Abstract
Alzheimer’s disease (AD) is an untreatable cause of dementia, and new therapeutic approaches are urgently needed. AD pathology is defined by extracellular amyloid plaques and intracellular neurofibrillary tangles. Research of the past decades has suggested that neuroinflammation plays a critical role in the pathophysiology of AD. This has led to the idea that anti-inflammatory treatments might be beneficial. Early studies investigated non-steroidal anti-inflammatory drugs (NSAIDS) such as indomethacin, celecoxib, ibuprofen, and naproxen, which had no benefit. More recently, protective effects of diclofenac and NSAIDs in the fenamate group have been reported. Diclofenac decreased the frequency of AD significantly compared to other NSAIDs in a large retrospective cohort study. Diclofenac and fenamates share similar chemical structures, and evidence from cell and mouse models suggests that they inhibit the release of pro-inflammatory mediators from microglia with leads to the reduction of AD pathology. Here, we review the potential role of diclofenac and NSAIDs in the fenamate group for targeting AD pathology with a focus on its potential effects on microglia.
Keywords: Alzheimer’s disease, diclofenac, microglia, neuroinflammation, NLRP3 inflammasome, NSAID
Introduction
Alzheimer’s disease (AD) is a chronic neurodegenerative condition leading to a progressive dementia syndrome. Currently, approximately 35.6 million people world-wide are affected, and 7.7 million new cases are diagnosed yearly, making AD the most common form of dementia.1 There is no therapeutic option to substantially improve, halt, or cure the disease, which results in a critical social and economic impact for aging societies. New therapeutic approaches are therefore urgently needed. The majority of patients (more than 95%) develop symptoms above the age of 65 (late-onset or sporadic AD), while a minority of patients bear familial mutations driving an earlier onset of the disease (early-onset AD).2 The etiology of sporadic AD is poorly understood and likely multi-factorial, with a complex set of contributory genetic and environmental factors.2 Clinically, AD is characterized by a progressive impairment of episodic memory, language, visuospatial abilities, decision-making, and executive function.1 Neuropsychiatric assessment as well as imaging, serum, and cerebrospinal fluid (CSF) biomarkers are helpful tools to assess for possible AD in an individual; however, these are not sensitive and/or specific enough to allow for a definitive diagnosis. Diagnostic certainty can only be obtained through post-mortem neuropathological brain analysis based on the presence of classic features described by Alois Alzheimer more than a hundred years ago; these features consist of extracellular amyloid plaques and intracellular neurofibrillary tangles (NFTs).3 Research in the past several decades has implicated neuroinflammatory signaling in the pathophysiology of AD, with activated microglia and reactive astrocytes as key cellular contributors. Microglia activation has two potential roles: an increase of Aβ clearance via phagocytosis and degradation and the release of pro-inflammatory cytokines, which might be neurotoxic or contribute to resultant AD pathology.4 Early studies on therapeutic options in AD investigated the effect of non-steroidal anti-inflammatory drugs (NSAIDS), including indomethacin, celecoxib, ibuprofen, and naproxen. However, no clear beneficial effect was found for the pathology and progression of AD.5,6 NSAIDs have distinct structures, mechanisms of action, and brain penetrance. A recent retrospective cohort study found a significantly lower frequency of AD in patients exposed to diclofenac compared to etodolac and naproxen based on review of patient charts.7 Diclofenac has a similar chemical structure to meclofenamic acid, an NSAID in the fenomate class. Fenomates are implicated in several pathways including downregulation of interleukin-1β (IL-1β), tumor necrosis factor α (TNFα), and the transcription factor specificity protein 1 (SP1), with subsequent reduction of neuroinflammation and AD pathology.7,8 These findings could hold great promise for future therapeutic strategies in AD. Here, we provide a comprehensive and narrative review of the available literature on the potential role of diclofenac and NSAIDs in the fenamate group for targeting AD pathology.
Aβ and tau are the classic hallmarks of AD pathology
The definitive diagnosis of AD requires neuropathological examination of multiple post-mortem brain regions to detect the hallmark features consisting of extracellular senile plaques (SPs) formed by accumulation of Aβ aggregates and intracellular NFTs containing aggregates of the hyperphosphorylated tau protein.3
In healthy subjects, Aβ is cleaved from its transmembrane Aβ precursor protein (APP) by β-site amyloid precursor protein-cleaving enzyme 1 (BACE-1), a β-secretase, as well as γ-secretase, a protein complex with presenilin 1 or 2 (PSEN1, PSEN2) at its catalytic core.9 Aβ is subsequently released into the extracellular space, where rapid degradation or removal by microglia takes place. Under pathological conditions, APP metabolism and Aβ peptide degradation are impaired, leading to chronic accumulation. Aβ peptides consist of 39–43 amino acids, with Aβ40 and Aβ42 being the major species in AD. An increase of Aβ42 and/or increase of the ratio of Aβ42/Aβ40 triggers the formation of insoluble fibrils and plaques.10 Soluble oligomers and intermediate-size aggregates are the most neurotoxic Aβ forms, and the severity of cognitive deficits in AD correlates with the number of oligomers and not the total Aβ burden.9,11 In familial AD, pathogenic mutations are found in the APP gene on chromosome 21 near the β-secretase and γ-secretase cleavage sites, which leads to increased accumulation of Aβ early in life.10 Other familial AD mutations are located in PSEN1/2, which are components of the γ-secretase.10 The increased expression of APP, for example, due to gene duplication, can lead to a familial form of AD as well.10 The lack of substantial clinical effects in trials targeting Aβ pathology (e.g. anti-Aβ monoclonal antibodies, BACE-1 inhibitors) suggest that other components such as tau pathology and neuroinflammation are crucial for AD pathogenesis.10
Tau is a microtubule-associated protein (MAP), encoded by the MAPT gene on chromosome 17, and regulates the stability of tubulin assemblies in the cell.10 Hyperphosphorylated tau forms aggregates called NFTs. Tau staging in AD is performed according to the classification by Braak & Braak, whereby NFT initially appear in the transentorhinal cortex (stage I and II), followed by spread to the limbic regions (stages III and IV) and neocortical regions (stages V and VI).12 The spread of tau pathology correlates directly with the progression of cognitive decline and patterns of atrophy.10 It has been reported that tau lesions can precede Aβ pathology, thus emphasizing the importance of tau for AD progression.13 Aggregation and formation of intracellular tau is not unique to AD and can be found in many other (albeit less frequent) tauopathies such as progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), Pick’s disease (PiD), and frontotemporal dementia and Parkinsonism linked to chromosome 17 (FTDP-17).10 Tau pathology induces significant cytotoxicity, resulting in mitochondrial dysfunction, synaptic impairment, defective cellular transport mechanisms, and ultimately neuronal death.10
The role of neuroinflammation for AD pathology
Phenotypic and transcriptional heterogeneity of microglia and astrocytes in AD pathology
In the past decades, glia cells including astrocytes and microglia emerged as key players of AD pathophysiology. A recent study using positron emission tomography (PET) tracers in 130 individuals across the AD clinical spectrum showed that the co-occurrences of Aβ, tau, and microglia most strongly predicted cognitive impairment, underlining the importance of microglia and neuroinflammation for AD pathology.14 Microglia are the resident phagocytic cells of the central nervous system (CNS) and responsible for maintaining neuronal homeostasis by the release of trophic factors, synaptic pruning, and removal of cellular debris, thus contributing to the remodeling and maintenance of synapses and memory. Importantly, microglia are a highly diverse population of cells and can adapt a broad spectrum of phenotype states, ranging from anti-inflammatory to pro-inflammatory stages. To add more complexity, studies in AD mouse models and human post-mortem tissue from AD patients showed that microglia may play distinct roles depending on disease stage and spatial distribution in the brain.15 Microglia have ramified processes that allow them to survey their surroundings, a state called ramified or surveying micoglia.16 Environmental triggers such as cellular debris or pathogens induce microglia activation and transformation into an ameboid state with a large, round cell body.17 Microglia in post-mortem hippocampus of patients with AD have a similar ameboid morphology, indicating chronic activation and proliferation.18 Various other microglial states have been described, including (1) hypertrophic microglia (large cell bodies with short and thick processes) in aged patients with AD with Braak stage IV-VI, (2) dystrophic or senescent microglia (with spherical swellings in their processes, lipofuscin deposits, and positive staining for L-ferritin) predominantly near tau pathology in AD human brain, and (3) dark microglia (with ramified dark processes due to accumulation of markers indicating oxidative stress, e.g., dilated endoplasmic reticulum and altered mitochondria) near axon terminals and dendritic spines in brains of 14-month-old APP/PS1 mice (AD mouse model expressing chimeric human/mouse APP and mutant PSEN1).15 In addition to the phenotypic heterogeneity, microglia in AD are characterized by a vast heterogeneity of genetic signatures and cytokine expression profiles. Genome-wide association studies identified triggered receptor expressed on myeloid cells (Trem2) as a crucial driver of microglial activation and appearance of disease-associated microglia (DAM) in AD.19 In 3-, 6-, and 8-month-old 5XFAD mice, DAM activation is a two-step process, involving a Trem2-independent decrease of homeostatic genes (e.g. purinergic receptor P2Y, G-protein coupled, 12 = P2ry12; transmembrane protein 119 = Tmem119) and increase of DAM-associated genes (e.g. apolipoprotein E = Apoe, TYRO protein tyrosine kinase binding protein = Tyrobp), followed by a Trem2-dependant step with increase of DAM-associated genes such as lipoprotein lipase (Lpl), integrin alpha X (Itgax), and C-type lectin domain containing 7a (Clec7a).20 These findings were confirmed in another study in 7-month-old 5XFAD mice (AD mouse model expressing four separate APP and two PSEN1 mutations) where lack of Trem2 resulted in reduced expression of DAM genes after intracerebral injection of tau.21 Since the initial paper by Keren-Shaul et al., the DAM signature was confirmed in several other AD mouse models with Aβ and/or tau pathology, including 9-month-old APP knockin mice,22,23 and 9- to 12-month-old P301S mice (a mouse model overexpressing human tau with the disease-causing mutation P301S).24 Subsequent studies identified multiple sub-states of DAM including pro-inflammatory, anti-inflammatory, and senescent signatures, with additional variability depending on the mouse strain.25 Besides the intra-state heterogeneity, other signatures were described. For example, the activated response microglia (ARM) signature appears in the hippocampus of APP knockin mice and overlaps partially with DAM.26 Additional microglial states in AD mouse models include lipid droplet accumulating microglia (LDAM), microglial amyloid beta response proteins (MARP), and neurodegenerative disease phenotype (MGnD) microglia.15 The presence of the DAM signature and other microglial signatures in human brain remains controversial. Gerrits et al.27 described enrichment of DAM genes (e.g, Lpl, Itgax) in tissues derived from AD patients compared to healthy controls. Maeda et al.28 showed decreased expression of the homeostatic marker P2ry12 in human post-mortem AD brain especially in brain areas with tau pathology in comparison to control brains. However, other authors reported that DAM are not conserved in human brain. Thus, Srinivasan et al.29 found little resemblance between the DAM profile and human AD microglia (HAM) from AD brain tissue. Interestingly, the HAM profile was characterized by enhanced expression of genes related to human aging rather than DAM genes.29 Taken together, the interspecies differences between microglia from non-primate models and human tissue remain a major challenge for the field. New techniques such as microglial PET tracers may provide valuable insight into the in vivo microglial signatures in humans in the future.
