Abstract
Objectives:
To determine if higher artificially sweetened beverage intake is associated with higher prevalence of urinary incontinence symptoms.
Methods:
We conducted a secondary analysis of data from the Women’s Health Initiative Observational Study. Our analytic cohort included 80,388 women. Participants who answered questions about beverage consumption and urinary incontinence symptoms at a 3 year follow up visit were included. Demographic characteristics were compared between three groups of beverage consumers: never to <1 serving/week, 1–6 servings/week, and ≥1 serving/day. Multivariable logistic regression models were constructed to estimate odds and type of urinary incontinence and adjust for potential confounders.
Results:
Most participants (64%) were rare consumers of artificially sweetened beverages, with 13% (n= 10,494) consuming ≥1 serving/day. The unadjusted odds of reporting urinary incontinence were 10–12% higher in women consuming 1–6 servings/week (OR 1.10, 95% CI 1.06–1.14) or ≥1 serving/day (OR 1.12, 95% CI 1.07– 1.18) vs never to <1 serving/week. In multivariable analyses, women consuming ≥1 serving/day (ref: never to <1 serving/week) had 10% higher odds of reporting mixed urinary incontinence (OR 1.10, 95% CI 1.02– 1.19). There were no significant differences for stress or urgency urinary incontinence symptoms between groups.
Conclusions:
When compared to never to <1 serving/week, women consuming ≥1 serving/day of artificially sweetened beverages had 10% greater odds of reporting mixed urinary incontinence after adjustments. Amount of artificially sweetened beverage consumption was not associated with stress or urgency urinary incontinence symptoms.
Keywords: incontinence, urinary incontinence, artificial sweeteners, artificially sweetened beverages, mixed incontinence, stress incontinence
Introduction:
Lower urinary tract symptoms (LUTS) affect approximately 30% of women (1,2), and urinary incontinence (UI) affects nearly 20% of women over age 50 (3,4). UI is also associated with significant comorbidities, including cognitive impairment, functional decline, falls, fractures, stroke, depression, and poor quality of life (5–7). Collectively, UI is thought to account for more than $60 billion in annual direct costs in the United States (8), and efforts to reduce this burden would have a significant impact on health care.
Anecdotally, several foods and drinks such as artificially sweetened beverages (ASB)s are thought to have adverse effects on the bladder and lower urinary tract. Thus, many clinicians recommend avoiding ASBs to reduce LUTS and UI, though evidence to support this recommendation is lacking. In rat models, artificial sweeteners have been shown to enhance detrusor muscle contraction (16), but limited evidence exists establishing a relationship between ASB intake and UI symptoms in humans. While studies have investigated the link between LUTS and UI with fluid intake and carbonated drinks (9–15), the relationship between ASBs and UI has been understudied.
The objective of this study was to examine the association between ASB consumption and UI symptoms. We hypothesized that higher levels of reported ASB consumption would be associated with higher prevalence of reported UI symptoms. We also aimed to identify whether ASB consumption was differentially associated with different types of UI (stress urinary incontinence, or SUI; urgency urinary incontinence, or UUI; and mixed urinary incontinence, or MUI).
Methods:
The Women’s Health Initiative Observational Study (WHI-OS) is a prospective, multicenter cohort study of 93,676 postmenopausal women. Detailed methods have been previously published elsewhere (17). Briefly, women between the ages of 50 and 79 years old were identified at 40 clinical centers across the United States and enrolled from 1993 to 1998. Women completed several self-administered questionnaires and WHI staff collected anthropometric measures at enrollment and throughout follow-up. In a follow up visit 3 years after enrollment, participants completed a questionnaire that asked them to estimate their consumption of ASB. Women also completed self-administered UI questionnaires at baseline and after 3 years of follow-up. The overall WHI protocol was approved by the institutional review boards of participating institutions, and all participants provided written informed consent for their study activities.
Ascertainment of ASB consumption and UI symptoms:
In this study, we included data from all women who participated in the WHI-OS who answered questions about ASB consumption and UI symptoms at their year 3 follow-up visit (n = 80,388). We excluded participants if they did not complete the incontinence questions or did not complete the annual follow-up question at year 3 about ASB consumption (n = 13,288). The question regarding ASBs was as follows: “During the past three months, how often did you drink these beverages?” (Beverages refer to “diet drinks such as diet [soda] or diet fruit drinks,” with a 12 fl. oz. can as a reference serving size.) Frequency of ASB consumption was described in nine categories: never or less than one serving per month (reference), 1–3 per month, 1 per week, 2–4 per week, 5–6 per week, 1 per day, 2–3 per day, 4–5 per day, 6 or more per day. These categories were collapsed for our analysis into 3 categories: never or less than one per week (reference), 1–6 per week, and 1 or more per day.
