Abstract
Purpose of Review
The aim of this review is to provide an overview of the menopause-related changes in microbiota and their role in the pathogenesis of menopause-related diseases. In addition, evidence on probiotic supplementation as a therapeutic strategy is discussed.
Recent Findings
The human microbiota is a complex community that lives in a mutualism relationship with the host. Menopause is associated with dysbiosis, and these changes in the composition of microbiota in different sites (gut, vaginal, and oral microbiota) might play a role in the pathogenesis of menopause-related diseases (i.e., osteoporosis, breast cancer, endometrial hyperplasia, periodontitis, and cardiometabolic diseases).
Summary
The present review highlights the pivotal role of microbiota in postmenopausal women health, in particular it (a) may increase intestinal calcium absorption thus preventing osteoporosis, (b) is associated with reduced risk of breast cancer and type 1 endometrial hyperplasia, (c) reduces gingival inflammation and menopausal periodontitis, and (d) beneficially affects multiple cardiometabolic risk factors (i.e., obesity, inflammation, and blood glucose and lipid metabolism). However, whether oral probiotic supplementation might be used for the treatment of menopause-related dysbiosis requires further clarification.
Keywords: Menopause, Dysbiosis, Menopause-related diseases, Probiotic therapy
Introduction
Microbiota consists of a community of microbes (bacteria, fungi, and viruses) that live inside and outside of the human body [1]. In the gut, microbial species live in a harmonic symbiosis with the host, contributing to [2] (1) increase the metabolic ability to ferment indigestible carbohydrates; (2) produce vitamins, i.e., B2, B12, K, and folic acid; (3) protect against the colonization of pathogenic bacteria; and (4) promote the maturation of immune cells and the normal development of their functions, as well as the inhibition of toxins and carcinogens [3]. According to microbial taxonomy at the phylum level, the following gut bacteria have been identified: Firmicutes (60–80%, i.e., Ruminococcus, Clostridium, Lactobacillus, Enterococcus), Bacteroidetes (20–30%, i.e., Bacteroides, Prevotella, Xylanibacter), Actinobacteria (less than 10%, i.e., Bifidobacterium), and Proteobacteria (less than 1%, i.e., Escherichia, Enterobacteriaceae) [3, 4]. Nevertheless, the composition of gut microbiota may change according to host-related factors (age, gender, latitude, ethnicity, diseases) [5], lifestyle (physical exercise, habitual diet, use of prebiotics and/or probiotics), and antibiotic therapy [4, 6, 7]. Dramatic changes of the composition of gut microbiota—known as dysbiosis—have been appointed as major contributors to several diseases such as asthma [8], eczema [8], obesity [9], type 2 diabetes [10], non-alcoholic fatty liver disease [11], colon cancer [12], heart disease [13], and neurological or neuropsychiatric diseases [14]. Among factors that can affect the composition of gut microbiota, the role of gender and sex hormones has not yet been sufficiently investigated.
Mounting evidence has shown that gender and sex hormones can play a pivotal role in modulating human response to external factors, likely through a different effect on microbiota. For example, in the study by Org et al. [15], male and female mice exhibited a significant difference in the abundance of several microbial species. Interestingly, this sex-related microbiota composition explained the variability of metabolic response when mice underwent an 8-week high-fat high-sucrose diet. In addition, to determine whether these findings were mediated by sex hormones, gonadectomized and hormone-treated mice underwent the same diet. The results showed that the hormonal status affected the composition of microbiota more on the chow diet in males, whereas in females this effect was more evident after the high-fat diet. Therefore, these experiments highlighted the role of gender on targeting gut microbiota composition and the response to dietary interventions.
In other studies [16, 17], estrogens have been shown to affect gut microbiota which can, in turn, significantly influence estrogen levels. Indeed, some microbial species (also known as estrabolome) can regulate circulating estrogens through the secretion of beta-glucuronidase, a bacterial enzyme that deconjugates estrogens and phytoestrogens in their active forms which can be reabsorbed in the intestine and enter the bloodstream [18].
Dysbiosis can reduce estrabolome, and consequently, the deconjugation of estrogen and phytoestrogen into their circulating active forms with the impairment of estrogen-receptor activation [19]. This condition can induce a wide range of diseases, such as polycystic ovary syndrome (PCOS) [20], obesity and obesity-associated metabolic diseases [7, 21], cardiovascular disease (CVD) [22], cognitive decline [23], type 1 endometrial hyperplasia, and endometrial and breast cancer (BC) [24]. Moreover, estrogens regulate the microbiological environment of the female reproductive tract by maintaining epithelial thickness, glycogen levels, mucus secretion, and decreasing vaginal pH through the promotion of Lactobacilli colonization and lactic acid production [25]. Consequently, during menopause, the abundance of vaginal Lactobacilli decreases along with hormonal and epithelial changes [26]. Finally, during the normal women life cycle, menopause is characterized by a dramatic reduction in estrogens and other female sex hormones [27, 28]. Overall, this evidence suggests that the composition of the microbiota could play a pivotal role in the onset or progression of some menopause-related clinical conditions [29].
Therefore, the aim of this review was to give an overview of the relationship between microbial dysbiosis and the most common menopause-related diseases (postmenopausal osteoporosis, BC, endometrial hyperplasia, periodontitis, obesity, and CVD). In addition, evidence on the effects of probiotic supplementation in postmenopausal women was discussed to evaluate whether it might be used as a therapeutic strategy for the prevention/management of menopause-related diseases.
Rationale for the Use of Probiotics in the Treatment of the Comorbidities Associated with Menopause
The evidence that changes in the composition of the gut microbiota might have a role in the pathogenesis of a heterogenous group of human diseases suggests that these conditions could be either prevented or ameliorated by therapeutic interventions aiming to correct gut dysbiosis [30]. The standard tool to achieve this goal is the administration of probiotics. According to the World Health Organization, probiotics are live microorganisms that when administered in adequate amounts will confer a health benefit on the host and this definition has been retained with only minimal grammatical changes in the consensus statement issued by the International Scientific Association for Probiotics and Prebiotics in 2016 [31]. Commercial probiotic products contain various combinations of bacteria and yeasts belonging to the following genuses: Lactobacillus, Bifidobacterium, Saccharomyces, Streptococcus, Enterococcus, Escherichia, and Bacillus. In many cases, these preparations also include vitamins, amino acids, or essential minerals and are marketed as dietary supplements. The basic idea behind the use of probiotics in clinics is that, upon oral administration, they could populate the gut replacing dysbiotic microorganisms and restoring the normal functional activities of gut microbiota. While this could seem an obvious consequence of probiotic therapy, the evidence that it really happens is not solid [32]. What has been observed is that, in general, the microorganisms contained in probiotics only transiently colonize the gut in a manner that is highly individually variable [33]. Long-term persistence and, even more importantly, stable changes in the resident intestinal microflora seem, instead, to occur only rarely [32]. The practical consequence of these data is that continued, long-term administration is probably required to maintain the benefits of probiotic treatment.
Different mechanisms concur to determine the beneficial effects of probiotics in different human diseases also including the comorbidities of menopause, as we will discuss in detail in the following sections. In particular, these microorganisms (1) improve gut barrier function, (2) modulate immune responses, (3) release biologically active extracellular mediators, and (4) generate biologically active substances by metabolizing either endogenous molecules or molecules taken with food (see Suez et al. [32] for a comprehensive review).
In the normal healthy gut, the intestinal epithelial cells are covered by a thin layer of mucus that they synthesize and release to form a physical and functional barrier isolating the intestinal mucosa and, more in general, the systemic circulation from the content of the gut lumen. The disruption of this barrier is an important causative factor of intestinal diseases such as inflammatory bowel disease and may grant the diffusion to distant sites of antigenic or toxic substances responsible for the genesis of non-intestinal diseases such as hepatic steatosis or parodontitis.
