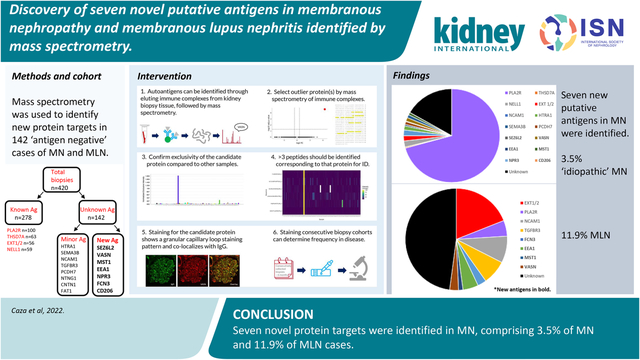
Abstract
Multiple autoantigens have been identified in membranous nephropathy (MN) by tissue-based proteomics. However, antigenic targets of disease are unknown for over 10% of patients with MN and over half of those with membranous lupus nephritis (MLN). Here, we identified multiple new targets in PLA2R-/THSD7A-/EXT-/NELL1-quadruple negative MN biopsies through mass spectrometry of immune complexes recovered from biopsy tissue of patients with MN. Patients with MN negative for these four antigens were identified from Arkana Laboratories case archives. Protein G immunoprecipitation recovered immune complexes from frozen biopsy tissue from 142 quadruple-negative cases and 278 cases of known antigen type, followed by interrogation by mass spectrometry. Potential putative antigens were confirmed through paraffin immunofluorescence and co-localization with IgG within immune deposits. Consecutive series of 165 cases of PLA2R-negative MN and 142 MLN biopsies were screened to determine the frequency for each potential antigen. Seven putative antigens were discovered within immune complexes from biopsies of patients with MN including FCN3, CD206, EEA1, SEZ6L2, NPR3, MST1, and VASN. Peptides from these proteins were not enriched in the 278 cases of known antigen type. Between three to 30 unique peptides were detected for each new target. Frequencies of each biomarker, determined by staining consecutive case series, ranged from under 1 to 4.9%. NPR3 and CD206 were only positive in index cases. All cases showed co-localization of IgG within the immune deposits. Thus, seven putative antigens were newly identified in MN and MLN. Due to the number of antigens identified, it is becoming impractical to type PLA2R-negative MN or MLN cases through immunostaining alone. A multiplex approach is needed for subtyping of these diseases.
Keywords: Membranous nephropathy, kidney biopsy, antigen, biomarker, mass spectrometry
Graphical Abstract
Introduction
Membranous nephropathy (MN) is a leading cause of nephrotic syndrome in adults and carries a high disease burden, with up to 16–40 percent of patients developing end-stage kidney disease at 5 to 15 years1–3. It is characterized by the presence of subepithelial immune complex deposits within glomeruli, which disrupt the glomerular filtration barrier and results in proteinuria. Traditionally, this disease has been designated as ‘primary’ when there are no known associated diseases or ‘secondary’ when certain diseases such as systemic lupus erythematosus or malignancy coincide. The main etiologies of ‘primary MN’ were due to autoantibodies directed against the podocyte proteins phospholipase A2 receptor (PLA2R) in nearly 70% of cases4, and thrombospondin type 1 domain containing 7A (THSD7A) in approximately 3% of total MN5. These renal-limited ‘primary’ forms of MN were identified in 2009 and 2014, respectively. Prior to these discoveries, only one other antigenic type of MN was identified, MN due to anti-neutral endopeptidase (NEP) antibodies, a very rare form that manifests in the antenatal and perinatal period6.