We defined prevalent UI as answering “yes” to the question “Have you ever leaked even a very small amount of urine involuntarily and you couldn’t control it?” on the year 3 follow-up questionnaire. We further categorized UI subtype using responses to the question “When do you usually leak urine?” Participants were defined as having urgency UI (UUI) if they selected only “When I feel the need to urinate and can’t get to the toilet fast enough,” stress UI (SUI) if they selected only “When I cough, laugh, sneeze, lift, stand up or exercise,” and mixed UI (MUI) if they selected multiple responses to this question. Those who answered that they leaked urine but did not have answers that fit into the above categories were classified as having “unknown/other” incontinence.
We collected data on demographic variables of participants including age, race, ethnicity, neighborhood socioeconomic status (nSES), body mass index (BMI), parity, use of diuretic medications, diabetes, hypertension, hormone therapy use, history of myocardial infarction, and history of congestive heart failure that was reported either during the participant’s initial screening visit or at their year 3 follow up visit. We also abstracted data on additional dietary or activity variables that could possibly relate to UI symptoms, including smoking history, alcohol intake, recreational physical activity, diet quality or healthy eating index (HEI), caffeine intake, and water consumption. NSES was defined based on U.S. census tracts from the 2000 census, with index ranges from 0 to 100 where higher scores indicate more affluent tracts (18). Diet quality was assessed using the Healthy Eating Index 2015 score, which is a measure of diet quality that assesses conformity to US Dietary Guidelines 2015 (19). Self-reported recreational physical activity was assessed using information about duration, frequency, and intensity of activity, as previously described (20).
Descriptive statistics were reported by ASB consumption groups, and comparisons were made using chi-square tests for categorical variables and ANOVA for continuous variables. A multivariable logistic regression model was constructed to estimate odds of reporting UI and adjusted for potential confounders including age, race, ethnicity, nSES, smoking, alcohol, caffeine, parity, diuretic use, diabetes, water consumption, BMI, hormone therapy use, physical activity, and diet quality (HEI). Cases with incomplete data were not included in adjusted models. All analyses were performed using SAS statistical software version 9.4 (SAS Institute Inc, Cary, NC), and all statistical testing with a significance level of 0.05.
Results:
Demographic features of the cohort are shown in Table 1. Most participants (64%) were infrequent consumers of ASBs, with 13% (n= 10,494) consuming ≥1 serving/day. Women who consumed a higher number of ASBs were younger, had lower neighborhood socioeconomic status, were more likely to be White and not of Hispanic origin, had higher BMIs and lower parity. They were also more likely to have diabetes, hypertension, congestive heart failure (CHF), a history of a myocardial infarction (MI), and use diuretic medications and hormone therapy. Women with higher ASB consumption were more commonly smokers, were less physically active, and had poorer quality diets; they also drank less alcohol and water but consumed more caffeine than women with lower ASB consumption.
Table 1a:
Characteristics of Participants by Frequency of Artificially Sweetened Beverage Consumption- Demographics
| Frequency of Artificially Sweetened Beverage Consumption | |||||
|---|---|---|---|---|---|
|
| |||||
| Overall | Never or <1 serving /week | 1–6 serving/week | ≥1 serving/day | ||
|
| |||||
| 80,388 | 51,480 | 18,414 | 10,494 | ||
|
| |||||
| Mean (SD) or N (%) | Mean (SD) or N (%) | Mean (SD) or N (%) | Mean (SD) or N (%) | P-value | |
|
| |||||
| Age (Mean (SD)) | 66.6 (7.3) | 67.3 (7.3) | 66.0 (7.1) | 64.4 (7.0) | <.001 |
|
| |||||
