Abstract
Purpose of review:
Recent research suggest that binge eating may be more prevalent among women in midlife than previously believed. The menopausal transition, an important developmental stage during midlife, is characterized by substantial fluctuations and eventual decreases in ovarian hormones that may contribute to increased risk. This narrative review summarizes findings from studies of binge eating during midlife and menopause and discusses the potential role of ovarian hormones in binge eating risk.
Recent findings:
Studies are few in number and findings are mixed, with only some studies showing increased binge eating during midlife and the menopausal transition. Sensitivity to ovarian hormones, potentially through gene x hormone interactions, may influence who experiences increased binge eating risk and could help explain mixed findings in the field.
Summary:
Future studies of hormone sensitivity and gene x hormone interactions are needed to further elucidate midlife and menopausal risk for binge eating in women.
Keywords: menopause, midlife, binge eating, estradiol, women, ovarian hormones
Introduction
Midlife is typically considered to occur between ages 40 and 60 [1] and is associated with increased risk for mood disorders and other forms of psychopathology in women1 (e.g., depression, psychosis [2, 3]). Significant shifts in midlife such as changing occupational status, increased risk for health problems, changes in marital status, and loss of loved ones may contribute negative impacts on mental health during this developmental period [4]. Women in midlife may also experience additional stressors related to caring for their children in addition to their aging parents or other family members [5].
Importantly, women in midlife also experience major biological changes during menopause. The menopausal transition is characterized by dramatic variations in ovarian hormones (e.g., estrogen and progesterone), followed by decreases in these same hormones as the woman transitions into menopause/post-menopause with the cessation of ovulation and menstruation [6]. Changes in ovarian hormones during menopause have been shown or hypothesized to be related to midlife increases in depression [7] and psychosis [2].
Ovarian hormone fluctuations and levels during menopause may also influence binge eating in midlife. Binge eating is defined as eating large amounts of food, in a short period of time, while a feeling loss of control over eating [8]. Binge eating is a transdiagnostic symptom that is present in bulimia nervosa (BN), binge eating disorder (BED), anorexia nervosa binge eating/purging subtype (AN-BP) and some types of other specified feeding and eating disorders (OSFED). Ovarian hormones have been shown to be particularly important for binge eating in adolescence and adulthood [9] and may show similar effects during menopause [10].
The purpose of this narrative review is to synthesize and evaluate studies examining binge eating risk during midlife and menopause. First, we will review studies examining the prevalence of binge eating and binge-related eating disorders during midlife. Second, we will review findings from the few studies that have examined binge eating symptoms across menopausal stages and/or associations between binge eating and menopausal symptoms. Finally, we will discuss ovarian hormones as mechanisms of potential effects during menopause and review the small number of studies that have examined hormone effects during menopause. Finally, we will provide areas for future work in this burgeoning area of research.
Prevalence of Binge Eating in Midlife
Although binge eating and binge-related eating disorders are more commonly studied in adolescent and young adult samples, researchers have increasingly begun to examine the prevalence of binge eating and binge eating-related disorders in women in midlife. These studies have examined rates of binge eating in community/population-based samples as well as rates of binge-related disorders (i.e., BN, BED) in clinical and community populations.
Findings overall have been somewhat inconsistent, although there appears to be a small, yet significant, increase in binge eating during midlife. For example, rates of binge eating in community and population-based samples of women in midlife (i.e., ages 40–60) have ranged from 10–22% [11–16], although one large, population-based study showed lower prevalence (4.4% in women ages 45–60 [17]). On average (Mean rate = 14.47%), these rates are somewhat higher than those observed in previous community-/population-based samples of older adolescents and young adult women (8.6% [18]). Unfortunately, none of these studies differentiated between women who began their binge eating in midlife versus those whose binge eating was a continuation or relapse of binge eating that began in an earlier life stage. Thus, it is unclear whether midlife is a period of increased or exacerbated risk for binge eating versus a continuation of risk from earlier developmental periods.
A small number of studies directly compared rates of binge eating in women in early versus middle adulthood. These studies also did not differentiate between new onset versus more chronic/remitting binge eating, but the direct comparisons across age provide some assurance that age differences may reflect changes in binge eating during midlife. Christian et al. [19] examined over 30,000 women across five different stages of development (i.e., early adolescence (ages 11–14), “late adolescence” (ages 15–18), “young adulthood” (ages 19–25), “early-middle adulthood” (ages 26–45), middle-late adulthood (ages 46 and up)) and found that binge eating scores were significantly higher in the early-middle adulthood and mid-late adulthood groups as compared to the other age groups. However, in a longitudinal, 30-year follow-up study, Brown et al. [20] observed significantly less binge eating during midlife (age range 48–52) as compared to baseline (age range 18–22) [20].
