Abstract
For over 25 years our group has used regenerative medicine strategies to develop improved biomaterials for use in congenital heart surgery. Among other applications, we developed a tissue engineered vascular graft (TEVG) by seeding tubular biodegradable polymeric scaffolds with autologous bone marrow-derived mononuclear cells. Results of our first-in-human study demonstrated feasibility as the TEVG transformed into a living vascular graft having an ability to grow, making it the first engineered graft with growth potential. Yet, outcomes of this first FDA-approved clinical trial evaluating safety revealed a prohibitively high incidence of early TEVG stenosis, preventing the widespread use of this promising technology. Mechanistic studies in mouse models provided important insight into the development of stenosis and enabled advanced computational models. Computational simulations suggested both a novel inflammation-driven, mechano-mediated process of in vivo TEVG development and an unexpected natural history, including spontaneous reversal of the stenosis. Based on these in vivo and in silico discoveries, we have been able to rationally design strategies for inhibiting TEVG stenosis that have been validated in preclinical large animal studies and translated to the clinic via a new FDA-approved clinical trial. This progress would not have been possible without the multi-disciplinary approach, ranging from small to large animal models and computational simulations. This same process is expected to lead to further advances in scaffold design, and thus next generation TEVGs.
Graphical Abstract
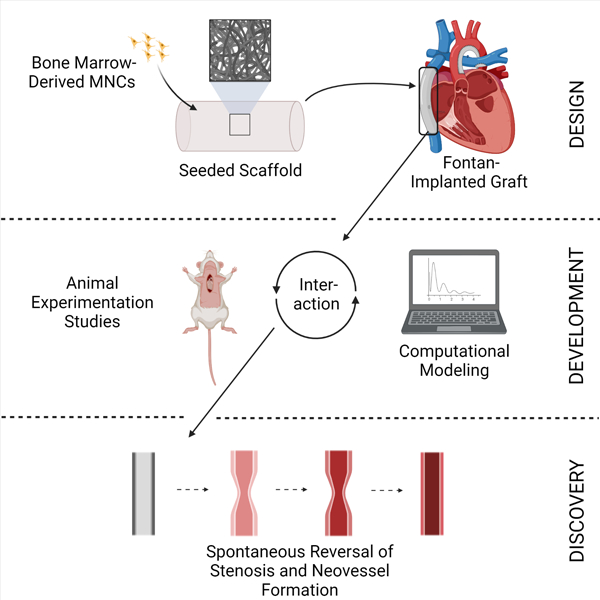
Clinical Challenge
Congenital cardiac anomalies represent the most common birth defect, affecting approximately 1% of live births.1 Severe forms of congenital heart disease comprise about one-third of these anomalies and are life-threatening without complex surgical intervention.2 Despite advances in the surgical and medical management of these conditions, they remain a leading cause of death in newborns.1–2 A significant source of postoperative morbidity and mortality in this patient population arises from the use of prosthetic biomaterials in the form of vascular grafts, cardiovascular patches, and replacement heart valves, which associate with an increased risk of thromboembolic events, poor durability due to neointimal hyperplasia, pathological development of ectopic calcification, susceptibility to infection, and somatic overgrowth (a term used to describe outgrowing a prosthesis).3
All currently available prosthetic biomaterials suffer from adverse events arising from poor biocompatibility.4 In contradistinction to prosthetic biomaterials, use of autologous tissue significantly reduces the risk of these complications.5 Thus, autologous tissue represents the best option for performing a major reconstructive cardiac operation when treating pediatric patients with congenital heart disease due to the decreased risk of graft-related complications and increased growth capacity of autologous tissue.5 Regrettably, there is rarely sufficient autologous tissue to enable a major congenital heart surgery, thus resulting in a need for engineered biomaterials. Given their availability, it is for this reason that prosthetic biomaterials are widely used in congenital heart surgeries.5 In order to reduce the risk of somatic overgrowth, surgeons have also developed strategies to minimize the need for graft replacement that include placement of oversized grafts and delaying surgery until patients are older.6–7 While these strategies limit the need for revisional surgery and graft replacement, they come at a cost. Oversized grafts can disrupt normal hemodynamics, which can increase the risk of thromboembolic complications, the most common serious adverse event following congenital heart surgery. 6–7 Delaying surgery exposes the patient to prolonged chronic hypoxia, which has adverse developmental consequences in addition to exposing the heart to prolonged volume overload which increases the risk of heart failure.6–7 Due to the lack of an alternative (a conduit with growth capacity) these risks are simply assumed and deemed unavoidable.
Development of an improved vascular conduit would also have important implications for other non-pediatric applications including use in adult cardiovascular applications including use in arterial bypass for peripheral vascular disease and angioaccess for dialysis where currently used prosthetic grafts are a significant source of thrombo-embolic complications and have limited durability due to neointimal hyperplasia. While the development of an improved vascular graft for use in coronary artery bypass grafting (CABG) would enable a paradigm shift since there are currently no prosthetic conduits with suitable performance to recommend use in CABG surgery. Thus, providing additional treatment options and reduced morbidity for the leading cause of death in the western word.
Tissue Engineering: A Potential Solution
Tissue engineering provides a potential strategy for creating improved biomaterials for use in congenital heart surgery.8 One tissue engineering approach is to implant a biodegradable scaffold within which an individual’s cells are seeded. The scaffold provides sites for cell attachment and space for neotissue formation. After implantation, the scaffold degrades as neotissue forms, ultimately creating an autologous biological construct without any synthetic components.9 The resulting tissue engineered construct is a viable, living bioprosthetic and as such possesses the ability to self-repair, remodel, and grow.8–9 The fundamental premise underlying our work is that tissue engineering can augment congenital heart surgery by regenerating autologous cardiovascular neotissue and thereby repair or replace tissues that are congenitally malformed or absent. In this way, it is expected that tissue engineered constructs will perform better than currently available prosthetic biomaterials.
