Abstract
Background:
Global indices of RV function provide limited insights into mechanisms underlying RV remodeling in pulmonary hypertension (PH). While RV myocardial architectural remodeling has been observed in PH, its effect on RV adaptation is poorly understood.
Methods:
Hemodynamic assessments were performed in two rodent models of PH. RV free wall (RVFW) myoarchitecture was quantified using generalized Q-space imaging and tractography analyses. Computational models were developed to predict RV wall strains. Data from animal studies were analyzed to determine the correlations between hemodynamic measurements, RV strains, and structural measures.
Results:
In contrast to the PH rats with severe RV maladaptation, PH rats with mild RV maladaptation showed a decrease in helical range of fiber orientation in the RVFW (139° vs 97°; p=0.029), preserved global circumferential strain (GCS), and exhibited less reduction in RV-PA coupling (0.029 vs 0.017 mm/mmHg; p=0.037). Helical range correlated positively with coupling (p=0.036) and stroke volume index (p<0.01). Coupling correlated with GCS (p<0.01) and global radial strain (GRS; p<0.01), but not global longitudinal strain (GLS).
Conclusions:
Data analysis suggests that adaptive RV architectural remodeling could improve RV function in PH. Our findings suggest the need to assess RV architecture within routine screenings of PH patients to improve our understanding of its prognostic and therapeutic significance in PH.
Keywords: Pulmonary hypertension, remodeling, right ventricle, adaptation
INTRODUCTION
The right ventricular (RV) pressure overload in patients with pulmonary hypertension (PH) triggers progressive RV remodeling at the fiber, tissue, and organ levels 1. Studies suggest that the morbidity and mortality in PH patients are not determined by the degree of pressure overload but rather by the response of the RV to the increased pressure 2–4. While global indices of RV function, such as ejection fraction, stroke volume (SV), and right ventricular-pulmonary arterial (RV-PA) coupling, have been used to assess prognosis in PH 5,6, there remains a need for novel RV remodeling-based markers that may provide insight into the transition of adaptive to maladaptive RV remodeling 7. Chronic RV remodeling events associated with PH (across various WHO groups) include hypertrophy, altered contractility, stiffening in the RV free wall (RVFW), and myoarchitecture alterations 7,8. Although RV hypertrophy and increased contractility are considered important adaptations to maintain RV function 7, prolonged pathological hypertrophy leads to maladaptive RV function and subsequently RV failure. However, the extent and the effect of RV architectural changes on RV function and its relationship with PH outcome remain poorly understood.
The normal myo-architecture in the heart consists of highly organized helices that work in unison to produce optimal blood ejection and a fair distribution of load on the fibers. Helical orientation in the myocardium plays a deterministic role in contractile and relaxation behavior of the RVFW 7. Previous studies have shown that altered fiber architecture in response to chronic pressure overload in the RV is a major factor in disturbing the contractile pattern of the RV that ultimately translates to the deterioration of RV function 9–11. Myo-architecture is a key driver of wall strains; thus, maladaptive changes in architecture may impair cardiac motion leading to RV-PA uncoupling, whereas adaptive changes can maintain proper cardiac contractile patterns and preserve RV-PA coupling.
Diffusion-weighted imaging 12 is a non-invasive modality of cardiac magnetic resonance imaging (CMR) 13–15 that identifies local fiber orientation with subvoxel angular resolution based on diffusion distribution in Q-space (represented by generalized Q-space imaging) and can be displayed as multi-voxel constructs through tractography 16,17. DWI modalities are advantageous over other techniques used for myocardial structural quantifications (e.g., polarized light microscopy and optical coherence imaging) as they allow accurate reconstructions of full three-dimensional (3-D) fiber tractography and ventricular geometry within the same scan that could be valuably used for subject-specific in-silico modeling of cardiac motion and function.
The objective of this study was to determine the effect of RV architectural remodeling on RV function and RV-PA coupling. Using observations from two PH rat models with different PH severity and RV function 18,19 coupled with in-silico modeling, we determined the effects of changes in helical range of myofibers on global cardiac strains and their associations with RV-PA coupling. Our findings suggest that RV architectural remodeling plays an important role in the adaptation of RV transverse motion and RV-PA coupling in PH.
METHODS
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
ETHICAL APPROVAL.
All animal protocols were approved by the Institutional Animal Care and Use Committee at the Providence VA Medical Center and conformed with the Health Research Extension Act, US Public Health Service, and US National Institutes of Health policy. The use of human data was approved by the Providence VA Medical Center Institutional Review Board.
SUGEN-HYPOXIA (SUHX) MODELS OF PH.
Male adult Sprague-Dawley (SD) rats (176–200 g, strain: 001) and Fischer (CDF) 344 rats (150–175 g, strain: 002) were purchased from Charles River Laboratory (Wilmington, MA). PH was induced as previously described 20 by a single subcutaneous injection of a vascular endothelial growth factor inhibitor (SU5416, 20 mg/kg body weight; APExBIO, Houston, TX) that was dissolved in a diluent containing 0.5% carboxymethylcellulose, 0.9% NaCl, 0.4% Polysorbate 80, and 0.9% benzyl alcohol, followed by 3 weeks of normobaric hypoxia exposure (10% FiO2; A-Chamber Animal Cage Enclosure with ProOx 360 High Infusion Rate O2 Controller, BioSpherix, Ltd., Parish, NY) and subsequently housing them at normoxic conditions for one additional week. The control group received a diluent injection and was housed in normoxic conditions until the end of the study. At the end of week 4, the animals underwent a transthoracic echocardiogram followed by invasive hemodynamics and were then euthanized under isoflurane by exsanguination for tissue collection.
IN-VIVO FUNCTIONAL ASSESSMENTS.
Echocardiographic measurement.
Animals (SD: n=11 Control, 16 PH; CDF: n=12 Control, 16 PH) were anesthetized with continuous isoflurane inhalation (1.5–2%) and transthoracic echocardiography was performed as previously described 21. A 9–18 MHz ultra-high frequency MS250 linear array rodent transducer was used on the Vevo 2100 (FUJIFILM VisualSonics, Toronto, ON, Canada) to obtain recordings. Two-dimensional, Doppler, and M-mode recordings were obtained to measure left ventricle (LV) fractional shortening and LV and RV dimensions. The pulsed-wave Doppler recording at the RV overflow tract was used to measure pulmonary acceleration time (PAT). Tricuspid annular plane systolic excursion (TAPSE) was measured by use of M-mode across the tricuspid valve annulus at the RVFW. TAPSE was determined by measuring the excursion of the tricuspid annulus from its highest position to the peak descent during ventricular systole. Tissue Doppler was used to measure the early diastolic velocity of the septum (at mitral annulus) and RV lateral wall (at tricuspid annulus). Global RV longitudinal strain was calculated on contiguous long-axis cine images using feature tracking software (Vevo LAB, FUJIFILM VisualSonics), although strain measurements proved unreliable in a small number of rats due to difficult views and were excluded from strain analysis.
Invasive hemodynamic measurements.
Using an open-chest technique and a high-fidelity Millar 2.0F catheter (SPR-869), RV end-diastolic pressure (RVEDP) and RV systolic pressure (RVSP) were measured in a cohort of rats (SD: n=6 Control, 10 PH; CDF: n=10 Control, 9 PH) under isoflurane anesthesia (1.5–2%) that were not assigned for ex-vivo DWI. Steady-state recordings were acquired for at least 30 seconds with a sampling rate of 2 kHz using PowerLab Data Acquisition system together with LabChart 8 software (ADInstruments, Colorado Springs, CO). The ratio TAPSE/RVSP was used as the measure of RV-PA coupling. For the cohort of rats that were assigned for ex-vivo DWI without RV catheterization, TAPSE/RVSP was calculated using an estimated RVSP from a linear regression between RVSP and PAT derived from echocardiography.