In contrast to these somewhat mixed findings, most studies of binge eating-related disorders found increased rates in midlife. For example, several cross-sectional studies found that BED in particular was more common in women in midlife (ages 40 and up) as compared to younger women (ages 18–39) [21, 22]. In large samples where women self-reported their diagnosis, 2–5% of women in midlife reported a binge-related eating disorder in the past 12 months (i.e., BN, BED, sub-threshold BN or BED [19, 23]). This later estimate is higher than the DSM-5 lifetime prevalence for BN (0.46–1.5%) and BED (1.25–3.5%) in adult women [8]. Unfortunately, these studies did not differentiate between new onset versus chronic/remitting cases, although Micali et al. [23] reported that 42% of all eating disorder cases (not just binge eating-related disorders) were new onset cases within the last 12 months. Notably, however, Brown et al. [20] reported lower rates of BN (8.3% at age 20 down to 0.2% at age 50) and BED (4.0% at age 20 down to 2.0% at age 50) in midlife in a longitudinal study, although statistical comparisons of age differences in these percentages across time were not conducted. Other studies have examined the prevalence of all eating disorders in midlife [20, 24], but they did not examine binge eating-related disorders separately, making it difficult to interpret the lower midlife prevalence in these studies. Ovarian hormones and other reproductive stages exhibit specific associations with binge eating rather than other eating disorder symptoms (e.g., weight and shape concerns – see Hildebrandt et al. [25] and Rolan et al. [26]), suggesting that it may be binge eating-related disorders in particular that show midlife spikes in risk as a result of hormonal changes.
In summary, although studies are few in number and findings are somewhat inconsistent, results indicate that rates of binge eating and binge-eating related disorders may be higher in midlife than previously believed. Binge eating-related disorders in particular may be more prevalent during midlife as compared to earlier life stages, although it remains unclear if these disorders represent new onset, chronic, or remitting cases from earlier stages of development. Additional studies are needed to replicate initial results and clarify the specificity of midlife as a period of increased risk relative to other life stages.
Binge Eating Risk during Menopause
A significant change that occurs in midlife in women is the menopausal transition. Menopause is typically broken down into four main stages: premenopause, perimenopause, menopause, and postmenopause [27]. Premenopause is characterized by the occurrence of regular menstrual cycles before changes in the cycle begin to take place. Some researchers may also refer to this period as the “late reproductive stage” [6], in order to differentiate women in this life stage from younger adults who may be in their peak reproductive years. Perimenopause defines the transition from pre-menopause to menopause and is characterized by dramatic fluctuations in ovarian hormones [27]. These fluctuations consist of both rising and falling levels of both estrogen and progesterone (see Figure 1). Vasomotor symptoms (e.g., hot flashes, night sweats), psychological symptoms (e.g., mood lability), and sleep difficulties are associated with these hormonal changes and most commonly occur in late perimenopause (i.e., 1–3 years prior to the last menstrual cycle) and the 24 months after the last menstrual period (i.e., late perimenopause and the first year post-menopause) [27]. Menstrual cycles become irregular during perimenopause, such that individuals may start to miss periods or experience changes in menstruation. Menopause is reached when a woman has not had a period for 12 consecutive months [27]. Following this, women are considered to be in the postmenopausal stage where ovarian hormone levels are low, although it is common for menopausal symptoms and hormone fluctuations to continue in the first few years [27]. Importantly, although women, on average, enter perimenopause around age 40, women who are the same age can vary dramatically in menopausal stage and duration [28, 29], highlighting the need to directly examine menopausal stage (rather than just age) in studies of midlife.
