Abstract
Research in humans and animals shows differences in impulsive choice, which is a failure to wait for larger, delayed rewards, when comparing males and females. It is possible that fluctuations in sex hormones (estradiol and progesterone) across the reproductive cycle contribute to sex differences in impulsive choice. The current study delivered an impulsive choice task with peak interval trials to female rats while estrous cycles, the rodent reproductive cycle, were tracked over the course of the task. Female rats were more sensitive to changes in delay in the proestrus phase of the estrous cycle and made more larger-later choices when in estrus, particularly when the delay to the smaller reward was short. Estradiol increases dramatically during proestrus while progesterone peaks during estrus, suggesting that estradiol and progesterone may affect impulsive choice through mechanisms such as delay discounting, delay aversion, and/or timing processes. Analyses of timing of the choice task delays showed inconsistent effects of the estrous cycle across delays, suggesting that reward-timing interactions may have complicated how hormone fluctuations affected interval timing. Further research is needed to determine the mechanism underlying increased larger-later choices during the estrus phase, increased delay sensitivity during the proestrus phase, and variability in interval timing across delays and estrous cycle stages.
Keywords: estrous cycle, impulsive choice, timing, female, rat
An impulsive choice involves selecting a smaller-sooner (SS) option over a larger-later (LL) option. In impulsive choice paradigms, choices involve trade-offs between reward amounts and delays and can be affected by many variables. Several studies have reported correlations between impulsive choice and maladaptive behaviors such as obesity (Rasmussen et al., 2010) and drug use (Bickel & Marsch, 2001). Multiple studies have also shown sex differences in impulsive choice in humans and animals (see Weafer & de Wit, 2014 for a review). For example, men show larger decreases in subjective value associated with reward compared to women when rewards are real rather than hypothetical (Kirby & Marakovic, 1996; Rasmussen et al., 2010; Weafer & de Wit, 2014). In our laboratory, male and female rats completed multiple impulsive choice tasks after a time-based intervention was delivered to improve self-control. Females were less impulsive than males, particularly showing a shallower slope in their choice functions (Panfil et al., 2020). However, these effects appeared to be task-dependent such that the degree to which the time-based intervention improved self-control was more variable in females. One possible explanation for sex differences in impulsive choice may be hormone fluctuations.
Hormones change across the human menstrual cycle and the estrous cycle, the reoccurring cycle of fertility in rodents. Rats cycle through stages in the estrous cycle: proestrus, estrus, metestrus, and diestrus. Cycles can be tracked through vaginal lavage, a technique where saline is flushed in and out of the vaginal cavity, capturing cells. Cell morphology is then assessed to determine the current estrous phase. Changes in cell morphology are a result of cyclical changes in estradiol and progesterone, female sex hormones (Cora et al., 2015). Briefly, proestrus is characterized by increased estradiol and decreased progesterone (Yoest et al., 2018). Estrus is marked by increased progesterone and reduced estradiol (Yoest et al., 2018). Metestrus has relatively low levels of estradiol and progesterone, lasting only a few hours (Hubscher et al., 2005). Finally, during diestrus, estradiol slowly increases and progesterone gradually decreases, which signals proestrus to begin again (Yoest et al., 2018).
To our knowledge, few animal studies have measured estrous cycles during an impulsive choice assessment without also administering drugs that could potentially alter decision making. Sex differences in choice behavior are often observed in preclinical studies designed to simulate disease states (Anker & Carroll, 2011; Becker & Hu, 2008; Carroll & Anker, 2010; Lynch et al., 2002), but few studies have specifically examined estrous cycles. Lukkes et al. (2016) examined the effects of ovariectomy in female rats in conjunction with other variables and found no effects of ovariectomy on impulsive choices, suggesting that the removal of a major source of sex hormones does not alter choice behavior. Along the same lines, there were no effects of estrous cycle on a delayed punishment impulsive choice task (Liley et al., 2019). However, performance on the delayed punishment task was not correlated with performance on a more traditional assessment of impulsive choice. This suggests any effect of estrous cycle on delayed punishment does not necessitate an estrous cycle effect on impulsive choice. In a more traditional assessment of impulsive choice behavior, Hernandez et al. (2020) reported no difference in choices based on estrous cycle in naturally cycling female rats. While these studies suggest little to no effect of estrous cycles or sex hormones on impulsive choice, it is important to note that rats were offered choices involving 0-s delays to rewards in those studies. This procedural detail may influence choice behavior to bias rats towards the SS option (Smith et al., 2022).
In addition to the preclinical literature, there have been observations of hormonal effects on impulsive choices in humans. In women, when estradiol was low, impulsive choice also decreased (Smith et al., 2014). Diekhof (2015) corroborated these results but also tested exclusively in the follicular phase, when progesterone levels are salient, reducing interactions of other hormones on choice behavior. These studies suggest a role for sex hormones in choice behavior, but it is still unclear which mechanisms of choice behavior may be affected.
Multiple cognitive mechanisms may underlie impulsive choice. One possible mechanism of impulsive choice is delay discounting, which is the decrease of subjective reward value as a function of time to delivery (Mazur, 2000). Subjects that discount at a shallower rate demonstrate greater self-control, evidenced by more larger-later choices (Baumann & Odum, 2012). Delay discounting has many parallels with delay aversion. Delay aversion is a motivated behavior to avoid delays (Sonuga-Barke et al., 1992). If a subject is averse to waiting or discounts steeply, they may be motivated to select immediate rewards, limiting the opportunity to experience long delays. Limited experience with long delays may negatively affect timing ability. Thus, delay discounting and/or delay aversion may lead to deficits in timing ability.