Besides microglia, astrocytes emerged as a major player in AD pathology. Healthy mouse and human brain disposes of diverse astrocyte populations, and additional phenotypes and genetic signatures can be found in disease states, adding to the complexity.30 Astrocytes are characterized by star-like branches that have the ability to ramify further in response to micro-environmental changes.15 In humans, reactive astrogliosis around SPs is found in early and moderate stages of AD, whereas astrocytic atrophy and paralysis is associated with late stages of disease.31 On a molecular level, astrocytes are heterogenous and undergo multiple distinct transcriptional states. In 7-month-old 5XFAD mice, Habib et al.32 identified six separate transcriptional states based on upregulation of genes associated with APP processing, Aβ clearance, and inflammation, using single neuron RNA sequencing. The group identified similar astrocyte profiles in aging wild-type (WT) mice and aging human brain.32 However, similar to microglia, the translation of astrocyte states from mouse models to humans is controversial. Several studies used single-neuron RNA sequencing from human AD samples and identified multiple clusters of astrocytes based on expression of homeostatic, pro- and anti-inflammatory genes.33,34 Taken together, further studies are needed to better understand the role of various astrocyte states in AD.
Activation of microglia in AD
Pathological triggers and danger signals, including cellular debris or pathogens induce microglia activation, migration to the site of the lesion, and initiation of an innate immune response.35,36 In AD, Aβ species bind microglia via specific cell surface receptors including scavenger receptor class A member 1 (SCARA1), cluster of differentiation (CD) 36, 14, and 47, and Toll-like receptors (TLRs) 2, 4, 6, and 9.37 This triggers phagocytosis and endolysosomal degradation of Aβ by the proteases neprilysin and insulin-degrading enzyme (IDE), as well as activation of the Nod-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome, and the release of pro-inflammatory cytokines and chemokines such as IL-1β, IL-6, TNFα, and chemokine (C-C motif) ligand 2 (CCL2).37 The persistent local increase of pro-inflammatory cytokines and exposure to Aβ species compromises microglial health and renders Aβ clearance inefficient, causing subsequent Aβ accumulation and deposition, which in turn maintains chronic inflammation.37–39 This concept is supported by the finding that mutations in TREM2 and CD33, both microglial receptors mediating phagocytosis, increase the risk for AD, and Aβ accumulation.37,40 Emerging data identified TREM2 as a potential ligand for neuronal ApoE, linking neuroinflammation and microglia activity directly to the ApoE ε4 allele, the strongest known genetic risk factor of late-onset AD.40
The pathways connecting the innate immune system and microglial activation with tau pathology were studied to a lesser degree. Similar to Aβ pathology, misfolded tau leads to microglia activation.41,42 In tau mouse models, synaptic loss and microglial activation precede neuronal loss, suggesting that soluble tau species may drive neuroinflammation rather than NFTs.43 In large genome-wide association studies, tau pathology was associated with a number of genes related to the regulation of the immune system, for example, CD33, ATP-binding cassette subfamily A member 7 (ABCA7), and cortactin-CD2-associated protein (CD2AP).44 The microglial receptor TREM2 is associated with enhancement of tau pathology as well, although its exact role for tau pathophysiology remains to be clarified.42 ApoE ε4 significantly contributed to exacerbation of tau pathology and neuroinflammation in a tau mouse model.45 Along these lines, in tau mouse models, microglial CX3C motif chemokine receptor 1 (CX3CXR1) was implicated in tau phagocytosis, and genetic deficiency led to elevated levels of hippocampal tau phosphorylation and aggregation, loss of synaptic integrity, and behavioral impairment.46 Recent data from tauopathy mouse models suggested that tau pathology promotes IL-1β secretion via activation of the NLRP3 inflammasome similar to Aβ.47
The role of IL-1β and the NLRP3 inflammasome in AD
IL-1β is a major driver of AD pathology. The IL-1 superfamily consists of 11 cytokines that have a similar gene structure, including IL-1α, IL-1β, IL-1Ra, IL-18, IL-33, IL-36Ra, IL-36α, IL-36β, IL-36γ, IL-37, and IL-38.48 IL-1β is a ubiquitous pro-inflammatory cytokine and involved in the host defense against external pathogens and internal tissue repair in various organ systems.48 In general, cell surface pattern recognition receptors (PRRs) detect external pathogens (e.g. microbes) via pathogen-associated molecular patterns (PAMPs), and internal stimuli such as protein aggregates, cell metabolites, or nucleic acids via endogenous damage-associated molecular patterns (DAMPs).37 PRR binding of PAMPs and DAMPs leads to activation and assembly of the canonical NLRP3 inflammasome, which can recruit pro-caspase-1 either directly or indirectly through recruitment of apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC). The proteolytic activation of caspase-1 results in cleavage of pro-IL-1β and production of mature IL-1β.48,49 In AD, microglial receptors recognize Aβ peptides as a DAMP with subsequent NLRP3 inflammasome activation and IL-1β secretion.50 A recent study in a human tau mouse model of tauopathy demonstrated that hyperphosphorylated tau can induce the NLRP3 pathway as well, leading to enhanced IL-1β release.51 Elevated levels of IL-1β can be detected in the brain tissue and CSF of AD patients.49,52 Caspase-1 expression is strongly enhanced in human brain tissue from patients with mild cognitive impairment (MCI) and AD.53 In rat and human neuronal cultures, IL-1β induced aberrant release and accumulation of the excitatory neurotransmitter glutamate, which has cytotoxic effects on neighboring neurons.54 This effect could be alleviated by treatment with N-methyl-D-aspartate (NMDA) receptors antagonists.54 In a mouse model of AD pathology, intraperitoneal administration of an IL-1 receptor (IL-1R) blocking antibody significantly improved brain inflammatory responses, alleviated cognitive deficits and attenuated tau pathology.55 Several studies in cell and mouse models showed that IL-1β over-expression can worsen tau phosphorylation and NFT formation.56,57 Knockout of NLRP3 in a tau mouse model reduced the levels of hyperphosphorylated tau and rescued spatial memory deficits in these mice.47 The injection of Aβ containing brain homogenate from APP/PS1 mice into the hippocampus of the same tau mice led to tau hyperphosphorylation in an NLRP3-dependant manner, supporting a model where the NLRP3/IL-1β pathway connects Aβ and tau pathology in AD.47 Some reports also suggest a neuroprotective role for IL-1β. The delivery of human IL-1β complementary DNA (cDNA) via adeno-associated virus into the hippocampus of APP/PS1 mice resulted in upregulation of microglia which led to a reduction of Aβ plaques.58 Several other groups reported similar findings in AD mouse models.59
The role of cyclooxygenases 1 and 2 (COX-1 and COX-2) in AD
COX enzymes catalyze the synthesis of prostanoids such as prostaglandins (PG, e.g. PGE2), prostacyclin (PGI2), and thromboxane A2 (TXA2) from arachnoid acids. COX enzymes are bi-functional with a homodimeric structure carrying out cyclooxygenation followed by peroxidation.60 There are three COX isoforms, the constitutively expressed COX-1, the inducible COX-2, and COX-3, a splice variant of COX-1.61,62 COX-1 and COX-2 are polypeptides with a size of 70 kDa and carry >60% identity.60 They are both located as membrane proteins in the nuclear envelope and the endoplasmic reticulum, while COX-2 is also found in the Golgi apparatus.63 In human brain, COX-1 can be located in the cytosol as well.64 Under physiological conditions, COX-1 is constitutively expressed in the majority of tissues, while COX-2 expression is restricted to a limited number of tissues (brain, kidneys, and female reproductive organs).60 Upon discovery of COX enzymes, the distinct expression patterns seemed to suggest that COX-1 is predominantly driving homeostatic functions such as gastric protection, renal perfusion, and hemostasis, while COX-2 is the main player in pathological states such as inflammation and tumor formation. However, subsequent studies revealed that the COX functions are more complex, and both isoforms play roles in tissue homeostasis and inflammation.60 Thus, Deininger and Schluesener65 observed COX-1 expression in microglia and COX-2 expression in neurons and endothelial cells in healthy rat brain and rat models of autoimmune encephalitis (EAE) and tumor growth. Yamagata et al.66 provided evidence that COX-2 induction in neurons may play an important physiological role for activity-dependent neuronal plasticity. Interestingly, COX-1 and COX-2 may have opposing roles in some scenarios. For example, Choi and colleagues demonstrated that the pharmacological inhibition or genetic deletion of COX-1 mitigates the inflammatory response to the injection of intra-cerebroventricular lipopolysaccharide (LPS) in mice,67 while Aid et al.68 found that pharmacological inhibition or genetic deletion of COX-2 led to an exacerbation of inflammation upon injection of LPS into cerebral ventricles of mice. In cell culture studies with human- and mouse-derived neuroblastoma and glioblastoma cell lines, Wang et al.69 found that COX-2 can regulate the synthesis of IL-1β in a prostaglandin-dependent manner, connecting the COX pathway with other pro-inflammatory pathways. Both COX-1 and COX-2 are implicated in AD pathophysiology in rodent and human brain. COX-1-deficient mice showed a pronounced reduction of neuronal damage, oxidative stress, and glial cell activation in response to Aβ injection into the lateral ventricle compared to WT controls.70 The intraventricular injection of soluble Aβ in rats induced increased expression of COX-2 in the hippocampus, accompanied by cognitive impairment.71 The treatment of these animals with the COX-2 inhibitor celecoxib prevented the upregulation of COX-2 and cognitive impairment.71 In an AD mouse model expressing both mutant APP and PSEN1, COX-2 over-expression in neurons was followed by increased Aβ production, plaque formation, and impaired learning ability.72 In human AD brain, COX-1 is expressed in microglia surrounding amyloid plaques, while COX-2 is up-regulated in neuronal cells, which fueled discussions in the field whether both COX-1 and COX-2 inhibition is necessary to effectively interfere with AD pathology in humans.73–75 COX-2 expression in human brain tissue is increased in early AD stages and decreases with disease progression to low expression levels in end-stage AD cases, suggesting that the effect of COX inhibitors in humans may depend on the disease stage.73 Importantly, interspecies differences may result in discrepancies between studies in rodents and humans, warranting cautious interpretation of study results. For example, a PET tracer for COX-1 successfully detected enzyme expression in mouse models of neuroinflammation, however failed to do so in humans.76 Similarly, the treatment of patients with NSAIDs did not replicate the data from animal studies and resulted in controversial effects on the progression of AD pathology. This will be discussed in more detail in the paragraphs below.