Our original tissue constructs were made via biopsy of a peripheral blood vessel, explanting the tissue to isolate the cellular components (endothelial, smooth muscle, and fibroblasts), and then expanding the cells in culture.10 The autologous cells were then seeded within a biodegradable scaffold fabricated from polyglycolic acid (PGA) fibers and incubated ex vivo prior to implantation.11 Using this approach, we were able to develop tissue engineered constructs for a variety of cardiovascular procedures, including valve replacement, surgical angioplasty, and vascular bypass. Results of our initial large animal pilot studies confirmed the feasibility of using tissue engineering methods to create neotissue for use in reconstructive cardiovascular procedures.10–12 We were also able to confirm the growth potential of these constructs, by demonstrating that they increase in size without becoming aneurysmal thus making our tissue engineered biomaterials the first engineered biomaterials with growth potential and thus the first with promise of avoiding additional surgeries due to somatic overgrowth.12 We subsequently performed the first implantation of a tissue engineered vascular graft (TEVG) in a human by augmenting a portion of the pulmonary artery that was congenitally hypoplastic (Figure 1).13
Figure 1.

Classic Tissue Engineering Paradigm. A biopsy from the saphenous vein allows cellular components, including endothelial cells, smooth muscle cells, and fibroblasts, to be isolated and expanded by serially passaging the cells over an 8-week period. 12 × 106 cells were then seeded onto a biodegradable tubular scaffold. The seeded construct was incubated for 10 days and then used to reconstruct the pulmonary artery. An angiogram performed 7-months after surgery demonstrated a widely patent TEVG without evidence of stenosis or aneurysmal dilatation. 13 The patient remains alive and well with the TEVG intact more than 20-years after implantation.
From [N Engl J Med, Shin’oka T, Imai Y, Ikada Y, Transplantation of a tissue-engineered pulmonary artery, 344(7)., 552–533. Copyright © (2001) Massachusetts Medical Society.] Reprinted with permission.
Despite demonstrating safety and efficacy in this first-in-human study, we abandoned this technique due to challenges in manufacturing the TEVG. The manufacture and assembly of this TEVG required an additional procedure (biopsy) to obtain autologous tissue. The subsequent cell expansion was very time consuming (6–10 weeks) and labor intensive, which added risk via the potential for contamination or even malignant dedifferentiation of the cells during ex vivo culture.14–15 However, the most significant downside to this methodology was its lack of reliability in diseased patients. We were unable to culture adequate numbers of cells from the vascular biopsy in a significant percentage of patients enrolled in the study, hence limiting our ability to make the TEVG.
We thus sought alternative cell sources and discovered that bone marrow-mononuclear cells could be used, instead of the endothelial cells, smooth muscle cells, and fibroblasts obtained from the vessel biopsy, to seed the scaffold and promote vascular neotissue formation.14 Results of our large animal studies confirmed the feasibility of this new technique to create neotissue and confirmed the growth capacity of this TEVG.16 The bone marrow-derived cells had the advantage that enough cells were available from a single bone marrow harvest, thus enabling adequate seeding of a scaffold without the need for cell culture and expansion ex vivo.15 This increased the utility of the methodology by dramatically reducing the time needed to obtain adequate numbers of cells for seeding the scaffold while improving reliability since adequate numbers of cells could be obtained from virtually any patient regardless of their disease status.
We also refined the design of our original TEVG scaffold by introducing a sealant consisting of a 50:50 copolymer of polycaprolactone and polylactic acid (PCLA), to create a degradable porous tube with a knitted PGA fiber core.17 The scaffold was designed to degrade by hydrolysis and to lose its mechanical integrity by approximately two months post-surgery while total scaffold degradation required approximately 6 months. The resulting vascular conduits demonstrated better surgical handling and improved neotissue formation while maintaining growth capacity in preclinical studies (Figure 2).14–17
Figure 2.

Scaffold Characterization: (A) Photograph of tubular scaffold with low and high magnification scanning electron microscope (SEM) images of cross-sections of the scaffold showing poly(glycolic acid), or PGA, fiber bundles, outlined in white. (B) Low and high magnification SEM images of the inner and outer surfaces of the scaffold reveal their porous structure while an image of the middle layer, obtained by dividing the wall sagittally, reveals the PGA fiber core embedded in a porous 50:50 PCLA sealant. 18
From [Drews JD, Pepper VK, Best CA, et al. Spontaneous reversal of stenosis in tissue-engineered vascular grafts. Sci Transl Med. 2020 Apr 1;12(537):eaax6919.]. Reprinted with permission from AAAS
Initial Clinical Trial
We developed a TEVG specifically for use in children with single ventricle disease requiring a Fontan surgery in which a vascular conduit is used to connect the inferior vena cava to the pulmonary artery.19 We view this TEVG as the archetype for tissue engineered constructs designed for use in congenital heart surgery. We contend that the lessons learned developing and translating this technology will facilitate the development and translation of other tissue engineered constructs and will open the door to the development of additional tissue engineered products, such as valved-conduits, which have the potential to improve further the outcomes of children born with congenital cardiac anomalies and advance the field of congenital heart surgery.
As our initial clinical target, we selected the extracardiac Fontan because it is the most commonly performed congenital heart operation requiring the use of a vascular conduit. In addition, it represents an opportunity to evaluate the growth potential of the TEVG.20 More importantly, the extracardiac Fontan conduit represents a reasonably safe initial clinical application because the large caliber conduit is used in a high-flow, low pressure circulation. Thus, the theoretical risks of catastrophic graft failure due to either acute thrombosis or aneurysmal dilatation and rupture are low compared to other clinical applications.
