Abstract
Apigenin is a kind of flavonoid with many beneficial biological effects. It not only has direct cytotoxicity to tumor cells, but also can boost the antitumor effect of immune cells by modulating immune system. The purpose of this study was to investigate the proliferation of NK cells treated with apigenin and its cytotoxicity to pancreatic cancer cells in vitro, and explore its potential molecular mechanism. In this study, the effect of apigenin on NK cell proliferation and killing pancreatic cancer cells were measured by CCK-8 assay. Perforin, granzyme B (Gran B), CD107a, and NKG2D expressions of NK cells induced with apigenin were detected by flow cytometry (FCM). The mRNA expression of Bcl-2, Bax and protein expression of Bcl-2, Bax, p-ERK, and p-JNK in NK cells were evaluated by qRT-PCR and western blotting analysis, respectively. The results showed that appropriate concentration of apigenin could significantly promote the proliferation of NK cells in vitro and enhance the killing activity of NK cells against pancreatic cancer cells. The expressions of surface antigen NKG2D and intracellular antigen perforin and Gran B of NK cells were upregulated after treating with apigenin. Bcl-2 mRNA expression was increased, while Bax mRNA expression was decreased. Similarly, the expression of Bcl-2, p-JNK, and p-ERK protein was upregulated, and the expression of Bax protein was downregulated. The molecular mechanism of the immunopotentiation effects of apigenin may be that it up-regulates Bcl-2 and down-regulates Bax expression at the gene and protein levels to facilitate NK cell proliferation, and up-regulates the expression of perforin, Gran B, and NKG2D through the activation of JNK and ERK pathways to enhance NK cell cytotoxicity.
Keywords: Apigenin, NK cell, immunopotentiation, pancreatic cancer, killing activity
Introduction
Pancreatic cancer is a highly malignant tumor of the digestive tract with rapid progress and poor prognosis characteristics. The global incidence of pancreatic cancer is on the rise over recent years. Cancer statistics in the United States shows that the 5-year relative survival rate is lowest for cancers of the pancreas (10%).1 However, in China, the 5-year survival rate is lower, only 7.2%.2 At present, pancreatic cancer is mainly treated by surgery in clinical practice, combined with chemotherapy, radiotherapy, and targeted therapy, but the therapeutic effect is still not very good. Recently the emergence of immunotherapy has provided a new direction for the treatment of pancreatic cancer.
Apigenin is a natural bioactive flavone with rich sources, which is known to exist in many kinds of vegetables and fruits, such as parsley, onion, grape, and apple. Apigenin has many beneficial biological activities, such as antitumor, antivirus, anti-inflammation, anti-oxidation, and neuroprotective effects, while it has low cytotoxicity to human normal cells.3–6 The direct cytotoxic effect of apigenin on tumor cell proliferation, metastasis, and invasion has been confirmed by many studies.7 In recent years, more and more evidences indicate that apigenin has important immunomodulatory effects and shows positive regulatory effects in a variety of antitumor effector cells. It is reported that apigenin exerts its immunoregulatory effect in vivo through modulating NF-κB.8 Apigenin can restrict melanoma growth by suppressing programmed cell death ligand-1 (PD-L1) expression in melanoma and host dendritic cells to elicit synergistic therapeutic effects.9 Villalobos-Ayala et al.10 reported that apigenin could increase Src Homology-2 (SH2) domain-containing inositol 5′-Phosphatase-1(SHIP-1)expression which correlated with the expansion of tumoricidal macrophages (TAM) and improve antitumor immune responses in the TME of mice with pancreatic cancer. New evidence confirms that apigenin can promote the proliferation and activation of NK cells and CD8+T cells, proving its ability to stimulate antitumor immunity.11 Lee et al. revealed that apigenin can enhance NK cell cytotoxicity to hepatocellular carcinoma (HCC) cells expressing HIF-1α through high expression of CD95 L on the surface of NK cells.12
NK cells are important effector cells to exert innate immunity and are the first line of defense against viruses and tumors for its recognizing antigens without prior sensitization, despite their small number in the peripheral blood.13 NK cell activation does not depend on antigen processing and presentation. However, it exerts cytotoxic activity similar to that of CD8+T cells against tumor cells and target cells infected by viruses, that is, it lyse target cells by releasing cytotoxic granules (perforin and granzyme). In addition, activated NK cells regulate adaptive immune response through secreting tumor necrosis factor- α and interferon- γ or other cytokines. With the development of NK cell research, the safety and efficacy of tumor immunotherapy based on NK cell have been continuously verified.14
In the present study, we attempted to investigate the immunoregulatory effect of apigenin on NK cell in terms of proliferation and killing effect on pancreatic cancer cells and explore the potential molecular mechanisms. The results showed that apigenin is an immunopotentiator of NK cells, which can facilitate the proliferation of NK cells and enhance their killing activity against pancreatic cancer cells. The current research adds new reference data for further understanding the immunoregulatory effect of apigenin.
Materials and methods
Reagents and antibodies
Apigenin was purchased from Sigma-Aldrich. Recombinant human IL-2 (rhIL-2) and recombinant human IL-15 (rhIL-15) were purchased from Xiamen Amoytop Biotech Co., Ltd Stem cell growth medium (SCGM) was purchased from CellGenix GmbH. Dulbecco’s modified eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from Thermo scientific. FITC-anti-human CD56, PerCP-Cy5.5-anti-human CD3, APC-anti-human CD107a, PE-anti-human Gran B, PE-anti-human Perforin, PE-anti-human NKG2D, and isotype controls were obtained from BD Biosciences. Trizol and SYBR Green PCR Master Mix were purchased from Thermo Fisher Scientific. PrimeScriptTMRT reagent Kit was purchased from Takara Bio Inc. The antibodies of Bcl-2, Bax, and GAPDH were obtained from Proteintech Group, Inc. phospho-SAPK/JNK and phospho-ERK antibodies were got from Cell Signaling Technology, Inc. AP-labed Goat anti-Rabbit IgG (H + L), AP-labed Goat anti-Mouse IgG (H + L), and CCK-8 kit were got from Beyotime Institute of Biotechnology.
