Abstract
Objective:
To examine the association of anti-Mullerian hormone (AMH) with indicators of concurrent and prospective measures of adiposity over ~9 years of follow-up.
Methods:
Participants were 697 parous women from the Project Viva pre-birth cohort without polycystic ovarian syndrome. We measured AMH at ~3 years postpartum (baseline). Outcomes were weight, body mass index (BMI), and waist circumference (WC) assessed at baseline, 4, and 9 years later; % body fat was assessed by bioimpedance at the 4- and 9-year visit. We used linear mixed-effect models including all outcome time-points and accounting for age across follow-up, hormonal contraception prescription, and in an additional model, we further adjusted for height.
Results:
Median AMH was 1.97 ng/ml (IQR 0.83, 4.36), 29.1% had AMH <1.0 ng/ml, and mean age at AMH measurement was 36.7 years (SD 4.9; range 20–48). AMH was inversely associated with average weight, BMI, and WC over follow-up. In age adjusted models, women with AMH <1.0 vs. ≥1.0 ng/ml were 4.92 kg (95% CI 2.01 to 7.82) heavier, had a 2.51 cm (95% CI 0.12 to 4.89) greater WC, and a 1.46 kg/m2 (95% CI 0.44 to 2.48) greater BMI across the 9 years of follow-up. Findings were similar after covariate adjustment and when AMH was modeled continuously. AMH was also inversely associated with higher fat mass %, however the CIs crossed the null.
Conclusion:
Low AMH at baseline was associated with greater adiposity concurrently and across ~ 9 years of follow-up. Whether low AMH is a useful marker of metabolic risk across mid-life requires further research.
Keywords: anti-Mullerian hormone (AMH), obesity, weight, body mass index, perimenopause
Introduction
Markers of fertility and ovarian reserve have been associated with cardiometabolic risk in females1,2. Decline in estradiol, which is primarily produced in the ovaries, has been associated with the development of obesity-related disease via its role in mediating cardiomyocyte function and energy homeostasis. However, as a marker of ovarian reserve, Anti-Mullerian hormone (AMH) which is predominantly produced by the ovarian granulosa cell and is partially regulated by adipoinsular axis hormones (e.g., insulin, leptin)3–9, is used more often in clinical fertility settings over other markers10. The preferred use of AMH is because it is less affected by menstrual cycle phase than to other circulating markers such as follicle stimulating hormone or estradiol11, and less prone to the types of measurement error that impact ultrasonographic assessment of antral follicle count12. At the ovary, AMH is thought to primarily act to downregulate folliculogensis, as well as mediating preantral follicular recruitment and dominant follicle selection12.
The role of circulating systemic AMH is less clear; however, given that it is a valid proxy of overall ovarian reserve and thus reflecting the production of ovarian endocrine hormones, AMH may also serve as a marker for future obesity-related cardiometabolic disease risk across midlife. Distilling the relationship between AMH levels and adiposity is challenging as increasing age is linked to both a lower ovarian follicular pool and thus lower AMH, as well as a predisposition toward weight gain and changes in body composition3. Therefore, in the absence of robust direct measures of the ovarian follicular pool, careful consideration of the age range of the study population is important when interpreting the relationship between AMH and body composition. Further, current understanding of the directionality of the association between AMH and adiposity is hampered by the cross-sectional design of most published papers on this topic, and indirect measures of adiposity such as BMI. For instance, two studies have reported a positive correlation of AMH and BMI among younger women of normal weight13,14, whereas studies among women with a greater age range and higher prevalence of obesity have reported either an inverse association with BMI15,16 or no association with BMI17–19 when assessed cross-sectionally. Longitudinal studies that capture the prospective relationship between AMH levels and subsequent repeated assessment of adiposity across middle adulthood are limited. In one longitudinal study of 1,015 Iranian women aged 20 to 50 years at enrollment, there were no differences in baseline BMI across AMH levels, and no difference in the trajectory of adiposity over 16 years of follow-up between the lowest versus highest age-adjusted AMH levels20.
In the current study, we aimed to describe the association of AMH levels and adiposity by modeling both concurrent associations and differences in adiposity indicators over time. To achieve these objectives, we leveraged longitudinal measures of adiposity from a large cohort of parous women in the Project Viva study with baseline AMH measured at approximately 3 years postpartum, with follow-up over an average of 9 years.
Methods
Study population
This study used data from women participating in Project Viva, an ongoing prospective cohort of women and their children. Details on recruitment and eligibility are described elsewhere21. Briefly, Project Viva was initially established to examine associations of prenatal nutrition with maternal and child outcomes. Subsequent a priori funded grant aims focused on other questions including the association of infertility markers with women’s health long-term, the topic of this analysis. Project Viva recruited pregnant women at Atrius Harvard Vanguard Medical Associates, a multispecialty group practice in eastern Massachusetts, between 1999 and 2002 at ~10 weeks gestation. Following delivery, women returned for follow-up visits at 3 years postpartum (considered the baseline visit for the present analysis), and subsequently 4 and 9 years later. At baseline, women provided a blood sample. We excluded from the current analysis women without blood collection (n=1081), women without AMH measured (n=228), and women who were pregnant at blood collection (n=53) (Figure 1). Given that polycystic ovarian syndrome (PCOS) is related to higher AMH, faster AMH decline, as well as greater BMI22,23, we also excluded women with PCOS (n=41). We based PCOS categorization on 3 sources, 1) prenatal medical records from study enrollment, 2) self-report at the 9-year visit, or 3) irregular menstrual cycle with a baseline free testosterone that exceeded the 90th percentile of regular menstruating women (1.824 pg/mL). Testosterone was measured using an immunoassay from Roche Diagnostics, Indianapolis, IN USA). The analytic sample thus included 697 women. We excluded data on adiposity parameters at a the 4 and 9 year visits the woman reported being pregnant at the time of the visit. Women who were included (n=697) vs. excluded (n=1403) from the present analysis were on average one year older, had higher educational attainment, and were more likely to be White. All participants provided written informed consent at enrollment and at each study visit. The institutional review board of Harvard Pilgrim Health Care approved all study protocols.
