Branched-chain amino acids (BCAAs), i.e., leucine, isoleucine, and valine, are essential amino acids involved in glucose regulation, immunity, and cell signaling.1 Excessive circulating BCAAs predict type 2 diabetes mellitus and subsequent cardiovascular disease (CVD) in the general population.2 Chronic kidney disease (CKD) alters circulating metabolites and is a strong CVD risk factor. We leveraged the African American Study of Kidney Disease and Hypertension (AASK) to evaluate BCAAs’ cardiovascular effects in CKD outside the context of diabetes.
AASK is a multi-center randomized trial studying the effects of three antihypertensives and two blood pressure control goals on hypertensive-attributed CKD progression in African Americans.3 Between 1995 and 1998, 1094 patients with hypertension-attributed CKD (baseline measured glomerular filtration rate [GFR] 20-65 ml/min/1.73m2) and without diabetes mellitus were enrolled. They were followed until renal replacement therapy (RRT) or death. Follow-up was extended among 691 individuals not needing RRT upon completion of the trial from 2002 to 2007. The study was approved by an institutional review committee. All participants gave informed consent. Data, methods, and materials supporting findings can be made available upon request.
Fasting serum samples at baseline were used for metabolomic profiling at Metabolon, Inc (Morrisville, NC). Metabolites were quantified with area under the curve of liquid chromatography-mass spectrometry peaks. Quality control processes were previously described.4 Circulating BCAA levels were available for analysis in 962 participants. Coefficient of variation in blind duplicates was 4.8%, 5.3%, and 6.8% for leucine, isoleucine, and valine, respectively.
Outcomes of this study were incident CVD (composite of hospitalization for nonfatal myocardial infarction, cardiac revascularization procedure, heart failure, stroke, or cardiovascular death, all adjudicated by the AASK Cardiovascular Outcomes Committee), cardiovascular death, and all-cause mortality.
Continuous variable correlations were assessed with Spearman coefficients. To evaluate associations between BCAAs and outcomes, we used multivariable Cox models with adjustment for baseline covariates including age, sex, randomized groups, body mass index (BMI), history of smoking, history of heart disease, measured GFR, proteinuria, fasting blood glucose, low-density lipoprotein cholesterol (LDLc), and C-reactive protein (CRP). Missing data (n=4 for proteinuria, n=206 for LDLc) were imputed with median values. Competing risk of death was assessed with Fine-Gray models adjusting for the same covariates. The cutoff for statistical significance was a Bonferroni-corrected two-sided-p-value of 0.017 (0.05/3 metabolites evaluated). Analyses were performed using Stata 17.0 (StataCorp, College Station, TX) and R (R Foundation, Vienna, Austria).
Among 962 participants (mean age 56 [S.D., 11] years, 39% women, mean GFR 48 [S.D., 13] ml/min/1.73m2, mean fasting blood glucose 94 [S.D., 15] mg/dl), BCAAs were mutually highly correlated, but less so with other covariables (Figure). During follow-up (median 7.4 years), there were 192 CVD events (myocardial infarction or revascularization, n=57; stroke, n=74; heart failure, n=74) and 220 deaths of which 54 were cardiovascular deaths. Adjusted for covariates, BCAAs were not associated with incident CVD with or without accounting for the competing risk of death. However, higher leucine and isoleucine were associated with lower cardiovascular death (per doubling, HR=0.52, 95%CI 0.31-0.87; p=0.013 for isoleucine; HR=0.30, 95%CI 0.13-0.68, p=0.004 for leucine). Higher valine was associated with lower cardiovascular death (HR=0.39, 95%CI 0.16-0.94, p=0.036), leucine and isoleucine were associated with lower all-cause mortality (HR=0.70, 95%CI 0.49-1.00, p=0.049 for isoleucine; HR=0.57, 95%CI 0.36-0.96, p=0.035 for leucine) with nominal statistical significance.
Figure 1.