Figure 1.
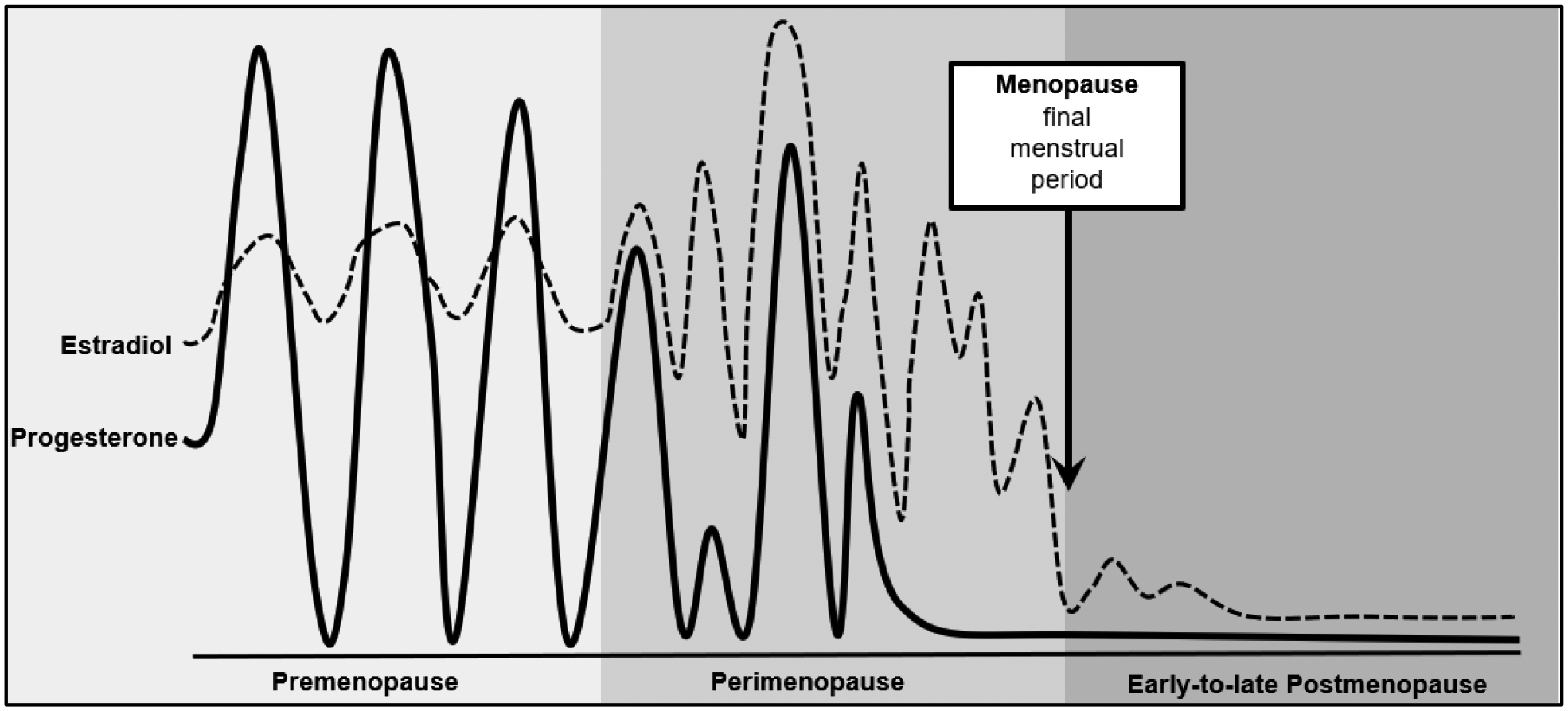
Changes in Ovarian Hormones across Menopausal Stage.
“Changes in ovarian hormones across reproductive stages” from Risk for midlife psychosis in women: Critical gaps and opportunities in exploring perimenopause and ovarian hormones as mechanisms of risk, by Culbert, Klump, & Thakkar, 2022 Psychological Medicine, 52(9), https://www.doi.org/10.1017/S0033291722001143. Licensed under Creative Commons CC BY 4.0.
Unfortunately, relatively few studies have examined differences in binge eating or binge-related disorders across menopausal stage, and findings are relatively inconsistent. Studies with large, population-based samples found small, yet significant, increases in binge eating symptoms [30] and eating disorders [31] during perimenopause as compared to premenopause. Although Mangweth-Matzek et al. [31] could not statistically compare differences in the prevalence of binge eating-related disorders due to low rates of overall eating disorder diagnoses, rates of BED were somewhat higher in perimenopause (3.6%) as compared to pre- and post-menopause (.5% and 1.5%, respectively). Once again, however, it is unclear whether these increases reflect new onset binge eating or chronic/remitting symptoms. Moreover, studies relying on smaller, convenience samples (e.g., women in breast exam waiting rooms, mothers of college students) typically found no significant differences in rates of binge eating across menopausal stage [31–35], despite some indication that rates were higher in perimenopause [35].
Unfortunately, many of the studies conducted thus far relied on very limited assessments of menopausal stage (e.g., women’s self-report of menopausal status, with no objective assessment) [30, 34, 35] and/or failed to compare women across all three different menopausal stages [32, 36]. The use of small, convenience samples also decreases confidence in findings. It is likely that these methodological limitations contribute to mixed findings obtained thus far.
However, it may be that variability in observed effects reflects real variance and important etiological mechanisms. Discrepant results may signal the presence of moderation of menopause and binge eating associations by other variables that make some women more vulnerable during menopause (and midlife – given inconsistent results for that variable as well) than others. When such moderation is present, findings are typically modest/non-significant or more variable, as samples would naturally vary in the extent to which individuals who are most at risk are included. Interestingly, a handful of studies have examined associations between binge eating and menopausal symptoms (e.g., changes in mood, hot flashes, sleeping difficulties, vaginal dryness, etc.) to determine whether women who are more sensitive to the physical/biological changes of menopause exhibit more binge eating. These studies found significant associations between levels of binge eating and menopausal symptoms [33, 37] that, in some cases, were stronger than associations with menopausal stage (i.e., premenopause, perimenopause, postmenopause) [33]. Stronger and more consistent associations with menopausal symptoms may reflect individual differences in vulnerability and sensitivity to the changes of menopause that contribute to binge eating in some, but not all, women. Given that changes in ovarian hormones drive menopausal symptoms and stages, it seems likely that at least some of this vulnerability may be differential sensitivity to ovarian hormones and their changes during the menopausal transition.