EX-VIVO FUNCTIONAL ASSESSMENTS.
Diffusion-weighted imaging.
DWI measures delimited molecular diffusivity of protons within the tissue resulting in voxel-based probability diffusion tensor (PDF) of fiber orientation based on principals of Q-space depiction (known as generalized Q-space imaging) representing regional fiber architecture. Separate groups of rats were randomly selected for the DWI study (SD: n=5 Control, 7 PH; CDF: n=4 Control, 8 PH). The whole heart was flash-frozen in liquid nitrogen after the harvest and scanned by DWI using multiple gradient pulses with varying orientations and diffusion sensitivities in 3D space 17. Scans were conducted at a 7T Bruker Biospec (Bruker Corp, Billerica, MA) using a pulse sequence with a high-angular resolution magnet (512 gradient directions, b=750 s/mm2, 8 k-space segments, scan time≈ 8hrs). All scans were performed at an isotropic 175 μm resolution. An accurate reconstruction of both heart anatomy and the regional myocardial architecture was obtained. Patterns of inter-voxel coherence (tractography) were reconstructed, and transmural helix angle distribution were assessed in the entire RVFW region. Myofiber orientations were obtained through post-processing the local diffusion PDFs using DSI Studio 22. Architectural measurements were found to be unreliable in a small number of rats due to distortion of the hearts and excluded from helical angle analysis.
IN-SILICO ASSESSMENTS.
Computational rodent heart model.
Rat-specific finite-element (FE) biventricular heart models were developed from DWI scans for control and PH hearts that were suitable for reconstruction (SD: n=5 Control, 5 PH; CDF: n=4 Control, 4 PH). The general steps to develop these models have been described in detail previously 23. Briefly, the development consisted of four main steps: (i) reconstruction and meshing of the biventricular geometry from DWI scan, (ii) registration of the regional architecture to the meshed geometry, (iii) incorporation of the passive and active behaviors to simulate full cardiac cycle, and (iv) using rat-specific pressure-volume (P-V) measurement as a target to obtain regional cardiac strains through solving an inverse problem 24.
Geometry reconstruction and architecture registration.
The segmentation and reconstruction of the 3-D full heart geometry from DWI scans were performed using Mimics (Materialise, Leuven, Belgium). The geometries for both control and PH hearts were truncated below the valve plane and meshed using quadratic tetrahedral elements. LV and RV regions in the FE models were separated to allow different stiffness for each region. The LV region consisted of the LV free wall (LVFW) and the interventricular septum, while the RV included the RVFW only. The myofiber architecture, reconstructed from regional directional diffusivity at each voxel, was registered to the corresponding ventricular mesh from the same heart. The RVFW region was divided into three layers ranging from endocardium to epicardium by splitting the RVFW thickness equally. Average fiber orientation in each layer was computed by taking the average of the principal direction from voxels in a central region of the RVFW, measuring approximately 4 mm x 4 mm. Transmural helical range was calculated as the difference between the average fiber angles at the endocardium and at the epicardium. Regional helical ranges were calculated as the difference between the average fiber angles in (i) endocardium and midwall and (ii) midwall and epicardium.
Inverse problem and RV strain calculations.
Wall stiffness and contractile tension were estimated in the LVFW and RVFW for control and PH rats through reproducing the P-V measurements 23. The fitted model was used to assess the 3-D strain distributions in the RVFW. Strain tensors were projected onto longitudinal, circumferential, and radial directions. Global strains were calculated by averaging the strain over a central area of the free wall with an area of approximately 10 mm2.
STATISTICAL ANALYSIS.
Animal data are presented as mean ± SEM. Comparison between SD and CDF groups was accomplished by using two-way ANOVA followed by a Tukey post hoc test and unpaired 2-tailed Student’s t-test when appropriate. Regression analysis was performed to assess the correlation between strain measures and hemodynamic and architectural parameters. Association between variables was measured with bivariate correlation, and trend lines were least-square fittings of straight-line models. For all analyses, a p value of < 0.05 was considered statistically significant. All statistical analyses were performed using Prism 9 software (GraphPad Software, San Diego, CA, USA) and MATLAB R2019b (Mathworks Inc., Natick, MA, USA). Figure legends specify the tests used for each experiment.
RESULTS
PH SEVERITY AND RV FUNCTION IN TWO PH MODELS.
PH development was confirmed in both SD-PH and CDF-PH models by echocardiography and invasive hemodynamic measurements. PH rats in both models showed a significant increase in RVSP (Figure 1A), RVEDP (Figure 1B), and RVFW thickness (as a measure of RV hypertrophy, Figure 1C) in comparison to their respective controls. RV function and ventricular-arterial coupling were also reduced in both PH rat models, indicated by a decrease in TAPSE (Figure 1D) and TAPSE/RVSP (Figure 1E; Figure S1 shows the evaluation of PAT-RVSP regression to calculate the coupling parameter). In addition, key differences in hemodynamic and coupling measurements were noted between the two PH rat models. RV SV was significantly decreased in CDF-PH rats (Figure 1F). In comparison to SD-PH rats, CDF-PH rats showed a greater reduction in TAPSE/RVSP (Figure 1E) and a higher RVSP (Figure 1A). The restrictive filling in the RV, characterized by increases in E/A ratio, was evident in CDF-PH rats (Figure 1G). Our findings thus demonstrate that CDF-PH model has a more severe hemodynamic profile in comparison to SD-PH model, similar to the previous reports 18,25. Together, these two models provided a contrastive range of RV function and were used for further analyses in this study.
Fig. 1. Characterization of PH Models.