Probiotics may improve the intestinal barrier through different mechanisms. First, they promote the secretion and release of mucus and enhance the formation of tight junctions [34, 35]. These effects are at least partially dependent on the release of soluble mediators such as indoles, which bind to pregnane X receptors, and hydroxycis-12-octadecenoic acid, which binds to GPR40 and activates the MAPK cascade [36]. In addition, probiotics reduce dysbiotic microorganism binding to intestinal epithelial cells both by competing with them for mucosal binding sites and by reducing their number through their killing via the release of antibacterial substances such as organic acids, like acetic acid and lactic acid, and bacteriocins [37, 38]. Microbial-associated molecular patterns of probiotic microorganisms, such as flagellin, pilin surface layer protein, capsule polysaccharide, lipopolysaccharide, or lipoteichoic acid, bind to specific pattern recognition receptors, including Toll-like receptors-2, 4, and 5, not only on dendritic cells but also on epithelial intestinal cells and on M-cells, a specialized cell type involved in the transcytosis of antigens to the cells of the gut-associated lymphoid tissue [39, 40]. The binding to epithelial cells promotes the synthesis and release of defensins, and several cytokines, including interleukin (IL)-6, IL-8, IL-10, tumor necrosis factor (TNF)-α, IL-1β, and interferon (IFN)-γ, increase the formation of tight junctions and exert antiapoptotic and anti-inflammatory effects. The interaction with dendritic cell receptors regulates the differentiation of naive T cells and, ultimately, the relative balance between TH1, TH2, TH17, and Treg lymphocytes [41]. Probiotics may also regulate immune responses through the release of small soluble mediators, which are generated through the metabolism of dietary fibers. This is the case of small chain fatty acids (SCFA) [42] such as butyrate and propionate which are generated in the gut and may diffuse with general circulation to exert their immunoregulatory and anti-inflammatory effects at distant sites in particular controlling Treg expansion [43–46]. Importantly, the immunomodulating effects of probiotics are not limited to the intestinal mucosa but impact immune responses systemically as it has been demonstrated in allergic disorders [47–49]. The immunomodulating and anti-inflammatory effects of probiotics and their ability to normalize gut mucosa permeability may partly explain their beneficial effects in some of the comorbidities of menopause such as osteoporosis and parodontitis. In fact, the increase in permeability which occurs in the intestinal dysbiosis of menopause prompts the activation of Th17 lymphocytes and the release of TNF-α and RANKL, ultimately leading to enhanced osteoclastogenesis and bone resorption [50]. Similar mechanisms are effective at the level of alveolar bone where they are responsible for bone resorption and the progression of the disease [51]. By reducing the plasma levels of cytokines, probiotics may also positively impact on cardiovascular risk which is increased by systemic microinflammation [52–54]. An additional important mechanism that could be responsible for the beneficial effects of probiotics on cardiovascular risk also in menopause is related to their ability to deconjugate bile salts such as lithocholic in a reaction catalyzed by the enzyme bile salt hydrolase [55]. The resulting deconjugated bile salts cannot be recycled back to the liver as efficiently as their conjugated counterparts and this leads to higher hepatic consumption of cholesterol by liver cells to synthesize new bile salts and, ultimately, to a decrease in plasma cholesterol levels [56]. Importantly, probiotics may also improve insulin resistance via SCFA and this further contributes to reducing cardiovascular risk [57].
The decrease in cytokine release and systemic inflammation induced by probiotics by the mechanisms described above might be relevant also in explaining their beneficial effect on BC whose development and progression are promoted by the inflammatory microenvironment caused by dysbiosis [58]. Another important mechanism that could have a role in determining the proposed protective role of normal microbiota and, possibly, of probiotics on breast cancer is related to the ability of these microorganisms to produce substances with anticancer activity [59]. Normal gut microbiota synthetizes small molecules with anticancer activity such as indole derivatives, indole propionic acid, and indoxyl sulfate [60, 61]. In addition, cadaverine and the bile salt metabolite lithocholic acid may also decrease cancer cell proliferation by interacting respectively with trace amino acid receptors and TGR5/FXR [62–65]. To what extent the intake of probiotics could increase the production of these compounds with anticancer properties is still uncertain. As mentioned before, probiotics express bile salt hydrolase and increase bile salt metabolism in the gut; an increase in indole-3-propionic acid was observed in rats treated with probiotics [66] but not in children affected with type I diabetes [67]. Increased levels of polyamines have been demonstrated in the elderly after treatment with bifidobacteria-containing synbiotics [68].
As mentioned above “Reciprocal interactions between estrogens and microbiota: implications in menopause” the estrabolome controls circulating levels of estrogens by metabolizing in the gut endogenous and exogenous molecules with estrogenic activity. In particular, besides deconjugating endogenous estrogens, gut microbiota also metabolizes plant lignans, the major source of phytoestrogen in Western populations, to generate enterolignans, enterolactone, and enterodiol [69]. These metabolites have a higher bioavailability than parental compounds and are responsible for most of the systemic effect of lignans; acting as modulators of estrogen receptors, these compounds exert agonist effects in certain tissues such as the bone and antagonist effects in others such as the breast. Dysbiosis may reduce enterolignan generation, cause the loss of their agonist/antagonist effect on estrogen-dependent tissues, and consequently increase the risk of osteoporosis and BC [59, 70]. A similar bioactivating role of gut microbiota has been described also for other phytoestrogens such as ellagitannins and isoflavones, which are converted respectively into urolithins and equol; these metabolites also have potent anticancer activity independent from their effects on estrogen receptors [71–73]. It has been suggested that probiotics could restore the impaired phytoestrogen bioactivation in the dysbiotic gut and such a mechanism could partly account for their beneficial effects in menopausal comorbidities such as osteoporosis and BC and give a rationale basis for the combined treatment of these conditions with probiotics plus phytoestrogens [74]. Evidence has been reported that several probiotic microorganisms including lactobacilli and bifidobacterial may perform in vitro some of the enzymatic reactions involved in lignan and isoflavone bioactivation [75–78]. Nonetheless, the clinical studies performed so far showed inconsistent results and, therefore, the relevance of this mechanism in probiotic therapeutic effects remains uncertain [79–82].
In the next sections, we will analytically review the available evidence on the role of dysbiosis and the benefits of probiotics in the main comorbidities of menopause.
Menopausal Dysbiosis and Osteoporosis
Osteoporosis is a clinical condition with a great impact on women's health [83]. Indeed, according to the Study of Women’s Health Across the Nation (SWAN) carried out in postmenopausal women (approximately 6 years after the last menstrual period), one in six women had one or more fractures, with a rate of 11 first fractures/1000 person/years [84].
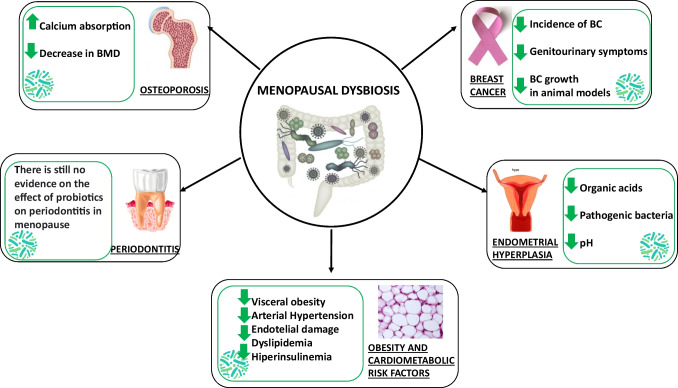
Recent studies have found a strict relationship between menopause, microbiota, and bone health suggesting novel implications for the prevention and/or therapeutic strategies for osteoporosis [85, 86••] (Fig. 1).
Fig. 1.
Mechanism explaining the association between dysbiosis and menopause-related diseases
In a double-blind, randomized, crossover acute trial carried out in 20 postmenopausal women, the addition of Lactobacillus helveticus to fermented milk was showed to rapidly increase serum calcium while decreasing parathormone concentrations, as compared to conventional milk [87]. These findings suggest that probiotics may promote intestinal calcium absorption. In a 12 month-double-blind, placebo-controlled study, 90 elderly women (75–80 years) with osteopenia (defined as a t-score between − 1 and − 2.5) were randomized to daily oral supplementation with Lactobacillus reuteri (LR 6475) or placebo. At the end of the study, LR 6475 reduced the loss of total volumetric body mass density (BMD) compared to placebo, thus representing a useful supplementation in elderly women with osteopenia [88]. Furthermore, in a medium-term (6 months) double-blind, randomized, clinical trial, 78 postmenopausal women at risk of osteoporosis or with untreated osteopenia were assigned to daily consumption of yoghurt enriched with bioactive compounds (calcium, vitamin D, vitamin K, vitamin C, zinc, magnesium, L-leucine) and probiotics (Lactobacillus plantarum 3547) or control yoghurt [89]. After 6 months, women consuming enriched yogurt showed a significantly increased BMD compared to controls. Moreover, increased N-terminal propeptide of type I collagen while decreased C-telopeptide of type I collagen concentrations—a bone formation and a bone resorption marker, respectively—were observed in the women consuming enriched yoghurt as compared to control [89].
All these studies support the feasibility and usefulness of probiotic supplementation—over the standard therapy with calcium and vitamin D—to improve bone health in menopausal women at risk of osteoporosis.
Menopausal Dysbiosis and Breast Cancer
Scientific evidence on the association between dysbiosis and the pathogenesis of BC has been poorly investigated [90, 91•] (Fig. 1).