The potential role of peripheral inflammation for AD pathology
The role of systemic inflammation for AD pathology is less well studied compared to CNS inflammation. Systemic inflammation is associated with accelerated cognitive decline.37,77 Severe infections requiring hospital treatment increase the risk for dementia including AD and vascular dementia.78 In a cohort of 300 AD patients, acute systemic inflammatory events were associated with increased serum levels of pro-inflammatory cytokines including TNFα and a two-fold increase of cognitive decline within 6 months.79 The level of TNFα correlated directly with cognitive decline in these patients – high TNFα baseline resulted in a four-fold increase of the rate of cognitive decline, whereas patients with low TNFα levels did not exhibit any cognitive decline.79 Individuals with AD show alteration in gut microbiota with increased abundance of pro-inflammatory microbiota (Escherichia and Shigella) and associated increased inflammatory markers in the blood (IL-1β, NLRP3, and CXCL2).80
The cellular and molecular pathways connecting peripheral with CNS inflammation are still under investigation. Multiple mechanisms have been proposed, including (1) the induction of blood–brain barrier leakage due to circulating cytokines with secondary cytokine access to the brain, (2) the access of circulating cytokines to the CNS through circumventricular organs, (3) the activation and migration of inflammatory immune cells from the periphery to the CNS, and (4) access of inflammatory mediators through transporter protein channels in the endothelium of the blood brain barrier.81 In AD, recent data provided evidence that peripheral immune cells can infiltrate the CNS and exert pro- or anti-inflammatory effects.82 Peripheral T-cells from AD patients have up-regulated expression of CXC chemokine receptor 2 (CXCR2) which guides their migration across the blood–brain barrier.82 This effect can be blocked in AD rat models with CXCR2 antagonists and TNFα antibodies, suggesting that T-cell migration and associated inflammation in AD is TNFα dependent.82 In addition, bone marrow–derived myeloid cells can infiltrate brain tissue via the chemoattractant protein CCL2 and its corresponding receptor CCR2 (C-C chemokine receptor type 2) and mitigate cognitive impairment and Aβ deposition in AD mouse models.42,77 Bone marrow–derived monocytes isolated from AD patients (however not from patients with MCI or healthy donors) produced IL-1β when stimulated with Aβ in vitro.83 The peripheral injection of LPS induced a transient increase of pro-inflammatory cytokines including IL-1β in both the CNS and the periphery (liver) as well as decreased microglial clearance of Aβ in APP/PS1 mice.84,85 Knockout of the NLRP3 inflammasome blocked these effects efficiently, highlighting a possible role of the NLRP3/IL-1β pathway for connecting peripheral and CNS inflammation in this model.84,85 In another study, mice underwent injection of intraperitoneal LPS after pre-treatment with either a COX inhibitor or a steroid. LPS injection caused behavioral changes as well as systemic inflammation with increased production of IL-6, IL-1β, TNFα, and prostaglandin E2 in the serum and the CNS.86 Interestingly, selective COX-1 inhibition with piroxicam (but not selective COX-2 inhibition with nimesulide) reversed the behavioral changes without affecting the peripheral or central cytokine levels.86 Dexamethasone effectively depleted cytokine production, however did not reverse the behavioral changes.86 Thus, it remained unclear if peripheral COX inhibition played a role in preventing CNS inflammation in this experiment. Taken together, further studies are clearly needed to elucidate the mechanisms connecting peripheral inflammation and neuroinflammation in AD and to identify appropriate therapeutic targets.
Summary
The data summarized above unanimously shows that neuroinflammation is a crucial component of AD pathology and progression. However, further studies are needed to clarify whether inflammation initiates AD pathology, or whether it is rather a bystander or downstream effect that chronically contributes to neuronal disease and cell death.87 Various activation and transcriptional states of glia cells including identification of multiple relevant pathways in the context of AD-associated neuroinflammation add another layer of complexity to the picture. Thus, neuroinflammation could play different (and even opposing) roles depending on the stage of disease, which may be highly relevant for the timing of therapeutic approaches. In addition, peripheral inflammation may play a role for initiation and/or chronic maintenance of neuroinflammation.
NSAIDs – definition and classes
NSAIDs have been used in early attempts to treat inflammation associated with AD pathology. NSAIDs are one of the most frequently prescribed classes of medications for pain relief, fever, and inflammatory conditions, and can be divided into eight classes based on their chemical structure and selectivity for inhibition (Table 1).88 The principal mechanism of action of all NSAIDs is the inhibition of cyclooxygenases (COX), which is required for the conversion of arachidonic acid to thromboxane (mediating platelet adhesion), prostaglandins (inducing vasodilation and hippocampal temperature regulation) and prostacyclin (involved in nociception).89 The pharmacological effects of NSAIDs are the direct results of reduction of these mediators, thus inhibiting platelet adhesion and modulating vasodilation, fever response, and pain perception. Of note, there are two main COX isoenzymes named COX-1 and COX-2, with distinct expression profiles and roles as discussed above.88 NSAID classes 1–7 are generally considered non-selective and inhibit COX-1 and COX-2, while class 8 NSAIDs selectively inhibit COX-2.88 The main adverse effects of NSAIDs include gastrointestinal (GI) mucosa damage and ulcers, renal damage, cardiovascular adverse effects (myocardial infarct and thromboembolic events), hepatotoxicity and hematological side effects related to platelet inhibition. GI side effects are reduced for COX-1 sparing NSAIDs given the selective inhibition of COX-2.88
Table 1.
NSAID classes and examples.
| Class | Examples |
|---|---|
| 1. Acetylated salicylates | Aspirin |
| 2. Non-acetylated salicylates | Diflunisal, salsalate |
| 3. Propionic acids | Naproxen, ibuprofen |
| 4. Acetic acids | Diclofenac, indomethacin |
| 5. Enolic acids | Meloxicam, piroxicam |
| 6. Anthranilic acids (fenamates) | Meclofenamic acid, mefenamic acid |
| 7. Naphthylalanine | Nabumetone |
| 8. Selective COX-2 inhibitors | Celecoxib, etoricoxib |
COX-2, cyclooxygenase 2; NSAID, non-steroidal anti-inflammatory drugs.
NSAIDs in AD – epidemiological studies, clinical trials, and meta-analyses
Multiple epidemiological studies, randomized controlled trials (RCT), and meta-analyses have addressed the potential therapeutic effect of NSAIDs in AD, with the results remaining controversial (Table 2). Importantly, the quality and level of evidence varies substantially depending on the type and scope of each study, and caution is warranted when interpreting the results. Information about the type of study and number of participants is provided in Table 2 and the text when appropriate. A Cochrane review from 2012 (including 14 RTC) evaluated treatment with aspirin, other NSAIDs (naproxen, indomethacin, ibuprofen, piroxicam, and nimesulide) and COX-2 inhibitors in AD patients, and concluded that there was no significant improvement in cognitive decline for any group.5 Importantly, patients receiving NSAIDs had more side effects and a trend toward higher death rates in this review. It should be noted that the review did not include a subgroup analysis for single NSAIDs.5 A recent meta-analysis included 16 cohort studies with a total of 236,022 study participants between 1995 and 2016 and showed that current or former NSAID use was associated with a significant reduction of developing AD in comparison with participants who did not take NSAIDs.6 The subgroup analysis for the effect of aspirin compared to acetaminophen or other NSAIDs, however, did not reveal a significant risk reduction for AD. The study did not include separate subgroup analyses for specific NSAIDs other than aspirin.6 In a large retrospective case–control study in the veteran population with inclusion of 246,199 veterans between 1998 and 2005, 49,349 individuals received a new diagnosis of AD during this time period. The odds ratio (OR) for AD decreased significantly for patients who received NSAIDs for more than 5 years. In subgroup analyses, this effect was more pronounced for ibuprofen and the OR decreased from 1.03 (1.00–1.06) to 0.56 (0.42–0.75), while results for other NSAIDs classes were inconsistent.90 The study did not report hazard ratios (HRs) to evaluate time-to-event data, and information about the progression of AD was therefore not available.90 The results of epidemiological studies triggered a number of clinical trials with variable quality due to short trial duration and low patient numbers.91 In a small double-blind, placebo-controlled study over 6 months with 160 AD patients, indomethacin slowed down cognitive decline in AD patients.92 Pasqualetti et al.93 followed 130 patients with mild to moderate AD taking ibuprofen versus placebo for the duration of 12 months and did not observe a difference in the Alzheimer’s Disease Assessment Sale (ADAS) or the mini–mental state examination (MMSE) in the treatment group. Other and larger RCTs (between 130 and 1649 participants) in patients with mild to moderate AD did not document benefits from NSAID use, for example, in several studies between 6 and 18 months, naproxen, rofecoxib, celecoxib, tarenflurbil, and nimesulide did not have any effects on cognitive function.94–98 The Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT), an RCT for 6 months in 2528 asymptomatic individuals with a family history of AD-like dementia, did not reveal a significant effect on cognitive function from naproxen or celecoxib, albeit a trend toward efficacy was found for both drugs.99 A follow-up study in the same patient cohort several years later did not document any protection from for either drug.100 Taken together, the data from epidemiological studies, clinical trials and meta-analyses in the past remains controversial, with some epidemiological studies and meta-analyses suggesting a positive effect of NSAIDs on AD progression, while clinical trials were mostly negative.91
Table 2.