| nSES (Mean (SD)) | 76.3 (8.3) | 76.4 (8.2) | 76.2 (8.3) | 76.0 (8.2) | <.001 |
| Missing | 8,345 | 5,298 | 1,927 | 1,120 | |
|
| |||||
| Race/Ethnicitya | |||||
| American Indian or Alaskan Native | 314 (0.4) | 195 (0.4) | 63 (0.3) | 56 (0.5) | <.001 |
| Asian or Pacific Islander | 2,293 (2.9) | 1,727 (3.4) | 387 (2.1) | 179 (1.7) | |
| Black or African American | 5,552 (6.9) | 3,610 (7.0) | 1,267 (6.9) | 675 (6.4) | |
| Hispanic/Latino | 2,557 (3.2) | 1,661 (3.2) | 572 (3.1) | 324 (3.1) | |
| White (not of Hispanic origin) | 68,590 (85.3) | 43,542 (84.6) | 15,908 (86.4) | 9,140 (87.1) | |
| Unknown or not listed above | 1,082 (1.3) | 745 (1.4) | 217 (1.2) | 120 (1.1) | |
|
| |||||
| BMI (kg/m2) | |||||
| <25 | 29,425 (39.7) | 21,636 (45.4) | 5,353 (31.6) | 2,436 (25.5) | <.001 |
| 25–<30 | 25,747 (34.7) | 16,041 (33.6) | 6,313 (37.3) | 3,393 (35.5) | |
| ≥30 | 18,984 (25.6) | 9,996 (21.0) | 5,271 (31.1) | 3,717 (38.9) | |
| Missing | 6,232 | 3,807 | 1,477 | 948 | |
|
| |||||
| Number of Live Births | |||||
| Never pregnant | 8,116 (10.2) | 5,342 (10.4) | 1,721 (9.4) | 1,053 (10.1) | <.001 |
| None | 2,307 (2.9) | 1,507 (2.9) | 488 (2.7) | 312 (3.0) | |
| 1 | 7,374 (9.2) | 4,755 (9.3) | 1,647 (9.0) | 972 (9.3) | |
| 2–4 | 52,485 (65.7) | 33,338 (65.2) | 12,230 (66.8) | 6,917 (66.3) | |
| 5+ | 9,590 (12.0) | 6,195 (12.1) | 2,214 (12.1) | 1,181 (11.3) | |
| Missing | 516 | 343 | 114 | 59 | |
Data expressed as N (%) unless otherwise indicated. P-value is chi-square for categorical variables and ANOVA for continuous variables. Measures were collected or updated to year 3 except race/ethnicity.
WHI Race and Ethnicity Variables for this analysis were defined and collected at baseline enrollment (1993–1998) on Form 2, Question #15, which asked participants to “describe your race or ethnic group.” 6 categories were options: (1) American Indian or Alaska Native; (2) Asian or Pacific Islander (ancestry is Chinese, Indo- Chinese, Korean, Japanese, Pacific Islander, Vietnamese); (3) Black or African-American (not of Hispanic origin); (4) Hispanic/Latino (ancestry is Mexican, Cuban, Puerto Rican, Central American, or South American); (5) White (not of Hispanic origin); and, (“6”) Other (Specify). [Notes: “Other” was checked by 1849 participants; 413 participants left the question blank; therefore, the data are “missing”.] For this analysis, the group that selected “other” is listed as “unknown or not listed above.”
BMI: body mass index
Kg: kilograms
M2: meters squared
nSES: neighborhood socioeconomic status
SD: standard deviation
Most women (74.7%) in the cohort reported UI symptoms, with 27.2% reporting SUI, 27.3% reporting UUI, 14.4% reporting MUI, and 5.8% reporting other/unknown type of incontinence (Table 2). Women with higher ASB consumption were more likely to report UI symptoms, specifically SUI and MUI, but not UUI.
Table 2:
Urinary Incontinence Symptoms of Participants by Frequency of Artificially Sweetened Beverage Consumption
| Frequency of Artificially Sweetened Beverage Consumption | |||||
|---|---|---|---|---|---|
| Overall | Rare (Never or <1 serving /week) | Frequent (1–6 serving /week) | Daily (≥1 serving /day) | ||
| 80,388 | 51,480 | 18,414 | 10,494 | ||
| N (%) | N (%) | N (%) | N (%) | P-value | |
| Urinary Incontinence (UI) | |||||
| No | 20,319 (25.3) | 13,364 (26.0) | 4,452 (24.2) | 2,503 (23.9) | <.001 |
| Yes | 60,069 (74.7) | 38,116 (74.0) | 13,962 (75.8) | 7,991 (76.1) | |
| Urinary Incontinence Type | |||||
| None | 20,319 (25.3) | 13,364 (26.0) | 4,452 (24.2) | 2,503 (23.9) | <.001 |
| Stress (SUI) | 21,876 (27.2) | 13,835 (26.9) | 5,077 (27.6) | 2,964 (28.2) | |
| Urge (UUI) | 21,967 (27.3) | 14,061 (27.3) | 5,143 (27.9) | 2,763 (26.3) | |
| Mixed (MUI) | 11,550 (14.4) | 7,096 (13.8) | 2,755 (15.0) | 1,699 (16.2) | |
| Unknown/Other | 4,676 (5.8) | 3,124 (6.1) | 987 (5.4) | 565 (5.4) | |
Data expressed as N (%) unless otherwise indicated. P-value is chi-square for categorical variables and ANOVA for continuous variables. Measures were collected or updated to year 3.