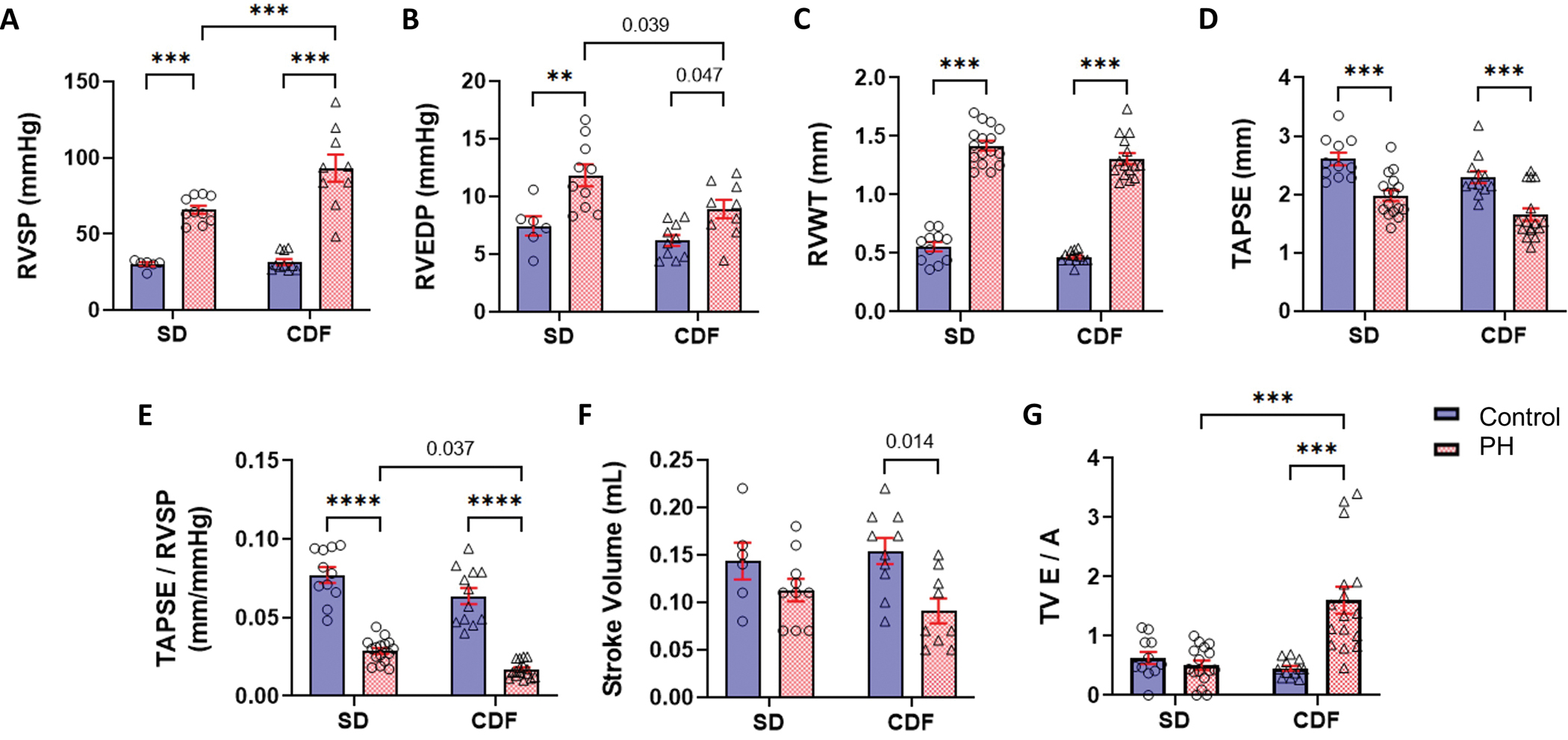
PH was confirmed in both models by hemodynamic (A-B) and echocardiographic measurements, as PH rats showed higher RV wall thickness (C), and RV systolic function measured by TAPSE (D) and TAPSE/RVSP (E) were significantly reduced in PH rats. Both PH models showed reductions in stroke volume (F) which were significant only for CFD-PH rats. In addition, the CDF-PH model had lower TAPSE/RVSP (E) and more severe RV dysfunction (G) in comparison to SD-PH rats. CDF: Fischer rat model; E/A: ratio of the early (E) to late (A) RV filling velocities; PH: pulmonary hypertension; RV: right ventricle; RVEDP: right ventricle end-diastolic pressure; RVSP: right ventricle systolic pressure; RVWT: right ventricle wall thickness; SD: Sprague-Dawley rat model; TAPSE: tricuspid annular plane systolic excursion; TAPSE/RVSP: measure of RV-pulmonary artery coupling; TV: tricuspid valve. **p<0.01; ***p<0.001. SD: n=6–11 Control, 10–16 PH; CDF: n=10–12 Control, 9–16 PH. Two-way ANOVA followed by Tukey post hoc test.
MYOARCHITECTURAL REMODELING ASSESSMENT USING DWI.
The transmural variation of the average fiber direction across the RVFW thickness with respect to the circumferential-longitudinal coordinate system showed a significant difference in helicity between the two PH models (Figure 2A–E). The helical range was preserved in the CDF-PH model, while it decreased significantly in the SD-PH model (Figure 2C–E). Regional analysis of helical range indicated that the majority of architectural remodeling took place in the RVFW endocardium-midwall region (Figure 2F–G; 3-D visualization examples of helical range are shown in Figures S2 and S3), with fibers in this region re-orienting towards the circumferential direction. No significant change in helical slope was noted in the CDF-PH model, whereas a significant decrease was noted in the SD-PH group (Figure 2H). The overall range and trend of the transmural variation were consistent with previous histological studies in rodents 11,24.
Fig 2: Architectural Remodeling in Right Ventricle.
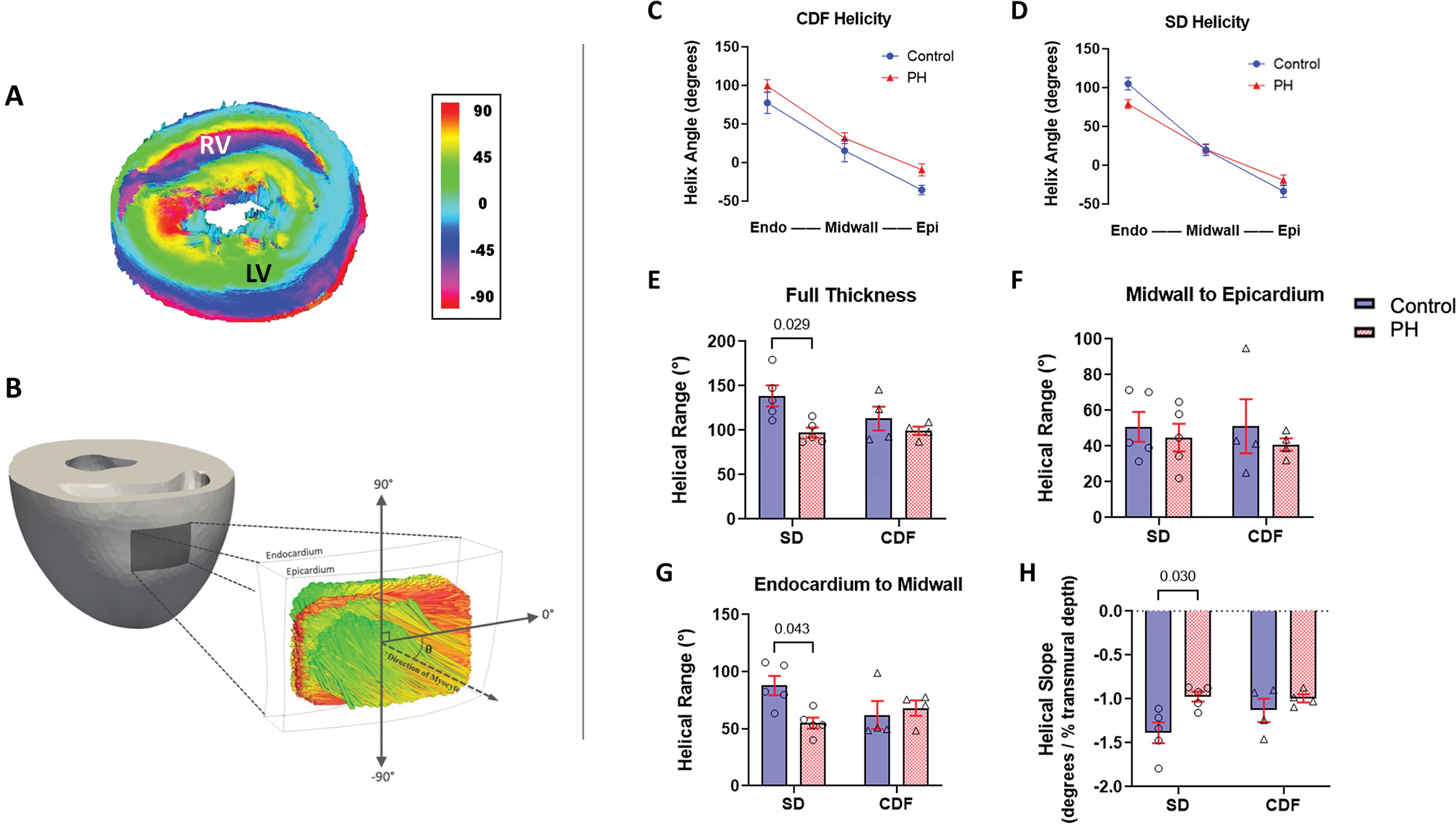
(A) DWI visualization example of a SD-control whole heart. Schematic showing the region of the heart from which DWI fiber data was gathered and the convention to calculate helical angle. (C-D) Myocardial fiber helix angles are shown at the endocardium, midwall, and epicardium. (E-G) Mean helical range were calculated across the full and partial transmural RVFW thickness. (H) Mean helical slope. CDF: Fischer rat model; DWI: Diffusion-weighted Imaging; LV: left ventricle; PH: pulmonary hypertension; RV: right ventricle; RVFW: right ventricle free wall; SD: Sprague-Dawley rat model. SD: n=5 Control, 5 PH; CDF: n=4 Control, 4 PH. Two-way ANOVA followed by Tukey post hoc test.