Alternatively, timing ability may contribute to impulsive choice independently (Smith et al., 2015). If an individual is unable to perceive time correctly, they may not judge the value associated with a large reward correctly. Three important metrics of timing ability are accuracy, precision, and confidence. Accuracy can be seen when individuals expect reward at the correct time. Precision is the interval over which responding occurs surrounding reward expectancy, with narrower windows of responding reflecting greater precision. Confidence is the rate of responding at the expected time of reward, an indication of how strongly an individual expects the reward (Stuebing et al., 2018). Smith et al. (2015) found a positive correlation between timing precision and decreased impulsive choice. However, other studies did not show a relationship between impulsive choice and timing (Eckard & Kyonka, 2018; Fox et al., 2019; Rung et al., 2018). While informative, these studies were conducted in male rats only. One study examined the effects of a time-based intervention on impulsive choice and interval timing in a group of female rats and found that the intervention resulted in decreased impulsive choices and increased confidence in timing the delays delivered during the choice task (Stuebing et al., 2018). However, this experiment did not include any males for comparison or measure estrous cycles. Altogether, timing ability may play a casual role in impulsive choice, but the effects of sex require further study.
To date, few studies have assessed sex differences in timing in humans and animals. Across studies in humans, men discriminate delays in the milliseconds to seconds range better than females (Rammsayer & Lustnauer, 1989; Wittmann & Szelag, 2003). In the animal literature, sex differences were assessed using supra-second durations in ovariectomized rats. Rats that received chronic estradiol treatment for 14 days prior to a temporal discrimination task showed decreased timing precision (Ross & Santi, 2000). Along the same lines, ovariectomized female, but not gonadectomized male rats underestimated intervals after a single estradiol injection (Pleil et al., 2011; Sandstrom, 2007). Overall, estradiol may alter time perception in females, which may be a driving factor of sex differences observed in humans and animals. However, this finding has not been examined in naturally cycling female rats. In the current study, female rats completed a 30-day impulsive choice task with peak interval trials to assess time perception within an impulsive choice task. Estrous cycle samples were collected daily over the entirety of testing. The ultimate goal of this study was to assess the relationship between impulsive choice, temporal processing, and the estrous cycle.
Method
Animals
Twenty-four female Sprague Dawley rats (Charles River, Stone Ridge, NY) arrived at the facility (Kansas State University, Manhattan, KS) at 21 postnatal days (PND) of age. Experimentation began at PND 61. The rats were pair-housed and maintained on 12-hr reverse light: dark schedule. The rats were tested during the dark phase of the cycle. There was ad libitum access to water in the home cages and experimental chambers. Rats were food restricted to approximately 90% of their ad libitum weight.
Apparatus
The experiment was conducted in 24 operant chambers (Med-Associates, St. Albans, VT) as described in Smith et al. (2015). Briefly, each operant chamber was equipped with a stainless-steel grid floor, two stainless steel walls (front and back), and a transparent polycarbonate side wall, ceiling, and door. Two pellet dispensers (ENV-203) delivered 45-mg food pellets (Bio-Serv, Flemington, NJ) to a food cup (ENV-200R7). Two retractable levers (ENV-112CM) were located on opposite sides of the food cup. The chamber was also equipped with a house light (ENV-227M) as well as two nose-poke key lights (ENV-119M-1). Experimental events were controlled and recorded with 1-ms resolution by the software program MED-PC V.
Procedure
Vaginal Lavage.
Starting on PND 55 (following onset of adolescence at ~PND 50), estrous cycles were tracked via vaginal lavage daily. Sterile saline (0.9%; ~0.2 mL) was drawn up with a sterile pipette tip and inserted 5-10 mm into the vaginal opening. The solution was flushed in and out until cloudy and then plated on microscope slides. The collection procedure occurred 30 minutes before behavioral testing.
Pre-Training.
Magazine training and lever training were delivered as described in Smith et al. (2015). Briefly, magazine training delivered food pellets on a random 60-s schedule and lasted one session. Lever training included fixed ratio 1, random ratio (RR) 3 and RR 5 schedules on both levers and lasted 3 sessions.
Impulsive Choice Task.
The impulsive choice task was delivered as described in Panfil et al. (2020). Briefly, the task consisted of three phases, during which rats chose between a SS (smaller-sooner) reward (1 pellet) and a LL (larger-later) reward (2 pellets). SS and LL levers were counterbalanced across all 24 rats, and lever assignments remained the same for the duration of the study. Each session involved a mixture of free-choice, forced-choice, and peak interval trials. On free-choice trials, both levers were available. After one lever was pressed, the other lever retracted, the cue light above the lever was illuminated, and the corresponding delay began. The first lever press after the delay elapsed resulted in food delivery, cue light offset, and the onset of the 60-s inter-trial interval. Forced-choice trials were identical to free-choice trials but only one lever was inserted during forced-choice trials. During peak interval trials, only one lever was available, and food was not delivered. Peak interval trials lasted for 90 s, and all responses made on the available lever were recorded. Each session contained 78 trials delivered in three 26-trial blocks. Each block contained 14 free-choice, 4 SS forced-choice, 4 LL forced-choice, 2 SS peak, and 2 LL peak trials. Sessions lasted for approximately 2 hr and delivered a maximum of 120 reinforcers. The SS delay increased from 5→10→20 s across three phases (10 consecutive sessions each). The LL delay remained at 30 s in all phases.
Estrous Sample Processing and Evaluation.
Estrous sample processing followed the procedure used in McLean et al. (2012). Heat-fixed samples were immersed in crystal violet solution for 1 min and distilled water twice for 1 min each. The slides were air-dried, and a coverslip was placed over the samples. Samples were evaluated by three individuals who were blind to the experimental conditions. An intra-class coefficient was calculated to confirm interrater reliability.
