Abstract
When titanium dioxide (TiO2) is irradiated with near-UV light, this semiconductor exhibits strong bactericidal activity. In this paper, we present the first evidence that the lipid peroxidation reaction is the underlying mechanism of death of Escherichia coli K-12 cells that are irradiated in the presence of the TiO2 photocatalyst. Using production of malondialdehyde (MDA) as an index to assess cell membrane damage by lipid peroxidation, we observed that there was an exponential increase in the production of MDA, whose concentration reached 1.1 to 2.4 nmol · mg (dry weight) of cells−1 after 30 min of illumination, and that the kinetics of this process paralleled cell death. Under these conditions, concomitant losses of 77 to 93% of the cell respiratory activity were also detected, as measured by both oxygen uptake and reduction of 2,3,5-triphenyltetrazolium chloride from succinate as the electron donor. The occurrence of lipid peroxidation and the simultaneous losses of both membrane-dependent respiratory activity and cell viability depended strictly on the presence of both light and TiO2. We concluded that TiO2 photocatalysis promoted peroxidation of the polyunsaturated phospholipid component of the lipid membrane initially and induced major disorder in the E. coli cell membrane. Subsequently, essential functions that rely on intact cell membrane architecture, such as respiratory activity, were lost, and cell death was inevitable.
The use of photocatalysts to destroy organic compounds in contaminated air or water has been extensively studied for the last 25 years. The P25 formulation of titanium dioxide (TiO2) from Degussa Chemical Company (Teterboro, N.J.) is the most widely used photocatalyst. TiO2 in the anatase crystal form is a semiconductor with a band gap of 3.2 eV or more. Upon excitation by light whose wavelength is less than 385 nm, the photon energy generates an electron hole pair on the TiO2 surface. The hole in the valence band can react with H2O or hydroxide ions adsorbed on the surface to produce hydroxyl radicals (OH·), and the electron in the conduction band can reduce O2 to produce superoxide ions (O2−). Both holes and OH· are extremely reactive with contacting organic compounds. Detection of other reactive oxygen species (ROS), such as hydrogen peroxide (H2O2) and singlet oxygen, has also been reported. Complete oxidation of organic compounds and Escherichia coli cells to carbon dioxide can be achieved (17, 19). In the absence of O2 or a suitable electron acceptor, no photocatalytic reaction occurs due to the extremely deleterious electron hole recombination processes (34). The detailed mechanism of the TiO2 photochemical reaction and the various ROS produced have been well-documented (3, 14, 22).
In 1985, Matsunaga and coworkers reported that microbial cells in water could be killed by contact with a TiO2-Pt catalyst upon illumination with near-UV light for 60 to 120 min (20). Later, the same group of workers successfully constructed a practical photochemical device in which TiO2 powder was immobilized on an acetylcellulose membrane. An E. coli suspension flowing through this device was completely killed (21). The findings of Matsunaga et al. created a new avenue for sterilization and resulted in attempts to use this novel photocatalytic technology for disinfecting drinking water and removing bioaerosols from indoor air environments (5, 12, 16, 25, 30, 34). Killing of cancer cells with the TiO2 photocatalyst for medical applications has also been reported (6). The previous work on photocatalytic disinfection and cell killing has recently been reviewed (3). Because of the widespread use of antibiotics and the emergence of more resistant and virulent strains of microorganisms, there is an immediate need to develop alternative sterilization technologies. The TiO2 photocatalytic process is a conceptually simple and promising technology.
Although a wealth of information has demonstrated the efficacy of the biocidal actions of the TiO2 photocatalyst, the fundamental mechanism underlying the photocatalytic killing process has not been well-established yet. An in-depth understanding of the mechanism is essential in order to devise a strategy and apply the technology in a practical system to efficiently kill a wide array of microorganisms. The first mechanism proposed was the mechanism proposed by Matsunaga and coworkers, who believed that direct photochemical oxidation of intracellular coenzyme A to its dimeric form was the root cause of decreases in respiratory activities that led to cell death (20, 21). They reported that the extent of killing was inversely proportional to the thickness and complexity of the cell wall. Saito and workers (25) proposed that the TiO2 photochemical reaction caused disruption of the cell membrane and the cell wall of Streptococcus sobrinus AHT, as shown by leakage of intracellular K+ ions that paralleled cell death. Leakage of intracellular Ca2+ ions has also been observed with cancer cells (26, 27). Perhaps more direct evidence that outer membrane damage occurs was described recently by Sunada et al. (31), who studied E. coli and found that the endotoxin, an integral component of the outer membrane, was destroyed under photocatalytic conditions when TiO2 was used.
