Abstract
On May 4–5, 2022, a meeting of multidisciplinary stakeholders in the prevention and treatment of venous thromboembolism (VTE) after trauma was convened by the Coalition for National Trauma Research (CNTR), funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH/NHLBI), and hosted by the American College of Surgeons (ACS) in Chicago, Illinois. This consensus conference gathered more than 40 in-person and 80 virtual attendees, including trauma surgeons, other physicians, thrombosis experts, nurses, pharmacists, researchers, and patient advocates. The objectives of the meeting were two-fold: 1) to review and summarize the present state of the scientific evidence regarding VTE prevention strategies in injured patients, and 2) to develop consensus on future priorities in VTE prevention implementation and research gaps.
To achieve these objectives, the first part of the conference consisted of talks from physician leaders, researchers, clinical champions, and patient advocates to summarize the current state of knowledge of VTE pathogenesis and prevention in patients with major injury. Video recordings of all talks and accompanying slides are freely available on the conference website. (https://www.nattrauma.org/research/research-policies-templates-guidelines/vte-conference/) Following this curriculum, the second part of the conference consisted of a series of small-group breakout sessions on topics potentially requiring future study. Through this process, research priorities were identified and plans of action to develop and undertake future studies were defined.
The 2022 Consensus Conference to Implement Optimal VTE Prophylaxis in Trauma answered the National Trauma Research Action Plan call to define a course for future research into preventing thromboembolism after trauma. A multidisciplinary group of clinical champions, physicians, scientists, and patients delineated clear objectives for future investigation to address important, persistent key knowledge gaps. The series of papers from the conference outline the consensus based on the current literature and a roadmap for research to answer these unanswered questions.
Keywords: Venous Thromboembolism, Pulmonary Embolism, Deep Vein Thrombosis, Blood Clots, Prevention
On May 4–5, 2022, a meeting of multidisciplinary stakeholders in the prevention and treatment of venous thromboembolism (VTE) after trauma was convened by the Coalition for National Trauma Research (CNTR), funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH/NHLBI), and hosted by the American College of Surgeons (ACS) in Chicago, Illinois. This consensus conference gathered more than 40 in-person and 80 virtual attendees, including trauma surgeons, other physicians, thrombosis experts, nurses, pharmacists, researchers, and patient advocates. The objectives of the meeting were two-fold: 1) to review and summarize the present state of the scientific evidence regarding VTE prevention strategies in injured patients, and 2) to develop consensus on future priorities in VTE prevention implementation and research gaps.
To achieve these objectives, the first part of the conference consisted of talks from physician leaders, researchers, clinical champions, and patient advocates to summarize the current state of knowledge of VTE pathogenesis and prevention in patients with major injury (Table 1). Video recordings of all talks and accompanying slides are freely available on the conference website. (https://www.nattrauma.org/research/research-policies-templates-guidelines/vte-conference/) Following this curriculum, the second part of the conference consisted of a series of small-group breakout sessions on topics potentially requiring future study (Table 2). Fostering future scientific leaders in the field, these breakout sessions were facilitated by early-career trauma surgeon recipients of four CNTR Young Investigator Travel Awards and two early-career trauma surgeons supported by the American Association for the Surgery of Trauma (AAST) and the Eastern Association for the Surgery of Trauma (EAST) in conjunction with established surgeon-scientist mentors. Through this process, research priorities were identified and plans of action to develop and undertake future studies were defined.
Table 1.
Overview of Organizers, Funded Early-Career Trauma Surgeon Travel Awardees, Speakers and Topics at the 2022 Consensus Conference to Implement Optimal Venous Thromboembolism (VTE) Prophylaxis in Trauma
| Conference Co-Directors | ||
|---|---|---|
| Todd Costantini, MD | University of California San Diego | |
| Elliott R. Haut, MD PhD | Johns Hopkins University | |
| Funded Early-Career Trauma Surgeon Travel Awardees | ||
| Navpreet Dhillon, MD | University of Maryland | NIH/NHLBI Funded |
| Ashanthi Ratnasekera, MD | Christiana Hospital | NIH/NHLBI Funded |
| Morgan Schellenberg, MD | University of Southern California | NIH/NHLBI Funded |
| Amanda Teichman, MD | Rutgers Robert Wood Johnson | NIH/NHLBI Funded |
| Lucy Z. Kornblith, MD | University of California San Francisco | AAST Funded |
| James Byrne, MD, PhD | Johns Hopkins University | EAST Funded |
| Speakers | Organization | Topic |
| Eileen Bulger, MD | Harborview Medical Center | Welcome and Opening Remarks |
| William Geerts, MD | Sunnybrook Health Sciences Centre | Overview of VTE in Trauma: Where have we been? Where are we going? |
| Eric Ley, MD | Cedars-Sinai Medical Center | Current Evidence-based Guidelines for VTE Prophylaxis |
| Ajai Malhotra, MD | University of Vermont | VTE as a Hospital Quality Indicator |
| Truman Milling, MD | University of Texas at Austin | Restarting Anticoagulation after Intracranial Hemorrhage |
| Bellal Joseph, MD | University of Arizona | DVT in Trauma: The Good, The Bad, and The Future |
| Thomas Beaumont, MD | University of California San Diego | VTE Prophylaxis: Neurosurgery Perspectives |
| Anna Miller, MD | Washington University in St. Louis | VTE Prophylaxis: Orthopedic Surgery Perspectives |
| M. Margaret Knudson, MD | University of California San Francisco | Overview and Update on CLOTT Studies |
| Elliott R. Haut, MD PhD | Johns Hopkins University | Overcoming Barriers to Delivering VTE Prophylaxis |
| Alok Khorana, MD | Case Western Reserve University | VTE Prophylaxis Post-Discharge |
| Mary Cushman, MD | University of Vermont | Health Disparities in VTE |
| Leslie Lake | National Blood Clot Alliance | The Patient Perspective |
| Rachel Kruer, PharmD | Indiana University Health | The Future of Pharmacologic VTE Prophylaxis: Are Direct Oral Anticoagulants the Answer? |
| Deborah Stein, MD | University of Maryland | The Aspirin Study: From ADAPT to PREVENT CLOT |
| John Fanikos, RPh MBA | Brigham & Women’s Hospital | Does Aspirin Have a Role in VTE Prevention in Trauma? |
| Todd Costantini, MD | University of California San Diego | Current Research Gaps: Review of National Trauma Research Agenda (NTRAP) Delphi Survey Results |
| Avery Nathens, MD PhD | Sunnybrook Health Sciences Centre | Utilizing the TQIP Infrastructure to Measure Outcomes and Implementation |
| Elliott R. Haut, MD PhD | Johns Hopkins University | Implementation Science Approaches and Outcome Measurement |
| Andrei Kindzelski, MD PhD Thuy-Vy Do, PhD Margie Shofer, BSN MBA COL Andrew Cap, MD |
NHLBI – NIH PCORI AHRQ US Army Inst. of Surgical Research |
Extramural Funding Opportunities and Priorities |
VTE- Venous Thromboembolism
TQIP- Trauma Quality Improvement Program
CLOTT - Consortium of Leaders in the Study of Traumatic Thromboembolism
NIH/NHLBI- National Institutes of Health / National Heart, Lung, and Blood Institute
PCORI- The Patient Centered Outcomes Research Institute
AHRQ- Agency for Healthcare Research & Quality
Table 2.