Data Analysis
Data was compiled with MATLAB 2020a (The MathWorks) and included the last 5 sessions of each phase of the impulsive choice task. Choice and timing behaviors were analyzed with multi-level repeated measures regressions. This analysis method assesses differences at the group level (fixed effects) and at the individual level (Bolker et al., 2008; Hoffman & Rovine, 2007; Young et al., 2013). In this analysis framework, individual choices and lever press responses were incorporated as correlated observations within individuals which increases precision of the confidence intervals surrounding the effect size estimates (Cnaan et al., 1997). These qualities of multi-level analyses decrease Type I error rates (assuming the models are not overparametrized; Bates et al., 2015; Matuschek et al., 2017). An α level of .05 was set for determining significant effects. Planned comparisons and post-hoc analyses probed significant interactions using the emmeans package in R (Lenth, 2022), and p-values were adjusted for multiple comparisons using the Tukey method.
Across analyses of impulsive choice and peak interval timing, estrous stage was entered as a three-level categorical variable with sum-to-zero coding. Proestrus was coded as 1, 0; estrus was coded as 0, 1; met/diestrus was coded as −1, −1. Metestrus and diestrus phases were collapsed together because metestrus lasts only a few hours, and there were few metestrus samples that did not show signs of transition to diestrus. In both choice and peak analyses, nine animals were removed from the analyses because they had no choice or peak interval data during proestrus. Vaginal lavage samples were obtained from the rats 30 minutes before they were loaded into the operant chambers for 2-hr sessions. The proestrus phase is relatively short, lasting 12-14 hr, so these nine rats may have cycled through the proestrus phase between sessions. Analyses only included the last 5 sessions of each phase as well (when behavior was relatively stable), so rats may have been in the proestrus phase outside of the 5-session window. Removal of the animals that were missing proestrus observations ensured a similar number of observations for each phase of the estrous cycle. This removes a possible source of bias as all rats contributed data from all three cycles to the analysis.
Impulsive Choice Behavior.
A multi-level repeated-measures logistic regression was conducted on free-choice trials using the lme4 package in R (Pinheiro et al., 2018). SS delay, estrous stage, and their interaction were included as fixed effects, and intercept was included as a random effect. Delay was treated as a continuous variable and mean-centered. Additional planned comparisons examined the rats’ preferences by extrapolating the model fits to test differences at two SS delays outside of the data range. To predict rats’ preferences for an immediate reward, a planned comparison was conducted at an SS delay of 0 s. To predict rats’ preference for the larger reward in the absence of any difference in delay, a planned comparison was conducted at an SS delay of 30 s. The final model was conducted on 8,398 choices from 15 rats.
Peak Trial Behavior.
Multi-level repeated-measures nonlinear regressions were conducted on peak interval trials only using the nlme package in R (Pinheiro et al., 2018) using a three-parameter modified Gaussian distribution to fit responses per minute:
| (1) |
where t represented the time in the peak trial, a was the time of the peak (accuracy), and p was the standard deviation of the peak (precision; see Fox et al., 2019 for a similar analysis). The function also included m to model the rate of responding at the peak (peak rate).
The models were conducted on peak trials received in the last 5 sessions of each SS delay in the impulsive choice task. The three parameters a, p, and m, were entered as fixed effects as well as estrous stage and SS delay. The same three parameters were entered as random effects that were allowed vary at the individual subject level. For replication purposes, the seed was set to 2019 in all peak interval analyses. Starting values for the model were chosen based on the average peak functions across all rats. The SS and LL peak trial models were conducted on 18,900 total observations.
Results
Impulsive Choice Behavior
There were no differences in LL choices across estrous stage at the mean SS delay. Planned comparisons at an SS delay of 0 s showed that when rats were in estrus (b = −4.66), they made more LL choices than when in proestrus (b = −5.19), t = −2.47, p = .036, and met/diestrus (b = −5.19), t = 2.53, p = .031. There were no differences in LL choices at 30 s. This indicated that when rats were in estrus, they preferred the LL choice at shorter delays to reward compared to when they were in proestrus and met/diestrus (Figure 1).
Figure 1.