Differential Sensitivity to Ovarian Hormones as Potential Mechanisms
Past data during earlier periods of development show significant associations between ovarian hormones and binge eating in women [9, 38, 39]. These studies indicate that lower levels of estradiol, in particular, are associated with increased food intake and binge eating in both animals and humans. For example, significantly increased food intake [40] and binge eating [41, 42] are observed in ovariectomized adult female rats, and these decreases are reversed with exogenous administration of estradiol [42, 43]. Studies in adult women show similar types of effects, as lower levels of estradiol significantly predict binge eating episodes [44], and menstrual cycle phases characterized by the highest levels of estradiol are associated with low levels of dysregulated eating [45, 46]. There are also critical estrogen x progesterone interactions in the prediction of binge eating in adult women, where higher levels of both estradiol and progesterone are associated with increased binge eating. Although seemingly contradictory at first glance, these findings are consistent with those above, as increased binge eating in this hormonal milieu is due to the antagonizing effects of progesterone on estrogen and estrogen’s anorexic effects on food intake [40, 44, 46].
Importantly, like the variable findings during midlife/menopause, data suggest that not all animals engage in binge eating with estrogen removal [41], and not all women engage in binge eating with hormone changes across the menstrual cycle [44]. There appears to be differential susceptibility to hormone changes that make some women/females more (or less) vulnerable across these hormonal changes. Like findings for menopausal symptoms, previous studies show that women with significant premenstrual symptoms are more likely to engage in higher levels of binge eating than those without these premenstrual symptoms (e.g., concentration issues, fatigue, decreased interest in usual activities, anxiety [47, 48]), suggesting that differential sensitivity to ovarian hormones may contribute to individual differences in binge eating across multiple reproductive phases.
Taken together, these data suggest that decreases in both estrogen and progesterone across the menopausal transition could significantly increase binge eating, particularly in women who are sensitive to hormonal changes. To our knowledge, only one pilot study has examined associations between ovarian hormones and binge eating during menopause. Baker et al. [10] examined a small sample of women (N = 8; ages 42–52; M age = 45.9, SD = 5.0) who engaged in two more episodes of loss of control over eating in the past month and were in early perimenopause, as defined by the STRAW+10 criteria (i.e., they experienced ≥ 7-day change in menstrual cycle length but no missed periods in the last 3 months [49]). Women collected daily levels of salivary hormones and daily behavioral measures for one full menstrual cycle (or 40 days, whichever came first). Findings replicated those described above in young adult women; higher levels of estradiol were associated with binge eating when progesterone levels were also high but not when progesterone levels were low [10]. Results remained unchanged after controlling for BMI and levels of negative affect [10].
This study had many strengths (e.g., daily assessments of hormones and binge eating, menopausal staging by STRAW+10 criteria), but the smaller sample size and limited number of menopausal stages highlight the need for larger-scale studies of hormone effects. There is also a need to include more heterogeneous samples of women with a larger range of binge eating risk. Baker et al. [10] focused on women with pre-existing binge-related pathology (i.e., loss of control over eating) to maximize the chances of finding hormone/binge eating associations. However, if these associations depend upon a woman’s differential susceptibility to hormone effects, then the study may have inadvertently selected women with this hormonal sensitivity, as women who are engaging in loss of control eating during menopause may do so precisely because of their increased susceptibility to hormonal changes. Additional studies are needed to examine the full range of binge eating pathology and the extent to which differential sensitivity to hormonal changes underlie binge eating risk during the menopausal transition.
Conclusion
Binge eating is present in women in midlife. Findings regarding increased risk for binge eating (relative to other stages) during midlife are inconsistent, although data suggest that there may be increased rates of binge eating and binge-related disorders during the menopausal transition as compared to other life stages. Notably, a couple of studies have found that binge eating was the highest in women who experience the most menopausal symptoms [33, 37], and the ovarian hormones that drive menopause have been significantly associated with binge eating in pilot studies [10]. Thus, it possible that the menopausal transition represents a period of vulnerability only in women who are particularly sensitive to the fluctuations and decreases in ovarian hormones that characterize the menopausal transition.
Overall, however, studies are few in number, and findings are constrained by a number of methodological limitations (e.g., small sample sizes). Larger-scale studies examining changes in binge eating during midlife and menopause are therefore needed. Researchers should first consider how menopausal stage is assessed and defined. The “gold standard” for assessing menopausal stage was proposed by Harlow and colleagues [27, 49] via the STRAW +10 criteria and includes assessing the number of skipped periods, changes in flow, persistent differences in length of cycle, and the assessment of vasomotor symptoms. Despite this, the STRAW +10 criteria were only used in one of the studies above [10]. Future research should use these criteria to increase the reliability and validity of menopausal staging.