Currently available prosthetic vascular grafts for use in the Fontan operation include both biological and synthetic conduits. Synthetic grafts are used more commonly than biological conduits due to the high incidence of ectopic calcification in biological vascular conduits in the first few years after implantation.21 Synthetic options include polytetrafluoroethylene (PTFE) grafts or polyethylene terephthalate (PETE) grafts. PTFE grafts have supplanted the use of PETE and represent the current standard of care given the high incidence of stenosis due to neointimal hyperplasia associated with PETE grafts.22–23 Nevertheless, the use of PTFE grafts is still associated with a risk for graft-related complications.
While the midterm performance of the use of the PTFE graft as an extracardiac conduit in the Fontan operation appears adequate,24 it comes with the caveat that the morbidity and mortality associated with oversized grafts or delaying surgery to avoid somatic overgrowth must be accounted for but are difficult to quantitate and remain poorly studied because there are currently no vascular conduits with growth capacity. In addition, reports of late term (>10 years) graft-related complications are on the rise.25–26 There is a growing body of literature highlighting the development of ectopic calcification in PTFE grafts in the Fontan circulation.27 Since the Fontan operation is typically performed between 2–3 years of age and the average survival of a post-Fontan patient is currently estimated to be >40 years, there is a pressing need to develop a vascular conduit with growth capacity and improved durability for use in this patient population.25
Multiple teams have advanced the clinical translation of TEVGs for use in the clinic; however, there are currently no TEVGs approved for use in the United States. To date most of the translational work has been performed on small-diameter TEVGs designed for arterial bypass or arteriovenous angioaccess in adults.28–34 These TEVGs are made using different materials and fabrication methods and typically suffer from different complications (aneurysmal dilation or thrombosis rather than stenosis) than our large-diameter TEVGs designed for use in the Fontan circulation. The TEVG most comparable to ours is the unseeded, bioabsorbable TEVG manufactured by Xeltis,35 which has been implanted as an extracardiac conduit in 5 patients undergoing modified Fontan procedures in Russia. Results of this pilot study revealed no graft-related complications within 2 years after implantation; however, this study failed to demonstrate growth potential of the Xeltis TEVG in children.36–37 Currently, our clinical trial (IDE 18703) represents the only FDA-approved clinical investigation evaluating the use of a TEVG with growth potential designed specifically for use in children.
We have completed two clinical trials evaluating the use of TEVGs as an extracardiac vascular conduits connecting the IVC to the pulmonary artery in patients undergoing modified Fontan surgery.18,38 Results of the initial clinical study performed at Tokyo Women’s Hospital in Japan confirmed the feasibility of using the TEVG in humans and demonstrated that the TEVGs possessed growth potential since the TEVGs increased in size over time without evidence of aneurysmal dilation (Figure 3).39 Results of this study demonstrated no graft-related deaths, with stenosis the only graft-related complication.40–41 All patients who developed stenosis were successfully treated with angioplasty or angioplasty and stenting.39–41 Based on the promising early results of the first clinical trial, we initiated a second clinical trial in the United States (IDE 14127), the first FDA-approved trial to evaluate the use of a TEVG for congenital heart patients. The incidence of early stenosis was significantly higher in our second study: 0 of 25 patients developed stenosis in the first year after implantation in the Japanese trial whereas 3 of 4 patients developed stenosis within the first year after implantation in the US trial (P<0.01, two-sided Fisher’s exact test).18 Fortunately, all patients who developed stenosis were successfully treated with angioplasty, but the clinical trial was closed early due to the unexpectedly high incidence of stenosis.18
Figure 3:

Clinical Performance of the TEVG- In our first clinical trial a total of 25 patients had TEVGs implanted as extracardiac Fontan conduits. There were no graft failures or graft-related deaths, however, late term follow-up revealed that stenosis was the most common graft related complication. Three-dimensional CT 1 year after implantation. The graft is patent and there is no aneurysmal dilation. Red arrows denote TEVG.40
This article was published in J Thorac Cardiovasc Surg, 139(2), Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, Shinoka T, Late-term results of tissue-engineered vascular grafts in humans, 431–436.e1–2, Copyright Elsevier (2010).