Cell culture
On the basis of the NK cell culture method previously established by our research group, we slightly improved the NK cell culture technology and added IL-15 to obtain higher amplification efficiency.15 In brief, peripheral blood mononuclear cells (PBMCs) isolated from 20 mL of heparin anticoagulant of healthy donors were cultured in a stem cell growth medium (SCGM) supplemented with 500U/ml rhIL-2, 10 ng/mL rhIL-15 and 10% autologous serum at 37°C in the humidified atmosphere of 5% CO2 for 12 d. The SCGM was replaced every 2 days. After 12 days of culture, the morphological characteristics of cells were observed and photographed under an inverted phase contrast microscope. Pancreatic cancer cells (PANC-1, SW1990, BxPC-3) were purchased from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM supplemented with 10% FBS at 37°C in 5% CO2. The culture medium was replaced every 2 days.
Purification of NK cells
After 12 days, the cells cultured in SCGM were collected and washed twice by sterile phosphate buffered saline (PBS), and then were resuspended in sterile PBS containing 0.5% bovine serum albumin and 2 mm EDTA. Subsequent negative selection and positive selection were carried out using magnetic beads coated with anti-human CD3 and anti-human CD56 according to the cell selection protocol, respectively (Miltenyi Biotec Inc).16 The cells before and after purification were stained with FITC-anti-human CD56, PerCP-Cy5.5-anti-human CD3 for flow cytometry (FCM) analysis. Corresponding fluorescent labeled mouse-IgG1 or mouse-IgG2b was used as isotype control.
Proliferation assay
The purified NK cells were collected and resuspended in SCGM containing IL-2 and IL-15 with a cell density of 2.5 × 105/ml. Different concentrations of apigenin obtained by multiple dilution method were incubated with previously purified NK cells in a 96-well plate at 37°C in 5% CO2 for 24 h, 48 h, and 72 h, respectively. The final apigenin concentrations of apigenin in different experimental groups were 40, 20, 10, 5, 2.5, 1.25, and 0.62 μM, respectively. Control group without apigenin was set at the same time and each group was set with three repeated wells. According to the operating instructions, 20 μL of CCK-8 solution was added into each well and cultured for another 4 h in the dark at 37°C in 5% CO2. To evaluate cell proliferation rate of NK cells, the OD value was measured with a microplate reader at the 450 nm wavelength.17 According to cell proliferation results, NK cell treated with apigenin (final concentration: 2.5, 5, 10, and 20 μM) for 72 h was selected in the next study.
Cytotoxicity assay
NK cells incubated with various concentrations of apigenin for 72 h were harvested and used as effector cells after being washed three times with PBS. Pancreatic cancer cells (PANC-1, SW1990, BxPC-3 cells) in logarithmic growth phase were used as target cells. NK cells induced with various concentrations of apigenin were co-incubated with pancreatic cancer cells (effector/target ratio = 10:1) in a 96-well plate at 37°C in 5% CO2 for 24 h. The experimental group, effector cell control group, and target cell control group were, respectively, provided with three wells. Then, 24 h later, 20 μL of CCK-8 solution was added into each well and incubated for further 4 h. The value of OD could be assessed with a microplate reader at a wavelength of 450 nm. Cytotoxicity was calculated with the following formula.17
Flow cytometric analysis
NK cells induced with various concentrations of apigenin for 72 h in a 6-well plate at the density of 2.5 × 105 cells/ml were harvested by centrifugation. NK cells were stained with 20 μL FITC–anti-CD56 and 20 μL PerCP-Cy5.5-anti-CD3 in a dark place at room temperature for 15 min, and then fixed and permeabilized by treating with Cell Permeabilization Kit (ANDER GRUB, Austria) for 15 min. According to manufacturer’s instructions, 5 μL PE-anti-Gran B or 5 μL PE-anti-perforin and isotype-matched control were added together with permeabilization reagents and incubated for 15 min. In the same way, NK cells were incubated with 20 μL FITC–anti-CD56, 20 μL PerCP-Cy5.5-anti-CD3, 5 μL PE-anti-NKG2D, or 5 μL APC-anti-CD107a in the dark for 15 min. Fluorescent stained cells were washed twice by PBS and detected by FCM.18
Quantitative real time-PCR (qRT-PCR)
The qRT PCR and primer sequences were referenced from Tano et al.19 After incubation with different concentrations of apigenin for 72 h, NK cells in each group were collected and washed with PBS, and then total RNA of NK cells harvested was extracted with Trizol. According to the instructions of reverse transcription kit purchased from ThermoFisher Scientific, Inc, cDNA was synthetized. The mRNA levels of Bcl-2 and Bax were detected using SYBR Green PCR Master Mix with real-time PCR system (Bio-Rad, CFX96 Real-Time system). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) acted as a housekeeping gene and internal control. The primer pairs of Bcl-2, Bax, and GAPDH mRNA were as follows: Bcl-2 forward, 5′-GTGGAGGAGCTCTTCAGGGA-3′, and reverse, 5′-AGGCACCCAGGGTGAGCAA-3′, Bax forward, 5′-GGCCCACCAGCTCTGAGCAGA-3′ and reverse, 5′-GCCACGTGGGCGGTCCCAAAGT-3′, GAPDH forward, 5′-GAAATCCCAGCACCATCTTCCCAGG-3′ and reverse, 5′-GTGGTGGACCTCATGGCCCACCATG-3′. The 2−ΔΔCt method was utilized to analyze relative gene expression levels.20 The expression level of Bcl-2 and Bax mRNA was evaluated with GAPDH mRNA expression as the standard.