Future studies should also use longitudinal methods to examine within-woman changes in binge eating from pre-menopause through post-menopause to help differentiate changes in pre-existing eating disorder symptoms versus new onset binge eating. These studies should assess for binge eating levels, as well as binge-eating related disorders, to determine if associations vary by severity of pathology. Menopausal symptoms should also be measured to continue investigating whether sensitivity to hormonal changes underlie observed associations. These studies should include direct assessment of estradiol and progesterone to elucidate the mechanisms of effects and the extent to which individual differences in hormone sensitivity predict binge eating during menopause. It would also be helpful for these studies to assess heightened stress responsivity, which can be modulated by ovarian hormones [50], as well as the myriad of stressors that women experience during midlife to disentangle the effects of hormonal changes from psychological (e.g., body shame, weight stigma; Wilfred et al. [16] and psychosocial (e.g., changing caregiver roles [51]) factors that impact women’s mental health and well-being. Careful assessments of other psychological symptoms that may also increase during midlife and the menopausal transition (e.g., negative affect; body weight/shape concerns [7, 52–54] are also needed to identify the specificity of effects for binge eating.
Finally, future studies should also identify the mechanisms that may underlie women’s differential hormonal sensitivity to menopausal changes. One potential pathway of effects is through gene x hormone interactions. Gene x hormone interactions occur when a person’s response to a risk factor (e.g., ovarian hormones) varies by their level of genetic risk. Individuals with genetic predispositions for the behavior will be most sensitive to the hormonal risk and most vulnerable to developing the behavior [55]. Individuals who are not at genetic risk are unlikely to develop the behavior, even in the presence of hormonal risk. In the case of menopause and ovarian hormones, it may be that women who are at genetic risk for binge eating are most sensitive to ovarian hormones and most likely to develop binge eating during the hormonal changes of the menopausal transition. Indirect data suggest this is the case – associations between menopausal stage and binge eating appear to be strongest in women who are most likely to be at genetic risk for binge eating (i.e., those with clinical, binge eating-related disorders). Moreover, past studies during puberty and adulthood show differential heritability of binge eating by ovarian hormone concentrations – with stronger genetic influences at lower estradiol [44, 56, 57] levels. These data suggest that differential susceptibility to ovarian hormones may be due to differential genetic risk for binge eating.
The exact way in which this genetic risk may translate into differential susceptibility to hormones remains unknown. However, ovarian hormones are steroid hormones that regulate gene transcription and protein synthesis in several key systems for binge eating [58], including the dopamine [58, 59] and serotonin system [60]. Although progesterone’s effects on gene transcription have been studied less frequently [61, 62], it may serve to modify or interact with the regulatory effects of estrogen [63–65]. Theoretically, interactions between risk variants in these systems and ovarian hormones could result in differential production of neurotransmitters, neuromodulators, their receptors, or their signal transduction mechanisms. Variants of estrogen and/or progesterone receptors may also result in different patterns of gene regulation by the hormones, leading to distinct cellular and behavioral responses. In all of these scenarios, two key individual difference variables impact binge eating risk - the presence/absence of risk alleles in hormonally responsive genes/systems and the presence of a risky hormonal environment. Increased binge eating would be evident in individuals who carry risk variants for binge eating and who are exposed to changing levels of ovarian hormones during menopause. Indeed, women with genetic risk for binge eating would be more likely to experience the perimenopausal hormonal environment as “risky.”
All of these hypotheses are speculative, but they highlight the need for additional studies of hormonal sensitivity and gene x environment interactions. These studies could be conducted in several ways. For example, the use of twin data would allow for the examination of differential heritability of binge eating across changing hormone levels during periomenopause. Alternatively, future polygenic risk scores (PRSs; one score that indexes number of risky variants across a multitude of genes) from genome-wide association studies (GWAS) could be used to examine differential associations between binge eating, these risk genes, and levels of hormones during the menopausal transition. Although clearly complex (and likely time consuming), these studies may provide the strongest evidence for the differential hormone sensitivity hypothesis and identify key gene x hormone mechanisms in midlife risk for binge eating in women.
Funding and/or Conflicts of interests/Competing interests.
This work was supported by a grant from the National Institute of Mental Health (R01 MH128196) awarded to KMC and KLK, a Global Foundation for Eating Disorders grant awarded to KMC and KLK, and a Graduate Research Fellowship from the National Science Foundation (NSF) awarded to CA. The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Science Foundation, or the Global Foundation for Eating Disorders. The authors have no additional competing interests to declare that are relevant to the content of this article.
Footnotes
When we refer to “women” and “female(s)”, we are describing sex assigned at birth. No studies of midlife or the menopausal transition have examined gender or gender identity.