Mechanistic Discoveries Based on Mouse Models
We subsequently returned to the bench to investigate cellular and molecular mechanisms underlying the formation of TEVG stenosis with the goal of using this information to rationally design strategies to reduce this complication in patients. We developed a murine model to take advantage of the power of genetic models not currently matched in other species.42 As a first step towards developing a murine model, we developed methods to fabricate scaffolds on a scale small enough to enable implantation of a TEVG in a mouse (3 mm in length, 0.8 mm in diameter).42 Since we cannot perform a Fontan operation in mice, we developed an IVC interposition graft model as a surrogate for the extracardiac modified Fontan operation.43 While there are hemodynamic differences between the IVC interposition graft model (including lower blood pressure) and the Fontan circulation, our model faithfully recapitulates many aspects of the process of neovessel formation that occurs in humans following Fontan operations, though over a shorter time course, which is experimentally advantageous. The murine model has proven to be a relevant model, since stenosis is the primary graft-related complication.44–45
In particular, we have evaluated more thoroughly the mechanisms that affect neotissue and thus neovessel formation: the process by which our biodegradable scaffold seeded with bone marrow-derived cells transforms into an endothelial-lined tube containing vascular smooth muscle cells and extracellular matrix, resembling a native blood vessel in structure and function.44 We discovered that cell seeding is not essential for neovessel formation, but it reduces the incidence of stenosis in a dose-dependent manner.45–46 By seeding more bone marrow-derived cells onto to the scaffold, we could significantly reduce narrowing, however the maximum density of cells that can be seeded within the scaffold is limited by scaffold saturation.46 Interestingly, the seeded cells rapidly disappeared from the TEVG after implantation, suggesting that they exert their effect via a paracrine mechanism.44 This important discovery challenged the classic tissue engineering paradigm that the cells seeded onto the scaffold were the ultimate source of the vascular neotissue. This finding shifted our focus towards the host immunological response to the TEVG.45 This paradigm shift led to the discovery that neovessel formation is a macrophage-mediated regenerative process, with the neovessel arising from ingrowth of vascular cells (endothelial and smooth muscle) from the neighboring vascular wall along the luminal surface of the scaffold and not from cells derived from the circulation or periadventitial space.45,47 We found that host-derived monocytes infiltrate the scaffold and that the degree of macrophage infiltration and the phenotype of these cells correlates with degree of TEVG narrowing.45,48 We also demonstrated that we could stop the process of neotissue formation by blocking macrophage infiltration into the scaffold.45 In other words, macrophage infiltration is essential for vascular neotissue formation, but when this process is excessive it leads to TEVG stenosis. There is, therefore, a need to achieve a proper balance of macrophage infiltration and function to promote successful neovessel formation.
We subsequently discovered that TGF-β signaling is a critical regulator of the formation of TEVG stenosis.49 We demonstrated that implantation of TEVGs into TGF-β receptor 1 (Tgfbr1) null mice significantly reduced the incidence of TEVG stenosis compared to wild-type mice. Our studies also showed that a Tgfbr1 inhibitor reduced monocyte infiltration into the scaffold, altered macrophage phenotype, and prevented the formation of TEVG stenosis without interfering with neovessel formation.48 Using a splenic transplant model in the mouse, we discovered that the spleen is a significant reservoir for the inflammatory monocytes that infiltrate the TEVG scaffold upon implantation.50 The angiotensin II type 1 (AT1) receptor had previously been demonstrated to control the release of monocytes from the spleen, so we then evaluated the utility of losartan (an AT1 receptor antagonist) on blocking the formation of TEVG stenosis.50 We found that short-term treatment with losartan provided sustained inhibition of TEVG stenosis.50 Furthermore, we demonstrated that short-term administration of losartan coupled with cell seeding eliminated the formation of TEVG stenosis without adversely impacting neovessel formation up to one year after implantation in the murine model.50 The short-term use of losartan to inhibit the formation of TEVG stenosis in patients undergoing modified Fontan surgery is a particularly attractive option since losartan is an FDA-approved medication (unlike the Tgfbr1 inhibitors) that is widely used with an excellent safety profile in the post-Fontan single ventricle patient population. (Figure 4).50
Figure 4.

Losartan Inhibits TEVG Stenosis: In order to evaluate effects of losartan on the formation of TEVG stenosis we implanted TEVGs as IVC interposition grafts in mice. We compared five groups: (1) unseeded TEVG, (2) unseeded TEVG treated with losartan (0.6g per L drinking water), (3) unseeded TEVG treated with a 2-week course of losartan, (4) seeded TEVG, and (5) seeded TEVG treated with a 2-week course of losartan (N=25/group). TEVGs were evaluated using ultrasound 6-months after implantation and up to 12 months in the short-term losartan + cell seeding group. The results were confirmed using histologic morphometry. We found that both cell seeding and short-term administration of losartan provided sustained inhibition of TEVG stenosis (p<0.01). In addition, the combination of cell seeding and short-term losartan administration eliminated TEVG stenosis. These findings suggest that cell seeding and short-term administration of losartan is a viable strategy for inhibiting the formation of TEVG stenosis in our clinical trial. 50
Computational Model
More recently we developed a computational model that describes and predicts in vivo growth and remodeling (G&R) of the TEVG.51–55 The model is predicated on the notion that neotissue forms via two primary processes: 1) inflammation driven neotissue formation, which is primarily induced by the foreign body reaction resulting from the implantation of the seeded scaffold, and 2) mechano-mediated remodeling arising from the inherent property of vascular tissue to promote mechanical homeostasis. We thus built on previous models of G&R developed and validated for native blood vessels by adding descriptors of inflammation driven neotissue formation. This computational model was developed and informed based on data generated using our mouse model of neotissue formation.51–53 We then performed a series of parametric studies in an attempt to gain additional insight into our clinical results.18 Results of the parametric studies accurately predicted the findings of our clinical trial, including the high incidence of early stenosis.18 Unexpectedly, the computational model also predicted that TEVG narrowing is a normal stage of the natural history of neotissue formation and that it would spontaneously reverse later during the process of neovessel formation Figure 5).18
Figure 5.