ARCHITECTURAL REMODELING INFLUENCES STRAIN AND RV-PA COUPLING.
Rodent-specific FE models were used to simulate global circumferential, longitudinal, and radial strains (denoted by GCS, GLS, and GRS, respectively). Representative simulated regional strains are shown in Figures S4 and S5. In comparison to respective controls, GRS and GLS were significantly decreased in both CDF-PH and SD-PH animals (Figure 3A, B); while GCS was reduced in CDF-PH group but not in the SD-PH animals (Figure 3C). In addition, good agreement was found between the simulated GLS and the corresponding longitudinal strain from echocardiography, with no significant difference between the measured GLS and simulated GLS (Figure 3D; Figure S6 shows the quantitative comparisons between measured and simulated GLS).
Fig 3: Right Ventricular Strains in Pulmonary Hypertension.
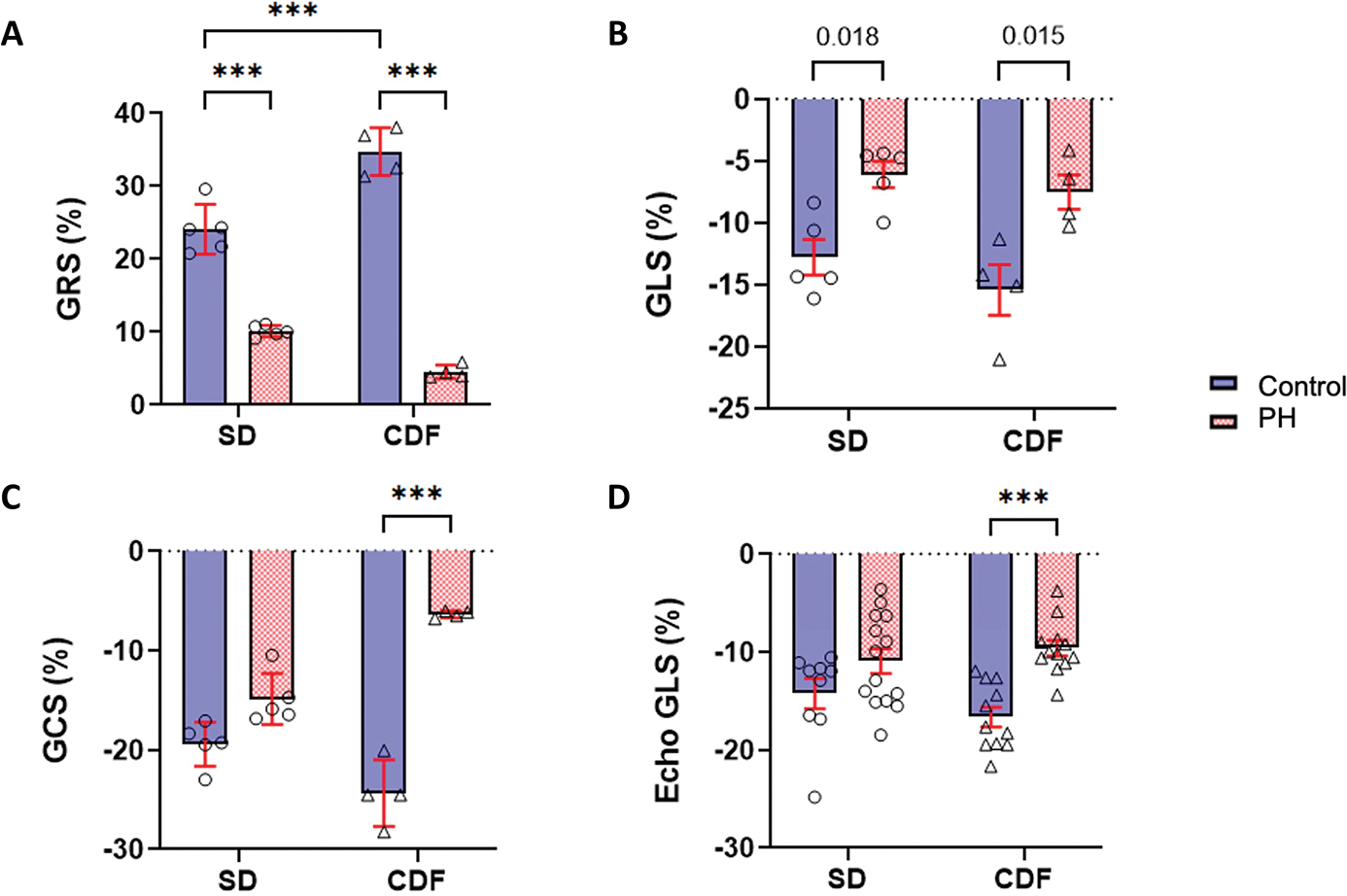
(A-C) Mean and SD of simulated global circumferential (GCS), longitudinal (GLS), and radial (GRS) RVFW strains from finite-element modeling. (D) Mean GLS calculated from echocardiography. CDF: Fischer rat model; PH: pulmonary hypertension; RVFW: right ventricle free wall; SD: Sprague-Dawley rat model. ***p<0.001. SD: n=5–9 Control, 5–14 PH; CDF: n=4–11 Control, 4–12 PH. Two-way ANOVA followed by Tukey post hoc test.
Next, we performed correlation analyses to ascertain if the helical range relates to hemodynamic measurements, such as RV-PA coupling, and global strains. All three strain measures, GRS, GCS, and GLS, were significantly correlated with the helical range (Figure 4A). Our analyses also showed that GCS and GRS significantly correlate to RV-PA coupling while GLS did not (Figure 4B). In contrast to the GCS data for CDF-Control and CDF-PH groups that tended to cluster at lower and upper bounds of the strain, respectively, the SD animals tended to cluster in a middle range of strain while showing stronger variations in the helical range (Figure 4A). These distinct patterns of the GCS-helical range highlight the impact of alterations in the helical range on preserving the transverse motion of the RV.
Fig 4: Correlation Between Strains, Myoarchitecture, and RV-PA Coupling.