References
- 1.Colarusso CA. Middle adulthood (ages 40–60). In: Child and Adult Development. Springer: Boston, MA; 1992. [Google Scholar]
- 2.Culbert KM, Thakkar KN, Klump KL. Risk for midlife psychosis in women: critical gaps and opportunities in exploring perimenopause and ovarian hormones as mechanisms of risk. Psychol Med. 2022:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soares CN. Depression and menopause: An update on current knowledge and clinical management for this critical window. Med Clin N Am. 2019;103(4):651–67. [DOI] [PubMed] [Google Scholar]
- 4.Winterich JA, Umberson D. How women experience menopause: the importance of social context. J Women Aging. 1999;11(4):57–73. [DOI] [PubMed] [Google Scholar]
- 5.Ballard KD, Kuh DJ, Wadsworth ME. The role of the menopause in women’s experiences of the ‘change of life’. Sociol Health Illn. 2001;23(4):397–424. [Google Scholar]
- 6.Santoro N, Roeca C, Peters BA, Neal-Perry G. The menopause transition: signs, symptoms, and management options. J Clin Endocrinol Metab. 2021;106(1):1–5. [DOI] [PubMed] [Google Scholar]
- 7.Gordon JL, Peltier A, Grummisch JA, Sykes Tottenham L. Estradiol fluctuation, sensitivity to stress, and depressive symptoms in the menopause transition: a pilot study. Front Psychol. 2019;10:1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed., text rev.) American Psychiatric Association. 2022. [Google Scholar]
- 9.Mikhail ME, Anaya C, Culbert KM, Sisk CL, Johnson A, Klump KL. Gonadal hormone influences on sex differences in binge eating across development. Curr Psychiatry Rep. 2021;23(11):1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker JH, Eisenlohr-Moul T, Wu YK, Schiller CE, Bulik CM, Girdler SS. Ovarian hormones influence eating disorder symptom variability during the menopause transition: A pilot study. Eat Behav. 2019;35:101337. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This was the first study to examine the association between within-person fluctuations in ovarian hormones and binge eating in perimenopausal women. Findings from this study suggest that higher levels of estradiol are associated with higher levels of binge eating symptoms only when progesterone levels are also high, which was consistent with previous studies examining dysregulated eating across the menstrual cycle.
- 11.Drobnjak S, Atsiz S, Ditzen B, Tuschen-Caffier B, Ehlert U.. Restrained eating and self-esteem in premenopausal and postmenopausal women. J Eat Disord. 2014; 2(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Freitas SR, Appolinario JC, de Moura Souza A, Sichieri R. Prevalence of binge eating and associated factors in a Brazilian probability sample of midlife women. Int J Eat Disord. 2008;41(5):471–8. [DOI] [PubMed] [Google Scholar]
- 13.Gagne DA, Von Holle A, Brownley KA, Runfola CD, Hofmeier S, Branch KE, Bulik CM. Eating disorder symptoms and weight and shape concerns in a large web‐based convenience sample of women ages 50 and above: Results of the gender and body image (GABI) study. Int J Eat Disord. 2012;45(7):832–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcus MD, Bromberger JT, Wei HL, Brown C, Kravitz HM. Prevalence and selected correlates of eating disorder symptoms among a multiethnic community sample of midlife women. Ann Behav Med. 2007;33(3):269–77. [DOI] [PubMed] [Google Scholar]
- 15.Santana DD, Mitchison D, Mannan H, Griffiths S, Appolinario JC, Da Veiga GV, Touyz S, Hay P. Twenty-year associations between disordered eating behaviors and sociodemographic features in a multiple cross-sectional sample. Psychol Med. 2022;14:1–10. [DOI] [PubMed] [Google Scholar]
- 16.Wilfred SA, Becker CB, Kanzler KE, Musi N, Espinoza SE, Kilpela LS. Binge eating among older women: prevalence rates and health correlates across three independent samples. J Eat Disord. 2021;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson JI, Hiripi E, Pope HG Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reichborn‐Kjennerud T, Bulik CM, Kendler KS, Røysamb E, Maes H, Tambs K, Harris JR. Gender differences in binge‐eating: A population‐based twin study. Acta Psychiatr Scand. 2003;108(3):196–202. [DOI] [PubMed] [Google Scholar]
- 19.Christian C, Perko VL, Vanzhula IA, Tregarthen JP, Forbush KT, Levinson CA. Eating disorder core symptoms and symptom pathways across developmental stages: A network analysis. J Abnorm Psychol. 2020;129(2):177. [DOI] [PubMed] [Google Scholar]; *This large-scale study examined differences in eating disorder symptoms and symptom pathways across development, spanning from early adolescence to late adulthood. Findings suggest that binge eating symptoms in particular may be more common in older women, including women in midlife, as compared to women in adolescence and early young adulthood. The study’s findings emphasize the importance of examining eating disorder risk in women in mid-to-late life.
- 20.Brown TA, Forney KJ, Klein KM, Grillot C, Keel PK. A 30-year longitudinal study of body weight, dieting, and eating pathology across women and men from late adolescence to later midlife. J Abnorm Psychol. 2020;129(4):376. [DOI] [PMC free article] [PubMed] [Google Scholar]; **To our knowledge, this is the only longitudinal study that has followed women from young adulthood through midlife and examined changes in both eating disorder symptoms and diagnoses. The study found overall decreases in the prevalence of both binge eating and eating disorder diagnoses as women aged, and found that most women who met diagnostic criteria for an eating disorder at an earlier time point were in recovery by age 50.