Topics for Break-out Sessions to Determine Future Research Priorities at the 2022 Consensus Conference to Implement Optimal Venous Thromboembolism (VTE) Prophylaxis in Trauma
| Timing of initiation of VTE prophylaxis for patients with high-risk injuries |
| Implementation of best practice early VTE prevention |
| Novel pharmacologic agents for VTE prevention |
| New approaches to optimizing VTE prevention |
| Extending VTE prophylaxis after acute hospitalization |
| Treatment of trauma-associated de novo pulmonary thrombosis |
The Patient Perspective: Central to the Mission
The patient must remain central to every mission in health care. Leslie Lake, the volunteer President of the National Blood Clot Alliance (NBCA), joined the conference to share her experience. When Leslie was struck by the sudden onset of shortness of breath in 2018, she did not expect ever to be standing before a room of medical professionals to tell her story four years later. Her interaction with the healthcare system would be formative. She presented to a busy emergency department in New York City, where she would subsequently undergo nine hours of waiting punctuated by evaluations that seemed unrelated to her symptoms, including three gynecologic examinations. On the verge of being discharged home with a diagnosis of fibroids, one provider prompted a CT angiogram of the chest which identified a pulmonary embolism (PE). She was promptly admitted to the intensive care unit.
Leslie passionately told her story of the considerable disconnect between her experience as a patient and the medical infrastructure around her. She received minimal information and so set about educating herself about blood clots. Discharged on apixaban, she found that her outpatient supports were few and opinions regarding her care plan were conflicting. Her journey of self-education led her to the NBCA. This organization’s website gave her the knowledge and language to advocate for herself (https://www.stoptheclot.org). She also found that as a finance professional, she was in a position that enabled her to navigate a complex health system to gain access to the ongoing medical care she needed. Through this process, she was struck by despair and anger that many people would not have been in a similar position to advocate for themselves. Leslie chose to act, to work so that others might not have to experience the disconnect that she had. She joined the NBCA Board of Directors and soon became its volunteer President.
The NBCA is a non-profit, patient-led advocacy organization founded in 2003 (1). Its formation stemmed from a collaboration between patients and researchers at the Centers for Disease Control (CDC), and work with the CDC is ongoing. Four-fifths of the NBCA’s Board of Directors are survivors or family members of those afflicted by VTE, and it is part of the organization’s charter that 50% of board members are patients. The organization’s mission is to advance the prevention, early diagnosis, and treatment of life-threatening thromboembolism. Supported by a Medical and Scientific Advisory Board, public engagement and education are the primary part of this mission.
Since 2015, the signature Stop the Clot, Spread the Word® initiative has reached more than 100 million people through its online resources (1). Several aspects of NBCA’s initiatives seek to target disparities in the incidence, treatment, and outcomes of VTE. Specifically, the Health Disparities Initiative is a quality improvement initiative aimed at improving health outcomes for African Americans affected by blood clots in rural areas, and the Health Equity Institute consists of an advisory council of Black VTE patients and healthcare providers focused on improving care in predominantly Black communities (2). The Women and Blood Clots initiative is dedicated to women’s unique risks and care needs, including VTE associated with hormone replacement therapy, birth control, and pregnancy. The Sports and Wellness Institute seeks to raise awareness that athletes are a vulnerable, often underdiagnosed population.
Patients remain at the center of the NBCA mission. Patients Educating Patients (PEP Talk) is a patient-driven support community. The Critical Look at Understanding the Emotional Suffering (CLUES) Blood Clot Study seeks to evaluate the emotional well-being of VTE patients, recognizing that post-traumatic stress is common among survivors (3).
Taken together, through the advocacy and work of patients like Leslie Lake, the NBCA provides a forum for VTE researchers to remain connected with the patient experience to ensure that this remains central to their mission. NBCA has provided the critically important patient voice to numerous PCORI funded projects related to VTE in trauma and non-trauma patient populations.
A Brief History of Venous Thromboembolism Research in Injured Patients
Dr. William Geerts led off the scientific curriculum of the conference, providing context with his nearly 30-year experience as one of the pre-eminent researchers in the field of VTE after trauma. Thromboembolism as a life-threatening complication after injury was first described long before the current era of trauma specialization. Paul von Bruns reported the occurrence of PE after fracture in 30 patients, many of which were fatal, in 1885 (4). Sevitt and Gallagher, in an autopsy series from the Birmingham Accident Hospital published in 1961, found deep vein thromboses (DVT) in 81% of trauma decedents (5). Of most significant concern, the authors concluded that more than 1% of all trauma admissions died of PE. In one of the first venography studies of trauma survivors published in 1967, Freeark et al. found that DVT occurred in 35% of immobilized patients, many of which were sub-clinical (6). Despite these findings, the pervasive opinion of the time was that “routine prophylactic treatment with anticoagulation may produce more trouble than it prevents” due to residual bleeding risk. These early investigations culminated in a call for action in the first thromboprophylaxis guidelines published by the National Institutes of Health Consensus Panel on VTE in 1986 (7). It was recognized that the “risks of thromboembolic diseases… have not been defined for the general trauma population” and the “efficacy and safety of various forms of prophylaxis have not been evaluated in patients with multisystem trauma.”
Based on the recognized need for high-quality investigation, Geerts et al. performed their landmark prospective study using venography in 349 consecutive admissions with severe injury (8). No mechanical or pharmacologic thromboprophylaxis was administered in this cohort that had a mean injury severity score (ISS) of 27. Venography identified DVT in 58% of patients, 18% of which were proximal DVT. This study identified independent risk factors for VTE in trauma, a list further built upon in subsequent studies (8–12) (Table 3). These data, confirming that major trauma patients are at very high risk for developing VTE, coincided with a time of increasing recognition of trauma as a unique disease process requiring sub-specialized care. As a result, the investigation into the subject has blossomed over the past three decades (Figure 1A).
Table 3.
Risk Factors for Venous Thromboembolism (VTE) in Trauma
| Age |
| Transfusion |
| Surgery |
| Injury severity score (ISS) |
| Pelvic or lower extremity fracture |
| Spinal cord injury |
| Major head injury |
| Major venous injury |
| Femoral venous catheter |
| Reduced mobility |
| Delay in VTE prophylaxis initiation |
| Missed doses of VTE prophylaxis |
Figure 1.