Figure 1: Participant flow chart.
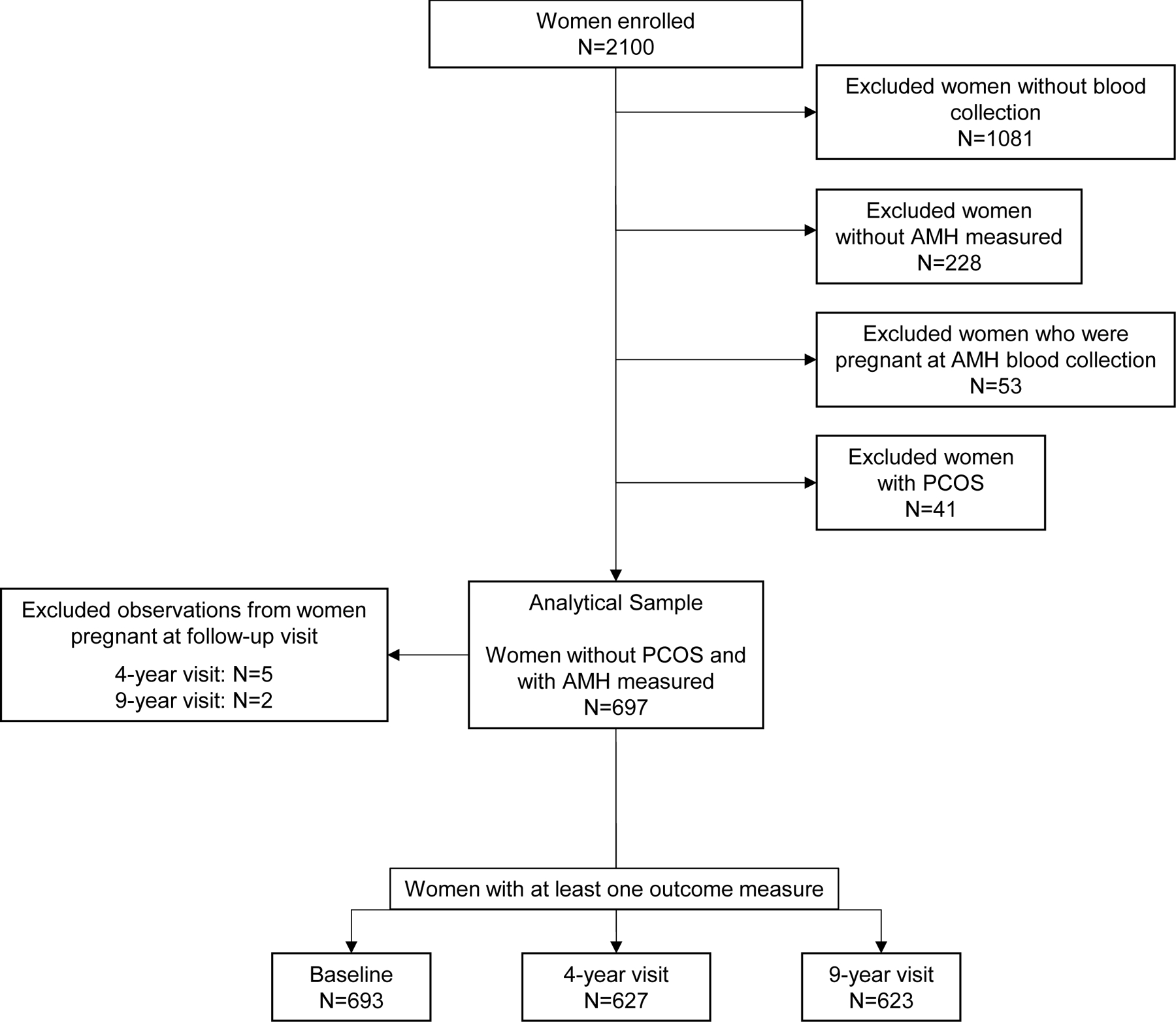
Pregnant women were enrolled in project viva between 1999–2002. After delivery women completed three in-person follow-up visits: baseline (3 years postpartum), and 4, and 9 years after baseline.
Abbreviations: AMH, anti-Mullerian hormone; PCOS, Polycystic ovarian syndrome
AMH assessment
Women’s blood was collected at baseline, and we measured plasma AMH using an ultra-sensitive ELISA assay from ANSH Labs (Webster, TX, USA).
Adiposity indicators
Trained research assistants measured weight, height, and waist circumference at baseline, and at the 4- and 9-year follow-up visits. We calculated body BMI as weight (kg)/ [height (m)]2. Research assistants also measured body fat in kg and as a % of total body weight via bioimpedance (Tanita scale model TBF-300A, Tanita Corporation of America, Arlington Heights, IL USA) at the 4- and 9-year visits.
Covariates
At study enrollment, women reported their age, race/ethnicity, education level, marital status, annual household income, and history of type 1 or type 2 diabetes via interview and self-administered questionnaire. At this time, women also reported on lifestyle behaviors including smoking habits (former, current, or never). We abstracted from women’s outpatient medical records the dosing, duration, and refill date of hormonal contraceptives and derived a variable to indicate whether women had an active hormonal contraceptive prescription within 3 months of AMH measurement.