Computational Model of G&R Predicts Reversible Nature of TEVG Stenosis: The G&R model was used to perform parametric studies, which revealed the reversible nature of TEVG stenosis – normalized luminal diameter (d) decreasing after implantation, then increasing. Furthermore, simulations performed using 5 different values of a model parameter for inflammation (K) revealed that the time course of recovery depends on inflammatory burden.18
We tested this computational model-based prediction experimentally using a pre-clinicical ovine IVC vascular interposition graft model to investigate further the in vivo development of the TEVG over more than 1 year post-operatively.18 Associated computational simulations again predicted a high incidence of early stenosis with spontaneous reversal similar to that experienced in our clinical studies.18 Results from the long-term ovine model confirmed the reversible nature of early TEVG stenosis.18 These ovine studies further demonstrated that the early stenosis was not only reversible, but also well tolerated, suggesting that we over-treated (with angioplasty) the patients in our clinical study who developed stenosis during the first year after implantation (Figure 6).18,58
Figure 6:

Natural history of in vivo neovessel formation: Serial evaluation of TEVGs implanted as intrathoracic IVC interposition grafts in a juvenile ovine model (N=24) using 3D angiography and IVUS confirm the transient formation of stenosis, with spontaneous reversal without intervention confirming the computational model-based prediction. (A) Representative 3D angiographic images of the TEVG (outlined in yellow) demonstrate the formation of stenosis by 6 weeks with significant improvement by 6 months. (B) Representative histological images (H&E low magnification) reveal that the stenosis arises from wall thickening within region of the scaffold and luminal encroachment with inward remodeling. (C) After the scaffold has degraded (52 weeks), the change in graft size occurs more gradually at a rate similar to the native IVC.18
Adapted from Blum KM, Zbinden JC, Ramachandra AB, et al. Tissue engineered vascular grafts transform into autologous neovessels capable of native function and growth. Commun Med (Lond). 2022 Jan 10;2:3. eCollection 2022.
Insights from Animal and Computational Model
Our experimental-computational approach, using murine models to identify underlying mechanisms and ovine models to validate computational predictions, has revealed that the in vivo development of a neovessel from an implanted polymeric construct is a complex, dynamic process governed by a simultaneous degradation of polymeric scaffold and elaboration of neotissue.48–49,56 Early on, the size, shape, and performance of a graft are dominated by the chemical, morphometric, and mechanical properties of the scaffold. As scaffold degrades, however, cell-mediated deposition and organization of neotissue progressively determines the size, shape, and performance of the graft.18,46,57 Indeed, the neotissue can continue to grow and remodel in response to mechanical or inflammatory cues following scaffold degradation. 19,48,56 Such “growth potential” is highly desired and unique to our TEVGs for congenital heart intervention. We have also shown (and verified computationally) that the early phase of neotissue formation is immunologically driven, due to the foreign body response to the scaffold, but increasingly mechanobiologically mediated as the polymer degrades and intramural cells sense and respond to the increased loading of the neotissue.18,46,57
The computational model has accelerated our discovery and return to the clinic by providing cost- and time-efficient insights into the process of neovessel formation, which we confirmed experimentally.18 Perhaps the most important model-based discovery is that once the TEVG scaffold is completely degraded, the resulting neovessel undergoes growth and remodeling processes that mimic the behavior of a native vessel, as ultimately desired. Thus, over time the TEVG transforms into a living vascular conduit that behaves more like a native vessel than like a prosthetic vascular graft. In addition to the development of growth capacity, this includes formation of a compliant wall (Figure 7) that is vasoreactive (Figure 8) and thus similar to the native IVC.
Figure 7:

Evolution of histo-mechanical properties of the TEVG implanted as IVC interposition grafts in lambs: The biomechanical properties of the TEVG arise from the sum of the mechanical properties of the scaffold and the biomechanical properties of the cells and extracellular matrix (ECM) of the neotissue. Upon implantation the scaffold is stiff compared to the compliance of the native IVC. (A) Histological photomicrographs of TEVG harvested at 1 week, 6 weeks, 6 months, and 1 year after implantation and stained with picro-sirius red (PSR). The photomicrographs are obtained using polarized light to visualize the PGA and collagen fibers. Note how the polymeric fibers degrade over a 6-month period while total scaffold degradation occurs over year. Note, too, that there is scant ECM at 1 week, but at 6 weeks there is a large amount of immature (green), poorly organized collagen, at 6 months the collagen begins to mature (orange) and become more organized, and by 1 year the collagen is mature (red) and highly organized. As the stiff scaffold degrades and the ECM matures and remodels, the TEVG becomes more compliant. (B) Circumferential stress-stretch curves for the 6-week TEVG (N=3) (red dots), 78-week TEVGs (N=3) (black dots) and native IVC (N=1) (open dots). Quantitative analysis and comparison of the 6-week TEVG to the 78-week TEVG and native IVC reveal that the (C) circumferential stress and (D) axial stress increase significantly and approach, but do not reach, the biomechanical properties of the native IVC.18
Adapted from Drews JD, Pepper VK, Best CA, et al. Spontaneous reversal of stenosis in tissue-engineered vascular grafts. Sci Transl Med. 2020 Apr 1;12(537):eaax6919. Reprinted with permission from AAAS
Adapted from Blum KM, Zbinden JC, Ramachandra AB, et al. Tissue engineered vascular grafts transform into autologous neovessels capable of native function and growth. Commun Med (Lond). 2022 Jan 10;2:3. eCollection 2022.
