Abstract
Background:
Guideline-directed medical therapy (GDMT) for heart failure with reduced ejection fraction (HFrEF) improves clinical outcomes and quality of life. Optimizing GDMT in the hospital is associated with greater long-term use in HFrEF. This study aimed to describe the efficacy of a multidisciplinary virtual HF intervention on GDMT optimization among patients with HFrEF admitted for any cause.
Methods:
In this pilot randomized, controlled study, consecutive patients with HFrEF admitted to non-cardiology medicine services for any cause were identified at a large academic tertiary care hospital between May to September 2021. Major exclusions were end-stage renal disease, hemodynamic instability, concurrent COVID-19 infection, and current enrollment in hospice care. Patients were randomized to a clinician-level virtual peer-to-peer consult intervention providing GDMT recommendations and information on medication costs vs. usual care. Primary endpoints included 1) proportion of patients with new GDMT initiation or use, and 2) changes to HF optimal medical therapy (OMT) scores which included target dosing (range 0–9).
Results:
Of 242 patients identified, 91 (38%) were eligible and randomized to intervention (N=52) or usual care (N=39). Baseline characteristics were similar between intervention and usual care (mean age 63 vs. 67 years, 23% vs. 26% female, 46% vs. 49% Black, mean EF 33% vs. 31%). GDMT use on admission was also similar. There were greater proportions of patients with GDMT initiation or continuation with the intervention compared with usual care. After adjusting for OMT score on admission, changes to OMT score at discharge were higher for the intervention group compared with usual care (+0.44 vs. −0.31, absolute difference +0.75, adjusted estimate 0.86 ± 0.42; p=0.041).
Conclusions:
Among eligible patients with HFrEF hospitalized for any cause on non-cardiology services, a multidisciplinary pilot virtual HF consultation increased new GDMT initiation and dose optimization at discharge.
Keywords: Heart failure with reduced ejection fraction, heart failure, implementation science, virtual care, medical therapy
Introduction
Heart failure (HF) with reduced ejection fraction (HFrEF) is a leading cause of hospitalization and contributes to significant morbidity and mortality.1 Once hospitalized for HF, patients with HFrEF are at an exceedingly high risk for 30-day mortality and rehospitalization.2 Despite the availability of multiple approved oral medications proven to reduce risk of mortality and HF hospitalization,3–7 the use of these evidence-based therapies is often significantly delayed, and many eligible patients never receive these therapies resulting in missed opportunities to extend survival, prevent hospitalizations, and improve quality of life.8,9
The in-hospital initiation of guideline-directed medical therapy (GDMT) has been established as an important and potentially impactful strategy for improving post-discharge outcomes and medication use.10,11 The hospital environment particularly offers the opportunity for multispecialty care and case management and social work assistance to afford care teams and patients resources to address clinical and patient-associated barriers towards prescribing of GDMT. Observational data have suggested that hospitalizations for any cause may provide additional opportunities to optimize GDMT while patients with HFrEF receive inpatient care for cardiovascular and non-cardiovascular conditions.12
Accordingly, the purpose of this study was to examine the efficacy of a multidisciplinary virtual peer-to-peer HF consult intervention designed to increased GDMT use among patients hospitalized to non-cardiology medical services at a large academic medical center. This study additionally sought to understand how the intervention would increase GDMT prescribing towards target dosages and their continued use among patients prescribed GDMT prior to admission.
Methods
This study was a quality improvement initiative to characterize and improve multidisciplinary care for patients with HF across medical services. Data on patient characteristics and the quality improvement-based intervention were collected for clinical care and quality improvement rather than primarily for research. Due to the sensitive nature of the data collected for this study, the authors cannot provide the data, statistical analysis code, or other study materials to other researchers.
Study Design and Population
In this single-center pilot randomized controlled trial, consecutive patients with HFrEF (HF with left ventricular ejection fraction ≤40%) admitted to non-cardiology medicine services for any cause were identified at a large academic tertiary care referral center between May to September 2021. The hospital was a large academic tertiary care referral center with over twenty primary inpatient medical services, eight primary cardiology services, and cardiology and HF consult teams. All patients admitted to non-cardiology medicine services were screened daily to determine study eligibility by HF clinicians using patient’s clinical characteristics, reason for admission, estimated length of stay, and estimated discharge date as recorded in the electronic health record by primary medicine clinicians. Length of stay was not uniform for all patients as it varied based on primary reasons for admission as well as post-hospitalization disposition. Screening occurred for all admitted patients within 5 days of estimated date of discharge as documented by primary medical teams into the electronic health record. For patients whose estimated length of stay was ≤3 days, the intervention was applied immediately upon admission for eligible patients in order to 1) minimize any chance of missed patient screenings, and 2) offer sufficient time during a patient’s hospital stay for primary clinicians to enact the recommendations prior to discharge given the patient’s shorter lengths of stay. Major exclusions were end-stage renal disease, concurrent COVID-19 infection, hemodynamic instability, active systemic infections, ongoing treatment for cancer, and hospice care. All in-hospital medical clinicians received didactics on GDMT prior to study start. Clinicians were offered an opportunity to opt out of the study, but there were none who declined participation. Clinician teams were subsequently randomized to receiving usual care or a clinician-level virtual peer-to-peer consult intervention. Clinical characteristics and medication prescribing on admission and discharge were recorded for patients in the usual care and intervention groups (Figure 1). Patients who were previously randomized and later re-hospitalized were not re-screened or included into the study during the subsequent hospitalizations. Attending and house staff clinicians had varying durations of inpatient medical service with the possibility that many would not rotate onto inpatient medical services during the study period, and thus randomization occurred at the clinician team level than at the individual clinician level. This study was approved following Institutional Review Board review and received waiver to obtain informed consent.