Statistical analysis
Preliminary analyses
Prior to formal analysis, we examined univariate distributions of AMH as well as bivariate associations of AMH in relation to background characteristics of the participants. Because AMH is an age-dependent biomarker that is inversely associated with age, we age-adjusted AMH levels. To do this we used linear regressions centered on mean participant age and then used this adjusted variable in bivariate analyses to identify covariates for the main analysis (AMH and adiposity across follow-up) while controlling for extraneous variation by age at AMH assessment.
To assess for a non-linear relationship between concurrent measures of AMH, age, and the four adiposity measures at baseline, we tested the significance (p-value<0.05) of linear, quadratic, and cubic age terms. With this approach, we identified significant linear and quadratic terms for age and therefore, included them in all models. We also assessed for a nonlinear relationship between continuous AMH and repeated adiposity measures across follow-up using restricted cubic splines and adjusting for a linear and quadratic term for age at AMH measurement, as well as longitudinal age at the 4- and 9-year visit. These age terms (age and age2 at time of AMH measurement, longitudinal age at the 4- and 9-year visit) are referred to as Model 1 covariates from here on forth. We tested 21 different spline values using an automatic selection process and specifying p-value=0.05 for retaining the spline value in the model. No spline variables were selected (test for curvature p-value=0.53), therefore we assessed continuous AMH as a linear term in regression analyses. We described the association of AMH with adiposity at baseline and each follow-up visit by plotting the least square mean difference and 95% CI of each adiposity indicators by AMH status (<1.0 vs. ≥1.0 ng/ml) while adjusting for Model 1 covariates.
AMH levels and adiposity across follow-up
For the main analysis, we considered AMH continuously and dichotomously as <1 vs. ≥1 ng/ml given that this cutoff has been associated with women’s reproductive success and as such may have biological relevance24. We used separate unadjusted, and covariate adjusted, multivariable mixed-effects linear regression models to assess the association of AMH with repeated measures of the four adiposity indicators of interest across follow-up (3 repeated assessments of weight, waist circumference, BMI; and 2 assessments of % fat mass). In these models, the explanatory variables included AMH (continuous or dichotomized), a random slope and intercept for each woman, an unstructured correlation matrix, and covariates. Estimates from these models can be interpreted as the average difference in the adiposity indicator outcome across follow-up for each 1 ng/ml increase in AMH, or for AMH <1 vs. ≥ 1 ng/ml as the referent. We tested for an interaction between AMH and age (linear term only) at each visit as an indicator for time to determine whether outcome trajectories (i.e., the rate of change in adiposity indicators across the follow-up) differed by AMH status. The interaction term was not significant; therefore, we did not include it in the final mixed-effects models.
We also modeled the association of AMH with repeated adiposity parameters measured at the 4- and 9-year visits while adjusting for the baseline value of that parameter as a covariate. We used this approach rather than adjusting for baseline adiposity and modeling outcomes at all 3 visits to avoid introducing bias into the estimate of association between an exposure and repeated measures of an outcome over time 25,26. Estimates from these models can be interpreted as the difference in average adiposity across 4 to 9 years of follow-up with respect to baseline AMH levels that is not explained by differences in adiposity at the time of AMH assessment.
In covariate adjusted analyses, we considered three sequential models. Model 1 included only age terms. In Model 2, we further included hormonal contraceptive prescription given that this variable was associated with AMH levels and hormonal contraceptive use is associated with slightly lower AMH27. Finally, given that a major determinant of between-person variation in weight is height, we included height in Model 3. We used a complete case analysis given that at least 95% of the participants had complete data on all outcomes and covariates. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all other analyses.
Sensitivity analyses
To assess the robustness of our findings we conducted a subgroup analysis excluding women with type 1 or type 2 diabetes due to the interrelationships among AMH secretion, the adipoinsular axis, and adiposity.
Results
The median (range) of AMH was 1.97 ng/ml (0.01, 25.48) and the mean (standard deviation) age at AMH measurement was 36.7 years (4.96). At baseline, 18% of women had obesity (BMI ≥ 30.0 kg/m2), and approximately half of the women (53%) had a normal BMI (18.5-<25.0 kg/m2). AMH levels were inversely associated with age (Table 1). Age-adjusted AMH levels did not differ by race/ethnicity, education, history of smoking, or diabetes. Women with an active hormonal contraceptive prescription had lower levels of AMH ng/ml (mean ± SE) (2.81 ± 0.20 vs. 3.09 ± 0.44).
Table 1:
Bivariate associations of Anti-Müllerian hormone (AMH) with baseline characteristics of 697 women in Project Viva
| Crude AMH (ng/ml) | Full sample | ||
|---|---|---|---|
| Characteristic | Median (range) | Pd | N (%) |
| AMH ng/ml | 1.97 (0.01, 25.48) | - | 697 |
| Age at blood draw, years | |||
| <25 | 4.68 (1.15 to 9.90) | <.001 | 18 (2.6) |
| 25–29 | 5.36 (0.42 to 11.58) | 42 (6.0) | |
| 30–34 | 2.77 (0.18 to 19.64) | 175 (25.1) | |
| 35–39 | 1.97 (0.01 to 25.48) | 274 (39.3) | |
| ≥40 | 0.91 (0.01 to 11.85) | 188 (27.0) | |
| Age-adjusted AMH (ng/ml) | |||
| Mean ± SE | |||
| Race/ethnicity | |||
| Asian | 2.79 ± 0.12 | 0.44 | 30 (4.3) |
| Black | 2.90 ± 0.52 | 91 (13.1) | |
| Hispanic | 3.80 ± 0.33 | 40 (5.7) | |
| Other a | 2.79 ± 0.46 | 28 (4.0) | |
| White | 2.99 ± 0.12 | 508 (72.9) | |
| Education status b | |||
| High School/GED or less | 2.25 ± 0.98 | 0.35 | 40 (5.7) |
| Some college/ assoc. degree | 3.24 ± 0.55 | 144 (20.7) | |
| 4 years of college | 2.84 ± 0.30 | 266 (38.2) | |
| Graduate degree | 3.16 ± 0.18 | 247 (35.4) | |
| Annual Household Income b | |||
| <$70,000 | 2.82 ± 0.14 | 0.20 | 227 (35.6) |
| ≥$70,000 | 3.13 ± 0.14 | 424 (60.8) | |
| Active hormonal birth-control prescription at blood draw | |||
| No | 3.09 ± 0.44 | <.001 | 504 (72.3) |
| Yes | 2.81 ± 0.20 | 193 (27.7) | |
| History of smoking b | |||
| No | 3.03 ± 0.43 | 0.79 | 497 (71.3) |
| Yes | 2.97 ± 0.20 | 198 (28.4) | |
| History of diabetes b, c | |||
| No | 3.01 ± 2.50 | 0.77 | 692 (99.3) |
| Yes | 2.64 ± 1.25 | 5 (0.7) |
Abbreviations: Anti-mullerian hormone (AMH), general education development (GED), associate (assoc.)
