occurs, may lead to circadian disruption particularly if it occurs at a time in conflict with the biological clock. Sleep-wake patterns can influence the synchronization of our central clock, particularly through alterations in light exposure, which is reduced when individuals are sleeping. Further, an irregular sleep schedule could lead to circadian disruption due to the irregular exposure to these synchronizing signals. Of course, sleep timing and regularity are related to characteristics described above, such as shift work and chronotype, and there will be some overlap between these concepts. Nonetheless, some studies have specifically examined sleep timing and regularity in relation to cardiometabolic risk factors.
Similar to chronotype, having a later sleep timing is associated with worse cardiometabolic health profiles. For example, a large observational study of Hispanic/Latino adults found that later sleep-wake timing was associated with greater estimated insulin resistance and higher systolic and diastolic blood pressure measures (46, 47). A systematic review reported that some studies observed significant associations between later sleep timing or greater variability in sleep timing and higher rates of diabetes and metabolic syndrome, greater weight gain and adiposity, and more cardiometabolic risk factors, however not all studies reviewed observed significant associations (48). Of note, some studies observed gender differences in these associations, which emphasizes again the importance of analyses stratified by gender. Finally, greater sleep regularity in patients with type 1 diabetes was associated with better glucose control (49), demonstrating the potential importance of sleep patterns for patient populations.
Associations between sleep regularity and cardiometabolic health have been examined. In a cohort study of women (mean age 51 years), the SWAN study, average bedtime was not significantly associated with BMI or estimated insulin resistance (HOMA-IR), however, greater variability in bedtime was associated with higher BMI and higher HOMA-IR values (50). Similarly, in a sample of elderly women (>80 years of age), average bedtime was not associated with anthropometric measures, but greater variability in bedtime was significantly associated with higher BMI, higher body fat percentage, and lower lean mass percentage (51). In older adults (>55 years) with a high risk of CVD, greater sleep variability was associated with a higher prevalence of diabetes, but not with measures of anthropometry and glucose control (52). In prospective analysis, greater sleep timing variability was associated with both incident CVD events and incident metabolic syndrome over approximately 5 years in the MESA study, which is a large, ethnically diverse cohort study of older adults with a mean age of 70 years (53, 54). These studies suggest that significant associations exist between greater sleep timing variability and greater prevalence, incidence, or risk of cardiometabolic health across the life course.
There are several metrics developed to capture sleep regularity. One is the concept of “social jet lag” (SJL) (55), which refers to variability in sleep timing between work/school days and free days, and studies have reported associations between greater SJL and cardiometabolic risk (48). For example, one study found that in younger adults (<61 years), greater SJL was associated with an increased prevalence of metabolic syndrome and diabetes/prediabetes but no association was observed in older adults (56). However, another study of young adults, 21–35 years, found no association between SJL and anthropometric or blood pressure measures (57). Another novel metric used to quantify sleep regularity is the Sleep Regularity Index. Sleep Regularity Index assesses the percentage probability of an individual being at the same asleep vs awake at any two-time points 24 hours apart (58). Lower Sleep Regularity Index values have been associated with adverse cardiometabolic outcomes (59), impaired daytime function (60), and delayed circadian sleep/wake timing (58). Therefore, these metrics may be useful methods to characterize the regularity of sleep.
Irregular sleep patterns could cause circadian disruption due to the inability of the biological clocks to synchronize accurately to the light-dark cycle because of inconsistent timing cues. Thus, regular sleep schedules should be encouraged to promote optimal health.
Meal timing
Diet plays a significant role in preventing and managing cardiometabolic diseases. Although the quantity and quality of one’s diet are important for health, some focus is shifting to the importance of meal timing. Food serves as a synchronizer of our peripheral clocks thus meal timing may affect circadian rhythms in metabolic organs (61). Chrononutrition is the study of the interaction between the body’s circadian rhythm and nutrients to influence human health (62).
Recent research (63, 64, 65, 66) has demonstrated the benefits of meal timing, including the duration of eating and its timing, for cardiometabolic health. This has led to an increased interest in a dietary intervention called intermittent fasting. In intermittent fasting, there is an alternation between fasting and normal eating periods a few times a week (about 1–3 days per week) (67). Time-restricted eating (TRE) is a form of intermittent fasting where individuals are restricted to an eating window where nutrient intake is limited to a period of 4 to 10 hours in a day without an overt attempt to restrict caloric or dietary intake. In some studies, TRE resulted in significant improvement in body weight, waist circumference, insulin sensitivity, beta cell function, and blood pressure (66, 68, 69).
