Abstract
Purpose of Review
This review evaluates cow milk’s impact on breast carcinogenesis by linking recent epidemiological evidence and new insights into the molecular signaling of milk and its constituents in breast cancer (BCa) pathogenesis.
Recent Findings
Recent prospective cohort studies support the association between cow’s milk consumption and the risk of estrogen receptor-α-positive (ER+) BCa. Milk is a complex biological fluid that increases systemic insulin-like growth factor 1 (IGF-1), insulin and estrogen signaling, and interacting hormonal promoters of BCa. Further potential oncogenic components of commercial milk include exosomal microRNAs (miR-148a-3p, miR-21-5p), bovine meat and milk factors, aflatoxin M1, bisphenol A, pesticides, and micro- and nanoplastics. Individuals with BRCA1 loss-of-function mutations and FTO and IGF1 gain-of-function polymorphisms enhancing IGF-1/mTORC1 signaling may be at increased risk for milk-induced ER+ BCa.
Summary
Recent prospective epidemiological and pathobiochemical studies identify commercial milk consumption as a critical risk factor of ER+ BCa. Large meta-analyses gathering individuals of different ethnic origins with milk derived from dairy cows of varying genetic backgrounds and diverse feeding procedures as well as missing data on thermal processing of milk (pasteurization versus ultra-heat treatment) make multi-national meta-analyses unsuitable for BCa risk estimations in susceptible populations. Future studies are required that consider all vulnerable periods of breast carcinogenesis to cow’s milk exposure, beginning during the perinatal period and puberty, since these are the most critical periods of mammary gland morphogenesis. Notwithstanding the need for better studies including detailed information on milk processing and vulnerable periods of human breast carcinogenesis, the available evidence suggests that dietary guidelines on milk consumption may have to be reconsidered.
Keywords: BRCA1, Breast cancer, Cow’s milk consumption, Estrogens, Exosomal microRNAs, Fat mass and obesity-associated gene, Insulin-like growth factor 1
Introduction
In 2020, 2.3 million women were diagnosed with breast cancer (BCa) with 685,000 deaths globally. At the end of 2020, 7.8 million were diagnosed with BCa in the past 5 years, making BCa the world’s most prevalent cancer [1]. The estimated new total BCa cases in the United States (US) for 2022 are 287,750 for females and 2710 for males, respectively [2]. BCa prevalence is high in industrialized countries, where cow’s milk and dairy consumption are major dietary components. From 1947 to 1997, the age-standardized death rate of BCa in Japan increased about 2-fold, and the respective intake of milk increased 20-fold [3]. This review aims to interpret the latest epidemiology in context with recent insights into the molecular signaling of milk linking cow’s milk consumption and BCa risk.
Milk is not a simple dietary food, but a growth-promoting endocrine system enhancing the synthesis of insulin-like growth factor-1 (IGF-1) [4], which activates the nutrient- and growth factor-sensitive kinase mechanistic target of rapamycin complex 1 (mTORC1) [5]. Physiologically, milk signaling is restricted to the period of lactation in all mammals except Neolithic and modern humans, who may be persistently exposed to cow’s milk. And even in societies who adopted dairy early on, milk was preferentially consumed in fermented forms until the widespread implementation of pasteurization and refrigeration technology [6]. In industrialized countries, cow’s milk intake in non-fermented forms may be an exposure over lifetime, beginning with maternal cow’s milk consumption during pregnancy, continued by cow’s milk intake in infancy, childhood, adolescence, and pre- and postmenopausal life [7•], which, as it will be discussed below, may influence BCa risk, particularly during certain vulnerable periods involved in breast carcinogenesis.
Search Strategy and Selection Criteria
PubMed was searched for original research articles, retrospective and prospective cohort studies, case-control studies, and meta-analyses/systematic reviews conducted in humans over the last 5 years relating cow’s milk consumption to the risk of BCa in humans. Search terms included “cow milk,” “dairy,” “diet,” “milk,” “non-fermented milk,” “fermented milk,” “breast cancer,” “mammary tumor,” and “breast cancer risk.” Milk-related compounds like “insulin-like growth factor 1,” “estrogens,” “bovine meat and milk factors,” “aflatoxins,” “bisphenol A,” “pesticides,” “microplastics,” and “nanoplastics” were linked to known pathogenic pathways in breast carcinogenesis. Factors associated with BCa risk including “birthweight,” “menarche,” “body mass index” “juvenile myopia,” “acne vulgaris,” and “linear growth” were also considered.
Increased Fetal Growth and Birthweight
Humans are the only mammalian species consuming the milk of another mammal during pregnancy, recommended by health professionals and dietary guidelines because milk is a rich source of calcium and vitamin D, the latter in countries with vitamin D fortification schemes. Milk contains a moderate amount of protein composed of essential branched-chain amino acids (BCAAs) thought to have beneficial effects for nutrition during pregnancy. The Generation R Study, a population-based prospective cohort study from fetal life until young adulthood in Rotterdam, investigated 3405 mothers during pregnancy [8]. Maternal cow’s milk consumption of > 3 glasses (450 ml of milk) per day was associated with greater fetal weight gain in the third trimester of pregnancy [8]. Worldwide studies confirmed an increase in fetal growth and birthweight in relation to milk consumption during pregnancy [9, 10]. Compared to women who had a normal birthweight (2500–3999 g), women who weighed ≥ 4000 g at birth had a 20 percent to fivefold increased risk of premenopausal BCa [11]. Birthweight is positively associated with BCa risk [12] as well as mammographic density among postmenopausal and less among premenopausal women [13].
Cow’s milk consumption enhances growth hormone (GH) levels in children and peak GH levels in adults [14, 15], as well as circulating IGF-1 levels in children and adults [4, 14–18, 19••, 20•]. There are two mechanisms leading to milk-mediated elevations of circulatory IGF-1 levels of the milk recipient: (1) uncertain proportions of bovine milk IGF-1, which shares an identical amino acid sequence with human IGF-1 [21], may be absorbed in the human intestine. (2) milk components, especially milk protein-derived amino acids (Trp, Arg, Met), may induce the synthesis and secretion of pituitary GH and hepatic IGF-1 into the circulation [5, 7•].
Of note, the administration of IGF-1 to pregnant mice resulted in significantly heavier birth and postnatal bodyweights of the offspring when compared to untreated controls. Morphometric analyses revealed that a prenatal dose of 5 μg IGF-1 resulted in significantly longer ductal elongation and higher breast density in the offspring. Furthermore, 5 μg IGF-1 also resulted in the highest number of breast stem/progenitor cells in the offspring when compared to controls whose mothers were not treated with IGF-1 during pregnancy [22]. These findings provide evidence for a prenatal IGF-1-mediated modulation of breast stem cell composition and breast density in the offspring [22]. Thus, the GH/IGF-1 axis may play an important role in regulating breast stem cell numbers during a prenatal developmental window [23].
Breastfeeding Versus Artificial Formula Feeding
For infants and nursing women, breastfeeding provides protection against BCa [24–26]. An inverse association was found between an increase in body mass index (BMI) and the duration of exclusive breastfeeding (EXBF) among carriers of the risk allele of the fat mass and obesity-associated gene (FTO) rs9939609 [27]. EXBF antagonizes the FTO rs9939609 risk allele and by the age of 15 years, the predicted reduction in BMI after 5 months of EXBF was 0.56 kg/m2 (95% CI: 0.11–1.01; p = 0.003) and 1.14 kg/m2 (95% CI: 0.67–1.62; p < 0.0001) in boys and girls, respectively [28]. Compared to infants, who received EXBF, FTO levels in blood mononuclear cells of infants fed artificial formula were excessively overexpressed [29••]. Notably, infant formula is deficient in human milk exosomes and microRNAs (miRs) including miR-30b [30••], which targets and suppresses FTO expression [31]. FTO is an N6-methyladenosine (m6A) demethylase and participates in the epigenetic regulation of adipogenesis and tumorigenesis thus changing mRNA expression networks and through interaction with mTORC1 [32, 33]. Similar molecular mechanisms play a role in the development of obesity and BCa [34, 35•], which exhibit overexpression of FTO [36]. Thus, the nutrigenomic and epigenetic regulation of FTO during postnatal life appears to be a critical window for breast carcinogenesis.
Early-Life BMI, Menarche, and Thelarche
BMI is critically related to the initiation of puberty. In concordance with total and percentage body fat, all pubertal stages began earlier in females with BMI ≥ 85th percentile comparable to females with average BMI [37]. The National Health and Nutrition Examination Survey (NHANES) observed that among children 5–10 years of age, those in the highest quartile (Q-IV) for milk intake had higher BMIs than those in lower Q-II [38]. Of note, milk had more consistent positive associations with BMI than did any other dairy product [38]. Every 5 kg/m2 increase in early-life BMI was associated with an elevated risk of BCa in a recent dose–response meta-analysis [39]. A recent systematic review and meta-analysis of cross-sectional and prospective cohort studies assessed the associations between total dairy consumption and its different subtypes with the prevalence and incidence of overweight, obesity, and overweight/obesity in children and adolescents [40]. Regarding prospective studies, total milk consumption was positively associated with overweight prevalence (OR (95% CI): 1.13 (1.01–1.26)) and incidence (RR (95%CI): 1.17 (1.01–1.35)) risk [40].
The NHANES and the Tehran Lipid and Glucose Study reported an association between cow’s milk consumption and early onset of menarche [41, 42], a further recognized risk factor for BCa [43], which correlates with breast density [44]. When considering early thelarche (< 10 years) and early menarche (< 12 years) together, women with both had a 30% higher risk of BCa compared with women with neither risk factor [45]. IGF-1 plays a crucial role in hypothalamic-pituitary-ovarian hormone-controlled metabolic processes that influence the onset of menarche [46]. In fact, serum levels of IGF-1 increase with age and pubertal development [47]. Noticeably, a more frequent consumption of milk-based drinks, which may increase circulating IGF-1 levels [4], was associated with a higher percentage of fibroglandular volume (FGV) measured at Tanner stage 4, whereas higher yogurt intake was associated with a lower FGV and delayed age at menarche in Chilean girls [48]. Earlier age of menarche promoted by cow’s milk consumption but not fermented dairy products may thus enhance the susceptibility to breast carcinogenesis during prepuberty and puberty.
Longitudinal Growth During Puberty
There is a well-established relationship between cow’s milk consumption during childhood and linear growth-enhancing height [49–51]. The consumption of milk, but not other dairy products, was associated with height among US preschool children in the NHANES 1999–2002 [49], whereas cow’s milk consumption was a significant predictor of the height of 12–18-year old adolescents [50]. The mitogen IGF-1 is the major inducer of bone growth [51–54]. During puberty, circulating IGF-1 promotes bone periosteal apposition [53]. Girls with higher serum IGF-1 levels in childhood enter puberty earlier [54]. Pubertal timing is influenced by IGF-1 promoting longitudinal growth earlier in childhood [54].
Biro et al. [55••] demonstrated that peak height velocity (PHV) was greatest in early, and least in late-maturing girls. The length of the pubertal growth spurt was longest in early, and shortest in late-maturing girls. Earlier onset of menarche was related to greater PHV. IGF-1 concentrations were tracked significantly during puberty and higher IGF-1 was related to earlier age of PHV, earlier age of menarche, greater PHV, and taller adult height [55••].
Acne and Juvenile-Onset Myopia–Indicators of Excessive IGF-1 Signaling
A visible indicator disease of exaggerated IGF-1 signaling is acne vulgaris, the most common inflammatory skin disease in industrialized countries, which is associated with increased height and BMI during puberty [56–58]. The consumption of dairy foods, particularly milk, and high glycemic carbohydrates, a common dietary pattern seen in acne patients of Westernized populations [59], increases circulatory levels of IGF-1 and insulin [60–63]. These findings point to accelerated IGF-1-mediated growth trajectories in acne pathogenesis leading to the hyperproliferation of sebaceous glands promoted by milk consumption [64–66]. In contrast, individuals with Laron syndrome exhibiting severe congenital IGF-1 deficiency do not develop acne vulgaris and are of small stature [67]. They are protected from common cancers including BCa [68]. Remarkably, the Sister Study recently showed that ever being diagnosed with severe acne before the age of 18 years was associated with a higher risk of BCa [69•].
Juvenile-onset myopia caused by increased vitreal chamber growth is another IGF-1-induced condition also related to increased height and BMI during adolescence [70]. According to a cross-sectional study of children 6–12 years in China, breastfeeding was associated with a decreased risk of myopia [71].
IGF-1 and Pubertal Mammary Gland Morphogenesis
Mammary development occurs almost entirely during puberty [72]. IGF-1 activates the proliferation of the ductal tree of the mammary gland [72]. IGF-1 is considered to be central to the process of ductal morphogenesis because neither estradiol (E2) nor progesterone (P) can act in the absence of IGF-1 [72]. Formation of the ductal tree is orchestrated by a specialized structure called the terminal end bud (TEB), which is responsible for the production of mature cell types leading to the elongation of the subtending duct. The TEB is also the regulatory control point for basement membrane deposition, branching, angiogenesis, and pattern formation [73]. It has been demonstrated in murine models that the earliest phase of pubertal mammary development (formation of TEBs) requires IGF-1. No other hormones have been shown to stimulate the formation of TEBs unless GH or IGF-1 is present. GH-induced IGF-1 is thus of major importance in ductal morphogenesis [74, 75]. It has been shown in the prepubertal mammary glands of BK5.IGF-1 transgenic (Tg) mice that IGF-1 preferentially activated the PI3K/AKT pathway via the formation of ERα/insulin receptor substrate 1 (IRS-1) complex [76]. Conversely, in postpubertal Tg glands, reduced ERα expression failed to stimulate the formation of the ERα/IRS-1 complex, allowing signaling to proceed via the alternate RAS/RAF/MAPK pathway [76]. Accordingly, changes in ERα expression at different stages of development direct IGF-1 signaling and the resulting tissue responses. As ERα levels are elevated during the prepubertal and postmenopausal stages, these may represent windows of susceptibility during which increased IGF-1 exposure maximally enhances BCa risk [76].