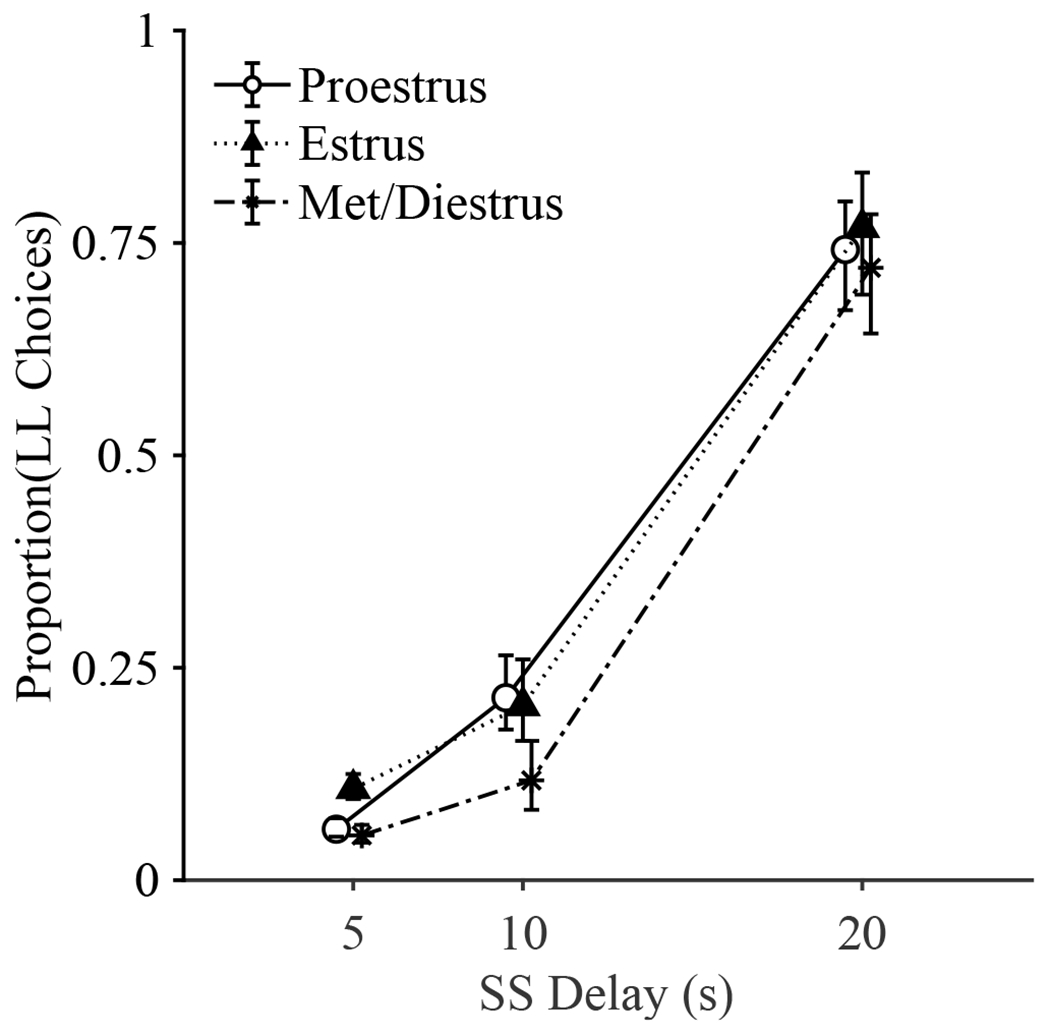
Mean proportion of LL (larger-later) choices across SS (smaller-sooner) delays with error bars (+/− SEM) computed based on the estimated marginal means of the multi-level logistic regression. Data points were jittered for readability.
Rats made more LL choices as the SS delay increased, b = 031, t = 44.31, p < .001, and sensitivity to delay (slope) was affected by estrous stage, b = −0.02, t = −2.69, p = .007. Rats had a steeper slope when in proestrus (b = 0.33) compared to when they were in estrus (b = 0.29; t = 2.53, p = .031) but not met/diestrus (b = 0.32). This suggests that rats in proestrus were more sensitive to delays (Figure 1).
LL Peak Trial Behavior
Across all estrous stages and SS delays, rats timed the 30-s LL delay accurately with an average peak time of 30.1 s (see Table 1 for peak time values for each delay). Across SS delays, rats’ peak times were significantly different based on estrous stage, b = −1.71, t = −4.06, p < .001. Pairwise comparisons showed that peak times differed when rats were in met/diestrus compared to proestrus, t = 3.89 p < .001, and estrus, t = 6.35, p < .001. When in met/diestrus, average peak time was 31.2 s while average peak times were 30.0 s and 29.3 s when in proestrus and estrus, respectively. As SS delay increased in the impulsive choice task, rats’ average peak time showed a small but significant increase (29.68 s to 29.72 s), b = 0.04, t = 2.15, p = .03. There was a significant interaction between estrous stage and SS delay on peak time, b = 0.07, t = 2,82, p = .005. Pairwise comparisons showed that rats differed when in estrus compared to met/diestrus, t = 3.54, p = .001. Peak times shifted later across SS delays for rats when in estrus while peak times shifted earlier for rats when in met/diestrus (Figure 2).
Table 1.
LL (larger-later) peak time, spread, and maximum peak rate for each SS (smaller-sooner) delay and estrous cycle. Note that the LL delay was always 30 s.
| Delay | Phase | Peak Time (s) | Peak Spread (s) | Maximum Peak Rate |
|---|---|---|---|---|
| 5 s | Proestrus | 29.7 | 21.5 | 0.32 |
| Estrus | 28.5 | 21.9 | 0.31 | |
| Met/diestrus | 31.4 | 21.6 | 0.33 | |
| 10 s | Proestrus | 29.9 | 19.5 | 0.38 |
| Estrus | 29.1 | 19.8 | 0.36 | |
| Met/diestrus | 31.2 | 19.9 | 0.39 | |
| 20 s | Proestrus | 30.3 | 15.6 | 0.51 |
| Estrus | 30.2 | 15.5 | 0.45 | |
| Met/diestrus | 30.9 | 16.6 | 0.50 |
Figure 2.