Within the past three years alone, ten new target antigens have been described in MN, including exostosin 1/2 (EXT1/2)7, neural epidermal growth factor-like 1 (NELL1)8, serine protease HTRA1 (HTRA1)9, semaphorin 3B (SEMA3B)10, protocadherin 7A (PCDH7A)11, protocadherin FAT1 (FAT1)12, netrin G1 (NTNG1)13, contactin 1 (CNTN1)14, neural cell adhesion molecule-1 (NCAM1)15, transforming growth factor beta receptor 3 (TGFBR3)16, neural-derived neurotropic factor (NDNF)17, and proprotein convertase subtilisin/kexin type 6 (PCSK6)18. The ‘primary’ versus ‘secondary’ classification of MN is becoming obsolete with the discovery of these multiple new antigenic drivers of disease, some of which have unique clinical characteristics or ‘secondary’ associations19, 20. Despite the growing understanding about the pathogenesis of this disease, the target antigen remains unknown in approximately 15–20% of cases that were formerly known as ‘primary’ MN and approximately 55% of membranous lupus nephritis (MLN)21.
Knowing the antigenic target of disease can be helpful in monitoring disease activity and may even be useful for primary diagnosis, as has been established for PLA2R-positive MN22–26. Additionally, knowledge of the antigenic type often adds information about the etiology of the patient’s disease. For example, patients with antibodies directed against CNTN1 often have a chronic inflammatory demyelinating polyneuropathy14. Likewise, patients with MN with EXT1/2, NCAM1, or TGFBR3 as a putative antigen often will have a corresponding autoimmune disease, such as systemic lupus erythematosus7, 15, 16. EXT1/2 positivity also carries prognostic value, as patients with EXT1/2-positivity have a reduced risk of progression to ESKD27, 28. Identification of other antigenic targets, like NELL1, indicate a higher likelihood of cancer or use of certain medications, such as lipoic acid, traditional indigenous medicines, or exposure to toxic substances such as mercury29–32. Like many diseases that are diagnosed based upon a histopathologic pattern, we now know that MN actually represents many different underlying disease states at the molecular pathophysiologic level.
The major antigens have now been identified in idiopathic MN, with the remaining being minor antigens in a small subset of patients with the disease. For MLN, most cases are not of known antigen type. All known antigens and putative antigens in MN and MLN were identified by mass spectrometry (MS), the majority of which employed tissue-based approaches. These utilize residual kidney biopsy tissue to enrich for glomerular proteins by laser capture microdissection and/or through elution of immune complexes through immunoprecipitation with protein G that binds IgG21. Here, we identified multiple novel biomarkers in PLA2R-/THSD7A-/EXT-/NELL1-quadruple negative MN biopsies and MLN biopsies through protein G immunoprecipitation and MS. Each putative antigen was confirmed by confocal microscopy and the prevalence was screened in cohorts of MN and MLN biopsies.
Materials and Methods
Case selection.
All cases used in this study were obtained from the clinical case archives at Arkana Laboratories. All data were collected according to a study protocol approved by Solutions institutional review board and all ethical principles and guidelines for the protection of human subjects in research were followed.
Kidney biopsies were identified for the mass spectrometry discovery cohorts from a biopsy database according to the following inclusion criteria 1) diagnosis of membranous glomerulopathy on kidney biopsy, 2) ≥ 10 glomeruli by light microscopy and ≥ 5 glomeruli by immunofluorescence microscopy, and 3) ≤ 50% interstitial fibrosis and tubular atrophy. Cases where MN was not the dominant pathology, including IgG4-related kidney disease, anti-brush border antibody disease, and ANCA-associated glomerulonephritis were excluded from analysis. We included cases that were PLA2R-positive, THSD7A-positive, EXT1/2-positive, and NELL1-positive as controls for known antigen type. Biopsies negative for all four antigens were used for discovery.
Mass spectrometry data was collected from two cohorts of cases. Discovery cohort 1 consisted of a total of 59 cases including 24 quadruple antigen-negative (PLA2R-/THSD7A-/EXT1/2-/NELL1-negative) MN cases. Discovery cohort 2 consisted of a 364 case cohort that included 118 ‘quadruple negative’ cases evaluated in parallel to 265 cases of known antigen type. Details of each cohort can be found in Figure 1. Due to differences in mass spectrometry methods it is not possible to combine these discovery cohorts.
Figure 1.