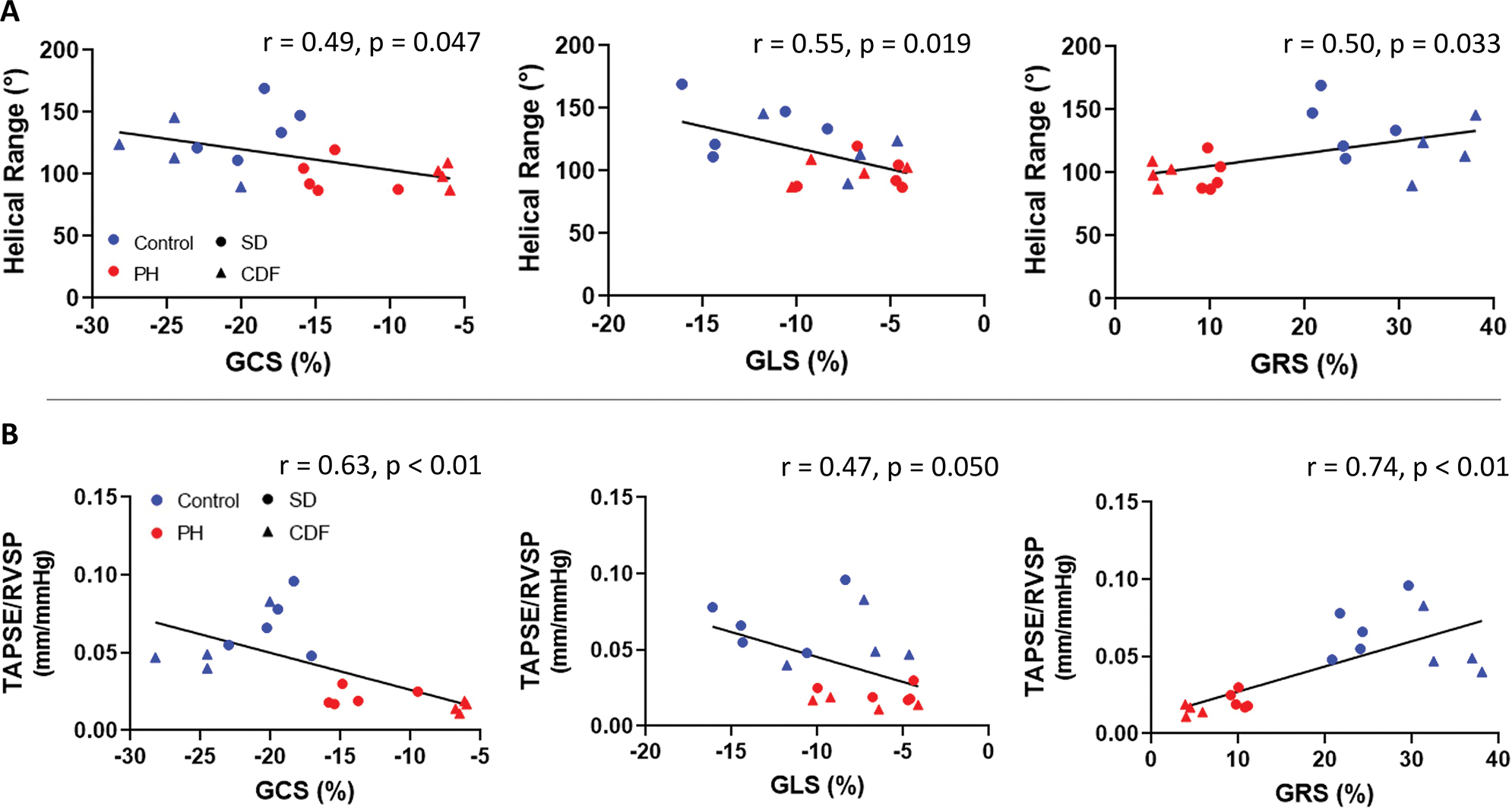
(A) RV global circumferential (GCS), longitudinal (GLS), and radial (GRS) strains were associated with helical range. (B) RV circumferential and radial strains were associated with RV-PA coupling, measured by TAPSE/RVSP, while longitudinal strain was not. CDF: Fischer rat model; PA: pulmonary artery; PH: pulmonary hypertension; RV: right ventricle; RVSP: right ventricle systolic pressure; SD: Sprague-Dawley rat model; TAPSE: tricuspid annular plane systolic excursion; TAPSE/RVSP: measure of RV-PA coupling. SD: n=5 Control, 5 PH; CDF: n=4 Control, 4 PH. Linear regression model.
RVFW helical range was also correlated with RV cardiac index and SV index (Figure 5A, B). In addition, TAPSE/RVSP was negatively correlated with helical range (Figure 5C), suggesting that preservation of RV-PA coupling may result from decreased helical range. RV wall thickness (RVWT) also exhibited a significant correlation with helical ranges (Figure 5D), suggesting that RVFW hypertrophy could facilitate architectural remodeling.
Fig 5: Correlation Between Myoarchitecture and RV Function.
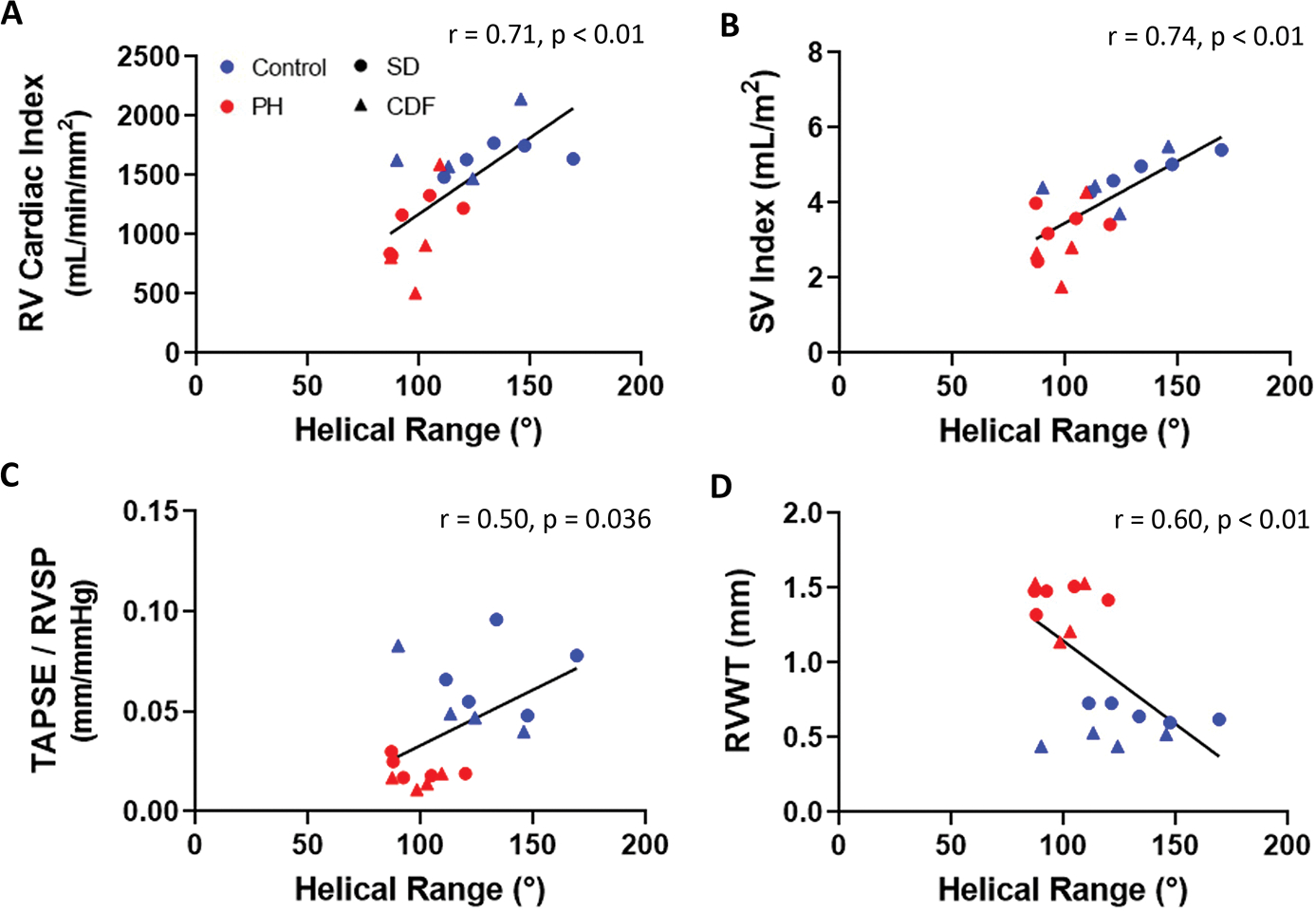
Helical range was associated with key RV functional metrics (A-B) and coupling (C), but not with RV wall thickness (D). CDF: Fischer rat model; PH: pulmonary hypertension; RV: right ventricle; RVSP: right ventricle systolic pressure; RVWT: right ventricle wall thickness; SD: Sprague-Dawley rat model; SV: stroke volume; TAPSE: tricuspid annular valve plane systolic excursion; TAPSE/RVSP: measure of RV-pulmonary artery coupling. SD: n=5 Control, 5 PH; CDF: n=4 Control, 4 PH. Linear regression model.