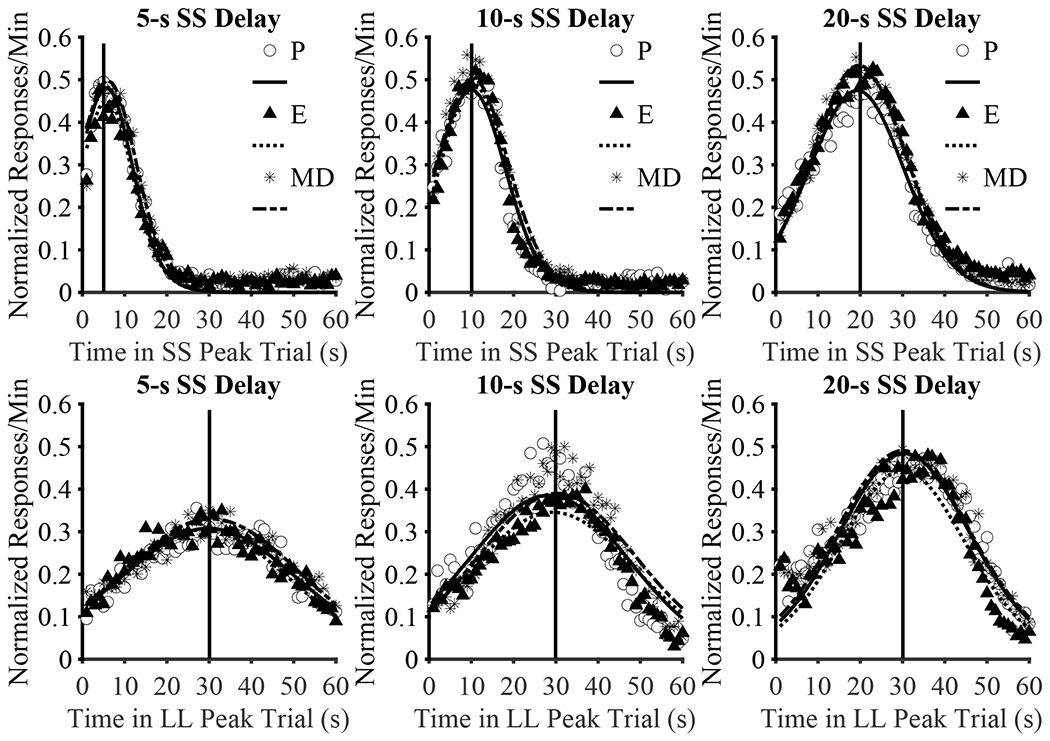
Mean responses per minute as a function of time into SS (smaller-sooner; top row) and LL (larger-later; bottom row) peak trials for all SS delays when rats were in proestrus (P), estrus (E), and met/diestrus (MD). Markers represent mean responses and lines represent fitted repeated measures multi-level nonlinear regression values. The solid vertical lines represent the target interval.
There were no differences in peak spread between estrous stages, so all rats timed the delay with similar levels of precision regardless of estrous stage (see Table 1 for peak spread values for each delay). However, as SS delay increased in the impulsive choice task, rats’ average peak spread decreased from 21.7 to 15.9 s, b = −0.39, t = −20.23, p < .001. Overall, rats timed the 30-s LL delay more precisely with more experience in the task (Figure 2).
There were no differences in peak rate between estrous stages. Peak rates increased as SS delay increased in the impulsive choice task, b = 0.01, t = 33.06, p < .001. Thus, the peak rate increased with more experience in the task, which suggests increased timing confidence (Figure 2). There were significant interactions between estrous stage and SS delay on peak rate, b = 0.002, t = 3.12, p = .002, and b = −0.002, t = −3.39, p = .001. Pairwise comparisons showed that when rats were in estrus they significantly differed from when they were in proestrus, t = −3.69, p = .001. Peak rate decreased less over training when rats were in estrus compared to when rats were in proestrus, suggesting smaller increases in timing confidence when rats were in estrus.
SS Peak Trial Behavior
Apart from a modest overestimation of the 5-s delay, rats timed the SS delays (5, 10, and 20 s) accurately with average peak times of 5.4, 10.2, and 19.9 s (see Table 2 for peak time values per delay). Across SS delays, rats differed in peak time based on estrous stage, b = −0.85, t = −4.41, p < .001. Pairwise comparisons showed that peak times differed when rats were in proestrus compared to estrus, t = −5.41, p < .0014, and met/diestrus, t = −7.39, p < .001. When rats were in proestrus, they underestimated time across delays compared to when they were in estrus and met/diestrus. As SS delay increased in the impulsive choice task, rats’ average peak time increased, b = 0.97, t = 99.43, p < .001. However, there were significant interactions between estrous stage and SS delay on peak time, b = −0.05, t = −3.81, p < .001, and b = 0.09, t = 6.50, p < .001. Pairwise comparisons showed that rats in estrus significantly differed from rats in proestrus, t = 5.80, p < .001, and rats in met/diestrus, t = 5.42, p < .001. When rats were in estrus, peak times shifted more with changes in delay compared to when rats were in proestrus and met/diestrus (Figure 2). Thus, estrus was associated with better tracking of the SS delays.
Table 2.
SS (smaller-sooner) peak time, spread, and maximum peak rate for each SS delay and estrous cycle.
| Delay | Phase | Peak Time (s) | Peak Spread | Maximum Peak Rate |
|---|---|---|---|---|
| 5 s | Proestrus | 5.1 | 7.0 | 0.47 |
| Estrus | 5.0 | 7.1 | 0.44 | |
| Met/diestrus | 6.1 | 7.3 | 0.47 | |
| 10 s | Proestrus | 9.6 | 8.3 | 0.48 |
| Estrus | 10.2 | 8.5 | 0.46 | |
| Met/diestrus | 10.8 | 8.6 | 0.49 | |
| 20 s | Proestrus | 18.8 | 11.0 | 0.49 |
| Estrus | 20.8 | 11.4 | 0.51 | |
| Met/diestrus | 20.1 | 11.2 | 0.53 |
Like the LL peak trials, there were no significant differences in peak spread between estrous stages (see Table 2 for peak spread at each delay). As SS delay increased in the impulsive choice task, rats’ average peak spread increased from 7.1 to 11.2, b = 0.27, t = 27.54, p < .001. Rats timed longer SS delays less precisely. Altogether, rats’ peak spread widened as the delay increased (Figure 2), consistent with the scalar property of timing (i.e., timing error increases with delay).