In a metagenomic study [92], 18 women with premenopausal BC showed no significant taxonomic differences when compared to 25 premenopausal healthy controls. However, in the same study when 44 patients with postmenopausal BC were compared to 46 healthy postmenopausal controls, 45 species differed significantly between the two groups. More in details, patients with postmenopausal BC exhibited a higher abundance of Escherichia coli, Klebsiella sp_1_1_55, Enterococcus gallinarum, Actinomyces sp HPA0247, Shewanella putrefaciens, and Erwinia amylovora, whereas there was less abundance of Eubacterium eligens and Lactoisacus. These results are in line with previous studies suggesting that the intestinal metagenomes in patients with postmenopausal BC are rich in genes that code for the biosynthesis of lipopolysaccharide that is a powerful trigger of systemic inflammation that could play a role in promoting neoplastic transformation [93]. In addition, it has been hypothesized that the microbial estrogen metabolism could play a role in the association between dysbiosis and BC. Indeed, one study demonstrated that microbiota diversity—the opposite of dysbiosis—is associated with the production of hydroxylated metabolites of estrogens in 60 postmenopausal women. In particular, compounds hydroxylated in positions 2 and 4 have been associated with a lower risk of BC [94].
As for human intervention studies, no specific studies in postmenopausal women or with probiotic supplementation on BC are available so far.
In a case–control study, 306 patients with BC and 662 healthy controls filled in a self-administered questionnaire to evaluate the consumption of beverages containing Lactobacillus casei shirota and of soy isoflavone-containing products (i.e., miso-soup and tofu) [95]. The survey showed that habitual consumption of Lactobacillus casei shirota and soy isoflavones was inversely associated with early BC incidence. Similarly, in a case–control study, 1010 patients with BC and 1950 controls were interviewed about the consumption of dairy products. The results showed that the risk of BC decreased significantly with a higher intake of yogurt, likely for the presence of probiotics [96]. Finally, a case–control study that also included a subgroup of postmenopausal women (55–64 years) reported that the consumption of fermented milk products was higher in the control group (n = 289) than in patients with BC (n = 133), suggesting a protective role in both pre- and postmenopausal women [97].
As for the potential mechanisms underlying the association between microbiota and BC, animal models demonstrated that probiotics could inhibit tumor growth and reduce tumor size, probably due to immunomodulatory, anti-angiogenesis, and anti-metastatic properties [98, 99]. Indeed, the oral administration of milk fermented by Lactobacillus casei CRL 431 to tumor-harboring BALB/c mice produced lower rates of tumor growth, angiogenesis, and metastasis and higher survival rates among the treatment group. In addition, the cytokine profile showed decreased IL-6 and increased monocyte chemoattractant protein-1 levels, a chemotactic cytokine [43]. In a similar study, reduced concentrations of IL-10, IL-6, and mammary glands TNF-α with clinical improvements (i.e., reduced tumor growth and angiogenesis) were detected after probiotic supplementation [99].
Of note, probiotic supplementation was effective also on the improvement of genitourinary symptoms in women treated for BC as consequences of chemotherapy and estrogen deprivation [100].
A 2-week supplementation with four Lactobacillus species (2 capsules/day) positively influenced the colonization of vaginal microbiota (evaluated by Nugent score) in 22 postmenopausal patients with BC receiving chemotherapy [101].
Therefore, probiotic supplementation seems to have a potential role both in the prevention of BC and in the management of chemotherapy-induced side effects in BC. However, more clinical studies are needed to elucidate their efficacy and safety.
Menopausal Dysbiosis and Type 1 Endometrial Hyperplasia
Type 1 endometrial hyperplasia is a precancerous condition characterized by a non-physiological and non-invasive endometrial growth, sustained by an increased estrogen/progesterone ratio [102]. During the fertile age, the risk of endometrial hyperplasia is associated with intermittent or absent ovulation, as PCOS. After menopause, endometrial hyperplasia is more common in women with estrogen-increasing conditions, such as obesity or hormone replacement therapy (HRT).
Endometrial hyperplasia could be also influenced by vaginal microbiota [103] (Fig. 1). Menopause is known to increase vaginal pH—due to the lack of estrogen, thus targeting microbial colonization. Interestingly, in endometrial carcinoma induced by type 1 endometrial hyperplasia, the uterine microbiota is characterized by the presence of Atopobium vaginae and Porphyromonas sp. with a pH > 4.5 [104]. These findings raise the possibility of (1) further investigating the microbiome role in the etiology or progression of endometrial cancer and (2) targeting specific bacterial strains that could favor lowering vaginal pH to reduce potentially pathogenic bacteria in the urogenital tract.
Some in vitro studies have shown that Lactobacillus rhamnosus BPL005 reduces pH levels by producing lactic acid and other organic acids, thus preventing endometrial infections by inhibiting some microbial species (i.e., Atopobium vaginae, Gardnerella vaginalis, Propionibacterium acnes, and Streptococcus agalactiae) [105].
However, meager evidence is available from human studies.
In a recent study, 130 healthy postmenopausal women suffering from menopausal symptoms were randomized to receive (a) 60 mg of soy isoflavones and 1 billion spores of Lactobacillus sporogenes or (b) calcium and vitamin D3 for 1 year. At the end of the study, menopausal symptoms significantly improved in the group consuming soy isoflavones plus Lactobacillus sporogenes versus the other group (calcium plus vitamin D3). No differences in endometrial thickness between groups were observed [106]. This study suggested that lactic bacteria might improve the absorption of soy isoflavones through the hydrolyzation of genistin and daidzin into the active aglycons by glycosidases, thus increasing the bioavailability of soy isoflavones.
Menopausal Dysbiosis and Periodontitis
Postmenopausal women have shown an increased risk of xerostomia (dry mouth), tooth mobility, and periodontitis (infection of the gums), likely related to reduced estrogen levels [29]. Indeed, oral mucosa and salivary glands present estrogen receptors and hypoestrogenemia has shown to activate polymorphonucleated and lymphocytes, increase cytokine levels, and modify the oral microbiota with an increase in gram-negative bacteria. In particular, some bacterial species such as Porphyromonas gingivalis and Tannerella forsythensis have been specifically associated with periodontitis in postmenopausal women [107]. Moreover, a 2-year open follow-up study in 400 postmenopausal women aged 50–58 years investigated the association between HRT and the composition of oral microbiota. After 2 years, in postmenopausal women on HRT (n = 200), there was a significant reduction in the abundance of Porphyromonas gingivalis and Tannerella forsythensis compared to the baseline. Conversely, in contrast, no changes in the oral microbiota were observed in the control group (n = 200) not treated with HRT [108]. Although some bacterial species have been specifically associated with periodontitis in postmenopausal women, to date, there is no evidence on the effect of probiotics in the prevention and treatment of periodontitis in this target group (Fig. 1). Therefore, further studies are required to evaluate whether probiotic supplementation could represent a useful strategy to modulate the oral microbiota in postmenopausal women.
Menopausal Dysbiosis and Obesity
Menopause is highly associated with obesity, and increased adiposity is the main risk factor for increased cardiometabolic alterations in postmenopausal women [109, 110]. Indeed, a 4-year observational study investigated changes in body weight and body fat during the menopausal transition in 156 healthy perimenopausal women. The results showed that subcutaneous abdominal fat increased in all participants; however, only women who enter menopause had a significant increase in visceral abdominal fat suggesting a redistribution of body fat mostly as central adiposity [111].
A recent meta-analysis of 11 longitudinal studies (n = 2.472 women) where participants were premenopausal at baseline and postmenopausal at follow-up highlighted significant differences in body weight and body fat distribution between premenopausal and postmenopausal periods. More in detail, as compared to baseline (premenopausal period), body mass index (BMI), percentage of body fat, waist and hip circumference, and visceral and trunk fat significantly increase in postmenopausal women [112]. In a more recent prospective cohort study with a 15-year follow-up, menopause and aging were independently correlated with increased BMI in 929 women who entered menopause during follow-up [113].
Body fat accumulation during menopause seems to be related to several mechanisms, including hormonal imbalance, reduction of energy expenditure, sedentary life, and increase in food intake [28].
Animal experiments demonstrated that the murine model of menopause (ovariectomized rats) had increased body weight and visceral fat [114], potentially due to increased food intake [115], decreased lipolysis [116], and reduced energy expenditure [117]. In particular, the expression of uncoupling proteins (UCPs) in brown and white adipose tissue could play a role in estrogen-mediated changes in body weight and energy expenditure. As a matter of fact, ovariectomized rats have a decreased UCP1 and UCP2 expression in brown and white adipose tissue, respectively, which translates into reduced energy expenditure [117]. Interestingly, when ovariectomized Sprague–Dawley rats were treated with estrogen, they exhibited a reduced weight gain and intra-abdominal fat accumulation. Nevertheless, estrogen therapy has been shown to induce uterine hypertrophy in the mouse model that makes it unsuitable for the prevention of weight gain in postmenopausal women [118].