Elevations of plasma GH and IGF-1 concentrations by cow’s milk intake may thus enhance the physiological magnitude of circulating GH/IGF-1 during puberty deviating sebaceous gland, eye growth, and bone homeostasis (displaying acne, early-onset juvenile myopia, pubertal skeletal overgrowth) but indiscernibly disturbing mammary gland maturation and TEB formation potentially increasing the risk of BCa.
Epidemiological Evidence
Cow’s Milk Exposure in Pre- and Postmenopausal Women
There are controversial results with regard to the reported epidemiological study type, i.e., national cohort studies (retrospective versus prospective), case-control studies, and large multi-national meta-analyses including worldwide “umbrella” studies, concerning the outcomes of BCa risk among adult women consuming cow’s milk.
Cohort Studies
According to the prospective study of the Norwegian Cancer Registry (n = 25,892), daily intake of > 750 ml whole cow’s milk compared to < 150 ml daily enhanced the risk of BCa by a factor of 2.91 [77]. A retrospective hospital-based case-control study (n = 1857 cases and 1202 controls) in the US found a positive association between cow’s milk intake and the risk of ER-negative BCa (OR: 1.58; 95% CI: 1.05–2.37) [78]. Fraser et al. [79••] recently reported an increase in BCa risk (HR = 1.50; 95% CI: 1.22–1.84) related to cow’s milk consumption independent from milk fat content in a Californian prospective cohort study of 52,795 women recruited from the Adventist Health Study-2, a large cohort of North American Adventists followed for 7.9 years. They found a stronger association between cow’s milk consumption with ER+ and PR+ tumors. The daily intake of 158 ml of milk already enhanced BCa risk, whereas the consumption of cheese and yogurt did not affect BCa risk [79••]. In contrast, Nilsson et al. [80] assessed the consumption of fermented milk, non-fermented milk, cheese, and butter, estimated from semiquantitative food frequency questionnaires, in relation to prospective risk of breast, prostate, colorectal, smoking-, and obesity-related cancers in 101,235 subjects, including 1921 BCa cases in the population-based Northern Sweden Health and Disease Study. They observed no consistent association between milk/dairy intake and BCa risk, whereas an increased BCa risk was observed for women in the third vs. lowest quintile of non-fermented milk intake [80]. Recently, Kaluza et al. [81••] presented the results of a population-based Swedish Mammography Cohort including 33,780 women (88.2% postmenopausal) and showed that high and continuous consumption of two daily servings of non-fermented milk compared to no milk consumption, significantly increased the incidence of ER+/PR+ BCa (HR = 1.30; 95% CI: 1.02–1.65).
These studies were derived from populations known for high dietary exposure to cow’s milk and dairy products and a high frequency of adult lactase persistence alleles thus allowing the consumption of higher quantities of milk compared to Asian populations, where lactase persistence is rare. A large cohort study including 22,788 subjects from the Swedish Cancer Registry with lactose intolerance (more common in individuals with an absence of lactase persistence, which is the ancestral human genetic trait [82]), and thus a lower milk and dairy intake, has found a lower risk of BCa (standardized incidence ratio = 0.79) [83].
Cow’s milk consumption was traditionally low in China (high genetic prevalence of lactase non-persistence). However, cow’s milk consumption in China increased due to governmental promotion. To investigate BCa risk factors in Chinese women residing in urban and rural areas of eastern China, a large-scale cross-sectional survey, which included 122,058 women, identified an increased risk of BCa associated with milk consumption in rural areas [84]. The prospective China Kadoorie Biobank Study recruited ~500,000 adults from ten diverse (five urban, five rural) areas across China during 2004–2008 with a mean follow-up of 10.8 years. A significant positive association was found between BCa and dairy consumption (predominantly milk) with an adjusted HR per 50 g/day consumption of 1.19 (95% CI: 1.01–1.41) (n = 2582) [85••].
Case-control Studies
An Iranian population-based case-control study on 350 BCa patients and 700 age-matched controls found a positive association between total milk intake (OR 1.76; 95% CI: 1.16–2.65) and BCa, whereas no significant associations between yogurt and cheese consumption and BCa risk were observed [86•]. In contrast, a case-control study from Poland including 823 BCa cases using a semiquantitative food frequency questionnaire, which retrospectively evaluated the consumption of milk and dairy products for a time period of 10–15 years prior to BCa diagnosis, reported that high consumption of milk decreased the risk of BCa for both premenopausal and postmenopausal women [87]. Conversely, a case-control study in a Western Mexican population (97 BCa patients, 104 controls) showed that high milk consumption increased the BCa risk by 7.2 times [88]. Another case-control study in Uruguay reported that a high intake of whole milk was associated with a significant increased risk of BCa, whereas ricotta cheese and skim yogurt were associated with significant decreased risks [89].
Meta-analyses Including International Studies
In 2015, Zang et al. [90] analyzed 22 prospective cohort studies (1,566,940 participants) and five case-control studies (33,372 participants) published between 1989 and 2013. High milk consumption was not found to have a preventive effect on BCa compared to low milk consumption (RR, 0.94; 95% CI: 0.86–1.03) and there was no evidence of a linear or nonlinear relationship between milk consumption and risk of BCa. The meta-analysis of Chen et al. [91] was reported in 2019 and selected data from 8 studies published between 1989 and 2009, and thus dismissed studies of a whole successive decade during which increasingly positive associations between milk intake and BCa risk have been reported [78, 79••, 81••, 85••, 86•, 88]. The authors concluded that all milk models and the “available epidemiologic evidence” do not support a strong association between the consumption of cow’s milk or milk products and BCa risk.
In 2021, He et al. [92]. performed a meta-analysis considering 36 articles with 1,019,232 participants including individuals of European and Asian populations. Although the authors did not present exclusive data for milk, non-fermented dairy product intake exhibited no significant associations for BCa in premenopausal (HR = 1.03; 95%CI: 0.97–1.09) and postmenopausal women (HR = 1.03; 95%CI: 0.98–1.08), respectively.
To evaluate the BCa risk of milk consumption during childhood and adolescence, Gil et al. [93] in 2022 provided summary RRs for the highest vs. lowest milk intake of 0.83 (95% CI: 0.69–1.00; p = 0.05; I2 = 60%) involving 6 studies published between 2001 and 2010, which relied on differing retrospective food questionnaires reporting milk consumption during childhood and adolescence and their potential risk association with BCa in adult life. One study reported on dietary habits in adolescence and midlife and the risk of BCa in postmenopausal women with a mean age of 77 years. Remarkably, milk intake during childhood and adolescence was evaluated retrospectively in these studies with nonuniform food questionaires which occurred in the 1950s, and thus recall of dietary behavior dated back for several decades pointing to serious limitations of this recently presented meta-analysis [93].
Pathobiochemical Evidence
Milk Components with Carcinogenic Potential
Several components in commercial cow’s milk may foster malignant transformation and may promote BCa initiation and progression. These factors are milk-derived and milk-induced IGF-1, estrogens, exosomal microRNAs, bovine meat and milk factors (BMMFs), and other contaminants like aflatoxins, bisphenol A, and micro- and nanoplastics as well as environmental pesticides [94•].
Bovine Milk IGF-1
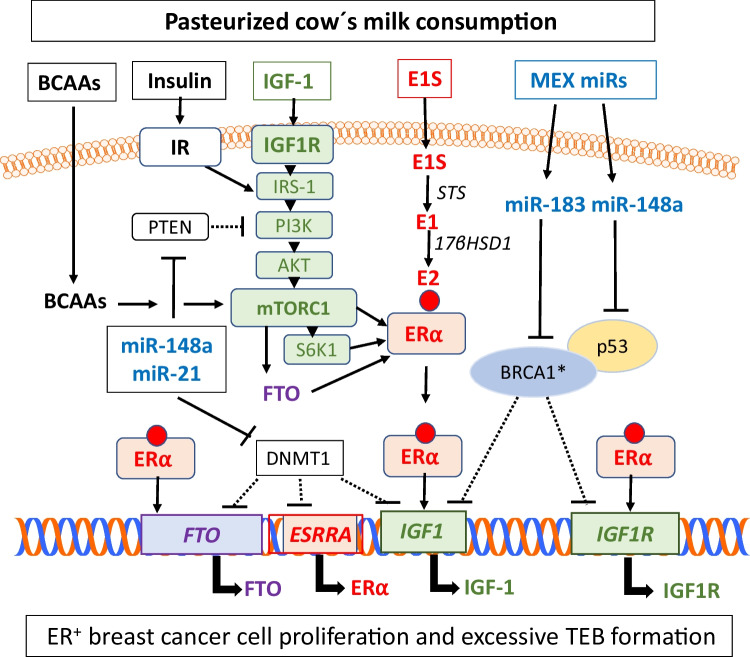
IGF-1 is a component of bovine milk and is not destroyed by pasteurization [95]. The natural concentration of IGF-1 in milk is 1.27–8.10 ng/ml [95], but IGF-1 concentration in 5777 random milk samples from Bavarian dairy cows ranged from 1.0 to 83.0 ng/ml [96]. According to a recent study in the US, median bovine GH and IGF-1 concentrations in conventional milk were 9.8 and 3.5 ng/ml, respectively, twenty and three times that found in organic samples [97]. Treatment of dairy cows with bovine somatotropin (bST) (forbidden in the European Union) to increase milk yield enhances IGF-1 concentrations in milk [96]. Notably, mastitis, a common inflammatory disease of lactating dairy cows, increases IGF-1 levels in milk whey [98]. Single-nucleotide polymorphisms (SNPs) of the bovine IGF1 gene have been associated with milk yield [99]. IGF-1 is of pivotal importance for the maintenance of cow’s milk production during lactation and thus milk yield [100]. Accordingly, the breeding selection of dairy cows for the enhancement of lactation performance may have increased milk IGF-1 levels over decades. The highest milk IGF-1 content was observed for whole milk, followed by reduced-fat and low-fat milk, respectively [101], indicating that the milk fat fraction also contributes to total milk IGF-1 levels. However, it is not the bovine milk-derived IGF-1 that increases circulatory IGF-1 levels in human milk consumers but the milk-induced intrinsic synthesis and secretion of IGF-1 by the milk consumer [5, 7•] (Fig. 1).
Fig. 1.
Synergistic interaction of milk-induced signaling pathways between branched-chain amino acids (BCAAs), insulin, insulin-like growth factor 1 (IGF-1), estrone sulfate (E1S), and milk exosome (MEX)–derived microRNAs (miRs) in breast cancer (BCa). Consumption of pasteurized cow’s milk increases circulatory levels of BCAAs, IGF-1, E1S, miR-148a, and miR-21 that may reach mammary gland epithelial and stromal cells. BCAAs and IGF-1 activate the mechanistic target of rapamycin complex 1 (mTORC1), which promotes the translation of estrogen receptor-α (ERα) and fat mass and obesity-associated gene (FTO). Phosphorylation of ERα by the kinase S6K1 and estradiol (E2) ligand binding activates ERα which stimulates the expression of IGF-1 and IGF-1 receptor (IGF1R). BCa cells are able to convert E1S to E2 via the action of steroid sulfatase (STS) and 17β-hydroxysteroid dehydrogenase type 1 (17 HSD1). Physiologically, BRCA1 via direct interaction with p53 inhibits gene expression of IGF1 and IGF1R. However, loss-of-function mutations of BRCA1* enhance IGF-1 and IGF1R expression. MiR-148a signaling via suppression of p53 and DNA methyltransferase 1 (DNMT1) enhances the expression of IGF-1, ERα, and FTO, a m6A RNA demethylase, that further enhances ERα expression. In addition, E2 promotes FTO expression. Individuals with adipogenic SNPs of FTO may exhibit increased susceptibility for milk-mediated FTO expression. MEX miR-148a-induced suppression of p53 and MEX miR-183-mediated suppression of BRCA1 may further enhance IGF-1 signaling in BCa
Commercial Milk Estrogens
Between 1850 and 1910, milk production was highly seasonal. Peak milk volumes occurred in the spring after calving and cows were not milked during the winter months [102]. Milk produced from “persistently” pregnant cows–the current routine praxis of the dairy industry–to increase commercial milk yield enhances milk estrogen concentrations. In pregnant cows, the predominant estrogen is estrone (E1) sulfate, which passes into milk. Heat treatment (70 °C and 95 °C) does not affect E1 and E2 concentrations compared to unprocessed raw milk [103]. The concentration of E1 sulfate increases from 30 pg/ml in non-pregnant cows to 151 pg/ml in pregnant cows at 40–60 days of gestation, and to a maximum level of 1000 pg/ml in cows at 220 days of gestation [104]. As milk of pregnant dairy cows is pooled, commercial milk contains higher estrogen amounts compared to former times, when lactation of cows was synchronized and cows gave birth only in spring time. Maruyama et al. [105] analyzed the exposure to exogenous estrogen through the intake of commercial milk produced from pregnant cows in children and adults. Urine concentrations of E1, E2, E3, and pregnanediol significantly increased in all adults and children after intake of 600 ml/m2 of commercial cow’s milk. In prepubertal children, urinary excretion volumes of estrogens and pregnanediol significantly increased within 1–3 h. The net increase of E2 excretion from the basal E2 levels in urine (before the intake) was 39–109 ng/4 h in this study. These data indicate that the intake of estrogens from cow’s milk corresponds to the daily estrogen production rate in prepubertal boys. Maruyama et al. [105] concluded that the intake of cow’s milk may be one of the major causes of early sexual maturation in prepubertal children. Peaker of the Hannah Dairy Research Foundation [106] did not consider these pediatric concerns [105] and stated that “even in worst case scenarios, oestrogen consumption in milk is considerably less than regulatory bodies regard as entirely safe.” Nevertheless, BCa cells are able to convert E1 sulfate into the 10 times more biologically active E2 [107]. Additionally, it is well accepted that even low doses of estrogens can both initiate as well as promote the growth of existing BCa [108, 109].
Furthermore, the molecular crosstalk between IGF-1 and E2 has potentiating synergistic effects in breast carcinogenesis. IGF-1 is a key activator of mTORC1 [5, 7•]. mTORC1 emerged as a critical node in estrogenic signaling in BCa cells. Estrogens rapidly and potently activate mTORC1, which is a crucial activator of ERα transcriptional activity [110]. mTORC1 and its downstream kinase S6K1 directly phosphorylate and activate ERα upon estrogen stimulation, which implicates activated mTORC1 signaling in the pathogenesis of ER+ BCa [111, 112]. There is a close interaction between ERα and insulin/IGF signaling in BCa [113, 114]. IGF-1 and IGF-2 are among the most potent mitogens for mammary epithelial cells and there is accumulating evidence that they interact with the E2 axis to regulate mitogenesis, apoptosis, adhesion, migration, and differentiation of mammary epithelial cells. Such interactions are bi-directional and E2 has been shown to regulate the expression and activity of IGF genes with the general effect of sensitizing breast epithelial cells to the actions of IGFs and insulin [113].