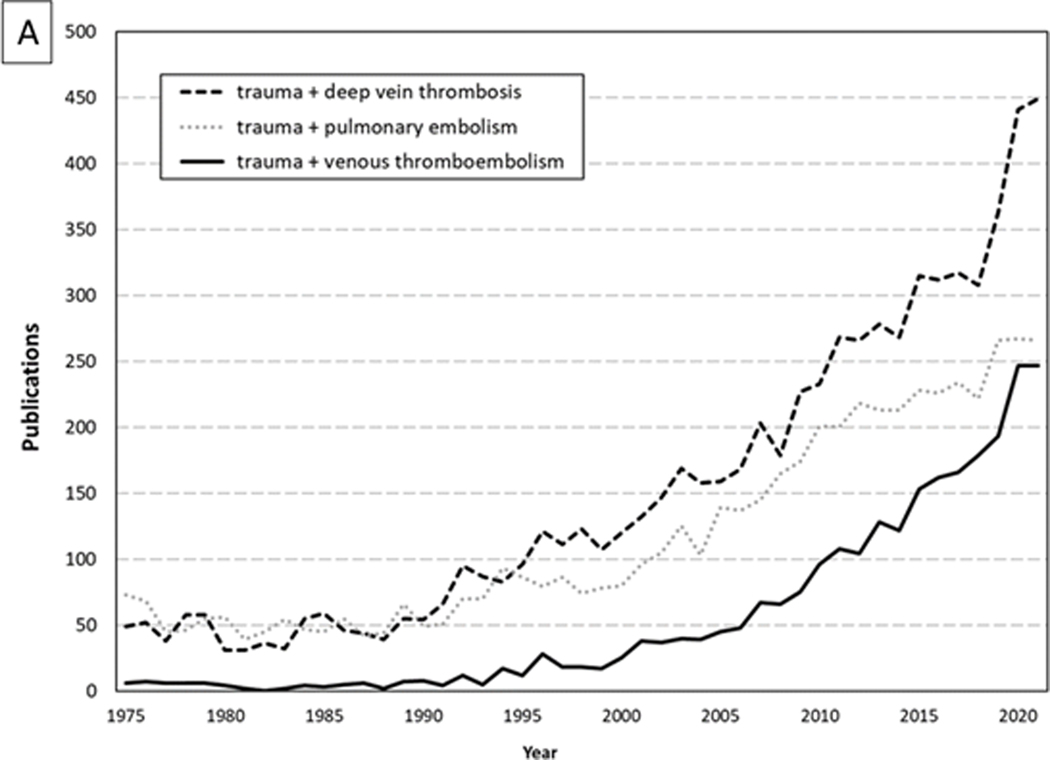
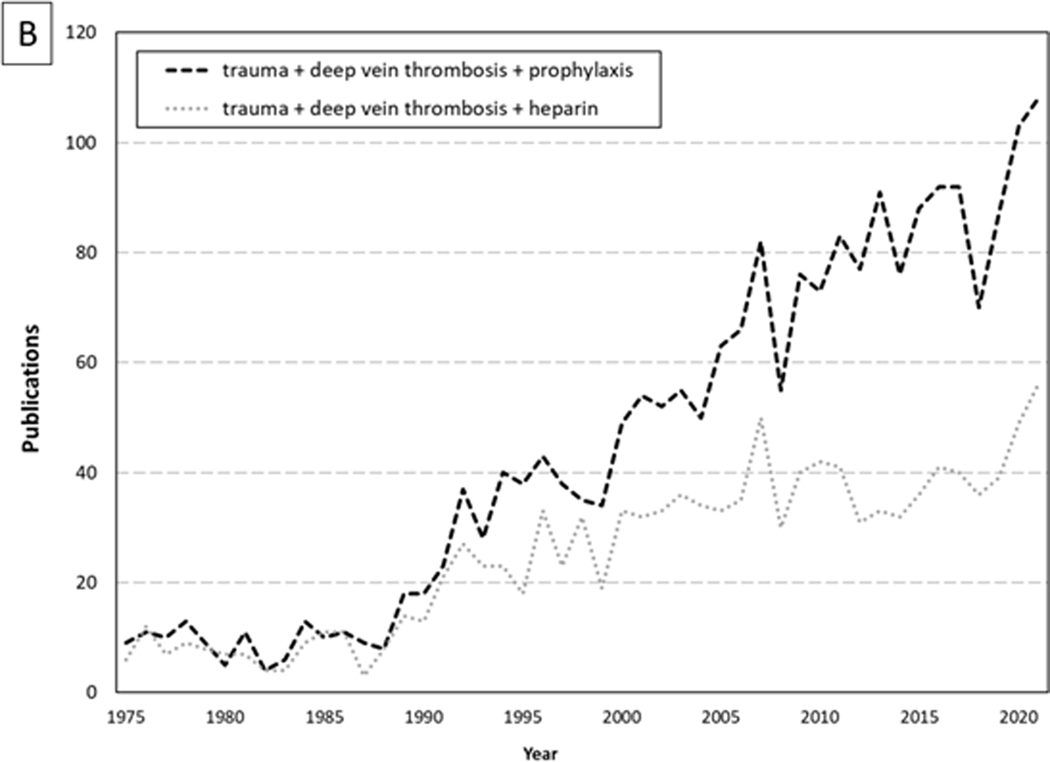
Dramatic Increases Over Time for Papers Published about (A) Venous Thromboembolism (VTE) and (B) (VTE) Prophylaxis in Trauma Patients
Effective prevention of thromboembolic complications became a leading priority in trauma research (Figure 1B). Early literature proposing use of pharmacologic agents for prophylaxis against VTE (13–15), while previously viewed with skepticism out of concern for persistent risk of hemorrhage after trauma, became the focus of renewed interest. Geerts et al. compared the effectiveness of low molecular weight heparin (LMWH; enoxaparin 30mg every 12 hours) to low-dose unfractionated heparin (UH; 5000 units every 12 hours) in their randomized controlled trial of consecutive patients with severe injury (16). Enoxaparin was associated with a 30% reduced risk of DVT overall, and 58% reduced risk of proximal DVT (number needed to treat = 11), as identified on venography. Major bleeding complications occurred in only 6 of 344 patients and were comparable between groups. Subsequent observational studies reconfirm that LMWH is superior to UH for prophylaxis against VTE in trauma patients (17–19) which is suggested by most guidelines (20, 21).
With pharmacologic VTE prophylaxis being the most effective means of prevention, the safe timing of initiation of prophylaxis has become a subject of vigorous evaluation. Geerts et al. demonstrated that bleeding risk is low in the majority of patients (16). Furthermore, many VTE events occur early during the acute admission. In one mixed trauma cohort from 34 level I and II trauma centers, the median times to identify DVT and PE were 7 and 4 days, respectively (22). Similarly, Sing et al. reported that over 50% of pulmonary emboli occurred within seven days of injury (23). This risk increases with each successive day that prophylaxis is delayed (17, 22). For this reason, early initiation within 24 hours of injury should be the standard for most injured patients. Exceptions to this practice have included specific subgroups in which the perceived risk of hemorrhage or its consequences persist. These groups include patients with traumatic brain injury, spinal cord injury, vertebral or pelvic fracture, and solid organ injury. While there is evidence that timely initiation of pharmacologic VTE prophylaxis is feasible without precipitating hemorrhagic complications in these populations (24–27), this continues to be an area of ongoing clinical debate and investigation.
As thromboprophylaxis practices evolved, inferior vena cava (IVC) filters have been used as an adjunctive primary prophylactic measure in patients with perceived contraindications to prophylactic anticoagulation. While some studies have indicated that IVC filters may reduce the risk of fatal PE (28), others have shown no association with patient survival (29). Ho et al. subsequently showed in their randomized controlled trial of 240 severely injured patients that prophylactic IVC filter use did not reduce the risk of symptomatic PE or mortality (30). Added to concerns of cost, variable utilization, low rates of removal (31), and potential morbidity, these data support that IVC filter use for primary prophylaxis should be limited and highly-selective. Guidelines now recommend against the use of IVC filters as primary prophylaxis in almost all cases (20, 21, 30). The guidelines emphasize that IVC filters should be used as part of adjunctive treatment for patients diagnosed with VTE in highly-select instances, such as patients with PE or proximal DVT in whom therapeutic anticoagulation is contraindicated. In these cases, structured follow-up is required to determine when resumption of anticoagulation is safe, to ensure filter retrieval, and detect complications (32).