As for the relationship between obesity and microbiota composition, the Firmicutes/Bacteroidetes ratio was directly associated with BMI in both animal models and studies in humans [119, 120]. In particular, women with obesity had a higher Firmicutes/Bacteroidetes ratio than men and increased plasma concentration of bacterial lipopolysaccharide—a well-known mediator of systemic inflammation [121]. Furthermore, it has been shown that obesity might affect the metabolic activity of some microbial species, including the hydrolyzation of isoflavones, that exhibit estrogen-like properties. As an example, a cross-sectional study of 355 women with overweight and obesity (n = 137 peri- and n = 218 postmenopausal women) who consumed at least 3 servings/week of soy (a source of isoflavones) demonstrated that women with higher BMI exhibited lower urinary concentrations of daidzein and its metabolites (equol and O-desmethylangolensin). This finding suggests an association between obesity and the alteration of microbiota composition and activity [122].
Overall, evidence available so far on the link between menopause, obesity, and microbiota composition is rather scarce. However, it rises some intriguing insights on novel strategies for body weight control tailoring microbial species that can metabolize estrogens and compound with estrogen-like properties.
Menopausal Dysbiosis and Cardiometabolic Risk
As mentioned in the previous section, menopause-related central obesity is a risk factor for cardiometabolic diseases [28, 123]. Indeed, menopause and early hormonal deprivation have been independently associated with a higher risk of metabolic syndrome, CVDs, stroke, heart failure, and total and heart disease mortality [123].
Over obesity, postmenopausal women exhibit a microbiota-dependent production of metabolites that may increase their cardiometabolic risk. More in detail, a metagenomic study in postmenopausal women demonstrated a strict association between several gut microbial species (i.e., Clostridium bolteae, Eubacterium ramulus, Ruminococcus torques, Catenibacterium mitsuokai, Holdemanella biformis) and markers of insulin resistance, dyslipidemia, and inflammation, independently from body weight [124].
Human intervention studies with probiotic supplementation in postmenopausal women have already shown a favorable effect on some cardiovascular risk factors. In a 12-week randomized placebo-controlled trial, 81 Caucasian women with obesity were assigned to a low dose or a high dose of a probiotic containing Bifidobacterium and Lactobacillus. At the end of the study, high dose significantly improved endothelial dysfunction, systolic blood pressure, and markers of inflammation (IL-6, TNF-α) and angiogenesis (vascular endothelial growth factor and thrombomodulin) [125]. In a similar study, high dose significantly improved body fat (waist circumference, fat mass, subcutaneous fat) and metabolic markers (uric acid, total cholesterol, triglycerides, low-density lipoprotein cholesterol, glucose, insulin, and homeostatic model assessment for insulin resistance) [126].
Several mechanisms could explain the pleiotropic effects of probiotic supplementation on multiple cardiometabolic risk factors [127, 128] (Fig. 1). Indeed, it is known that microbiota has a pivotal role in maintaining the integrity of the intestinal barrier, thus reducing bacteria translocation and, consequently, systemic inflammation. On the other hand, from the fermentation of polysaccharides and undigested proteins, some microbial species can produce SCFA (namely acetate, propionate, and butyrate), which can influence several metabolic pathways. Briefly, SCFA act as a mediator of transcriptional regulations and post-translational modifications, by the inhibition of lysine and histone deacetylase, thus influencing important transcription factors (in particular, peroxisome proliferator-activated receptor γ and aryl hydrocarbon receptor). SCFA can also activate signaling transduction pathways, thus activating multiple free fatty acid receptors.
Although further studies are needed, supplementation with probiotics could represent a useful and safe tool to control several cardiometabolic risk factors in postmenopausal women.
Probiotic and Prebiotic Safety and Study Limitations
Human supplementation with probiotics and prebiotics is usually considered to be safe [129]. However, although probiotics and prebiotics are generally considered safe in healthy adults, their use has been linked to a higher risk of infection and/or morbidity in critically ill adults in intensive care units, and postoperative, hospitalized, or immunocompromised patients [129]. So far, few cases of bacteremia, sepsis, and endocarditis caused by L. rhamnosus GG or L. casei lactobacilli have been reported [130]. Infections with Bifidobacteria are considered rare. However, bacteraemias, sepsis, and cholangitis induced by Bacillus subtilis [131] and fungal sepsis caused by Saccharomyces boulardii [132] have been reported. Of note, the association between probiotic and prebiotic use and increased risk of infection in immunocompromised patients needs to be further evaluated [133]. Overall, probiotic and prebiotic supplementation is considered safe in general when administered to immunocompetent individuals.
Certainly, the currently available studies on probiotics and prebiotics have a number of limitations that cannot be underestimated when drawing conclusions on their use. For instance, many studies have evaluated populations that are too small or have considered durations of use that are too short. Another limitation is the lack of microbial analyses of feces, which could demonstrate the influence of probiotic bacteria on the composition of the gut microbiota. It would also be interesting to perform mechanistic studies (similar to animal models) to explain the favorable effects of the metabolic activity of probiotics and prebiotics.
Conclusions
Table 1 summarizes the randomized studies reported on the use of probiotics in postmenopausal women. Although evidence from human intervention studies is limited so far, probiotic supplementation in postmenopausal women could represent a feasible and safe strategy to manage the menopause-related disease. In particular, oral probiotic formulations—especially those including Lactobacillus ssp. casei, helveticus, rhamnosus, and reuteri—might have pleiotropic beneficial effects on health by:
Promoting intestinal calcium absorption and reducing a further decrease in BMD in women at risk of osteoporosis or with osteopenia, thus potentially delaying bone damage
Reducing the incidence of BC and by improving the genitourinary symptoms associated with BC therapy
Promoting the reduction of vaginal pH, through the production of organic acids and the reduction of pathogenic bacteria which are risk factors for type 1 endometrial hyperplasia in in vitro models
Improving insulin resistance, dyslipidemia and inflammation, thus reducing the cardiometabolic risk of the postmenopausal woman
Table 1.
Randomized controlled trials of probiotics in postmenopausal women
| References | Design | Subjects | Probiotics strains (amount) | Probiotic form and dose | Duration | Outcomes | Main findings |
|---|---|---|---|---|---|---|---|
| Lambert et al. [74] | Randomized, double-blind, placebo-controlled trial | 78 postmenopausal women with osteopenia (40 probiotic; 38 placebo) | Heterogeneous culture (proprietary) of probiotic lactic acid bacteria | Powder twice daily | 12 months | Primary: effect of red clover extracts rich in isoflavone aglycones and probiotics against bone mass density loss. Secondary: effects of red clover extract on bone turnover markers, estrogen metabolites, plasma isoflavone concentration, equol-producer status, plasma lipid concentrations, and blood pressure | Red clover extracts significantly attenuated bone mineral density loss at the L2–L4 lumbar spine vertebra, femoral neck, and trochanter compared with the control group. Plasma concentrations of collagen type 1 cross-linked C-telopeptide were significantly decreased in the red clover extract group compared with the control group. Red clover extracts significantly elevated plasma isoflavone concentration, urinary 2-hydroxyestrone (2-OH) to 16a-ydroxyestrone (16a-OH) ratio, and equol-producer status compared with the control group |