Samoli et al. [115] provided evidence in support of an interaction of IGF-1 with the expression of ERα in the non-malignant mammary tissue in the context of BCa pathogenesis. As shown in ovarian cancer cell lines, E2 induces gene expression of IGF-1 and c-myc and increases the binding of ERα to the promoters of IGF-1 and c-myc [116]. The activation of ERα in BCa upregulates the expression of IGF-1, IRS-1, and IGF1R, which results in the amplification of IGF-1 responses. Reciprocally, stimulation of IGF1R increases the phosphorylation and activity of ERα [117]. In ER+ BCa cells (MCF-7 cells), E2 treatment significantly activated the IGF1R promoter [118] (Fig. 1). Unfortunately, IGF1R expression is not controlled in the routine pathology of BCa.
The association between fat mass and obesity (FTO) gene polymorphisms and BCa is influenced by the status of ERs. Estrogens may promote BCa cell proliferation through upregulation of FTO gene expression and activation of the PI3K/AKT signaling pathway in ER+ BCa patients [119•]. Translational evidence indicates that milk intake enhances FTO-mediated transcription [120]. There appears to be a significant difference in the magnitude of FTO induction between human and bovine milk [120].
Impact of BRCA1 on IGF-1/IGF1R Signaling
BRCA1, the well-established susceptibility gene for BCa and ovarian cancer [121], increases intratumoral IGF-1 protein expression in BRCA mutation carriers suggesting an involvement of the IGF-1/IGF1R axis in BCa pathogenesis [122]. Kang et al. [123] showed that in human BCa cells, IGF1 gene expression is negatively regulated by BRCA1 at the transcriptional level. The IGF1R gene is also under negative control by BRCA1 and p53, which both physically interact with the IGF1R promoter [124] (Fig. 1). Thus, the loss of BRCA1 function can overstimulate IGF-1/IGF1R/PI3K/AKT/mTORC1 signaling, which significantly contributes to an increase in cell survival and proliferation thus promoting BCa. Elevated circulatory IGF-1 levels via milk consumption may thus have potentiating effects on the initiation and promotion of BCa. In fact, a recent observational and Mendelian randomization study confirmed a causal role of circulatory IGF-1 in BCa [125•].
Cow Milk’s Exosomal MicroRNAs with Oncogenic Activity
Cow´s milk contains abundant exosomes (nanoparticles of ~100 nm diameter) carrying microRNAs (miRs) that survive pasteurization but not ultra-heat treatment (UHT) of milk [126•, 127•]. Abundant signature miRs of pasteurized cow’s milk, milk fat, and milk exosomes (MEX) are bovine miR-148a-3p and miR-21-5p [128, 129]. These share identical nucleotide sequences with the corresponding human miRs. Notably, MIR148A is a domestication gene of dairy cattle, which increases milk yield [130, 131]. After oral administration, bovine MEX stays bioavailable and reach the systemic circulation and the tissues of mice and humans [132•, 133•, 134•]. MEX miR-148a-3p targets the mRNA of DNA methyltransferase 1 (DNMT1) [129], thereby enhancing the expression of ERα in BCa cells [135] and stimulating IGF-1 expression [136]. In addition, MEX miR-148a-3p attenuates the expression of the tumor suppressor p53 [137•], which is a negative transcriptional regulator of IGF1R [138, 139] (Fig. 1). Accordingly, increased serum levels of miR-148a-3p in humans were positively correlated with the presence of BCa [140•].
Regarding miR-21-5p, it is overexpressed in BCa compared with normal breast tissue and can promote the proliferation and invasion of BCa cell lines, and suppresses its downstream target gene phosphatase and tensin homolog (PTEN) [141] (Fig. 1). Via suppressing PTEN, miR-148a-3p and miR-21-5p enhance PI3K/AKT/mTORC1 signaling. Interestingly, labeled bovine MEX and miR-21-5p accumulate in murine placenta and embryos following oral gavage [133•]. Notably, increased levels of miR-21-5p have been detected in the placenta of infants with increased birthweight [142, 143]. Additionally, placental miR-21 overexpression is associated with increased fetal growth [144•, 145]. Thus, bovine MEX miR signaling during pregnancy via cow’s milk intake may also affect fetal mammary gland growth trajectories.
MiR-21-5p is significantly upregulated in BCa tissue, cells, and exosomes [146•]. Notably, after the consumption of 1 L of commercial 1% fat cow’s milk, miR-21-5p plasma levels significantly increased by 147% in human volunteers for 3.2 h postprandially [134•]. Of importance, mastitis, a common inflammatory complication of lactating dairy cows, is associated with enhanced expression of miR-21 in the milk of affected cows [147], thus increasing the load of this oncogenic miR in milk [148].
Cancer cell metabolism is characterized by a shift from an oxidative to a glycolytic bioenergetic pathway, a process known as the Warburg effect. Both miR-148a-3p and miR-21-5p promote hypoxia-inducible factor (HIF)–induced glycolysis via targeting hypoxia-inducible factor 1α inhibitor (HIF1AN) and von Hippel-Lindau tumor suppressor (VHL), respectively. Of note, HIF-dependent hallmarks of cancer include angiogenesis and metabolic rewiring, which are well-established drivers of BCa aggressiveness, therapy resistance, and poor prognosis [149]. Furthermore, it has been reported in BCa that overexpression of miR-378* suppresses estrogen-related receptor-γ (ESRRG) [150], a key transcription factor regulating mitochondrial oxidative phosphorylation. ESRRG is also a target gene of miR-148a-3p (targetscan.org), which may thus synergize with miR-378* in promoting glycolysis-induced BCa cell proliferation [150]. Thus, bioactive oncogenic miRs of cow’s milk that survive pasteurization and are increased by breeding selection and bovine mastitis may epigenetically promote BCa carcinogenesis. Figure 1 illustrates the potential molecular crosstalk of milk-induced IGF-1, estrogen, and miR signaling in the pathogenesis of BCa. Of importance, MEX miR-183, which is overexpressed in the milk of cows with Staphylococcus aureus–induced mastitis [151], has been shown to aggravate BCa [152•, 153, 154, 155•]. Furthermore, BRCA1 is a predicted target gene of miR-183-5p.2 (targetscan.org). MiR-183-5p via targeting BRCA1 may thus increase IGF-1 expression, which is in fact upregulated in the milk of cows suffering from mastitis [98].
Bovine Meat and Milk Factors
Uptake of dairy products of Bos taurus–derived dairy cows, particularly consumed at an early age, is suggested to represent one of the main risk factors for the development of BCa [156]. Virus-like circular DNAs of bovine meat and milk factors (BMMFs) have been recently isolated from commercial milk [157] and BCa tissue [158•]. Transcriptome profiling upon BMMF expression identified host cellular gene expression changes related to cell cycle progression pointing to a potential pathogenic involvement of BMMFs in BCa [159•]. Interestingly, certain oncogenic viruses operate via the activation of IGF-1 signaling [160].
Milk Aflatoxins
Ruminants hydroxylate aflatoxin B1 (AFB1), ingested by contaminated food, to aflatoxin M1 (AFM1), which is excreted into cow’s milk [161]. AFM1 in milk is among the most carcinogenic compounds, especially when relatively high levels are consumed in vulnerable age groups, i.e., infants and the elderly [161]. The increase of AFM1 concentrations in the milk of maize-fed cows due to climate change is a recent matter of concern [162•]. Concentrations of AFB1 and AFM1 of pasteurized and UHT milk were detected in the range of 0.7–1.5 μg/l [163]. High concentrations of AFM1 have recently been detected in human breast milk [164]. As shown in hepatocytes, AFB1 stimulates PI3K/AKT signaling [165] and may thus converge with milk-induced IGF-1/PI3K/AKT signaling.
Milk Contamination with Bisphenol A
Recently, the endocrine disruptor bisphenol A (BPA), a synthetic compound with estrogenic activity, has been detected in raw and processed milk [166•, 167•]. The average concentration of bisphenol A found in milk from cartons (0.87 ng/ml) was greater than in milk from plastic bottles (0.35 ng/ml) [168•]. The maximal probable daily intake (PDI) of BPA by (ng/kg per capita) by milk consumption has been presented for Norway (0.73), Austria (3.21), France (7.75), Italy (7.87), and Belgium (15.16), respectively [169]. These PDI values are in the range for other BPA-contaminated foodstuffs like beverages, seafood, and meat [169]. A recent Nigerian study showed that in the category of dairy products, the highest daily intake of BPA was in canned evaporated milk, followed by packaged cheese while raw cheese did not contribute to the total estimated daily intake [170]. Thus, milk consumption significantly contributes to the total estimated daily dietary BPA intake. This is relevant, because even low-dose BPA exposure is a matter of concern for BCa development and may affect vulnerable periods of breast development [171]. In mice, perinatal exposure to BPA increased the number of TEBs and the progesterone response of mammary epithelial cells [172]. In rats, perinatal exposure to BPA induced ductal hyperplasia, ductal carcinoma in situ, and malignant tumors [173, 174]. In rhesus monkeys, fetal exposure to BPA increased the density of mammary buds and accelerated mammary epithelial development [175]. In ovarian cancer cells, BPA enhanced the crosstalk between ERα and IGF1R signaling pathways [176]. Thus, milk-derived estrogens and BPA in concert with further dietary BPA exposure may have potentiating effects in BCa carcinogenesis.
Milk Contamination with Xenobiotics, Pesticides, Microplastics, and Nanoplastics
Pesticides are among the most commonly found contaminants, not only in raw cow’s milk but also after milk pasteurization and UHT processing [94•]. Oxidative stress caused by pesticides is an important mechanism through which pesticides exert their harmful effects. Many pesticides have been shown to modulate gene expression at the level of non-coding RNAs, histone deacetylases, and DNA methylation patterns suggesting their role in epigenetic deviations [177]. It is impossible to oversee the impact and interaction of all pesticide and xenobiotic contaminations in commercial milk that may as well modulate the GH-IGF axis [178].
Humans are estimated to ingest tens of thousands to millions of microplastic (MP) particles annually, in the order of several milligrams daily [179]. Available information suggests that inhalation of indoor air and ingestion of drinking water bottled in plastic are the major sources of MP exposure. However, little is known about dietary MP exposure in humans. In newborns and infants, bottle feeding and medical devices can contribute to MP ingestion [179]. The detection of MP in seafood, honey, milk, beer, table salt, drinking water, and air is a recent matter of concern [180]. The concentration of MPs identified in cow´s milk samples ranged from 204 to 1004 MPs per 100 ml exhibiting a surface area mainly ≤ 50 μm2 [181•]. The increased paraben-induced proliferation of estrogen-sensitive BCa cells was augmented in the presence of plastic nanoparticles. The mechanism may be related to the translocation and adsorption properties of nanoplastics acting as a Trojan horse to expose cells to parabens more efficiently [182•]. The frequently used microplastic-derived plasticizer organophosphate ester tri-o-cresyl phosphate interacts with ERα in MCF-7 BCa cells promoting cancer cell growth [183•].
Table 1 summarizes the spectrum of potential milk-derived carcinogenic compounds that may vary in relation to many genetic and environmental factors.
Table 1.
Milk-derived potential carcinogenic compounds that vary in relation to genetic and environmental factors of dairy cows, milk processing and distribution
| Carcinogenic factors | Potential effects on breast carcinogenesis |
|---|---|
| Dairy cow selection by IGF1 gene polymorphisms associated with increased lactation performance | Increased milk levels of IGF-1, which enhances mitogenic IGF-1 signaling |
| Treatment of dairy cows with bovine somatotropin (bST) to increase milk yield | Increased milk levels of mitogenic IGF-1 |
| Modification of milk compounds with oncogenic potential by infectious mastitis | Increased concentrations of IGF-1, miR-21 and miR-183 in milk thus promoting IGF-1/AKT/mTORC1 signaling |
| Whole milk compared to skim milk | Higher milk levels of IGF-1 in whole milk |
| Increased MIR148A expression increasing milk yield | Increased levels of miR-148a-3p in milk enhancing PI3K/AKT/mTORC1 signaling, glycolysis, and IGF-1 and ERα expression via suppression of DNMT1 |
| Dairy cow strains transfected with BMMFs | Stimulatory effects on cell proliferation |
| Increased levels of aflatoxin M1 (AFM1) in maize-fed cows compared to grass-fed cows | Synergistic effects of AFM1 on PI3K/AKT/mTORC1 signaling |
| Milk contamination with bisphenol A (BPA) | Synergistic effect of BPA on mitogenic IGF-1 and ERα signaling |
| Milk microplastics and nanoplastics | Potential catalytic action on estrogen signaling |
| Variations in thermal milk processing | Increased survival of oncogenic exosomal miRs by pasteurization compared to cooking and ultra-heat treatment (UHT) |
| Variations in microbial milk processing | Bioactivity of exosomal miRs in non-fermented pasteurized milk compared to decreased bioactivity of oncogenic exosomal miRs by microbial fermentation |
Conclusions
Two recent prospective cohort studies in California [79••] and Sweden [81••] identified cow’s milk consumption as a nutritional risk factor for ER+ BCa in North American and European populations. Epidemiological evidence, albeit conflicting, is supported by pathobiochemical pathways of milk-derived signaling (Fig. 1). The molecular crosstalk between BRCA1, FTO, IGF-1, IGF1R, E2, MEX miRs, AFM1, BMMFs, BPA, and MP/nanoplastics may potentiate oncogenic signal transduction pathways promoting BCa initiation and progression. According to NHANES III, adult IGF-1 levels and IGF-1/IGFBP-3 molar ratio had significant inverse associations with adolescent milk intake in non-Hispanic White men, but not in men of other ethnicities or in women [184•]. The sequential impact of cow’s milk signaling during vulnerable periods of human life in the pathogenesis of BCa has never been investigated by epidemiological research. The majority of epidemiological studies focus on pre- and/or postmenopausal women in relation to retrospectively estimated milk consumption missing the most critical periods of breast morphogenesis during fetal and puberty-associated breast tissue development and TEB formation (Table 2).
