Premature atrial contractions (PACs) frequently initiate atrial fibrillation (AF).1 The pulmonary veins (PVs) are the most common origin of PAC-triggered AF, with two possible explanations:1 either PACs generally lead to AF, and the PVs are the most common origin; or, PV PACs are particularly arrhythmogenic. PAC frequency predicts AF risk,2 suggesting that PAC eradication might effectively prevent AF and compelling the identification of the most culpable PAC type. Leveraging data from the HOLIDAY (How Alcohol Induces Atrial Arrhythmias) trial, we tested the hypothesis that PACs arising from the PVs are more likely to induce AF.
PACs were administered during sinus rhythm to patients undergoing PVI ablation in a prospective protocol as part of HOLIDAY, a randomized, double-blind trial of 100 patients that evaluated atrial electrophysiology during infusion of alcohol versus placebo.3 PACs were administered using paced extra-stimuli in order from the proximal coronary sinus (CS), distal CS, high crista terminalis (HCT), left upper (LU) PV and right upper (RU) PVs. If the intended upper PV was electrically isolated, the ipsilateral lower PV was used; if both were isolated, the captured site closest to the intended PV was utilized. A 600 ms 8 beat drive train (S1) was administered, and, using a “step-up” approach, S2 was administered at 140 ms and increased by 10 ms until atrial capture occurred. The S2 coupling interval of the first captured beat constituted the PAC delivered from that site, also defining that site’s atrial effective refractory period (AERP). Non-sustained (<30 seconds) and sustained (>30 seconds) AF was documented after each PAC. The study infusion was then initiated, and the same protocol was repeated. The study was approved by the University of California-San Francisco Institutional Review Board. Participants provided informed consent. The authors will make the data, methods, and materials available to investigators upon reasonable request.
Logistic models using robust standard errors (to address clustering within individuals) and adjusted for study infusion were performed to calculate the odds of AF induction by PAC location. Sensitivity analyses included examining sustained AF as the outcome and after restricting to pacing before any study infusions. “Percent treatment explained” analyses (determining confidence intervals using bootstrapping resampling with 500 repetitions) was performed to test the hypothesis that shorter AERPs mediated differences in frequency of AF induction by site.4 Two-tailed p values <0.05 were considered statistically significant. Statistical analyses were performed using Stata version 16 (StataCorp, College Station, TX).
One hundred patients, mean age 60 ± 11 years, 26% women, 80% with paroxysmal AF, 42% with prior ablation, mean baseline heart rate 64 ± 22 bpm were included. PACs induced 41 AF events in 22 patients. No statistically significant differences were observed between those with and without PAC-induced AF. The PV PAC location was in the intended LUPV 88% of the time and RUPV 89% of the time, with the remaining PACs administered from the lower vein or just outside the intended vein.
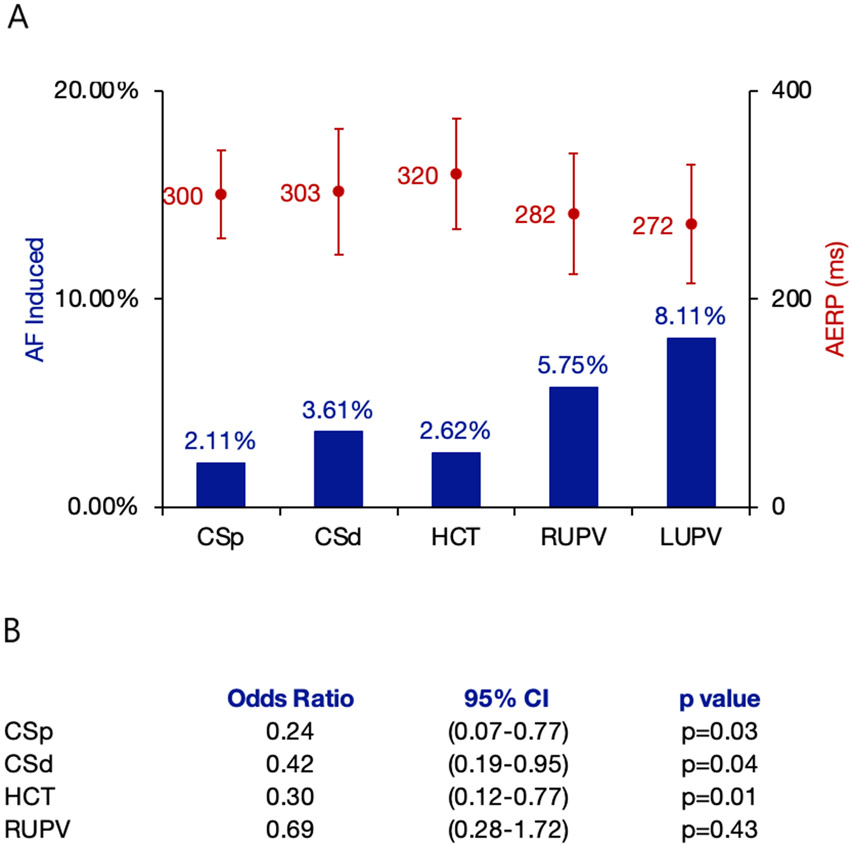
Most AF events were initiated by pulmonary vein PACs (Figure Panel A). After multivariable adjustment, PV PACs were significantly more likely to induce AF than other PACs (OR 2.62, 95% CI 1.37-5.00, p=0.004). No meaningful differences were observed in the sensitivity analyses.