There were differences in peak rate between estrous stages, b = 0.02, t = 2.35, p = .019 and b = −0.03, t = −3.51, p = .001 in that rats had lower peak rates in met/diestrus compared to proestrus, t = −2.43, p = .04, and estrus, t = −4.84, p < .001. Peak rates increased as SS delay increased in the impulsive choice task, b = 0.003, t = 7.93, p < .001. There were significant interactions between estrous stage and SS delay on peak rate, b = −0.002, t = −3.05, p = .002 and b = 0.001, t = 2.17, p = .03. Pairwise comparisons showed that peak rates of rats in proestrus significantly differed from rats in estrus, t = −2.98, p = .008, and rats in met/diestrus, t = −2.43, p = .040. Peak rates of rats in proestrus increased less with SS delay compared to when rats in estrus and met/diestrus (Figure 2).
Discussion
We assessed estrous cycle effects on impulsive choices and interval timing in naturally cycling female rats. When females were in estrus, they made more LL choices when testing the model predictions at a 0-s SS delay, indicating that female rats were more resistant to the temptation of immediacy when in estrus. In contrast, when females were in estrus and met/diestrus, they were less sensitive to delay compared to when they were in proestrus. This suggests that the different SS delays were more salient during proestrus. Overall, female rats’ choices were likely influenced by fluctuations in sex hormones across the estrous cycle, which may have bearing on the interpretation of sex differences in impulsive choice.
Progesterone peaks at the beginning of the estrus stage, which was when rats were predicted to make more LL choices at 0 s. Previous research where progesterone was administered to ovariectomized female rats reported increased self-control in a marble burying task (Llaneza & Frye, 2009) and a go/no-go task (Swalve et al., 2016). However, progesterone administration to intact female rats decreased impulsive choices only when rats chose between amounts of cocaine, not sucrose pellets (Smethells et al., 2016). Altogether, progesterone levels may affect the value of reinforcers, altering choice behavior. The present results suggest that progesterone may improve self-control in naturally cycling female rats when choosing between amounts of standard grain pellets, but further research is needed where progesterone is directly measured and multiple types of reinforcers are compared. Changes in choice behavior across the estrous cycle could occur through multiple cognitive mechanisms including reduced delay discounting, increased delay tolerance, and/or altered time perception. Delay discounting is the decrease in subjective reward value as time to reward increases (Mazur, 2000). During estrus, rats may discount at a shallower rate due to increased progesterone. This could explain increased self-control coupled with lower delay sensitivity. Along the same lines, increased progesterone that occurs during estrus may increase delay tolerance, or the ability to wait for longer delays (Sonuga-Barke et al., 1992), which may explain increased LL choices in the face of temptation by the short SS.
The difference in delay sensitivity during proestrus versus estrus (and met/diestrus) could be due to alterations in timing ability, which may be affected by fluctuating sex hormones. Timing ability was evaluated in the current study using peak trials delivered during the impulsive choice task. Across delays, rats timed accurately, but underestimation and overestimation patterns were not consistent between SS and LL delays. Rats in proestrus were more accurate on some delays while rats in estrus were more accurate on others. There were no differences in peak spread, a measure of timing precision, based on estrous stage. Similar to peak time, differences in peak rate were inconsistent across SS and LL delays. Altogether, there were no clear patterns in timing behavior based on estrous cycle. The timing results did not relate to choice behavior in a straightforward way, suggesting that timing processes were not a major contributor to the choice results. Instead, proestrus may have increased the delay discounting rate.
However, it is important to note that complexities in timing analyses may have occurred because of the interaction between choice and timing. We measured timing during the choice task to depict how the female rats were timing the delays within the task and to assess timing and choice behavior during the same stage of an individual estrous cycle. However, a weakness of this method is that the choice task parameters likely interact with timing because of the different reward amounts associated with the SS and LL options. Previous research examining the effects of reward value on timing using peak trials showed that varying the reward amount associated with a delay can alter timing accuracy, precision, and confidence (Galtress, Garcia, et al., 2012; Galtress & Kirkpatrick, 2009, 2010; Galtress, Marshall, et al., 2012). This suggests that timing processes may have been affected by the contrasting reward amounts associated with the SS and LL options, and the estrous cycle could have moderated the reward-timing interactions. Future research should measure peak timing outside of the choice task to address this issue.
The neurobiology of the timing system is another key factor. One key circuit in the timing system is the prefrontal-striatal pathway. The prefrontal cortex, specifically the prelimbic region, tracks multiple delays during impulsive choice tasks as evident in differential neural activity in response to SS and LL choices (Sackett et al., 2019). In addition, the dorsal striatum is involved in interval timing, which is impaired after dopaminergic lesions (Meck, 2006). Neurons in the prelimbic region send projections to the dorsal striatum (Vertes, 2004). There are estrogen receptors in both the prefrontal cortex and dorsal striatum (Kuiper et al., 1997; Mermelstein et al., 1996; Shughrue et al., 1997). Thus, estradiol may act within the dorsal striatum, prelimbic cortex, and/or the projections between regions to result in altered temporal perception.
Altogether, the current study suggested that the estrous cycle can affect impulsive choice behavior. These data add to the literature by demonstrating that both estrus and proestrus stages of the estrous cycle may affect choices and interval timing in naturally cycling female rats. Measurement of estrogen and progesterone present in blood samples collected before and after impulsive choice and timing assessments may provide further evidence to support the effects of the estrous cycle on choice and timing. Given that the relationships reported here were only explored in a correlational manner, future research with causal manipulations is warranted to understand the specific mechanisms behind these effects. For example, future studies could employ progesterone administration coupled with choice and timing tasks to better understand how progesterone may affect reward valuation and timing processes. The current data provide some clues to guide those future research efforts.
Highlights.