- 21.Elran-Barak R, Fitzsimmons-Craft EE, Benyamini Y, Crow SJ, Peterson CB, Hill LL, Crosby RD, Mitchell JE, Le Grange D. Anorexia nervosa, bulimia nervosa, and binge eating disorder in midlife and beyond. J Nerv Ment Dis. 2015;203(8):583–90. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins PE, Price T. Eating pathology in midlife women: Similar or different to younger counterparts?. Int J Eat Disord. 2018. Jan;51(1):3–9. [DOI] [PubMed] [Google Scholar]
- 23.Micali N, Martini MG, Thomas JJ, Eddy KT, Kothari R, Russell E, Bulik CM, Treasure J. Lifetime and 12-month prevalence of eating disorders amongst women in mid-life: a population-based study of diagnoses and risk factors. BMC Med. 2017;15(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward ZJ, Rodriguez P, Wright DR, Austin SB, Long MW. Estimation of eating disorders prevalence by age and associations with mortality in a simulated nationally representative US cohort. JAMA Netw. 2019;2(10):e1912925-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hildebrandt BA, Racine SE, Keel PK, Burt SA, Neale M, Boker S, Sisk CL, Klump KL. The effects of ovarian hormones and emotional eating on changes in weight preoccupation across the menstrual cycle. Int J Eat Disord. 2015;48(5):477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolan EP, Mikhail ME, Culbert KM, Burt SA, Klump KL. (submitted). Estrogen moderation of genetic influences on eating disorders symptoms during gonadarche in girls: Specific effects on binge eating. [DOI] [PMC free article] [PubMed]
- 27.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ, STRAW+ 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop+ 10: Addressing the unfinished agenda of staging reproductive aging. J. Clin. Endocrinol. Metab 2012;97(4):1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan S, Gomes A, Singh RS. Is menopause still evolving? Evidence from a longitudinal study of multiethnic populations and its relevance to women’s health. BMC Women’s Health. 2020;20(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38(3):425–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khalil J, Boutros S, Kheir N, Kassem M, Salameh P, Sacre H, Akel M, Obeid S, Hallit S. Eating disorders and their relationship with menopausal phases among a sample of middle-aged Lebanese women. BMC Women’s Health. 2022;22(1):1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study is one of the few large, population-based studies to examine changes in eating disorder symptoms across pre-, peri-, and post-menopause. Findings from this study suggest that women in perimenopause engage in higher levels of binge eating than women in premenopause.
- 31.Mangweth‐Matzek B, Hoek HW, Rupp CI, Kemmler G, Pope HG Jr, Kinzl J. The menopausal transition—A possible window of vulnerability for eating pathology. Int J Eat Disord. 2013;46(6):609–16. [DOI] [PubMed] [Google Scholar]
- 32.Baker JH, Peterson CM, Thornton LM, Brownley KA, Bulik CM, Girdler SS, Marcus MD, Bromberger JT. Reproductive and appetite hormones and bulimic symptoms during midlife. Eur Eat Disord Rev. 2017;25(3):188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mangweth-Matzek B, Rupp CI, Vedova S, Dunst V, Hennecke P, Daniaux M, Pope HG. Disorders of eating and body image during the menopausal transition: associations with menopausal stage and with menopausal symptomatology. Eat Weight Disord. 2021;26(8):2763–9. [DOI] [PubMed] [Google Scholar]; **This is the first study to examine differences in eating disorder prevalence and disordered eating symptoms across both menopausal stage and levels of menopausal symptoms (e.g., changes in mood, hot flashes, difficulty sleeping). Findings from this study suggested that menopausal symptoms, rather than menopausal stage, are associated with higher levels of disordered eating symptoms. Importantly, findings provide indirect support for the role of ovarian hormone sensitivity in binge eating risk during midlife.
- 34.Thompson KA, Bardone-Cone AM. Evaluating attitudes about aging and body comparison as moderators of the relationship between menopausal status and disordered eating and body image concerns among middle-aged women. Maturitas. 2019;124:25–31. [DOI] [PubMed] [Google Scholar]
- 35.Thompson KA, Bardone‐Cone AM. Menopausal status and disordered eating and body image concerns among middle‐aged women. Int J Eat Disord. 2019;52(3):314–8. [DOI] [PubMed] [Google Scholar]
- 36.Copeland AL, Martin PD, Geiselman PJ, Rash CJ, Kendzor DE. Predictors of pretreatment attrition from smoking cessation among pre- and postmenopausal, weight-concerned women. Eat Behav. 2006;7(3):243–251. [DOI] [PubMed] [Google Scholar]
- 37.Hooper SC, Marshall VB, Becker CB, LaCroix AZ, Keel PK, Kilpela LS. Mental health and quality of life in postmenopausal women as a function of retrospective menopause symptom severity. Menopause. 2022;29(6):707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Culbert KM, Sisk CL, Klump KL. Sex steroid hormones and differential risk for eating pathology: A review of genetic and phenotypic effects across development. Curr Opin Behav Sci. 2018;23:124–30. [Google Scholar]
- 39.Klump KL, Culbert KM, Sisk CL. Sex differences in binge eating: Gonadal hormone effects across development. Annu Rev Clin Psychol. 2017;13:183–207. [DOI] [PubMed] [Google Scholar]