includes 27 participants with >1 race/ethnicity and 1 person who indicated race/ethnicity as “other”.
assessed at enrollment.
Type 1 diabetes: n=3, Type 2 diabetes: n=2.
For categories of age, education, and pre-pregnancy BMI the p-value represents a test for linear trend.
Association of age-adjusted AMH and adiposity indicators at baseline and each follow-up visit
Low AMH (< 1.0 vs. ≥ 1.0 ng/ml) was associated with greater weight and BMI at baseline, the 4-year, and 9-year visits (Figure 2), adjusting for age at baseline and follow-up. For instance, women with AMH < 1.0 ng/ml were 5.07 kg (95% CI: 2.06 to 8.09) heavier and had 2.83 cm (95% C1: 0.05 to 5.60) larger waist circumference and 1.33 kg/m2 (95% CI: 0.18 to 2.48) higher BMI at the 9-year visit. AMH < 1 ng/ml was minimally related to greater fat mass % at the 4-year visit.
Figure 2: Least square mean differences and 95% confidence intervals for each adiposity indicator with respect to AMH < 1 vs. ≥ 1 ng/ml across follow-up (N=697).
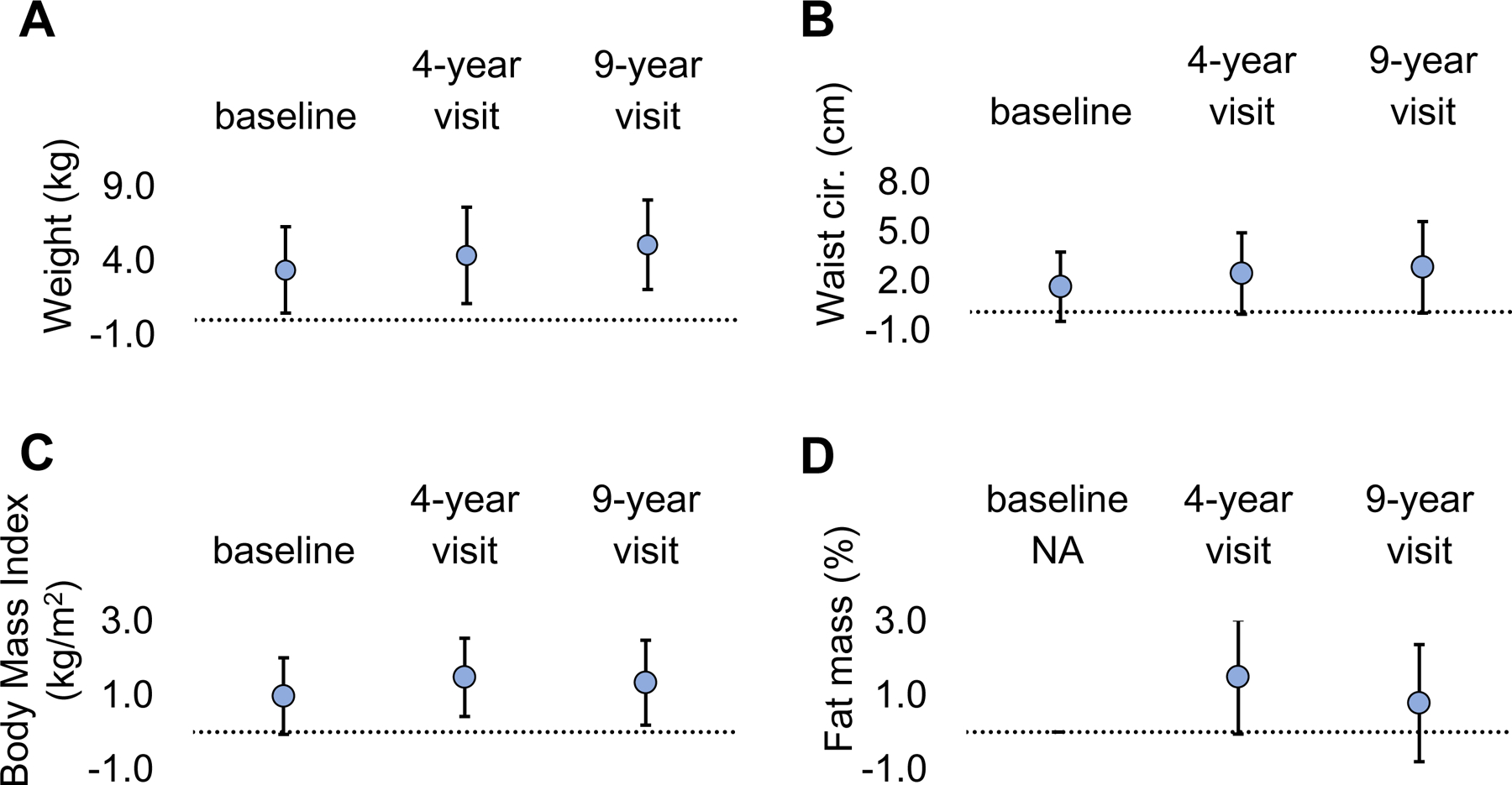
Abbreviations: AMH, anti-Mullerian hormone; BMI, body mass index; NA, not applicable
P-values = Type 3 tests for main effects derived from generalized linear models adjusted for age + age2.