| Nettleton et al. [82] | Randomized, crossover trial | 40 postmenopausal women (20 with no cancer history; 20 breast cancer survivors) | Lactobacillus acidophilus DDS1 and Bifidobacterium longum (109 CFU) | Capsules once daily | 6 weeks | Effect of probiotic consumption together with soy protein on the bioavailability and plasma concentrations of phytoestrogens, particularly the isoflavone metabolite equol | Plasma phytoestrogen concentrations and the number of equol-producers did not differ between the soy and soy plus probiotics diets |
| Narva et al. [87] | Randomized, double-blind, crossover trial | 20 postmenopausal women | Lactobacillus helveticus (not reported) | Fermented milk (220 ml) or peptide orange juice (400 ml) once daily | 22 days | Effect of milk fermented with L. helveticus and small peptides formed by the bacterium on acute changes in calcium metabolism and bone resorption in postmenopausal women | L. helveticus-fermented milk reduced serum parathormone and increased serum calcium compared to the control milk. L. helveticus-derived peptides had no significant acute effect on calcium metabolism; in fact, ionized calcium was lower and parathormone higher after the juice containing peptides compared to the control juice |
| Nilsson et al.[88] | Randomized, double-blind, placebo-controlled trial | 70 postmenopausal women with low bone mass density (34 probiotic; 36 placebo) | Lactobacillus reuteri ATCCPTA 6475 (5 × 109 CFU) | Stick packs (freeze-dried probiotics mixed with maltodextrin powder) twice daily | 12 months | Primary: changes after 12 months in tibia total volumetric bone mass density. Secondary: relative changes after 12 months in areal bone mass density measured at the hip and spine; trabecular bone volume fraction; cortical volumetric bone mass density; cortical thickness; serum markers for bone turnover; serum markers for inflammation; serum glycated hemoglobin; and body composition | L. reuteri 6475 reduced loss of total volumetric bone mass density compared to placebo both in the intention-to-treat analysis and per protocol analysis |
| Morato-Martínez et al. [89] | Randomized, double-blind, placebo-controlled trial | 65 postmenopausal women at risk of osteoporosis or untreated osteopenia (33 probiotic; 32 placebo) | Lactobacillus plantarum 3547 (1010 CFU) | Mix packs with water (120 ml) and lyophilized product (30 g) separated by a membrane once daily | 24 weeks | Effect of regular consumption of a dairy product enriched with bioactive nutrients (calcium, vitamin D, vitamin K, vitamin C, zinc, magnesium, L-leucine, and L. plantarum 3547) on bone metabolism markers of postmenopausal women at risk of osteoporosis | The intervention group showed a significantly increased bone mass compared to the control group. The intervention group maintained bone mass density compared to the control group, whose bone mass density significantly decreased at the end of the study. For biochemical markers, the intervention group significantly increased serum levels of the N-terminal propeptide of type I collagen bone formation marker and decreased the carbo-terminal telopeptide of type I collagen bone resorption marker compared to the control group |
| Marschalek et al.[101] | Randomized, double-blind, placebo-controlled pilot trial | 22 postmenopausal women with breast cancer undergoing chemotherapy (11 probiotic; 11 placebo) | Lactobacillus crispatus LbV 88, Lactobacillus rhamnosus LbV 96, Lactobacillus jensenii LbV 116, Lactobacillus gasseri LbV 150N (2.5 × 109 CFU for each one) | Capsules twice daily | 2 weeks | Effect of an orally administered preparation of 4 L. spp. on the vaginal microbiota of postmenopausal women with breast cancer undergoing chemotherapy | There was a positive influence on the vaginal microbiota in 7/11 (63%) women in the intervention group and 4/11 (36%) women in the control group. There was a shift in the Nugent score towards normal microbiota levels in the intervention group and a significant deterioration of the Nugent score in the control group |
| Colacurci et al. [106] | Randomized, placebo-controlled trial | 124 healthy postmenopausal women suffering from menopausal symptoms (62 probiotic; 62 placebo) | Lactobacillus sporogenes (1 bilion spores) | Tablets once daily | 12 months | Effect of a nutraceutical compound containing isoflavones and L. sporogenes compared to calcium and vitamin D alone on endometrium, breast, and liver function | After 12 months of treatment mammographic density, endometrial thickness and hepatic function did not show significant differences between groups, while menopausal symptoms were progressively and significantly reduced in severity and frequency during treatment with soy isoflavones plus L. sporogenes vs calcium plus vitamin D3 |
| Szulińska et al.[125] | Randomized, placebo-controlled trial | 81 postmenopausal women with obesity (23 high probiotic dose; 24 low probiotic dose; 24 placebo) | Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19, and Lactococcus lactis W58 (2.5 × 109 CFU for low-dose group; 1010 CFU for high-dose group) | Powder twice daily | 12 weeks | Primary: effects of different doses of supplemented multispecies probiotics on the functional parameters of endothelial and vascular dysfunction. Secondary: effects of different doses of supplemented multispecies probiotics on the biochemical parameters of endothelial and vascular dysfunction | High doses of probiotic supplementation decreased systolic blood pressure, vascular endothelial growth factor, pulse wave analysis systolic pressure, pulse wave analysis pulse pressure, pulse wave analysis augmentation index, pulse wave velocity, interleukin-6, tumor necrosis factor alpha, and thrombomodulin. Low doses of probiotic supplementation decreased systolic blood pressure and interleukin-6 levels. The mean changes in the estimated parameters, compared among the three groups, revealed significant differences in vascular endothelial growth factor, pulse wave analysis systolic pressure, pulse wave analysis augmentation index, pulse wave velocity, tumor necrosis factor alpha, and thrombomodulin |
| Szulińska et al. [126] | Randomized, placebo-controlled trial | 81 postmenopausal women with obesity (23 high probiotic dose; 24 low probiotic dose; 24 placebo) | Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19, and Lactococcus lactis W58 (2.5 × 109 CFU for low-dose group; 1010 CFU for high-dose group) | Powder twice daily | 12 weeks | Primary: effects of different doses of supplemented multispecies probiotics on the lipopolysaccharide level. Secondary: effects of different doses of supplemented multispecies probiotics on cardiometabolic parameters | There were significant favorable changes (mostly large or medium effects) in evaluated parameters in both the high- and low-dose groups but not in the placebo group. In the high-dose group, lipopolysaccharide, waist, fat mass, subcutaneous fat, uric acid, total cholesterol, triglycerides, low-density lipoprotein cholesterol, glucose, insulin, and insulin-resistant index were improved. Similar changes were observed in the low-dose group, except for lipopolysaccharide, uric acid, triglycerides, and glucose levels. Additionally, significant differences were observed in both groups in terms of fat percentage and visceral fat. When the mean changes were compared between the three groups, statistically significant differences in lipopolysaccharide levels, uric acid, glucose, insulin, and insulin-resistant index were found |
CFU colony-forming unit
Abbreviations
- PCOS
Polycystic ovary syndrome
- CVD
Cardiovascular disease
- BC
Breast cancer
- IL
Interleukin
- TNF
Tumor necrosis factor
- IFN
Interferon
- SCFA
Short-chain fatty acids
- BMD
Body mass density
- HRT
Hormone replacement therapy
- BMI
Body mass index
- UCP
Uncoupling protein
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Compliance with Ethical Standards
Conflict of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Luigi Barrea, Ludovica Verde, and Renata Simona Auriemma equally contributed to this paper.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Gomes AC, Hoffmann C, Mota JF. The human gut microbiota: metabolism and perspective in obesity. Gut Microbes. 2018. [DOI] [PMC free article] [PubMed]
- 2.Berg G, Rybakova D, Fischer D, Cernava T, Vergès M-CC, Charles T, et al. Correction to: Microbiome definition re-visited: old concepts and new challenges. Microbiome. 2020. [DOI] [PMC free article] [PubMed]
- 3.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017. [DOI] [PMC free article] [PubMed]