However, one randomized control trial of time-restricted eating did not observe significantly greater weight loss compared to the control group who ate 3 structured meals per day (70), however, the TRE group did not begin eating until noon (12 pm to 8 pm eating window). Additionally, results from interventional studies (71, 72) showed that caloric intake decreased when eating duration was reduced, thus these beneficial outcomes of TRE may be a result of the reduced energy intake. In a recent RCT, Liu et al found that people with obesity who were assigned to TRE did not have additional benefits of a decrease in body weight or metabolic risk factors as compared to those on a daily calorie restriction (73). Whether TRE at specific times of day has cardiometabolic health benefits remains an important area of study.
Research has suggested that early time-restricted eating, i.e., when the eating interval starts earlier in the day, has been reported to have some health benefits while a later time of intake is associated with risk factors for metabolic diseases (63, 74). Results from RCT showed that early (8-hour TRE; choose to eat between 06:00 and 15:00) time TRE showed a greater reduction in insulin resistance in early TRE (Δ = −1.08 ± 1.59) as compared to mid-day (8-hour between 11:00 and 20:00) TRE (Δ = 0.39 ± 0.71, p < 0.001) or the control group (i.e., eating ad libitum over more than 8 hours per day; Δ = −0.05 ± 0.75, p = 0.002) in healthy participants without obesity (75). Results from a recent systematic review (n= 19 articles) suggested that early TRE (eating ends before 17:00) and late (eating starts after 10:00 and ends before 23:00) had similar metabolic health effects on study participants (76). Consumption of breakfast, the first meal of the day, is often associated with metabolic outcomes. Frequent consumption of breakfast has been associated with a reduced risk of metabolic conditions while breakfast skippers are more likely to have a higher body mass index and more at risk for cardiometabolic diseases (77, 78). For example, in the Adventist Health Study 2 (n=50,660; mean follow-up of approximately 7 years), those who ate breakfast had a significant decline in their body mass index as compared to breakfast skippers (−0.03 kg/m2/y, 95% CI −0.04- −0.01) (79). Also, a meta-analysis of observational studies (n= 15 cohort studies) showed that frequent breakfast consumers (i.e., > 3 times /week) had a reduced risk of type 2 diabetes, obesity, metabolic syndrome, CVD, and hypertension as compared to those who consumed breakfast three or fewer times in a week (80). Results from a large cross-sectional study from the 2013–2017 Korea National Health and Nutrition Examination Survey (KNHANES) (n=14, 279) showed that eating in the morning was associated with a decreased prevalence of metabolic syndrome (OR 0.71, 95% CI 0.59–0.87) and abdominal obesity (OR 0.82, 95% C.I 0.70–0.95) in women (81). Results from a meta-analysis (n=9 trials) observe higher total energy intake among breakfast eaters as compared to breakfast skippers (mean difference 259.79 kcal/day) (82). The better metabolic outcomes associated with breakfast intake could be a result of food acting as a synchronizer of the peripheral clocks. While the “optimal time” for food intake may not be well understood, regular breakfast consumption can affect many physiological processes, such as glucose homeostasis and regulation of plasma lipids thus resulting in the alignment between the central and peripheral clocks (83). However, none of these studies assessed internal circadian timing so the timing of meals relative to the internal clock could not be ascertained. Nonetheless, these studies suggest a benefit of early morning eating in the metabolic health of individuals.
Eating late at night may be detrimental to metabolic health and it has been associated with an increase in body fat, which may result in metabolic diseases such as diabetes, obesity, and cardiovascular diseases. Late eating may result in a misalignment of central and peripheral clocks leading to metabolic diseases (84). A study by Sakai et al. (85) examined the relationship between late-night dinner and glycemic control in people living with type 2 diabetes and showed an independent association between late dinner and higher hemoglobin A1c, a marker of glucose control. Another study by Reid et al. showed that late eating and eating closer to bedtime were associated with higher caloric intake (86). Also results from the KNHANES showed that late-night eating was associated with an increase in the prevalence of metabolic syndrome (OR 1.25, 95% CI 1.04–1.49) and a reduced level of high-density cholesterol (OR 1.18, 95% CI 1.01–1.38) (81).