MUI: mixed urinary incontinence; SUI: stress urinary incontinence; UI: urinary incontinence; UUI: urge urinary incontinence
Adjusted odds of reporting UI symptoms relative to level of ASB consumption are shown in Table 3. The unadjusted odds of reporting UI were 10–12% higher in women who consumed 1 serving of ASB at least weekly vs rare consumption (OR 1.10, 95% CI 1.06–1.14 for those who consumed 1–6 servings of ASBs/week, and OR 1.12, 95% CI 1.07– 1.18 for those who consumed ≥1 servings of ASBs daily). These associations were no longer seen after adjustments (Figure 1). In multivariable adjusted models investigating type of UI, women consuming ≥1 ASB serving per day compared to <1 serving/week had 10% higher odds of reporting MUI (OR 1.10, 95% CI 1.02– 1.19) but no significant differences for SUI or UUI symptoms were seen between those consuming <1 serving/week vs 1–6 servings/week or ≥1 servings/day.
Table 3:
Odds Ratios and 95% Confidence Intervals for Artificially Sweetened Beverage Consumption and Urinary incontinence
| Artificially Sweetened Beverage | ||||
|---|---|---|---|---|
|
| ||||
| Never or <1/week | 1–6/week | 1 or more/day | ||
|
| ||||
| Urinary Incontinence | N | 51,480 | 18,414 | 10,494 |
| Adjusted OR (95% CI) | ref | 1.01 (0.97–1.06) | 1.04 (0.98–1.11) | |
|
| ||||
| Type of incontinence | ||||
|
| ||||
| Urge | N | 14,061 | 5,143 | 2,763 |
| Adjusted OR (95% CI) | ref | 1.05 (1.00–1.11) | 1.05 (0.98–1.13) | |
|
| ||||
| Stress | N | 13,835 | 5,077 | 2,964 |
| Adjusted OR (95% CI) | ref | 0.99 (0.94–1.05) | 1.02 (0.95–1.09) | |
|
| ||||
| Mixed | N | 7,096 | 2,755 | 1,699 |
| Adjusted OR (95% CI) | ref | 1.02 (0.96–1.09) | 1.10 (1.01–1.19) | |
|
| ||||
| Unknown | N | 3,124 | 987 | 565 |
| Adjusted OR (95% CI) | ref | 0.90 (0.82–0.98) | 0.98 (0.87–1.10) | |
Type of incontinence compared to No urinary incontinence in polytomous logistic regression. The model adjusted for age, race/ethnicity, NSES (neighborhood socioeconomic status), smoking, alcohol, caffeine, # live births, diuretic use, diabetes, water consumption, BMI, Hormone Therapy use, physical activity, diet quality (HEI). Cases with incomplete data were not included in adjusted models (N=64,464)
Figure 1:
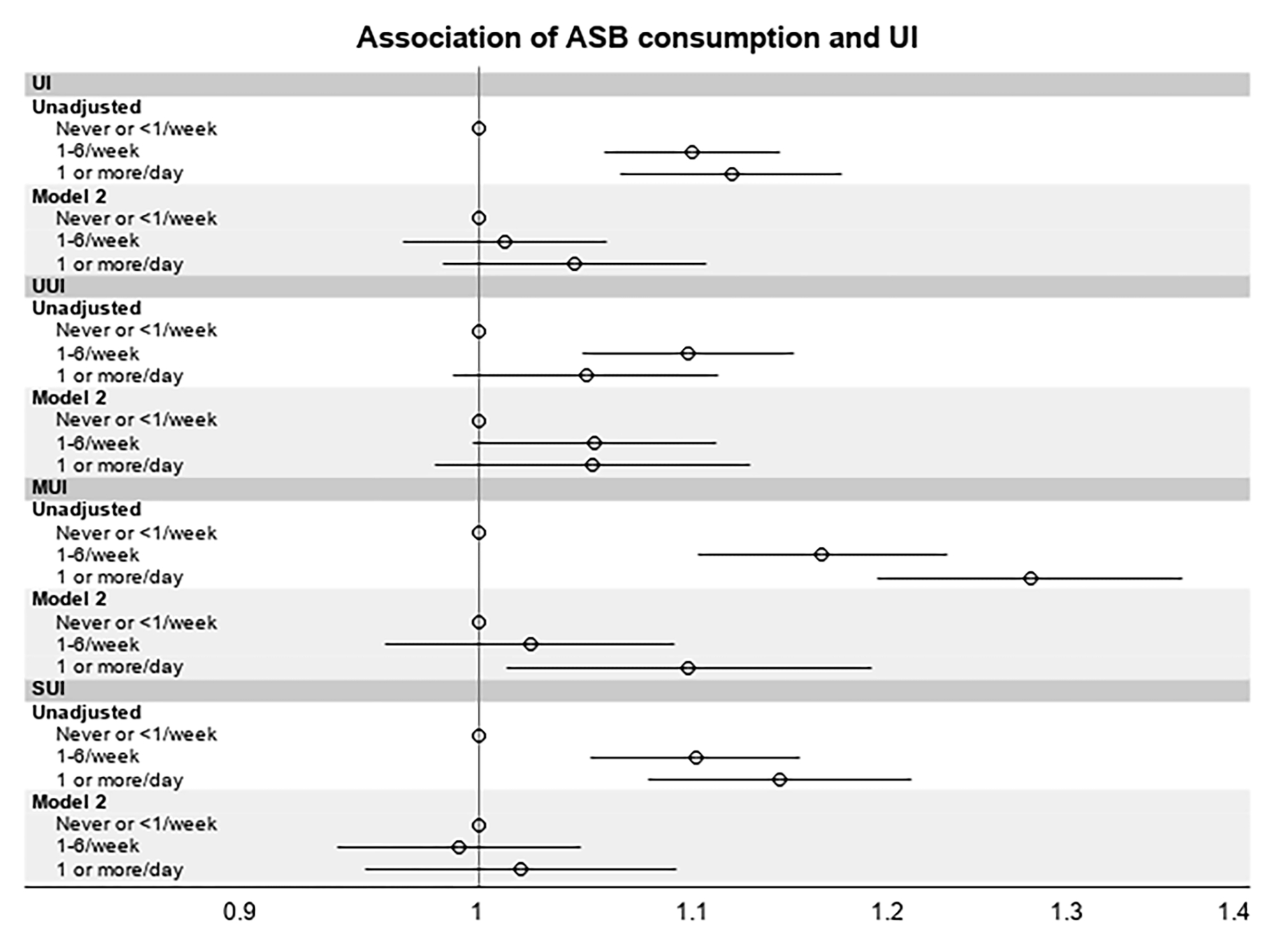
Relationship Between ASB Consumption Frequency and Odds of Reporting UI Symptoms