Further efforts to prevent potentially fatal PE have included screening high-risk, asymptomatic patients with duplex ultrasound for early identification of DVT. Research to determine the effectiveness of this approach has been confounded by wide practice variation (33). Higher screening utilization leads to surveillance bias in rates of DVT detection (12, 34), a hospital quality indicator, and does not appear to translate into lower rates of PE in mixed trauma cohorts (35, 36). However, routine duplex screening in patients with high-risk injury patterns may be justified (37) and cost-effective (38). A prospective randomized trial published in 2021 found that screening in patients with high risk-assessment scores was associated with fewer PE (39). The added benefit of this approach over and above a rigorous protocol for timely pharmacologic prophylaxis remains uncertain. Both WTA and AAST/COT guidelines recommend that routine screening only be performed in this high-risk patient subgroup.
Further optimization of pharmacologic VTE prophylaxis through dose adjustment has become a subject of great interest. Sub-therapeutic levels of anti-Factor Xa are found in the majority of trauma patients at conventional doses of LMWH (40, 41). Protocols utilizing a higher starting dose LMWH (typically 40mg every 12 hours) and dose adjustment based on anti-Factor Xa levels have been associated with reduced rates of VTE (42). However, some studies have shown no benefit to dose adjustment using anti-Factor Xa levels (43, 44), and weight-based adjustment protocols may represent a simpler, less costly approach to achieving the goal of prophylactic effect (45–47).
Current Guidelines for Optimal Venous Thromboembolism Prophylaxis After Trauma
Based on the scientific work of the past 30 years, it should now be feasible to minimize the risk of major VTE events (symptomatic or fatal PE and extensive DVT) without precipitating hemorrhage complications. This can be achieved through up-to-date, evidence-based protocols. The first published recommendations by a trauma professional organization for the prevention of VTE were the Eastern Association for the Surgery of Trauma (EAST) practice management guidelines in 2002 (48). Twenty years later, the Western Trauma Association (WTA) (21) and jointly the American Association for the Surgery of Trauma (AAST) with the American College of Surgeons Committee on Trauma (COT) (20) have published their own evidence-based algorithms. Dr. Eric Ley provided an overview of similarities and differences between these guidelines.
Agent Selection, Dose, and Timing of Initiation
Both WTA and AAST/COT guidelines recommend LMWH over UH for thromboprophylaxis. This is due to the high-quality level I and subsequent observational data that LMWH is likely superior to UH for VTE prevention (16–18). Studies that reported equivalence were likely either underpowered or could not control important factors related to patient selection (49, 50). The use of LMWH also dramatically reduces, if not eliminates, the risk of heparin-induced thrombocytopenia, which is not insignificant with UH (51, 52).
Both guidelines now recommend LMWH 40mg every 12 hours. This is an increase from the previous standard dosing (30mg every 12 hours) due to data demonstrating that the majority of patients require at least 40mg every 12 hours to achieve target levels of anti-Factor Xa (41).
Both guidelines emphasize the early initiation of pharmacologic VTE prophylaxis to reduce risk of VTE due to delay (17, 22). The WTA recommends initiation within 24 hours of injury, while the AAST/COT recommends immediate initiation in patients without active bleeding or high-risk injuries (TBI, spinal cord injury, or solid organ injury).
High Risk Injuries
While WTA and AAST/COT guidelines both prioritize early initiation of thromboprophylaxis, their approaches to achieve this in patients with TBI, spinal cord injury, or solid organ injury differ. Timely initiation in all injuries appears feasible without increasing risk of hemorrhagic complication (26, 53–59). The WTA guideline recommends initiation of prophylaxis within 24 hours of bleeding cessation, spinal fixation, or stability of intracranial hemorrhage. In contrast, the AAST/COT recommends following the Modified Berne-Norwood Criteria for TBI (54), and initiating prophylaxis within 48 hours after spinal cord or solid organ injury (58, 59). Both guidelines recommend commencing a lower dose of pharmacologic prophylaxis for these patients (LMWH 30mg every 12 hours), reflecting a lack of data to demonstrate safety of higher dosing in these populations.
Missed Doses of Pharmacologic VTE Prophylaxis
Both societies emphasize that thromboprophylaxis should be continuous and without interruption. Missed doses of VTE prophylaxis are significantly associated with increased risk of VTE (60, 61). Furthermore, the practice of holding pharmacologic prophylaxis for surgical procedures out of fear of bleeding complication is not based on evidence. In Geerts’ 1996 landmark trial, prophylaxis was not withheld for surgical procedures except for spinal fixation (for which only one pre-operative dose was held) (16).
Dose Adjustment and Special Populations
Both the WTA and AAST/COT discuss dose adjustment based on weight and anti-Factor Xa levels. The AAST/COT recommends weight-based dosing first for patients with body mass index greater than 30 kg/m2 using LMWH 0.5mg/kg every 12 hours (62), a provision that is not made by the WTA. Both guidelines recommend adjusting LMWH dosing to achieve target anti-Factor Xa levels (peak 0.2 – 0.4 IU/mL or trough 0.1 – 0.2 IU/mL) after initiation, citing literature to support lower rates of VTE with this approach (41, 42, 63).
Specific populations of patients may require lower dosing of LMWH to achieve goal anti-Factor Xa levels. Creatinine clearance (CrCl) should dictate the dose required to achieve target prophylactic effect. For this reason, both guidelines agree that patients with impaired renal function (CrCl 30 – 60 mL/min) should receive lower dosing (30mg every 12 hours). This recommendation is also made for patients over 65 years of age, with very low body weight (< 50kg), or pregnant. Patients with CrCl < 30 mL/min are recommended to receive UH by both guidelines.
Deriving Consensus on Future Research Directions
Despite guidelines from two professional trauma associations based on nearly three decades of scientific evidence, the optimal practice for preventing VTE after trauma remains unclear. Practice variation persists owing to clinical uncertainty and barriers to implementing best-practice prevention into routine clinical care. Todd Costantini described results from a consensus process called the National Trauma Research Action Plan (NTRAP) (64) which has already identified four major research gaps of high priority in the area of VTE: 1) implementation of early best practice VTE prevention, 2) timing of initiation of VTE prophylaxis in patients with high-risk injuries, 3) approaches to optimizing VTE prophylaxis, and 4) evaluating use of novel pharmacologic agents for VTE prophylaxis.
Representatives from funding agencies, including the Agency for Healthcare Research & Quality (AHRQ), the National Institutes of Health / National Heart, Lung, and Blood Institute (NIH/NHLBI), the Patient-Centered Outcomes Research Institute (PCORI), and the United States Army Institute of Surgical Research, delivered a panel session titled “Extramural Funding Opportunities and Priorities.” Avery Nathens spoke about research opportunities to leverage the existing infrastructure of the American College of Surgeons Trauma Quality Improvement Program (TQIP) to perform studies (whether prospective cohort, randomized trials, or retrospective observational) and build additional data elements such as structural, process, or outcomes data points into TQIP for individual trauma centers to contribute additional data to answer these questions.