(A) Association of AMH < 1 vs. ≥ 1 ng/ml at baseline with weight (kg) at baseline, 4, and 9 years later. At each timepoint, AMH < 1ng/ml was associated with greater weight compared to AMH ≥ 1ng/ml
(B) Association of AMH < 1 vs. ≥ 1 ng/ml baseline with waist cir. (cm) at baseline, 4, and 9 years later.
(C) Association of AMH < 1 vs. ≥ 1 ng/ml baseline with BMI (kg/m2) at baseline, 4, and 9 years later. At the 4-year and 9-year visits, AMH < 1ng/ml was associated with higher BMI compared to AMH ≥ 1ng/ml
(D) Association of AMH < 1 vs. ≥ 1 ng/ml at baseline with fat mass % at 4 and 9 years later. Fat mass % was measured only at the 4-year and 9-year visits.
Longitudinal association of AMH levels and average adiposity across follow-up
Over nine years of follow-up, each 1ng/ml decrement in AMH was associated with higher weight and waist circumference in unadjusted analysis (Table 2). Prior to adjusting for age, AMH was not associated with BMI, likely because of extraneous variation in the relationship due to age, as indicated by the fact that after age-adjustment, the estimates of association were of greater magnitude and more precise. For instance, in age-adjusted models, women with AMH < 1.0 ng/ml weighed 4.92 kg (95% CI: 2.01 to 7.82) more, had 2.51 cm greater waist circumference (95% CI: 0.12 to 4.89), and 1.46 kg/m2 greater BMI (95% CI: 0.44 to 2.48) than those with AMH ≥1.0 ng/ml (Table 2). These estimates were minimally changed following additional adjustment for hormonal contraception prescription (Model 2), or adjustment for height (Model 3). AMH both continuously and dichotomously were inversely associated with higher fat mass %, however the confidence intervals crossed the null.
Table 2:
Longitudinal association of Anti-Mullerian Hormone (AMH) at baseline with adiposity indicators across follow-up (N=697)
| Model 0 | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| Per 1 ng/ml decrement in AMH | ||||
| Weight (kg) | 0.41 (0.01 to 0.82) | 0.67 (0.22 to 1.12) | 0.66 (0.21 to 1.11) | 0.59 (0.16 to 1.02) |
| Waist circumference (cm) | 0.25 (−0.05 to 0.56) | 0.46 (0.09 to 0.82) | 0.45 (0.08 to 0.82) | 0.42 (0.06 to 0.79) |
| Body mass index (kg/m2) | 0.12 (−0.02 to 0.26) | 0.21 (0.05 to 0.37) | 0.21 (0.05 to 0.37) | 0.21 (0.05 to 0.37) |
| Fat mass % a | 0.13 (−0.08 to 0.34) | 0.21 (−0.02 to 0.43) | 0.20 (−0.02 to 0.43) | 0.18 (−0.04 to 0.41) |
| AMH < 1 vs. ≥ 1 ng/ml | ||||
| Weight (kg) | 2.81 (0.14 to 5.48) | 4.92 (2.01 to 7.82) | 4.79 (1.84 to 7.73) | 4.13 (1.32 to 6.93) |
| Waist circumference (cm) | 1.04 (−0.95 to 3.04) | 2.51 (0.12 to 4.89) | 2.36 (−0.05 to 4.77) | 2.08 (−0.31 to 4.47) |
| Body mass index (kg/m2) | 0.73 (−0.21 to 1.66) | 1.46 (0.44 to 2.48) | 1.43 (0.40 to 2.46) | 1.48 (0.45 to 2.52) |
| Fat mass % a | 0.82 (−0.56 to 2.20) | 1.21 (−0.25 to 2.67) | 1.17 (−0.30 to 2.65) | 0.99 (−0.46 to 2.44) |
Follow-up measures of adiposity indicators were concurrently at baseline, 4, and 9 years later
Abbreviations: AMH, anti-Mullerian hormone
Model 0: Unadjusted
Model 1: Age and age2 at time of AMH measurement, and longitudinal age at the 4-year and 9-year visits
Model 2: Model 1 + hormonal contraceptive prescription at study enrollment.
Model 3: Model 2 + height
Assessed at 4 and 9 years only.
After accounting for differences in baseline adiposity, the estimates of association of AMH and the adiposity indicators across the 4- and 9-year visits were reduced by ~77% and no longer statistically significant (see Table, Supplemental Digital Content 1).
Sensitivity analyses
Exclusion of women with diabetes at enrollment (n=5) did not change the results.
Discussion
In this large prospective study of 697 women aged ~35 years at baseline, AMH <1.0 vs. ≥1 ng/ml was associated with greater weight, waist circumference, and BMI measured concurrently as well as across an average of 9 years of follow-up. These results suggest that lower AMH levels are associated with greater adiposity indicators across mid-life, which is a transitional life stage when many women gain weight and risk of many obesity-related chronic diseases emerge28, and therefore, a time when body composition may be especially important.