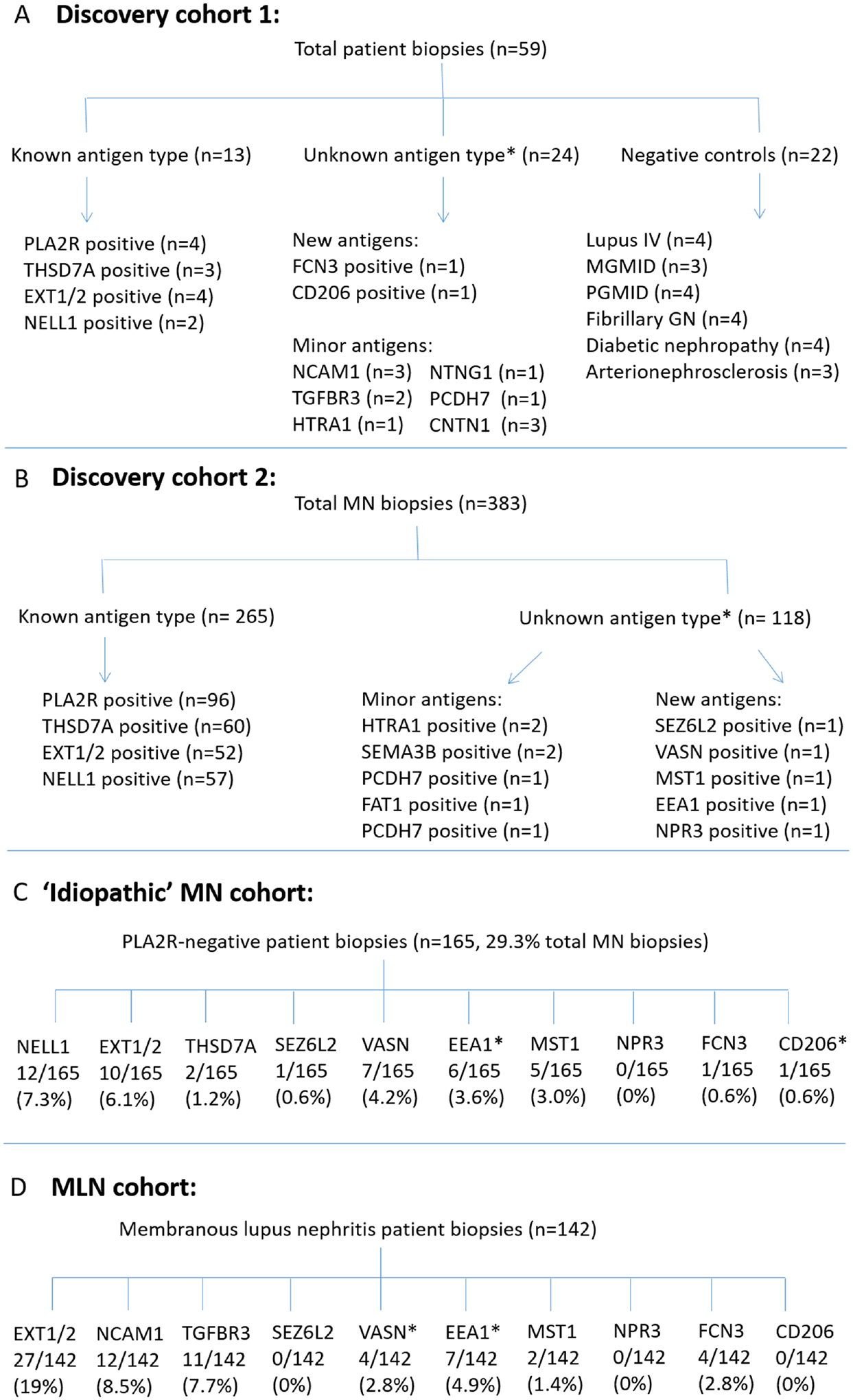
Cohort diagram of MN cases from A-B) Mass spectrometry cohorts; C) Consecutive case series of idiopathic MN patients; D) Consecutive case series of MLN patients.