Selected epidemiological studies, meta-analyses, and clinical trials for the use of NSAIDs in AD.
| Title | Type of study | Study population | NSAIDs investigated | Main conclusions | Comment |
|---|---|---|---|---|---|
| Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease5 | Cochrane review, included 14 randomized controlled trials | The number of participants was 352, 138, and 1745 for aspirin, steroid, and NSAID groups, respectively. | - Aspirin - Steroids - “traditional” NSAIDs (naproxen, indomethacin, ibuprofen, piroxicam, nimesulide) - selective COX-2 inhibitors |
There was no significant improvement of cognitive decline in AD patients for any NSAID. | Review did not include subgroup analysis for single NSAIDs. |
| NSAID Exposure and Risk of Alzheimer’s Disease: An Updated Meta-Analysis From Cohort Studies6 | Meta-analysis included 16 cohort studies | 236,022 participants | - Aspirin - Other NSAIDs |
Current or former NSAID use was associated with a significant risk reduction of developing AD in comparison with participants who did not take NSAIDs. | - There was no risk reduction for aspirin alone, or for other NSAIDs when analyzed separateley from aspirin. - The study did not include separate subgroup analyses for specific NSAIDs other than aspirin. |
| Protective effects of NSAIDs on the development of Alzheimer disease90 | Retrospective case control study | 246,199 veterans | - Ibuprofen - Other NSAIDs |
The odds ratio (OR) for AD decreased significantly for patients who received NSAIDs for more than 5 years. Effect was more pronounced for ibuprofen. | The study did not report hazard ratios (HR) to evaluate time-to-event data, and information about the progression of AD was therefore not available. |
| Clinical trial of indomethacin in Alzheimer’s disease92 | Double-blind, placebo-controlled study over 6 months; | AD patients, 160 participants | Indomethacin | Indomethacin slows down progression of cognitive decline in AD patients. | An important caveat of this study is the limited scale/ number of participants and study duration. |
| A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer’s disease93 | Randomized, controlled study over 12 months | Patients with mild to moderate AD, 130 participants | Ibuprofen versus placebo | There was no difference of cognitive decline between treatment and placebo group. | Participants who were ApoE ε4 carriers treated with ibuprofen did not have a significant cognitive decline. |
| Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial94 | Randomized, controlled study over 12 months | Mild to moderate AD, 351 participants | Rofecoxib or naproxen versus placebo | There was no benefit of treatment with NSAIDs for cognitive function. | — |
| Rofecoxib: no effect on Alzheimer’s disease in a 1-year, randomized, blinded, controlled study96 | Randomized, controlled trial over 12 months | Mild or moderate AD in 692 patients | Rofecoxib versus placebo | There was no benefit of Rofecoxib on cognitive function. | The results persisted after adjusting for severity of dementia at baseline, presence of ApoE ε4 allele, and donepezil use. |
| Long-term efficacy and safety of celecoxib in Alzheimer’s disease97 | Randomized, controlled trial over the course of 52 weeks | Mild to moderate AD, 308 patients completed treatment | Celecoxib versus placebo | There was no benefit of celecoxib on cognitive function. | — |
| Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial98 | Randomized, controlled study over a duration of 18 months | Mild AD, 1649 patients were included in the study | Tarenflurbil versus placebo | There was no benefit of NSAID on cognitive decline or loss of activities of daily living. | — |
| Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial99 | ADAPT (Alzheimer’s Disease Anti-inflammatory Prevention Trial), randomized, controlled trial for duration of 6 months | Recruitment of 2528 asymptomatic individuals with at least one first-degree relative with AD-like dementia | Naproxen or celecoxib versus placebo | A trend toward efficacy for both NSAIDs was present, however there was no clear benefit of NSAIDs on cognitive function. | Recruitment started in early 2001. The study was suspended in 2004 due to significantly increased cardiovascular risk of celecoxib. |
| Non-steroidal anti-inflammatory drug (NSAID) use and Alzheimer disease in community-dwelling elderly patients101 | Cross-sectional retrospective study | 2708 study individuals | - Aspirin - Non-aspirin (diclofenac, ketorolac, piroxicam) |
NSAID users had a 50% lower risk for being affected by AD. In subgroup analyses, the most significant risk reduction was seen for diclofenac. | After correction for confounders, the association between NSAID use and lower risk for AD was significant only for non-aspirin NSAID use. |
| A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer’s disease102 | Randomized controlled pilot study over 25 weeks | Mild to moderate AD, 41 patients | Diclofenac 50 mg daily vs control group | Trend for improved cognition with diclofenac after 6 months (compared to declined MMSE in the control group) | An important caveat of this study is the very low number of study participants. |
| Diclofenac reduces the risk of Alzheimer’s disease: a pilot analysis of NSAIDs in two US veteran populations7 | Retrospective cohort study | AD patients (1431 receiving diclofenac, versus 14,646 receiving etodolac, and 12,203 receiving naproxen for at least 1 year) | - Diclofenac (average of 131.3 mg daily) versus naproxen or etodolac | The incidence rates for AD in both the naproxen and etodolac group were significantly higher than for diclofenac. | Study had some limitations: –Small sample size for diclofenac –Retrospective design –Inclusion of patients based on chart review only |
AD, Alzheimer’s Disease; COX-2, cyclooxygenase 2; MMSE, mini-mental state examination; NSAID, non-steroidal anti-inflammatory drug.
New insights into the effects of diclofenac
Several reasons for the discrepancies between epidemiological data, meta-analyses and clinical trials have been discussed: (1) It is possible that the efficacy of NSAIDs may require a long treatment period for any protective effect on AD, while most clinical trials were only 6–12 months long. This conclusion is supported by epidemiological studies that have documented an increasing effect of NSAID use with the number of years patients were exposed.90 (2) The number of study individuals in many trials was oftentimes too small to reveal meaningful statistical effects when compared with some epidemiological studies that included hundred thousands of patients.91 (3) The limited availability of biomarkers for pre-clinical AD and the diagnostic uncertainty of biomarkers (serum, CSF, imaging) for AD pathology makes it challenging to select clinically homogeneous patient groups for trials, which may introduce significant bias. (4) It has been proposed that inflammation is an upstream event in AD pathology, and it is possible that treatment with NSAIDs must occur early in the AD course (potentially before the onset of symptoms) to affect disease onset and progression.87 (5) NSAIDs were investigated based on their known properties of COX inhibition and modulation of levels of prostaglandins and other mediators of the COX pathway. However, preclinical research has shown that other pathways such as the NLRP3 inflammasome may be relevant to the anti-inflammatory effect of some NSAIDs in cell and mouse models, and this was not reflected in the selection of drugs tested in clinical trials.7,91 (6) The dose of NSAIDs used in different studies was not standardized and varied significantly between studies which may have contributed to the variability of the results.5,6
A recent case—control longitudinal retrospective study aimed to address some of these concerns by analyzing the association between specific NSAID use and cognitive decline (rather than assessing the incidence and prevalence) in MCI and AD patients.91 The study was based on the data from the Alzheimer’s disease Neuroimaging Initiative (ADNI) of 1619 individuals with MCI or early AD, who were followed at the time of enrollment, at 6 months, 12 months, and yearly thereafter up to a maximum of 120 months.91 Negative binominal generalized linear modeling was used for the analysis of association between treatment with NSAIDs and cognitive decline. Doses and total treatment duration for medications were not reported. Celecoxib, diclofenac, ibuprofen, aspirin, and naproxen were associated with a substantial reduction of AD prevalence compared to the control group. However, aspirin, ibuprofen, naproxen, and celecoxib did not have any significant effects on cognitive decline based on MMSE or ADAS. Diclofenac was the only NSAID associated with a significant reduction of cognitive decline over time based on MMSE scores. The effect did approach significance based on ADAS scores. Interestingly, there was a positive effect on ADAS scores when included as a main effect; however, this effect was not significant when time was included, suggesting that the primary effect of diclofenac is on cognitive decline rather than onset of disease.91 Regarding the other NSAIDs (as well as paracetamol, an endocannabinoid modulator that does not inhibit COX1 or COX2), the authors concluded that their similar effect on AD incidence (despite significant structural and functional differences) could be due to healthy use bias, that is, healthier individuals being more likely to take NSAIDs and to remain in the study.91 Of note, only 30 subjects were identified in this study who were taking diclofenac, leading to the cautious conclusion by the authors that further research is needed to confirm the effects seen for diclofenac.