The lack of data regarding a specific mechanism of cell death prompted us to investigate the effect of photocatalytic oxidation on cell membrane polyunsaturated phospholipids. Hydroxyl radicals generated by the TiO2 photocatalyst are very potent oxidants and are nonselective in reactivity (22). Because of their high levels of reactivity, they are also very short lived. When irradiated TiO2 particles are in direct contact with or close to microbes, the microbial surface is the primary target of the initial oxidative attack. Polyunsaturated phospholipids are an integral component of the bacterial cell membrane, and the susceptibility of these compounds to attack by ROS has been well-documented (13, 18). Many functions, such as semipermeability, respiration, and oxidative phosphorylation reactions, rely on an intact membrane structure. Lipid peroxidation is, therefore, detrimental to all forms of life. In this paper, we report for the first time that the TiO2 photocatalytic reaction indeed causes the lipid peroxidation reaction to take place and that, as a result, normal functions associated with an intact membrane, such as respiratory activity, are lost. We propose that the loss of membrane structure and, therefore, membrane functions is the root cause of cell death when photocatalytic TiO2 particles are outside the cell.
MATERIALS AND METHODS
Culture of microorganisms.
E. coli K-12 strain ATCC27325 was grown aerobically in 100 ml of Luria-Bertani broth at 30°C on a rotary shaker (200 rpm) for 18 h. The cells used for respiratory measurements were cultured at 25°C. E. coli cells were harvested by centrifugation at 7,800 × g for 15 min, washed, and suspended in sterile deionized water. The final optical density at 660 nm of the suspension was determined by measuring the turbidity with a Spectronic 21D spectrophotometer (Milton Roy Co.). The correlation between optical density at 660 nm and amount of cell mass produced was determined by measuring the dry weights of washed cells at different stages of cell growth.
Photocatalytic reaction.
TiO2 (P25 formulation; Degussa) particles with an average composition of 75% anatase and 25% rutile and a surface area of about 50 m2 g−1 were used for all experiments. A 100-mg ml−1 stock suspension was freshly prepared with deionized water and kept in the dark. TiO2 was added to cells in water immediately prior to the reaction. The final concentrations ranged from 0.1 to 1 mg ml−1. All experiments were conducted in continuously stirred aqueous slurry solutions to ensure maximal mixing and to prevent settling of the TiO2 particles. Overhead illumination by long-wavelength UV light was provided by two 40-W black light tubes (type F40/BL-B; Sylvania) with a spectral maximum at 356 nm. The light intensity reaching the surface at the center of the glass reaction vessel was approximately 8 W m−2; this was determined by using a Blak-Ray UV meter with the peak intensity at 365 nm (model J-221 long-wavelength UV meter; UVP Inc., San Gabriel, Calif.). The reaction was terminated by removing the reaction vessel from the light, and the reaction mixture was used immediately for various assays, as described below. Dark control samples were covered with black cloth and stirred under the same conditions.
Cell viability.
The numbers of viable cells in cell suspensions that were subjected to the TiO2-light treatment or were not subjected to the TiO2-light treatment were determined by plating 30- to 100-μl aliquots of serially diluted suspensions onto Luria-Bertani agar plates. The plates were incubated at 30°C for 24 h, and then the numbers of colonies on the plates were counted.
Determination of lipid peroxidation.
Formation of malondialdehyde (MDA) was used as an index to measure lipid peroxidation. MDA was quantified based on its reaction with thiobarbituric acid (TBA) to form a pink MDA-TBA adduct (10). One milliliter of a TiO2-cell slurry was mixed with 2 ml of 10% (wt/vol) trichloroacetic acid, and the solids were removed by centrifugation at 11,000 × g for 35 min and then for an additional 20 min to ensure that the TiO2 particles, cells, and precipitated proteins were completely removed. Three milliliters of a freshly prepared 0.67% (wt/vol) TBA (Sigma Chemical Co.) solution was then added to the supernatant. The samples were incubated in a boiling water bath for 10 min and cooled, and the absorbance at 532 nm was measured with a Cary 5E spectrophotometer (Varian Instruments, Sugar Lane, Tex.). The concentrations of the MDA formed were calculated based on a standard curve for the MDA (Sigma Chemical Co.) complex with TBA; the E532 was 49.5 mM−1 cm−1. The extent of lipid peroxidation was expressed in nanomoles of MDA per milligram (dry weight) of cells.
Determination of cellular respiration.