Table 2.
Ethnic, individual and environmental factors modifying the susceptibility to milk’s nutrigenomic effects
| Genetic and environmental predisposition factors of milk consumers | Nutrigenomic effects in milk consumers enhancing breast carcinogenesis |
|---|---|
| Lactase (LCT) persistence | Tolerance to higher quantities of cow’s milk intake compared to individuals with lactase non-persistence (Asian populations) |
| IGF1 single nucleotide polymorphisms with enhanced IGF-1 expression | Genetically predisposed IGF-1 hyper-responders may be at increased risk for milk-induced IGF-1 signal transduction |
| Variations in the prevalence of BRCA1 loss-of-function mutations | Enhanced IGF-1 signaling compared to ethnic groups with lower prevalence of BRCA1 mutations |
| Variations in the frequency of adipogenic FTO gene polymorphism | Synergism of milk-derived estrogen exposure and FTO-mediated estrogen signaling |
| Molecular heterogeneity of hormone receptor expression (ER+, ER−, PR+, PR−, HER2+, HER2−, triple negative BCa) | Milk signaling may preferentially promote ER-positive BCa |
| Variations in cow’s milk exposure during vulnerable periods of breast development, mammary branching morphogenesis, and tubular end bud formation | Enhanced risk of breast carcinogenesis by lifetime milk exposure including pregnancy (fetal overgrowth, increased birthweight), childhood and puberty (early menarche, increased longitudinal bone length), and pre- and postmenopausal periods |
| Variations in the dietary intake of high glycemic carbohydrates in combination with milk (milk + sugar); variations in the prevalence of type 2 diabetes | Western populations exposed to high glycemic load diets exhibit increased serum insulin and IGF-1 levels; diabetes type 2 is associated with increased risk of BCa |
| Quantitative and qualitative variations in protein intake | High milk protein (yogurt, cheese) and animal protein (meat) intake further increase serum IGF-1 concentrations |
| Variations in frequency and duration of artificial estrogen administration | Synergism of milk-induced and iatrogenic estrogen exposure enhancing estrogen and IGF-1 signaling |
| Variations in breastfeeding versus artificial formula feeding | Artificial formula feeding during the postnatal period may deviate postnatal breast morphogenesis via increased FTO expression |
| Variations in the mode and duration of breastfeeding | Reduced risk of BCa by prolonged breastfeeding of the offspring |
| Maternal variations in the duration of breastfeeding | Reduced risk of BCa in mothers who offer prolonged breastfeeding to their infants |
Milk is a highly complex bioactive fluid containing multiple biological and environmental effectors and thus is not a suitable single variable for epidemiological studies like blood pressure. Most published meta-analyses ignore the processing of milk, especially the thermal effects of pasteurization versus UHT, and thus do not provide information on the presence or absence of oncogenic MEX miRs [185]. Genetic variations of domestic cows like SNPs of the bovine IGF1 gene, breeding selections for lactation performance (enhanced bovine MIR148A expression), prevalence of mastitis, feeding procedures (grass versus corn), and environmental contaminations (aflatoxins, bisphenol A, pesticides, BMMFs, MPs, nanoplastics) are not and could never be taken into account by epidemiological studies, but may all have synergistic impacts on milk’s oncogenic signaling capacity (Table 1).
On the side of the milk consumer, there is as well a great genetic and nutrigenomic heterogeneity. Genetic susceptibility explains 5–10% of all BCa cases. BRCA1 and BRCA2 genes are the most common cause of hereditary BCa exhibiting ethnic differences [186•]. A recent multicenter study comprehensively describes the characteristics of the 1650 unique BRCA1 and 1731 unique BRCA2 deleterious (disease-associated) mutations identified in the CIMBA database and observed substantial variation in mutation type and frequency by geographical region and race/ethnicity [187••]. Women with BRCA1 loss-of-function mutations in milk-consuming Western societies may thus exhibit increased susceptibility to milk-derived IGF-1 signaling as mutant BRCA1 exhibits less inhibitory activity on IGF-1 and IGF1R expression [123, 124]. Furthermore, women with certain FTO and IGF1 gain-of-function gene polymorphisms appear to be at increased risk for BCa [26–28, 188, 189] (Table 2).
There is mounting epidemiological evidence of a robust association between type 2 diabetes (T2D) and an increased risk of common cancers including BCa [190•]. Current understanding of the complex signaling pathways underlying the obesity/T2D/BCa link focuses particularly on the insulin/IGF system [191, 192]. In fact, milk-induced insulin, IGF-1, and MEX miR-148a-3p/miR-21-5p-mediated signaling pathways have recently been linked to the pathogenesis of T2D [7•, 193, 194••, 195, 196] relating milk consumption to the pathogenesis of both T2D and BCa.
Large meta-analyses include populations of different ethnic origins like Europeans exhibiting lactase persistence (allowing high amounts of milk intake) and Asian populations with lactase non-persistence (naturally restricting milk consumption). We conclude that multi-national meta-analyses intended to present extremely high proband numbers are less reliable for an adequate estimation of BCa risk as they do not consider the genetic heterogeneity of BCa patients, i.e., prevalence of germline and somatic mutations in a milk-consuming population. In contrast, prospective studies including subjects derived from circumscribed homogenous ethnic populations with comparable genetic backgrounds as well as dairy cows of a given genetic background offer a more realistic evaluation of the potential carcinogenic effects of cow’s milk consumption.
In summary, available data from recent prospective cohort studies as well as pathobiochemical insights into synergies between milk and BCa signaling pathways identify commercial cow’s milk consumption as a critical risk factor for ER-positive BCa in close analogy with milk’s impact on the pathogenesis of prostate cancer, the most common cancer in men [197–199].
In our opinion, current guidelines for dairy milk consumption should be viewed with caution. They do not appropriately differentiate between the biological effects of pasteurized milk, UHT milk, and fermented dairy products [6]. They neglect the signaling effects induced by cow’s milk consumption during vulnerable periods on human mammary morphogenesis, especially during pregnancy [200], childhood and puberty (school milk programs) [201], and adulthood like US Dietary Guidelines 2015–2020 recommending a daily intake of ~ 300 ml of cow’s milk [202]. Even, the updated Dietary Guidelines for Americans 2020–2025 still recommend fat-free and low-fat milk as core elements of a healthy diet [203]. In contrast, Fraser et al. [79••] observed a significantly increased risk of BCa in US women by daily intake of ~ 160 ml milk independent of milk’s fat level. In accordance with Wehbe and Kreydiyyeh [204], it is time to reconsider dairy recommendations, especially milk intake.
Acknowledgements
We thank Harald zur Hausen, German Cancer Research Center Heidelberg, Germany, for his constructive continued dialog concerning milk-related cancer risk factors.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data Availability
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/11/2023
Missing Open Access funding information has been added in the Funding Note.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.World Health Organization. Fact Sheet. Breast cancer. https://www.who.int/news-room/fact-sheets/detail/breast-cancer. Released 26 Mar 2021. Accessed 18 Sept 18 2022.
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Li XM, Ganmaa D, Sato A. The experience of Japan as a clue to the etiology of breast and ovarian cancers: relationship between death from both malignancies and dietary practices. Med Hypotheses. 2003;60(2):268–275. doi: 10.1016/s0306-9877(02)00385-7. [DOI] [PubMed] [Google Scholar]
- 4.Qin LQ, He K, Xu JY. Milk consumption and circulating insulin-like growth factor-I level: a systematic literature review. Int J Food Sci Nutr. 2009;60(Suppl 7):330–340. doi: 10.1080/09637480903150114. [DOI] [PubMed] [Google Scholar]
- 5.Melnik BC. Milk–A nutrient system of mammalian evolution promoting mTORC1-dependent translation. Int J Mol Sci. 2015;16(8):17048–17087. doi: 10.3390/ijms160817048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melnik BC, Schmitz G. Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev. 2021;67:101270. doi: 10.1016/j.arr.2021.101270. [DOI] [PubMed] [Google Scholar]
- 7.• Melnik BC. Lifetime impact of cow’s milk on overactivation of mTORC1: from fetal to childhood overgrowth, acne, diabetes, cancers, and neurodegeneration. Biomolecules. 2021;11(3):404. 10.3390/biom11030404. This review identifies milk as an endocrine postnatal signaling system enhancing IGF-1 and mTORC1 signal transduction physiologically restricted to the period of lactation but not for persistent use, which may promote diseases of Western civilization including breast and prostate cancer. [DOI] [PMC free article] [PubMed]
- 8.Heppe DH, van Dam RM, Willemsen SP, den Breeijen H, Raat H, Hofman A, et al. Maternal milk consumption, fetal growth, and the risks of neonatal complications: the Generation R Study. Am J Clin Nutr. 2011;94(2):501–509. doi: 10.3945/ajcn.111.013854. [DOI] [PubMed] [Google Scholar]
- 9.Melnik BC, John SM, Schmitz G. Milk consumption during pregnancy increases birth weight, a risk factor for the development of diseases of civilization. J Transl Med. 2015;13:13. doi: 10.1186/s12967-014-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang D, Wu Q, Xu X, Ji C, Xia Y, Zhao Z, et al. Maternal consumption of milk or dairy products during pregnancy and birth outcomes: a systematic review and dose-response meta-analysis. Front Nutr. 2022;9:900529. doi: 10.3389/fnut.2022.900529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forman MR, Cantwell MM, Ronckers C, Zhang Y. Through the looking glass at early-life exposures and breast cancer risk. Cancer Invest. 2005;23(7):609–624. doi: 10.1080/07357900500283093. [DOI] [PubMed] [Google Scholar]
- 12.Michels KB, Trichopoulos D, Robins JM, Rosner BA, Manson JE, Hunter DJ, et al. Birthweight as a risk factor for breast cancer. Lancet. 1996;348(9041):1542–1546. doi: 10.1016/S0140-6736(96)03102-9. [DOI] [PubMed] [Google Scholar]
- 13.Cerhan JR, Sellers TA, Janney CA, Pankratz VS, Brandt KR, Vachon CM. Prenatal and perinatal correlates of adult mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1502–1508. doi: 10.1158/1055-9965.EPI-04-0762. [DOI] [PubMed] [Google Scholar]
- 14.Rich-Edwards JW, Ganmaa D, Pollak MN, Nakamoto EK, Kleinman K, Tserendolgor U, et al. Milk consumption and the prepubertal somatotropic axis. Nutr J. 2007;6:28. doi: 10.1186/1475-2891-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrea L, Di Somma C, Macchia PE, Falco A, Savanelli MC, Orio F, et al. Influence of nutrition on somatotropic axis: milk consumption in adult individuals with moderate-severe obesity. Clin Nutr. 2017;36(1):293–301. doi: 10.1016/j.clnu.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Rogers I, Emmett P, Gunnell D, Dunger D, Holly J, ALSPAC Study Team Milk as a food for growth? The insulin-like growth factors link. Public Health Nutr. 2006;9(3):359–368. doi: 10.1079/phn2006853. [DOI] [PubMed] [Google Scholar]
- 17.Norat T, Dossus L, Rinaldi S, Overvad K, Grønbaek H, Tjønneland A, et al. Diet, serum insulin-like growth factor-I and IGF-binding protein-3 in European women. Eur J Clin Nutr. 2007;61(1):91–98. doi: 10.1038/sj.ejcn.1602494. [DOI] [PubMed] [Google Scholar]
- 18.Crowe FL, Key TJ, Allen NE, Appleby PN, Roddam A, Overvad K, et al. The association between diet and serum concentrations of IGF-I, IGFBP-1, IGFBP-2, and IGFBP-3 in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1333–1340. doi: 10.1158/1055-9965.EPI-08-0781. [DOI] [PubMed] [Google Scholar]
- 19.•• Romo Ventura E, Konigorski S, Rohrmann S, Schneider H, Stalla GK, Pischon T, et al. Association of dietary intake of milk and dairy products with blood concentrations of insulin-like growth factor 1 (IGF-1) in Bavarian adults. Eur J Nutr. 2020;59(4):1413–20. 10.1007/s00394-019-01994-7. This cross-sectional German study of 526 men and women aged 18–80 years provided evidence that each 200 g increment in cow milk per day was associated with 10.0 µg/l higher IGF-1. In contrast, no association between cheese or yogurt intake and IGF-1 concentrations has been observed. [DOI] [PubMed]
- 20.• Lovell AL, Milne T, Matsuyama M, Hill RJ, Davies PSW, Grant CC, et al. Protein intake, IGF-1 concentrations, and growth in the second year of life in children receiving Growing Up Milk – Lite (GUMLi) or cow’s milk (CM) intervention. Front Nutr. 2021;8:666228. 10.3389/fnut.2021.666228. This New Zealand study confirmed that plasma IGF-1 concentrations and growth of children were independently associated with total protein intake from cow’s milk at the age of 2 years. [DOI] [PMC free article] [PubMed]