Figure 1:
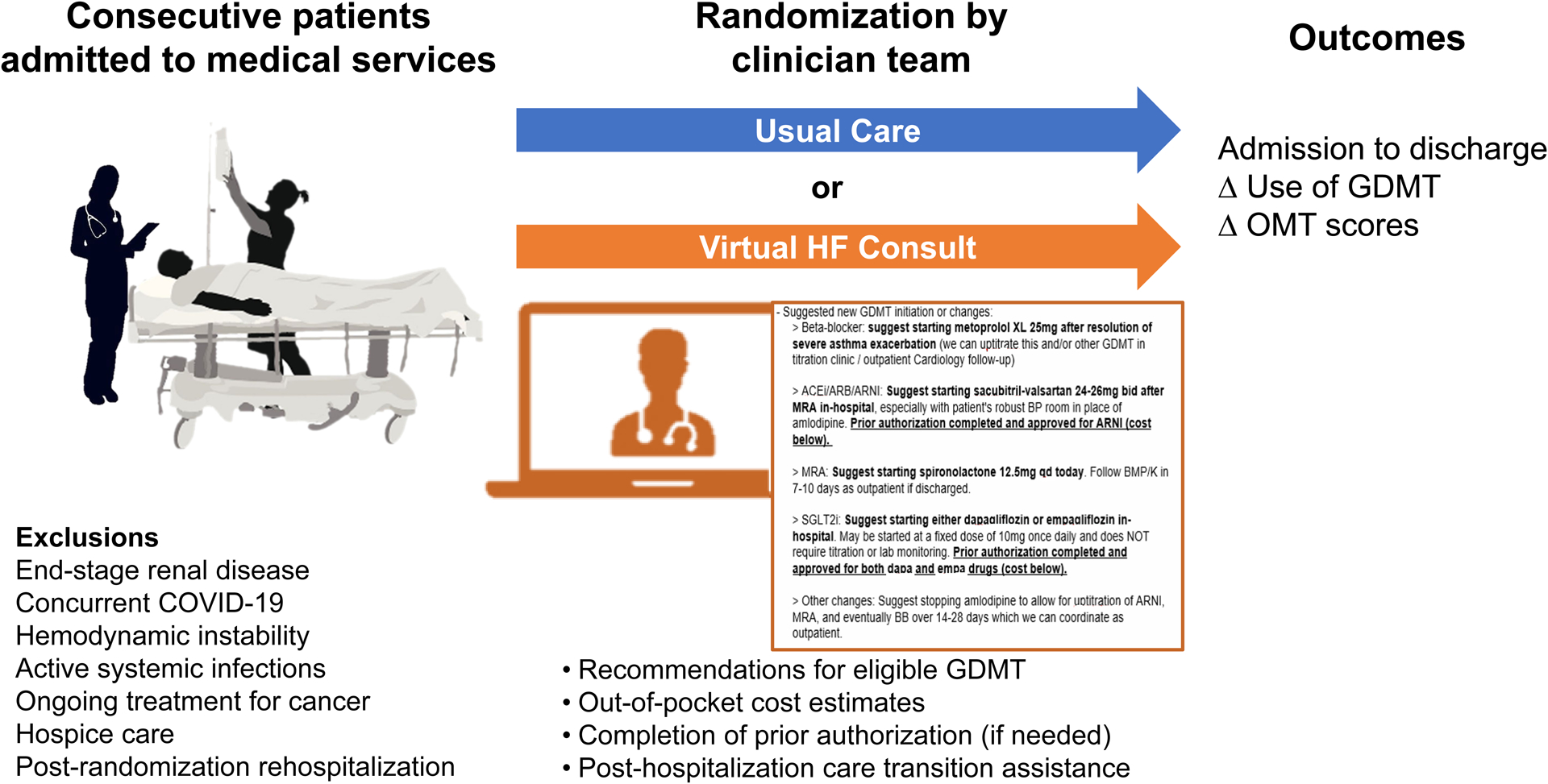
Study design of the in-hospital virtual peer-to-peer consult intervention pilot trial.
Intervention: A Virtual Peer-to-Peer Communication
A multidisciplinary team led by heart failure clinicians included advanced practice providers, pharmacists, and nurses. The intervention was an involuntary virtual peer-to-peer communication between a heart failure-led multidisciplinary specialty team and rounding medical teams. Eligibility for GDMT was determined based on patient clinical characteristics and previously reported intolerances to medications. Contemporary GDMT classes were available on hospital formulary. The suggested changes to GDMT were determined on an individual patient basis by a HF clinician based on the review of clinical characteristics, anticipated remaining length of stay prior to discharge, and overall hospital course. Due to varying lengths of stay, a universal protocol was not applied. Rather a stepwise approach to initiate/uptitrate β-blockers, angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB)/angiotensin receptor-neprilysin inhibitor (ARNI), mineralocorticoid receptor antagonists (MRA), and sodium-glucose co-transporter 2 inhibitor (SGLT2i) was suggested by the HF clinician based on clinical judgement in the context of other medical problems, blood pressure, laboratory studies, and remaining length of stay.
Virtual peer-to-peer communication included recommendations for eligible GDMT, out-of-pocket cost estimates, completion of medication prior authorization (if needed), and arrangements for HF clinic follow-up. Medication cost data was provided by a pharmacy technician and included estimated out-of-pocket costs for ARNI and SGLT2i medications for 30 and 90-day supply prescriptions based on the patient’s prescription drug coverage. If the patient met eligibility but required prior authorization or lacked prescription drug coverage, a HF clinician and pharmacy technician completed prior authorizations or assisted with pharmacy assistance program applications, as needed. Medical teams were offered assistance with arranging HF clinic follow up visits within 7 days of anticipated discharge either with a patient’s primary cardiologist or the hospital’s outpatient HF bridge clinic.
The virtual peer-to-peer communication provided a summary of all recommendations to primary team clinicians by a HF clinical fellow/advanced practice provider and an advanced HF and transplant cardiology attending through a signed virtual consult note (Figure S1) and by an accompanying clinical page/telephone call. Suggested changes to GDMT prescribing were ultimately left to the discretion of the primary medical rounding clinician. Further recommendations to initiate/uptitrate GDMT at follow-up were outlined and communicated for outpatient cardiology clinicians to consider. Clinician teams randomized to usual care did not receive the virtual peer-to-peer communication.
Outcomes
Co-primary endpoints included 1) proportion of patients with new GDMT initiation/continuation, and 2) changes to a modified HF optimal medical therapy (OMT) score. The composite modified HF OMT score was used to evaluate the prescribing of GDMT and titration towards target dosing among eligible patients (range 0–9),13 which included target dosing for evidence-based β-blockers, ACEI/ARB/ARNI, MRA, and SGLT2i. If patients were not eligible for a medication class (e.g., documented allergy or intolerance to a class of HF medications), that medication was excluded from the patient’s composite OMT score. Thus, denominators of prescribed GDMT reflected only those medications for which patients were eligible.
Statistical Analysis
Baseline patient and hospital characteristics were summarized as the mean (± standard deviation) for continuous variables, and as counts (percentages) for categorical variables. For patients eligible for the study, descriptive comparisons of baseline patient and hospital characteristics between patients in the usual care vs. intervention groups were conducted using the Wilcoxon rank-sum test for continuous variables and Pearson’s chi-square or Fisher’s exact test for categorical variables.
GDMT use on admission, at discharge, and the difference between discharge and admission were summarized as numerator/denominator and percentages by study group. Differences in changes in GDMT use between groups were compared by Pearson’s Chi-square or Fisher’s Exact test between groups. Linear regression adjusting for baseline composite OMT score was used to estimate the effect of the intervention on composite OMT score at discharge. A two-sided p-value ≤0.05 was considered statistically significant. Statistical analyses were performed using Stata 16 (StataCorp, College Station, Texas).