The exact mechanism by which TRE influences circadian rhythms and health is poorly understood. It could be that by restricting dietary intake, the body utilizes less glucose and more lipids and ketones for energy thus resulting in improving glucose levels and lipid homeostasis, which may improve metabolic health (87). Additionally, though TRE may not result in ketosis (i.e., when the body has inadequate carbohydrates and utilizes stored fat for energy), it may increase autophagy and antioxidant defenses in humans (88). The thermic effect of food may explain why later meal timing may influence some risk factors of cardiometabolic diseases. A lower thermic effect of food has been observed in the evening as compared to in the morning. This effect may be a result of circadian influence (89, 90). Additionally, endocrine factors may peak in humans depending on the time-of-day oscillations (91, 92, 93). For example, in the morning (~7 am- 8 am) during the active phase, there is a peak of the hormone cortisol which regulates the body’s energy and prepares it for the active phase (91). Ghrelin is a hormone that increases appetite and peaks at three-time points in the day i.e., ~8 am, 1 pm, and 6 pm (92). Additionally, there is a peak of the hormone leptin in the evening (~7 pm) which is responsible for decreasing appetite and fat breakdown (93). Thus, consuming meals during the active phase where many hormones peak may be optimal for health.
Diet plays a vital role in health and matching food intake with the individual’s internal circadian clock supports metabolic health. Novel dietary interventions, such as TRE, may help maintain or improve circadian alignment, which in turn may lead to a decrease in many metabolic risks (94, 95). Despite the benefits of intermittent fasting and TRE on health, there are some limitations. No consensus exists on the ideal timing for eating/fasting for optimal health thus there is heterogeneity in various studies on the timing of meals. Meal timing influences human physiology thus when there is a misalignment between feeding/fasting cycles and the endogenous circadian system, the health of individuals may be impacted. Meal timing, including TRE, is a novel and promising dietary strategy that is important for cardiometabolic health. More research is needed to understand optimal meal timing and dietary patterns for circadian health and cardiometabolic health.
Potential mediators between behavior, circadian disruption, and cardiometabolic disease
We have described evidence for associations between several potential circadian disruptors and markers of cardiometabolic health. The mechanisms underlying these associations are not fully understood. A limitation of large observational studies is the lack of direct measurements of the circadian system or circadian phase (i.e., the timing of the internal clock). None of the studies described above had a direct measure of the internal circadian system, such as dim light melatonin onset or body temperature rhythms, therefore it is not possible to assess the degree to which circadian rhythms were disrupted. The circadian system is hypothesized to be involved in these associations because both light and feeding serve as time cues to this system. Light exposure at night would be greater in night shift workers and in those who stay awake later and light is a major synchronizer of the central circadian clock. Metabolites from feeding also serve as time signals to some peripheral clocks and therefore eating at the “wrong” time could lead to circadian desynchrony between clocks in various tissues. There are, of course, other non-circadian mechanisms that may link these factors to poor health, including an unhealthy diet, poor sleep quality or reduced physical activity. A better understanding of the underlying mechanisms would provide critical information for determining if and how to intervene on the behaviors described above.
Future research
Additional research is needed to understand better the link between circadian disruption and cardiometabolic health in population-based studies. Although experimental studies are well designed to test cause and effect, they are short-term studies, and research is needed to identify which behaviors are risk factors for chronic diseases, which develop over longer periods of time. Therefore, the field would benefit from developing and refining methods to assess circadian disruption in population studies. This could include combining assessment methods, including activity levels, skin temperature, and light exposure, however, these measurements do need to be validated. Other methodological considerations for future research include how best to define shift work now that work shifts can be at all times of day (and not just day vs night). Finally, assessment of diet is challenging, but methods that include the timing of intake, particularly with respect to bed and wake times, are critical for this field. The Automated Self-Administered 24-hour Dietary Assessment Tool ( ASA24), for example, does allow for the timing of both meals and sleep (96). Overall, the addition of biomarkers (i.e., a biological marker for objectively evaluating the presence of circadian rhythm disruptors, e.g., melatonin or body temperature) of circadian disruption would improve the rigor and quality of the evidence for an association between these behaviors and cardiometabolic health.