- 21.Honegger A, Humbel RE. Insulin-like growth factors I and II in fetal and adult bovine serum. Purification, primary structures, and immunological cross-reactivities. J Biol Chem. 1986;261(2):569–575. doi: 10.1016/S0021-9258(17)36130-6. [DOI] [PubMed] [Google Scholar]
- 22.Chang CI, Low HP, Qiu L, Strohsnitter WC, Hsieh CC. Prenatal modulation of breast density and breast stem cells by insulin-like growth factor-1. Am J Stem Cells. 2012;1(3):239–252. [PMC free article] [PubMed] [Google Scholar]
- 23.Ginestier C, Wicha MS. Mammary stem cell number as a determinate of breast cancer risk. Breast Cancer Res. 2007;9(4):109. doi: 10.1186/bcr1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freudenheim JL, Marshall JR, Graham S, Laughlin R, Vena JE, Bandera E, et al. Exposure to breastmilk in infancy and the risk of breast cancer. Epidemiology. 1994;5(3):324–331. doi: 10.1097/00001648-199405000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Chen J, Li Q, Huang W, Lan H, Jiang H. Association between breastfeeding and breast cancer risk: evidence from a meta-analysis. Breastfeed Med. 2015;10(3):175–182. doi: 10.1089/bfm.2014.0141. [DOI] [PubMed] [Google Scholar]
- 26.Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, Lancet Breastfeeding Series Group et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 27.Abarin T, Yan WuY, Warrington N, Lye S, Pennell C, Briollais L. The impact of breastfeeding on FTO-related BMI growth trajectories: an application to the Raine pregnancy cohort study. Int J Epidemiol. 2012;41(6):1650–1660. doi: 10.1093/ije/dys171. [DOI] [PubMed] [Google Scholar]
- 28.Wu YY, Lye S, Briollais L. The role of early life growth development, the FTO gene and exclusive breastfeeding on child BMI trajectories. Int J Epidemiol. 2017;46(5):1512–1522. doi: 10.1093/ije/dyx081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.•• Cheshmeh S, Nachvak SM, Rezvani N, Saber A. Effects of breastfeeding and formula feeding on the expression level of FTO, CPT1A and PPAR-α genes in healthy infants. Diabetes Metab Syndr Obes. 2020;13:2227–37. 10.2147/DMSO.S252122. This research paper provides evidence for excessively deviated expression of fat mass and obesity-associated gene (FTO) by infant formula compared to breast feeding. FTO expression has recently been related to breast carcinogenesis. [DOI] [PMC free article] [PubMed]
- 30.•• Leiferman A, Shu J, Upadhyaya B, Cui J, Zempleni J. Storage of extracellular vesicles in human milk, and microRNA profiles in human milk exosomes and infant formulas. J Pediatr Gastroenterol Nutr. 2021;69(2):235–8. 10.1097/MPG.0000000000002363. Current infant formula is deficient in milk exosomes and their microRNAs, thus is unable to transfer the maternal RNA software for epigenetic programming. [DOI] [PMC free article] [PubMed]
- 31.Sun L, Gao M, Qian Q, Guo Z, Zhu P, Wang X, et al. Triclosan-induced abnormal expression of miR-30b regulates fto-mediated m6A methylation level to cause lipid metabolism disorder in zebrafish. Sci Total Environ. 2021;770:145285. doi: 10.1016/j.scitotenv.2021.145285. [DOI] [PubMed] [Google Scholar]
- 32.Wang JY, Chen LJ, Qiang P. The potential role of N6-methyladenosine (m6A) demethylase fat mass and obesity-associated gene (FTO) in human cancers. Onco Targets Ther. 2020;13:12845–12856. doi: 10.2147/OTT.S283417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan N, Lu Y, Zhang Y, Pu S, Xi H, Nie X, et al. FTO – a common genetic basis for obesity and cancer. Front Genet. 2020;11:559138. doi: 10.3389/fgene.2020.559138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akbari ME, Gholamalizadeh M, Doaei S, Mirsafa F. FTO gene affects obesity and breast cancer through similar mechanisms: a new insight into the molecular therapeutic targets. Nutr Cancer. 2018;70(1):30–36. doi: 10.1080/01635581.2018.1397709. [DOI] [PubMed] [Google Scholar]
- 35.• Azzam SK, Alsafar H, Sajini AA. FTO m6A demethylase in obesity and cancer: implications and underlying molecular mechanisms. Int J Mol Sci. 2022;23(7):3800. 10.3390/ijms23073800. This paper links increased FTO expression to obesity and common cancers including breast cancer and provides potential molecular modes of action between obesity and cancer. [DOI] [PMC free article] [PubMed]
- 36.Tan A, Dang Y, Chen G, Mo Z. Overexpression of the fat mass and obesity associated gene (FTO) in breast cancer and its clinical implications. Int J Clin Exp Pathol. 2015;8(10):13405–13410. [PMC free article] [PubMed] [Google Scholar]
- 37.Abou El Ella SS, Barseem NF, Tawfik MA, Ahmed AF. BMI relationship to the onset of puberty: assessment of growth parameters and sexual maturity changes in Egyptian children and adolescents of both sexes. J Pediatr Endocrinol Metab. 2020;33(1):121–128. doi: 10.1515/jpem-2019-0119. [DOI] [PubMed] [Google Scholar]
- 38.Wiley AS. Dairy and milk consumption and child growth: is BMI involved? An analysis of NHANES 1999–2004. Am J Hum Biol. 2010;22(4):517–525. doi: 10.1002/ajhb.21042. [DOI] [PubMed] [Google Scholar]
- 39.Byun D, Hong S, Ryu S, Nam Y, Jang H, Cho Y, et al. Early-life body mass index and risks of breast, endometrial, and ovarian cancers: a dose-response meta-analysis of prospective studies. Br J Cancer. 2022;126(4):664–672. doi: 10.1038/s41416-021-01625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babio N, Becerra-Tomás N, Nishi SK, López-González L, Paz-Graniel I, García-Gavilán J, et al. Total dairy consumption in relation to overweight and obesity in children and adolescents: a systematic review and meta-analysis. Obes Rev. 2022;23(Suppl 1):e13400. doi: 10.1111/obr.13400. [DOI] [PubMed] [Google Scholar]
- 41.Wiley AS. Milk intake and total dairy consumption: associations with early menarche in NHANES 1999–2004. PloS One. 2011;6(2):e14685. doi: 10.1371/journal.pone.0014685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramezani Tehrani F, Moslehi N, Asghari G, Gholami R, Mirmiran P, Azizi F. Intake of dairy products, calcium, magnesium, and phosphorus in childhood and age at menarche in the Tehran Lipid and Glucose Study. PloS One. 2013;8(2):e57696. doi: 10.1371/journal.pone.0057696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsieh CC, Trichopoulos D, Katsouyanni K, Yuasa S. Age at menarche, age at menopause, height and obesity as risk factors for breast cancer: associations and interactions in an international case-control study. Int J Cancer. 1990;46(5):796–800. doi: 10.1002/ijc.2910460508. [DOI] [PubMed] [Google Scholar]
- 44.Novotny R, Daida Y, Morimoto Y, Shepherd J, Maskarinec G. Puberty, body fat, and breast density in girls of several ethnic groups. Am J Hum Biol. 2011;23(3):359–365. doi: 10.1002/ajhb.21145. [DOI] [PubMed] [Google Scholar]
- 45.Goldberg M, D’Aloisio AA, O’Brien KM, Zhao S, Sandler DP. Pubertal timing and breast cancer risk in the Sister Study cohort. Breast Cancer Res. 2020;22(1):112. doi: 10.1186/s13058-020-01326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao J, Xiong DH, Guo Y, Yang TL, Recker RR, Deng HW. Polymorphism in the insulin-like growth factor 1 gene is associated with age at menarche in aucasian females. Hum Reprod. 2007;22(6):1789–1794. doi: 10.1093/humrep/dem052. [DOI] [PubMed] [Google Scholar]
- 47.Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jørgensen K, et al. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab. 1994;78(3):744–752. doi: 10.1210/jcem.78.3.8126152. [DOI] [PubMed] [Google Scholar]
- 48.Gaskins AJ, Pereira A, Quintiliano D, Shepherd JA, Uauy R, Corvalán C, et al. Dairy intake in relation to breast and pubertal development in Chilean girls. Am J Clin Nutr. 2017;105(5):1166–1175. doi: 10.3945/ajcn.116.150359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiley AS. Consumption of milk, but not other dairy products, is associated with height among US preschool children in NHANES 1999–2002. Ann Hum Biol. 2009;36(2):125–138. doi: 10.1080/03014460802680466. [DOI] [PubMed] [Google Scholar]
- 50.Wiley AS. Does milk make children grow? Relationships between milk consumption and height in NHANES 1999–2002. Am J Hum Biol. 2005;17(4):425–441. doi: 10.1002/ajhb.20411. [DOI] [PubMed] [Google Scholar]
- 51.Hoppe C, Mølgaard C, Michaelsen KF. Cow’s milk and linear growth in industrialized and developing countries. Annu Rev Nutr. 2006;26:131–173. doi: 10.1146/annurev.nutr.26.010506.103757. [DOI] [PubMed] [Google Scholar]
- 52.Wiley AS. Cow milk consumption, insulin-like growth factor-I, and human biology: a life history approach. Am J Hum Biol. 2012;24(2):130–138. doi: 10.1002/ajhb.22201. [DOI] [PubMed] [Google Scholar]
- 53.Xu L, Wang Q, Wang Q, Lyytikäinen A, Mikkola T, Völgyi E, et al. Concerted actions of insulin-like growth factor 1, testosterone, and estradiol on peripubertal bone growth: a 7-year longitudinal study. J Bone Miner Res. 2011;26(9):2204–2211. doi: 10.1002/jbmr.422. [DOI] [PubMed] [Google Scholar]
- 54.Upners EN, Busch AS, Almstrup K, Petersen JH, Assens M, Main KM, et al. Does height and IGF-I determine pubertal timing in girls? Pediatr Res. 2021;90(1):176–183. doi: 10.1038/s41390-020-01215-6. [DOI] [PubMed] [Google Scholar]
- 55.•• Biro FM, Huang B, Wasserman H, Gordon CM, Pinney SM. Pubertal growth, IGF-1, and windows of susceptibility: puberty and future breast cancer risk. J Adolesc Health. 2021;68(3):517–22. 10.1016/j.jadohealth.2020.07.016. This longitudinal study of pubertal maturation in 183 girls, recruited at ages 6–7, followed up between 2004 and 2018 relates higher circulatory IGF-1 concentrations, earlier menarche, and longer pubertal growth periods with breast cancer risk. There appears to be an IGF-1-sensitive pubertal window of susceptibility, which may be disturbed by milk-induced elevations of serum IGF-1 levels. The promotion of school milk may thus promote breast cancer development. [DOI] [PMC free article] [PubMed]
- 56.Robeva R, Assyov Y, Tomova A, Kumanov P. Acne vulgaris is associated with intensive pubertal development and altitude of residence–a cross-sectional population-based study on 6,200 boys. Eur J Pediatr. 2013;172(4):465–471. doi: 10.1007/s00431-012-1907-1. [DOI] [PubMed] [Google Scholar]
- 57.Di Landro A, Cazzaniga S, Parazzini F, Ingordo V, Cusano F, Atzori L, et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults. J Am Acad Dermatol. 2012;67(6):1129–1135. doi: 10.1016/j.jaad.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 58.Melnik BC, John SM, Plewig G. Acne: risk indicator for increased body mass index and insulin resistance. Acta Derm Venereol. 2013;93(6):644–649. doi: 10.2340/00015555-1677. [DOI] [PubMed] [Google Scholar]
- 59.Cordain L, Lindeberg S, Hurtado M, Hill K, Eaton SB, Brand-Miller J. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138(12):1584–1590. doi: 10.1001/archderm.138.12.1584. [DOI] [PubMed] [Google Scholar]
- 60.Hoyt G, Hickey MS, Cordain L. Dissociation of the glycaemic and insulinaemic responses to whole and skimmed milk. Br J Nutr. 2005;93(2):175–177. doi: 10.1079/bjn20041304. [DOI] [PubMed] [Google Scholar]
- 61.Hoppe C, Mølgaard C, Dalum C, Vaag A, Michaelsen KF. Differential effects of casein versus whey on fasting plasma levels of insulin, IGF-1 and IGF-1/IGFBP-3: results from a randomized 7-day supplementation study in prepubertal boys. Eur J Clin Nutr. 2009;63(9):1076–1083. doi: 10.1038/ejcn.2009.34. [DOI] [PubMed] [Google Scholar]
- 62.Jung JY, Yoon MY, Min SU, Hong JS, Choi YS, Suh DH. The influence of dietary patterns on acne vulgaris in Koreans. Eur J Dermatol. 2010;20(6):768–772. doi: 10.1684/ejd.2010.1053. [DOI] [PubMed] [Google Scholar]
- 63.Burris J, Shikany JM, Rietkerk W, Woolf K. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe Acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118(10):1874–1885. doi: 10.1016/j.jand.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 64.Smith TM, Gilliland K, Clawson GA, Thiboutot D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128(5):1286–1293. doi: 10.1038/sj.jid.5701155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18(10):833–841. doi: 10.1111/j.1600-0625.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- 66.Melnik BC, John SM, Schmitz G. Over-stimulation of insulin/IGF-1 signaling by western diet may promote diseases of civilization: lessons learnt from Laron syndrome. Nutr Metab. 2011;8:41. doi: 10.1186/1743-7075-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25(8):950–954. doi: 10.1111/j.1468-3083.2010.03896.x. [DOI] [PubMed] [Google Scholar]
- 68.Laron Z, Kauli R, Lapkina L, Werner H. IGF-I deficiency, longevity and cancer protection of patients with Laron syndrome. Mutat Res Rev Mutat Res. 2017;772:123–133. doi: 10.1016/j.mrrev.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 69.• Murphy JD, Sandler D, White AJ, O’Brien KM. Severe acne and risk of breast cancer. Breast Cancer Res Treat. 2019;177(2):487–95. 10.1007/s10549-019-05302-z. The Sister Study, a large prospective cohort of women who had a sister diagnosed with breast cancer, during an average follow-up of 8.4 years showed that ever being diagnosed with severe acne was associated with a higher risk of breast cancer, particularly in women who were diagnosed prior to the age of 18 years. Increased systemic IGF-1 signaling in acne (sebaceous gland hyperproliferation) may also induce IGF-1-mediated deviation in mammary morphogenesis during puberty, further aggravated by milk-induced elevations of IGF-1.