Implementation of Early Best Practice VTE Prevention
Delays in initiation and interruption of pharmacologic VTE prophylaxis significantly increase risk of DVT and potentially life-threatening PE (17, 22, 60). Furthermore, the use of VTE prophylaxis in patients with high-risk injuries remains highly variable (65). Education for clinicians, clinical decision support tools for risk assessment, patient-centered education, and computerized alerts have been demonstrated to improve prescription and adherence to VTE prophylaxis in medical and surgical patients (66–70). However, there remains a knowledge gap regarding the application, potential effectiveness, and cost of such interventions to improve implementation of VTE prophylaxis in trauma patients. Based on the breakout sessions at the VTE Consensus Conference, Ratnasekera et al. summarize the state of the literature on this subject and present their proposal for future research directions to improve implementation of VTE prophylaxis best practices (71).
Timing of Initiation of Pharmacologic VTE Prophylaxis in High-Risk Patients
Perhaps the subject of greatest clinical uncertainty is achieving timely but safe initiation of pharmacologic VTE prophylaxis in patients at high-risk for hemorrhagic complications. Patients with TBI and intracranial hemorrhage are at particularly high risk for hemorrhage expansion. For this reason, significant practice variation exists, both with respect to selection of prophylactic agent and timing. A major contributor to this practice variation is heterogeneity with respect to patterns of injury. While several studies support that timely initiation of prophylaxis can be achieved without risking harm due to hemorrhage expansion (53, 55–57), other data raise the potential for adverse events in patients with highest-risk injuries (72). However, these studies are predominantly observational in nature without adequate granularity to be conclusive. Similarly, studies that support the safety of early initiation of prophylaxis in patients with solid organ or spinal cord injuries are mainly single center or retrospective (26, 58, 59). Summarizing the evidence and discussion from the VTE Consensus Conference, Schellenberg et al. present their recommendations for future studies to clarify this knowledge gap (73).
Approaches to Optimizing VTE Prophylaxis Dosing in Trauma Patients
While the superiority of LMWH over UH for thromboprophylaxis in trauma patients has been established, the best approach to achieving optimum prophylactic effect remains unclear. Dose-adjustment based on anti-Factor Xa levels is currently recommended in WTA and AAST/COT guidelines (20, 21) based on studies that showing the benefit of reduced VTE rates (41, 42, 63). However, other studies show no association between adjustment based on anti-Factor Xa level and rates of VTE (43). There is evidence that differences in serum antithrombin III may be implicated in the association between observed anti-Factor Xa levels and effectiveness of heparinoid prophylaxis (74). Furthermore, there are studies that support a purely weight-based dosing approach to achieve adequate prophylaxis (45–47). Thromboelastographic findings have also been correlated with risk of VTE (75). A more in-depth summary of these data is presented by Teichman et al. who discuss their recommendations for future research into these mechanisms for the purpose of optimizing VTE prescription dosing prevention strategies (76).
Novel Pharmacologic Agents for VTE Prophylaxis in Trauma
While subcutaneous heparins have become the standard for pharmacologic VTE prophylaxis in trauma, there may be alternatives with similar effectiveness. Oral agents, such as aspirin or direct oral anticoagulant medications, may be preferred by patients if they could achieve the goal of prevention while avoiding repeated injections – a common reason for patient refusal (77, 78). Deborah Stein spoke about a large, ongoing PCORI-funded randomized trial comparing aspirin vs. LMWH in orthopedic trauma patients (79). Based on the VTE Consensus Conference, Dhillon et al. review the current evidence for aspirin or direct oral anticoagulant agents as VTE prophylaxis in trauma patients and makes recommendations for future research into their use (80).
Conclusions
The 2022 Consensus Conference to Implement Optimal VTE Prophylaxis in Trauma answered the National Trauma Research Action Plan call to define a course for future research into preventing thromboembolism after trauma. A multidisciplinary group of clinical champions, physicians, scientists, and patients delineated clear objectives for future investigation to address important, persistent key knowledge gaps. The series of papers from the conference outline the consensus based on the current literature and a roadmap for research to answer these unanswered questions (71, 73, 76, 80).
Disclosures
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R13HL158206 (“Consensus Conference to Implement Optimal VTE Prophylaxis in Trauma”). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Haut reports research funding from The Patient-Centered Outcomes Research Institute (PCORI) and the Agency for Healthcare Research and Quality (AHRQ).
Dr. Haut and Ms. Lake are unpaid board members of the National Blood Clot Alliance (NBCA).
Contributor Information
Elliott R. Haut, Division of Acute Care Surgery, Department of Surgery; Department of Anesthesiology and Critical Care Medicine; Department of Emergency Medicine The Johns Hopkins University School of Medicine, Baltimore, Maryland; The Armstrong Institute for Patient Safety and Quality, Johns Hopkins Medicine, Baltimore, Maryland; Department of Health Policy and Management The Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
James P. Byrne, Division of Acute Care Surgery, Department of Surgery.
Michelle A. Price, The Coalition for National Trauma Research, San Antonio, Texas.
Pamela Bixby, The Coalition for National Trauma Research, San Antonio, Texas.
Eileen M. Bulger, Department of Surgery, University of Washington, Seattle, WA.
Leslie Lake, The National Blood Clot Alliance Philadelphia, Pennsylvania.
Todd Costantini, Division of Trauma, Surgical Critical Care, Burns and Acute Care Surgery, Department of Surgery, University of California San Diego School of Medicine, San Diego, CA.