More research is also needed to understand optimal meal timing and dietary patterns for circadian health and cardiometabolic health. Time-restricted eating studies that compare various eating protocols (e.g., 8:16 vs. 10:16), as well as compliance to these dietary regimens, are needed. In addition, there are limited studies on TRE timing (i.e., early vs late TRE) and the optimal feeding/fasting window for better health outcomes. In particular, these studies need to be designed to distinguish the effects of TRE from caloric restriction. Also, while many studies have focused on individuals with metabolic disorders and shown some benefits, it will be important to outline the benefits of TRE for healthy participants without overweight or obesity. Other factors such as the long-term adherence to TRE, the diet quality of people on TRE, and the effects of possibly missing out on family meals or social events, especially at night should be explored.
Ultimately, research needs to determine whether modifying any of these behaviors, such as advancing or stabilizing bedtime, can have beneficial effects. This includes not only studies to determine if such an intervention is effective, but also implementation studies to assess whether the intervention is feasible and acceptable in real-world settings. Importantly, these implementation studies need to be conducted in various subgroups of the population, including genders, ages, and race and ethnicity, since sociocultural factors strongly influence behavior. Together, these future studies would help pave the way for a better understanding of these circadian disruptors in relation to cardiometabolic diseases and identify effective intervention strategies.
Conclusion
Circadian rhythms are essential for regulating the physiological processes of the human body. In the last decade, evidence from several observational studies shows a link between circadian disruption and cardiometabolic diseases. Lifestyle and environmental factors can lead to circadian disruption, but future research should design strategies to minimize exposure to these factors or mitigate their effects when unavoidable (e.g., shift work). As an emerging field, there are many unanswered questions that we hope future studies will address. Finally, there is a need to develop effective dissemination methods to teach the public about the importance of “circadian health” to overall health and well-being.
What is already known?
Synchrony between our internal clocks and the external world is essential for optimal health.
Disruption of circadian rhythms could increase the risk of cardiometabolic diseases.
Circadian rhythms can be disrupted through environmental and behavioral factors, such as light exposure, sleep timing, and meal timing.
What does this review add?
This review summarizes results from observational studies that examined associations between potential circadian disruptors, such as shift work, chronotype, sleep timing, sleep regularity and meal timing, and cardiometabolic disease risk.
Many, but not all these observational studies have observed the detrimental effect of circadian disruption on cardiometabolic function. Additionally, an association does not infer causality.
How will results change the direction of research or the focus of clinical practice?
Future research should fill the gaps discussed in this review, including more mechanistic studies, prospective studies, and clinical trials.
Researchers working in the field are encouraged to design effective strategies that minimize exposure to circadian disruption and develop dissemination methods to teach the public about the importance of “circadian health”.
Funding:
NIH (T32HL007909, P01AG011412)
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 2010;72: 517–549. [DOI] [PubMed] [Google Scholar]
- 2.Patke A, Murphy PJ, Onat OE, Krieger AC, Ozcelik T, Campbell SS, et al. Mutation of the Human Circadian Clock Gene CRY1 in Familial Delayed Sleep Phase Disorder. Cell 2017;169: 203–215 e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allada R, Bass J. Circadian Mechanisms in Medicine. N Engl J Med 2021;384: 550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol 2019;15: 75–89. [DOI] [PubMed] [Google Scholar]
- 5.Güldür T, Otlu HG. Circadian rhythm in mammals: time to eat & time to sleep. Biol Rhythm Res 2017;48: 243–261. [Google Scholar]
- 6.Reutrakul S, Knutson KL. Consequences of Circadian Disruption on Cardiometabolic Health. Sleep Med Clin 2015;10: 455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. BMJ 2016;355: i5210. [DOI] [PubMed] [Google Scholar]