Historical research data have provided some evidence that diclofenac could be a promising drug candidate in AD. In a cross-sectional study of 2708 individuals, logistic regression analysis showed that chronic use of diclofenac had the greatest risk reduction on AD compared to other NSAIDs with an OR of 0.21 (CI 95%: 0.05–0.90).101 The dose for diclofenac or other medications was not reported in this study.101 Another small study recruited 41 patients with mild to moderate AD with an MMSE of 11–25 and randomized into a treatment group receiving diclofenac at a dose of 50 mg daily and a control group receiving placebo. After 6 months, there was a trend for improved MMSE scores in the diclofenac group, compared to declined MMSE scores in the control group.102
More recently, one of us (Stuve) performed a large retrospective cohort study among veterans with AD receiving diclofenac at an average of 131.3 mg per day (1431 individuals), etodolac at an average of 851 mg per day (14,646 individuals) and naproxen at an average of 927 mg per day (12,203 individuals) for the duration of at least 1 year.7 The incidence of dementia in the diclofenac group was significantly reduced (0.28%; 95% CI: 0.076–0.714) compared to naproxen (1.66%; 95% CI 1.44–1.9) and etodolac (2.24%; 95% CI: 2.01–2.49).7 The incidence rates for AD in both the naproxen and etodolac group were significantly higher than for diclofenac, and Cox regression survival analysis of AD showed a protective effect of diclofenac compared to naproxen.7 Naproxen was used as a baseline in this study, since prior studies have documented its lack of effect on AD. The HR for AD was significantly lower for diclofenac (0.25) compared to naproxen after controlling for age, comorbidities, and study site effects.7 Despite some limitations of the study design, namely the small sample size for diclofenac compared to naproxen and etodolac, the retrospective design, and the lack of control regarding compliance by including prescription history only into the data set, these data hold great promise and will require future studies to test the effect of diclofenac in AD.
Pharmacology and mechanisms of action of diclofenac and related NSAIDs
Pharmacology
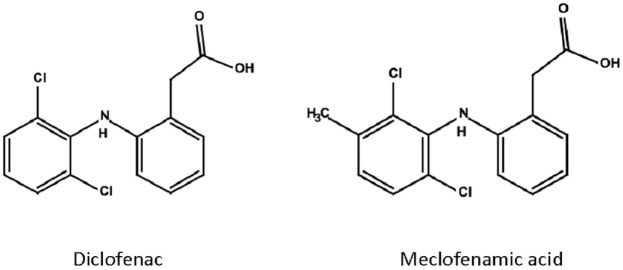
Diclofenac is one of the most frequently prescribed NSAIDs and belongs to the family of phenylacetic acids, derivatives of acetic acid. Diclofenac was first synthesized in 1973 and has since been US Food and Drugs Administration (FDA)-approved for multiple inflammatory conditions. It is a weak acid with an acidity constant of 4, indicating partial solubility in both aqueous and hydrophobic environments.103,104 Its structure consists of a phenylacetic acid group and a phenyl ring with two chlorine atoms (Figure 1), which causes twisting of the phenyl ring and binding to the substrate pocket of COX enzymes.103 Diclofenac is typically rapidly absorbed, and 60% reach the systemic blood circulation due to first-pass metabolism.103 Similar to other acidic NSAIDs, it has high affinity to plasma proteins, and accumulates in inflamed tissue with acidic environment.103 The short biological half-life of approximately 2 hours requires frequent administration, which led to the production of enteric-coated tablets and similar formulations with delayed absorption.103 NSAIDs have generally good capacity to cross the blood brain barrier and access the brain. In a study that compared diclofenac, mefenamic acid and acetaminophen in mice, both diclofenac and mefenamic acid had good brain penetration independent of systemic inflammation, which was carried out by the carboxyl groups and an anion transport system into the CNS.105,106 Diclofenac was shown to reach significant CSF levels in adults and children.107,108
Figure 1.
Chemical structures of meclofenamic acid and diclofenac. Prepared with JChemPaint.
Mechanisms of action beyond COX inhibition
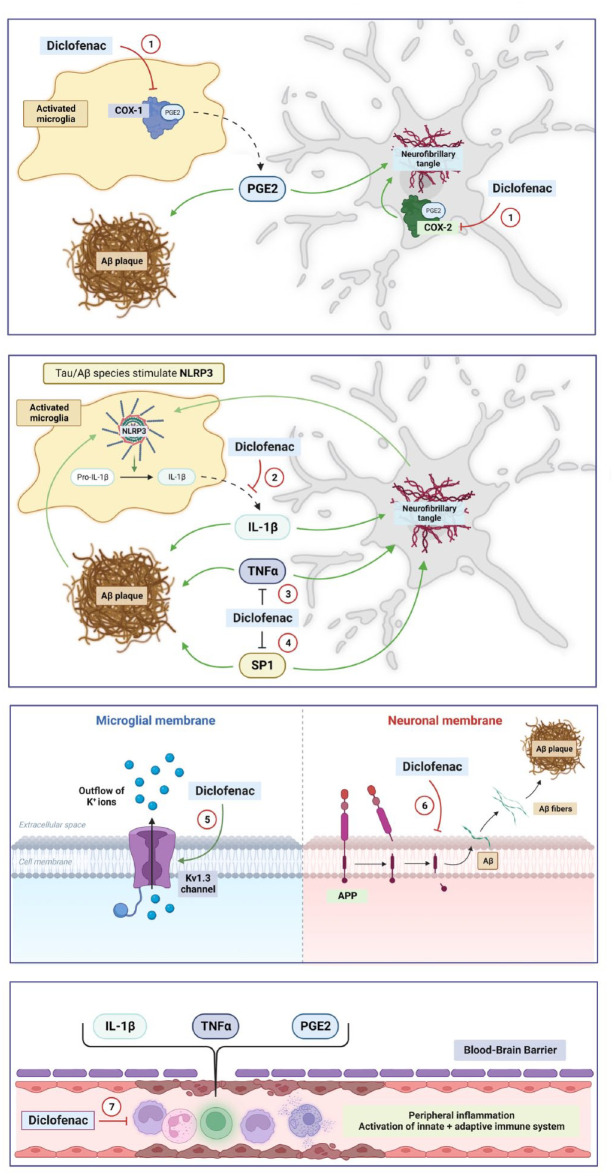
Unlike most traditional NSAIDs, diclofenac has a higher selectivity for COX-2 than for COX-1 (Figure 2, mechanism 1).104 Multiple other pathways have been described for diclofenac that could potentially have therapeutic effects on AD (Figure 2):
Figure 2.
Proposed mechanisms of action for diclofenac in AD in the CNS and the periphery. Multiple pathways have been described for diclofenac that could potentially have therapeutic effects in AD. (1) Inhibition of COX-1 (primarily in microglia), COX-2 (primarily in neurons). (2) Inhibition of NLRP3 and release of IL-1β from microglia. (3) Potassium channel modulation in glia cells with inhibitory effect on inflammatory responses. (4) Inhibition of APP processing and Aβ plaque formation. (5) Inhibition of Specificity Protein 1 (SP1), a zinc-finger transcription factor regulating the transcription of APP, MAPT, BACE1, and CDK5 in the brain. (6) TNFα inhibition. (7) Inhibition of systemic inflammation and secondary neuroinflammation. Green errors = activation. Red errors = inhibition. Prepared with BioRender. Please see text for more details on abbreviations and the proposed mechanisms.
NLRP3 and IL-1β inhibition: The chemical structure of diclofenac is similar to meclofenamic acid (Figure 1), an NSAID in the fenamate class. Other fenamates include flufenamic acid and mefenamic acid. In 2006, a study investigating the therapeutic potential of mefenamic acid in an AD rat model concluded that the drug upregulates expression of the anti-apoptotic protein Bcl-X(L), reduced free radical nitric oxide and cytochrome C from mitochondria, and attenuated Aβ-related neurotoxicity and memory impairment.109 A subsequent study by Daniels et al.8 identified fenamate NSAIDs as effective and selective inhibitors of the NLRP3 inflammasome independently of COX enzymes in immortalized bone-marrow-derived macrophages. Notably, NSAIDS of the fenamate class (flufenamic acid, mefenamic acid, and meclofenamic acid) tested in this study at a concentration of 100 uM were significantly more effective at inhibiting the release of IL-1β than other NSAIDs (celecoxib and ibuprofen).8 The authors used Western Blot analysis to demonstrate that fenamates specifically block caspase-1-dependent processing of IL-1β, without significant effect on cell death. Importantly, the fenamates did not inhibit IL-1β release by other inflammasomes.8 In addition, fenamates inhibited ASC speck formation and production of the active p10 subunit of caspase-1 in cell models. Further experiments showed that fenamates exert their effect via inhibition of the membrane standing chloride-transporting volume-regulated anion channel (VRAC). Of note, in the second part of the study, mefenamic acid was delivered via osmotic minipumps into the cerebral ventricles (28 days, 5 mg per kilogram per day) of 3x TgAD mice (AD mouse model harboring mutations of APP, MAPT, and PSEN1) and reduced neuroinflammation including microglial activation and IL-1β release to levels encountered in WT mice.8 Diclofenac was also tested and had a modest effect on IL-1β release in this study. However, diclofenac did not affect the chloride channel VRAC, and future work will be required to determine the exact molecular mechanism underlying the inhibition of IL-1β release by diclofenac.
Potassium channel modulation: Diclofenac and the structurally related meclofenamic acid (Figure 1) can act as voltage-gated potassium channel opener of Kv1.3 in macrophages and leucocytes with inhibitory effect on immune responses.104,110,111 In the 5XFAD mouse model of AD, pharmacological Kv1.3 inhibition with PAP-1 (5-(4-phenoxybutoxy)psoralen) led to a significant reduction of neuroinflammation, AD pathology, and cognitive deficits compared to control animals.112 Further studies are required to dissect the exact molecular mechanism of this effect, and to assess if diclofenac has a comparable impact on AD pathology via Kv1.3 inhibition.