Figure 8:

Neovessels Mimic the Structure and Function of Native Vessels: (A) Low and high magnification images reveal that neovessels (TEVG implanted in a lamb > 1 year) resemble native vessels (IVC) including a thin laminated wall composed of a monolayer of endothelial cells (CD31+) surrounded by concentric layers of smooth muscle cells (calponin+). (B) Pharmacologic stimulation of the ovine neovessels demonstrate that the neovessels (N=3) become vasoactive responding similarly as the IVC (N=3) when exposed to physiological doses of vasoconstrictors such as endothelin-1 (ET-1) and vasodilators such as sodium nitroprusside (SNP) ex vivo.18
Adapted from Blum KM, Zbinden JC, Ramachandra AB, et al. Tissue engineered vascular grafts transform into autologous neovessels capable of native function and growth. Commun Med (Lond). 2022 Jan 10;2:3. eCollection 2022
Return to the Clinic
Based on these discoveries, we recently initiated our next TEVG clinical trial (IDE 18703), at present the only active FDA-approved clinical investigation of the use of a TEVG with growth potential designed specifically for use in children. The purpose of this trial is to evaluate the safety of a second-generation TEVG. The design of our second generation TEVG builds upon findings from our preclinical studies in an effort to reduce the incidence of TEVG stenosis and to improve the overall performance of the TEVG (Figure 9). Critical design alterations include: (1) use of the new system for isolating bone marrow derived-mononuclear cells, (2) use of an optimized cell seeding dose and incubation period, (3) short-term use of losartan during the perioperative period, and (4) implementation of new angioplasty criteria designed to allow spontaneous reversal of stenosis in asymptomatic patients. This clinical trial is a prospective, single-arm, exploratory-confirmatory trial to evaluate the safety of the second-generation TEVG as a vascular conduit for extracardiac modified Fontan surgery in children with single ventricle cardiac anomalies. Successful completion of this trial will be necessary for obtaining regulatory approval for the TEVG using the orphan regulatory pathway and, if successful, could enable the widespread use of the first vascular graft with growth capacity and thereby avoid the need to oversize the graft at implantation, to unnecessarily delay surgery, and avoid somatic overgrowth, thus addressing a critical problem in the field of congenital heart surgery.
Figure 9:

Timeline describing the development and translation of a tissue engineered vascular graft made by seeding autologous bone marrow-derived mononuclear cells onto a biodegradable polymeric scaffold created for use as an extracardiac Fontan conduit.
Highlights.
Development of tissue-engineered vascular grafts offers a promising approach to treat children with congenital heart disease
Tissue engineered vascular grafts allow for the growth of native tissue as opposed to traditional reconstruction materials such as polytetrafluoroethylene
Computational studies reveal that implanted tissue engineered vascular grafts experience early stenosis that is then spontaneously reversed in the process of neovessel formation
Losartan is capable of inhibiting stenosis of tissue-engineered vascular grafts by blocking monocyte release from the spleen
Acknowledgements
The graphic abstract was created with BioRender.com.
Sources of Funding
Supported by National Institutes of Health (NIH) UH3HL148693 and NIH R01 HL139796.
Abbreviations:
- AT1
angiotensin II type 1
- CABG
coronary artery bypass grafting
- G&R
growth and remodeling
- IVC
inferior vena cava
- PCLA
polycaprolactone and polylactic acid
- PETE
polyethylene terephthalate
- PGA
polyglycolic acid
- PTFE
polytetrafluoroethylene
- TEVG
tissue-engineered vascular graft
- TGF-𝛽
transforming growth factor-beta
- Tgfbr1
transforming growth factor-beta receptor 1
Footnotes
The other authors report no conflicts.
Disclosures: Christopher Breuer and Toshiharu Shinoka receive grant support from Gunze Limited.
Contributor Information
Thomas Breuer, Nationwide Children’s Hospital, Columbus, OH.
Michael Jimenez, Nationwide Children’s Hospital, Columbus, OH.
Jay D. Humphrey, Yale University, School of Engineering and Applied Science, New Haven, CT
Toshiharu Shinoka, Nationwide Children’s Hospital, Columbus, OH.
Christopher K. Breuer, Nationwide Children’s Hospital, Columbus, OH
References
- 1.van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011. Nov 15;58(21):2241–7. [DOI] [PubMed] [Google Scholar]
- 2.Simeone RM, Oster ME, Cassell CH, Armour BS, Gray DT, Honein MA. Pediatric inpatient hospital resource use for congenital heart defects. Birth Defects Res A Clin Mol Teratol 2014. Dec;100(12):934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee AY, Mahler N, Best C, Lee YU, Breuer CK. Regenerative implants for cardiovascular tissue engineering. Transl Res 2014. Apr;163(4):321–41. [DOI] [PubMed] [Google Scholar]
- 4.Shoji T, Shinoka T. Tissue engineered vascular grafts for pediatric cardiac surgery. Transl Pediatr 2018. Apr;7(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Son JA, Reddy M, Hanley FL. Extracardiac modification of the Fontan operation without use of prosthetic material. J Thorac Cardiovasc Surg 1995. Dec;110(6):1766–8. [DOI] [PubMed] [Google Scholar]
- 6.Alexi-Meskishvili V, Ovroutski S, Ewert P, Dähnert I, Berger F, Lange PE, Hetzer R. Optimal conduit size for extracardiac Fontan operation. Eur J Cardiothorac Surg 2000. Dec;18(6):690–5. [DOI] [PubMed] [Google Scholar]