- 8.Rosa D, Terzoni S, Dellafiore F, Destrebecq A. Systematic review of shift work and nurses’ health. Occupational Medicine 2019;69: 237–243. [DOI] [PubMed] [Google Scholar]
- 9.Sun M, Feng W, Wang F, Li P, Li Z, Li M, et al. Meta-analysis on shift work and risks of specific obesity types. Obes Rev 2018;19: 28–40. [DOI] [PubMed] [Google Scholar]
- 10.Li W, Chen Z, Ruan W, Yi G, Wang D, Lu Z. A meta-analysis of cohort studies including dose-response relationship between shift work and the risk of diabetes mellitus. Eur J Epidemiol 2019;34: 1013–1024. [DOI] [PubMed] [Google Scholar]
- 11.Khosravipour M, Khanlari P, Khazaie S, Khosravipour H, Khazaie H. A systematic review and meta-analysis of the association between shift work and metabolic syndrome: The roles of sleep, gender, and type of shift work. Sleep Med Rev 2021;57: 101427. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Yu L, Gao Y, Jiang L, Yuan L, Wang P, et al. Association between shift work or long working hours with metabolic syndrome: a systematic review and dose–response meta-analysis of observational studies. Chronobiology International 2021;38: 318–333. [DOI] [PubMed] [Google Scholar]
- 13.Manohar S, Thongprayoon C, Cheungpasitporn W, Mao MA, Herrmann SM. Associations of rotational shift work and night shift status with hypertension: a systematic review and meta-analysis. J Hypertens 2017;35: 1929–1937. [DOI] [PubMed] [Google Scholar]
- 14.Gamboa Madeira S, Fernandes C, Paiva T, Santos Moreira C, Caldeira D. The Impact of Different Types of Shift Work on Blood Pressure and Hypertension: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health 2021;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritonja J, Aronson KJ, Day AG, Korsiak J, Tranmer J. Investigating cortisol production and pattern as mediators in the relationship between shift work and cardiometabolic risk. Canadian Journal of Cardiology 2018;34: 683–689. [DOI] [PubMed] [Google Scholar]
- 16.Dutheil F, Baker JS, Mermillod M, De Cesare M, Vidal A, Moustafa F, et al. Shift work, and particularly permanent night shifts, promote dyslipidaemia: A systematic review and meta-analysis. Atherosclerosis 2020;313: 156–169. [DOI] [PubMed] [Google Scholar]
- 17.Kim SW, Jang EC, Kwon SC, Han W, Kang MS, Nam YH, et al. Night shift work and inflammatory markers in male workers aged 20–39 in a display manufacturing company. Ann Occup Environ Med 2016;28: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwak HS, Park HO, Kim YO, Son JS, Kim CW, Lee JH, et al. The effect of shift work on high sensitivity C-reactive protein level among female workers. Annals of Occupational and Environmental Medicine 2019;31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson CY, Tanz LJ, Lawson CC, Schernhammer ES, Vetter C, Rich‐Edwards JW. Night shift work and cardiovascular disease biomarkers in female nurses. American journal of industrial medicine 2020;63: 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee W, Kang S-K, Choi W-J. Effect of long work hours and shift work on high-sensitivity C-reactive protein levels among Korean workers. Scandinavian Journal of Work, Environment & Health 2021;47: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloomfield D, Park A. Night time blood pressure dip. World J Cardiol 2015;7: 373–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marqueze EC, Ulhoa MA, Moreno CR. Effects of irregular-shift work and physical activity on cardiovascular risk factors in truck drivers. Rev Saude Publica 2013;47: 497–505. [DOI] [PubMed] [Google Scholar]
- 23.Souza RV, Sarmento RA, de Almeida JC, Canuto R. The effect of shift work on eating habits: a systematic review. Scand J Work Environ Health 2019;45: 7–21. [DOI] [PubMed] [Google Scholar]
- 24.Hulsegge G, Boer JM, van der Beek AJ, Verschuren WM, Sluijs I, Vermeulen R, et al. Shift workers have a similar diet quality but higher energy intake than day workers. Scandinavian journal of work, environment & health 2016: 459–468. [DOI] [PubMed] [Google Scholar]
- 25.McDowall K, Murphy E, Anderson K. The impact of shift work on sleep quality among nurses. Occup Med 2017;67: 621–625. [DOI] [PubMed] [Google Scholar]
- 26.Sondrup N, Termannsen AD, Eriksen JN, Hjorth MF, Faerch K, Klingenberg L, et al. Effects of sleep manipulation on markers of insulin sensitivity: A systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev 2022;62: 101594. [DOI] [PubMed] [Google Scholar]