ASB = artificially sweetened beverage; MUI = mixed urinary incontinence; SUI = stress urinary incontinence; UI = urinary incontinence; UUI = urgency urinary incontinence;
Frequency of ASB consumption is self-reported approximate number of 12 oz. servings consumed per unit time. Odds Ratios depicted are unadjusted and adjusted odds ratios for each type of UI symptoms comparing ASB consumption categories.
Discussion:
In this analysis of the Women’s Health Initiative Observational Study, the largest US cohort study of postmenopausal women, we found that higher ASB consumption was not associated with odds of reporting SUI or UUI symptoms. When compared to <1 serving/week, women consuming ≥1 ASB per day had 10% greater odds of reporting MUI after adjusting for potential confounders. This study is the largest study to date investigating the relationship between artificially sweetened beverages and urinary incontinence symptoms in postmenopausal women. While previous studies have shown a relationship between reduction in certain fluids and lower urinary tract symptoms (14), the specific relationship between ASBs and UI has been understudied, despite guidelines recommending avoidance of bladder irritants like ASBs to control LUTS (21).
In this cohort, we found higher ASB consumption to be associated with factors known to be associated with UI symptoms, including elevated BMI, increased caffeine consumption, diagnosis of diabetes, and diuretic use, among others. Since these variables were likely to be significant confounders in the association between ASB intake and UI symptoms, our multivariable logistic regression models were important in understanding the role that ASBs specifically may play in reporting incontinence symptoms. We constructed a model to adjust for variables like BMI, physical activity, diet quality, and other medical comorbidities known to be related to UI (22), and after adjustments neither SUI nor UUI symptoms were associated with ASB consumption. These findings suggest that the association of ASB intake with UI was confounded by BMI and comorbidities. It is possible that women with UI chose to limit beverage consumption, and reverse causation bias may have led to more UI in women reporting lower beverage consumption. The potential for reverse causation bias may mask any true association between increased ASB consumption and prevalence of UI, though the lack of association after adjustments for confounders remains notable.