Protein G Immunoprecipitation.
Protein lysates were prepared from residual kidney biopsy tissue frozen in optimized cutting temperature (OCT) medium. Biopsy cores were washed four times at 4°C with 1 mL phosphate-buffered saline (PBS) to remove OCT, with five minutes per wash. After the final PBS wash, the PBS was replaced with 500 μl Pierce IP lysis buffer (Thermo-Fisher, Waltham MA, catalog #87787) with the addition of protease and phosphatase inhibitors (Halt protease and phosphatase inhibitor cocktail, Thermo-Fisher, catalog #78440). 150 μl of 0.1 mm glass beads were added to the samples, followed by mechanical disruption of the tissue cores using a Biospec mini-bead beater. Three cycles of ‘bead beating’ were performed, two minutes each. After the final ‘bead beating’ step, the samples were centrifuged using a benchtop microcentrifuge to remove large tissue fragments. The supernatant was transferred to low protein binding microfuge tubes containing 50 μl PBS-washed magnetic protein G Dynabeads (Thermo-Fisher, Waltham MA, catalog #1003D). Immunoprecipitations were performed by incubation at room temperature for 1 hour with shaking. The samples were exposed to a magnet, with the supernatant removed following incubation. The beads were then washed with PBS four times to reduce non-specific protein binding. After the final wash, the beads were frozen at −20°C until further analysis.
Preparation of Laser Capture Microdissected Glomeruli for Mass Spectrometry
Paraffin-embedded tissue was cut at 10 μm thickness onto Leica PET-membrane frame slides. Twenty to forty glomeruli were dissected per case. These slides were then stained with Meyer’s hematoxylin. The glomeruli were microdissected with a Leica DM6000B microscope. Microdissected glomeruli were lysed in 2% SDS and 0.1M DTT at 99°C for 1h and processed by filter assisted sample preparation. Clarified lysate was concentrated using Vivacon 500 concentrators with a molecular weight cut-off of 30kDa (Sartorius, Gottingen, Germany). Washes with 8M Urea in 0.1M Tris/Cl, pH 8.5 were used to remove SDS, and the samples alkylated with 0.05M iodoacetamide. Iodoacetamide was removed by 3 washes with 8 M urea /0.1 M Tris/Cl, pH 8.5, followed by 3 washes with 0.05 M ammonium bicarbonate. Proteins digestion was performed using sequencing-grade trypsin (Promega, Madison WI) at a 40:1 w/w ratio at 37°C for 16 h. Peptides were collected by centrifugation, then desalted on C18-Stage tips (Thermo-Fisher Scientific, Waltham, MA).
Mass Spectrometry.
Peptides were produced through trypsin digestion of the immune complexes adhered to protein G beads post-immunoprecipitation. Mass spectrometry was performed with data-dependent acquisition (DDA) for the 59 case cohort and with data-independent acquisition (DIA) from the 364 case cohort. DDA MS methods were previously described33.
DIA mass spectrometry was used for analysis as follows. An UltiMate 3000 RSLC nano system (Thermo Scientific) was used to separate tryptic peptides on a 150 × 0.075 mm column packed with a reverse phase XSelect CSH C18 2.5 μm resin. The peptides were eluted with a gradient of buffer A (0.1% formic acid + 0.5% acetonitrile)/buffer B (0.1% formic acid + 99.9% acetonitrile), from a 97:3 ratio to a 60:40 ratio over 1 hour. Electrospray (2.25 kV) was used to ionize the eluted peptides on an Orbitrap Exploris 480 mass spectrometer (Thermo Scientific). Six gas-phase fractions of a pooled sample were acquired on the Orbitrap Exploris mass spectrometer with 4 m/z DIA spectra (30,000 resolution, normalized AGC target 100%, maximum inject time 66 ms) in a staggered window pattern to assemble a chromatogram library34. Precursor spectra were acquired after each DIA duty cycle, spanning the m/z range of the gas-phase fraction (i.e. 496–602 m/z, 596–702 m/z). For wide-window acquisitions, precursor spectra from each DIA cycle were acquired (385–1015 m/z, 60,000 resolution, normalized AGC target 100%, maximum injection time 50 ms), followed by 50× 12 m/z DIA spectra (12 m/z precursor isolation windows at 15,000 resolution, normalized AGC target 100%, maximum injection time 33 ms) using a staggered window pattern with optimized window placements.