- 4.Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019. [DOI] [PMC free article] [PubMed]
- 5.Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019. [DOI] [PMC free article] [PubMed]
- 6.Gupta VK, Paul S, Dutta C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front Microbiol. 2017. [DOI] [PMC free article] [PubMed]
- 7.Lynch S V., Pedersen O. The human intestinal microbiome in health and disease. New England J Med. 2016. [DOI] [PubMed]
- 8.Zimmermann P, Messina N, Mohn WW, Finlay BB, Curtis N. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: a systematic review. J Allergy Clin Immunol. 2019. [DOI] [PubMed]
- 9.Tokarek J, Gadzinowska J, Młynarska E, Franczyk B, Rysz J. What is the role of gut microbiota in obesity prevalence? A few words about gut microbiota and its association with obesity and related diseases. Microorganisms. 2022. [DOI] [PMC free article] [PubMed]
- 10.Zhou Z, Sun B, Yu D, Zhu C. Gut microbiota: an important player in type 2 diabetes mellitus. Front Cell Infect Microbiol. 2022. [DOI] [PMC free article] [PubMed]
- 11.Bauer KC, Littlejohn PT, Ayala V, Creus-Cuadros A, Finlay BB. Nonalcoholic fatty liver disease and the gut-liver axis: exploring an undernutrition perspective. Gastroenterology [Internet]. 2022;162:1858–1875.e2. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0016508522002037 [DOI] [PubMed]
- 12.Rebersek M. Gut microbiome and its role in colorectal cancer. BMC Cancer. 2021. [DOI] [PMC free article] [PubMed]
- 13.Kim M, Huda MN, Bennett BJ. Sequence meets function - microbiota and cardiovascular disease. Cardiovasc Res. 2022. [DOI] [PMC free article] [PubMed]
- 14.Barrio C, Arias-Sánchez S, Martín-Monzón I. The gut microbiota-brain axis, psychobiotics and its influence on brain and behaviour: a systematic review. Psychoneuroendocrinology. 2022. [DOI] [PubMed]
- 15.Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016. [DOI] [PMC free article] [PubMed]
- 16.Huang G, Xu J, Lefever DE, Glenn TC, Nagy T, Guo TL. Genistein prevention of hyperglycemia and improvement of glucose tolerance in adult non-obese diabetic mice are associated with alterations of gut microbiome and immune homeostasis. Toxicol Appl Pharmacol. 2017. [DOI] [PMC free article] [PubMed]
- 17.Flores R, Shi J, Fuhrman B, Xu X, Veenstra TD, Gail MH, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012. [DOI] [PMC free article] [PubMed]
- 18.Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen–gut microbiome axis: physiological and clinical implications. Maturitas. 2017. [DOI] [PubMed]
- 19.Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. 2011. [DOI] [PMC free article] [PubMed]
- 20.Charalampakis V, Tahrani AA, Helmy A, Gupta JK, Singhal R. Polycystic ovary syndrome and endometrial hyperplasia: an overview of the role of bariatric surgery in female fertility. Eur J Obstetr Gynecol Reproduct Biol. 2016. [DOI] [PubMed]
- 21.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015. [DOI] [PMC free article] [PubMed]
- 22.Richards EM, Li J, Stevens BR, Pepine CJ, Raizada MK. Gut microbiome and neuroinflammation in hypertension. Circ Res. 2022. [DOI] [PMC free article] [PubMed]
- 23.Vuong HE, Yano JM, Fung TC, Hsiao EY. The microbiome and host behavior. Ann Rev Neurosci. 2017. [DOI] [PMC free article] [PubMed]
- 24.Alizadehmohajer N, Shojaeifar S, Nedaeinia R, Esparvarinha M, Mohammadi F, Ferns GA, et al. Association between the microbiota and women’s cancers – cause or consequences? Biomed Pharmacother. 2020. [DOI] [PubMed]
- 25.Muhleisen AL, Herbst-Kralovetz MM. Menopause and the vaginal microbiome. Maturitas. 2016. [DOI] [PubMed]
- 26.Homma H, Hoy E, Xu DZ, Lu Q, Feinman R, Deitch EA. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am J Physiol Gastrointestinal Liver Physiol. 2005. [DOI] [PubMed]
- 27.Pugliese G, Barrea L, Laudisio D, Aprano S, Castellucci B, Framondi L, et al. Mediterranean diet as tool to manage obesity in menopause: a narrative review. Nutrition. 2020. [DOI] [PubMed]
- 28.Barrea L, Pugliese G, Laudisio D, Colao A, Savastano S, Muscogiuri G. Mediterranean diet as medical prescription in menopausal women with obesity: a practical guide for nutritionists. Crit Rev Food Sci Nutr. 2020. [DOI] [PubMed]
- 29.Vieira AT, Castelo PM, Ribeiro DA, Ferreira CM. Influence of oral and gut microbiota in the health of menopausal women. Front Microbiol. 2017. [DOI] [PMC free article] [PubMed]
- 30.Chen Y, Zhou J, Wang L. Role and mechanism of gut microbiota in human disease. Front Cell Infect Microbiol. 2021. [DOI] [PMC free article] [PubMed]
- 31.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014. [DOI] [PubMed]
- 32.Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019. [DOI] [PubMed]
- 33.Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018. [DOI] [PubMed]
- 34.Dai C, Zhao DH, Jiang M. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int J Mol Med. 2012. [DOI] [PubMed]
- 35.Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut. 2003. [DOI] [PMC free article] [PubMed]
- 36.Miyamoto J, Mizukure T, Park SB, Kishino S, Kimura I, Hirano K, et al. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway. J Biol Chem. 2015. [DOI] [PMC free article] [PubMed]
- 37.van Zyl WF, Deane SM, Dicks LMT. Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microbes. 2020. [DOI] [PMC free article] [PubMed]
- 38.Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A. Probiotic mechanisms of action. Ann Nutr Metabol. 2012. [DOI] [PubMed]
- 39.Liu Q, Yu Z, Tian F, Zhao J, Zhang H, Zhai Q, et al. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb Cell Factories. 2020. [DOI] [PMC free article] [PubMed]
- 40.Tyrer P, Foxwell AR, Cripps AW, Apicella MA, Kyd JM. Microbial pattern recognition receptors mediate M-cell uptake of a gram-negative bacterium. Infect Immun. 2006. [DOI] [PMC free article] [PubMed]
- 41.Maldonado Galdeano C, Cazorla SI, Lemme Dumit JM, Vélez E, Perdigón G. Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab. 2019. [DOI] [PubMed]
- 42.Barrea L, Muscogiuri G, Annunziata G, Laudisio D, Pugliese G, Salzano C, et al. From gut microbiota dysfunction to obesity: could short-chain fatty acids stop this dangerous course? Hormones. 2019. [DOI] [PubMed]
- 43.Arpaia N, Campbell C, Fan X, Dikiy S, Van Der Veeken J, Deroos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013. [DOI] [PMC free article] [PubMed]
- 44.Colonic T, Homeostasis C, Smith PM, Howitt MR, Panikov N, Michaud M, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013. [DOI] [PMC free article] [PubMed]
- 45.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013. [DOI] [PubMed]
- 46.Chen J, Vitetta L. The role of butyrate in attenuating pathobiont-induced hyperinflammation. Immune Netw. 2020. [DOI] [PMC free article] [PubMed]
- 47.Anania C, Brindisi G, Martinelli I, Bonucci E, D’Orsi M, Ialongo S, et al. Probiotics function in preventing atopic dermatitis in children. Int J Mol Sci. 2022;23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/35628229 [DOI] [PMC free article] [PubMed]
- 48.Huang J, Zhang J, Wang X, Jin Z, Zhang P, Su H, et al. Effect of probiotics on respiratory tract allergic disease and gut microbiota. Front Nutr. 2022. [DOI] [PMC free article] [PubMed]
- 49.Farahmandi K, Mohr AE, McFarland L V. Effects of probiotics on allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. Am J Rhinol Allergy. 2022. [DOI] [PubMed]
- 50.Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016. [DOI] [PMC free article] [PubMed]
- 51.Jia L, Tu Y, Jia X, Du Q, Zheng X, Yuan Q, et al. Probiotics ameliorate alveolar bone loss by regulating gut microbiota. Cell Prolif. 2021. [DOI] [PMC free article] [PubMed]
- 52.Farrokhian A, Raygan F, Soltani A, Tajabadi-Ebrahimi M, Sharifi Esfahani M, Karami AA, et al. The effects of synbiotic supplementation on carotid intima-media thickness, biomarkers of inflammation, and oxidative stress in people with overweight, diabetes, and coronary heart disease: a randomized, double-blind, placebo-controlled trial. Probiotics Antimicrob Proteins. 2019. [DOI] [PubMed]
- 53.Malik M, Suboc TM, Tyagi S, Salzman N, Wang J, Ying R, et al. Lactobacillus plantarum 299v supplementation improves vascular endothelial function and reduces inflammatory biomarkers in men with stable coronary artery disease. Circ Res. 2018. [DOI] [PMC free article] [PubMed]