Results
Among 20 medical teams randomized to the clinician-level intervention or usual, 242 consecutively admitted patients with HFrEF were screened for study eligibility. Common reasons for exclusions included end-stage renal disease (N=52) and concurrent COVID-19 infection (N=12). Among 91 (38%) patients who met eligibility, 52 patients were admitted to medical teams randomized to intervention and 39 patients were admitted to medical teams randomized to receive usual care. Mean (±SD) length of stay was similar between intervention and usual care groups (9.6 ± 8.5 vs. 11.2 ± 9.8 days; p=0.61).
Baseline Characteristics
Compared with the usual care group, baseline characteristics were similar among patients who received the intervention: mean age 62.7 ± 14.1 vs. 66.8 ± 13.0 years (p=0.15), 23% vs. 26% women (p=0.78), 46% vs. 49% Black race (p=0.97), 51% vs. 44% ischemic cardiomyopathy (p=0.54), 46% vs. 50% diabetes (p=0.72), 46% vs. 44% chronic kidney disease (p=0.85), and mean ejection fraction 33.1 ± 8.4 vs. 31.2 ± 1.3% (p=0.28) (Table 1).
Table 1:
Characteristics on admission of patients with heart failure with reduced ejection fraction admitted to non-cardiology services and eligible for the virtual peer-to-peer consultation pilot trial.
| Characteristic | Usual Care (N = 39) |
Intervention (N = 52) |
Total (N = 91) |
P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 66.8±13.0 | 62.7±14.1 | 64.4±13.7 | 0.15 |
| Female Sex | 10 (26) | 12 (23) | 22 (24) | 0.78 |
| Race | ||||
| White | 17 (44) | 24 (46) | 41 (45) | 0.97 |
| Black | 19 (49) | 24 (46) | 43 (47) | |
| Other | 3 (8) | 4 (8) | 7 (8) | |
| Hispanic Ethnicity | 1 (3) | 1 (2) | 2 (2) | 0.84 |
| Insurance | 0.42 | |||
| Private | 7 (18) | 12 (23) | 19 (21) | |
| Medicare/Medicare Advantage | 23 (59) | 23 (44) | 46 (51) | |
| Medicaid | 6 (15) | 8 (15) | 14 (15) | |
| None/Self-pay | 3 (8) | 9 (17) | 12 (13) | |
| Comorbidities | ||||
| Heart failure subtype | ||||
| Ischemic | 20 (51) | 23 (44) | 43 (47) | 0.54 |
| Nonischemic | 10 (26) | 19 (37) | 29 (32) | |
| Unknown | 9 (23) | 10 (19) | 19 (21) | |
| Coronary artery disease | 23 (59) | 26 (50) | 49 (54) | 0.39 |
| Hypertension | 34 (87) | 44 (84) | 78 (86) | 0.73 |
| Hyperlipidemia | 28 (72) | 38 (73) | 66 (73) | 0.89 |
| Diabetes mellitus | 18 (46) | 26 (50) | 44 (48) | 0.72 |
| Obesity | 11 (35) | 17 (37) | 28 (36) | 0.89 |
| Atrial fibrillation/flutter | 15 (38) | 21 (40) | 36 (40) | 0.85 |
| Anemia | 22 (56) | 32 (63) | 54 (60) | 0.54 |
| Chronic kidney disease | 18 (46) | 23 (44) | 41 (45) | 0.85 |
| Cancer | 6 (15) | 6 (12) | 12 (13) | 0.59 |
| Chronic obstructive pulmonary disease | 8 (21) | 19 (37) | 27 (30) | 0.098 |
| Obstructive sleep apnea | 5 (13) | 7 (13) | 12 (13) | 0.93 |
| Cerebrovascular accident | 10 (26) | 10 (19) | 20 (22) | 0.46 |
| Implantable cardioverter-defibrillator | 6 (15) | 13 (25) | 19 (21) | 0.26 |
| Chronic resynchronization therapy | 3 (8) | 5 (10) | 8 (9) | 0.75 |
| Clinical Characteristics | ||||
| Left ventricular ejection fraction, % | 31.2±1.3 | 33.1±8.4 | 32.3±8.3 | 0.28 |
| Left ventricular end-diastolic diameter, cm | 5.37±0.76 | 5.33±0.94 | 5.35±0.86 | 0.81 |
| Heart rate, bpm | 89.9±29.0 | 86.5±20.3 | 88.0±24.3 | 0.51 |
| Systolic blood pressure, mmHg | 125.5±24.2 | 125.8±22.3 | 125.6±23.0 | 0.95 |
| Diastolic blood pressure, mmHg | 75.6±18.6 | 76.0±15.9 | 75.8±17.0 | 0.92 |
| Body mass index, kg/m2 | 26.9±7.3 | 28.5±8.3 | 27.9±7.9 | 0.37 |
| Sodium, mmol/L | 137.0±4.7 | 136.9±4.8 | 137.0±4.7 | 0.92 |
| Potassium, mmol/L | 4.1±0.6 | 4.2±0.9 | 4.1±0.8 | 0.70 |
| Creatinine, mg/dL | 1.9±1.4 | 1.9±1.9 | 1.9±1.7 | 0.98 |
| Estimated glomerular filtration rate, mL/min/1.73m2 | 51.9±28.5 | 50.1±26.6 | 50.8±27.3 | 0.76 |
| GDMT use on Admission | ||||
| β-blocker | 26 (67) | 31 (60) | 57 (63) | 0.49 |
| ACEI/ARB/ARNI | 20 (53) | 32 (62) | 52 (58) | 0.40 |
| ACEI/ARB | 18 (47) | 25 (48) | 43 (48) | |
| ARNI | 2 (5) | 7 (13) | 9 (10) | |
| MRA | 10 (26) | 17 (33) | 27 (30) | 0.51 |
| SGLT2i | 6 (16) | 10 (20) | 16 (18) | 0.64 |
Data are reported as mean ± standard deviation for continuous variables or number (percentage) for categorical variables. Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; MRA, mineralocorticoid antagonist; SGLT2i, sodium-glucose cotransporter 2 inhibitor.
Objective vital signs and admission laboratory results were similar between the intervention and usual care cohorts (Table 1). As compared with usual care, patients in the intervention group had similar mean heart rate of 86.5 ± 20.3 vs. 89.9 ± 29.0 bpm (p=0.51), mean systolic blood pressure 125.8 ± 22.3 vs. 125.5 ± 24.2 mmHg (p=0.95), mean potassium 4.2 ± 0.9 vs. 4.1 ± 0.6 mmol/L (p=0.70), and mean estimated glomerular filtration rate 50.1 ± 26.6 vs. 51.9 ± 28.5 mL/min/1.73sq m (p=0.76).
Use of GDMT on Admission
Use of GDMT on admission among eligible patients without contraindications was also similar between intervention and usual care groups: β-blocker 67% vs. 60% (total N=91; p=0.49); ACEI/ARB/ARNI 53% vs. 62% (N=90; p=0.40); MRA 26% vs 33% (N=90; p=0.51); SGLT2i 16% vs. 20% (N=89; p=0.64) (Table 1).