Previous studies on the relationship between AMH and adiposity have yielded conflicting findings13,14,18,19. Discrepancies in the literature are due, in part, to the predominantly cross-sectional designs, as well as variation in multiple study sample characteristics related to the ovarian follicular pool, such as age, menstrual cycle, population-level obesity prevalence, and the adipoinsular axis which regulates weight and metabolism. Therefore, in the sections that follow we discuss prior findings in the context of these factors and the characteristics of the study sample.
Similar to the direction of association in our study, low AMH (e.g., lowest AMH tertile <1.47 ng/ml) has been associated with current obesity status in two cross-sectional studies in which participants had mean ages ≥35 years and >28% of the sample had obesity15,16. In another study among younger women aged 23–34 years, with >80% prevalence of overweight/obesity and median AMH of 3.2 ng/ml, continuous AMH was inversely associated with current BMI29. Additionally, the women were asked to recall their weight at 18 years of age, and it was found that AMH was significantly lower among women with obesity at both 18 years and at study enrollment compared to women who developed obesity later on29. The finding that AMH was lower among women who maintained obesity as they approached prime reproductive age, in conjunction with our finding that lower AMH levels was associated with greater adiposity across mid-life, indicate that lower AMH levels are an important characteristic of sustained excess adiposity.
On the other hand, in two studies where women had a mean AMH level of 2.4 ng/ml, AMH was positively correlated with BMI only in subgroup analyses of younger women with a BMI in the normal range13,14. Three studies have reported no difference in AMH levels across BMI strata13,18,19. These three studies were conducted among women with a range of ages (mean ages 24 – 46) and a range of prevalence of BMI ≥30 kg/m2 (14%−51%). In these prior studies, the positive association or the lack of difference in AMH levels across BMI strata may be due to how age was accounted for (e.g., stratification or lack of statistical adjustment). Indeed, in the current analysis we found that prior to adjusting for age, AMH was not associated with BMI, likely because of extraneous variation in the relationship due to age, as indicated by the fact that after age-adjustment, the estimates of association were of greater magnitude and more precise. This latter point highlights the importance of accounting for age and potentially a non-linear relationship of age and AMH when examining BMI related outcomes..
Of note, when we examined the association of AMH with adiposity across the 4-year and 9-year visits while adjusting for adiposity at baseline, the magnitude of the association estimate of AMH and adiposity over follow-up was reduced. This was expected given that the inverse relationship between AMH and adiposity was apparent at baseline. This finding is similar to those from a large longitudinal study of 1,015 Iranian women, wherein the authors found that low AMH was not associated with adiposity over 16 years of follow-up after adjusting for baseline BMI 20. These findings suggest that AMH is likely to be a marker of ongoing processes related to higher adiposity sustained throughout midlife, rather than a causal agent of subsequent adiposity gain.
In considering the biological context of our findings, we acknowledge that the consequences of low AMH and adiposity are inextricably linked with the adipoinsular axis as well as ovarian hormones. Low AMH and sustained higher weight as women age may reflect declines in ovarian hormone production, and the alterations of glucose metabolism that is a typical result of aging and changes in adipose deposition30,31. AMH synthesis and secretion are partially regulated by levels of insulin, leptin, and adiponectin4–9, all of which are interconnected with increased adiposity32. In humans, higher levels of serum leptin have been associated with lower AMH29. Although leptin directly impacts the efficiency of glucose metabolism, paradoxically in obese states, higher circulating concentrations of leptin and reductions in the soluble leptin receptor are observed and thought to represent leptin resistance, which has been analogized to insulin resistance 33,34. Of particular interest to future studies may be how AMH production, and specifically reductions in AMH, are impacted by chronically high circulating levels of leptin, which may reflect a longer period of sustained elevations in weight in a woman’s life. Beyond the role of the adipoinsular axis in maintaining energy balance, estrogens have been implicated in regulating energy homeostasis via their action on the hypothalamus, with higher levels of estrogens related to a protective impact on the development of metabolic complications35,36. Thus, the association of lower AMH and greater weight across mid-life may reflect the crosstalk between the reproductive and adipoinsular axis and serve as a marker of ovarian endocrine aging37,38.
Strengths and limitations
A primary strength of the current study was our ability to examine prospective associations of AMH with precisely measured adiposity across mid-life, a time when substantial changes in weight and women’s lifestyle can occur. This unique data enabled us to capture associations that persist over nearly a decade, as opposed to those that may be transient and/or affected by temporary lifestyle factors such as the adoption of less healthy dietary choices. In addition, we had access to rich covariate data that allowed us to examine subgroup analyses, such as those among women without a prescription for hormonal contraception that may introduce extraneous variability into the association of interest.
There are a few limitations of the current study that are important to consider. First, we did not have a direct measure of follicle reserve such as antral follicle count. Regardless, AMH is a valid biomarker of follicle count11,12 and is particularly useful in large epidemiologic studies for which regression analyses capitalize on ranking of individuals within a population rather than absolute accuracy. Second, we excluded women with PCOS, which we defined as having an irregular menstrual cycle lengths and free testosterone >1.82 pg/ml, these criteria may be imperfect proxies for PCOS and therefore we may have excluded some women without PCOS but who have irregular menstrual cycles. Third, the results reported herein are likely only directly generalizable to women with AMH measured while still regularly menstruating, to women of a similar age demographic, and whom have a comparable prevalence of obesity. We emphasize this latter point as prior findings appear to be dependent on the underlying characteristics of the study sample. Finally, we note that since all participants were women recruited during pregnancy, our findings may be less be generalizable to women with low fertility who never achieve a pregnancy.