DISCUSSION
RV function is a major determinant of survival in PH patients 26. However, uncertainty regarding how myocardial structure influences organ performance persists. The data presented here show for the first time a significant association between image-derived architectural remodeling and key hemodynamic and coupling parameters in the RV in PH. RV-PA coupling, characterized by TAPSE/RVSP 27, is a notable global determinant of RV adaptation to PH and was shown to be significantly correlated with the helical range. Myoarchitecture is a key driver of wall strains and a critical input for in-silico modeling of RV motion. In turn, the range and pattern of RV wall strains are expected to govern RV function and directly influence RV-PA coupling. The helical range was reduced in SD-PH rats with an adaptive remodeling resulting in the preservation of GCS and, in turn, leading to a smaller reduction in the coupling, whereas the lack of adaptive changes in helical range in the CDF-PH animals did not restore the diminishing GCS leading to a significant reduction in coupling and severe maladaptation. In particular, CDF-PH rats showed no significant change in helical slope and, instead, exhibited a nearly uniform shift in fibers orientation towards the longitudinal direction while preserving the helical range. This event appears to exacerbate the maladaptation in the CDF-PH animals, leading to further impairment of transverse motion and RV-PA uncoupling. Expectedly, helical range showed positive correlations with RV functional parameters of cardiac index and SV index as well. Overall, our findings suggest that architectural remodeling in the RVFW is a key structural change behind the adaptations of circumferential and longitudinal RVFW contractile patterns in PH at the tissue level that could translates to the curtailment or advancement of RV-PA uncoupling at the organ level (Figure 6).
Fig 6: Architectural remodeling influences RV-PA coupling.
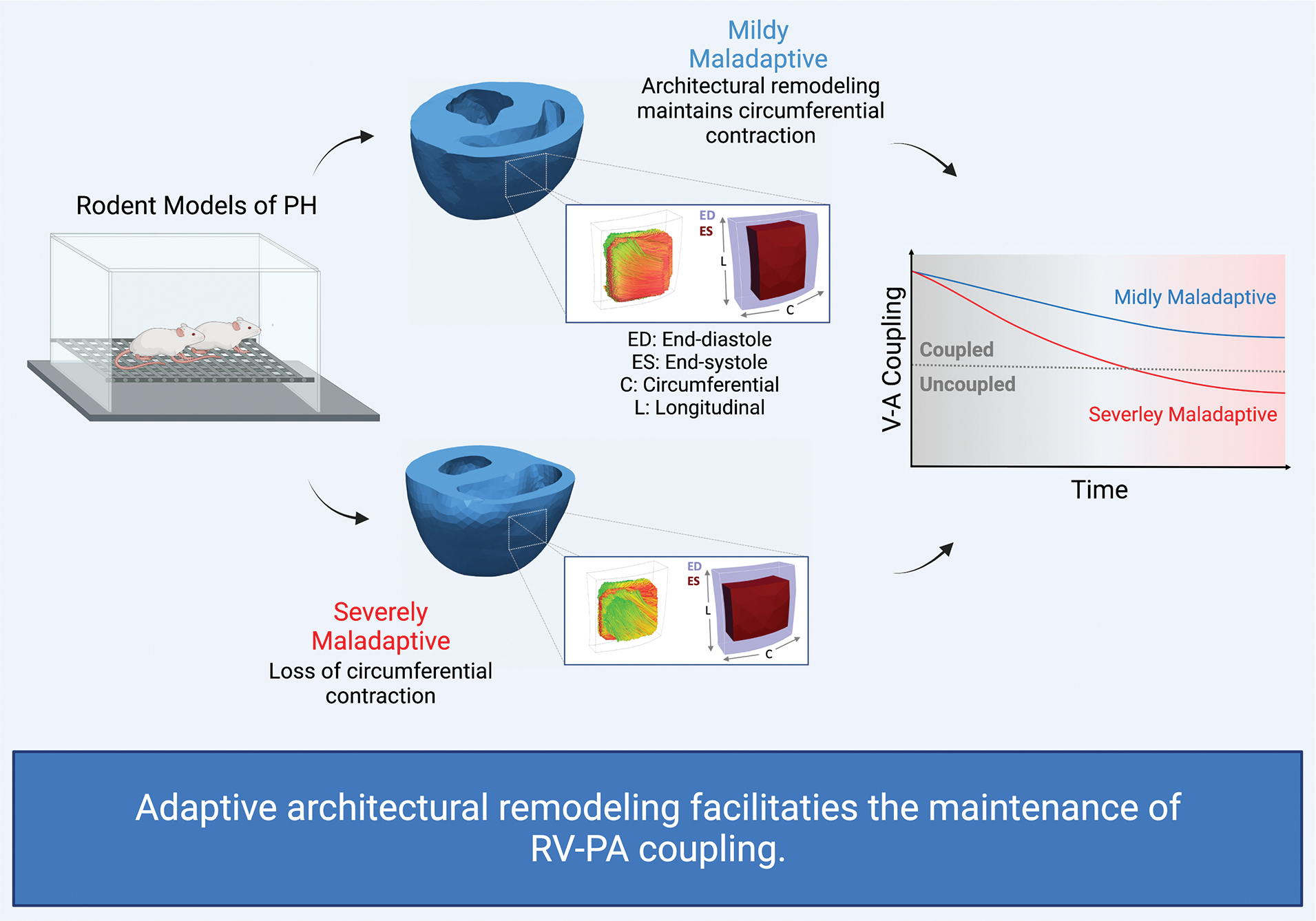
Adaptive architectural remodeling contributes to preserving transverse (circumferential) contraction that enhances active elastance of the RV and facilitates the maintenance of RV-PA coupling. Theoretically, architectural remodeling can improve RV contractility independent of changes in intrinsic active properties of myofibers. PA: pulmonary artery; PH: pulmonary hypertension; RV: right ventricle; V-A: ventricle-arterial.
Impairments in radial, circumferential, and longitudinal motions in the RV can separately undermine RV function; however, the adaptation of each motion and its significance to RV function in PH remain unclear. Longitudinal RVFW motion assessed by TAPSE, RV S’, and global longitudinal strain has been traditionally considered as the primary index of RV motion and is more easily quantified in the RV via echocardiography and CMR. While longitudinal RVFW strains have been shown to correlate with functional indices in PH 5,28,29, we found strong correlation between transverse wall motion (characterized by circumferential and radial strains) in PH rodents. Previous studies noted the importance of RV transverse motion in RV ejection fraction 30–32; however, the mechanistic relationship of this motion with RV function in PH is yet to be understood 33. In this study, we found that GCS and GRS have a better correlation with the RV-PA coupling parameter than GLS. Preservation of GCS was likely mediated through adaptive architectural remodeling differentiating between mildly maladaptive and severely maladaptive rodent groups. Previous clinical studies support the findings from our animal work on the incremental value of GCS 33. Larger human studies measuring RV strains via CMR, echocardiography, or computed tomography and RVFW architecture via DWI sequences are warranted to fully establish the links among RVFW architectural remodeling, impairments in RV transverse and longitudinal motions, and key functional adaptations including changes in RV-PA coupling. Should transverse RVFW motion be found to play a determining role in RV function in PH, GCS and GRS could prove useful as structurally informed stratification tools in PH patients.