Western blotting analysis
NK cells treated with apigenin for 72 h were harvested and washed twice with precooled PBS. Cells were lysed in protein extraction regent. The lysate samples were separated by SDS-PAGE, and blotted onto a polyvinylidene difluoride membrane. After being blocked with 1% BSA in TBST at room temperature, the membranes were incubated with primary antibodies for Bcl-2, Bax, p-JNK, and p-ERK overnight at 4°C. The membranes were then incubated with an alkaline phosphatase peroxidase-conjugated secondary antibody. Detection was performed by the BCIP/NBT Alkaline Phosphatase Color Development Kit. The recorded protein bands were captured by a digital camera. The gray value was analyzed with Image J software.21
Statistical analysis
Data were described as mean ± standard deviation (SD), no less than triplicate determinations were independently carried out in all experiments. Statistical analysis was performed by one-way ANOVA using SPSS 16.0 software, Duncan’s test was employed for multiple comparisons, and Dunnett’s test was used to compare the differences between experimental groups and the control group. GraphPad Prism 6.0 software was utilized to draw statistical graph. p values <0.05 were considered statistically significant.
Results
Identification of NK cells
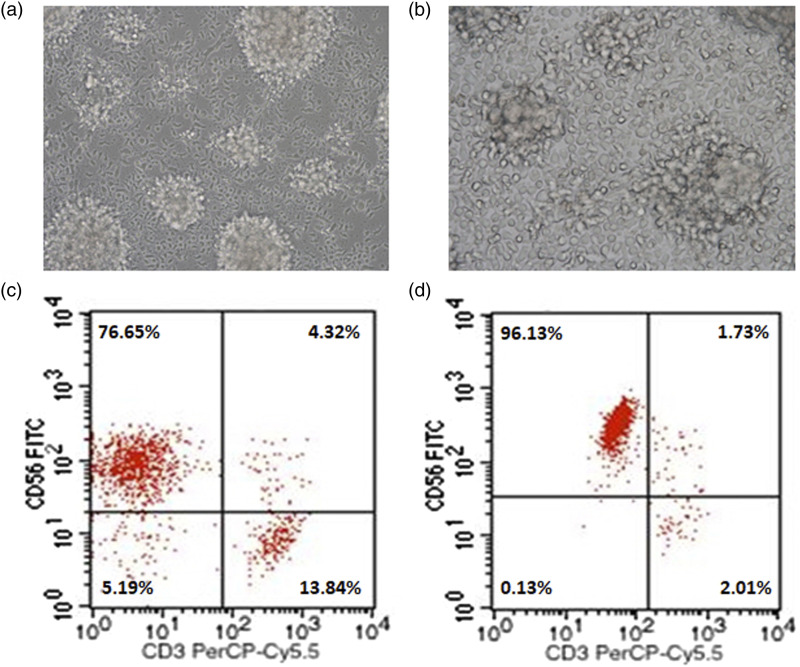
PBMCs derived from healthy donors were induced in vitro with rhIL-2 and rhIL-15 in a stem cell growth medium (SCGM) for 12 days. Under the inverted phase contrast microscope, the cultured cells were crystal clear, showing different morphologies such as spherical, ovoid, fusiform, and a large number of cell colonies of different sizes. A small number of cells adhered to the culture flask and had adhesion ability (Figure 1(a) and 1(b)). CD3−CD56+ is the characteristic molecular marker of NK cells. The results of flow cytometry analysis showed that the average percentage of cells expressing CD3−CD56+ surface molecules was 74.25% ± 3.37% (Figure 1(c)), suggesting that NK cells were efficiently expanded through the above culture protocol. After magnetic cell sorting, the desired NK cell population with purity of 96.31% ± 2.07% was obtained. This will be conducive to subsequent experimental research. (Figure 1(d)).
Figure 1.
Morphological characteristics and phenotypic identification of NK cells after expansion. a: Cellular morphologies of NK cells in suspension growth state after 12 days of expansion (200×). b: Cellular morphologies of NK cells adhering to the wall of the culture flask (400×). c: Representative dot plot depicting the percentage of NK cells within the cultured PBMCs. d: Representative dot plot of NK cell percentage after magnetic cell sorting.
Effect of apigenin on proliferation of NK cells
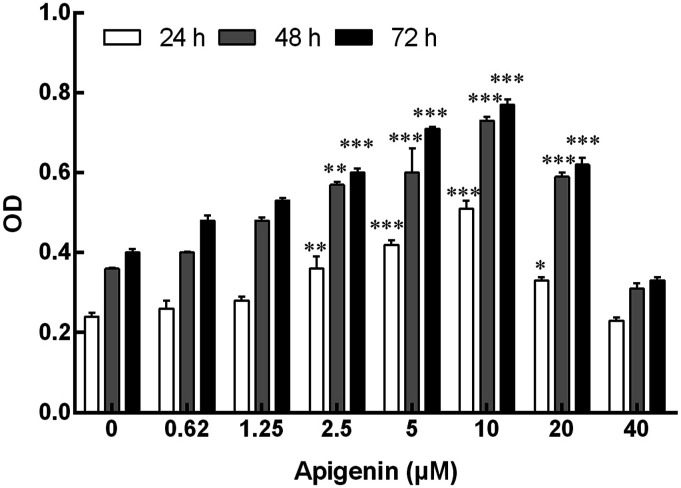
NK cells were co-incubated with apigenin at different doses for 24 h, 48 h, and 72 h. The results showed that apigenin-induced NK cell proliferation was shown to be a bell-shape response. Specifically, apigenin could promote NK cell proliferation within the concentration range of 2.5 μM–20 μM (Figure 2); within the concentration range of 2.5 μM–10 μM, the cell proliferation effect became more and more obvious with the increase of dose. However, the proliferation effect decreased at 20 μM. Compared with 24 h and 48 h, 72 h was more significant in cell proliferation induced by the same dose of apigenin. Therefore, in the following study, 2.5 μM–20 μM concentration and 72 h were used as the concentration range and time point to study the effect of apigenin on NK cell function change.
Figure 2.
Effect of apigenin on proliferation of NK cells. NK cells were cultured with apigenin at concentrations of 0, 2.5, 5, 10, 20, 40 μM for 24 h, 48 h, and 72 h. Data are presented as mean ± standard deviation (SD). *p < 0.05, **p < 0.01, ***p < 0.001 compared with the respective control group (0 μM group). OD, optical density.