- 54.Moludi J, Kafil HS, Qaisar SA, Gholizadeh P, Alizadeh M, Vayghyan HJ. Effect of probiotic supplementation along with calorie restriction on metabolic endotoxemia, and inflammation markers in coronary artery disease patients: a double blind placebo controlled randomized clinical trial. Nutr J. 2021. [DOI] [PMC free article] [PubMed]
- 55.Begley M, Hill C, Gahan CGM. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol. 2006. [DOI] [PMC free article] [PubMed]
- 56.Jones ML, Tomaro-Duchesneau C, Martoni CJ, Prakash S. Cholesterol lowering with bile salt hydrolase-active probiotic bacteria, mechanism of action, clinical evidence, and future direction for heart health applications. Exp Opin Biol Ther. 2013. [DOI] [PubMed]
- 57.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015. [DOI] [PubMed]
- 58.Rosean CB, Bostic RR, Ferey JCM, Feng TY, Azar FN, Tung KS, et al. Preexisting commensal dysbiosis is a host-intrinsic regulator of tissue inflammation and tumor cell dissemination in hormone receptor–positive breast cancer. Cancer Res. 2019. [DOI] [PMC free article] [PubMed]
- 59.Eslami-S Z, Majidzadeh-A K, Halvaei S, Babapirali F, Esmaeili R. Microbiome and breast cancer: new role for an ancient population. Front Oncol. 2020. [DOI] [PMC free article] [PubMed]
- 60.Sári Z, Mikó E, Kovács T, Boratkó A, Ujlaki G, Jankó L, et al. Indoxylsulfate, a metabolite of the microbiome, has cytostatic effects in breast cancer via activation of AHR and PXR receptors and induction of oxidative stress. Cancers. 2020. [DOI] [PMC free article] [PubMed]
- 61.Sári Z, Mikó E, Kovács T, Jankó L, Csonka T, Lente G, et al. Indolepropionic acid, a metabolite of the microbiome, has cytostatic properties in breast cancer by activating AHR and PXR receptors and inducing oxidative stress. Cancers. 2020. [DOI] [PMC free article] [PubMed]
- 62.Kovács P, Csonka T, Kovács T, Sári Z, Ujlaki G, Sipos A, et al. Lithocholic acid, a metabolite of the microbiome, increases oxidative stress in breast cancer. Cancers. 2019. [DOI] [PMC free article] [PubMed]
- 63.Kovács T, Mikó E, Vida A, Sebő É, Toth J, Csonka T, et al. Cadaverine, a metabolite of the microbiome, reduces breast cancer aggressiveness through trace amino acid receptors. Sci Rep. 2019. [DOI] [PMC free article] [PubMed]
- 64.Luu TH, Bard JM, Carbonnelle D, Chaillou C, Huvelin JM, Bobin-Dubigeon C, et al. Lithocholic bile acid inhibits lipogenesis and induces apoptosis in breast cancer cells. Cell Oncol. 2018. [DOI] [PubMed]
- 65.Mikó E, Vida A, Kovács T, Ujlaki G, Trencsényi G, Márton J, et al. Lithocholic acid, a bacterial metabolite reduces breast cancer cell proliferation and aggressiveness. Biochimica et Biophysica Acta - Bioenergetics. 2018. [DOI] [PubMed]
- 66.Abildgaard A, Elfving B, Hokland M, Wegener G, Lund S. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology. 2017. [DOI] [PubMed]
- 67.Mondanelli G, Orecchini E, Volpi C, Panfili E, Belladonna ML, Pallotta MT, et al. Effect of probiotic administration on serum tryptophan metabolites in pediatric type 1 diabetes patients. Int J Tryptophan Res. 2020. [DOI] [PMC free article] [PubMed]
- 68.Manzoni MSJ, Rossi EA, Pauly-Silveira ND, Pinto RA, Roselino MN, Carlos IZ, et al. Consumption effect of a synbiotic beverage made from soy and yacon extracts containing Bifidobacterium animalis ssp. lactis BB-12 on the intestinal polyamine concentrations in elderly individuals. Food Res Int. 2017. [DOI] [PubMed]
- 69.Clavel T, Henderson G, Alpert CA, Philippe C, Rigottier-Gois L, Doré J, et al. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl Environ Microbiol. 2005. [DOI] [PMC free article] [PubMed]
- 70.Kuhnle GGC, Ward HA, Vogiatzoglou A, Luben RN, Mulligan A, Wareham NJ, et al. Association between dietary phyto-oestrogens and bone density in men and postmenopausal women. British J Nutr. 2011. [DOI] [PubMed]
- 71.Al-Harbi SA, Abdulrahman AO, Zamzami MA, Khan MI. Urolithins: the gut based polyphenol metabolites of ellagitannins in cancer prevention, a review. Front Nutr. 2021. [DOI] [PMC free article] [PubMed]
- 72.Landete JM, Arqués J, Medina M, Gaya P, de Las Rivas BD, Muñoz R. Bioactivation of phytoestrogens: intestinal bacteria and health. Crit Rev Food Sci Nutr. 2016. [DOI] [PubMed]
- 73.Zhang J, Ren L, Yu M, Liu X, Ma W, Huang L, et al. S-equol inhibits proliferation and promotes apoptosis of human breast cancer MCF-7 cells via regulating miR-10a-5p and PI3K/AKT pathway. Arch Biochem Biophys. 2019. [DOI] [PubMed]
- 74.Lambert MNT, Thybo CB, Lykkeboe S, Rasmussen LM, Frette X, Christensen LP, et al. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: a randomized controlled trial. Am J Clin Nutr. 2017. [DOI] [PubMed]
- 75.Gaya P, Peirotén Á, Medina M, Landete JM. Bifidobacterium adolescentis INIA P784: the first probiotic bacterium capable of producing enterodiol from lignan extracts. J Funct Foods. 2017.
- 76.Gaya P, Peirotén Á, Medina M, Landete JM. Isoflavone metabolism by a collection of lactic acid bacteria and bifidobacteria with biotechnological interest. Int J Food Sci Nutr. 2016. [DOI] [PubMed]
- 77.Landete JM, Gaya P, Rodríguez E, Langa S, Peirotén Á, Medina M, et al. Probiotic bacteria for healthier aging: immunomodulation and metabolism of phytoestrogens. BioMed Res Int. 2017. [DOI] [PMC free article] [PubMed]
- 78.Polia F, Pastor-Belda M, Martínez-Blázquez A, Horcajada MN, Tomás-Barberán FA, García-Villalba R. Technological and biotechnological processes to enhance the bioavailability of dietary (poly)phenols in humans. J Agric Food Chem. 2022. [DOI] [PMC free article] [PubMed]
- 79.Bonorden MJL, Greany KA, Wangen KE, Phipps WR, Feirtag J, Adlercreutz H, et al. Consumption of Lactobacillus acidophilus and Bifidobacterium longum do not alter urinary equol excretion and plasma reproductive hormones in premenopausal women. Eur J Clin Nutr. 2004. [DOI] [PubMed]
- 80.Larkin TA, Price WE, Astheimer LB. Increased probiotic yogurt or resistant starch intake does not affect isoflavone bioavailability in subjects consuming a high soy diet. Nutrition. 2007. [DOI] [PubMed]
- 81.McMullen MH, Hamilton-Reeves JM, Bonorden MJL, Wangen KE, Phipps WR, Feirtag JM, et al. Consumption of Lactobacillus acidophilus and Bifidobacterium longum does not alter phytoestrogen metabolism and plasma hormones in men: a pilot study. J Altern Complement Med. 2006. [DOI] [PubMed]
- 82.Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. Plasma phytoestrogens are not altered by probiotic consumption in postmenopausal women with and without a history of breast cancer. J Nutr. 2004. [DOI] [PubMed]
- 83.Jackson RD, Mysiw WJ. Insights into the epidemiology of postmenopausal osteoporosis: the women’s health initiative. Semin Reprod Med. 2014. [DOI] [PubMed]
- 84.Cauley JA, Danielson ME, Greendale GA, Finkelstein JS, Chang YF, Lo JC, et al. Bone resorption and fracture across the menopausal transition: the Study of Women’s Health Across the Nation. Menopause. 2012. [DOI] [PMC free article] [PubMed]
- 85.Huidrom S, Beg MA, Masood T. Post-menopausal osteoporosis and probiotics. Curr Drug Targets. 2020. [DOI] [PubMed]
- 86.•• Lorenzo J. From the gut to bone: connecting the gut microbiota with Th17 T lymphocytes and postmenopausal osteoporosis. J Clin Invest. 2021. The results of this study argue that interactions of the gut microbiota with the immune system are involved in the effects of estrogen withdrawal on trabecular bone. [DOI] [PMC free article] [PubMed]
- 87.Narva M, Nevala R, Poussa T, Korpela R. The effect of Lactobacillus helveticus fermented milk on acute changes in calcium metabolism in postmenopausal women. Eur J Nutr. 2004. [DOI] [PubMed]
- 88.Nilsson AG, Sundh D, Bäckhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med. 2018. [DOI] [PubMed]
- 89.Morato-Martínez M, López-Plaza B, Santurino C, Palma-Milla S, Gómez-Candela C. A dairy product to reconstitute enriched with bioactive nutrients stops bone loss in high-risk menopausal women without pharmacological treatment. Nutrients. 2020. [DOI] [PMC free article] [PubMed]
- 90.Samkari AA, Alsulami M, Bataweel L, Altaifi R, Altaifi A, Saleem AM, et al. Body microbiota and its relationship with benign and malignant breast tumors: a systematic review. Cureus. 2022; Available from: https://www.cureus.com/articles/99350-body-microbiota-and-its-relationship-with-benign-and-malignant-breast-tumors-a-systematic-review [DOI] [PMC free article] [PubMed]