In addition to architectural remodeling, the RV attempts to cope with pressure overload via myocardial stiffening and hypertrophy 2,34. However, the distinction between adaptive and maladaptive stiffening as well as how intrinsic stiffening, volumetric hypertrophy, and architectural remodeling influence each other 9 remain unclear. In this study, we observed distinct adaptive and maladaptive events in SD-PH and CDF-PH animals. Although significant RVFW hypertrophy was observed in both SD-PH and CDF-PH animals, RV diastolic filling stiffness (indicated by tricuspid valve E/A) was nearly preserved in SD-PH rats but significantly increased in CDF-PH. This observation suggests that the absence of adaptive changes in helical range in CDF-PH may be linked to excessive myocardial stiffening in this group. It remains to be determined if increased stiffness results in lack of fiber remodeling and preserved helical range and worse uncoupling. Our findings underscore the importance of adopting tissue-level RVFW remodeling markers as precursors of RV dysfunction for improved, mechanistic, and early prognosis of PH.
The current practice to evaluate RV function in PH includes RHC and standard CMR using FLASH sequences. However, these measurements reflect only the “net” effects of underlying remodeling events providing limited insights into the contribution of each event, offer inadequate sensitivity to underlying mechanisms, and become evident only after substantial progression of RV remodeling. Our study with preclinical animal models provides mechanistic insights into the significance of architectural remodeling as a key adaptive mechanism to maintain RV transverse motion and limit impairments in RV function in PH. Our animal study highlights the need to investigate the extent, contribution, and adaptiveness of architectural remodeling events in PH patients in future studies. As DWI is becoming more feasible in humans, this study underscores the need to develop DWI sequences tailored to the RV anatomy and motion, and to perform large-scale DWI in human PH population to determine the contribution of RV architectural remodeling in the PH progression and its prognostic significance. Finally, mechanisms of architectural remodeling remain unknown. Although a portion of this remodeling is expected to be merely due to fibers conformation to geometric adaptation, regionally heterogeneous elevated wall stress is expected to lead to anisotropic hypertrophy in the RV that could contribute to architectural remodeling. These mechanisms remain to be identified.
Myocardial strains were estimated via animal-specific in-silico models that were enforced to match hemodynamic measurements for each animal. Although we validated the longitudinal strains against the strains derived from B-mode echocardiographic acquisitions, in-vivo strains measured by CMR can serve as additional validation of our in-silico strain estimates in future studies. Although TAPSE/RVSP and SV/ESV as measures of RV-PA coupling are not considered as accurate as multi-beat RV pressure-volume analysis, the use of these measures and their strong correlation with multi-beat-derived coupling parameters have been extensively reported in prior imaging studies. Lastly, validation of these preclinical findings regarding the importance of transverse motion and myoarchitecture in RV remodeling in well-characterized cohort of PH patients is needed.
Supplementary Material
CLINICAL PERSPECTIVE.
What’s new?
We use preclinical models of pulmonary hypertension coupled with state-of-the-art diffusion-weighted imaging and in-silico modeling to demonstrate that reductions in the helical range of myofibers in the right ventricular free wall are associated with the preservation of circumferential strain and less reduction in right ventricular-pulmonary arterial coupling. Resulting data and analysis show for the first time a significant association between image-derived architectural remodeling and key hemodynamic and coupling parameters in the right ventricle and pulmonary hypertension.
What are the clinical implications?
Architectural remodeling of the right ventricle remains a central, yet overlooked, remodeling event. Our analysis indicates underlying architectural remodeling is a principal driver of right ventricular adaptation to pressure overload. These findings point to a pressing need to develop diffusion-weighted imaging sequences tailored to right ventricular anatomy and motion to assess myofiber architecture in the right ventricle in pulmonary hypertension patients as the existing diffusion-weighted imaging sequence primarily focuses on the left ventricle.
Acknowledgments
Some of the material of this work was supported with resources and the use of facilities at the VA Providence Healthcare System and the CPVB COBRE (under NIGMS grant P20GM103652). The views expressed in this article are those of the authors and do not reflect the position nor policy of the Department of Veterans Affairs and the NIH.
Sources of Funding
National Institutes of Health grant R00HL138288 (RA)
National Heart, Lung, and Blood Institute grant R01HL128661 (GC)
Veterans Affairs Clinical Science Research and Development grant I01CX001892 (GC)
National Heart, Lung, and Blood Institute grant R01HL148727 (GC)
National Heart, Lung, and Blood Institute grant RO1/R56 HL139680 (RJG)
National Institute of General Medical Sciences/National Institutes of Health grant 5P20GM103652 (PZ)
NON-STANDARD ABREVIATIONS AND ACRONYMS
- CDF
Fischer
- CMR
Cardiac magnetic resonance imaging
- DWI
Diffusion-weighted imaging
- FE
Finite-element
- LV
Left ventricle
- LVFW
Left ventricle free wall
- PH
Pulmonary hypertension
- P-V
Pressure-volume
- RV
Right ventricle
- RVEDP
Right ventricle end-diastolic pressure
- RVEDV
Right ventricle end-diastolic volume
- RVFW
Right ventricle free wall
- RHC
Right heart catheterization
- RV-PA
Right ventricle – pulmonary artery
- RVSP
Right ventricle systolic pressure
- RVWT
Right ventricle wall thickness
- SD
Sprague-Dawley
- SV
Stroke volume
- TAPSE
Tricuspid annular plane systolic excursion
Footnotes
Disclosures
Authors declare that they have no conflicts of interest.
Supplemental Materials:
Figures S1 – S6
References
- 1.Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. Journal of the American College of Cardiology. 2013;62:D22–D33. [DOI] [PubMed] [Google Scholar]
- 2.Bogaard HJ, Abe K, Noordegraaf AV, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 2009;135:794–804. [DOI] [PubMed] [Google Scholar]
- 3.Dong Y, Pan Z, Wang D, Lv J, Fang J, Xu R, Ding J, Cui X, Xie X, Wang X. Prognostic Value of Cardiac Magnetic Resonance–Derived Right Ventricular Remodeling Parameters in Pulmonary Hypertension: A Systematic Review and Meta-Analysis. Circulation: Cardiovascular Imaging. 2020;13:e010568. [DOI] [PubMed] [Google Scholar]
- 4.Vélez-Rendón D, Zhang X, Gerringer J, Valdez-Jasso D. Compensated right ventricular function of the onset of pulmonary hypertension in a rat model depends on chamber remodeling and contractile augmentation. Pulmonary circulation. 2018;8:2045894018800439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tello K, Dalmer A, Vanderpool R, Ghofrani HA, Naeije R, Roller F, Seeger W, Wilhelm J, Gall H, Richter MJ. Cardiac magnetic resonance imaging-based right ventricular strain analysis for assessment of coupling and diastolic function in pulmonary hypertension. JACC: Cardiovascular Imaging. 2019;12:2155–2164. [DOI] [PubMed] [Google Scholar]