Cytotoxicity of NK cells to pancreatic cancer cells
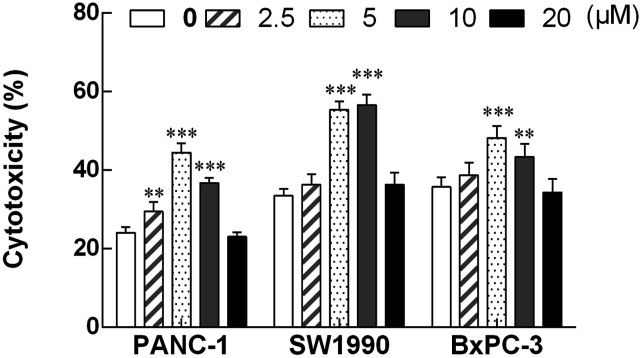
After treatment with different concentrations of apigenin for 72 h, NK cells were co-incubated with pancreatic cancer cells at the effect: target ratio of 10:1. As shown in Figure 3, the killing activity of NK cells induced by apigenin to three pancreatic cancer cell lines (PANC-1 cells, SW1990 cells, and BxPC-3 cells) was increased to varying degrees. Compared with the control group, the concentration range of apigenin that significantly enhanced NK cell killing activity was 2.5–10 μM, 5–10 μM, and 5–10 μM, respectively. After treatment with apigenin, the killing activity of NK cells was enhanced within a certain concentration range. The cytotoxic activity of NK cells induced by apigenin to three pancreatic cancer cell lines (PANC-1 cells, SW1990 cells, BxPC-3 cells) was increased to varying degrees. The concentration range of apigenin that significantly enhances the cytotoxicity of NK cells against PANC-1 cells, SW1990 cells, and BxPC-3 cells was 2.5 μM–10 μM, 5 μM–10 μM, and 5 μM–10 μM, respectively. The killing efficiency of NK cells at these concentrations was significantly higher than that of the corresponding control group (24.1% ± 1.3%, 33.6% ± 1.8%, and 35.8% ± 2.4%, respectively). At the concentration of 5 μM apigenin, the cytotoxic activity of NK cells to PANC-1 cells and BxBC-3 cells was 44.5% ± 1.9% and 48.2% ± 3.0%, respectively, reaching the peak value. Similarly, the cytotoxic activity of NK cells to SW1990 cells reached a peak value of 56.6% ± 2.2% at the concentration of 10 μM of apigenin, and compared with cytotoxic activity of NK cells at 5 μM of apigenin (55.4% ± 2.1%), there is no significant difference between the two groups.
Figure 3.
The cytotoxic activity of NK cells treated with apigenin for 72 h to three pancreatic cancer cell lines (PANC-1, SW1990 and BxPC-3). NK cells treated with various concentrations of apigenin were used as effector cells, and pancreatic cancer cells (PANC-1, SW1990, and BxPC-3) were used as target cells (E: T=10: 1). The cytotoxicity of NK cells against three pancreatic cancer cell lines was markedly enhanced after treatment with apigenin at the dose of 5 μM and 10 μM, and significantly higher than that of the corresponding control group.**p < 0.01, ***p < 0.001.
Surface and intracellular antigen expression of NK cells
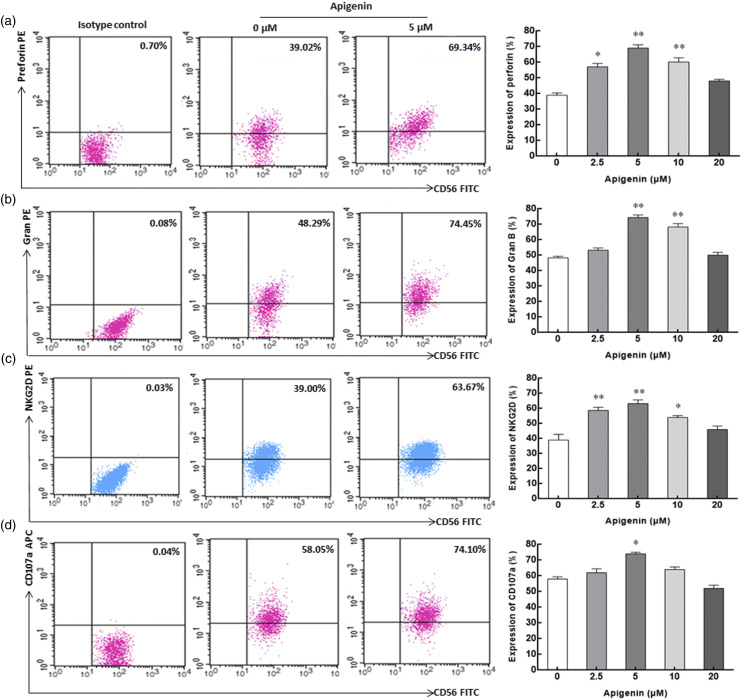
Perforin and granzyme B are important effector molecules for NK cells to exert cytotoxic function, and their expression level reflects the cytotoxic activity of NK cells. After incubation with apigenin for 72 h, the expression of perforin in NK cells increased significantly within the concentration range of 2.5 μM–10 μM, and the expression of granzyme B increased significantly within the concentration range of 5 μM–10 μM. Both of them reached the peak at the concentration of 5 μM, which were 74.4% ± 1.5% and 69.2% ± 1.8%, respectively, significantly higher than the corresponding control group (39.0% ± 1.3% and 48.3% ± 0.9%) without apigenin induction (Figures 4(a) and (b)). NK cells can exert cytotoxic effects on target cells through the interaction of NKG2D and its ligand NKG2DL. In the range of 2.5 μM–10 μM, the expression of NKG2D on NK cells induced by apigenin was significantly higher than that in the control group. At the concentration of 5 μM, the expression of NKG2D was the highest, reaching 63.2% ± 2.3%, while the control group was only 39.0% ± 3.4% (Figure 4(c)). CD107a has been proved to be an important marker of NK cell cytotoxicity. Our results showed that the expression of CD107a on the surface of NK cells was 74.1% ± 0.9%, significantly higher than that of the control group (58.2% ± 1.3%). Although the expression of CD107a was also higher than that of the control group at 2.5 μM and 10 μM concentrations, it was not statistically significant (Figure 4(d)).