- 70.Cordain L, Eaton SB, Brand Miller J, Lindeberg S, Jensen C. An evolutionary analysis of the aetiology and pathogenesis of juvenile-onset myopia. Acta Ophthalmol Scand. 2002;80(2):125–135. doi: 10.1034/j.1600-0420.2002.800203.x. [DOI] [PubMed] [Google Scholar]
- 71.Liu S, Ye S, Wang Q, Cao Y, Zhang X. Breastfeeding and myopia: a cross-sectional study of children aged 6–12 years in Tianjin, China. Sci Rep. 2018;8(1):10025. doi: 10.1038/s41598-018-27878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kleinberg DL, Barcellos-Hoff MH. The pivotal role of insulin-like growth factor I in normal mammary development. Endocrinol Metab Clin North Am. 2011;40(3):461–471. doi: 10.1016/j.ecl.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 73.Paine IS, Lewis MT. The terminal end bud: the little engine that could. J Mammary Gland Biol Neoplasia. 2017;22(2):93–108. doi: 10.1007/s10911-017-9372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ruan W, Kleinberg DL. Insulin-like growth factor I is essential for terminal end bud formation and ductal morphogenesis during mammary development. Endocrinology. 1999;140(11):5075–5081. doi: 10.1210/endo.140.11.7095. [DOI] [PubMed] [Google Scholar]
- 75.Kleinberg DL, Feldman M, Ruan W. IGF-I: an essential factor in terminal end bud formation and ductal morphogenesis. J Mammary Gland Biol Neoplasia. 2000;5(1):7–17. doi: 10.1023/a:1009507030633. [DOI] [PubMed] [Google Scholar]
- 76.Tian J, Berton TR, Shirley SH, Lambertz I, Gimenez-Conti IB, DiGiovanni J, et al. Developmental stage determines estrogen receptor alpha expression and non-genomic mechanisms that control IGF-1 signaling and mammary proliferation in mice. J Clin Invest. 2012;122(1):192–204. doi: 10.1172/JCI42204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gaard M, Tretli S, Løken EB. Dietary fat and the risk of breast cancer: a prospective study of 25,892 Norwegian women. Int J Cancer. 1995;63(1):13–17. doi: 10.1002/ijc.2910630104. [DOI] [PubMed] [Google Scholar]
- 78.McCann SE, Hays J, Baumgart CW, Weiss EH, Yao S, Ambrosone CB. Usual consumption of specific dairy foods is associated with breast cancer in the Roswell Park Cancer Institute Data Bank and BioRepository. Curr Dev Nutr. 2017;1(3):e000422. doi: 10.3945/cdn.117.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.•• Fraser GE, Jaceldo-Siegl K, Orlich M, Mashchak A, Sirirat R, Knutsen S. Dairy, soy, and risk of breast cancer: those confounded milks. Int J Epidemiol. 2020;49(5):1526–37. 10.1093/ije/dyaa007. This prospective cohort study of 52,795 North American women, initially free of cancer, was followed for 7.9 years. Higher intake of dairy milk was associated with a hazard ratio of 1.50 (95% CI: 1.22–1.84) for breast cancer. Full-fat and reduced-fat milks produced similar results, whereas no associations were noted with cheese and yogurt. Milk intake preferentially correlates with ER+/PR+ breast cancer. [DOI] [PMC free article] [PubMed]
- 80.Nilsson LM, Winkvist A, Esberg A, Jansson JH, Wennberg P, van Guelpen B, et al. Dairy products and cancer risk in a Northern Sweden population. Nutr Cancer. 2020;72(3):409–420. doi: 10.1080/01635581.2019.1637441. [DOI] [PubMed] [Google Scholar]
- 81.•• Kaluza J, Komatsu S, Lauriola M, Harris HR, Bergkvist L, Michaëlsson K, et al. Long-term consumption of non-fermented and fermented dairy products and risk of breast cancer by estrogen receptor status – population-based prospective cohort study. Clin Nutr. 2021;40(4):1966–73. 10.1016/j.clnu.2020.09.013. This prospective population-based Swedish mammography cohort including 33,780 women during a follow-up of 16.6 years related long-term non-fermented milk consumption with increased ER+/PR+ breast cancer incidence (HR = 1.30, 95%CI: 1.02–1.65) for the average of milk intake ≥2 vs. 0 servings/day. [DOI] [PubMed]
- 82.Comerford KB, Pasin G. Gene-dairy food interactions and health outcomes: a review of nutrigenetic studies. Nutrients. 2017;9(7):710. doi: 10.3390/nu9070710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ji J, Sundquist J, Sundquist K. Lactose intolerance and risk of lung, breast and ovarian cancers: aetiological clues from a population-based study in Sweden. Br J Cancer. 2015;112(1):149–152. doi: 10.1038/bjc.2014.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang F, Yu L, Wang F, Liu L, Guo M, Gao D, et al. Risk factors for breast cancer in women residing in urban and rural areas of eastern China. J Int Med Res. 2015;43(6):774–789. doi: 10.1177/0300060515592901. [DOI] [PubMed] [Google Scholar]
- 85.•• Kakkoura MG, Du H, Guo Y, Yu C, Yang L, Pei P, et al. Dairy consumption and risks of total and site-specific cancers in Chinese adults: an 11-year prospective study of 0.5 million people. BMC Med. 2022;20(1):134. 10.1186/s12916-022-02330-3. This prospective China Kadoorie Biobank study recruited ~0.5 million adults from 10 diverse areas across China during 2004–2008. Significant positive associations of dairy consumption (predominantly milk) with risks with adjusted HRs per 50 g/day usual consumption being 1.19 (95%CI: 1.01–1.41) for female breast cancer. [DOI] [PMC free article] [PubMed]
- 86.• Dashti F, Soltani S, Benisi-Kohansal S, Azadbakht L, Esmaillzadeh A. Consumption of dairy products and odds of breast cancer: an Iranian case-control study. Breast Cancer. 2022;29(2):352–60. 10.1007/s12282-021-01317-x. This population-based Iranian case-control study on 350 patients with confirmed breast cancer and 700 controls reported a positive association between total milk intake (OR 1.76; 95% CI: 1.16–2.65) and breast cancer. [DOI] [PubMed]
- 87.Wajszczyk B, Charzewska J, Godlewski D, Zemła B, Nowakowska E, Kozaczka M, et al. Consumption of dairy products and the risk of developing breast cancer in Polish women. Nutrients. 2021;13(12):4420. doi: 10.3390/nu13124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Galván-Salazar HR, Arreola-Cruz A, Madrigal-Pérez D, Soriano-Hernández AD, Guzman-Esquivel J, Montes-Galindo DA, et al. Association of milk and meat consumption with the development of breast cancer in a Western Mexican population. Breast Care. 2015;10(6):393–396. doi: 10.1159/000442230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ronco AL, De Stéfani E, Dáttoli R. Dairy foods and risk of breast cancer: a case-control study in Montevideo, Uruguay. Eur J Cancer Prev. 2002;11(5):457–463. doi: 10.1097/00008469-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 90.Zang J, Shen M, Du S, Chen T, Zou S. The association between dairy intake and breast cancer in Western and Asian populations: a systematic review and meta-analysis. J Breast Cancer. 2015;18(4):313–322. doi: 10.4048/jbc.2015.18.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen L, Li M, Li H. Milk and yogurt intake and breast cancer risk: a meta-analysis. Medicine. 2019;98(12):e14900. doi: 10.1097/MD.0000000000014900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.He Y, Tao Q, Zhou F, Si Y, Fu R, Xu B, Xu J, Li X, Chen B. The relationship between dairy products intake and breast cancer incidence: a meta-analysis of observational studies. BMC Cancer. 2021;21(1):1109. doi: 10.1186/s12885-021-08854-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gil H, Chen QY, Khil J, Park J, Na G, Lee D, et al. Milk intake in early life and later cancer risk: a meta-analysis. Nutrients. 2022;14(6):1233. doi: 10.3390/nu14061233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.• Calahorrano-Moreno MB, Ordoñez-Bailon JJ, Baquerizo-Crespo RJ, Dueñas-Rivadeneira AA, B S M Montenegro MC, Rodríguez-Díaz JM. Contaminants in the cow’s milk we consume? Pasteurization and other technologies in the elimination of contaminants. F1000Res. 2022;11:91. 10.12688/f1000research.108779.1. This review details the main contaminants found in cow milk, refers to the sources of contamination and their impact on human health. [DOI] [PMC free article] [PubMed]
- 95.Collier RJ, Miller MA, Hildebrandt JR, Torkelson AR, White TC, Madsen KS, et al. Factors affecting insulin-like growth factor-I concentration in bovine milk. J Dairy Sci. 1991;74(9):2905–2911. doi: 10.3168/jds.S0022-0302(91)78473-7. [DOI] [PubMed] [Google Scholar]
- 96.Daxenberger A, Breier BH, Sauerwein H. Increased milk levels of insulin-like growth factor 1 (IGF-1) for the identification of bovine somatotropin (bST) treated cows. Analyst. 1998;123(12):2429–2435. doi: 10.1039/a804923h. [DOI] [PubMed] [Google Scholar]
- 97.Welsh JA, Braun H, Brown N, Um C, Ehret K, Figueroa J, et al. Production-related contaminants (pesticides, antibiotics and hormones) in organic and conventionally produced milk samples sold in the USA. Public Health Nutr. 2019;22(16):2972–2980. doi: 10.1017/S136898001900106X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shuster DE, Kehrli ME, Jr, Baumrucker CR. Relationship of inflammatory cytokines, growth hormone, and insulin-like growth factor-I to reduced performance during infectious disease. Proc Soc Exp Biol Med. 1995;210(2):140–149. doi: 10.3181/00379727-210-43933. [DOI] [PubMed] [Google Scholar]
- 99.Mullen MP, Lynch CO, Waters SM, Howard DJ, O’Boyle P, Kenny DA, et al. Single nucleotide polymorphisms in the growth hormone and insulin-like growth factor-1 genes are associated with milk production, body condition score and fertility traits in dairy cows. Genet Mol Res. 2011;10(3):1819–1830. doi: 10.4238/vol10-3gmr1173. [DOI] [PubMed] [Google Scholar]
- 100.Plath-Gabler A, Gabler C, Sinowatz F, Berisha B, Schams D. The expression of the IGF family and GH receptor in the bovine mammary gland. J Endocrinol. 2001;168(1):39–48. doi: 10.1677/joe.0.1680039. [DOI] [PubMed] [Google Scholar]
- 101.Marín-Quiroga A, Villanueva-Fierro I, Rodríguez-Pérez MA. Lares-Asseff A, Cháirez-Hernández I, Proal-Nájera JB. Association of IGF-1 content with whole, reduced-fat, and low-fat milk in México. Agrociencia. 2015;49(2):113–23. https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-31952015000200001. online version ISSN 2521–9766. Accessed 18 Sept 2022
- 102.Bateman F. Improvement in American dairy farming, 1850–1910: a quantitative analysis. J Econ Hist. 1968;28(2):255–273. doi: 10.1017/S0022050700069321. [DOI] [Google Scholar]
- 103.Snoj T, Zuzek MC, Cebulj-Kadunc N, Majdic G. Short communication: heat treatment and souring do not affect milk estrone and 17β-estradiol concentrations. J Dairy Sci. 2018;101(1):61–65. doi: 10.3168/jds.2017-13205. [DOI] [PubMed] [Google Scholar]
- 104.Heap RB, Hamon M. Oestrone sulphate in milk as an indicator of a viable conceptus in cows. Br Vet J. 1979;135(4):355–363. doi: 10.1016/s0007-1935(17)32838-5. [DOI] [PubMed] [Google Scholar]
- 105.Maruyama K, Oshima T, Ohyama K. Exposure to exogenous estrogen through intake of commercial milk produced from pregnant cows. Pediatr Int. 2010;52(1):33–38. doi: 10.1111/j.1442-200X.2009.02890.x. [DOI] [PubMed] [Google Scholar]
- 106.Peaker M. Oestrogens in milk and breast cancer: a cause for concern…or not? J Dairy Res. 2020;87(2):266–269. doi: 10.1017/S0022029920000370. [DOI] [PubMed] [Google Scholar]
- 107.Sasano H, Suzuki T, Nakata T, Moriya T. New development in intracrinology of breast carcinoma. Breast Cancer. 2006;13(2):129–136. doi: 10.2325/jbcs.13.129. [DOI] [PubMed] [Google Scholar]
- 108.Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocr Rev. 2000;21(1):40–54. doi: 10.1210/edrv.21.1.0386. [DOI] [PubMed] [Google Scholar]
- 109.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 110.Torres AS, Holz MK. Unraveling the multifaceted nature of the nuclear function of mTOR. Biochim Biophys Acta Mol Cell Res. 2021;1868(2):118907. doi: 10.1016/j.bbamcr.2020.118907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alayev A, Salamon RS, Berger SM, Schwartz NS, Cuesta R, Snyder RB, et al. mTORC1 directly phosphorylates and activates Erα upon estrogen stimulation. Oncogene. 2016;35(27):3535–3543. doi: 10.1038/onc.2015.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yamnik RL, Digilova A, Davis DC, Brodt ZN, Murphy CJ, Holz MK. S6 kinase 1 regulates estrogen receptor alpha in control of breast cancer cell proliferation. J Biol Chem. 2009;284(10):6361–6369. doi: 10.1074/jbc.M807532200. [DOI] [PubMed] [Google Scholar]