Outcomes
The proportion of patients with GDMT initiation/continuation was greater with intervention compared with usual care for ACEI/ARB/ARNI (71% vs. 49%; p=0.04), and nominally higher for MRA (41% vs. 21%; p=0.05) and SGLT2i (26% vs. 14%; p=0.19) (Table 2). Rates of GDMT discontinuation or dose reduction were similar between groups, although nominally with less de-escalation in the intervention group (Figure 2).
Table 2:
Characteristics on discharge of patients with heart failure with reduced ejection fraction admitted to non-cardiology services and eligible for the virtual peer-to-peer consultation pilot trial.
| Discharge Characteristics | Control (N = 39) |
Intervention (N = 52) |
Total (N = 91) |
P-value |
|---|---|---|---|---|
| Length of stay | 11.2±19.8 | 9.6±8.5 | 10.3±14.4 | 0.61 |
| Received intensive care during admission | 8 (21) | 11 (21) | 19 (21) | 0.94 |
| Clinical characteristics | ||||
| Heart rate, bpm | 75.4±14.3 | 80.9±13.3 | 78.5±13.9 | 0.06 |
| Systolic blood pressure, mmHg | 122.3±20.8 | 125.5±18.4 | 124.1±19.4 | 0.43 |
| Diastolic blood pressure, mmHg | 71.7±14.3 | 72.6±14.2 | 72.2±14.2 | 0.78 |
| Sodium, mmol/L | 137.2±3.4 | 136.4±3.1 | 136.8±3.2 | 0.25 |
| Potassium, mmol/L | 4.2±0.5 | 4.1±0.4 | 4.1±0.5 | 0.73 |
| Creatinine, mg/dL | 1.5±1.2 | 1.4±0.6 | 1.4±0.9 | 0.41 |
| Estimated glomerular filtration rate, mL/min/1.73m2 | 62.8±28.8 | 61.7±25.8 | 62.2±27.0 | 0.84 |
| GDMT use at discharge | ||||
| β-blocker | 30 (77) | 39 (78) | 69 (78) | 0.90 |
| ACEI/ARB/ARNI | 17 (49) | 32 (71) | 49 (61) | 0.04 |
| ACEI/ARB | 14 (40) | 22 (49) | 36 (45) | |
| ARNI | 3 (9) | 10 (22) | 13 (16) | |
| MRA | 8 (21) | 20 (40) | 28 (32) | 0.05 |
| SGLT2i | 5 (14) | 12 (26) | 17 (20) | 0.19 |
| Prior authorizations submitted and completed | 0 (0) | 7 (13) | 7 (8) | N/A |
Data are reported as mean ± standard deviation for continuous variables or number (percentage) for categorical variables. Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; MRA, mineralocorticoid antagonist; SGLT2i, sodium-glucose cotransporter 2 inhibitor.
Figure 2: Guideline-directed medical therapy at discharge among patients admitted for any cause randomized by non-cardiology clinician teams to an in-hospital virtual peer-to-peer consult intervention.
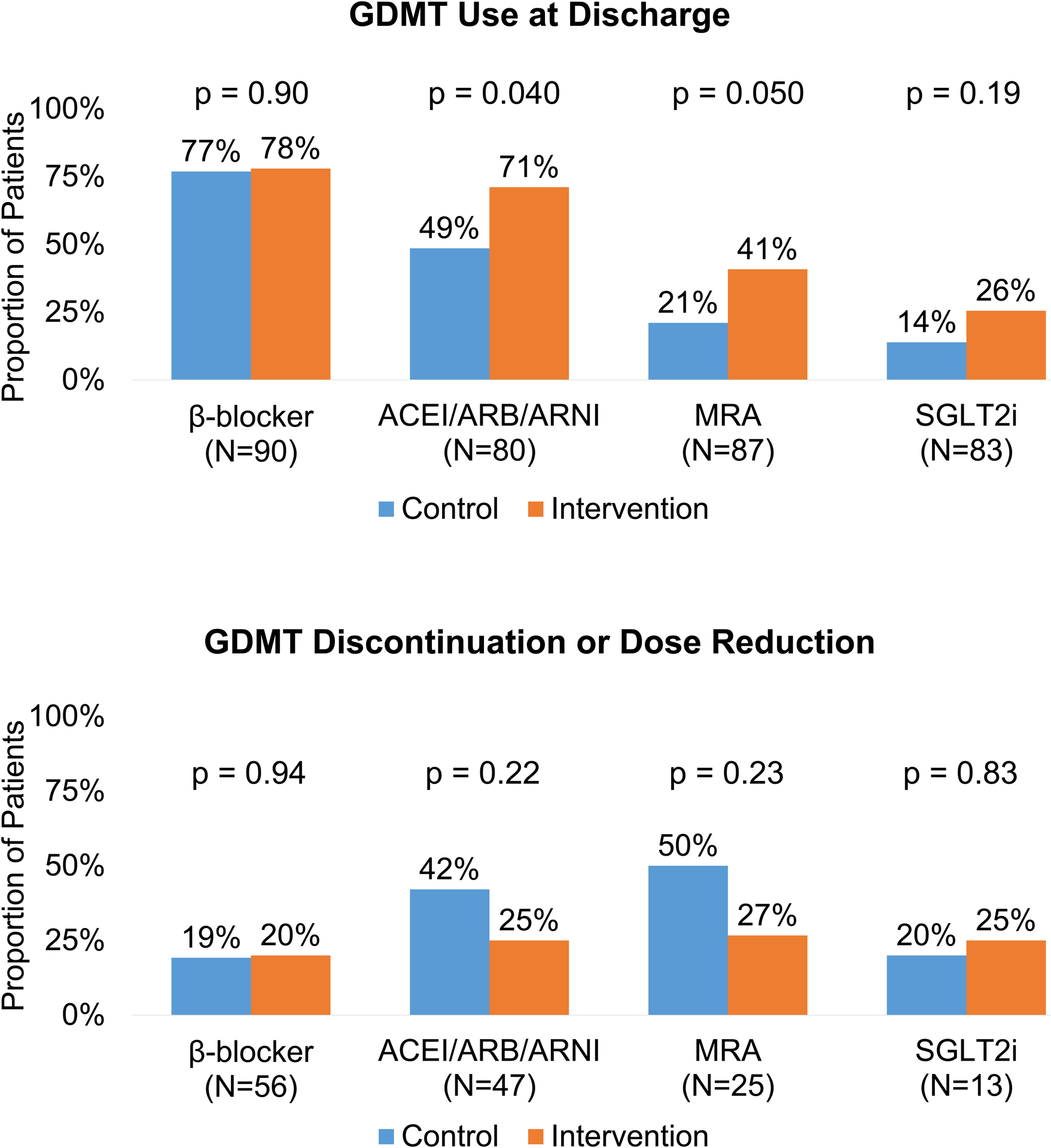
The top panel presents the proportion of all patients either initiated or continued on GDMT by discharge. The bottom panel presents the proportion of patients who had GDMT discontinued or reduced in dose without having other GDMT classes initiated in place of these changes. Abbreviations: ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor-neprilysin inhibitors; GDMT, guideline-directed medical therapy; MRA, mineralocorticoid receptor antagonists; SGLT2i, sodium-glucose cotransporter-2 inhibitors.