Conclusions
Our study adds to the limited prior prospective data by providing a comprehensive report of the cross-sectional, prospective, and longitudinal association of AMH and several measures of body composition. We found that low levels of AMH (<1.0 ng/ml) were associated with higher average weight, waist circumference, and BMI across nearly a decade of follow-up. Therefore, low AMH may serve as a flag for women who maintain a higher average adiposity over a transitional period during which many chronic metabolic diseases begin to manifest. The extent to which lower AMH is a useful marker of future development of adiposity and metabolic risk across mid-life requires further research, including studies starting earlier in the lifecourse.
Supplementary Material
Supplemental Digital Content 1 (Table) which shows the association of AMH and adiposity adjusted for baseline adiposity indicator
Sources of Funding:
R01 HD096032 NICHD/NIH, UH3 OD023286 OD/NIH, R01 HD034568 NICHD/NIH, Harvard Pilgrim Health Care Institute, KL2-TR002534/the Center for Clinical and Translational Sciences Institute, K99 HD108272 NICHD/NIH
Footnotes
Financial disclosures/Conflicts of interest: None reported.
References
- 1.Zhu D, Chung HF, Dobson AJ, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health. Nov 2019;4(11):e553–e564. doi: 10.1016/s2468-2667(19)30155-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brand JS, van der Schouw YT, Onland-Moret NC, et al. Age at menopause, reproductive life span, and type 2 diabetes risk: results from the EPIC-InterAct study. Diabetes Care. Apr 2013;36(4):1012–9. doi: 10.2337/dc12-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update May-Jun 2014;20(3):370–85. doi: 10.1093/humupd/dmt062 [DOI] [PubMed] [Google Scholar]
- 4.Su HI, Sammel MD, Freeman EW, Lin H, DeBlasis T, Gracia CR. Body size affects measures of ovarian reserve in late reproductive age women. Menopause Sep-Oct 2008;15(5):857–61. doi: 10.1097/gme.0b013e318165981e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nestler JE. Insulin regulation of human ovarian androgens. Human reproduction (Oxford, England) Oct 1997;12 Suppl 1:53–62. doi: 10.1093/humrep/12.suppl_1.53 [DOI] [PubMed] [Google Scholar]
- 6.Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Human Reproduction Update 2016;22(6):709–724. doi: 10.1093/humupd/dmw027 [DOI] [PubMed] [Google Scholar]
- 7.Merhi Z, Buyuk E, Berger DS, et al. Leptin suppresses anti-Mullerian hormone gene expression through the JAK2/STAT3 pathway in luteinized granulosa cells of women undergoing IVF. Human reproduction (Oxford, England) Jun 2013;28(6):1661–9. doi: 10.1093/humrep/det072 [DOI] [PubMed] [Google Scholar]
- 8.Merhi Z, Bazzi AA, Bonney EA, Buyuk E. Role of adiponectin in ovarian follicular development and ovarian reserve. Biomed Rep 2019;1(1):1–5. doi: 10.3892/br.2019.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chabrolle C, Tosca L, Ramé C, Lecomte P, Royère D, Dupont J. Adiponectin increases insulin-like growth factor I-induced progesterone and estradiol secretion in human granulosa cells. Fertility and sterility Dec 2009;92(6):1988–96. doi: 10.1016/j.fertnstert.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 10.Broer SL, van Disseldorp J, Broeze KA, et al. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update Jan-Feb 2013;19(1):26–36. doi: 10.1093/humupd/dms041 [DOI] [PubMed] [Google Scholar]
- 11.Toner JP, Seifer DB. Why we may abandon basal follicle-stimulating hormone testing: a sea change in determining ovarian reserve using antimüllerian hormone. Fertility and Sterility 2013;99(7):1825–1830. doi: 10.1016/j.fertnstert.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 12.Parry JP, Koch CA. Ovarian Reserve Testing. In: Feingold KR, Anawalt B, Boyce A, et al. , eds. Endotext MDText.com, Inc. Copyright © 2000–2022, MDText.com, Inc.; 2000. [Google Scholar]
- 13.Skałba P, Cygal A, Madej P, et al. Is the plasma anti-Müllerian hormone (AMH) level associated with body weight and metabolic, and hormonal disturbances in women with and without polycystic ovary syndrome? Eur J Obstet Gynecol Reprod Biol Oct 2011;158(2):254–9. doi: 10.1016/j.ejogrb.2011.06.006 [DOI] [PubMed] [Google Scholar]
- 14.Albu D, Albu A. The relationship between anti-Müllerian hormone serum level and body mass index in a large cohort of infertile patients. Endocrine Jan 2019;63(1):157–163. doi: 10.1007/s12020-018-1756-4 [DOI] [PubMed] [Google Scholar]
- 15.Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF, 3rd. Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertility and sterility Jan 2007;87(1):101–6. doi: 10.1016/j.fertnstert.2006.05.074 [DOI] [PubMed] [Google Scholar]
- 16.Bleil ME, Gregorich SE, McConnell D, Rosen MP, Cedars MI. Does accelerated reproductive aging underlie premenopausal risk for cardiovascular disease? Menopause Nov 2013;20(11):1139–46. doi: 10.1097/GME.0b013e31828950fa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, Kim JJ, Kim MJ, et al. Relationship between serum anti-Mullerian hormone with vitamin D and metabolic syndrome risk factors in late reproductive-age women. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology Apr 2018;34(4):327–331. doi: 10.1080/09513590.2017.1397113 [DOI] [PubMed] [Google Scholar]