REFERENCES
- 1.National Blood Clot Alliance: Stop The Clot 2022. https://www.stoptheclot.org/. Updated November 1, 2022. [Google Scholar]
- 2.National Blood Clot Alliance: Partnership to Address Health Inequities 2022. https://www.stoptheclot.org/partnership-to-address-health-inequities/. Updated November 1, 2022. [Google Scholar]
- 3.National Blood Clot Alliance: Critical Look at Understanding Emotional Suffering of Blood Clot Survivors 2021. https://www.stoptheclot.org/news/clues-blood-clot-study/. Updated November 1, 2022. [Google Scholar]
- 4.Bruns P. Ueber plotzliche Todesfalle nach knockenbruchen in Folge von Venenthrombose und Embolie. Klin Chir. 1886; 2:1–18. [Google Scholar]
- 5.Sevitt S, Gallagher N. Venous thrombosis and pulmonary embolism. A clinico-pathological study in injured and burned patients. Br J Surg. 1961; 48:475–89. [DOI] [PubMed] [Google Scholar]
- 6.Freeark RJ, Boswick J, Fardin R. Posttraumatic venous thrombosis. Arch Surg. 1967; 95(4):567–75. [DOI] [PubMed] [Google Scholar]
- 7.NIH Consensus Panel. Prevention of venous thrombosis and pulmonary embolism. JAMA. 1986; 256(6):744–9. [PubMed] [Google Scholar]
- 8.Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. NEJM. 1994; 331(24):1601–6. [DOI] [PubMed] [Google Scholar]
- 9.Shackford SR, Davis JW, Hollingsworth-Fridlund P, Brewer NS, Hoyt DB, Mackersie RC. Venous thromboembolism in patients with major trauma. Am J Surg. 1990; 159(4):365–9. [DOI] [PubMed] [Google Scholar]
- 10.Knudson MM, Lewis FR, Clinton A, Atkinson K, Megerman J. Prevention of venous thromboembolism in trauma patients. J Trauma. 1994; 37(3):480–7. [DOI] [PubMed] [Google Scholar]
- 11.Knudson MM, Gomez D, Haas B, Cohen MJ, Nathens AB. Three thousand seven hundred thirty-eight posttraumatic pulmonary emboli: a new look at an old disease. Ann Surg. 2011; 254(4):625–32 [DOI] [PubMed] [Google Scholar]
- 12.Haut ER, Chang DC, Pierce CA, Colantuoni E, Efron DT, Haider AH, et al. Predictors of posttraumatic deep vein thrombosis (DVT): Hospital practice versus patient factors-an analysis of the National Trauma Data Bank. J Trauma. 2009; 66(4):994–9. [DOI] [PubMed] [Google Scholar]
- 13.Murray GD, Best CH. The Use of Heparin in Thrombosis. Ann Surg. 1938; 108(2):163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crafoord C, Jorpes E. Heparin as a Prophylactic Against Thrombosis. JAMA. 1941; 116(26):2831–5. [Google Scholar]
- 15.Sevitt S, Gallagher NG. Prevention of venous thrombosis and pulmonary embolism in injured patients. A trial of anticoagulant prophylaxis with phenindione in middle-aged and elderly patients with fractured necks of femur. Lancet. 1959; 2(7110):981–9. [DOI] [PubMed] [Google Scholar]
- 16.Geerts WH, Jay RM, Code KI, Chen E, Szalai JP, Saibil EA, et al. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. NEJM. 1996; 335(10):701–7. [DOI] [PubMed] [Google Scholar]
- 17.Byrne JP, Geerts W, Mason SA, Gomez D, Hoeft C, Murphy R, et al. Effectiveness of low-molecular-weight heparin versus unfractionated heparin to prevent pulmonary embolism following major trauma: A propensity-matched analysis. J Trauma Acute Care Surg. 2017; 82(2):252–62. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs BN, Cain-Nielsen AH, Jakubus JL, Mikhail JN, Fath JJ, Regenbogen SE, et al. Unfractionated heparin versus low-molecular-weight heparin for venous thromboembolism prophylaxis in trauma. J Trauma Acute Care Surg. 2017; 83(1):151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran A, Fernando SM, Carrier M, Siegal DM, Inaba K, Vogt K, et al. Efficacy and Safety of Low Molecular Weight Heparin Versus Unfractionated Heparin for Prevention of Venous Thromboembolism in Trauma Patients: A Systematic Review and Meta-analysis. Ann Surg. 2022; 275(1):19–28. [DOI] [PubMed] [Google Scholar]
- 20.Yorkgitis BK, Berndtson AE, Cross A, Kennedy R, Kochuba MP, Tignanelli C, et al. American Association for the Surgery of Trauma/American College of Surgeons-Committee on Trauma Clinical Protocol for inpatient venous thromboembolism prophylaxis after trauma. J Trauma Acute Care Surg. 2022; 92(3):597–604. [DOI] [PubMed] [Google Scholar]
- 21.Ley EJ, Brown CVR, Moore EE, Sava JA, Peck K, Ciesla DJ, et al. Updated guidelines to reduce venous thromboembolism in trauma patients: A Western Trauma Association critical decisions algorithm. J Trauma Acute Care Surg. 2020; 89(5):971–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hecht JP, Han EJ, Cain-Nielsen AH, Scott JW, Hemmila MR, Wahl WL. Association of timing of initiation of pharmacologic venous thromboembolism prophylaxis with outcomes in trauma patients. J Trauma Acute Care Surg. 2021; 90(1):54–63. [DOI] [PubMed] [Google Scholar]
- 23.Sing RF, Camp SM, Heniford BT, Rutherford EJ, Dix S, Reilly PM, et al. Timing of pulmonary emboli after trauma: implications for retrievable vena cava filters. J Trauma. 2006; 60(4):732–4. [DOI] [PubMed] [Google Scholar]
- 24.Ahlquist S, Park HY, Kelley B, Holly L, Shamie AN, Park DY. Venous Thromboembolism Chemoprophylaxis Within 24 Hours of Surgery for Spinal Cord Injury: Is It Safe and Effective? Neurospine. 2020; 17(2):407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skarupa D, Hanna K, Zeeshan M, Madbak F, Hamidi M, Haddadin Z, et al. Is early chemical thromboprophylaxis in patients with solid organ injury a solid decision? J Trauma Acute Care Surg. 2019; 87(5):1104–12. [DOI] [PubMed] [Google Scholar]
- 26.Schellenberg M, Inaba K, Biswas S, Heindel P, Benjamin E, Strumwasser A, et al. When is It Safe to Start VTE Prophylaxis After Blunt Solid Organ Injury? A Prospective Study from a Level I Trauma Center. World J Surg. 2019; 43(11):2797–803. [DOI] [PubMed] [Google Scholar]
- 27.Byrne JP, Mason SA, Gomez D, Hoeft C, Subacius H, Xiong W, et al. Timing of Pharmacologic Venous Thromboembolism Prophylaxis in Severe Traumatic Brain Injury: A Propensity-Matched Cohort Study. J Am Coll Surg. 2016; 223(4):621–31.e5. [DOI] [PubMed] [Google Scholar]
- 28.Haut ER, Garcia LJ, Shihab HM, Brotman DJ, Stevens KA, Sharma R, et al. The effectiveness of prophylactic inferior vena cava filters in trauma patients: a systematic review and meta-analysis. JAMA Surg. 2014; 149(2):194–202. [DOI] [PubMed] [Google Scholar]
- 29.Sarosiek S, Rybin D, Weinberg J, Burke PA, Kasotakis G, Sloan JM. Association Between Inferior Vena Cava Filter Insertion in Trauma Patients and In-Hospital and Overall Mortality. JAMA Surg. 2017; 152(1):75–81. [DOI] [PubMed] [Google Scholar]
- 30.Ho KM, Rao S, Honeybul S, Zellweger R, Wibrow B, Lipman J, et al. A Multicenter Trial of Vena Cava Filters in Severely Injured Patients. NEJM. 2019; 381(4):328–37. [DOI] [PubMed] [Google Scholar]