Figure 4.
The expression of surface and intracellular antigen molecules on/in NK cells incubated with apigenin for 72h. a, b, c, and d represent the expression of perforin, Gran B, NKG2D, and CD107a in/on NK cells with apigenin treatment, respectively. Representative dot plot depicting the expression percentage of surface and intracellular antigen molecules on/in NK cells in the three columns on the left (from left to right, isotype control, 0 μM control group, and 5 μM treatment group). In the rightmost column, histograms describing the expression of perforin, Gran B, NKG2D, and CD107a on/in NK cells treated with various concentrations of apigenin are shown. Data are displayed in mean ± SD. *p < 0.05, **p < 0.01 compared with the 0 μM control group.
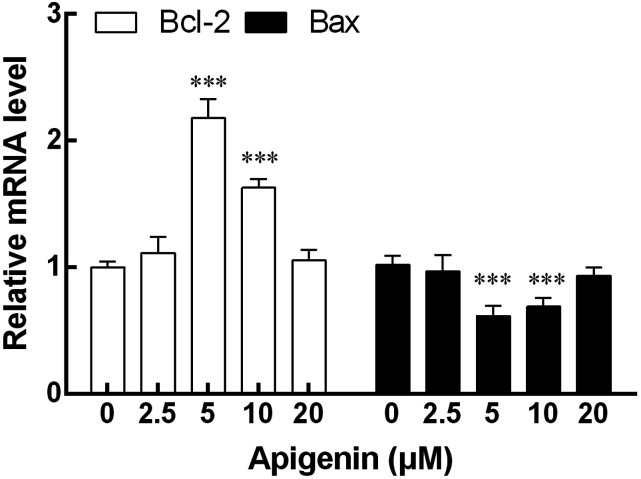
Expression of Bcl-2 and Bax mRNA in NK cells
The expression of Bcl-2 and Bax mRNA in NK cells induced by apigenin was detected by qRT-PCR. The results showed that the expression of Bcl-2 mRNA in NK cells was significantly enhanced by the concentration of 5 μM and 10 μM apigenin compared with NK cells without apigenin treatment. On the contrary, the same concentration of apigenin showed a significant down-regulation effect on the expression of Bax mRNA in NK cells. The changes of Bcl-2 and Bax mRNA expression were most significant at 5 μM (Figure 5).
Figure 5.
The mRNA expression of Bax and Bcl-2 in NK cells after treatment with apigenin for 72 h. The mRNA levels of Bcl-2 at 5 μM and 10 μM doses of apigenin were significantly higher than that of the control group. The change of Bax mRNA expression was contrary to that of Bcl-2, and the expression level decreased significantly at 5 μM and 10 μM doses. Results were expressed as mean ± SD, ***p < 0.001 VS compared with the 0 μM control group.
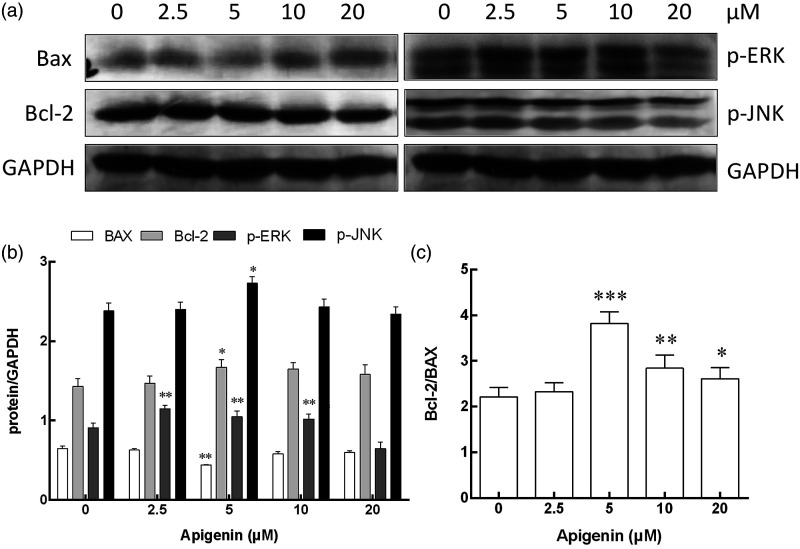
Protein expression of Bcl-2, p-JNK, and p-ERK in NK cells
Bcl-2 and Bax are proteins associated with apoptosis. As shown in Figure 6, anti-apoptotic protein Bcl-2 expression was significantly enhanced by apigenin at a dose of 5 μM, while pro-apoptotic protein Bax expression was decreased at a dose of 5 μM. The Bcl-2 to Bax protein ratio in NK cells treated with apigenin of 5 μM was significantly higher than that in the control group. Although the ratio of Bcl-2 to Bax protein in the 10 μM group and the 20 μM group was lower than that in the 5 μM group, it was still significantly higher than that in the control group. The JNK and ERK pathways play an important role in many physiological and pathological processes. The expression of p-ERK protein in NK cells was significantly higher than that in the untreated control group after being treated with apigenin at a concentration of 2.5 μM–10 μM for 72 h, while the expression of p-JNK protein was significantly higher than that in the control group only at a concentration of 5 μM.
Figure 6.
The protein expression of Bax, Bcl-2, p-ERK, p-JNK, and GAPDH in NK cells after treatment with apigenin for 72 h. a: Western blot analysis for Bax, Bcl-2, p-ERK, p-JNK, and GAPDH expression in NK cells treated with apigenin at concentrations of 0, 2.5, 5, 10, and 20 μM, respectively. b: Protein activation analysis of NK cells treated with apigenin at different concentrations, the means and standard deviation are depicted as diagrams. c: The ratio of Bcl-2 and Bax in NK cells. *p < 0.05, **p < 0.01, ***p < 0.001 versus the 0 μM control group.