Composite OMT scores on admission were similar between both groups, with usual care achieving 2.28 (95% CI: 0.61, 1.22) and intervention group achieving 2.48 (95% CI: 0.66, 1.32). By discharge, composite OMT scores achieved varied by each group, with usual care 1.97 (95% CI: 0.58, 1.16) and intervention group 2.92 (95% CI: 0.68, 1.35). After adjusting for OMT score on admission, changes to OMT score at discharge were higher following intervention (+0.44) compared with usual care (−0.31), with absolute difference +0.75 (adjusted estimate 0.86 ± 0.42; p=0.04) (Figure 3).
Figure 3 (and Central Illustration): Changes in optimal medical therapy scores by discharge among patients admitted for any cause randomized by non-cardiology clinician teams to an in-hospital virtual peer-to-peer consult intervention.
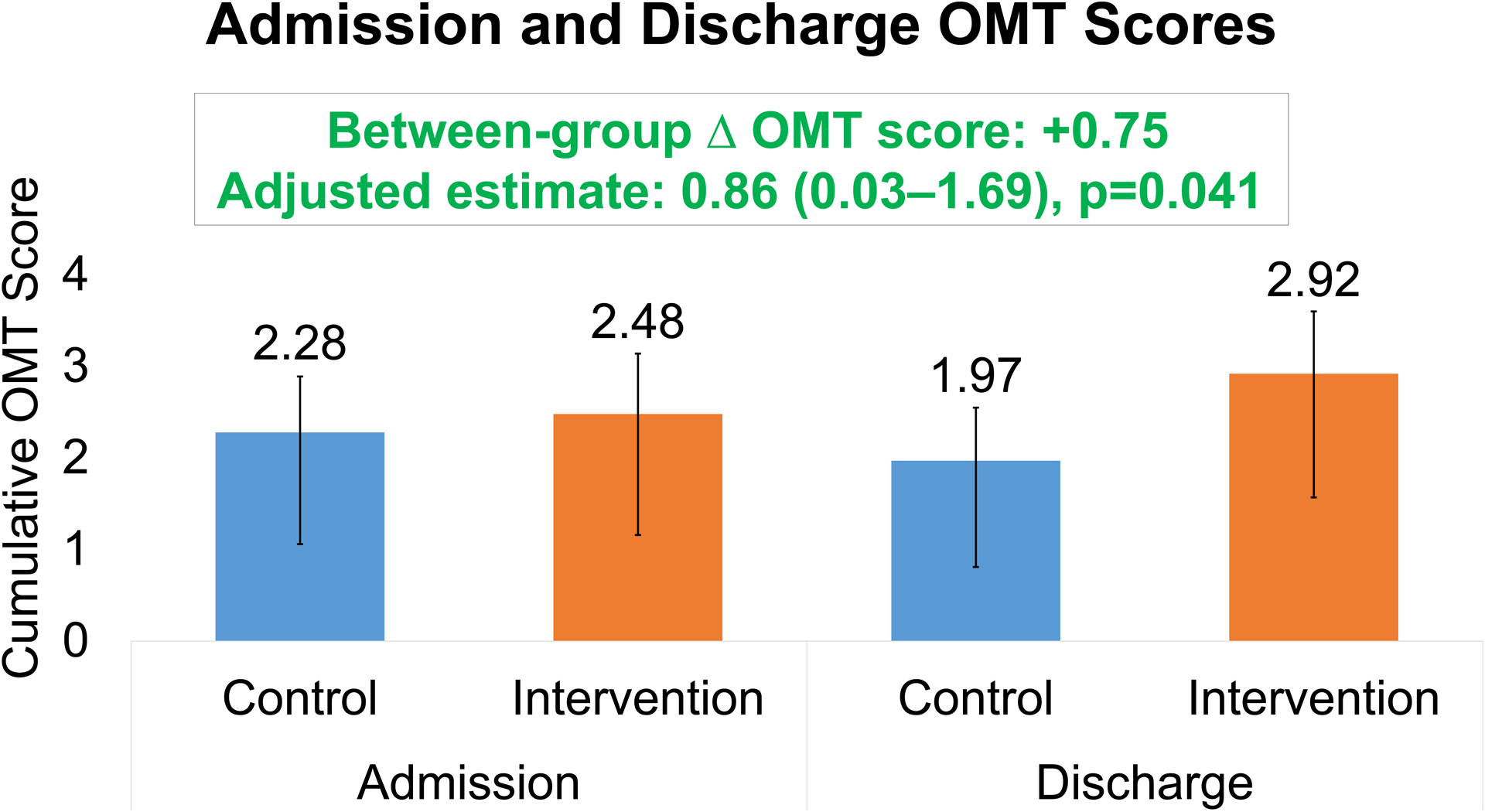
Optimal medical therapy (OMT) scores at discharge adjusted for baseline scores were overall favorable among patients receiving the virtual intervention compared with usual care. OMT score developed by the Heart Failure Collaboratory.
Post-Discharge Follow-up
Follow-up at 30-days did not differ between the usual care and intervention groups, with 33 (63%) vs. 25 (64%) (p=0.95) patients having attended primary care follow-up visits within 30 days of discharge, and only 17 (33%) vs. 12 (31%) (p=0.85) patients having attended heart failure or general cardiology visits within 30 days of discharge. Mortality at 30 days was lower for the intervention cohort compared to usual care, 0 (0%) vs 3 (7.7%).
Pharmacy Component of Intervention
Cost estimates were provided when applicable to patients in the intervention arm not already prescribed ARNI or SGLT2i and without contraindications. Of the 52 patients, 25 (48%) and 32 (62%) had 30-day cost estimates for ARNI and SGLT2i, respectively. The mean and median expected 30-day cost for ARNI was $60.01 and $21.00, respectively. The mean and median expected 30-day cost for SGLT2i was $50.60 and $11.27, respectively. Likewise, prior authorizations were required and submitted for patients in the intervention arm for 7 (13%) patients, all of whom received approval.
Discussion
In this single-center pilot randomized controlled trial of consecutive patients with HFrEF admitted to non-cardiology medical services, less than half of patients were eligible for the virtual peer-to-peer consult intervention with primary limitations including end-stage renal disease and concurrent COVID-19 infections. GDMT use on admission among eligible patients with HFrEF was similar between study groups but overall low. The multidisciplinary virtual consult intervention significantly increased composite OMT scores by discharge, notably through greater new initiations/continuations and fewer discontinuations of ACEI/ARB/ARNI, MRA, and SGLT2i therapies.