Inhibition of APP processing and Aβ plaque formation: Several studies demonstrate that some NSAIDs including diclofenac affect APP processing by modulation of γ-secretase activity, inhibition of Aβ fibril formation and stimulation of α-secretase, thus leading to a reduced production of amyloidogenic Aβ species.113,114
Specifity Protein 1 (SP1) inhibition: Tolfenamic acid (TA), an NSAID in the fenamate group, was proposed to have yet another mechanism that could be relevant in AD, that is, by promoting Specificity Protein 1 (SP1) degradation.115 SP1 is a zinc-finger transcription factor regulating the transcription of APP, MAPT, BACE1, and cyclin-dependent kinase-5 (CDK5) in the brain.115,116 SP1 levels are elevated in the frontal cortex of AD transgenic mice and AD patients.115 TA lowers the expression of SP1-regulated genes, thus decreasing levels of pathological Aβ and hyperphosphorylated tau species in AD mouse models.115 In tau transgenic mouse models, the administration of TA led to significant improvement in cognition tests.117 In the mouse studies, TA was given at concentrations between 5 and 50 mg/kg per oral gavage for 34 days.115,117 A randomized, double-blind, placebo-controlled phase 2a trial to assess the effects of TA in individuals with PSP is ongoing (NCT04253132). TA will be administered at doses of 50, 300, and 600 mg daily. Primary outcome measures are safety and tolerance. Other outcome measures will include CSF and plasma biomarkers as well as clinical rating scales. Based on studies in cell models, diclofenac could affect SP1 similar to TA. In a cell model of pancreatic cancer, TA was used at a concentration of 50 µM and induced degradation of several transcription factors including SP1, SP3, and SP4, which in turn decreased vascular endothelial growth factor (VEGF) expression and tumor growth. In this study, diclofenac was tested as well and had moderate enhancing effect on SP1, SP3, and SP4 degradation, albeit less pronounced than TA.118 These results will require further confirmation in AD models.
TNFα inhibition: The reduction of the pro-inflammatory cytokine TNFα may be an additional mechanism of interest in AD.119 TNFα contributes to abnormal Aβ processing, synaptic loss, neuronal dysfunction, and cognitive decline in AD.119 In rats, the intraperitoneal delivery of diclofenac and derivatives at a dose of 47.2 μmol/kg decreased the plasma levels of both IL-1β and TNFα.119,120 The administration of intraperitoneal diclofenac in rats at 5 or 10 mg/kg for 15 days led to a modest reduction of TNFα in selected brain areas.121 Further studies are needed to clarify the potential effects of diclofenac on TNFα levels in AD.
Inhibition of systemic inflammation: In recent years, data emerged that peripheral acute and chronic inflammation may have a significant impact on AD pathology as well,81 as described in detail in earlier sections of this review. Thus, in addition to the direct effects in the brain, diclofenac could have indirect effects on cerebral AD pathology by reducing prostaglandin-mediated peripheral inflammation via COX-1/2 inhibition and secondary neuroinflammation.
Conclusion
AD remains one of the main medical challenges of the 21st century with major impact on our aging societies, and new therapeutic strategies are desperately needed. Neuroinflammation has a significant impact on AD pathology and anti-inflammatory medications could be a powerful tool to delay or prevent disease onset and progression. Past studies concluded that NSAIDs do not have a significant impact on AD onset of progression. However, neither diclofenac nor fenamate NSAIDs were included in prior large AD studies.7 One reason may have been the lack of marketing of fenamates to physicians and patients after their release on the market in the 1960s.7 In addition, in the United States, fenamates were primarily prescribed for menstrual symptoms in women below the age of 50 years, who have a low likelihood to develop AD.7,122 Recent data in cell and animal models as well as in large epidemiological studies suggest diclofenac and fenamates as promising candidates to modify AD progression due to their unique pharmacological properties on the NLRP3/ IL-1β pathway (Figure 2). Other mechanisms and pathways such as enhancement of transcription factor SP1 degradation and attenuation of peripheral inflammation could play a role as well, albeit pending further investigation (Figure 2). Diclofenac has been FDA approved for multiple inflammatory conditions since 2002 and is generally well tolerated, making it a very attractive candidate for upcoming trials and therapeutic approaches.
Outlook
Future studies should address the exact pharmacological and molecular mechanisms of diclofenac and fenamates in AD. For example, prior studies suggest that fenamates (but not diclofenac) decrease IL-1β release by inhibiting the membrane chloride channel VRAC. However, it is unknown if diclofenac and/or fenamates can physically bind/interact with components of the NLRP3 inflammasome. In the same way, the molecular mechanism driving the potential effect of diclofenac and fenamates on potassium channels, SP1, and TNFα are unknown. Taken together, careful studies in appropriate in vitro and cell/animal systems are required to elucidate the details of how diclofenac and fenamates affect specific pathways in AD pathology.
In addition, large and well-controlled prospective trials are needed to document the effect of diclofenac in humans, preferentially prior to disease onset or early during the disease course. Specifically, a randomized placebo-controlled trial should be performed in a large study cohort of at least several hundred participants with MCI or early stages of AD. Ideally, the selection of patients would be achieved based on a combination of clinical, blood, CNS, and imaging biomarkers of AD (volumetric MRI studies, PET imaging) to allow the inclusion of a homogeneous population, and serial testing of biomarkers should be continued throughout the study in intervals of 3–6 months. We propose a standard dose of diclofenac 75 mg twice daily, with careful monitoring for side effects, for example, on the gastrointestinal tract and the cardiovascular system. Important endpoints include onset of cognitive deficits, cognitive decline over time, changes of AD biomarkers in blood and CSF, changes of imaging parameters, and changes of inflammatory markers such as IL-1β in CSF and serum. Importantly, an appropriate study duration of at least 1–2 years seems crucial to detect cognitive decline. Ultimately, the data on diclofenac and fenamates may guide the way for the development of other drugs that interfere with the NLRP3/IL-1β pathway, with the prospect of extending our anti-inflammatory toolkit in the battle against AD.
Supplemental Material
Supplemental material, sj-pdf-1-tan-10.1177_17562864231156674 for Microglia as a cellular target of diclofenac therapy in Alzheimer’s disease by Barbara E. Stopschinski, Rick A. Weideman, Danni McMahan, David A. Jacob, Bertis B. Little, Hsueh-Sheng Chiang, Nil Saez Calveras and Olaf Stuve in Therapeutic Advances in Neurological Disorders
Acknowledgments
Ther authors would like to thank Dr. Marc Diamond for helpful discussions and comments.
Footnotes
ORCID iDs: Barbara E. Stopschinski  https://orcid.org/0000-0002-5715-4567
https://orcid.org/0000-0002-5715-4567
Nil Saez Calveras  https://orcid.org/0000-0002-9153-9700
https://orcid.org/0000-0002-9153-9700
Olaf Stuve  https://orcid.org/0000-0002-0469-6872
https://orcid.org/0000-0002-0469-6872
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Barbara E. Stopschinski, Department of Neurology, University of Texas Southwestern Medical Center, Dallas, TX, USA Center for Alzheimer’s and Neurodegenerative Diseases, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Rick A. Weideman, Pharmacy Service, Dallas VA Medical Center, Dallas, TX, USA
Danni McMahan, Pharmacy Service, Dallas VA Medical Center, Dallas, TX, USA.
David A. Jacob, Veterans Integrated Service Network 17, Arlington, TX, USA
Bertis B. Little, School of Public Health and Information Sciences, University of Louisville, Louisville, KY, USA
Hsueh-Sheng Chiang, Department of Neurology, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Nil Saez Calveras, Department of Neurology, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Olaf Stuve, Department of Neurology, University of Texas Southwestern Medical Center, Dallas, TX, USA; Peter O’Donnell Jr. Brain Institute, University of Texas Southwestern Medical Center, Dallas, TX, USA; Neurology Section, Dallas VA Medical Center, 4500 South Lancaster Road, Dallas, TX 75216, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Barbara E. Stopschinski: Conceptualization; Data curation; Formal analysis; Visualization; Writing – original draft; Writing – review & editing.
Rick A. Weideman: Conceptualization; Data curation; Formal analysis; Writing – original draft; Writing – review & editing.
Danni McMahan: Conceptualization; Writing – review & editing.
David A. Jacob: Conceptualization; Writing – review & editing.
Bertis B. Little: Conceptualization; Data curation; Formal analysis; Writing – review & editing.
Hsueh-Sheng Chiang: Conceptualization; Writing – review & editing.
Nil Saez Calveras: Conceptualization; Visualization; Writing – review & editing.