- 31.Leeper WR, Murphy PB, Vogt KN, Leeper TJ, Kribs SW, Gray DK, et al. Are retrievable vena cava filters placed in trauma patients really retrievable? Eur J Trauma Emerg Surg. 2016; 42(4):459–64. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman JA, Barnes GD, Chaer RA, Cuschieri J, Eberhardt RT, Johnson MS, et al. Society of Interventional Radiology Clinical Practice Guideline for Inferior Vena Cava Filters in the Treatment of Patients with Venous Thromboembolic Disease: Developed in collaboration with the American College of Cardiology, American College of Chest Physicians, American College of Surgeons Committee on Trauma, American Heart Association, Society for Vascular Surgery, and Society for Vascular Medicine. J Vasc Interv Radiol. 2020; 31(10):1529–44. [DOI] [PubMed] [Google Scholar]
- 33.Haut ER, Schneider EB, Patel A, Streiff MB, Haider AH, Stevens KA, et al. Duplex ultrasound screening for deep vein thrombosis in asymptomatic trauma patients: a survey of individual trauma surgeon opinions and current trauma center practices. J Trauma. 2011; 70(1):27–33. [DOI] [PubMed] [Google Scholar]
- 34.Haut ER, Pronovost PJ. Surveillance bias in outcomes reporting. JAMA. 2011; 305(23):2462–3. [DOI] [PubMed] [Google Scholar]
- 35.Pierce CA, Haut ER, Kardooni S, Chang DC, Efron DT, Haider A, et al. Surveillance bias and deep vein thrombosis in the national trauma data bank: the more we look, the more we find. J Trauma. 2008; 64(4):932–6. [DOI] [PubMed] [Google Scholar]
- 36.Dietch ZC, Edwards BL, Thames M, Shah PM, Williams MD, Sawyer RG. Rate of lower-extremity ultrasonography in trauma patients is associated with rate of deep venous thrombosis but not pulmonary embolism. Surgery. 2015; 158(2):379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen CJ, Murray CR, Meizoso JP, Ginzburg E, Schulman CI, Lineen EB, et al. Surveillance and Early Management of Deep Vein Thrombosis Decreases Rate of Pulmonary Embolism in High-Risk Trauma Patients. J Am Coll Surg. 2016; 222(1):65–72. [DOI] [PubMed] [Google Scholar]
- 38.Malhotra AK, Goldberg SR, McLay L, Martin NR, Wolfe LG, Levy MM, et al. DVT surveillance program in the ICU: Analysis of cost-effectiveness. PLoS One. 2014; 9(9):e106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kay AB, Morris DS, Woller SC, Stevens SM, Bledsoe JR, Lloyd JF, et al. Trauma patients at risk for venous thromboembolism who undergo routine duplex ultrasound screening experience fewer pulmonary emboli: A prospective randomized trial. J Trauma Acute Care Surg. 2021; 90(5):787–96. [DOI] [PubMed] [Google Scholar]
- 40.Walker CK, Sandmann EA, Horyna TJ, Gales MA. Increased Enoxaparin Dosing for Venous Thromboembolism Prophylaxis in General Trauma Patients. Ann Pharmacother. 2017; 51(4):323–31. [DOI] [PubMed] [Google Scholar]
- 41.Ko A, Harada MY, Barmparas G, Chung K, Mason R, Yim DA, et al. Association Between Enoxaparin Dosage Adjusted by Anti-Factor Xa Trough Level and Clinically Evident Venous Thromboembolism After Trauma. JAMA Surg. 2016; 151(11):1006–13. [DOI] [PubMed] [Google Scholar]
- 42.Dhillon NK, Barmparas G, Lin TL, Linaval NT, Yang AR, Sekhon HK, et al. A Systems-based Approach to Reduce Deep Venous Thrombosis and Pulmonary Embolism in Trauma Patients. World J Surg. 2021; 45(3):738–45. [DOI] [PubMed] [Google Scholar]
- 43.Karcutskie CA, Dharmaraja A, Patel J, Eidelson SA, Padiadpu AB, Martin AG, et al. Association of Anti-Factor Xa-Guided Dosing of Enoxaparin With Venous Thromboembolism After Trauma. JAMA Surg. 2018; 153(2):144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karcutskie CA, Dharmaraja A, Patel J, Eidelson SA, Martin AG, Lineen EB, et al. Relation of antifactor-Xa peak levels and venous thromboembolism after trauma. J Trauma Acute Care Surg. 2017; 83(6):1102–7. [DOI] [PubMed] [Google Scholar]
- 45.Kay AB, Majercik S, Sorensen J, Woller SC, Stevens SM, White TW, et al. Weight-based enoxaparin dosing and deep vein thrombosis in hospitalized trauma patients: A double-blind, randomized, pilot study. Surgery. Epub 2018. Apr 23. [DOI] [PubMed] [Google Scholar]
- 46.Taylor A, Huang E, Waller J, White C, Martinez-Quinones P, Robinson T. Achievement of goal anti-Xa activity with weight-based enoxaparin dosing for venous thromboembolism prophylaxis in trauma patients. Pharmacotherapy. 2021; 41(6):508–14. [DOI] [PubMed] [Google Scholar]
- 47.Stutsrim AE, Eady JM, Collum M, Rebo GJ, Rebo KA, Miller PR, et al. Weight-Based Enoxaparin Achieves Adequate Anti-Xa Levels More Often in Trauma Patients: A Prospective Study. Am Surg. 2021; 87(1):77–82. [DOI] [PubMed] [Google Scholar]
- 48.Rogers FB, Cipolle MD, Velmahos G, Rozycki G, Luchette FA. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group. J Trauma. 2002; 53(1):142–64. [DOI] [PubMed] [Google Scholar]
- 49.Olson EJ, Bandle J, Calvo RY, Shackford SR, Dunne CE, Van Gent JM, et al. Heparin versus enoxaparin for prevention of venous thromboembolism after trauma: A randomized noninferiority trial. J Trauma Acute Care Surg. 2015;79(6):961–8. [DOI] [PubMed] [Google Scholar]
- 50.Checchi KD, Costantini TW, Badiee J, Berndtson AE, Calvo RY, Rooney AS, et al. A tale of two centers: Is low-molecular-weight heparin really superior for prevention of posttraumatic venous thromboembolism? J Trauma Acute Care Surg. 2021; 91(3):537–41. [DOI] [PubMed] [Google Scholar]
- 51.Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005; 106(8):2710–5. [DOI] [PubMed] [Google Scholar]
- 52.Warkentin TE, Levine MN, Hirsh J, Horsewood P, Roberts RS, Gent M, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. NEJM. 1995; 332(20):1330–5. [DOI] [PubMed] [Google Scholar]
- 53.Störmann P, Osinloye W, Freiman TM, Seifert V, Marzi I, Lustenberger T. Early Chemical Thromboprophylaxis Does not Increase the Risk of Intracranial Hematoma Progression in Patients with Isolated Severe Traumatic Brain Injury. World J Surg. 2019; 43(11):2804–11. [DOI] [PubMed] [Google Scholar]
- 54.Phelan HA, Wolf SE, Norwood SH, Aldy K, Brakenridge SC, Eastman AL, et al. A randomized, double-blinded, placebo-controlled pilot trial of anticoagulation in low-risk traumatic brain injury: The Delayed Versus Early Enoxaparin Prophylaxis I (DEEP I) study. J Trauma Acute Care Surg. 2012; 73(6):1434–41. [DOI] [PubMed] [Google Scholar]
- 55.Norwood SH, Berne JD, Rowe SA, Villarreal DH, Ledlie JT. Early venous thromboembolism prophylaxis with enoxaparin in patients with blunt traumatic brain injury. J Trauma. 2008;65(5):1021–6. [DOI] [PubMed] [Google Scholar]
- 56.Meyer RM, Larkin MB, Szuflita NS, Neal CJ, Tomlin JM, Armonda RA, et al. Early venous thromboembolism chemoprophylaxis in combat-related penetrating brain injury. J Neurosurg. 2017; 126(4):1047–55. [DOI] [PubMed] [Google Scholar]