ScaffoldDIA (Proteome software) was used to configure DIA library searches. Narrow-window DIA files were searched against the human predicted spectral library from Prosit (2019/04 Uniprot) with 10 ppm precursor and fragment ion tolerances to generate an empirically corrected chromatogram library with a peptide and protein false discovery rate of 1%. Wide-window DIA files were subsequently searched against the generated empirically corrected chromatogram library.
Immunostaining for Target Proteins.
PLA2R and EXT2 staining was performed on frozen tissue by immunohistochemistry. THSD7A, NELL1, EXT1, NCAM1, TGFBR3, VASN, FCN3, MST1, NPR3, EEA1, CD206, and SEZ6L2 staining was performed on formalin-fixed paraffin-embedded (FFPE) tissue sections by paraffin immunofluorescence. These primary antibodies were applied following deparaffinization and antigen retrieval. THSD7A, EXT1, NCAM1, TGFBR3, FCN3, CD206, NPR3, SEZ6L2, and MST1 underwent heat-based antigen retrieval at 95°C using the PT-link system (Leica Biosystems, Buffalo Grove IL). NELL1, EEA1, and VASN were retrieved with proteinase K.
The secondary antibody Alexa Fluor 488 goat anti-mouse IgG was used with the NELL1 antibody. The secondary antibody Rhodamine Red X Affinipure goat anti-rabbit IgG was used for tissue sections exposed to the other primary antibodies (EXT1, NCAM1, TGFBR3, VASN, FCN3, MST1, EEA1, CD206, SEZ6L2, and NPR3).
When sufficient frozen tissue was available, staining for IgG subclasses was performed. 4 μM frozen tissue sections were washed in PBS to remove OCT medium. Tissue sections were incubated with FITC-conjugated IgG1, IgG2, IgG3, and IgG4 antibodies for 30 minutes at room temperature in the dark. Slides were coverslipped in aqueous mounting medium and imaged immediately.
Antibodies
All antibodies were commercially available and the supplier information is as follows: PLA2R rabbit polyclonal antibody (Sigma-Aldrich, cat # HPA012657, 1:50), THSD7A rabbit polyclonal antibody (Atlas Antibodies, cat #AMAB91234, 1:50 dilution), EXT2 rabbit polyclonal antibody (Abcam, cat # ab102843, 1:50), EXT1 rabbit polyclonal antibody (Invitrogen, cat # PA5–60699 1:50 dilution), NELL1 mouse monoclonal antibody (Invitrogen, cat # PA5–27958, 1:50 dilution), NCAM1 rabbit polyclonal antibody (Sigma, cat # HPA039835), TGFBR3 rabbit polyclonal antibody, (1:25 dilution, Thermo-Fisher), FCN3 rabbit polyclonal antibody (Invitrogen PA5–71727, 1:50 dilution), CD206 rabbit polyclonal antibody (Invitrogen PA5–82136, 1:50 dilution), VASN rabbit polyclonal antibody (Invitrogen PA5–98236, dilution), MST1/MST1 rabbit polyclonal antibody (Invitrogen cat# PA5–42762, 1:50), EEA1 rabbit polyclonal antibody (Invitrogen, cat # PA5–29013, 1:25 dilution), NPR3 rabbit polyclonal antibody (Invitrogen, cat # PA5–85282, 1:200 dilution), SEZ6L2 rabbit polyclonal antibody (Invitrogen, cat # PA5–64172, 1:50), Alexa Fluor 488 goat anti-rabbit IgG (Jackson Immunoresearch, cat #111–035-045, 1:100 dilution), FITC-conjugated goat anti-mouse IgG (Jackson Immunoresearch 111–095-003, 1:100 dilution), Rhodamine Red X Affinipure goat anti-rabbit IgG (Jackson Immunoresearch, cat # 111–295-144, 1:100 dilution), Peroxidase-conjugated goat anti-rabbit IgG (Jackson Immunoresearch, cat # 111–035-045, 1:100 dilution),and polyclonal (fluorescein isothiocyanate-conjugated) rabbit anti-human IgG (Agilent, 1:40 dilution). IgG subclass antibodies were all direct FITC conjugates from Jackson Immunoresearch (IgG1, catalog #115–095-205; IgG2, catalog # 115–095-207; IgG3, catalog # F4641; and IgG4, catalog # F9889, all used at 1:25 dilution).