After the photocatalytic reaction, a 300-ml TiO2-cell slurry containing 0.5 mg of TiO2 ml−1 and 1.2 × 108 CFU ml−1 was centrifuged at 5,000 × g for 45 min, and the pellet was resuspended in 15 ml of sterile H2O and used for the following assays. An oxygen uptake assay was conducted in a 2-ml water-jacketed chamber fitted with a model 5331 Clark type oxygen electrode (Yellow Springs Instrument Co., Yellow Springs, Ohio). The reaction mixture contained 2 ml of resuspended TiO2-cell slurry and 12.5 mM potassium phosphate buffer (pH 7.0). The reaction was initiated by injecting 50 μl of either 1 M sodium succinate (pH 7.0) or 1 M glucose as the electron donor. The reduction of 2,3,5-triphenyltetrazolium chloride (TTC) to its reduced product, 2,3,5-triphenyltetrazolium formazan (TTF), was measured as described by Smith and Pugh (29), with minor modifications. A 1-ml aliquot of the resuspended TiO2-cell slurry was mixed with 1 ml of a 1% (wt/vol) TTC (Sigma Chemical Co.) solution, and then 50 μl of 0.5 M potassium phosphate buffer (pH 7.0) and 50 μl of 1 M sodium succinate (pH 7.0) were added. The mixture was incubated for 60 min at 20°C in the dark. After incubation, samples were centrifuged at 8,000 × g for 15 min, and the pellets were extracted with 3 ml of methanol for 15 min with shaking. The extracted cells were then removed by centrifugation at 8,000 × g for 15 min, and the absorbance at 485 nm of the red supernatant was measured with a Cary 5E spectrophotometer. The concentrations of the TTF formed were determined based on a standard curve for freshly prepared TTF (Sigma Chemical Co.) in methanol, which had an E485 of 27.5 mM−1 cm−1. The rate of O2 or TTC reduction was expressed in nanomoles of O2 or TTF per minute per milligram (dry weight) of cells.
RESULTS
Effects of cell and TiO2 concentrations on disinfection.
In order to study the killing mechanism, a high concentration of E. coli cells is required to examine any changes in cellular processes resulting from TiO2 biocidal action. To determine the optimal dose of TiO2 for a certain cell concentration, photocatalytic reactions were carried out with cell concentrations ranging from 9.1 × 102 to 5 × 108 CFU ml−1 and TiO2 concentrations ranging from 0.1 to 1 mg ml−1 (Table 1). After 30 min of irradiation with near-UV light in the presence of 0.1 mg of TiO2 ml−1, 92 to 98% of the E. coli cells were killed when the initial cell concentration was less than 105 CFU ml−1. This low dose of TiO2, however, did not effectively kill the cells in a suspension containing 108 CFU ml−1. However, when this cell concentration was used and the TiO2 dose was increased to 0.5 or 1 mg ml−1, there was a significant improvement in the killing efficiency. At a still higher cell concentration (5 × 108 CFU ml−1), the killing efficiency observed with 1 mg of TiO2 ml−1 was much lower. TiO2 concentrations greater than 1 mg ml−1 resulted in decreases in the killing efficiency. This was probably due to shading of the cells by the TiO2 particles so that light in the TiO2-cell slurry became limiting. Thus, the most effective TiO2 concentration for killing E. coli cells at concentrations ranging from 103 to 108 CFU ml−1 was 1 mg ml−1. Nonetheless, due to TiO2 interference with various cellular assays, a lower TiO2 concentration had to be used in several of the studies described below.
TABLE 1.
Effects of various cell and TiO2 concentrations on the killing of E. coli
| Cell concn (CFU ml−1) | TiO2 concn (mg ml−1) | Survival ratio (%)a |
|---|---|---|
| 9.1 × 102 | 0.1 | 2.2 |
| 9.1 × 104 | 0.1 | 8.4 |
| 1 × 108 | 0.1 | 51.1 |
| 1 × 108 | 0.5 | 21.5 |
| 1 × 108 | 1 | 3.7 |
| 5 × 108 | 1 | 30.8 |
Ratio of the cell concentration after 30 min in the light to the corresponding cell concentration in the dark.
Effect of irradiated TiO2 on lipid peroxidation.