- 6.Lahm T, Douglas IS, Archer SL, Bogaard HJ, Chesler NC, Haddad F, Hemnes AR, Kawut SM, Kline JA, Kolb TM. Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward. An Official American Thoracic Society Research Statement. American journal of respiratory and critical care medicine. 2018;198:e15–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharifi Kia D, Kim K, Simon MA. Current Understanding of the Right Ventricle Structure and Function in Pulmonary Arterial Hypertension. Frontiers in physiology. 2021;12:752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill MR, Simon MA, Valdez-Jasso D, Zhang W, Champion HC, Sacks MS. Structural and mechanical adaptations of right ventricle free wall myocardium to pressure overload. Ann Biomed Eng. 2014;42:2451–2465. doi: 10.1007/s10439-014-1096-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avazmohammadi R, Mendiola EA, Li DS, Vanderslice P, Dixon RAF, Sacks MS. Interactions Between Structural Remodeling and Hypertrophy in the Right Ventricle in Response to Pulmonary Arterial Hypertension. Journal of Biomechanical Engineering. 2019;141:1–13. doi: 10.1115/1.4044174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharifi Kia D, Benza E, Bachman TN, Tushak C, Kim K, Simon MA. Angiotensin Receptor-Neprilysin Inhibition Attenuates Right Ventricular Remodeling in Pulmonary Hypertension. Journal of the American Heart Association. 2020;9:e015708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avazmohammadi R, Hill M, Simon M, Sacks M. Transmural remodeling of right ventricular myocardium in response to pulmonary arterial hypertension. APL Bioengineering. 2017;1:016105. doi: 10.1063/1.5011639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spinner GR, Stoeck CT, Mathez L, von Deuster C, Federau C, Kozerke S. On probing intravoxel incoherent motion in the heart-spin-echo versus stimulated-echo DWI. Magnetic resonance in medicine. 2019;82:1150–1163. [DOI] [PubMed] [Google Scholar]
- 13.Mekkaoui C, Sosnovik DE. Diffusion magnetic resonance imaging tractography of the heart. In: Cardiac Mapping. 2019:1113–1123. [Google Scholar]
- 14.Sosnovik DE. Magnetic Resonance-Based Characterization of Myocardial Architecture. Heart Failure Clinics. 2021;17:85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mekkaoui C, Reese TG, Jackowski MP, Cauley SF, Setsompop K, Bhat H, Sosnovik DE. Diffusion tractography of the entire left ventricle by using free-breathing accelerated simultaneous multisection imaging. Radiology. 2017;282:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor EN, Hoffman MP, Aninwene II GE, Gilbert RJ. Patterns of intersecting fiber arrays revealed in whole muscle with generalized Q-space imaging. Biophysical journal. 2015;108:2740–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor EN, Hoffman MP, Barefield DY, Aninwene GE, Abrishamchi AD, Lynch IV TL, Govindan S, Osinska H, Robbins J, Sadayappan S. Alterations in multi-scale cardiac architecture in association with phosphorylation of myosin binding protein-C. Journal of the American Heart Association. 2016;5:e002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang B, Deng Y, Suen C, Taha M, Chaudhary KR, Courtman DW, Stewart DJ. Marked strain-specific differences in the SU5416 rat model of severe pulmonary arterial hypertension. American journal of respiratory cell and molecular biology. 2016;54:461–468. [DOI] [PubMed] [Google Scholar]
- 19.Suen CM, Chaudhary KR, Deng Y, Jiang B, Stewart DJ. Fischer rats exhibit maladaptive structural and molecular right ventricular remodelling in severe pulmonary hypertension: a genetically prone model for right heart failure. Cardiovascular research. 2019;115:788–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clements RT, Vang A, Fernandez-Nicolas A, Kue NR, Mancini TJ, Morrison AR, Mallem K, McCullough DJ, Choudhary G. Treatment of Pulmonary Hypertension With Angiotensin II Receptor Blocker and Neprilysin Inhibitor Sacubitril/Valsartan. Circ Heart Fail. 2019;12:e005819. doi: 10.1161/CIRCHEARTFAILURE.119.005819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vang A, Bos DdSG, Fernandez-Nicolas A, Zhang P, Morrison AR, Mancini TJ, Clements RT, Polina I, Cypress MW, Jhun BS. ⍺7 nicotinic acetylcholine receptor mediates right ventricular fibrosis and diastolic dysfunction in pulmonary hypertension. JCI insight. 2021;6:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeh F-C, Wedeen VJ, Tseng W-YI. Generalized q-Sampling Imaging. IEEE Transactions on Medical Imaging. 2010;29:1626–1635. doi: 10.1109/TMI.2010.2045126 [DOI] [PubMed] [Google Scholar]
- 23.Avazmohammadi R, Mendiola EA, Soares JS, Li DS, Chen Z, Merchant S, Hsu EW, Vanderslice P, Dixon RAF, Sacks MS. A Computational Cardiac Model for the Adaptation to Pulmonary Arterial Hypertension in the Rat. Ann Biomed Eng. 2019;47:138–153. doi: 10.1007/s10439-018-02130-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avazmohammadi R, Li DS, Leahy T, Shih E, Soares JS, Gorman JH, Gorman RC, Sacks MS. An integrated inverse model-experimental approach to determine soft tissue three-dimensional constitutive parameters: application to post-infarcted myocardium. Biomech Model Mechanobiol. 2018;17:31–53. doi: 10.1007/s10237-017-0943-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zungu-Edmondson M, Shults NV, Melnyk O, Suzuki YJ. Natural reversal of pulmonary vascular remodeling and right ventricular remodeling in SU5416/hypoxia-treated Sprague-Dawley rats. PLoS One. 2017;12:e0182551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonelli AR, Arelli V, Minai OA, Newman J, Bair N, Heresi GA, Dweik RA. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188:365–369. doi: 10.1164/rccm.201209-1640OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tello K, Wan J, Dalmer A, Vanderpool R, Ghofrani HA, Naeije R, Roller F, Mohajerani E, Seeger W, Herberg U, et al. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Circ Cardiovasc Imaging. 2019;12:e009047. doi: 10.1161/CIRCIMAGING.119.009047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moceri P, Duchateau N, Baudouy D, Schouver ED, Leroy S, Squara F, Ferrari E, Sermesant M. Three-dimensional right-ventricular regional deformation and survival in pulmonary hypertension. Eur Heart J Cardiovasc Imaging. 2018;19:450–458. doi: 10.1093/ehjci/jex163 [DOI] [PubMed] [Google Scholar]
- 29.Hulshof HG, Eijsvogels TMH, Kleinnibbelink G, van Dijk AP, George KP, Oxborough DL, Thijssen DHJ. Prognostic value of right ventricular longitudinal strain in patients with pulmonary hypertension: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2019;20:475–484. doi: 10.1093/ehjci/jey120 [DOI] [PubMed] [Google Scholar]
- 30.Sakuma M, Ishigaki H, Komaki K, Oikawa Y, Katoh A, Nakagawa M, Hozawa H, Yamamoto Y, Takahashi T, Shirato K. Right Ventricular Ejection Function Assessed by Cineangiography Importance of Bellows Action. Circulation Journal. 2002;66:605–609. doi: 10.1253/circj.66.605 [DOI] [PubMed] [Google Scholar]
- 31.Anavekar NS, Gerson D, Skali H, Kwong RY, Kent Yucel E, Solomon SD. Two-Dimensional Assessment of Right Ventricular Function: An Echocardiographic–MRI Correlative Study. Echocardiography. 2007;24:452–456. doi: 10.1111/j.1540-8175.2007.00424.x [DOI] [PubMed] [Google Scholar]
- 32.Jone PN, Schäfer M, Pan Z, Bremen C, Ivy DD. 3D echocardiographic evaluation of right ventricular function and strain: a prognostic study in paediatric pulmonary hypertension. Eur Heart J Cardiovasc Imaging. 2018;19:1026–1033. doi: 10.1093/ehjci/jex205 [DOI] [PubMed] [Google Scholar]
- 33.Kind T, Mauritz G-J, Marcus JT, van de Veerdonk M, Westerhof N, Vonk-Noordegraaf A. Right ventricular ejection fraction is better reflected by transverse rather than longitudinal wall motion in pulmonary hypertension. J Cardiovasc Magn Reson. 2010;12:35–35. doi: 10.1186/1532-429X-12-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vélez-Rendón D, Zhang X, Gerringer J, Valdez-Jasso D. Compensated right ventricular function of the onset of pulmonary hypertension in a rat model depends on chamber remodeling and contractile augmentation. Pulm Circ. 2018;8:2045894018800439. doi: 10.1177/2045894018800439 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