While the association between ASB consumption and MUI symptoms was significant, potential reasons for this link while other incontinence types lack a relationship are not clear. It is possible that the association of ASBs with bladder irritation may be synergistic when multiple UI pathologies exist, and it is also possible that additional confounders were present in the group that reported MUI which were not measured and influenced our results. Additionally, a 10% increased odds of reporting MUI symptoms with high ASB consumption is of uncertain clinical significance, and this study utilized a sample with extremely high statistical power to detect even small differences between groups. Since the lower bound of the confidence interval was low (1.01), it is possible that the clinical significance of this finding is limited. As behavior change is often difficult to achieve, particularly regarding reduction of fluid intake and reduction in consumption of bladder irritants (14), these findings suggest that clinicians may find greater utility in focusing behavior change counseling on behaviors likely to have more of an impact on UI symptoms like total volume of fluid intake rather than beverage type. Additionally, as consumption of ASBs can also be important in avoiding sugar-sweetened beverages (which may have more deleterious effects on health that also worsen incontinence, including weight gain, development/worsening of diabetes, etc.), clinicians could consider redirecting counseling efforts away from avoiding ASBs for this reason.
Strengths of this study include the use of a large sample of postmenopausal women with detailed information on numerous demographic and behavioral variables. The ability to adjust for multiple potential confounders, including variables not commonly available in most databases like diet quality and physical activity, makes this study uniquely suited to isolating the potential relationship between ASBs and UI symptoms. However, there are also several limitations to this study. The primary limitation of this study is the cross-sectional design, as causation and directionality of the results cannot be determined. Additionally, while the WHI-OS has detailed information about a large number of variables and behaviors, additional variables that were not measured or included in our models may have influenced our findings. Specifically, dietary factors known to influence urinary incontinence symptoms such as carbonated beverages may have varied among groups, and this could have confounded our results. We also did not have data regarding potentially confounding factors for incontinence treatment, including use of overactive bladder medications or history of surgical treatment for SUI that may have reduced UI prevalence. ASB consumption was also self-reported, and thus this data may contain inaccuracies from recall bias. Also, the urinary incontinence question on the year 3 questionnaire included all participants who reported ever having a urinary incontinence episode, rather than participants who reported frequent or regular UI episodes. Thus, the association of ASBs may have been more or less pronounced in women with more frequent incontinence episodes. Our analysis also did not investigate whether higher daily ASB intake (e.g. 2 or more servings daily) correlates more substantially with UI symptoms. Lastly, this study was also observational rather than a clinical trial, and observed associations do not necessarily indicate causation. Future studies should include prospective data collection in order to clarify the relationship between ASB consumption and prevalence or incidence of UI symptoms.
Conclusions:
In this study of postmenopausal women in the United States, consumption of ASBs was not significantly associated with UI symptoms, a finding that may directly impact patient counseling with respect to beverage and fluid management. Further study is needed in order to quantify the association of other beverages thought to be associated with LUTS and UI symptoms, which will offer patients more accurate information on the relative impact that fluid management is likely to have on their UI symptoms.
Table 1b:
Characteristics of Participants by Frequency of Artificially Sweetened Beverage Consumption- Medical Comorbidities
| Frequency of Artificially Sweetened Beverage Consumption | |||||
|---|---|---|---|---|---|
|
| |||||
| Overall | Never or <1 serving /week | 1–6 serving/week | ≥1 serving/day | ||
|
| |||||
| 80,388 | 51,480 | 18,414 | 10,494 | ||
|
| |||||
| N (%) | N (%) | N (%) | N (%) | P-value | |
|
| |||||
| Treated Diabetes | 4,390 (5.5) | 1,804 (3.5) | 1,450 (7.9) | 1,136 (10.8) | <.001 |
| Missing | 75 | 51 | 11 | 13 | |
|
| |||||
| Treated Hypertension | 28,655 (36.0) | 17,611 (34.6) | 6,975 (38.3) | 4,069 (39.1) | <.001 |
| Missing | 786 | 516 | 184 | 86 | |
|
| |||||
| History of MI | 2,202 (2.7) | 1,342 (2.6) | 537 (2.9) | 323 (3.1) | 0.006 |
| Missing | |||||
|
| |||||
| History of CHF | 1,192 (1.5) | 675 (1.3) | 312 (1.7) | 205 (2.0) | <.001 |
| Missing | |||||
| Hormone Therapy use | |||||
| Never used | 26,654 (33.2) | 17,685 (34.4) | 5,691 (30.9) | 3,278 (31.2) | <.001 |
| Past user | 11,198 (13.9) | 7,354 (14.3) | 2,471 (13.4) | 1,373 (13.1) | |
| Current user | 42,519 (52.9) | 26,429 (51.4) | 10,250 (55.7) | 5,840 (55.7) | |
| Missing | 17 | 12 | 2 | 3 | |
|
| |||||
| Diuretic medication use (current) | 14,059 (18.4) | 8,426 (17.2) | 3,499 (20.1) | 2,134 (21.6) | <.001 |
| Missing | 4,157 | 2,547 | 980 | 630 | |
Data expressed as N (%) unless otherwise indicated. P-value is chi-square for categorical variables and ANOVA for continuous variables. Measures were collected or updated to year 3.