- 7.Itatani K, Miyaji K, Tomoyasu T, Nakahata Y, Ohara K, Takamoto S, Ishii M. Optimal conduit size of the extracardiac Fontan operation based on energy loss and flow stagnation. Ann Thorac Surg 2009. Aug;88(2):565–72; discussion 572–3. [DOI] [PubMed] [Google Scholar]
- 8.Patterson JT, Gilliland T, Maxfield MW, Church S, Naito Y, Shinoka T, Breuer CK. Tissue-engineered vascular grafts for use in the treatment of congenital heart disease: from the bench to the clinic and back again. Regen Med 2012. May; 7(3):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langer R, Vacanti JP. Tissue engineering. Science 1993. May; 260(5110):920–926. [DOI] [PubMed] [Google Scholar]
- 10.Shinoka T, Ma PX, Shum-Tim D, Breuer CK, Cusick RA, Zund G, Langer R, Vacanti JP, Mayer JE Jr. Tissue-engineered heart valves. Autologous valve leaflet replacement study in a lamb model. Circulation 1996. Nov 1; 94(9 Suppl):II164–8. [PubMed] [Google Scholar]
- 11.Breuer CK, Shinoka T, Tanel RE,et al. Tissue engineering lamb heart valve leaflets. Biotechnol Bioeng 1996. Jun 5;50(5):562–7. [DOI] [PubMed] [Google Scholar]
- 12.Shinoka T, Shum-Tim D, Ma PX, Tanel RE, Isogai N, Langer R, Vacanti JP, Mayer JE Jr. Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiovasc Surg 1998. Mar; 115(3):536–45; discussion 545–6. [DOI] [PubMed] [Google Scholar]
- 13.Shin’oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med 2001. Feb 15; 344(7):532–3. [DOI] [PubMed] [Google Scholar]
- 14.Matsumura G, Miyagawa-Tomita S, Shin’oka T, Ikada Y, Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation 2003. Oct; 108(14):1729–1734. [DOI] [PubMed] [Google Scholar]
- 15.Hibino N, Shinoka T, Matsumura G, Ikada Y, Kurosawa H. The tissue-engineered vascular graft using bone marrow without culture. J Thorac Cardiovasc Surg 2005. May;129(5):1064–1070. [DOI] [PubMed] [Google Scholar]
- 16.Matsumura G, Ishihara Y, Miyagawa-Tomita S, Ikada Y, Matsuda S, Kurosawa H, Shin’oka T. Evaluation of tissue-engineered vascular autografts. Tissue Eng 2006. Nov;12(11):3075–3083. [DOI] [PubMed] [Google Scholar]
- 17.Brennan MP, Dardik A, Hibino N, Roh JD, Nelson GN, Papademitris X, Shinoka T, Breuer CK. Tissue-engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann Surg 2008. Sep; 248(3):370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drews JD, Pepper VK, Best CA, et al. Spontaneous reversal of stenosis in tissue-engineered vascular grafts. Sci Transl Med 2020. Apr 1;12(537):eaax6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naito Y, Imai Y, Shin’oka T,et al. Successful clinical application of tissue-engineered graft for extracardiac Fontan operation. J Thorac Cardiovasc Surg 2003. Feb;125(2):419–20. [DOI] [PubMed] [Google Scholar]
- 20.Matsumura G, Hibino N, Ikada Y, Kurosawa H, Shin’oka T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials 2003. Jun;24(13):2303–8. [DOI] [PubMed] [Google Scholar]
- 21.Monro JL, Salmon AP, Keeton BR. The outcome of antibiotic sterilised aortic homografts used in the Fontan procedure. Eur J Cardiothorac Surg 1993;7(7):360–3; discussion 364. [DOI] [PubMed] [Google Scholar]
- 22.van Brakel TJ, Schoof PH, de Roo F, Nikkels PG, Evens FC, Haas F. High incidence of Dacron conduit stenosis for extracardiac Fontan procedure. J Thorac Cardiovasc Surg 2014. May;147(5):1568–72. [DOI] [PubMed] [Google Scholar]
- 23.Careddu L, Petridis FD, Angeli E, Balducci A, Mariucci E, Egidy Assenza G, Donti A, Gargiulo GD. Dacron Conduit for Extracardiac Total Cavopulmonary Anastomosis: A Word of Caution. Heart Lung Circ 2019. Dec;28(12):1872–1880. [DOI] [PubMed] [Google Scholar]
- 24.Lee C, Lee CH, Hwang SW, Lim HG, Kim SJ, Lee JY, Shim WS, Kim WH. Midterm follow-up of the status of Gore-Tex graft after extracardiac conduit Fontan procedure. Eur J Cardiothorac Surg 2007. Jun;31(6):1008–12. [DOI] [PubMed] [Google Scholar]
- 25.Kelly JM, Mirhaidari GJM, Chang YC, Shinoka T, Breuer CK, Yates AR, Hor KN. Evaluating the Longevity of the Fontan Pathway. Pediatr Cardiol 2020. Dec;41(8):1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagler DJ, Miranda WR, Haggerty BJ, Anderson JH, Johnson JN, Cetta F, Said SM, Taggart NW. Fate of the Fontan connection: Mechanisms of stenosis and management. Congenit Heart Dis 2019. Jul;14(4):571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayabuchi Y, Mori K, Kitagawa T, Sakata M, Kagami S. Polytetrafluoroethylene graft calcification in patients with surgically repaired congenital heart disease: evaluation using multidetector-row computed tomography. Am Heart J 2007. May;153(5):806.e1–8. [DOI] [PubMed] [Google Scholar]
- 28.L’Heureux N, Pâquet S, Labbé R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J 1998. Jan;12(1):47–56. [DOI] [PubMed] [Google Scholar]
- 29.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science 1999. Apr 16;284(5413):489–93. [DOI] [PubMed] [Google Scholar]
- 30.L’Heureux N, Dusserre N, Konig G, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med 2006. Mar;12(3):361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A 2011. May 31;108(22):9214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syedain ZH, Meier LA, Bjork JW, Lee A, Tranquillo RT. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials 2011. Jan;32(3):714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Syedain ZH, Graham ML, Dunn TB, O’Brien T, Johnson SL, Schumacher RJ, Tranquillo RT. A completely biological “off-the-shelf” arteriovenous graft that recellularizes in baboons. Sci Transl Med 2017. Nov 1;9(414):eaan4209. [DOI] [PubMed] [Google Scholar]
- 34.Dahl SL, Kypson AP, Lawson JH, et al. Readily available tissue-engineered vascular grafts. Sci Transl Med 2011. Feb 2;3(68):68ra9. [DOI] [PubMed] [Google Scholar]