- 27.Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C. Circadian typology: a comprehensive review. Chronobiol Int 2012;29: 1153–1175. [DOI] [PubMed] [Google Scholar]
- 28.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 2003;18: 80–90. [DOI] [PubMed] [Google Scholar]
- 29.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 1976;4: 97–110. [PubMed] [Google Scholar]
- 30.Santisteban JA, Brown TG, Gruber R. Association between the munich chronotype questionnaire and wrist actigraphy. Sleep Disorders 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003;26: 342–392. [DOI] [PubMed] [Google Scholar]
- 32.Knutson KL, von Schantz M. Associations between chronotype, morbidity and mortality in the UK Biobank cohort. Chronobiol Int 2018;35: 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu JH, Yun CH, Ahn JH, Suh S, Cho HJ, Lee SK, et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J Clin Endocrinol Metab 2015;100: 1494–1502. [DOI] [PubMed] [Google Scholar]
- 34.Baldanzi G, Hammar U, Fall T, Lindberg E, Lind L, Elmståhl S, et al. Evening chronotype is associated with elevated biomarkers of cardiometabolic risk in the EpiHealth cohort: a cross-sectional study. Sleep 2022;45: zsab226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merikanto I, Lahti T, Puolijoki H, Vanhala M, Peltonen M, Laatikainen T, et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol Int 2013;30: 470–477. [DOI] [PubMed] [Google Scholar]
- 36.Makarem N, Paul J, Giardina EV, Liao M, Aggarwal B. Evening chronotype is associated with poor cardiovascular health and adverse health behaviors in a diverse population of women. Chronobiol Int 2020;37: 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lotti S, Pagliai G, Colombini B, Sofi F, Dinu M. Chronotype Differences in Energy Intake, Cardiometabolic Risk Parameters, Cancer, and Depression: A Systematic Review with Meta-Analysis of Observational Studies. Adv Nutr 2022;13: 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vetter C, Devore EE, Ramin CA, Speizer FE, Willett WC, Schernhammer ES. Mismatch of Sleep and Work Timing and Risk of Type 2 Diabetes. Diabetes Care 2015;38: 1707–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan M, Sun D, Zhou T, Heianza Y, Lv J, Li L, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J 2020;41: 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nowak C, Sundstrom J, Gustafsson S, Giedraitis V, Lind L, Ingelsson E, et al. Protein Biomarkers for Insulin Resistance and Type 2 Diabetes Risk in Two Large Community Cohorts. Diabetes 2016;65: 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zuraikat FM, St-Onge M-P, Makarem N, Boege HL, Xi H, Aggarwal B. Evening chronotype is associated with poorer habitual diet in us women, with dietary energy density mediating a relation of chronotype with cardiovascular health. The Journal of nutrition 2021;151: 1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi Frisk M, Hedner J, Grote L, Ekblom O, Arvidsson D, Bergstrom G, et al. Eveningness is associated with sedentary behavior and increased 10-year risk of cardiovascular disease: the SCAPIS pilot cohort. Scientific reports 2022;12: 8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao Q, Garaulet M, Scheer F. Meal timing and obesity: interactions with macronutrient intake and chronotype. Int J Obes (Lond) 2019;43: 1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hittle BM, Caruso CC, Jones HJ, Bhattacharya A, Lambert J, Gillespie GL. Nurse Health: The Influence of Chronotype and Shift Timing. West J Nurs Res 2020;42: 1031–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loef B, Baarle Dv, Van Der Beek AJ, Beekhof PK, Van Kerkhof LW, Proper KI. The association between exposure to different aspects of shift work and metabolic risk factors in health care workers, and the role of chronotype. PLoS One 2019;14: e0211557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abbott SM, Weng J, Reid KJ, Daviglus ML, Gallo LC, Loredo JS, et al. Sleep timing, stability, and BP in the sueno ancillary study of the hispanic community health study/study of latinos. Chest 2019;155: 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knutson KL, Wu D, Patel SR, Loredo JS, Redline S, Cai J, et al. Association Between Sleep Timing, Obesity, Diabetes: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Cohort Study. Sleep 2017;40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaput JP, Dutil C, Featherstone R, Ross R, Giangregorio L, Saunders TJ, et al. Sleep timing, sleep consistency, and health in adults: a systematic review. Appl Physiol Nutr Metab 2020;45: S232–S247. [DOI] [PubMed] [Google Scholar]