Female rats were more sensitive to delay during proestrus
Estrus was associated with more LL choices at shorter delays
Increased estradiol, which occurs during proestrus, may alter delay sensitivity
Progesterone, which peaks during estrus, may affect LL choices
Acknowledgements
All authors have contributed significantly to the manuscript and consent to having their names on the manuscript. There are no conflicts of interest to declare for KP, AD, and KK. The authors would like to thank Carrie Bailey, Robert Small, and MacKenzie Gwinner for data collection support. Subsets of this data were presented at Mid-American Association for Behavior Analysis Annual Conference 2019. Data was included as preliminary data in Kelsey Panfil’s Master of Science thesis obtained at Kansas State University. This research was funded by grants MH085739 and GM113109 from the National Institutes of Health awarded to Kimberly Kirkpatrick and Kansas State University. The funding sources had no role other than financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anker JJ, & Carroll ME (2011). Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci, 8, 73–96. 10.1007/7854_2010_93 [DOI] [PubMed] [Google Scholar]
- Bates D, Kliegl R, Vasishth S, & Baayen H (2015). Parsimonious mixed models. arXiv preprint arXiv:1506.04967. [Google Scholar]
- Baumann AA, & Odum AL (2012). Impulsivity, risk taking, and timing. Behaviour Processes, 90(3), 408–414. 10.1016/j.beproc.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, & Hu M (2008). Sex differences in drug abuse. Front Neuroendocrinol, 29(1), 36–47. 10.1016/j.yfrne.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, & Marsch LA (2001). Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction, 96(1), 73–86. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11177521 [DOI] [PubMed] [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, & White J-SS (2008). Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology & Evolution, 24(3), 127–135. 10.1016/j.tree.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Carroll ME, & Anker JJ (2010). Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav, 58(1), 44–56. 10.1016/j.yhbeh.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Cnaan A, Laird NM, & Slasor P (1997). Tutorial in biostatistics: using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Statistics in Medicine, 16, 2349–2380. [DOI] [PubMed] [Google Scholar]
- Cora MC, Kooistra L, & Travlos G (2015). Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicologic Pathology, 43(6), 776–793. 10.1177/0192623315570339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Cheng R-K, & Meck WH (2011). Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology, 36, 3–25. 10.1038/npp.2010.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK (2015). Be quick about it. Endogenous estradiol level, menstrual cycle phase and trait impulsiveness predict impulsive choice in the context of reward acquisition. Hormones and Behavior, 74, 186–193. 10.1016/j.yhbeh.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Eckard ML, & Kyonka EGE (2018). Differential reinforcement of low rates differentially decreased timing precision. Behav Processes, 151, 111–118. 10.1016/j.beproc.2018.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AE, Visser EJ, & Nicholson AM (2019). Interventions aimed at changing impulsive choice in rats: Effects of immediate and relatively long delay to reward training. Behaviour Processes, 158, 126–136. 10.1016/j.beproc.2018.11.009 [DOI] [PubMed] [Google Scholar]
- Galtress T, Garcia A, & Kirkpatrick K (2012). Individual differences in impulsive choice and timing in rats. J Exp Anal Behav, 98(1), 65–87. 10.1901/jeab.2012.98-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtress T, & Kirkpatrick K (2009). Reward value effects on timing in the peak procedure. Learning and Motivation, 40(2), 109–131. 10.1016/j.lmot.2008.05.004 [DOI] [Google Scholar]
- Galtress T, & Kirkpatrick K (2010). Reward magnitude effects on temporal discrimination. Learning and Motivation, 41(2), 108–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtress T, Marshall AT, & Kirkpatrick K (2012). Motivation and timing: clues for modeling the reward system. Behaviour Processes, 90, 142–153. 10.1016/j.beproc.2012.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Orsini C, Wheeler AR, Ten Eyck TW, Betzhold SM, Labiste CC, Wright NG, Setlow B, & Bizon JL (2020). Testicular hormones mediate robust sex differences in impulsive choice in rats. Elife, 9. 10.7554/eLife.58604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L, & Rovine MJ (2007). Multilevel models for the experimental psychology: foundations and illustrative examples. Behavior Research Methods, 39(1), 101–117. 10.3758/BF03192848 [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Brooks DL, & Johnson JR (2005). A quantitative method for assessing stages of the rat estrous cycle. Biotechnic and Histochemistry, 80(2), 79–87. 10.1080/10520290500138422 [DOI] [PubMed] [Google Scholar]
- Kirby KN, & Marakovic NN (1996). Delay-discounting probabilistic rewards: Rates decrease as amounts increase. Psychonomic Bulletin and Review, 3(1), 100–104. 10.3758/BF03210748 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, & Gustafsson JA (1997). Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology, 138(3), 863–870. 10.1210/endo.138.3.4979 [DOI] [PubMed] [Google Scholar]
- Lenth R (2022). emmeans: Estimated Marginal Means, aka Least-Squares. https://CRAN.R-project.org/package=emmeans
- Liley AE, Gabriel DBK, Sable HJ, & Simon NW (2019). Sex Differences and Effects of Predictive Cues on Delayed Punishment Discounting. eNeuro, 6(4). 10.1523/ENEURO.0225-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llaneza DC, & Frye CA (2009). Progestogens and estrogen influence impulsive burying and avoidant freezing behavior of naturally cycling and ovariectomized rats. Pharmacol Biochem Behav, 93(3), 337–342. 10.1016/j.pbb.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Sonntag KC, Freund N, Thompson BS, Meda S, & Andersen SL (2016). Erratum to “Preventative treatment in an animal model of DHD: Behavioral and biochemical effects of methylphenidate and its interactions with ovarian hormones in female rats” [Eur. Neuropsychopharmacol. 26 (2016) 1496-1506]. Eur Neuropsychopharmacol, 26(11), 1843. 10.1016/j.euroneuro.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, & Carroll ME (2002). Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl), 164(2), 121–137. 10.1007/s00213-002-1183-2 [DOI] [PubMed] [Google Scholar]