Discussion
Traditional Chinese medicines (TCMs) have a long history in treating diseases in China and other Asian countries. They have been identified to possess functions of antioxidant, immunoregulation, and reducing the risk of chronic disease.22 Evidences have shown that some TCMs may activate or inhibit human immune cells and induce immune cells to produce cytokines with different effects in participating in the human immunity.23 NK cells, one of the important members of innate immunity, play a vital role in monitoring and resisting tumor and virus infection. Many studies have revealed that TCMs can regulate the immune function of NK cells. Luo et al.24 found that Yu-ping-feng could promote NK cells infiltration into tumor, increase the number of NK cells in spleen, and enhance antitumor effect of NK cells. Li et al.25 reported that shikonin could increase the expression of Gran B and perforin in NK cells, which was related to the activation of p-ERK1/2 and P-Akt. Apigenin, recognized as a kind of bioactive flavonoid, shows to possess various pharmacological activities, not only can restrict tumor growth through multiple mechanisms but also modulate immune cell function under different body conditions.9,26,27 Martínez et al.28 considered that apigenin is the most potent flavonoid in inflammation that can modify immune responses. Therefore, the question of whether apigenin has immunopotentiation effects on NK cells and its possible molecular mechanism has attracted our attention. In this study, the immunomodulation of apigenin on NK cells has been discussed.
Cell counting kit-8 (CCK-8) has been used by many researchers to evaluate cell proliferation.29,30 Hence, based on the successful expansion of NK cells in vitro, we observed the effect of apigenin on the proliferation of NK cells by CCK-8 method and found that apigenin promoted the proliferation of NK cells within the dose range of 2.5 μM–20 μM. Bcl-2 is one of the most important oncogenes in cell apoptosis study, exhibiting inhibition of apoptosis, while Bax is reverse.31 Bcl-2 and Bax are important factors affecting the survival and proliferation of NK cells, as well as other lymphocytes.32,33 In order to understand the proliferation-related signaling pathway, we detected the expression of Bcl-2 and Bax both in mRNA and protein levels in NK cells induced by apigenin, and the Bcl-2 to Bax ratio was analyzed in this study. The results of qRT-PCR and Western blotting analysis showed that apigenin could inhibit gene and protein expression of Bax in NK cells after 72 h induction, especially in 5 μM and 10 μM dose groups. On the contrary, the gene and protein expression of Bcl-2 were increased. Not only that, the ratio of Bcl-2 to Bax in NK cells was significantly elevated in 5 μM and 10 μM treated groups. The expression of these related genes and proteins was consistent with the results of proliferation analysis, which could be considered as the molecular mechanism.
Apigenin has direct antitumor effect. Woo et al.35 reported that apigenin could inhibit the proliferation of melanoma cells through regulating Akt and mitogen-activated protein kinase signal pathways to induce apoptosis. However, our study showed that apigenin increased the killing activity of NK cells against pancreatic cancer cells, suggesting that apigenin exerts indirect antitumor effect by activating NK cells. Activated NK cells release perforin and granzyme after contacting with target cells. Perforin makes the target cells to appear with holes, which creates a prerequisite for the granzyme to enter the target cells. After entering the target cell, granzyme starts the apoptosis program of the target cell by combining with the key substrates in the target cell, leading to the apoptosis of the target cell.35,36 Perforin/granzyme pathway is one of the important pathways for cytotoxic lymphocytes to induce apoptosis and kill tumor cells and virus infected cells.37 Fisher et al.38 revealed that while increasing the intracellular level of perforin and Gran B in NK cells, the killing effect of NK cells was improved, which proved the relationship between perforin/granzyme pathway and cytotoxic activity. NKG2D molecule is an important factor that determines the activation state of NK cells.39 Through binding to NKG2D ligands, expressing mainly on tumor cells, NKG2D plays an important role in the immune response, including immune surveillance, antimicrobial, and antitumor effects. The activation of NKG2D can enhance the killing activity of NK cells against many tumor cells expressing corresponding ligands.40 In our study, the expression of perforin, Gran B, and NKG2D in/on NK cells was significantly upregulated after treatment with apigenin at doses of 2.5 μM–10 μM. The results suggested that apigenin could regulate the activation and killing function of NK cell. Studies have shown that CD107a is a marker of NK cell functional activity.41 Our results showed that the expression of CD107a on NK cells was obviously increased after apigenin treatment, which was consistent with its killing effect to pancreatic cancer cells.
As the intermediate link of MAPK signaling pathway, ERK, JNK, and p38 pathways are closely related to the granular polarization in NK cells.42–44 Furthermore, some previous studies have shown that NKG2D-mediated antitumor activity is achieved by activating ERK and JNK MAP kinases.42,45,46 Teng et al.47 proved the correlation between ERK pathway and NK cytotoxic activity from the opposite perspective, that is, the expression level of phosphorylated ERK (p-ERK) decreased, and the cytotoxic activity of NK cells decreased. Western blotting analysis was used to examine the effect of apigenin on NK cytotoxicity-related signal pathway. As observed in this study, apigenin can activate ERK and JNK signal pathways in NK cell within a certain concentration range, which preliminarily revealed the molecular mechanism of apigenin enhancing the cytotoxicity of NK cell against cancer cell. However, the secretion of immunoregulation-related cytokines has not been detected, which needs further research. Of course, in addition to apigenin, there are many flavonoids with similar biological activities and pharmacological effects, such as quercetin, baicalin, myricetin, and luteolin. Hence, another limitation of this study is that only apigenin has been studied, and several flavonoids have not been selected for comparative study at the same time, which needs further research in the next step.
Conclusion
To sum up, the current study confirmed that apigenin in a certain concentration range can promote proliferation of NK cells and enhance its cytotoxicity to pancreatic cancer cells, playing a positive immunoregulation. Apigenin may play a critical role in promoting NK cell proliferation by upregulating Bcl-2 and downregulating Bax expression at both mRNA and protein levels. Moreover, apigenin can upregulate the expression of perforin, Gran B, and NKG2D, possibly by activating JNK and ERK pathways, thereby enhancing the killing activity of NK cells against pancreatic cancer cells.