- 49.Chontong S, Saetung S, Reutrakul S. Higher sleep variability is associated with poorer glycaemic control in patients with type 1 diabetes. Journal of sleep research 2016;25: 438–444. [DOI] [PubMed] [Google Scholar]
- 50.Taylor BJ, Matthews KA, Hasler BP, Roecklein KA, Kline CE, Buysse DJ, et al. Bedtime Variability and Metabolic Health in Midlife Women: The SWAN Sleep Study. Sleep 2016;39: 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim M, Sasai H, Kojima N, Kim H. Objectively measured night‐to‐night sleep variations are associated with body composition in very elderly women. Journal of sleep research 2015;24: 639–647. [DOI] [PubMed] [Google Scholar]
- 52.Rosique-Esteban N, Papandreou C, Romaguera D, Warnberg J, Corella D, Martinez-Gonzalez MA, et al. Cross-sectional associations of objectively-measured sleep characteristics with obesity and type 2 diabetes in the PREDIMED-Plus trial. Sleep 2018;41. [DOI] [PubMed] [Google Scholar]
- 53.Huang T, Mariani S, Redline S. Sleep Irregularity and Risk of Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol 2020;75: 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang T, Redline S. Cross-sectional and Prospective Associations of Actigraphy-Assessed Sleep Regularity With Metabolic Abnormalities: The Multi-Ethnic Study of Atherosclerosis. Diabetes Care 2019;42: 1422–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int 2006;23: 497–509. [DOI] [PubMed] [Google Scholar]
- 56.Koopman ADM, Rauh SP, van ‘t Riet E, Groeneveld L, van der Heijden AA, Elders PJ, et al. The Association between Social Jetlag, the Metabolic Syndrome, and Type 2 Diabetes Mellitus in the General Population: The New Hoorn Study. J Biol Rhythms 2017: 748730417713572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McMahon DM, Burch JB, Youngstedt SD, Wirth MD, Hardin JW, Hurley TG, et al. Relationships between chronotype, social jetlag, sleep, obesity and blood pressure in healthy young adults. Chronobiol Int 2019;36: 493–509. [DOI] [PubMed] [Google Scholar]
- 58.Phillips AJ, Clerx WM, O’Brien CS, Sano A, Barger LK, Picard RW, et al. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Scientific reports 2017;7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lunsford-Avery JR, Engelhard MM, Navar AM, Kollins SH. Author Correction: Validation of the Sleep Regularity Index in Older Adults and Associations with Cardiometabolic Risk. Sci Rep 2021;11: 24398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murray JM, Phillips AJ, Magee M, Sletten TL, Gordon C, Lovato N, et al. Sleep regularity is associated with sleep-wake and circadian timing, and mediates daytime function in delayed sleep-wake phase disorder. Sleep Medicine 2019;58: 93–101. [DOI] [PubMed] [Google Scholar]
- 61.Wehrens SMT, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, et al. Meal Timing Regulates the Human Circadian System. Curr Biol 2017;27: 1768–1775 e1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henry CJ, Kaur B, Quek RYC. Chrononutrition in the management of diabetes. Nutr Diabetes 2020;10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McHill AW, Phillips AJ, Czeisler CA, Keating L, Yee K, Barger LK, et al. Later circadian timing of food intake is associated with increased body fat. Am J Clin Nutr 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med 2016;375: 919–931. [DOI] [PubMed] [Google Scholar]
- 65.Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2011;35: 714–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meng H, Zhu L, Kord-Varkaneh H, Santos HO, Tinsley GM, Fu P. Effects of intermittent fasting and energy-restricted diets on lipid profile: A systematic review and meta-analysis. Nutrition 2020;77: 110801. [DOI] [PubMed] [Google Scholar]
- 67.Patterson RE, Laughlin GA, LaCroix AZ, Hartman SJ, Natarajan L, Senger CM, et al. Intermittent Fasting and Human Metabolic Health. J Acad Nutr Diet 2015;115: 1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 2014;20: 991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab 2018;27: 1212–1221 e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern Med 2020;180: 1491–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell metabolism 2015;22: 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, et al. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab 2020;32: 366–378 e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu D, Huang Y, Huang C, Yang S, Wei X, Zhang P, et al. Calorie restriction with or without time-restricted eating in weight loss. New England Journal of Medicine 2022;386: 1495–1504. [DOI] [PubMed] [Google Scholar]