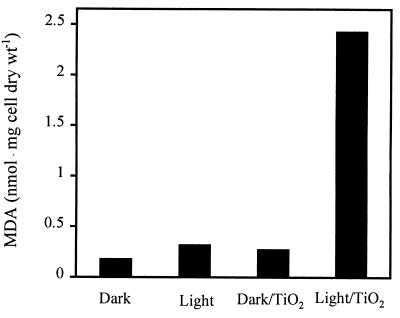
To estimate membrane damage, we examined production of MDA, a product of lipid peroxidation, by E. coli cells. The effects of irradiated TiO2 on MDA formation in E. coli cells under various conditions were determined (Fig. 1). When E. coli cells (2.5 × 108 CFU ml−1) were incubated with 0.1 mg of TiO2 ml−1 in a slurry and were subjected to illumination (8 W m−2) for 30 min with continuous stirring, approximately 2.4 nmol of MDA per mg of cell mass was extracted. However, when the TiO2 slurry was not illuminated, only 0.28 nmol of MDA per mg was detected. When no TiO2 was present, control cells in the dark and in the light produced comparable low levels of MDA, indicating that the amount of preexisting MDA was negligible and that UV light alone at the wavelength and duration used did not result in a significant level of lipid peroxidation. The lipid peroxidation process, therefore, depends on the presence of both light and TiO2. A photocatalysis experiment in which an aged TiO2 solution stored in the presence of room light resulted in a lower level of MDA in the light and a higher background value in the dark. As a result, a freshly prepared TiO2 solution was used for subsequent experiments in which the effect of photocatalytic activity was examined. Although a large amount of TiO2 yielded more MDA in the light, it also resulted in an elevated background value in the dark control. As expected, when a low level of TiO2 (0.1 mg ml−1) was used along with a high cell concentration (Fig. 1), only 44% of the viable cells were killed within 30 min, yet the amount of MDA produced was nearly nine times the amount produced in the TiO2 dark control.
FIG. 1.
Effects of light and TiO2 on lipid peroxidation of E. coli. Cells (2.5 × 108 CFU ml−1) were incubated in the dark, in UV light, in the dark with TiO2 (0.1 mg ml−1), and in UV light with TiO2 (0.1 mg ml−1) for 30 min with continuous stirring. The light intensity was 8 W m−2. MDA was quantified by the TBA assay.
The validity of using the amount of MDA as an index to assess lipid peroxidation has been challenged due the complexity of determining amounts of MDA (2, 9). To prove that MDA was indeed a product of lipid peroxidation under photocatalytic conditions and that it did not arise as an artifact or as a decomposition product from other macromolecules in whole cells, we used phosphatidylethanolamine as a model E. coli membrane phospholipid and studied its peroxidation. Since phosphatidylethanolamine is one of the predominant phospholipids in most bacterial cell membranes (35), using this compound could also confirm that lipid peroxidation occurred and could support the hypothesis that this pathway is involved in the biocidal action of TiO2. When phosphatidylethanolamine (0.2 mg ml−1; Sigma Chemical Co.) and TiO2 (1 mg ml−1) were subjected to UV illumination for 1 h, approximately 0.72 μM MDA was detected based on the standard MDA-TBA method. The concentration of MDA obtained with the dark control was only 0.21 μM and was probably the result of preexisting oxidized products in the sample. Both the validity of using MDA as an index compound for the assay and the efficacy of the TiO2 photocatalyst for initiating the lipid peroxidation reaction were manifested by this experiment.
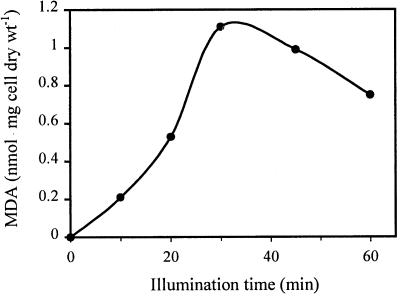
To determine how the lipid peroxidation process affects cell survival and to correlate this process with losses of other cellular functions normally associated with an intact membrane, we carried out experiments to determine the kinetics of lipid peroxidation (Fig. 2). A 10-ml suspension containing 1.8 × 109 CFU ml−1 and TiO2 (1 mg ml−1) was subjected to illumination with continuous stirring. Due to the nature of the ROS, once initiated, the TiO2-mediated reaction cannot be terminated even by placing the reaction mixture on ice or in the dark. To ensure accuracy, we first subjected a sample to 60 min of illumination and then after 15 min started a 45-min sample and so on. For the zero-time sample we mixed the cells with TiO2 in the dark and started the MDA-TBA analysis immediately. Within 10 min, the MDA levels started to increase, and then they increased steadily over time and reached a maximum value of 1.1 nmol · mg (dry weight) of cells−1 after 30 min, indicating that peroxidation of membrane lipid was occurring. A slight decrease in MDA production was observed during prolonged illumination.
FIG. 2.
Kinetics of lipid peroxidation in E. coli induced by TiO2 photocatalysis. Cell suspensions (1.8 × 109 CFU ml−1) were treated with TiO2 (1 mg ml−1) and UV light (8 W m−2) for various periods of time. MDA was quantified by the TBA assay.