Olaf Stuve: Conceptualization; Data curation; Formal analysis; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Pardo-Moreno T, González-Acedo A, Rivas-Domínguez A, et al. Therapeutic approach to Alzheimer’s disease: current treatments and new perspectives. Pharmaceutics 2022; 14: 1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mendiola-Precoma J, Berumen LC, Padilla K, et al. Therapies for prevention and treatment of Alzheimer’s disease. Biomed Res Int 2016; 2016: 2589276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 2019; 14: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang WY, Tan MS, Yu JT, et al. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 2015; 3: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jaturapatporn D, Isaac MG, McCleery J, et al. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. Cochrane Database Syst Rev 2012; 2: CD006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang C, Wang Y, Wang D, et al. NSAID exposure and risk of Alzheimer’s disease: an updated meta-analysis from cohort studies. Front Aging Neurosci 2018; 10: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stuve O, Weideman RA, McMahan DM, et al. Diclofenac reduces the risk of Alzheimer’s disease: a pilot analysis of NSAIDs in two US veteran populations. Ther Adv Neurol Disord 2020; 13: 1756286420935676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daniels MJ, Rivers-Auty J, Schilling T, et al. Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer’s disease in rodent models. Nat Commun 2016; 7: 12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med 2010; 362: 329–344. [DOI] [PubMed] [Google Scholar]
- 10. Kametani F, Hasegawa M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front Neurosci 2018; 12: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lue LF, Kuo YM, Roher AE, et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol 1999; 155: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82: 239–259. [DOI] [PubMed] [Google Scholar]
- 13. Braak H, Del Tredici K. Are cases with tau pathology occurring in the absence of Abeta deposits part of the AD-related pathological process? Acta Neuropathol 2014; 128: 767–772. [DOI] [PubMed] [Google Scholar]
- 14. Pascoal TA, Benedet AL, Ashton NJ, et al. Microglial activation and tau propagate jointly across Braak stages. Nat Med 2021; 27: 1592–1599. [DOI] [PubMed] [Google Scholar]
- 15. St-Pierre MK, VanderZwaag J, Loewen S, et al. All roads lead to heterogeneity: the complex involvement of astrocytes and microglia in the pathogenesis of Alzheimer’s disease. Front Cell Neurosci 2022; 16: 932572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 2005; 8: 752–758. [DOI] [PubMed] [Google Scholar]
- 17. Giulian D. Ameboid microglia as effectors of inflammation in the central nervous system. J Neurosci Res 1987; 18: 155–171, 132–133. [DOI] [PubMed] [Google Scholar]
- 18. Marlatt MW, Bauer J, Aronica E, et al. Proliferation in the Alzheimer hippocampus is due to microglia, not astroglia, and occurs at sites of amyloid deposition. Neural Plast 2014; 2014: 693851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krasemann S, Madore C, Cialic R, et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 2017; 47: 566–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keren-Shaul H, Spinrad A, Weiner A, et al. A unique microglia type associated with restricting development of Alzheimer’s Disease. Cell 2017; 169: 1276–1290. [DOI] [PubMed] [Google Scholar]
- 21. Delizannis AT, Nonneman A, Tsering W, et al. Effects of microglial depletion and TREM2 deficiency on Abeta plaque burden and neuritic plaque tau pathology in 5XFAD mice. Acta Neuropathol Commun 2021; 9: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saito T, Matsuba Y, Mihira N, et al. Single App knock-in mouse models of Alzheimer’s disease. Nat Neurosci 2014; 17: 661–663. [DOI] [PubMed] [Google Scholar]
- 23. Swartzlander DB, Propson NE, Roy ER, et al. Concurrent cell type-specific isolation and profiling of mouse brains in inflammation and Alzheimer’s disease. JCI Insight 2018; 3: e121109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Romero-Molina C, Navarro V, Sanchez-Varo R, et al. Distinct microglial responses in two transgenic murine models of TAU pathology. Front Cell Neurosci 2018; 12: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang HS, Onos KD, Choi K, et al. Natural genetic variation determines microglia heterogeneity in wild-derived mouse models of Alzheimer’s disease. Cell Rep 2021; 34: 108739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sala Frigerio C, Wolfs L, Fattorelli N, et al. The major risk factors for Alzheimer’s disease: age, sex, and genes modulate the microglia response to Abeta plaques. Cell Rep 2019; 27: 1293–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gerrits E, Brouwer N, Kooistra SM, et al. Distinct amyloid-beta and tau-associated microglia profiles in Alzheimer’s disease. Acta Neuropathol 2021; 141: 681–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maeda J, Minamihisamatsu T, Shimojo M, et al. Distinct microglial response against Alzheimer’s amyloid and tau pathologies characterized by P2Y12 receptor. Brain Commun 2021; 3: fcab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Srinivasan K, Friedman BA, Etxeberria A, et al. Alzheimer’s patient microglia exhibit enhanced aging and unique transcriptional activation. Cell Rep 2020; 31: 107843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. John Lin CC, Yu K, Hatcher A, et al. Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci 2017; 20: 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verkhratsky A, Rodrigues JJ, Pivoriunas A, et al. Astroglial atrophy in Alzheimer’s disease. Pflugers Arch 2019; 471: 1247–1261. [DOI] [PubMed] [Google Scholar]
- 32. Habib N, McCabe C, Medina S, et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat Neurosci 2020; 23: 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou Y, Song WM, Andhey PS, et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat Med 2020; 26: 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith AM, Davey K, Tsartsalis S, et al. Diverse human astrocyte and microglial transcriptional responses to Alzheimer’s pathology. Acta Neuropathol 2022; 143: 75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wolf SA, Boddeke HW, Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol 2017; 79: 619–643. [DOI] [PubMed] [Google Scholar]
- 36. Kettenmann H, Hanisch UK, Noda M, et al. Physiology of microglia. Physiol Rev 2011; 91: 461–553. [DOI] [PubMed] [Google Scholar]
- 37. Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol 2015; 14: 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krabbe G, Halle A, Matyash V, et al. Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS ONE 2013; 8: e60921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Streit WJ, Braak H, Xue QS, et al. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol 2009; 118: 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qin Q, Wang M, Yin Y, et al. The specific mechanism of TREM2 regulation of synaptic clearance in Alzheimer’s disease. Front Immunol 2022; 13: 845897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bolos M, Llorens -Martin M, Perea JR, et al. Absence of CX3CR1 impairs the internalization of Tau by microglia. Mol Neurodegener 2017; 12: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Laurent C, Buée L, Blum D. Tau and neuroinflammation: what impact for Alzheimer’s disease and tauopathies. Biomed J 2018; 41: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yoshiyama Y, Higuchi M, Zhang B, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007; 53: 337–351. [DOI] [PubMed] [Google Scholar]
- 44. Hollingworth P, Harold D, Sims R, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet 2011; 43: 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shi Y, Yamada K, Liddelow SA, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 2017; 549: 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bhaskar K, Konerth M, Kokiko-Cochran ON, et al. Regulation of tau pathology by the microglial fractalkine receptor. Neuron 2010; 68: 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ising C, Venegas C, Zhang S, et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019; 575: 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mendiola AS, Cardona AE. The IL-1beta phenomena in neuroinflammatory diseases. J Neural Transm 2018; 125: 781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cacabelos R, Alvarez XA, Fernández-Novoa L, et al. Brain interleukin-1 beta in Alzheimer’s disease and vascular dementia. Methods Find Exp Clin Pharmacol 1994; 16: 141–151. [PubMed] [Google Scholar]
- 50. Luciunaite A, McManus RM, Jankunec M, et al. Soluble Abeta oligomers and protofibrils induce NLRP3 inflammasome activation in microglia. J Neurochem 2020; 155: 650–661. [DOI] [PubMed] [Google Scholar]
- 51. Jiang S, Maphis NM, Binder J, et al. Proteopathic tau primes and activates interleukin-1beta via myeloid-cell-specific MyD88- and NLRP3-ASC-inflammasome pathway. Cell Rep 2021; 36: 109720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Griffin WS, Stanley LC, Ling C, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A 1989; 86: 7611–7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Heneka MT, Kummer MP, Stutz A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013; 493: 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ye L, Huang Y, Zhao L, et al. IL-1beta and TNF-alpha induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. J Neurochem 2013; 125: 897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kitazawa M, Cheng D, Tsukamoto MR, et al. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal beta-catenin pathway function in an Alzheimer’s disease model. J Immunol 2011; 187: 6539–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sheng JG, Zhu SG, Jones RA, et al. Interleukin-1 promotes expression and phosphorylation of neurofilament and tau proteins in vivo. Exp Neurol 2000; 163: 388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li Y, Liu L, Barger SW, et al. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci 2003; 23: 1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cherry JD, Olschowka JA, O’Banion, et al. Arginase 1+ microglia reduce Abeta plaque deposition during IL-1beta-dependent neuroinflammation. J Neuroinflammation 2015; 12: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Matousek SB, Ghosh S, Shaftel SS, et al. Chronic IL-1beta-mediated neuroinflammation mitigates amyloid pathology in a mouse model of Alzheimer’s disease without inducing overt neurodegeneration. J Neuroimmune Pharmacol 2012; 7: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res 2009; 50(Suppl.): S29–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Feng L, Sun W, Xia Y, et al. Cloning two isoforms of rat cyclooxygenase: differential regulation of their expression. Arch Biochem Biophys 1993; 307: 361–368. [DOI] [PubMed] [Google Scholar]
- 62. Chandrasekharan NV, Dai H, Roos KL, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A 2002; 99: 13926–13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yuan C, Smith WL. A cyclooxygenase-2-dependent prostaglandin E2 biosynthetic system in the Golgi apparatus. J Biol Chem 2015; 290: 5606–5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kitamura Y, Shimohama S, Koike H, et al. Increased expression of cyclooxygenases and peroxisome proliferator-activated receptor-gamma in Alzheimer’s disease brains. Biochem Biophys Res Commun 1999; 254: 582–586. [DOI] [PubMed] [Google Scholar]
- 65. Deininger MH, Schluesener HJ. Cyclooxygenases-1 and -2 are differentially localized to microglia and endothelium in rat EAE and glioma. J Neuroimmunol 1999; 95: 202–208. [DOI] [PubMed] [Google Scholar]