- 113.Lanzino M, Morelli C, Garofalo C, Panno ML, Mauro L, Andò S, et al. Interaction between estrogen receptor alpha and insulin/IGF signaling in breast cancer. Curr Cancer Drug Targets. 2008;8(7):597–610. doi: 10.2174/156800908786241104. [DOI] [PubMed] [Google Scholar]
- 114.Hawsawi Y, El-Gendy R, Twelves C, Speirs V, Beattie J. Insulin-like growth factor – oestradiol crosstalk and mammary gland tumourigenesis. Biochim Biophys Acta. 2013;1836(2):345–353. doi: 10.1016/j.bbcan.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 115.Samoli E, Lagiou A, Zourna P, Barbouni A, Georgila C, Tsikkinis A, et al. Expression of estrogen receptors in non-malignant mammary tissue modifies the association between insulin-like growth factor 1 and breast cancer risk. Ann Oncol. 2015;26(4):793–797. doi: 10.1093/annonc/mdu583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sasaki H, Hayakawa J, Terai Y, Kanemura M, Tanabe-Kimura A, Kamegai H, et al. Difference between genomic actions of estrogen versus raloxifene in human ovarian cancer cell lines. Oncogene. 2008;27(19):2737–2745. doi: 10.1038/sj.onc.1210926. [DOI] [PubMed] [Google Scholar]
- 117.Surmacz E, Bartucci M. Role of estrogen receptor alpha in modulating IGF-I receptor signaling and function in breast cancer. J Exp Clin Cancer Res. 2004;23(3):385–394. [PubMed] [Google Scholar]
- 118.Maor S, Mayer D, Yarden RI, Lee AV, Sarfstein R, Werner H, et al. Estrogen receptor regulates insulin-like growth factor-I receptor gene expression in breast tumor cells: involvement of transcription factor Sp1. J Endocrinol. 2006;191(3):605–612. doi: 10.1677/joe.1.07016. [DOI] [PubMed] [Google Scholar]
- 119.• Gholamalizadeh M, Jarrahi AM, Akbari ME, Bourbour F, Mokhtari Z, Salahshoornezhad S, et al. Association between FTO gene polymorphisms and breast cancer: the role of estrogen. Expert Rev Endocrinol Metab. 2020;15(2):115–21. 10.1080/17446651.2020.1730176. This updated review aims to evaluate the impact of the FTO gene and its polymorphisms in the pathogenesis of breast cancer. Estrogens may promote breast cancer cell proliferation through the upregulation of FTO expression and activation of the PI3K/AKT signaling pathway in ER+ breast cancer patients. [DOI] [PubMed]
- 120.Melnik BC. Milk: an epigenetic amplifier of FTO-mediated transcription? Implications for Western diseases. J Transl Med. 2015;13:385. doi: 10.1186/s12967-015-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 122.Hudelist G, Wagner T, Rosner M, Fink-Retter A, Gschwantler-Kaulich D, Czerwenka K, Kroiss R, et al. Intratumoral IGF-I protein expression is selectively upregulated in breast cancer patients with BRCA1/2 mutations. Endocr Relat Cancer. 2007;14(4):1053–1062. doi: 10.1677/ERC-06-0075. [DOI] [PubMed] [Google Scholar]
- 123.Kang HJ, Yi YW, Kim HJ, Hong YB, Seong YS, Bae I. BRCA1 negatively regulates IGF-1 expression through an estrogen-responsive element-like site. Cell Death Dis. 2012;3(6):e336. doi: 10.1038/cddis.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Abramovitch S, Werner H. Functional and physical interactions between BRCA1 and p53 in transcriptional regulation of the IGF-IR gene. Horm Metab Res. 2003;35(11–12):758–762. doi: 10.1055/s-2004-814154. [DOI] [PubMed] [Google Scholar]
- 125.• Tan VY, Bull CJ, Biernacka KM, Teumer A, Richardson TG, Sanderson E, et al. Investigation of the interplay between circulating lipids and IGF-I and relevance to breast cancer risk: an observational and Mendelian randomization study. Cancer Epidemiol Biomarkers Prev. 2021;30(12):2207–16. 10.1158/1055-9965.EPI-21-0315. Mendelian randomization was conducted to estimate the relationship between lipids or IGF-I and breast cancer and suggest a causal role of IGF-1. [DOI] [PMC free article] [PubMed]
- 126.• Kleinjan M, van Herwijnen MJ, Libregts SF, van Neerven RJ, Feitsma AL, Wauben MH. Regular industrial processing of bovine milk impacts the integrity and molecular composition of extracellular vesicles. J Nutr. 2021;151(6):1416–25. 10.1093/jn/nxab031. This ultrastructural and RNA study found that ultra-heat processing of cow milk destroys milk’s extracellular vesicles (EVs) and their microRNAs, whereas pasteurization did not affect total EV numbers but reduced milk-EV-associated RNAs. MicroRNA-148a was still detectable after pasteurization. [DOI] [PubMed]
- 127.• Zhang Y, Xu Q, Hou J, Huang G, Zhao S, Zheng N, et al. Loss of bioactive microRNAs in cow’s milk by ultra-high-temperature treatment but not by pasteurization treatment. J Sci Food Agric. 2022;102(7):2676–85. 10.1002/jsfa.11607. This study revealed that the bioactive microRNAs in raw milk were lost after ultra-heat treatment but not after pasteurization. [DOI] [PubMed]
- 128.Chen X, Gao C, Li H, Huang L, Sun Q, Dong Y, et al. Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Res. 2010;20(10):1128–1137. doi: 10.1038/cr.2010.80. [DOI] [PubMed] [Google Scholar]
- 129.Golan-Gerstl R, Elbaum Shiff Y, Moshayoff V, Schecter D, Leshkowitz D, Reif S. Characterization and biological function of milk-derived miRNAs. Mol Nutr Food Res. 2017;61(10):1700009. doi: 10.1002/mnfr.201700009. [DOI] [PubMed] [Google Scholar]
- 130.Braud M, Magee DA, Park SD, Sonstegard TS, Waters SM, MacHugh DE, et al. Genome-wide microRNA binding site variation between extinct wild aurochs and modern cattle identifies candidate microRNA-regulated domestication genes. Front Genet. 2017;8:3. doi: 10.3389/fgene.2017.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Do DN, Dudemaine PL, Li R, Ibeagha-Awemu EM. Co-expression network and pathway analyses reveal important modules of miRNAs regulating milk yield and component traits. Int J Mol Sci. 2017;18(7):1560. doi: 10.3390/ijms18071560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.• Manca S, Upadhyaya B, Mutai E, Desaulniers AT, Cederberg RA, White BR, et al. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci Rep. 2018;8(1):11321. 10.1038/s41598-018-29780-1. This important study provides experimental evidence that cow milk exosomes administered orally to mice were detectable in various distant murine tissues. [DOI] [PMC free article] [PubMed]
- 133.• Sadri M, Shu J, Kachman SD, Cui J, Zempleni J. Milk exosomes and miRNA cross the placenta and promote embryo survival in mice. Reproduction. 2020;160(4):501–9. 10.1530/REP-19-0521. This study provides evidence that fluorophore-labeled milk exosomes, microRNA-21–5p, and microRNA-30d accumulate in murine placenta and embryos following oral gavage of cow milk-derived exosomes. [DOI] [PubMed]
- 134.• Mutai E, Ramer-Tait AE, Zempleni J. MicroRNAs in bovine milk exosomes are bioavailable in humans but do not elicit a robust pro-inflammatory cytokine response. ExRNA. 2020;2(2):1–9. 10.1186/s41544-019-0041-x. This study demonstrates that milk exosomal microRNAs like microRNA-21 significantly increased in the plasma of healthy individuals after consumption of 1 L of 1% fat commercial cow milk.
- 135.Xu Y, Chao L, Wang J, Sun Y. miRNA-148a regulates the expression of the estrogen receptor through DNMT1-mediated DNA methylation in breast cancer cells. Oncol Lett. 2017;14(4):4736–4740. doi: 10.3892/ol.2017.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ouni M, Gunes Y, Belot MP, Castell AL, Fradin D, Bougnères P. The IGF1 P2 promoter is an epigenetic QTL for circulating IGF1 and human growth. Clin Epigenetics. 2015;7(1):22. doi: 10.1186/s13148-015-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.• Guo MM, Zhang K, Zhang JH. Human breast milk-derived exosomal miR-148a-3p protects against necrotizing enterocolitis by regulating p53 and sirtuin 1. Inflammation. 2022;45(3):1254–68. 10.1007/s10753-021-01618-5. This experimental study shows that milk exosomal microRNA-148a-3p targets the key transcription factor p53, a critical suppressor of carcinogenesis. [DOI] [PubMed]
- 138.Werner H, Karnieli E, Rauscher FJ, LeRoith D. Wild-type and mutant p53 differentially regulate transcription of the insulin-like growth factor I receptor gene. Proc Natl Acad Sci U S A. 1996;93(16):8318–8323. doi: 10.1073/pnas.93.16.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Melnik BC. Milk disrupts p53 and DNMT1, the guardians of the genome: implications for acne vulgaris and prostate cancer. Nutr Metab. 2017;14:55. doi: 10.1186/s12986-017-0212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.• Li X, Tang X, Li K, Lu L. Evaluation of serum microRNAs (miR-9-5p, miR-17-5p, and miR-148a-3p) as potential biomarkers of breast cancer. Biomed Res Int. 2022;2022:9961412. 10.1155/2022/9961412. This study identifies serum microRNA-148a-3p, the most abundant microRNA of cow milk, as potential biomarker of breast cancer. [DOI] [PMC free article] [PubMed]
- 141.Kuang Y, Nie YJ. Exploration of the regulatory effect of miR-21 on breast cancer cell line proliferation and invasion as well as the downstream target genes. Asian Pac J Trop Med. 2016;9(5):470–473. doi: 10.1016/j.apjtm.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 142.Jiang H, Wu W, Zhang M, Li J, Peng Y, Miao TT, et al. Aberrant upregulation of miR-21 in placental tissues of macrosomia. J Perinatol. 2014;34(9):658–663. doi: 10.1038/jp.2014.58. [DOI] [PubMed] [Google Scholar]
- 143.Zhang JT, Cai QY, Ji SS, Zhang HX, Wang YH, Yan HT, et al. Decreased miR-143 and increased miR-21 placental expression levels are associated with macrosomia. Mol Med Rep. 2016;13(4):3273–3280. doi: 10.3892/mmr.2016.4892. [DOI] [PubMed] [Google Scholar]
- 144.• Kochhar P, Dwarkanath P, Ravikumar G, Thomas A, Crasta J, Thomas T, et al. Placental expression of miR-21-5p, miR-210-3p and miR-141-3p: relation to human fetoplacental growth. Eur J Clin Nutr. 2022;76(5):730–8.10.1038/s41430-021-01017-x. This study identifies microRNA-21–5p, a signature microRNA of cow milk, as a promoter of feto-placental growth. It may thus also affect fetal mammary gland development. [DOI] [PubMed]
- 145.Maccani MA, Padbury JF, Marsit CJ. miR-16 and miR-21 expression in the placenta is associated with fetal growth. PloS One. 2011;6(6):e21210. doi: 10.1371/journal.pone.0021210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.• Liu M, Mo F, Song X, He Y, Yuan Y, Yan J, et al. Exosomal has-miR-21-5p is a biomarker for breast cancer diagnosis. PeerJ. 2021;9:e12147. 10.7717/peerj.12147. This study identifies exosomal human microRNA-21-5p as a biomarker for breast cancer. Human and bovine microRNA-21-5p are identical in nucleotide sequence. [DOI] [PMC free article] [PubMed]
- 147.Lai YC, Fujikawa T, Maemura T, Ando T, Kitahara G, Endo Y, et al. Inflammation-related microRNA expression level in the bovine milk is affected by mastitis. PloS One. 2017;12(5):e0177182. doi: 10.1371/journal.pone.0177182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Selcuklu SD, Donoghue MT, Spillane C. miR-21 as a key regulator of oncogenic processes. Biochem Soc Trans. 2009;37(Pt 4):918–925. doi: 10.1042/BST0370918. [DOI] [PubMed] [Google Scholar]
- 149.de Heer EC, Jalving M, Harris AL. HIFs, angiogenesis, and metabolism: elusive enemies in breast cancer. J Clin Invest. 2020;130(10):5074–5087. doi: 10.1172/JCI137552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Eichner LJ, Perry MC, Dufour CR, Bertos N, Park M, St-Pierre J, et al. miR-378(∗) mediates metabolic shift in breast cancer cells via the PGC-1β/ERRγ transcriptional pathway. Cell Metab. 2010;12(4):352–361. doi: 10.1016/j.cmet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 151.Sun J, Aswath K, Schroeder SG, Lippolis JD, Reinhardt TA, Sonstegard TS. MicroRNA expression profiles of bovine milk exosomes in response to Staphylococcus aureus infection. BMC Genomics. 2015;16:806. doi: 10.1186/s12864-015-2044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.• Wu C, Tuo Y, Hu G, Luo J. miR-183-5p aggravates breast cancer development via mediation of RGS2. Comput Math Methods Med. 2021;2021:9664195. 10.1155/2021/9664195. This study implicates a critical role for microRNA-183-5p in breast cancer. This microRNA is upregulated in milk exosomes of cows affected by mastitis, a common inflammatory udder disease of dairy cows.