- 74.Qian J, Scheer F. Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trends Endocrinol Metab 2016;27: 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xie Z, Sun Y, Ye Y, Hu D, Zhang H, He Z, et al. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nature communications 2022;13: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie Z, He Z, Ye Y, Mao Y. Effects of time-restricted feeding with different feeding windows on metabolic health: A systematic review of human studies. Nutrition 2022;102: 111764. [DOI] [PubMed] [Google Scholar]
- 77.Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol Int 2014;31: 64–71. [DOI] [PubMed] [Google Scholar]
- 78.Odegaard AO, Jacobs DR Jr., Steffen LM, Van Horn L, Ludwig DS, Pereira MA. Breakfast frequency and development of metabolic risk. Diabetes Care 2013;36: 3100–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kahleova H, Lloren JI, Mashchak A, Hill M, Fraser GE. Meal Frequency and Timing Are Associated with Changes in Body Mass Index in Adventist Health Study 2. J Nutr 2017;147: 1722–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li ZH, Xu L, Dai R, Li LJ, Wang HJ. Effects of regular breakfast habits on metabolic and cardiovascular diseases: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021;100: e27629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ha K, Song Y. Associations of meal timing and frequency with obesity and metabolic syndrome among Korean adults. Nutrients 2019;11: 2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sievert K, Hussain SM, Page MJ, Wang Y, Hughes HJ, Malek M, et al. Effect of breakfast on weight and energy intake: systematic review and meta-analysis of randomised controlled trials. BMJ 2019;364: l42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pickel L, Sung HK. Feeding Rhythms and the Circadian Regulation of Metabolism. Front Nutr 2020;7: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, et al. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation 2017;135: e96–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sakai R, Hashimoto Y, Ushigome E, Miki A, Okamura T, Matsugasumi M, et al. Late-night-dinner is associated with poor glycemic control in people with type 2 diabetes: The KAMOGAWA-DM cohort study. Endocrine journal 2017: EJ17–0414. [DOI] [PubMed] [Google Scholar]
- 86.Reid KJ, Baron KG, Zee PC. Meal timing influences daily caloric intake in healthy adults. Nutr Res 2014;34: 930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Cabo R, Mattson MP. Effects of Intermittent Fasting on Health, Aging, and Disease. N Engl J Med 2019;381: 2541–2551. [DOI] [PubMed] [Google Scholar]
- 88.Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morris CJ, Garcia JI, Myers S, Yang JN, Trienekens N, Scheer FA. The Human Circadian System Has a Dominating Role in Causing the Morning/Evening Difference in Diet-Induced Thermogenesis. Obesity (Silver Spring) 2015;23: 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Romon M, Edme JL, Boulenguez C, Lescroart JL, Frimat P. Circadian variation of diet-induced thermogenesis. Am J Clin Nutr 1993;57: 476–480. [DOI] [PubMed] [Google Scholar]
- 91.Carroll T, Raff H, Findling JW. Late-night salivary cortisol measurement in the diagnosis of Cushing’s syndrome. Nat Clin Pract Endocrinol Metab 2008;4: 344–350. [DOI] [PubMed] [Google Scholar]
- 92.Espelund U, Hansen TK, Højlund K, Beck-Nielsen H, Clausen JT, Hansen BS, et al. Fasting unmasks a strong inverse association between ghrelin and cortisol in serum: studies in obese and normal-weight subjects. The Journal of Clinical Endocrinology & Metabolism 2005;90: 741–746. [DOI] [PubMed] [Google Scholar]
- 93.Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. Journal of Clinical Endocrinology & Metabolism 2003;88: 2838–2843. [DOI] [PubMed] [Google Scholar]
- 94.Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity 2019;27: 1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab 2020;31: 92–104 e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Subar AF, Kirkpatrick SI, Mittl B, Zimmerman TP, Thompson FE, Bingley C, et al. The Automated Self-Administered 24-hour dietary recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet 2012;112: 1134–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]