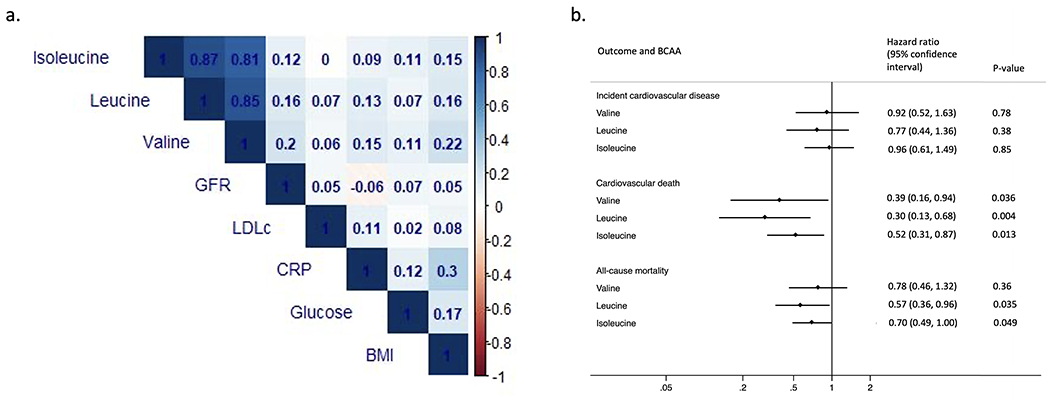
Circulating branched-chain amino acid, cross-sectional Spearman correlations, associations with incident cardiovascular event, cardiovascular death, and all-cause mortality in the African American Study of Kidney Disease and Hypertension.
BCAA, branched-chain amino acid. BMI, body mass index. CRP, C-reactive protein. GFR, glomerular filtration rate. LDLc, low-density lipoprotein cholesterol.
Hazard ratios are modeled per doubling of each BCAA and adjusted for baseline age, sex, randomized treatment groups, body mass index, history of smoking, history of heart disease, measured GFR, proteinuria, blood glucose, low-density lipoprotein cholesterol, and C-reactive protein. Proteinuria, LDLc, and CRP had skewed distributions and were log-transformed in all analyses.
Evidence supports a causal role of excessive circulating BCAAs in insulin resistance, diabetes, and obesity.5 In a general population (primarily White individuals with preserved kidney function), circulating BCAAs were positively associated with incident CVD with insulin resistance being the mediator.2 Outside the context of diabetes, relatively few studies have assessed the cardiovascular effects of BCAAs, with findings being less consistent. In animals, myocardial stress impaired myocardial BCAA catabolism, causing local accumulation of BCAAs and branched-chain α-keto acids (BCKAs), BCAA transamination products that can negatively impact the cardiovascular system.5 It Is less clear whether systemic alterations in these metabolites also have cardiovascular effects. Our study expands the evidence base with a unique study population. The lack of association between BCAAs and CVD here may be attributable to the population’s advanced kidney disease, a condition that substantially alters metabolite excretion. While excessive BCAAs can reflect catabolic defects and predict insulin resistance, a healthy level of BCAAs supports cellular metabolism, cardiomyocyte signaling, and may be anti-aging.1 We speculate that higher BCAAs in those with advanced CKD and without diabetes may be closer to the general population’s normal range, reflecting relative health.
This study’s observational nature limits assessment of causality. The relatively small sample size and metabolite quantification on a relative scale complicate comparison with other studies. This study also lacked measurements of BCKAs, potentially toxic byproducts of impaired BCAA catabolism.
In summary, we observed lower cardiovascular death and all-cause mortality, but not higher CVD risk, with higher circulating BCAAs in individuals with advanced non-diabetic CKD. These findings expand on current evidence of BCAAs and cardiovascular health.
Sources of Funding:
CMR is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; R03 DK128386) and the National Heart, Lung, and Blood Institute (NHLBI; R01 HL153178). JC and MEG are supported by the CKD Biomarkers Consortium (NIDDK U01 DK085689). MEG is supported by the NIDDK (R01DK108803 and K24HL155861).
Footnotes
Disclosures: None
References:
- 1.Huang Y, Zhou M, Sun H, Wang Y. Branched-chain amino acid metabolism in heart disease: an epiphenomenon or a real culprit? Cardiovasc Res. 2011;90:220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobias DK, Lawler PR, Harada PH, Demler OV, Ridker PM, Manson JE, Cheng S, Mora S. Circulating Branched-Chain Amino Acids and Incident Cardiovascular Disease in a Prospective Cohort of US Women. Circ Genom Precis Med. 2018;11:e002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gassman JJ, Greene T, Wright JT Jr., Agodoa L, Bakris G, Beck GJ, Douglas J, Jamerson K, Lewis J, Kutner M, et al. Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK). J Am Soc Nephrol. 2003;14:S154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo S, Coresh J, Tin A, Rebholz CM, Appel LJ, Chen J, Vasan RS, Anderson AH, Feldman HI, Kimmel PL, et al. Serum Metabolomic Alterations Associated with Proteinuria in CKD. Clin J Am Soc Nephrol. 2019;14:342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neinast M, Murashige D, Arany Z. Branched Chain Amino Acids. Annu Rev Physiol. 2019;81:139–164. [DOI] [PMC free article] [PubMed] [Google Scholar]