Since it is known that a wide range of organic compounds can be decomposed under photocatalytic conditions (14, 19, 22), it is possible that the product of lipid peroxidation, MDA, is also a target of oxidative degradation. To test this hypothesis, we illuminated an MDA solution (27.5 μM) containing TiO2 (0.1 mg ml−1) for 30 min and then determined the residual amount of MDA by the MDA-TBA method. Light alone or the TiO2 photocatalyst in the dark had no effect on the preexisting MDA. However, as we expected, the illuminated TiO2 preparation lost nearly 88% of the added MDA within 30 min.
Effect of irradiated TiO2 on cellular respiratory activity.
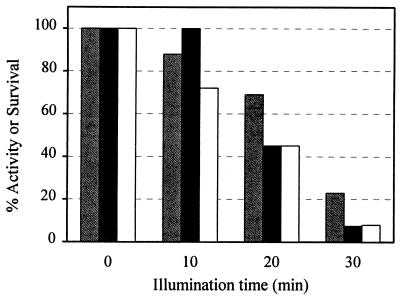
Since the bacterial cell membrane contains essential components of the respiratory chain, it was reasonable to investigate the effect of TiO2 photocatalysis on cellular respiratory activities. Respiration was monitored by determining the uptake of O2 with a Clark type oxygen electrode and by studying the reduction of TTC to TTF, a red precipitate. Succinate was used as the electron donor in both assays. When E. coli cells at a concentration of 1.2 × 108 CFU ml−1 were irradiated with TiO2 (0.5 mg ml−1) for various periods of time, the kinetic data (Fig. 3) revealed an apparent loss of respiratory activity with reaction time, and the kinetics coincided well with the loss of cell viability. After 30 min, both viability and respiratory activity were reduced drastically. Similar results were obtained when glucose was used instead of succinate as the electron donor. The progressive loss of viability and respiratory activity is in good agreement with the lipid peroxidation kinetics shown in Fig. 2.
FIG. 3.
Kinetic losses of respiratory activity and viability of E. coli induced by TiO2 photocatalysis. Cells (1.2 × 108 CFU ml−1) were treated with TiO2 (0.5 mg ml−1) and incubated under UV light (8 W m−2). Respiratory activity was determined by measuring the reduction of oxygen and the reduction of TTC to TTF. Viability was determined by the plate count method. Gray bars, oxygen uptake; black bars, TTC reduction; open bars, survival. The 100% activities at time zero were 16 nmol of O2 · min−1 · mg (dry weight) of cells−1 for oxygen uptake and 0.27 nmol of TTF · min−1 · mg (dry weight) of cells−1 for TTC reduction.
Light alone did not have a significant effect on cell viability or on O2 uptake and TTC reduction activities. Incubation of TiO2 with E. coli cells in the dark had only a slight impact on the O2 uptake rate and viability. However, we observed that TiO2 alone consistently caused a decrease in the whole-cell TTC reduction rate in the dark. After TiO2 was incubated with E. coli cells for 15 min in the dark, 27% of the TTC reduction rate was lost, and after 30 min, only 60% of the activity remained. However, Fig. 3 shows that when light was present along with TiO2, the residual TTC reduction activity was only 9% after 30 min of reaction. Even though TiO2 had an impact on TTC reduction activity in the dark, the additional decrease caused by light is significant. As observed with lipid peroxidation, the loss of respiratory activity depends on the presence of both light and TiO2.
DISCUSSION
The results of our viability study confirmed the previous findings of Matsunaga et al. (20, 21), Saito et al. (25), and Wei et al. (34) that illuminated TiO2 exhibits bactericidal activity and that disinfection is positively correlated with the TiO2 dose used up to a concentration of 1 mg ml−1. The survival ratios in Table 1 compare the levels of viability in the light with those in the dark at corresponding cell and TiO2 concentrations. Including TiO2 in the dark control was necessary since when the TiO2-cell slurry was stirred in the dark for 30 min, it yielded a slightly lower viable cell count than a similar sample without TiO2 would. We attributed this phenomenon to aggregation of TiO2 particles with cells in the dark. This could result in the formation of one colony from more than one cell on an agar plate. A similar observation was made by Saito et al. (25).