- 57.Byrne JP, Mason SA, Gomez D, Hoeft C, Subacius H, Xiong W, et al. Timing of Pharmacologic Venous Thromboembolism Prophylaxis in Severe Traumatic Brain Injury: A Propensity-Matched Cohort Study. J Am Coll Surg. 2016; 223(4):621–631. [DOI] [PubMed] [Google Scholar]
- 58.Murphy PB, de Moya M, Karam B, Menard L, Holder E, Inaba K, et al. Optimal timing of venous thromboembolic chemoprophylaxis initiation following blunt solid organ injury: meta-analysis and systematic review. Eur J Trauma Emerg Surg. 2022; 48(3):2039–46. [DOI] [PubMed] [Google Scholar]
- 59.Kim DY, Kobayashi L, Chang D, Fortlage D, Coimbra R. Early pharmacological venous thromboembolism prophylaxis is safe after operative fixation of traumatic spine fractures. Spine. 2015; 40(5):299–304. [DOI] [PubMed] [Google Scholar]
- 60.Louis SG, Sato M, Geraci T, Anderson R, Cho SD, Van PY, et al. Correlation of missed doses of enoxaparin with increased incidence of deep vein thrombosis in trauma and general surgery patients. JAMA Surg. 2014; 149(4):365–70. [DOI] [PubMed] [Google Scholar]
- 61.Godat LN, Haut ER, Moore EE, Knudson MM, Costantini TW. Venous thromboembolism risk after spinal cord injury: A secondary analysis of the CLOTT study. J Trauma Acute Care Surg. Epub 2022. Oct 7. [DOI] [PubMed] [Google Scholar]
- 62.Berndtson AE, Costantini TW, Lane J, Box K, Coimbra R. If some is good, more is better: An enoxaparin dosing strategy to improve pharmacologic venous thromboembolism prophylaxis. J Trauma Acute Care Surg. 2016; 81(6):1095–100. [DOI] [PubMed] [Google Scholar]
- 63.Singer GA, Riggi G, Karcutskie CA, Vaghaiwalla TM, Lieberman HM, Ginzburg E, et al. Anti-Xa-guided enoxaparin thromboprophylaxis reduces rate of deep venous thromboembolism in high-risk trauma patients. J Trauma Acute Care Surg. 2016; 81(6):1101–8. [DOI] [PubMed] [Google Scholar]
- 64.Costantini TW, Galante JM, Braverman MA, Phuong J, Price MA, Cuschieri J, et al. Developing a National Trauma Research Action Plan: Results from the acute resuscitation, initial patient evaluation, imaging, and management research gap Delphi survey. J Trauma Acute Care Surg. 2022; 93(2):200–8. [DOI] [PubMed] [Google Scholar]
- 65.Zarzaur BL, Kozar RA, Fabian TC, Coimbra R. A survey of American Association for the Surgery of Trauma member practices in the management of blunt splenic injury. J Trauma. 2011; 70(5):1026–31. [DOI] [PubMed] [Google Scholar]
- 66.Kahn SR, Morrison DR, Diendéré G, Piché A, Filion KB, Klil-Drori AJ, et al. Interventions for implementation of thromboprophylaxis in hospitalized patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2018; 4(4):Cd008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kahn SR, Diendéré G, Morrison DR, Piché A, Filion KB, Klil-Drori AJ, et al. Effectiveness of interventions for the implementation of thromboprophylaxis in hospitalised patients at risk of venous thromboembolism: an updated abridged Cochrane systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2019; 9(5):e024444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haut ER, Lau BD, Kraenzlin FS, Hobson DB, Kraus PS, Carolan HT, et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg. 2012; 147(10):901–7. [DOI] [PubMed] [Google Scholar]
- 69.Haut ER, Aboagye JK, Shaffer DL, Wang J, Hobson DB, Yenokyan G, et al. Effect of Real-time Patient-Centered Education Bundle on Administration of Venous Thromboembolism Prevention in Hospitalized Patients. JAMA Netw Open. 2018; 1(7):e184741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haut ER, Owodunni OP, Wang J, Shaffer DL, Hobson DB, Yenokyan G, et al. Alert-Triggered Patient Education Versus Nurse Feedback for Nonadministered Venous Thromboembolism Prophylaxis Doses: A Cluster-Randomized Controlled Trial. J Am Heart Assoc. 2022; 11(18):e027119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ratnasekera A, et al. Implementation Science Approaches to Optimizing Venous Thromboembolism Prevention in Patients with Traumatic Injuries: Findings from the 2022 Consensus Conference to Implement Optimal VTE Prophylaxis in Trauma. J Trauma Acute Care Surg. 2022. (Submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Byrne JP, Witiw CD, Schuster JM, Pascual JL, Cannon JW, Martin ND, et al. Association of Venous Thromboembolism Prophylaxis After Neurosurgical Intervention for Traumatic Brain Injury With Thromboembolic Complications, Repeated Neurosurgery, and Mortality. JAMA Surg. 2022; 157(3):e215794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schellenberg M, et al. Optimal Timing for Initiation of Pharmacologic Venous Thromboembolism Prophylaxis after Injury: Findings from the 2022 Consensus Conference to Implement Optimal VTE Prophylaxis in Trauma. J Trauma Acute Care Surg. 2022. (Submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Droege ME, Droege CA, Philpott CD, Webb ML, Ernst NE, Athota K, et al. Impact of antithrombin III and enoxaparin dosage adjustment on prophylactic anti-Xa concentrations in trauma patients at high risk for venous thromboembolism: a randomized pilot trial. J Thromb Thrombolysis. 2021; 52(4):1117–28. [DOI] [PubMed] [Google Scholar]
- 75.Cotton BA, Minei KM, Radwan ZA, Matijevic N, Pivalizza E, Podbielski J, et al. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J Trauma Acute Care Surg. 2012; 72(6):1470–5. [DOI] [PubMed] [Google Scholar]
- 76.Teichman AL, et al. Approaches for Optimizing Venous Thromboembolism (VTE) Prevention in Injured Patients: Findings from the 2022 Consensus Conference to Implement Optimal VTE Prophylaxis in Trauma. J Trauma Acute Care Surg. 2022. (Submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Popoola VO, Tavakoli F, Lau BD, Lankiewicz M, Ross P, Kraus P, et al. Exploring the impact of route of administration on medication acceptance in hospitalized patients: Implications for venous thromboembolism prevention. Thromb Res. 2017; 160:109–13. [DOI] [PubMed] [Google Scholar]
- 78.Wong A, Kraus PS, Lau BD, Streiff MB, Haut ER, Hobson DB, et al. Patient preferences regarding pharmacologic venous thromboembolism prophylaxis. J Hosp Med. 2015; 10(2):108–11. [DOI] [PubMed] [Google Scholar]
- 79.O’Toole RV, Stein DM, Frey KP, O’Hara NN, Scharfstein DO, Slobogean GP, et al. PREVENTion of CLots in Orthopaedic Trauma (PREVENT CLOT): a randomised pragmatic trial protocol comparing aspirin versus low-molecular-weight heparin for blood clot prevention in orthopaedic trauma patients. BMJ Open. 2021; 11(3):e041845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dhillon NK, et al. Novel Therapeutic Medications for Venous Thromboembolism Prevention in Trauma Patients: Findings from the 2022 Consensus Conference to Implement Optimal VTE Prophylaxis in Trauma. J Trauma Acute Care Surg. 2022. (Submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]