A substantial number of patients with HFrEF are admitted to non-cardiology services for treatment of HF and non-HF related conditions.14 The present study was designed to engage medical clinicians and suggest opportunities to increase GDMT while primary inpatient teams focused on treating the patient’s primary reason for hospitalization. Other recent trials on multidisciplinary patient care aimed to increase GDMT use and adherence through either patient/provider-facing electronic health record prompts or hospital-level audit feedback.10,15–17 The present study differed by engaging non-cardiology inpatient clinicians, and included pharmacy and case management input and post-discharge follow-up in the virtual intervention which mitigated system-level and patient-level barriers to GDMT prescribing in the in-hospital setting. The IMPLEMENT-HF study led by Bhatt et al. similarly studied virtual consultative care in HFrEF in a pre-post interventional analysis showing associated increases in β-blockers, ARNI, MRA, and triple therapy use hospital admission to discharge.12 Unlike in the present study, IMPLEMENT-HF occurred prior to SGLT2i approval for HFrEF, and included a broader population with end-stage renal disease eligible for β-blocker monotherapy.12 The present study confirmed the feasibility and efficacy of a virtual HF-specialty led multidisciplinary intervention through its randomization design among a population eligible for multiple GDMT classes. It further adds to the novelty of IMPLEMENT-HF by also increasing SGLT2i therapy use for HFrEF. This class of medications in particular carries unique implementation challenges in the in-hospital setting given that it is associated with multiple cardio-renal-metabolic conditions and has multiple clinician-level knowledge gaps in its use.11,18 Future in-hospital peer-to-peer consult investigations studying GDMT optimization may focus on identifying and intervening on suitable higher-risk patients with HFrEF and comorbid conditions, particularly those with advanced renal disease.
This study offers a strategy to mitigate gaps in GDMT use in HFrEF. In US clinical practice, nearly one-third of patients hospitalized for HFrEF are not prescribed target doses of β-blocker and nearly half or more are not prescribed target doses of ACEI/ARB/ARNI or MRA at the time of discharge.10,11,19 These trends in GDMT use in chronic ambulatory HFrEF similarly mirrored GDMT use on admission in the present study, albeit having a modest single-center population of patients on non-cardiology services. MRA and SGLT2i use were <50% overall, yet the intervention group demonstrated greater use of ACEI/ARB/ARNI, MRA, and SGLT2i by discharge. While approximately 40% of patients in both study arms were not on β-blockers on admission, the intervention yielded similar modest improvements β-blocker use at discharge to ~75% compared with usual care. While we were unable to study factors that contribute to GDMT sequencing, the overall higher use of β-blockers on admission and at discharge between both groups suggests that when clinicians are faced with GDMT initiation in-hospital, prescribing patterns may mirror the temporal sequence of the completed HFrEF trials, particularly with β-blocker and ACEI/ARB initiated sooner than MRA/ARNI.20–23 The familiarity in β-blocker use over other GDMT classes among medical clinicians may also have influenced the higher rates of use at discharge in the usual care arm. While factors contributing to in-hospital medication sequencing merit further investigation, trial data confirm that GDMT initiated in-hospital improves downstream prescribing and long-term adherence patterns,24–26 and targeting in-hospital care settings for GDMT optimization must be prioritized.
It is important to highlight several ongoing barriers to advancing GDMT that were observed in the present study. The vast majority of patients with HFrEF were excluded due to active problems or potential issues with medical stability that would interfere with initiation or titration of GDMT towards ideal target dosing. Unlike other observational studies of virtual consultation,12 patients with end-stage renal disease were excluded due to limited options for GDMT and variable hemodynamic stabilities to advance β-blocker dosing, particularly due to comorbid vascular conditions and hemodialysis needs. Despite actively arranging close HF or cardiology follow-up, nearly half of patients did not follow up with any provider and seldomly with cardiology clinicians, limiting the opportunity for further GDMT titration. Lastly, the mean length of stay was similarly ~9 days for both groups, and patients were identified within 5 days of estimated discharge, limiting the overall time available to initiate/titrate GDMT prior to discharge. Despite these identified barriers, the virtual peer-to-peer consult intervention increased overall GDMT use and may serve as a key component to longitudinal optimization of GDMT and engage non-cardiology clinicians in clinical practice.
In-hospital and post-discharge quality improvement initiatives in HF examining the use of pharmacy resources in inpatient HF care have provided important insight in the potential of multidisciplinary HF care. Inpatient comprehensive pharmacotherapeutic evaluation in geriatric patients with HF has been shown to be a feasible adjunct to optimizing medical therapy and identifying intolerances.27 Systematic pharmacy-led review of medical therapy in patients with HF prior to discharge, particularly in patients at high risk for increased healthcare utilization and low health literacy, is feasible but has not been shown to improve post-discharge outcomes when studied in isolation.28,29 The present study highlights the utility of early pharmacy input and providing real-time cost estimates to prescribing clinicians. Perhaps the pharmacy input on prescription drug coverage and assistance with prior authorizations may have served as a major advantage to this particular peer-to-peer consult intervention over other reported strategies. Future investigations in communication between provider and patients and peer-to-peer communication are warranted to explore the granularity in subcomponents of multidisciplinary HF care, to explore treatment gaps in order to overcome barriers GDMT optimization, and to understand feasibility of the pharmacy components to such interventions across health institutions with varying care delivery models.
Limitations
Results of this pilot trial must be interpreted in the context of the following limitations. Its single center design and small cohort may limit generalizability to broader HF populations with differences in care delivery, cost-related barriers, and electronic health record platforms that do not support virtual peer-to-peer consultation. Randomization occurred by clinician team, and not by individual clinicians or patients. Both attending and house staff clinicians may have later rotated independently onto teams randomized to the other study arm, potentially leading to contamination and attenuation of measured outcomes. However, the exact number of attending and house staff clinicians rotating on/off services (including day/night shifts) could not be quantified to estimate the effect of contamination. Additionally, randomization at the clinician team-level resulted in imbalance in the number of patients between both study arms, although this did not result in significant differences in patient characteristics between both groups. A proportion of patients were excluded with active COVID-19 infection due to the potential for harm at the start of the study, and recent data suggests tolerability of SGLT2i among patients with COVID-19 and cardiometabolic risk factors,30 offering greater opportunity to include these patients in the future. Due to variable lengths of stay, suggested GDMT changes were personalized to patient’s clinical characteristics, and a protocolized sequencing of GDMT could not be studied. While concurrent inpatient cardiology consultation was rare, the exact numbers of cardiology consultation are unavailable to report and may possible have further contributed to contamination risk. Due to the small sample size and randomization design, residual measured and unmeasured confounding may account for some or all of these findings.