Our results demonstrate for the first time that as determined with MDA as the index compound, lipid peroxidation of polyunsaturated phospholipids in E. coli occurs as a result of oxidative actions exerted by the TiO2 photocatalyst. The process requires the presence of both light and TiO2 (Fig. 1). It is apparent from the time course of MDA production that the initial phase of lipid peroxidation progresses at an exponential rate. The subsequent decrease in the MDA concentration after prolonged illumination is attributed to photocatalytic oxidation of MDA. Initiation of lipid peroxidation is known to require some form of radical attack. However, once initiated, the reaction propagates by generating a peroxy radical intermediate that, by itself, undergoes peroxidation with another unsaturated lipid molecule (13). It has also been suggested that superoxide ions, which are known to be produced on the irradiated TiO2 surface, react with the intermediate hydroperoxide to initiate new radical chain reactions (32, 33), assuming that the molecule can penetrate the cell membrane once its semipermeability is compromised. If not terminated, the cascades of autoxidation reactions explain the exponential increase in MDA production and ultimately lead to destruction of the lipid phase, which is the cell membrane itself.
Another serious effect of the lipid peroxidation process is that many of the intermediates in this process can react with important biological molecules to cause additional damage. It is thought that lipid peroxidation products may be mutagenic (1, 7, 8). Furthermore, MDA itself is quite reactive and is able to modify proteins via carbonylation or to form protein-MDA adducts (4, 24). Both pathways account for the disappearance of MDA from assay mixtures after 30 min (Fig. 2). Our data also establish that MDA is oxidatively destroyed by TiO2 photocatalysis. This is not surprising given the nonspecific nature of the oxidative attacks by ROS that occur under photocatalytic conditions. Our MDA values, therefore, were the net result of the rate of MDA production and the rate of MDA destruction that occurred concurrently by the same photocatalytic process or during the subsequent participation of MDA in other chemical reactions. Under prolonged illumination conditions, cell wall breakdown and cell membrane breakdown would presumably allow TiO2 particles to gain access to and attack the cell membrane directly. Eventually, the rate of MDA destruction exceeds the rate of MDA production, as observed after 30 min of reaction (Fig. 2). Based on this evidence, the rate and extent of lipid peroxidation in E. coli cells have very likely been underestimated previously, as has the severity of the impact of the TiO2 photocatalytic process. Consequently, the idea that ROS derived from the irradiated TiO2 reaction can disturb cell membrane phospholipids, lipoproteins, and nucleic acids, which places cells in a state of oxidative stress and eventually leads to cell death, is a viable concept.
Alterations in membrane architecture caused by lipid peroxidation ultimately lead to conformational changes in many membrane-bound proteins and electron mediators and to changes in how these compounds are oriented across the cell membrane. Consequently, functional changes are expected. Parallel research in our laboratory has also established that illuminated TiO2 has an adverse effect on the semipermeability of E. coli cell membranes (15). Our findings explain the observed leakage of K+ ions from Streptococcus sobrinus (25) and the leakage of Ca2+ ions from cancer cells (26, 27) following TiO2 photocatalytic treatments. Our results also confirm previous reports of Matsunaga et al. (20, 21) and provide additional evidence that the TiO2 photocatalytic reaction has a deleterious effect on cellular respiratory activity, the loss of which parallels cell death. Presumably, membrane disorder disrupts the spatial organization of the electron mediators that span the cell membrane and causes the electron transport pathway from succinate or glucose to oxygen or TTC to be short-circuited. Tetrazolium dyes, such as TTC in its oxidized form, are reducible by the cytochrome systems of bacteria during respiration (28). Reduction of TTC has been used frequently to assess metabolic activities in various microorganisms (23, 36). Failure to reduce an artificial acceptor, such as TTC, following TiO2 treatment implies that the damaged cell membrane can no longer generate or maintain a sufficiently negative redox potential. When Farr and coworkers subjected E. coli to oxidative stress, both radical-generating conditions and H2O2 treatments caused a rapid decrease in proton motive force-dependent and -independent transport across the cell membrane (11). These authors suggested that oxidative disruption of the membrane integrity reduces the proton motive force, which is the driving force for ATP synthesis.
Based on our findings, we propose that ROS, such as OH·, O2−, and H2O2 generated on the irradiated TiO2 surface, operate in concert to attack polyunsaturated phospholipids in E. coli. The lipid peroxidation reaction that subsequently causes a breakdown of the cell membrane structure and therefore its associated functions is the mechanism underlying cell death. All life forms have a cell membrane made up of a variety of lipids with various degrees of unsaturation and rely on their structures to carry out essential functions. Thus, the proposed killing mechanism is applicable to all cell types. Indeed, preliminary data for TiO2 photocatalysis of a gram-positive organism, Micrococcus luteus, demonstrated that lipid peroxidation occurred and that there was a simultaneous loss of cell viability. The attack by ROS generated by the photocatalytic process outside the cell is very likely the initial mode of killing that is observed for bacteria and other cell types. However, the findings reported here do not rule out the possibility of photocatalytic attack inside a cell after TiO2 particles are ingested via phagocytosis, as observed in eucaryotic cells (6).