- 18.Sahmay S, Usta T, Erel CT, et al. Is there any correlation between amh and obesity in premenopausal women? Arch Gynecol Obstet Sep 2012;286(3):661–5. doi: 10.1007/s00404-012-2363-x [DOI] [PubMed] [Google Scholar]
- 19.Halawaty S, ElKattan E, Azab H, ElGhamry N, Al-Inany H. Effect of obesity on parameters of ovarian reserve in premenopausal women. J Obstet Gynaecol Can Jul 2010;32(7):687–90. doi: 10.1016/s1701-2163(16)34573-x [DOI] [PubMed] [Google Scholar]
- 20.Amiri M, Ramezani Tehrani F, Rahmati M, Firouzi F, Azizi F. Do trends of adiposity and metabolic parameters vary in women with different ovarian reserve status? A population-based cohort study. Menopause Jun 2020;27(6):684–692. doi: 10.1097/gme.0000000000001513 [DOI] [PubMed] [Google Scholar]
- 21.Oken E, Baccarelli AA, Gold DR, et al. Cohort profile: project viva. Int J Epidemiol Feb 2015;44(1):37–48. doi: 10.1093/ije/dyu008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen MJ, Yang WS, Chen CL, Wu MY, Yang YS, Ho HN. The relationship between anti-Mullerian hormone, androgen and insulin resistance on the number of antral follicles in women with polycystic ovary syndrome. Human reproduction (Oxford, England) Apr 2008;23(4):952–7. doi: 10.1093/humrep/den015 [DOI] [PubMed] [Google Scholar]
- 23.Tehrani FR, Solaymani-Dodaran M, Hedayati M, Azizi F. Is polycystic ovary syndrome an exception for reproductive aging? Human reproduction (Oxford, England) Jul 2010;25(7):1775–81. doi: 10.1093/humrep/deq088 [DOI] [PubMed] [Google Scholar]
- 24.Gleicher N, Weghofer A, Barad DH. Anti-Mullerian hormone (AMH) defines, independent of age, low versus good live-birth chances in women with severely diminished ovarian reserve. Fertility and sterility 2010;94(7):2824–2827. doi: 10.1016/j.fertnstert.2010.04.067 [DOI] [PubMed] [Google Scholar]
- 25.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When Is Baseline Adjustment Useful in Analyses of Change? An Example with Education and Cognitive Change. American Journal of Epidemiology 2005;162(3):267–278. doi: 10.1093/aje/kwi187 [DOI] [PubMed] [Google Scholar]
- 26.Kramer MS, Zhang X, Bin Aris I, et al. Methodological challenges in studying the causal determinants of child growth. International Journal of Epidemiology 2016;45(6):2030–2037. doi: 10.1093/ije/dyw090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Briggs S, Hariton E, Shirazi T, Hershlag A. Hormonal Contraceptive use is Associated with Significantly Lower AMH Levels in Women of Reproductive Age. Fertility and sterility 2020;114(3):e172. doi: 10.1016/j.fertnstert.2020.08.490 [DOI] [Google Scholar]
- 28.Khoudary SRE, Aggarwal B, Beckie TM, et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation 2020;142(25):e506–e532. doi:doi: 10.1161/CIR.0000000000000912 [DOI] [PubMed] [Google Scholar]
- 29.Bernardi LA, Carnethon MR, de Chavez PJ, et al. Relationship between obesity and anti-Müllerian hormone in reproductive-aged African American women. Obesity (Silver Spring, Md) 2017;25(1):229–235. doi: 10.1002/oby.21681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morselli E, Santos RS, Criollo A, Nelson MD, Palmer BF, Clegg DJ. The effects of oestrogens and their receptors on cardiometabolic health. Nature Reviews Endocrinology 2017/06/01 2017;13(6):352–364. doi: 10.1038/nrendo.2017.12 [DOI] [PubMed] [Google Scholar]
- 31.Sasaki T Age-Associated Weight Gain, Leptin, and SIRT1: A Possible Role for Hypothalamic SIRT1 in the Prevention of Weight Gain and Aging through Modulation of Leptin Sensitivity. Review. Frontiers in Endocrinology 2015-July-16 2015;6doi: 10.3389/fendo.2015.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kieffer TJ, Habener JF. The adipoinsular axis: effects of leptin on pancreatic beta-cells. Am J Physiol Endocrinol Metab Jan 2000;278(1):E1–e14. doi: 10.1152/ajpendo.2000.278.1.E1 [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y, Rui L. Leptin signaling and leptin resistance. Frontiers of Medicine 2013/06/01 2013;7(2):207–222. doi: 10.1007/s11684-013-0263-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaab M, Kratzsch J. The soluble leptin receptor. Best Practice & Research Clinical Endocrinology & Metabolism 2015/10/01/ 2015;29(5):661–670. doi: 10.1016/j.beem.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Shi H. Regulation of Estrogen Receptor α Expression in the Hypothalamus by Sex Steroids: Implication in the Regulation of Energy Homeostasis. International Journal of Endocrinology 2015/09/27 2015;2015:949085. doi: 10.1155/2015/949085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roa-Díaz ZM, Raguindin PF, Bano A, Laine JE, Muka T, Glisic M. Menopause and cardiometabolic diseases: What we (don’t) know and why it matters. Maturitas 2021/10/01/ 2021;152:48–56. doi: 10.1016/j.maturitas.2021.06.013 [DOI] [PubMed] [Google Scholar]
- 37.Nestor CC, Kelly MJ, Rønnekleiv OK. Cross-talk between reproduction and energy homeostasis: central impact of estrogens, leptin and kisspeptin signaling. Horm Mol Biol Clin Investig Mar 2014;17(3):109–28. doi: 10.1515/hmbci-2013-0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CG, Carr MC, Murdoch SJ, et al. Adipokines, inflammation, and visceral adiposity across the menopausal transition: a prospective study. J Clin Endocrinol Metab Apr 2009;94(4):1104–10. doi: 10.1210/jc.2008-0701 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1 (Table) which shows the association of AMH and adiposity adjusted for baseline adiposity indicator