Figure:
A. Proportion of Induced Atrial Fibrillation and Atrial Effective Refractory Periods by Premature Atrial Contraction Location. B. Odds of Induced Atrial Fibrillation Compared to the Left Upper Pulmonary Vein
Blue bars indicate the percentage of induced atrial fibrillation with respect to total premature atrial contractions across all participants administered from each site. Red circles indicate the mean AERP (ms), and the red Y-error bars indicate standard deviations.
CSp denotes proximal coronary sinus; CSd denotes distal coronary sinus; HCT denotes high crista terminalis; RUPV and LUPV denote right and left upper pulmonary veins, respectively.
The LUPV was statistically significantly more likely to induce AF compared to each non-PV site, but not significantly different compared to the RUPV (Figure Panel B).
The mean PAC coupling interval, defined by the AERP at each site, was 276 ± 57 ms in the PVs compared to 307 ± 53 ms for non-PV sites. A statistically significant 65% of the greater proportion of AF inducibility from PV PACs was mediated by shorter delivered PAC coupling intervals (reflecting lower PV AERPs) compared to other sites (95% CI 10%-121%, p=0.021).
In a prospective and uniformly administered protocol, PV PACs were significantly more likely to induce AF compared to other sites in the left and right atria. The majority of this relationship was statistically explained by shorter AERPs in the PVs. There are several potential explanations for these findings. Influences of nearby ganglionic plexi may promote early afterdepolarizations and triggered activity in the pulmonary veins, contributing to AF initiation.5 The shorter-coupled PACs allowed by the shorter PV AERPs may have encountered more heterogenous atrial refractoriness, fostering the development of functional, partially reentrant circuits.
It is important to acknowledge several limitations. We cannot comment on relative arrhythmogenicity of different PAC sites given the same coupling interval. The findings may be because the PVs were tested last, but, given the step-up approach employed, it would appear unlikely that the atria were adequately pre-conditioned to fibrillate. Patients were under general anesthesia, but a uniformly administered protocol to minimize electrophysiologic effects was used.
PV PACs were significantly more likely to induce AF compared to PACs from other atrial sites, a relationship mediated by lower PV AERPs. These observations may sharpen the focus on the PAC type most useful for AF risk stratification and future investigations into AF prevention.
Sources of Funding:
This research was supported by R01AA022222 from the NIH and the NIAAA.
Nonstandard Abbreviations and Acronyms:
- AERP
atrial effective refractory period
- AF
atrial fibrillation
- CS
coronary sinus
- HOLIDAY
how alcohol induces atrial arrhythmias
- LU
left upper
- PAC
premature atrial contraction
- PV
pulmonary vein
- RU
right upper
Footnotes
Disclosures: Dr. Marcus is a consultant for Johnson and Johnson and InCarda, and holds equity in InCarda.
References:
- 1.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- 2.Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodenia N, Gottdiener JS, Marcus GM. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med. 2013;159:721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus GM, Dukes JW, Vittinghoff E, Nah G, Badhwar N, Moss JD, Lee RJ, Lee BK, Tseng ZH, Walters TE, et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Intravenous Alcohol to Assess Changes in Atrial Electrophysiology. JACC Clin Electrophysiol. 2021;7:662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med. 1997;16:1515–27. [DOI] [PubMed] [Google Scholar]
- 5.Stavrakis S, Nakagawa H, Po SS, Scherlag BJ, Lazzara R, Jackman WM. The role of the autonomic ganglia in atrial fibrillation. JACC Clin Electrophysiol. 2015;1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]