Conclusion
A multidisciplinary single-center pilot clinician-level virtual consultation for patients with HFrEF admitted to non-cardiology hospital services is feasible and offers additional opportunities to optimize GDMT for HFrEF. GDMT was particularly optimized through greater new initiations/continuations and fewer discontinuations of ACEI/ARB/ARNI, MRA, and SGLT2i therapies. Moreover, this study showed modest rates of post-hospitalization follow-up, a barrier to longitudinal GDMT optimization in HFrEF following hospitalizations for any cause. Our data suggest that virtual peer-to-peer consultation may serve as an adjunctive tool in broadly engaging multispecialty clinicians in the care of patients with HF. Further investigations and initiatives are needed to address clinical inertia towards in-hospital and post-discharge GDMT optimization in order to improve long-term clinical outcomes.
Supplementary Material
Figure S1: Example Virtual Consult Note of the Heart Failure Virtual Peer-to-Peer Consult Intervention.
Clinical Perspective:
What is new?
Use of evidenced-based practices and achievement of target guideline-directed medical therapy (GDMT) among eligible patients with HFrEF remains relatively rare in the in-hospital setting. Identifying patients with HFrEF on non-cardiology medical services provides an opportunity to optimize GDMT.
A multidisciplinary virtual peer-to-peer consult intervention significantly improved GDMT by discharge, notably through greater new initiations/continuations and fewer discontinuations of renin-angiotensin-aldosterone system and sodium-glucose cotransporter 2 inhibitors.
What are the clinical implications?
Larger randomized clinical trials are needed to examine electronic health record-based virtual peer-to-peer communication and associated quality of care and clinical outcomes.
Studies on in-hospital virtual consult tools may be challenging to execute and to measure long-term outcomes without identifying key system-level and patient-level barriers that limit patient engagement and outpatient clinic follow-up.
Acknowledgments and Sources of Funding
This analysis was independently funded by the Duke Heart Center and Duke Clinical Research Institute. The academic authors had full control in the development, writing, and submission of the manuscript contents.
Disclosures
VNR and MDK have received salary support from a National Institutes of Health (NIH) Training Grant. SGB serves on an advisory board for Cytokinetics. SJG has received research support from the Duke University Department of Medicine Chair’s Research Award, American Heart Association (#929502), National Heart Lung and Blood Institute, Amgen, AstraZeneca, Bristol Myers Squibb, Cytokinetics, Merck & Co., Inc., Novartis, Pfizer, and Sanofi; has served on advisory boards for Amgen, AstraZeneca, Boehringer Ingelheim/ Lilly, Bristol Myers Squibb, Cytokinetics, Roche Diagnostics, and Sanofi; serves as a consultant for Amgen, Bayer, Boehringer Ingelheim/ Lilly, Bristol Myers Squibb, Merck Sharp & Dohme LLC (a subsidiary of Merck & Co., Inc., Rahway, NJ, USA), PharmaIN, Roche Diagnostics, Sanofi, Tricog Health, Urovant Pharmaceuticals, and Vifor; and has received speaker fees from Boehringer Ingelheim and Cytokinetics. MF was supported by the National Heart, Lung, and Blood Institute (NHLBI) (K23HL151744), the American Heart Association (20IPA35310955), Doris Duke, Bayer, Bodyport, BTG Specialty Pharmaceuticals and Verily. He receives consulting fees from Abbott, Alio Health, Alleviant, Audicor, AxonTherapies, Bayer, Bodyguide, Bodyport, Boston Scientific, Cadence, Coridea, CVRx, Daxor, Deerfield Catalyst, Edwards LifeSciences, EKO, Feldschuh Foundation, Fire1, Gradient, Intershunt, Medtronic, NIMedical, NXT Biomedical, Pharmacosmos, PreHealth, ReCor, Shifamed, Splendo, Sumacor, SyMap, Verily, Vironix, Viscardia, Zoll. ADD reports research funding through his institution from the American Heart Association, Biofourmis, Bodyport, Cytokinetics, American Regent, Inc, the NHLBI, Novartis, and Story Health. He also provides consulting services for and/or receives honoraria from Abiomed, AstraZeneca, Cardionomic, InnaMed, LivaNova, Natera, Novartis, Procyrion, Story Health, Vifor, and Zoll. He has also received non-financial support from Abbott for educational and research activities.
Abbreviations
- ACEI
angiotensin-converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- ARNI
angiotensin receptor-neprilysin inhibitor
- GDMT
guideline-directed medical therapy
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- MRA
mineralocorticoid antagonist
- OMT
optimal medical therapy
- SGLT2i
sodium-glucose cotransporter 2 inhibitor
References
- 1.Murphy SP, Ibrahim NE, Januzzi JL. Heart Failure With Reduced Ejection Fraction: A Review. JAMA. 2020;324:488–504. doi: 10.1001/jama.2020.10262 [DOI] [PubMed] [Google Scholar]
- 2.Butler J, Yang M, Manzi MA, Hess GP, Patel MJ, Rhodes T, Givertz MM. Clinical Course of Patients With Worsening Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol. 2019;73:935–944. doi: 10.1016/j.jacc.2018.11.049 [DOI] [PubMed] [Google Scholar]
- 3.Greene SJ, O’Brien EC, Mentz RJ, Luo N, Hardy NC, Laskey WK, Heidenreich PA, Chang CL, Turner SJ, Yancy CW, et al. Home-Time After Discharge Among Patients Hospitalized With Heart Failure. Journal of the American College of Cardiology. 2018;71:2643–2652. doi: 10.1016/j.jacc.2018.03.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen LA, Gheorghiade M, Reid KJ, Dunlay SM, Chan PS, Hauptman PJ, Zannad F, Konstam MA, Spertus JA. Identifying patients hospitalized with heart failure at risk for unfavorable future quality of life. Circ Cardiovasc Qual Outcomes. 2011;4:389–398. doi: 10.1161/CIRCOUTCOMES.110.958009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambrosy AP, Hernandez AF, Armstrong PW, Butler J, Dunning A, Ezekowitz JA, Felker GM, Greene SJ, Kaul P, McMurray JJ, et al. The clinical course of health status and association with outcomes in patients hospitalized for heart failure: insights from ASCEND-HF. Eur J Heart Fail. 2016;18:306–313. doi: 10.1002/ejhf.420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krumholz HM, Normand SL, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999–2011. Circulation. 2014;130:966–975. doi: 10.1161/CIRCULATIONAHA.113.007787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018;72:351–366. doi: 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 9.Greene SJ, Fonarow GC, DeVore AD, Sharma PP, Vaduganathan M, Albert NM, Duffy CI, Hill CL, McCague K, Patterson JH, et al. Titration of Medical Therapy for Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol. 2019;73:2365–2383. doi: 10.1016/j.jacc.