CHF: congestive heart failure
MI: myocardial infarction
Table 1c:
Characteristics of Participants by Frequency of Artificially Sweetened Beverage Consumption- Behaviors
| Frequency of Artificially Sweetened Beverage Consumption | |||||
|---|---|---|---|---|---|
|
| |||||
| Overall | Never or <1 serving /week | 1–6 serving/week | ≥1 serving/day | ||
|
| |||||
| 80,388 | 51,480 | 18,414 | 10,494 | ||
|
| |||||
| N (%) | N (%) | N (%) | N (%) | P-value | |
|
| |||||
| Smoking status | |||||
| Never Smoked | 40,817 (51.4) | 26,872 (52.8) | 9,168 (50.4) | 4,777 (46.0) | <.001 |
| Past Smoker | 34,902 (43.9) | 21,554 (42.4) | 8,347 (45.9) | 5,001 (48.1) | |
| Current Smoker | 3,737 (4.7) | 2,438 (4.8) | 690 (3.8) | 609 (5.9) | |
| Missing | 932 | 616 | 209 | 107 | |
|
| |||||
| Recreational Physical Activity (MET hours/week) (Mean (SD)) | 13.6 (14.6) | 14.0 (14.8) | 13.4 (14.0) | 12.3 (14.0) | <.001 |
| Missing | 153 | 99 | 32 | 22 | |
|
| |||||
| HEI 2015 (Mean (SD)) | 67.8 (10.3) | 68.5 (10.3) | 67.5 (9.6) | 64.8 (10.4) | <.001 |
| Missing | 561 | 363 | 122 | 76 | |
|
| |||||
| Caffeine (mg) (Mean (SD)) | 143.6 (124.2) | 141.7 (123.2) | 147.1 (123.5) | 146.5 (129.7) | <.001 |
| Missing | 561 | 363 | 122 | 76 | |
|
| |||||
| Water consumption (8oz serving) | |||||
| <1/day | 8,597 (10.7) | 5,042 (9.8) | 2,154 (11.7) | 1,401 (13.4) | <.001 |
| 1–5/day | 53,292 (66.3) | 33,867 (65.8) | 12,231 (66.5) | 7,194 (68.6) | |
| ≥6/day | 18,457 (23.0) | 12,552 (24.4) | 4,017 (21.8) | 1,888 (18.0) | |
Data expressed as N (%) unless otherwise indicated. P-value is chi-square for categorical variables and ANOVA for continuous variables. Measures were collected or updated to year 3.
HEI: Healthy Eating Index
MET: Metabolic Equivalent of Task, where 1 MET = ratio of the rate of energy expended during an activity to the rate of energy expended at rest.
Mg: milligrams
Oz: ounces
SD: standard deviation
Acknowledgements:
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005.
Sources of funding:
The Women’s Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
Footnotes
Financial disclosures/Conflicts of interest: Cheryl B Iglesia reports the following disclosures: UpToDate contributor; SGS Soc Gyn Surgery Executive Board; NICHD Pelvic Floor Disorders Network Advisory Board, Chair, Editorial Board, Urogynecology; OBGManagement Medical Advisory Board: Patty Brisben Foundation; Foundation for Female Health Awareness; Healthy Women Foundation. The other authors have nothing to disclose.