Acknowledgements
The authors thank the Central Laboratory of the 71st Group Army Hospital of PLA Army(The Affiliated Huaihai Hospital of Xuzhou Medical University) for providing experimental conditions for this study and Prof. Xu-Ming Liu of Southeast University for her linguistic assistance in the process of revising the manuscript.
Footnotes
Authors' contributions: All the authors have made corresponding contributions to the work of this paper. Chun-Fang Xu, Fu-Xing Chen, and Yong-Bo Feng designed the research strategy. Yong-Bo Feng executed the experiments. Yong-Bo Feng and Zhong-Hai Zhou prepared the manuscript. Guo-Hua Chen and Ling Chen provided assistance in laboratory techniques and statistical analysis. All the authors read and agreed to the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Medical Science and Technology Innovation Research Foundation of Nanjing Military Area Command (NO.15MS045) and Suzhou social development project (NO.SYS201115).
Data availability: The data supporting the current research conclusion can be obtained from the corresponding author according to reasonable requirements.
Ethical approval: Ethical approval for this study was obtained from the Ethics Committee of the 71st group Army Hospital of PLA Army (APPROVAL NUMBER: LL-2020YX05). All volunteers involved in the study signed informed consent.
Informed consent: Written informed consent was obtained from all subjects before the study.
ORCID iDs
Yong-Bo Feng https://orcid.org/0000-0003-2460-4696
Yang Yang https://orcid.org/0000-0001-5618-3940
Zhong-Hai Zhou https://orcid.org/0000-0003-3683-7200
References
- 1.Siegel RL, Miller KD, Fuchs HE, et al. (2021) Cancer statistics, 2021. CA A Cancer J Clin 71(1): 7–33. [DOI] [PubMed] [Google Scholar]
- 2.He Y, Zhou X, Fan X, et al. (2022) Disease Burden of Pancreatic Cancer - China, 1990-2019. China CDC Weekly 4(24): 527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JA, Ha SK, Cho E, et al. (2015) Resveratrol as a Bioenhancer to Improve Anti-Inflammatory Activities of Apigenin. Nutrients 7(11): 9650–9661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sánchez-Marzo N, Pérez-Sánchez A, Ruiz-Torres V, et al. (2019) Antioxidant and Photoprotective Activity of Apigenin and its Potassium Salt Derivative in Human Keratinocytes and Absorption in Caco-2 Cell Monolayers. Int J Mol Sci 20(9): 2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng S, Zhu Y, Li JF, et al. (2017) Apigenin inhibits renal cell carcinoma cell proliferation. Oncotarget 8(12): 19834–19842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balez R, Steiner N, Engel M, et al. (2016) Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer's disease. Sci Rep 6: 31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardenas H, Arango D, Nicholas C, et al. (2016) Dietary Apigenin Exerts Immune-Regulatory Activity in Vivo by Reducing NF-κB Activity, Halting Leukocyte Infiltration and Restoring Normal Metabolic Function. Int J Mol Sci 17(3): 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu M, Wang S, Song YU, et al. (2016) Apigenin suppresses colorectal cancer cell proliferation, migration and invasion via inhibition of the Wnt/β-catenin signaling pathway. Oncol Lett 11(5): 3075–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu L, Zhang Y, Tian K, et al. (2018) Apigenin suppresses PD-L1 expression in melanoma and host dendritic cells to elicit synergistic therapeutic effects. J Exp Clin Cancer Res: CR (Clim Res) 37(1): 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villalobos-Ayala K, Ortiz Rivera I, Alvarez C, et al. (2020) Apigenin Increases SHIP-1 Expression, Promotes Tumoricidal Macrophages and Anti-Tumor Immune Responses in Murine Pancreatic Cancer. Cancers 12(12): 3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, Chen X, Chang Z, et al. (2023) Boosting anti-tumour immunity using adjuvant apigenin. Anti Cancer Agents Med Chem 23(3): 266–277. [DOI] [PubMed] [Google Scholar]
- 12.Lee HH, Cho H. (2022) Apigenin Increases Natural Killer Cytotoxicity to Human Hepatocellular Carcinoma Expressing HIF-1α through High Interaction of CD95/CD95L. J Microbiol Biotechnol 32(4): 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Galat V, Galat Y, et al. (2021) NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol 14(1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laskowski TJ, Biederstädt A, Rezvani K. (2022) Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer 22(10): 557–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu XT, Liu JQ, Lu XT, et al. (2013) The enhanced effect of lupeol on the destruction of gastric cancer cells by NK cells. Int Immunopharm 16(2): 332–340. [DOI] [PubMed] [Google Scholar]
- 16.Zhou ZH, Chen FX, Xu WR, et al. (2013) Enhancement effect of dihydroartemisinin on human γδ T cell proliferation and killing pancreatic cancer cells. Int Immunopharm 17(3): 850–857. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Liu JQ, Zhou ZH, et al. (2016) Enhancement of CD3AK cell proliferation and killing ability by α-Thujone. Int Immunopharm 30: 57–61. [DOI] [PubMed] [Google Scholar]
- 18.Asgari A, Sharifzadeh S, Ghaderi A, et al. (2019) In vitro cytotoxic effect of Trastuzumab in combination with Pertuzumab in breast cancer cells is improved by interleukin-2 activated NK cells. Mol Biol Rep 46(6): 6205–6213. [DOI] [PubMed] [Google Scholar]
- 19.Tano T, Okamoto M, Kan S, et al. (2013) Prognostic impact of expression of Bcl-2 and Bax genes in circulating immune cells derived from patients with head and neck carcinoma. Neoplasia 15(3): 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods 25(4): 402–408. [DOI] [PubMed] [Google Scholar]
- 21.Chen YQ, Zheng L, Aldarouish M, et al. (2018) Wnt pathway activator TWS119 enhances the proliferation and cytolytic activity of human γδT cells against colon cancer. Exp Cell Res 362(1): 63–71. [DOI] [PubMed] [Google Scholar]