- 153.Li P, Sheng C, Huang L, Zhang H, Huang L, Cheng Z, et al. MiR-183/-96/-182 cluster is up-regulated in most breast cancers and increases cell proliferation and migration. Breast Cancer Res. 2014;16(6):473. doi: 10.1186/s13058-014-0473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Macedo T, Silva-Oliveira RJ, Silva VAO, Vidal DO, Evangelista AF, Marques MMC. Overexpression of mir-183 and mir-494 promotes proliferation and migration in human breast cancer cell lines. Oncol Lett. 2017;14(1):1054–1060. doi: 10.3892/ol.2017.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.• Anderson O, Guttilla Reed IK. Regulation of cell growth and migration by miR-96 and miR-183 in a breast cancer model of epithelial-mesenchymal transition. PLoS One. 2020;15(5):e0233187. 10.1371/journal.pone.0233187. This study provides evidence that microRNA-183 promotes epithelial-mesenchymal transition in breast carcinogenesis. [DOI] [PMC free article] [PubMed]
- 156.zur Hausen H, de Villiers EM. Dairy cattle serum and milk factors contributing to the risk of colon and breast cancers. Int J Cancer. 2015;137(4):959–967. doi: 10.1002/ijc.29466. [DOI] [PubMed] [Google Scholar]
- 157.Falida K, Eilebrecht S, Gunst K, Zur Hausen H, de Villiers EM. Isolation of two virus-like circular DNAs from commercially available milk samples. Genome Announc. 2017;5(17):e00266-17. doi: 10.1128/genomeA.00266-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.• de Villiers EM, Zur Hausen H. Bovine meat and milk factors (BMMFs): their proposed role in common human cancers and type 2 diabetes mellitus. Cancers. 2021;13(21):5407. 10.3390/cancers13215407. This review provides a potential molecular link between virus-like circular DNA, called bovine meat and milk factors, in the pathogenesis of common cancers including breast cancer and type 2 diabetes. [DOI] [PMC free article] [PubMed]
- 159.• Eilebrecht S, Hotz-Wagenblatt A, Sarachaga V, Burk A, Falida K, Chakraborty D, et al. Expression and replication of virus-like circular DNA in human cells. Sci Rep. 2018;8(1):2851. 10.1038/s41598-018-21317-w. This study provides evidence that bovine meat and milk factors enhance cell proliferation. [DOI] [PMC free article] [PubMed]
- 160.Józefiak A, Larska M, Pomorska-Mól M, Ruszkowski JJ. The IGF-1 signaling pathway in viral infections. Viruses. 2021;13(8):1488. doi: 10.3390/v13081488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ismail A, Akhtar S, Levin RE, Ismail T, Riaz M, Amir M. Aflatoxin M1: prevalence and decontamination strategies in milk and milk products. Crit Rev Microbiol. 2016;42(3):418–427. doi: 10.3109/1040841X.2014.958051. [DOI] [PubMed] [Google Scholar]
- 162.• Van der Fels-Klerx HJ, Vermeulen LC, Gavai AK, Liu C. Climate change impacts on aflatoxin B1 in maize and aflatoxin M1 in milk: a case study of maize grown in Eastern Europe and imported to the Netherlands. PLoS One. 2019;14(6):e0218956. 10.1371/journal.pone.0218956. This study raises concerns that climate change-promoted increases in aflatoxin levels in maize fed to dairy cows may exceed critical milk aflatoxin concentrations. Aflatoxins have a high carcinogenic activity. [DOI] [PMC free article] [PubMed]
- 163.Scaglioni PT, Becker-Algeri T, Drunkler D, Badiale-Furlong E. Aflatoxin B1 and M1 in milk. Anal Chim Acta. 2014;829:68–74. doi: 10.1016/j.aca.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 164.Hussen Kabthymer R, Gebremeskel Kanno G, Aregu MB, Paixão S, Belachew T. Prevalence and concentration of Aflatoxin M1 in human breast milk in sub-Saharan Africa: a systematic review and meta-analysis, and cancer risk assessment. Int J Environ Health Res. 2022;15:1–17. doi: 10.1080/09603123.2022.2036330. [DOI] [PubMed] [Google Scholar]
- 165.Wang J, Chen Y, Mo PL, Wei YJ, Liu KC, Zhang ZG, et al. 1α,25-Dihydroxyvitamin D3 inhibits aflatoxin B1-induced proliferation and dedifferentiation of hepatic progenitor cells by regulating PI3K/Akt and Hippo pathways. J Steroid Biochem Mol Biol. 2018;183:228–237. doi: 10.1016/j.jsbmb.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 166.• Santonicola S, Ferrante MC, Leo GD, Murru N, Anastasio A, Mercogliano R. Study on endocrine disruptors levels in raw milk from cow’s farms: risk assessment. Ital J Food Saf. 2018;7(3):7668. 10.4081/ijfs.2018.7668. The authors raise concerns about the potential adverse health effects of endocrine disruptors like bisphenol A in commercial milk. [DOI] [PMC free article] [PubMed]
- 167.• Mercogliano R, Santonicola S, Albrizio S, Ferrante MC. Occurrence of bisphenol A in the milk chain: a monitoring model for risk assessment at a dairy company. J Dairy Sci. 2021;104(5):5125–32. 10.3168/jds.2020-19365. The investigators are concerned about the detection of bisphenol A in cow milk. The highest contamination levels were detected in raw milk from the storage tank. [DOI] [PubMed]
- 168.• Frankowski R, Grześkowiak T, Czarczyńska-Goślińska B, Zgoła-Grześkowiak A. Occurrence and dietary risk of bisphenols and parabens in raw and processed cow’s milk. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2022;39(1):116–29. 10.1080/19440049.2021.1986234. This study detected bisphenols mainly in the processed milk bought in stores while parabens were found in all samples of both raw and processed cow’s milk. [DOI] [PubMed]
- 169.Russo G, Barbato F, Mita DG, Grumetto L. Occurrence of bisphenol A and its analogues in some foodstuff marketed in Europe. Food Chem Toxicol. 2019;131:110575. doi: 10.1016/j.fct.2019.110575. [DOI] [PubMed] [Google Scholar]
- 170.Adeyi AA, Babalola BA. Bisphenol-A (BPA) in foods commonly consumed in Southwest Nigeria and its human health risk. Sci Rep. 2019;9(1):17458. doi: 10.1038/s41598-019-53790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang Z, Liu H, Liu S. Low-dose bisphenol A exposure: a seemingly instigating carcinogenic effect on breast cancer. Adv Sci. 2016;4(2):1600248. doi: 10.1002/advs.201600248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ayyanan A, Laribi O, Schuepbach-Mallepell S, Schrick C, Gutierrez M, Tanos T, et al. Perinatal exposure to bisphenol a increases adult mammary gland progesterone response and cell number. Mol Endocrinol. 2011;25(11):1915–1923. doi: 10.1210/me.2011-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod Toxicol. 2007;23(3):383–390. doi: 10.1016/j.reprotox.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Acevedo N, Davis B, Schaeberle CM, Sonnenschein C, Soto AM. Perinatally administered bisphenol A as a potential mammary gland carcinogen in rats. Environ Health Perspect. 2013;121(9):1040–1046. doi: 10.1289/ehp.1306734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tharp AP, Maffini MV, Hunt PA, VandeVoort CA, Sonnenschein C, Soto AM. Bisphenol A alters the development of the rhesus monkey mammary gland. Proc Natl Acad Sci U S A. 2012;109(21):8190–8195. doi: 10.1073/pnas.1120488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hwang KA, Park MA, Kang NH, Yi BR, Hyun SH, Jeung EB, et al. Anticancer effect of genistein on BG-1 ovarian cancer growth induced by 17 β-estradiol or bisphenol A via the suppression of the crosstalk between estrogen receptor α and insulin-like growth factor-1 receptor signaling pathways. Toxicol Appl Pharmacol. 2013;272(3):637–646. doi: 10.1016/j.taap.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 177.Sabarwal A, Kumar K, Singh RP. Hazardous effects of chemical pesticides on human health-cancer and other associated disorders. Environ Toxicol Pharmacol. 2018;63:103–114. doi: 10.1016/j.etap.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 178.Scarth JP. Modulation of the growth hormone-insulin-like growth factor (GH-IGF) axis by pharmaceutical, nutraceutical and environmental xenobiotics: an emerging role for xenobiotic-metabolizing enzymes and the transcription factors regulating their expression. A review Xenobiotica. 2006;36(2–3):119–218. doi: 10.1080/00498250600621627. [DOI] [PubMed] [Google Scholar]
- 179.Kannan K, Vimalkumar K. A Review of human exposure to microplastics and insights into microplastics as obesogens. Front Endocrinol. 2021;12:724989. doi: 10.3389/fendo.2021.724989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE. Human consumption of microplastics. Environ Sci Technol. 2019;53(12):7068–7074. doi: 10.1021/acs.est.9b01517. [DOI] [PubMed] [Google Scholar]
- 181.• Da Costa Filho PA, Andrey D, Eriksen B, Peixoto RP, Carreres BM, Ambühl ME, et al. Detection and characterization of small-sized microplastics (≥ 5 µm) in milk products. Sci Rep. 2021;11(1):24046. 10.1038/s41598-021-03458-7. This paper reports the presence of relatively low amounts of small-sized microplastics (≥ 5 µm) in raw milk collected at a farm just after the milking machine and in some processed commercial liquid and powdered cow milk products. [DOI] [PMC free article] [PubMed]
- 182.• Roje Ž, Ilić K, Galić E, Pavičić I, Turčić P, Stanec Z, Vrček IV. Synergistic effects of parabens and plastic nanoparticles on proliferation of human breast cancer cells. Arh Hig Rada Toksikol. 2019;70(4):310–14. 10.2478/aiht-2019-70-3372. This study shows synergistic effects of plastic nanoparticles and parabens found in cow milk in the promotion of the proliferation of estrogen-sensitive breast cancer cells. [DOI] [PubMed]
- 183.• Böckers M, Paul NW, Efferth T. Organophosphate ester tri-o-cresyl phosphate interacts with estrogen receptor α in MCF-7 breast cancer cells promoting cancer growth. Toxicol Appl Pharmacol. 2020;395:114977. 10.1016/j.taap.2020.114977. This research work identifies the contribution of microplastic-derived plasticizers in ER+ breast cancer cell proliferation. [DOI] [PubMed]
- 184.• Srinivasan V, Nimptsch K, Rohrmann S. Associations of current, childhood, and adolescent milk intake with serum insulin-like growth factor (IGF)-1 and IGF binding protein 3 concentrations in adulthood. Nutr Cancer. 2019;71(6):931–8. 10.1080/01635581.2019.1595044. This study identified long-term and short-term associations between cow milk intake and IGF-1 and IGFBP-3 levels, whereby associations varied by race, ethnicity, and sex. [DOI] [PubMed]
- 185.Melnik BC, Weiskirchen R, Schmitz G. Milk exosomal microRNAs: friend or foe?–a narrative review. ExRNA; 2022. [Google Scholar]
- 186.• Han SA, Kim SW. BRCA and breast cancer-related high-penetrance genes. Adv Exp Med Biol. 2021;1187:473–90. 10.1007/978-981-32-9620-6_25. High-penetrance breast cancer susceptibility genes deliberate a greater than tenfold relative risk of breast cancer. BRCA1 and BRCA2 genes are the most common cause of hereditary breast cancer. [DOI] [PubMed]
- 187.•• Rebbeck TR, Friebel TM, Friedman E, Hamann U, Huo D, Kwong A, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat. 2018;39(5):593–620. 10.1002/humu.23406. This multicenter study comprehensively describes the characteristics of the 1650 unique BRCA1 and 1731 unique BRCA2 deleterious (disease-associated) mutations identified in the CIMBA database and observed substantial variation in mutation type and frequency by geographical region and race/ethnicity. [DOI] [PMC free article] [PubMed]
- 188.Khoury-Shakour S, Lejbkowicz F, Barnett-Griness O, Tamir A, Pinchev M, Rennert G. Genetic variation in IGF-1 and breast cancer risk in Ashkenazi carriers and noncarriers of BRCA1/2 mutations. Eur J Cancer Prev. 2009;18(5):361–367. doi: 10.1097/CEJ.0b013e32832e0942. [DOI] [PubMed] [Google Scholar]
- 189.Costa-Silva DR, Barros-Oliveira MD, Borges RS, Tavares CB, Borges US, Alves-Ribeiro FA, et al. Insulin-like growth factor 1 gene polymorphism and breast cancer risk. An Acad Bras Cienc. 2016;88(4):2349–2356. doi: 10.1590/0001-3765201620160169. [DOI] [PubMed] [Google Scholar]
- 190.• Pearson-Stuttard J, Papadimitriou N, Markozannes G, Cividini S, Kakourou A, Gill D, et al. Type 2 diabetes and cancer: an umbrella review of observational and Mendelian randomization studies. Cancer Epidemiol Biomarkers Prev. 2021;30(6):1218–28. 10.1158/1055-9965.EPI-20-1245. This comprehensive review of observational and Mendelian randomization studies links type 2 diabetes to an increase risk of breast cancer, two disease entities related to milk consumption. [DOI] [PMC free article] [PubMed]
- 191.Belardi V, Gallagher EJ, Novosyadlyy R, LeRoith D. Insulin and IGFs in obesity-related breast cancer. J Mammary Gland Biol Neoplasia. 2013;18(3–4):277–289. doi: 10.1007/s10911-013-9303-7. [DOI] [PubMed] [Google Scholar]
- 192.Kang C, LeRoith D, Gallagher EJ. Diabetes, obesity, and breast cancer. Endocrinology. 2018;159(11):3801–3812. doi: 10.1210/en.2018-00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Melnik BC. The pathogenic role of persistent milk signaling in mTORC1- and milk-microRNA-driven type 2 diabetes mellitus. Curr Diabetes Rev. 2015;11(1):46–62. doi: 10.2174/1573399811666150114100653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.•• Melnik BC, Schmitz G. Milk exosomal microRNAs: postnatal promoters of β cell proliferation but potential inducers of β cell de-differentiation in adult life. Int J Mol Sci. 2022;23:11503. 10.3390/ijms231911503. This extensive review provides signaling pathways mediated by cow milk-derived exosomal microRNAs in promotingβcell de-differentiation, a recent concept of type 2 diabetes pathogenesis. [DOI] [PMC free article] [PubMed]
- 195.de Candia P, Spinetti G, Specchia C, Sangalli E, La Sala L, Uccellatore A, et al. A unique plasma microRNA profile defines type 2 diabetes progression. PLoS One. 2017;12(12):e0188980. doi: 10.1371/journal.pone.0188980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Doghish AS, Elsisi AM, Amin AI, Abulsoud AI. Circulating miR-148a-5p and miR-21-5p as novel diagnostic biomarkers in adult Egyptian male patients with metabolic syndrome. Can J Diabetes. 2021;45(7):614–618. doi: 10.1016/j.jcjd.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 197.Melnik BC, John SM, Carrera-Bastos P, Cordain L. The impact of cow’s milk-mediated mTORC1-signaling in the initiation and progression of prostate cancer. Nutr Metab. 2012;9(1):74. doi: 10.1186/1743-7075-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Melnik BC, John SM, Weiskirchen R, Schmitz G. The endocrine and epigenetic impact of persistent cow milk consumption on prostate carcinogenesis. J Transl Genet Genom. 2022;6:1–45. doi: 10.20517/jtgg.2021.37. [DOI] [Google Scholar]
- 199.Orlich MJ, Mashchak AD, Jaceldo-Siegl K, Utt JT, Knutsen SF, Sveen LE, et al. Dairy foods, calcium intakes, and risk of incident prostate cancer in Adventist Health Study-2. Am J Clin Nutr. 2022;116(2):314–324. doi: 10.1093/ajcn/nqac093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Johns Hopkins Medicine. Nutrition during pregnancy. https://www.hopkinsmedicine.org/health/wellness-and-prevention/nutrition-during-pregnancy. Accessed 18 Sept 2022
- 201.European Commission. Agriculture and rural development. School fruit, vegetables and milk scheme. https://agriculture.ec.europa.eu/common-agricultural-policy/market-measures/school-fruit-vegetables-and-milk-scheme_en. Accessed 18 Sept 2022
- 202.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th edn. Washington, DC: U.S. Department of Health and Human Services and U.S. Department of Agriculture; 2015. https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines/previous-dietary-guidelines/2015. Accessed 18 Sept 2022
- 203.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans 2020–2025. https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf. Accessed 18 Sept 2022
- 204.Wehbe Z, Kreydiyyeh S. Cow’s milk may be delivering potentially harmful undetected cargoes to humans. Is it time to reconsider dairy recommendations? Nutr Rev. 2022;80(4):874–888. doi: 10.1093/nutrit/nuab046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.