ACKNOWLEDGMENTS
This work was supported by the FIRST Program at the National Renewable Energy Laboratory and the Center for Indoor Air Research.
REFERENCES
- 1.Akasaka S, Yamamoto K. Mutagenesis resulting from DNA damage by lipid peroxidation in the supF gene of Escherichia coli. Mutat Res. 1994;315:105–112. doi: 10.1016/0921-8777(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 2.Aust S. Lipid peroxidation. In: Greenwald R A, editor. CRC handbook of methods for oxygen radical research. Boca Raton, Fla: CRC Press, Inc.; 1987. pp. 203–207. [Google Scholar]
- 3.Blake D M, Maness P-C, Huang Z, Wolfrum E J, Jacoby W A, Huang J. Application of the photocatalytic chemistry of titanium dioxide to disinfection and the killing of cancer cells. Sep Purif Methods. 1999;28:1–50. [Google Scholar]
- 4.Burcham P C, Kuhan Y T. Introduction of carbonyl groups into proteins by the lipid peroxidation product, malondialdehyde. Biochem Biophys Res Commun. 1996;220:996–1001. doi: 10.1006/bbrc.1996.0521. [DOI] [PubMed] [Google Scholar]
- 5.Byrne J A, Eggins B R, Brown N M D, McKinnery B, Rouse M. Immobilisation of TiO2 powder for the treatment of polluted water. Appl Catal B Environ. 1998;17:25–36. [Google Scholar]
- 6.Cai R, Hashimoto K, Itoh K, Kubota Y, Fujishima A. Photokilling of malignant cells with ultrafine TiO2 powder. Bull Chem Soc Jpn. 1991;64:1268–1273. [Google Scholar]
- 7.Cao E H, Liu X Q, Wang L G, Xu N F. Evidence that lipid peroxidation products bind to DNA in liver cells. Biochim Biophys Acta. 1995;1259:187–191. doi: 10.1016/0005-2760(95)00162-6. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhary A K, Nokubo M, Redy G R, Yeola S N, Morrow J D, Blair I A, Marnett L J. Detection of endogenous malondialdehyde-deoxyguanosine adducts in human liver. Science. 1994;265:1580–1582. doi: 10.1126/science.8079172. [DOI] [PubMed] [Google Scholar]
- 9.Draper H H, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186B:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 10.Esterbauer H, Cheeseman K H. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186B:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 11.Farr S B, Touati D, Kogoma T. Effects of oxygen stress on membrane functions in Escherichia coli: role of HPI catalase. J Bacteriol. 1988;170:1837–1842. doi: 10.1128/jb.170.4.1837-1842.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaswami D Y, Trivedi D M, Block S S. Photocatalytic disinfection of indoor air. J Sol Energy Eng. 1997;119:92–96. [Google Scholar]
- 13.Gutteridge J M C. Lipid peroxidation: some problems and concepts. In: Halliwell B, editor. Oxygen radicals and tissue injury. Proceedings of a Brook Lodge Symposium. Bethesda, Md: Upjohn Co.; 1987. pp. 9–19. [Google Scholar]
- 14.Hoffmann M R, Martin S T, Choi W, Bahnemann D W. Environmental applications of semiconductor photocatalysis. Chem Rev. 1995;95:69–96. [Google Scholar]
- 15.Huang Z, Maness P C, Smolinski S, Blake D M, Jacoby W A, Wolfrum E J. Abstracts of the 99th General Meeting of the American Society for Microbiology 1999. Washington, D.C: American Society for Microbiology; 1999. Effects of titanium dioxide photocatalytic reaction on the permeability of E. coli, abstr. Q-234; p. 578. [Google Scholar]
- 16.Ireland J C, Klostermann P, Rice E W, Clark R M. Inactivation of Escherichia coli by titanium dioxide photocatalytic oxidation. Appl Environ Microbiol. 1993;59:1668–1670. doi: 10.1128/aem.59.5.1668-1670.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacoby W A, Maness P C, Wolfrum E J, Blake D M, Fennel J A. Mineralization of bacterial cell mass on a photocatalytic surface in air. Environ Sci Technol. 1998;32:2650–2653. [Google Scholar]
- 18.Kappus H. Lipid peroxidation: mechanisms, analysis, enzymology and biological relevance. In: Sies H, editor. Oxidative stress. New York, N.Y: Academic Press, Inc.; 1985. pp. 273–310. [Google Scholar]
- 19.Legrini O, Oliveros E, Braun A M. Photochemical processes for water treatment. Chem Rev. 1993;93:671–698. [Google Scholar]