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeVore AD, Granger BB, Fonarow GC, Al-Khalidi HR, Albert NM, Lewis EF, Butler J, Piña IL, Allen LA, Yancy CW, et al. Effect of a Hospital and Postdischarge Quality Improvement Intervention on Clinical Outcomes and Quality of Care for Patients With Heart Failure With Reduced Ejection Fraction: The CONNECT-HF Randomized Clinical Trial. JAMA. 2021;326:314–323. doi: 10.1001/jama.2021.8844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao VN, Murray E, Butler J, Cooper LB, Cox ZL, Fiuzat M, Green JB, Lindenfeld J, McGuire DK, Nassif ME, et al. In-Hospital Initiation of Sodium-Glucose Cotransporter-2 Inhibitors for Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol. 2021;78:2004–2012. doi: 10.1016/j.jacc.2021.08.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatt AS, Varshney AS, Nekoui M, Moscone A, Cunningham JW, Jering KS, Patel PN, Sinnenberg LE, Bernier TD, Buckley LF, et al. Virtual optimization of guideline-directed medical therapy in hospitalized patients with heart failure with reduced ejection fraction: the IMPLEMENT-HF pilot study. Eur J Heart Fail. 2021;23:1191–1201. doi: 10.1002/ejhf.2163 [DOI] [PubMed] [Google Scholar]
- 13.Fiuzat M, Hamo CE, Butler J, Abraham WT, DeFilippis EM, Fonarow GC, Lindenfeld J, Mentz RJ, Psotka MA, Solomon SD, et al. Optimal Background Pharmacological Therapy for Heart Failure Patients in Clinical Trials: JACC Review Topic of the Week. J Am Coll Cardiol. 2022;79:504–510. doi: 10.1016/j.jacc.2021.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapelios CJ, Canepa M, Benson L, Hage C, Thorvaldsen T, Dahlström U, Savarese G, Lund LH. Non-cardiology vs. cardiology care of patients with heart failure and reduced ejection fraction is associated with lower use of guideline-based care and higher mortality: Observations from The Swedish Heart Failure Registry. Int J Cardiol. 2021;343:63–72. doi: 10.1016/j.ijcard.2021.09.013 [DOI] [PubMed] [Google Scholar]
- 15.Allen LA, Venechuk G, McIlvennan CK, Page RL, Knoepke CE, Helmkamp LJ, Khazanie P, Peterson PN, Pierce K, Harger G, et al. An Electronically Delivered Patient-Activation Tool for Intensification of Medications for Chronic Heart Failure With Reduced Ejection Fraction: The EPIC-HF Trial. Circulation. 2021;143:427–437. doi: 10.1161/CIRCULATIONAHA.120.051863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghazi L, Yamamoto Y, Riello RJ, Coronel-Moreno C, Martin M, O’Connor KD, Simonov M, Huang J, Olufade T, McDermott J, et al. Electronic Alerts to Improve Heart Failure Therapy in Outpatient Practice: A Cluster Randomized Trial. J Am Coll Cardiol. 2022;79:2203–2213. doi: 10.1016/j.jacc.2022.03.338 [DOI] [PubMed] [Google Scholar]
- 17.Rao VN, Kaltenbach LA, Granger BB, Fonarow GC, Al-Khalidi HR, Albert NM, Butler J, Allen LA, Lanfear DE, Ariely D, et al. The Association of Digital Health Application Use With Heart Failure Care and Outcomes: Insights From CONNECT-HF. J Card Fail. 2022. doi: 10.1016/j.cardfail.2022.07.050 [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, Peterson E, Pagidipati N. Barriers to prescribing glucose-lowering therapies with cardiometabolic benefits. Am Heart J. 2020;224:47–53. doi: 10.1016/j.ahj.2020.03.017 [DOI] [PubMed] [Google Scholar]
- 19.Greene SJ, Triana TS, Ionescu-Ittu R, Burne RM, Guérin A, Borentain M, Kessler PD, Tugcu A, DeSouza MM, Felker GM, et al. In-Hospital Therapy for Heart Failure With Reduced Ejection Fraction in the United States. JACC Heart Fail. 2020;8:943–953. doi: 10.1016/j.jchf.2020.05.013 [DOI] [PubMed] [Google Scholar]
- 20.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 21.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 22.McMurray JJV, Packer M. How Should We Sequence the Treatments for Heart Failure and a Reduced Ejection Fraction? A Redefinition of Evidence-Based Medicine. Circulation. 2020. doi: 10.1161/CIRCULATIONAHA.120.052926 [DOI] [PubMed] [Google Scholar]
- 23.Maddox TM, Januzzi JL, Allen LA, Breathett K, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld J, Masoudi FA, et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021. doi: 10.1016/j.jacc.2020.11.022 [DOI] [PubMed] [Google Scholar]
- 24.Gattis WA, O’Connor CM, Gallup DS, Hasselblad V, Gheorghiade M, Coordinators I-HIa. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534–1541. doi: 10.1016/j.jacc.2003.12.040 [DOI] [PubMed] [Google Scholar]
- 25.Mentz RJ, DeVore AD, Tasissa G, Heitner JF, Piña IL, Lala A, Cole RT, Lanfear DD, Patel CB, Ginwalla M, et al. PredischaRge initiation of Ivabradine in the ManagEment of Heart Failure: Results of the PRIME-HF Trial. Am Heart J. 2020;223:98–105. doi: 10.1016/j.ahj.2019.12.024 [DOI] [PubMed] [Google Scholar]
- 26.Wachter R, Senni M, Belohlavek J, Straburzynska-Migaj E, Witte KK, Kobalava Z, Fonseca C, Goncalvesova E, Cavusoglu Y, Fernandez A, et al. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. 2019;21:998–1007. doi: 10.1002/ejhf.1498 [DOI] [PubMed] [Google Scholar]
- 27.Walgraeve K, Van der Linden L, Flamaing J, Fagard K, Spriet I, Tournoy J. Feasibility of optimizing pharmacotherapy in heart failure patients admitted to an acute geriatric ward: role of the clinical pharmacist. Eur Geriatr Med. 2018;9:103–111. doi: 10.1007/s41999-017-0019-x [DOI] [PubMed] [Google Scholar]
- 28.Barker A, Barlis P, Berlowitz D, Page K, Jackson B, Lim WK. Pharmacist directed home medication reviews in patients with chronic heart failure: a randomised clinical trial. Int J Cardiol. 2012;159:139–143. doi: 10.1016/j.ijcard.2011.02.034 [DOI] [PubMed] [Google Scholar]
- 29.Bell SP, Schnipper JL, Goggins K, Bian A, Shintani A, Roumie CL, Dalal AK, Jacobson TA, Rask KJ, Vaccarino V, et al. Effect of Pharmacist Counseling Intervention on Health Care Utilization Following Hospital Discharge: A Randomized Control Trial. J Gen Intern Med. 2016;31:470–477. doi: 10.1007/s11606-016-3596-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosiborod MN, Esterline R, Furtado RHM, Oscarsson J, Gasparyan SB, Koch GG, Martinez F, Mukhtar O, Verma S, Chopra V, et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021;9:586–594. doi: 10.1016/s2213-8587(21)00180-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Example Virtual Consult Note of the Heart Failure Virtual Peer-to-Peer Consult Intervention.