- 22.Ma HD, Deng YR, Tian Z, et al. (2013) Traditional Chinese medicine and immune regulation. Clin Rev Allergy Immunol 44(3): 229–241. [DOI] [PubMed] [Google Scholar]
- 23.Huang CF, Lin SS, Liao PH, et al. (2008) The immunopharmaceutical effects and mechanisms of herb medicine. Cell Mol Immunol 5(1): 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Y, Wu J, Zhu X, et al. (2016) NK Cell-Dependent Growth Inhibition of Lewis Lung Cancer by Yu-Ping-Feng, an Ancient Chinese Herbal Formula. Mediat Inflamm 2016: 3541283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Lu H, Gu Y, et al. (2017) Enhancement of NK cells proliferation and function by Shikonin. Immunopharmacol Immunotoxicol 39(3): 124–130. [DOI] [PubMed] [Google Scholar]
- 26.Xu L, Zhang L, Bertucci AM, et al. (2008) Apigenin, a dietary flavonoid, sensitizes human T cells for activation-induced cell death by inhibiting PKB/Akt and NF-kappaB activation pathway. Immunol Lett 121(1): 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasr-Bouzaiene N, Sassi A, Bedoui A, et al. (2016) Immunomodulatory and cellular antioxidant activities of pure compounds from Teucrium ramosissimum Desf. Tumour biology: the Journal of the International Society for Oncodevelopmental Biology and Medicine 37(6): 7703–7712. [DOI] [PubMed] [Google Scholar]
- 28.Martínez G, Mijares MR, De Sanctis JB. (2019) Effects of Flavonoids and Its Derivatives on Immune Cell Responses. Recent Pat Inflamm Allergy Drug Discov 13(2): 84–104. [DOI] [PubMed] [Google Scholar]
- 29.Xu Z, Wu J, Cai P, et al. (2019) Effects of FHIT gene on proliferation and apoptosis of osteosarcoma cells. Oncol Lett 17(1): 877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geng J, Wang Y, Zhang L, et al. (2020) The cajanine derivative LJ101019C regulates the proliferation and enhances the activity of NK cells via Kv1.3 channel-driven activation of the AKT/mTOR pathway. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology 66: 153113. [DOI] [PubMed] [Google Scholar]
- 31.Czabotar PE, Lessene G, Strasser A, et al. (2014) Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 15(1): 49–63. [DOI] [PubMed] [Google Scholar]
- 32.Song S, Jacobson KN, McDermott KM, et al. (2016) ATP promotes cell survival via regulation of cytosolic [Ca2+] and Bcl-2/Bax ratio in lung cancer cells. Am J Physiol Cell Physiol 310(2): C99–C114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bin-Alee F, Arayataweegool A, Buranapraditkun S, et al. (2020) Evaluation of lymphocyte apoptosis in patients with oral cancer. J Appl Oral Sci: Revista FOB 28: e20200124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woo JS, Choo GS, Yoo ES, et al. (2020) Apigenin induces apoptosis by regulating Akt and MAPK pathways in human melanoma cell A375SM. Mol Med Rep 22(6): 4877–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oberoi P, Jabulowsky RA, Bähr-Mahmud H, et al. (2013) EGFR-targeted granzyme B expressed in NK cells enhances natural cytotoxicity and mediates specific killing of tumor cells. PLoS One 8(4): e61267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trapani JA. (2001) Granzymes: a family of lymphocyte granule serine proteases. Genome Biol 2(12): REVIEWS3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trapani JA, Smyth MJ. (2002) Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol 2(10): 735–747. [DOI] [PubMed] [Google Scholar]
- 38.Fisher L, Zinter M, Stanfield-Oakley S, et al. (2019) Vaccine-induced antibodies mediate higher antibody-dependent cellular cytotoxicity after interleukin-15 pretreatment of natural killer effector cells. Front Immunol 10: 2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu H, Wang S, Xin J, et al. (2019) Role of NKG2D and its ligands in cancer immunotherapy. American journal of cancer research 9(10): 2064–2078. [PMC free article] [PubMed] [Google Scholar]
- 40.Pahl JHW, Koch J, Götz JJ, et al. (2018) CD16A Activation of NK Cells Promotes NK Cell Proliferation and Memory-Like Cytotoxicity against Cancer Cells. Cancer Immunology Research 6(5): 517–527. [DOI] [PubMed] [Google Scholar]
- 41.Alter G, Malenfant JM, Altfeld M. (2004) CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 294(1–2): 15–22. [DOI] [PubMed] [Google Scholar]
- 42.Li C, Ge B, Nicotra M, et al. (2008) JNK MAP kinase activation is required for MTOC and granule polarization in NKG2D-mediated NK cell cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America 105(8): 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chini CC, Boos MD, Dick CJ, et al. (2000) Regulation of p38 mitogen-activated protein kinase during NK cell activation. Eur J Immunol 30(10): 2791–2798. [DOI] [PubMed] [Google Scholar]
- 44.Wei S, Gamero AM, Liu JH, et al. (1998) Control of lytic function by mitogen-activated protein kinase/extracellular regulatory kinase 2 (ERK2) in a human natural killer cell line: identification of perforin and granzyme B mobilization by functional ERK2. J Exp Med 187(11): 1753–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X, Allan DSJ, Krzewski K, et al. (2006) CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proceedings of the National Academy of Sciences of the United States of America 103(27): 10346–10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu CC, Chen JK. (2010) Resveratrol enhances perforin expression and NK cell cytotoxicity through NKG2D-dependent pathways. J Cell Physiol 223(2): 343–351. [DOI] [PubMed] [Google Scholar]
- 47.Teng R, Wang Y, Lv N, et al. (2020) Hypoxia Impairs NK Cell Cytotoxicity through SHP-1-Mediated Attenuation of STAT3 and ERK Signaling Pathways. Journal of Immunology Research 2020: 4598476. [DOI] [PMC free article] [PubMed] [Google Scholar]