- 20.Matsunaga T, Tomada R, Nakajima T, Wake H. Photochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol Lett. 1985;29:211–214. [Google Scholar]
- 21.Matsunaga T, Tomoda R, Nakajima Y, Nakamura N, Komine T. Continuous-sterilization system that uses photosemiconductor powders. Appl Environ Microbiol. 1988;54:1330–1333. doi: 10.1128/aem.54.6.1330-1333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills A, Le Hunte S. An overview of semiconductor photocatalysis. J Photochem Photobiol A Chem. 1997;108:1–35. [Google Scholar]
- 23.Parrington L J, Sharpe A N, Peterkin P I. Improved aerobic colony count technique for hydrophobic grid membrane filters. Appl Environ Microbiol. 1993;59:2784–2789. doi: 10.1128/aem.59.9.2784-2789.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Requena J R, Fu M X, Ahmed M J, Jenkins A J, Lyons T J, Baynes J M, Thorpe S R. Quantification of malondialdehyde and 4-hydroxynonenal adducts to lysine residue in native and oxidized human low-density lipoprotein. Biochem J. 1997;322:317–325. doi: 10.1042/bj3220317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito T, Iwase T, Morioka T. Mode of photocatalytic bactericidal action of powdered semiconductor TiO2 on mutans streptococci. J Photochem Photobiol B Biol. 1992;14:369–379. doi: 10.1016/1011-1344(92)85115-b. [DOI] [PubMed] [Google Scholar]
- 26.Sakai H, Cai R, Hashimoto K, Kato T, Hashimoto K, Fujishima A, Kubota Y, Ito E, Yoshioka T. Photocatalytic effect of TiO2 particles on tumor cells—study on mechanism of cell death by measuring concentration of intracellular calcium ion. Photomed Photobiol. 1990;12:135–138. [Google Scholar]
- 27.Sakai H, Ito E, Cai R-X, Yoshioka T, Hashimoto K, Fujishima A. Intracellular Ca+2 concentration change of T24 cell under irradiation in the presence of TiO2 ultrafine particles. Biochim Biophys Acta. 1994;1201:259–265. doi: 10.1016/0304-4165(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 28.Smith J J, McFeters G A. Mechanisms of INT (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl tetrazolium chloride), and CTC (5-cyano-2,3-ditolyl tetrazolium chloride) reduction in Escherichia coli K-12. J Microbiol Methods. 1997;29:161–175. doi: 10.1111/j.1365-2672.1996.tb03212.x. [DOI] [PubMed] [Google Scholar]
- 29.Smith S N, Pugh G J F. Evaluation of dehydrogenase as a suitable indicator of soil microflora activity. Enzyme Microb Technol. 1979;1:279–281. [Google Scholar]
- 30.Stevenson M, Bullock K, Lin W-Y, Rajeshwar K. Sonolytic enhancement of the bactericidal activity of irradiated titanium dioxide suspensions in water. Res Chem Intermed. 1997;23:311–323. [Google Scholar]
- 31.Sunada K, Kikuchi Y, Hashimoto K, Fujishima A. Bactericidal and detoxification effects of TiO2 thin film photocatalysts. Environ Sci Technol. 1998;32:726–728. [Google Scholar]
- 32.Sutherland M W, Gebicki J M. A reaction between the superoxide free radical and lipid peroxidation in sodium linoleate micelles. Arch Biochem Biophys. 1982;214:1–11. doi: 10.1016/0003-9861(82)90001-7. [DOI] [PubMed] [Google Scholar]
- 33.Thomas M J, Mehl K S, Pryor W A. The role of superoxide in xanthine oxidase-induced autooxidation of linoleic acid. J Biol Chem. 1982;257:8343–8347. [PubMed] [Google Scholar]
- 34.Wei C, Lin W-Y, Zaina Z, Williams N E, Zhu K, Kruzic A P, Smith R L, Rajeshwar K. Bactericidal activity of TiO2 photocatalyst in aqueous media: toward a solar-assisted water disinfection system. Environ Sci Technol. 1994;28:934–938. doi: 10.1021/es00054a027. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson S G. Gram-negative bacteria. In: Ratledge C, Wilkinson S G, editors. Microbial lipids. Vol. 1. San Diego, Calif: Academic Press; 1988. pp. 299–317. [Google Scholar]
- 36.Zimmermann R, Iturriaga R, Becker-Birck J. Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl Environ Microbiol. 1978;36:926–935. doi: 10.1128/aem.36.6.926-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]