- 40.Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol-Regul Integr Comp Physiol. 2013;305(11):R1215–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klump KL, Suisman JL, Culbert KM, Kashy DA, Keel PK, Sisk CL. The effects of ovariectomy on binge eating proneness in adult female rats. Horm Behav. 2011;59(4):585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Micioni Di Bonaventura MV, Lutz TA, Romano A, Pucci M, Geary N, Asarian L, Cifani C. Estrogenic suppression of binge‐like eating elicited by cyclic food restriction and frustrative‐nonreward stress in female rats. Int J Eat Disord. 2017;50(6):624–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu Z, Geary N, Corwin RL. Individual effects of estradiol and progesterone on food intake and body weight in ovariectomized binge rats. Physiol Behav. 2011;104(5):687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klump KL, Racine SE, Hildebrandt B, Burt SA, Neale M, Sisk CL, Boker S, Keel PK. Influences of ovarian hormones on dysregulated eating: A comparison of associations in women with versus women without binge episodes. Clin Psychol Sci. 2014;2(5):545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol Med. 2007;37(1):131–41. [DOI] [PubMed] [Google Scholar]
- 46.Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk CL, Boker S, Hu JY. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. J Abnorm Psychol. 2013;122(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Çoban ÖG, Karakaya D, Önder A, İşleyen Z, Adanır AS. Association of premenstrual dysphoric disorder and eating behaviors among nursing students: A cross-sectional study. J Pediatr Adolesc Gynecol. 2021;34(2):203–8. [DOI] [PubMed] [Google Scholar]
- 48.Hardin SL, Thornton LM, Munn‐Chernoff MA, Baker JH. Premenstrual symptoms as a marker of ovarian hormone sensitivity in eating disorders. Int J Eat Disord. 2020;53(2):296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harlow SD, Crawford S, Dennerstein L, Burger HG, Mitchell ES, Sowers MF, ReSTAGE Collaboration. Recommendations from a multi-study evaluation of proposed criteria for staging reproductive aging. Climacteric. 2007;10(2):112–9. [DOI] [PubMed] [Google Scholar]
- 50.Ter Horst GJ, Wichmann R, Gerrits M, Westenbroek C, Lin Y. Sex differences in stress responses: Focus on ovarian hormones. Physiol Behav. 2009;97(2):239–49. [DOI] [PubMed] [Google Scholar]
- 51.Samuels KL, Maine MM, Tantillo M. Disordered eating, eating disorders, and body image in midlife and older women. Curr Psychiatry Rep. 2019;21(8):1–9. [DOI] [PubMed] [Google Scholar]
- 52.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375–82. [DOI] [PubMed] [Google Scholar]
- 53.Karvonen-Gutierrez C, Kim C. Association of mid-life changes in body size, body composition and obesity status with the menopausal transition. Healthcare. 2016;4(3):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willi J, Süss H, Grub J, Ehlert U. Biopsychosocial predictors of depressive symptoms in the perimenopause—findings from the Swiss Perimenopause Study. Menopause. 2021;28(3):247–54. [DOI] [PubMed] [Google Scholar]
- 55.Ottman R Gene–environment interaction: definitions and study design. Prev Med. 1996;25(6):764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klump KL, Fowler N, Mayhall L, Sisk CL, Culbert KM, Burt SA. Estrogen moderates genetic influences on binge eating during puberty: Disruption of normative processes?. J Abnorm Psychol. 2018. Jul;127(5):458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klump KL, O’Connor SM, Hildebrandt BA, Keel PK, Neale M, Sisk CL, Boker S, Alexandra Burt S. Differential effects of estrogen and progesterone on genetic and environmental risk for emotional eating in women. Clin Psychol Sci. 2016;4(5):895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015;9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Becker JB, 2009. Sexual differentiation of motivation: A novel mechanism? Horm. Behav 55, 646–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Östlund H, Keller EV, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Ann NY Acad Sci. 2003;1007(1):54–63. [DOI] [PubMed] [Google Scholar]
- 61.Arbo BD, Andrade S, Osterkamp G, Gomez R, Ribeiro MF. Effect of low doses of progesterone in the expression of the GABA (A) receptor α4 subunit and procaspase-3 in the hypothalamus of female rats. Endocrine. 2014;46(3):561–7. [DOI] [PubMed] [Google Scholar]
- 62.Rivera HM, Oberbeck DR, Kwon B, Houpt TA, Eckel LA. Estradiol increases Pet-1 and serotonin transporter mRNA in the midbrain raphe nuclei of ovariectomized rats. Brain research. 2009;1259:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bethea CL, Reddy AP. Effect of ovarian hormones on genes promoting dendritic spines in laser-captured serotonin neurons from macaques. Mol Psychiatry. 2010;15(10):1034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jayaraman A, Pike CJ. Differential effects of synthetic progestagens on neuron survival and estrogen neuroprotection in cultured neurons. Mol Cell Endocrinol. 2014. Mar 25;384(1–2):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rivera HM, Bethea CL. Ovarian steroids increase PSD‐95 expression and dendritic spines in the dorsal raphe of ovariectomized macaques. Synapse. 2013;67(12):897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]