- 66. Yamagata K, Andreasson KI, Kaufmann WE, et al. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron 1993; 11: 371–386. [DOI] [PubMed] [Google Scholar]
- 67. Choi SH, Langenbach R, Bosetti F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J 2008; 22: 1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aid S, Langenbach R, Bosetti F. Neuroinflammatory response to lipopolysaccharide is exacerbated in mice genetically deficient in cyclooxygenase-2. J Neuroinflammation 2008; 5: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang P, Guan PP, Wang T, et al. Aggravation of Alzheimer’s disease due to the COX-2-mediated reciprocal regulation of IL-1beta and Abeta between glial and neuron cells. Aging Cell 2014; 13: 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70. Choi SH, Bosetti F. Cyclooxygenase-1 null mice show reduced neuroinflammation in response to beta-amyloid. Aging 2009; 1: 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mhillaj E, Morgese MG, Tucci P, et al. Celecoxib prevents cognitive impairment and neuroinflammation in soluble amyloid beta-treated rats. Neuroscience 2018; 372: 58–73. [DOI] [PubMed] [Google Scholar]
- 72. Xiang Z, Ho L, Yemul S, et al. Cyclooxygenase-2 promotes amyloid plaque deposition in a mouse model of Alzheimer’s disease neuropathology. Gene Expr 2002; 10: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hoozemans JJ, Rozemuller JM, van Haastert ES, et al. Cyclooxygenase-1 and -2 in the different stages of Alzheimer’s disease pathology. Curr Pharm Des 2008; 14: 1419–1427. [DOI] [PubMed] [Google Scholar]
- 74. Hoozemans JJ, Rozemuller AJ, Janssen I, et al. Cyclooxygenase expression in microglia and neurons in Alzheimer’s disease and control brain. Acta Neuropathol 2001; 101: 2–8. [DOI] [PubMed] [Google Scholar]
- 75. Yermakova AV, Rollins J, Callahan LM, et al. Cyclooxygenase-1 in human Alzheimer and control brain: quantitative analysis of expression by microglia and CA3 hippocampal neurons. J Neuropathol Exp Neurol 1999; 58: 1135–1146. [DOI] [PubMed] [Google Scholar]
- 76. Ohnishi A, Senda M, Yamane T, et al. Exploratory human PET study of the effectiveness of (11)C-ketoprofen methyl ester, a potential biomarker of neuroinflammatory processes in Alzheimer’s disease. Nucl Med Biol 2016; 43: 438–444. [DOI] [PubMed] [Google Scholar]
- 77. Bettcher BM, Tansey MG, Dorothee G, et al. Peripheral and central immune system crosstalk in Alzheimer disease – a research prospectus. Nat Rev Neurol 2021; 17: 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sipilä PN, Heikkilä N, Lindbohm JV, et al. Hospital-treated infectious diseases and the risk of dementia: a large, multicohort, observational study with a replication cohort. Lancet Infect Dis 2021; 21: 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Holmes C, Cunningham C, Zotova E, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology 2009; 73: 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cattaneo A, Cattane N, Galluzzi S, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging 2017; 49: 60–68. [DOI] [PubMed] [Google Scholar]
- 81. Sun Y, Koyama Y, Shimada S. Inflammation from peripheral organs to the brain: how does systemic inflammation cause neuroinflammation. Front Aging Neurosci 2022; 14: 903455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Liu YJ, Guo DW, Tian L, et al. Peripheral T cells derived from Alzheimer’s disease patients overexpress CXCR2 contributing to its transendothelial migration, which is microglial TNF-alpha-dependent. Neurobiol Aging 2010; 31: 175–188. [DOI] [PubMed] [Google Scholar]
- 83. Saresella M, La Rosa F, Piancone F, et al. The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Mol Neurodegener 2016; 11: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tejera D, Mercan D, Sanchez-Caro JM, et al. Systemic inflammation impairs microglial Abeta clearance through NLRP3 inflammasome. EMBO J 2019; 38: e101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Beyer MMS, Lonnemann N, Remus A, et al. Enduring changes in neuronal function upon systemic inflammation are NLRP3 inflammasome dependent. J Neurosci 2020; 40: 5480–5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Teeling JL, Cunningham C, Newman TA, et al. The effect of non-steroidal anti-inflammatory agents on behavioural changes and cytokine production following systemic inflammation: implications for a role of COX-1. Brain Behav Immun 2010; 24: 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med 2006; 12: 1005–1015. [DOI] [PubMed] [Google Scholar]
- 88. Ghlichloo I, Gerriets V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs). Treasure Island, FL: StatPearls, 2022. [PubMed] [Google Scholar]
- 89. Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 1971; 231: 232–235. [DOI] [PubMed] [Google Scholar]
- 90. Vlad SC, Miller DR, Kowall NW, et al. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 2008; 70: 1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rivers-Auty J, Mather AE, Peters R, et al. Anti-inflammatories in Alzheimer’s disease-potential therapy or spurious correlate? Brain Commun 2020; 2: fcaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rogers J, Kirby LC, Hempelman SR, et al. Clinical trial of indomethacin in Alzheimer’s disease. Neurology 1993; 43: 1609–1611. [DOI] [PubMed] [Google Scholar]
- 93. Pasqualetti P, Bonomini C, Dal Forno G, et al. A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer’s disease. Aging Clin Exp Res 2009; 21: 102–110. [DOI] [PubMed] [Google Scholar]
- 94. Aisen PS, Schafer KA, Grundman M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 2003; 289: 2819–2826. [DOI] [PubMed] [Google Scholar]
- 95. Aisen PS, Schmeidler J, Pasinetti GM. Randomized pilot study of nimesulide treatment in Alzheimer’s disease. Neurology 2002; 58: 1050–1054. [DOI] [PubMed] [Google Scholar]
- 96. Reines SA, Block GA, Morris JC, et al. Rofecoxib: no effect on Alzheimer’s disease in a 1-year, randomized, blinded, controlled study. Neurology 2004; 62: 66–71. [DOI] [PubMed] [Google Scholar]
- 97. Soininen H, West C, Robbins J, et al. Long-term efficacy and safety of celecoxib in Alzheimer’s disease. Dement Geriatr Cogn Disord 2007; 23: 8–21. [DOI] [PubMed] [Google Scholar]
- 98. Green RC, Schneider LS, Amato DA, et al. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA 2009; 302: 2557–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Group AR, Lyketsos CG, Breitner JC, et al. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology 2007; 68: 1800–1808. [DOI] [PubMed] [Google Scholar]
- 100. ADAPT-FS Research Group. Follow-up evaluation of cognitive function in the randomized Alzheimer’s disease anti-inflammatory prevention trial and its follow-up study. Alzheimers Dement 2015; 11: 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Landi F, Cesari M, Onder G, et al. Non-steroidal anti-inflammatory drug (NSAID) use and Alzheimer disease in community-dwelling elderly patients. Am J Geriatr Psychiatry 2003; 11: 179–185. [PubMed] [Google Scholar]
- 102. Scharf S, Mander A, Ugoni A, et al. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer’s disease. Neurology 1999; 53: 197–201. [DOI] [PubMed] [Google Scholar]
- 103. Altman R, Bosch B, Brune K, et al. Advances in NSAID development: evolution of diclofenac products using pharmaceutical technology. Drugs 2015; 75: 859–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Amanullah A, Upadhyay A, Dhiman R, et al. Development and challenges of diclofenac-based novel therapeutics: targeting cancer and complex diseases. Cancers 2022; 14: 4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fukuda M, Kitaichi K, Abe F, et al. Altered brain penetration of diclofenac and mefenamic acid, but not acetaminophen, in Shiga-like toxin II-treated mice. J Pharmacol Sci 2005; 97: 525–532. [DOI] [PubMed] [Google Scholar]
- 106. Menassé R, Hedwall PR, Kraetz J, et al. Pharmacological properties of diclofenac sodium and its metabolites. Scand J Rheumatol Suppl 1978; 22: 5–16. [DOI] [PubMed] [Google Scholar]
- 107. Zecca L, Ferrario P, Costi P. Determination of diclofenac and its metabolites in plasma and cerebrospinal fluid by high-performance liquid chromatography with electrochemical detection. J Chromatogr 1991; 567: 425–432. [DOI] [PubMed] [Google Scholar]
- 108. Kokki H, Kumpulainen E, Laisalmi M, et al. Diclofenac readily penetrates the cerebrospinal fluid in children. Br J Clin Pharmacol 2008; 65: 879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Joo Y, Kim HS, Woo RS, et al. Mefenamic acid shows neuroprotective effects and improves cognitive impairment in vitro and in vivo Alzheimer’s disease models. Mol Pharmacol 2006; 69: 76–84. [DOI] [PubMed] [Google Scholar]
- 110. Lam J, Wulff H. The lymphocyte potassium channels Kv1.3 and KCa3.1 as targets for immunosuppression. Drug Dev Res 2011; 72: 573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Peretz A, Degani N, Nachman R, et al. Meclofenamic acid and diclofenac, novel templates of KCNQ2/Q3 potassium channel openers, depress cortical neuron activity and exhibit anticonvulsant properties. Mol Pharmacol 2005; 67: 1053–1066. [DOI] [PubMed] [Google Scholar]
- 112. Maezawa I, Nguyen HM, Di Lucente J, et al. Kv1.3 inhibition as a potential microglia-targeted therapy for Alzheimer’s disease: preclinical proof of concept. Brain 2018; 141: 596–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hirohata M, Ono K, Naiki H, et al. Non-steroidal anti-inflammatory drugs have anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. Neuropharmacology 2005; 49: 1088–1099. [DOI] [PubMed] [Google Scholar]
- 114. Weggen S, Eriksen JL, Das P, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature 2001; 414: 212–216. [DOI] [PubMed] [Google Scholar]
- 115. Adwan L, Subaiea GM, Basha R, et al. Tolfenamic acid reduces tau and CDK5 levels: implications for dementia and tauopathies. J Neurochem 2015; 133: 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Adwan L, Subaiea GM, Zawia NH. Tolfenamic acid downregulates BACE1 and protects against lead-induced upregulation of Alzheimer’s disease related biomarkers. Neuropharmacology 2014; 79: 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Leso A, Bihaqi SW, Masoud A, et al. Loss in efficacy measures of tolfenamic acid in a tau knock-out model: relevance to Alzheimer’s disease. Exp Biol Med 2019; 244: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Abdelrahim M, Baker CH, Abbruzzese JL, et al. Tolfenamic acid and pancreatic cancer growth, angiogenesis, and Sp protein degradation. J Natl Cancer Inst 2006; 98: 855–868. [DOI] [PubMed] [Google Scholar]
- 119. Chang R, Yee KL, Sumbria RK. Tumor necrosis factor alpha Inhibition for Alzheimer’s Disease. J Cent Nerv Syst Dis 2017; 9: 1179573517709278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Li L, Rossoni G, Sparatore A, et al. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic Biol Med 2007; 42: 706–719. [DOI] [PubMed] [Google Scholar]
- 121. Vieira V, Glassmann D, Marafon P, et al. Effect of diclofenac sodium on seizures and inflammatory profile induced by kindling seizure model. Epilepsy Res 2016; 127: 107–113. [DOI] [PubMed] [Google Scholar]
- 122. Hill J, Zawia NH. Fenamates as potential therapeutics for neurodegenerative disorders. Cells 2021; 10: 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tan-10.1177_17562864231156674 for Microglia as a cellular target of diclofenac therapy in Alzheimer’s disease by Barbara E. Stopschinski, Rick A. Weideman, Danni McMahan, David A. Jacob, Bertis B. Little, Hsueh-Sheng Chiang, Nil Saez Calveras and Olaf Stuve in Therapeutic Advances in Neurological Disorders