- 35.Brugmans M, Serrero A, Cox M, Svanidze O, Schoen FJ. Morphology and mechanisms of a novel absorbable polymeric conduit in the pulmonary circulation of sheep. Cardiovasc Pathol 2019. Jan-Feb;38:31–38. [DOI] [PubMed] [Google Scholar]
- 36.Bockeria LA, Svanidze O, Kim A, Shatalov K, Makarenko V, Cox M, Carrel T. Total cavopulmonary connection with a new bioabsorbable vascular graft: First clinical experience. J Thorac Cardiovasc Surg 2017. Jun;153(6):1542–1550. doi: 10.1016/j.jtcvs.2016.11.071. Epub 2017 Feb 7. Erratum in: J Thorac Cardiovasc Surg. 2018 Mar;155(3):1348–1349. [DOI] [PubMed] [Google Scholar]
- 37.Bockeria L, Carrel T, Lemaire A, Makarenko V, Kim A, Shatalov K, Cox M, Svanidze O. Total cavopulmonary connection with a new restorative vascular graft: results at 2 years. J Thorac Dis 2020. Aug;12(8):4168–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isomatsu Y, Shin’oka T, Matsumura G, Hibino N, Konuma T, Nagatsu M, Kurosawa H. Extracardiac total cavopulmonary connection using a tissue-engineered graft. J Thorac Cardiovasc Surg 2003. Dec;126(6):1958–62. [DOI] [PubMed] [Google Scholar]
- 39.Shin’oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M, Kurosawa H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg 2005. Jun;129(6):1330–8. [DOI] [PubMed] [Google Scholar]
- 40.Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, Shinoka T. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 2010. Feb;139(2):431–6, 436.e1–2. [DOI] [PubMed] [Google Scholar]
- 41.Sugiura T, Matsumura G, Miyamoto S, Miyachi H, Breuer CK, Shinoka T. Tissue-engineered Vascular Grafts in Children With Congenital Heart Disease: Intermediate Term Follow-up. Semin Thorac Cardiovasc Surg 2018. Summer;30(2):175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roh JD, Nelson GN, Brennan MP, et al. Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model. Biomaterials 2008. Apr; 29(10):1454–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson GN, Mirensky T, Brennan MP, Roh JD, Yi T, Wang Y, Breuer CK. Functional small-diameter human tissue-engineered arterial grafts in an immunodeficient mouse model: preliminary findings. Arch Surg 2008. May; 143(5):488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roh JD, Sawh-Martinez R, Brennan MP, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A 2010. Mar 9;107(10):4669–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hibino N, Yi T, Duncan DR, et al. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J 2011. Dec; 25(12):4253–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee YU, Mahler N, Best CA, Tara S, Sugiura T, Lee AY, Yi T, Hibino N, Shinoka T, Breuer CK, Rational design of an improved tissue-engineered vascular graft: determining the optimal cell dose and incubation time. Regen Med 2016. Mar; 11(2):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hibino N, Villalona G, Pietris N, et al. Tissue-engineered vascular grafts form neovessels that arise from regeneration of the adjacent blood vessel. FASEB J 2011. Aug; 25(8):2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee YU, de Dios Ruiz-Rosado J, Mahler N, et al. TGF-β receptor 1 inhibition prevents stenosis of tissue-engineered vascular grafts by reducing host mononuclear phagocyte activation. FASEB J 2016. Jul;30(7):2627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duncan DR, Chen PY, Patterson JT, et al. TGFβR1 inhibition blocks the formation of stenosis in tissue-engineered vascular grafts. J Am Coll Cardiol 2015. Feb 10; 65(5):512–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruiz-Rosado JD, Lee YU, Mahler N, et al. Angiotensin II receptor I blockade prevents stenosis of tissue engineered vascular grafts. FASEB J 2018. Jun 15; 32(12):fj201800458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller KS, Lee YU, Naito Y, Breuer CK, Humphrey JD. Computational model of the in vivo development of a tissue engineered vein from an implanted polymeric construct. J Biomech 2014. Jun 27;47(9):2080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller KS, Khosravi R, Breuer CK, Humphrey JD. A hypothesis-driven parametric study of effects of polymeric scaffold properties on tissue engineered neovessel formation. Acta Biomater 2015. Jan;11:283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khosravi R, Ramachandra AB, Szafron JM, Schiavazzi DE, Breuer CK, Humphrey JD. A computational bio-chemo-mechanical model of in vivo tissue-engineered vascular graft development. Integr Biol (Camb) 2020. Apr 14;12(3):47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szafron JM, Khosravi R, Reinhardt J, Best CA, Bersi MR, Yi T, Breuer CK, Humphrey JD. Immuno-driven and Mechano-mediated Neotissue Formation in Tissue Engineered Vascular Grafts. Ann Biomed Eng 2018. Nov;46(11):1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szafron JM, Ramachandra AB, Breuer CK, Marsden AL, Humphrey JD. Optimization of Tissue-Engineered Vascular Graft Design Using Computational Modeling. Tissue Eng Part C Methods 2019. Oct;25(10):561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shinoka T, What is the best material for extracardiac Fontan operation, J Thorac Cardiovasc Surg 153, 1551–1552 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Khosravi R, Miller KS, Best CA, Shih YC, Lee YU, Yi T, Shinoka T, Breuer CK, J.D. Humphrey, Biomechanical diversity despite mechanobiological stability in tissue engineered vascular grafts two years post-implantation. Tissue Eng. Part A 21, 1529–38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blum KM, Zbinden JC, Ramachandra AB, et al. Tissue engineered vascular grafts transform into autologous neovessels capable of native function and growth. Commun Med (Lond) 10, 2:3 eCollection (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]