- 91.• Plaza-DÍaz J, Álvarez-Mercado AI, Ruiz-Marín CM, Reina-Pérez I, Pérez-Alonso AJ, Sánchez-Andujar MB, et al. Association of breast and gut microbiota dysbiosis and the risk of breast cancer: a case-control clinical study. BMC Cancer. 2019. This is the first time that the contribution of bacteria, archaea, viruses, and fungi together with their alteration by environmental contaminants to the risk of breast cancer will be evaluated in the same study. [DOI] [PMC free article] [PubMed]
- 92.Zhu J, Liao M, Yao Z, Liang W, Li Q, Liu J, et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome. 2018. [DOI] [PMC free article] [PubMed]
- 93.Fuhrman BJ, Feigelson HS, Flores R, Gail MH, Xu X, Ravel J, et al. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab. 2014. [DOI] [PMC free article] [PubMed]
- 94.Zhao H, Chen J, Li X, Sun Q, Qin P, Wang Q. Compositional and functional features of the female premenopausal and postmenopausal gut microbiota. FEBS Lett. 2019. [DOI] [PubMed]
- 95.Toi M, Hirota S, Tomotaki A, Sato N, Hozumi Y, Anan K, et al. Probiotic beverage with soy isoflavone consumption for breast cancer prevention: a case-control study. Curr Nutr Food Sci. 2013. [DOI] [PMC free article] [PubMed]
- 96.Lê MG, Moulton LH, Hill C, Kramar A. Consumption of dairy produce and alcohol in a case-control study of breast cancer. J Nat Cancer Inst. 1986. [DOI] [PubMed]
- 97.Veer van’t P, Dekker J, Lamers JW, Kok FJ, Schouter EG, Brants HAM, et al. Consumption of fermented milk products and breast cancer: a case-control study in the Netherlands. Cancer Res. 1989. [PubMed]
- 98.Aragón F, Carino S, Perdigón G, De Moreno De LeBlanc A. Inhibition of growth and metastasis of breast cancer in mice by milk fermented with Lactobacillus casei CRL 431. J Immunother. 2015. [DOI] [PubMed]
- 99.Aragón F, Carino S, Perdigón G, De Moreno de LeBlanc A. The administration of milk fermented by the probiotic Lactobacillus casei CRL 431 exerts an immunomodulatory effect against a breast tumour in a mouse model. Immunobiology. 2014. [DOI] [PubMed]
- 100.Krychman ML, Carter J, Aghajanian CA, Dizon DS, Castiel M. Chemotherapy-induced dyspareunia: a case study of vaginal mucositis and pegylated liposomal doxorubicin injection in advanced stage ovarian carcinoma. Gynecol Oncol. 2004. [DOI] [PubMed]
- 101.Marschalek J, Farr A, Marschalek ML, Domig KJ, Kneifel W, Singer CF, et al. Influence of orally administered probiotic Lactobacillus strains on vaginal microbiota in women with breast cancer during chemotherapy: a randomized placebo-controlled double-blinded pilot study. Breast Care. 2017. [DOI] [PMC free article] [PubMed]
- 102.Mills AM, Longacre TA. Endometrial hyperplasia. Semin Diag Pathol. 2010;27:199–214. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0740257010000997 [DOI] [PubMed]
- 103.Calleja-Agius J, Brincat MP. The urogenital system and the menopause. Climacteric. 2015. [DOI] [PubMed]
- 104.Walther-António MRS, Chen J, Multinu F, Hokenstad A, Distad TJ, Cheek EH, et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016. [DOI] [PMC free article] [PubMed]
- 105.Chenoll E, Moreno I, Sánchez M, Garcia-Grau I, Silva Á, González-Monfort M, et al. Selection of new probiotics for endometrial health. Front Cell Infect Microbiol. 2019. [DOI] [PMC free article] [PubMed]
- 106.Colacurci N, De Franciscis P, Atlante M, Mancino P, Monti M, Volpini G, et al. Endometrial, breast and liver safety of soy isoflavones plus Lactobacillus sporogenes in post-menopausal women. Gynecol Endocrinol. 2013. [DOI] [PubMed]
- 107.Mascarenhas P, Gapski R, Al-Shammari K, Wang HL. Influence of sex hormones on the periodontium. J Clin Periodontol. 2003. [DOI] [PubMed]
- 108.Tarkkila L, Kari K, Furuholm J, Tiitinen A, Meurman JH. Periodontal disease-associated micro-organisms in peri-menopausal and post-menopausal women using or not using hormone replacement therapy. A two-year follow-up study. BMC Oral Health. 2010. [DOI] [PMC free article] [PubMed]
- 109.Leeners B, Geary N, Tobler PN, Asarian L. Ovarian hormones and obesity. Human Reprod Update. 2017. [DOI] [PMC free article] [PubMed]
- 110.Laudisio D, Barrea L, Pugliese G, Aprano S, Castellucci B, Savastano S, et al. A practical nutritional guide for the management of sleep disturbances in menopause. Int J Food Sci Nutr. 2021. [DOI] [PubMed]
- 111.Lovejoy JC, Champagne CM, De Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes. 2008. [DOI] [PMC free article] [PubMed]
- 112.Ambikairajah A, Walsh E, Tabatabaei-Jafari H, Cherbuin N. Fat mass changes during menopause: a metaanalysis. Am J Obstetr Gynecol. 2019. [DOI] [PubMed]
- 113.Montazeri SA, Ramezani Tehrani F, Bidhendi Yarandi R, Erfani H, Mansournia MA, Azizi F. Effect of aging, menopause, and age at natural menopause on the trend in body mass index: a 15-year population-based cohort. Fertil Steril. 2019. [DOI] [PubMed]
- 114.Babaei P, Mehdizadeh R, Ansar MM, Damirchi A. Effects of ovariectomy and estrogen replacement therapy on visceral adipose tissue and serum adiponectin levels in rats. Menopause Int. 2010. [DOI] [PubMed]
- 115.Gray JM, Wade GN. Food intake, body weight, and adiposity in female rats: actions and interactions of progestins and antiestrogens. Am J Physiol - Endocrinol Metab. 1981. [DOI] [PubMed]
- 116.Darimont C, Delansorne R, Paris J, Ailhaud G, Negrel R. Influence of estrogenic status on the lipolytic activity of parametrial adipose tissue in vivo: an in situ microdialysis study. Endocrinology. 1997. [DOI] [PubMed]
- 117.Pedersen SB, Bruun JM, Kristensen K, Richelsen B. Regulation of UCP1, UCP2, and UCP3 mRNA expression in brown adipose tissue, white adipose tissue, and skeletal muscle in rats by estrogen. Biochem Biophys Res Commun. 2001. [DOI] [PubMed]
- 118.Rachoń D, Vortherms T, Seidlovä-Wuttke D, Wuttke W. Effects of dietary equol on body weight gain, intra-abdominal fat accumulation, plasma lipids, and glucose tolerance in ovariectomized Sprague-Dawley rats. Menopause. 2007. [DOI] [PubMed]
- 119.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Nat Acad Sci USA. 2005. [DOI] [PMC free article] [PubMed]
- 120.Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017. [DOI] [PMC free article] [PubMed]
- 121.Razavi AC, Potts KS, Kelly TN, Bazzano LA. Sex, gut microbiome, and cardiovascular disease risk. Biol Sex Diff. 2019. [DOI] [PMC free article] [PubMed]
- 122.Miller LM, Lampe JW, Newton KM, Gundersen G, Fuller S, Reed SD, et al. Being overweight or obese is associated with harboring a gut microbial community not capable of metabolizing the soy isoflavone daidzein to O-desmethylangolensin in peri- and post-menopausal women. Maturitas. 2017. [DOI] [PubMed]
- 123.El Khoudary SR, Thurston RC. Cardiovascular implications of the menopause transition: endogenous sex hormones and vasomotor symptoms. Obstetr Gynecol Clin North Am. 2018. [DOI] [PubMed]
- 124.Brahe LK, Le Chatelier E, Prifti E, Pons N, Kennedy S, Hansen T, et al. Specific gut microbiota features and metabolic markers in postmenopausal women with obesity. Nutr Diabetes. 2015. [DOI] [PMC free article] [PubMed]
- 125.Szulińska M, Łoniewski I, Skrypnik K, Sobieska M, Korybalska K, Suliburska J, et al. Multispecies probiotic supplementation favorably affects vascular function and reduces arterial stiffness in obese postmenopausal women—a 12-week placebo-controlled and randomized clinical study. Nutrients. 2018. [DOI] [PMC free article] [PubMed]
- 126.Szulińska M, Łoniewski I, van Hemert S, Sobieska M, Bogdański P. Dose-dependent effects of multispecies probiotic supplementation on the lipopolysaccharide (LPS) level and cardiometabolic profile in obese postmenopausal women: a 12-week randomized clinical trial. Nutrients. 2018. [DOI] [PMC free article] [PubMed]
- 127.Salamone D, Rivellese AA, Vetrani C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: the possible role of dietary fibre. Acta Diabetol. 2021. [DOI] [PMC free article] [PubMed]
- 128.Nogal A, Valdes AM, Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes. 2021. [DOI] [PMC free article] [PubMed]
- 129.Zawistowska-Rojek A, Tyski S. Are probiotic really safe for humans? Pol J Microbiol. 2018. [DOI] [PMC free article] [PubMed]
- 130.Boyle RJ; Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006. [DOI] [PubMed]
- 131.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013. [DOI] [PMC free article] [PubMed]
- 132.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008. [DOI] [PubMed]
- 133.Hempel S, Newberry S, Ruelaz A, Wang Z, Miles JN, Suttorp MJ, Johnsen B, Shanman R, Slusser W, Fu N, et al. Safety of probiotics used to reduce risk and prevent or treat disease. Evid Rep Technol Assess (Full Rep). 2011. [PMC free article] [PubMed]