- Matell MS, & Meck WH (2004). Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Cognitive Brain Research, 21(2), 139–170. 10.1016/j.cogbrainres.2004.06.012 [DOI] [PubMed] [Google Scholar]
- Matuschek H, Kliegl R, Vasishth S, Baayen H, & Bates D (2017). Balancing Type I error and power in linear mixed models. Journal of Memory and Language, 94, 305–315. 10.1016/j.jml.2017.01.001 [DOI] [Google Scholar]
- Mazur JE (2000). Tradeoffs among delay, rate, and amount of reinforcement. Behaviour Processes, 49(1), 1–10. 10.1016/s0376-6357(00)00070-x [DOI] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, & Bennett SA (2012). Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. Journal of Visualized Experiments(67), e4389. 10.3791/4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH (2006). Neuroanatomical localization of an internal clock: a functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Research, 1109(1), 93–107. 10.1016/j.brainres.2006.06.031 [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, & Surmeier DJ (1996). Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. The Journal of neuroscience : the official journal of the Society for Neuroscience, 16(2), 595–604. http://www.ncbi.nlm.nih.gov/pubmed/8551343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panfil K, Bailey C, Davis I, Mains A, & Kirkpatrick K (2020). A time-based intervention to treat impulsivity in male and female rats. Behavioural Brain Research, 379, 112316. 10.1016/j.bbr.2019.112316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, & Team RC (2018). nlme: Linear and nonlinear mixed effects models. R package version 3.1-137 https://CRAN.R-project.org/package=nlme [Google Scholar]
- Pleil KE, Cordes S, Meck WH, & Williams CL (2011). Rapid and acute effects of estrogen on time perception in male and female rats. Front Integr Neurosci, 5, 63. 10.3389/fnint.2011.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammsayer T, & Lustnauer S (1989). Sex differences in time perception. Percept Mot Skills, 68(1), 195–198. [DOI] [PubMed] [Google Scholar]
- Rasmussen EB, Lawyer SR, & Reilly W (2010). Percent body fat is related to delay and probability discounting for food in humans. Behaviour Processes, 83, 23–30. 10.1016/j.beproc.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Ross L, & Santi A (2000). The effects of estrogen on temporal and numerical processing in ovariectomized female rats. Psychobiology, 28(3), 394–405. [Google Scholar]
- Rung JM, Buhusi CV, & Madden GJ (2018). Reducing impulsive choice: V. The role of timing in delay-exposure training. Behaviour Processes, 157, 557–561. 10.1016/j.beproc.2018.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett DA, Moschak TM, & Carelli RM (2019). Prelimbic cortical neurons track preferred reward value and reflect impulsive choice during delay discounting behavior. The Journal of Neuroscience, 39(16), 3108–3118. 10.1523/JNEUROSCI.2532-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom NJ (2007). Estradiol modulation of the speed of an internal clock. Behav Neurosci, 121(2), 422–432. 10.1037/0735-7044.12L2.422 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, & Merchenthaler I (1997). Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol, 388(4), 507–525. [DOI] [PubMed] [Google Scholar]
- Smethells JR, Swalve NL, Eberly LE, & Carroll ME (2016). Sex differences in the reduction of impulsive choice (delay discounting) for cocaine in rats with atomoxetine and progesterone. Psychopharmacology (Berl), 233(15-16), 2999–3008. 10.1007/s00213-016-4345-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, Marshall AT, & Kirkpatrick K (2015). Mechanisms of impulsive choice: II. Time-based interventions to improve self-control. Behaviour Processes, 112, 29–42. 10.1016/j.beproc.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CT, Sierra Y, Oppler SH, & Boettiger CA (2014). Ovarian cycle effects on immediate reward selection bias in humans: a role for estradiol. The Journal of Neuroscience, 34(16), 5468–5476. 10.1523/JNEUROSCI.0014-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TS, Panfil K, & Kirkpatrick K (2022). Generalizability of time-based interventions: Effects of choice procedure and smaller-sooner delay. Behaviour Processes, 196, 104584. 10.1016/j.beproc.2022.104584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Taylor E, Sembi S, & Smith J (1992). Hyperactivity and delay aversion-I. the effect of delay on choice. Journal of Child Psychology and Psychiatry, 33(2), 387–398. [DOI] [PubMed] [Google Scholar]
- Stuebing SL, Marshall AT, Triplett A, & Kirkpatrick K (2018). Females in the forefront: Time-based intervention effects on impulsive choice and interval timing in female rats. Animal Cognition, 21(6), 759–772. 10.1007/s10071-018-1208-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swalve N, Smethells JR, & Carroll ME (2016). Progesterone attenuates impulsive action in a Go/No-Go task for sucrose pellets in female and male rats. Hormones and Behavior, 85, 43–47. 10.1016/j.yhbeh.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2004). Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse, 51(1), 32–58. 10.1002/syn.10279 [DOI] [PubMed] [Google Scholar]
- Weafer J, & de Wit H (2014). Sex differences in impulsive action and impulsive choice. Addictive Behaviors, 39(11), 1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, & Szelag E (2003). Sex Differences in Perception of Temporal Order. Perceptual and Motor Skills, 96(1), 105–112. 10.2466/pms.2003.96.L105 [DOI] [PubMed] [Google Scholar]
- Yoest KE, Quigley JA, & Becker JB (2018). Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Hormones and Behavior, 104, 119–129. 10.1016/j.yhbeh.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ME, Webb TL, Rung JM, & Jacobs EA (2013). Sensitivity to changing contingencies in an impulsivity task. Journal of the Experimental Analysis of Behavior, 99(3), 335–345. 10.1002/jeab.24 [DOI] [PMC free article] [PubMed] [Google Scholar]