Data from this study was presented at the 2021 American Urogynecologic Society’s Pelvic Floor Disorders Week Meeting
References
- 1.Moller L, Lose G, Jorgensen T. Incidence and remission rates of lower urinary tract symptoms at one year in women aged 40–60: longitudinal study. BMJ. 2000;320(7247):1429–32. doi: 10.1136/bmj.320.7247.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coyne K, Sexton C, Bell J, et al. The prevalence of (LUTS) and overactive bladder (OAB) by racial/ethnic group and age: results from OAB-POLL. Neurourol Urodyn. 2013; 32(3):230–237. doi: 10.1002/nau.22295. [DOI] [PubMed] [Google Scholar]
- 3.Gorina Y, Schappert S, Bercovitz A, Elgaddal N, Kramarow E. Prevalence of incontinence among older americans. Vital Health Stat 3. 2014;(36):1–33. [PubMed] [Google Scholar]
- 4.Wu J, Hundley A, Fulton R, Myers E. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstet Gynecol. 2009;114(6):1278–1283. doi: 10.1097/AOG.0b013e3181c2ce96. [DOI] [PubMed] [Google Scholar]
- 5.Perry S, Shaw C, Assassa P et al. An epidemiological study to establish the prevalence of urinary symptoms and felt need in the community: the Leicestershire MRC Incontinence Study. J Public Health Med 2000;22(3):427–34. doi: 10.1093/pubmed/22.3.427. [DOI] [PubMed] [Google Scholar]
- 6.Huang A, Brown J, Kanaya A, et al. Quality-of-life impact and treatment of urinary incontinence in ethnically diverse older women. Arch Intern Med. 2006;166(18):2000–2006. doi: 10.1001/archinte.166.18.2000. [DOI] [PubMed] [Google Scholar]
- 7.Brown J, Vittinghoff E, Wyman J, et al. Urinary incontinence: does it increase risk for falls and fractures? Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2000;48(7):721–725. doi: 10.1111/j.1532-5415.2000.tb04744.x. [DOI] [PubMed] [Google Scholar]
- 8.Coyne K, Wein A, Nicholson S, Kvasz M, Chen C, Milsom I. Economic burden of urgency urinary incontinence in the United States: a systematic review. J Manag Care Pharm. 2014;20(2):130–140. doi: 10.18553/jmcp.2014.20.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dallosso HM, McGrother CW, Matthews RJ, Donaldson MM; Leicestershire MRC Incontinence Study Group. The association of diet and other lifestyle factors with overactive bladder and stress incontinence: a longitudinal study in women. BJU Int. 2003;92(1):69–77. doi: 10.1046/j.1464-410x.2003.04271.x. [DOI] [PubMed] [Google Scholar]
- 10.Hashim H, Al Mousa R. Management of fluid intake in patients with overactive bladder. Curr Urol Rep. 2009;10(6):428–33. doi: 10.1007/s11934-009-0068-x. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald M, Ayuste D, Brubaker L. How do urinary diaries of women with an overactive bladder differ from those of asymptomatic controls? BJU Int. 2005;96(3):365–7. doi: 10.1111/j.1464-410X.2005.05632.x. [DOI] [PubMed] [Google Scholar]
- 12.Swithinbank L, Hashim H, Abrams P. The effect of fluid intake on urinary symptoms in women. J Urol. 2005;174(1):187–9. doi: 10.1097/01.ju.0000162020.10447.31. [DOI] [PubMed] [Google Scholar]
- 13.Robinson D, Giarenis I, Cardozo L. You are what you eat: the impact of diet on overactive bladder and lower urinary tract symptoms. Maturitas. 2014;79(1):8–13. doi: 10.1016/j.maturitas.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Miller JM, Garcia CE, Hortsch SB, Guo Y, Schimpf MO. Does instruction to eliminate coffee, tea, alcohol, carbonated, and artificially sweetened beverages improve lower urinary tract symptoms? A prospective trial. J Wound Ostomy Continence Nurs. 2016;43(1):69–79. doi: 10.1097/WON.0000000000000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradley CS, Erickson BA, Messersmith EE et al. Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN). Evidence of the impact of diet, fluid intake, caffeine, alcohol and tobacco on lower urinary tract symptoms: a systematic review. J Urol. 2017;198(5):1010–20. doi: 10.1016/j.juro.2017.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadgupta J, Elliott RA, Doshani A, Tincello DG. Enhancement of rat bladder contraction by artificial sweeteners via increased extracellular Ca2+ influx. Toxicol Appl Pharrmacol. 2006;217(2):216–24. doi: 10.1016/j.taap.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 18.Dubowitz T, Ghosh-Dastidar M, Eibner C et al. The Women’s Health Initiative: The food environment, neighborhood socioeconomic status, BMI, and blood pressure. Obesity (Silver Spring). 2012;20(4):862–71. doi: 10.1038/oby.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drewnowski A, Aggarwal A, Cook A, Stewart O, Moudon AV. Geographic disparities in Healthy Eating Index scores (HEI- 2005 and 2010) by residential property values: findings from Seattle Obesity Study (SOS). Prev Med. 2016:83:46–55. doi: 10.1016/j.ypmed.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson GL, Manson J, Wallace R et al. Impplementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9 supp;):S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 21.Gormley EA, Lightner DJ, Burgio KL et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline. J Urol 2012; 188:2455. doi: 10.1016/j.juro.2012.09.079. [DOI] [PubMed] [Google Scholar]
- 22.Bauer SR, Kenfield SA, Sorensen M et al. Physical activity, diet, and incident urinary incontinence in postmenopausal women: Women’s Health Initiative Observational Study. J Gerontol A Biol Sci Med Sci 2021; 76(9):1600–1607. doi: 10.1093/gerona/glab118. [DOI] [PMC free article] [PubMed] [Google Scholar]