Immunofluorescence imaging and confocal microscopy.
Staining specificity was established by staining 15 known negative control cases for each antigen, including 3 cases each of PLA2R-positive MN, THSD7A-positive MN, EXT1/2-positive MN, NELL1-positive MN, and diabetic nephropathy cases. Granular capillary loop staining was considered a positive result and cases were considered negative if there was an absence of staining along the glomerular capillary loops.
For confocal microscopy, tissue sections were co-stained with FITC-conjugated polyclonal rabbit anti-human IgG at 1:40 dilution. Slides were coverslipped in anti-fade mounting medium and imaged under a Leica SBA DMI8 confocal laser scanning microscope. PLA2R-positive MN cases were co-stained in parallel to cases of interest as negative controls.
Frequency cohorts to determine frequency of biomarkers in MN and MLN
Retrospective cohorts of consecutive kidney biopsies were evaluated to determine the frequency for each biomarker. These cohorts included 165 PLA2R-negative MN and 142 cases of MLN. Of all idiopathic MN biopsies in the time frame of this consecutive cohort, 29.3% were PLA2R-negative. All cases were stained for each biomarker, including SEZ6L2, VASN, EEA1, MST1, NPR3, FCN3, and CD206.In addition to immunostaining for each of the seven biomarkers, staining for NELL1, THSD7A, and EXT1 (or EXT2) was performed on the ‘idiopathic’ MN cohort. For the MLN cohort, immunostaining for EXT1 (or EXT2), NCAM1, and TGFBR3 was performed. These controls were used to evaluate for exclusivity or dual positivity with each of the biomarkers.
Results.
Mass Spectrometry Revealed Novel Putative Antigens in MN.
Patient biopsies negative for PLA2R, THSD7A, EXT1/2, and NELL1 (‘quadruple-negative’ cases, n=142 total from two discovery cohorts) were evaluated by mass spectrometry to identify new antigenic targets in MN following protein G immunoprecipitation to elute antibodies from frozen biopsy tissue. These were compared to 278 cases of known antigen type, including PLA2R (n=100), THSD7A (n=63), EXT1/2 (n=56), and NELL1 MN (n=59). These cases were from two discovery cohorts due to being within two separate mass spectrometry runs (Figure 1A + B).
To consider an enriched protein to be a potential target in MN in our quadruple-negative biopsies, the following four criteria were required: (1) Identification by mass spectrometry within protein G immunoprecipitates of frozen biopsy tissue with 3 or more unique peptides, (2) Granular Capillary loop staining of the target protein by paraffin immunofluorescence, (3) Confocal microscopy demonstrating IgG co-localization of the target protein along the glomerular capillary loops, and (4) Specificity of staining along glomerular capillary loops compared to PLA2R, THSD7A, EXT1/2, NELL1, and diabetic glomerulopathy controls. For protein candidates that met the above criteria, we screened consecutive case cohorts of PLA2R-negative idiopathic MN and MLN to evaluate the frequency of each putative antigen in disease.
Known Antigens in MN can be Identified by Protein G Immunoprecipitation and Laser Capture Microdissection.
To determine whether protein G immunoprecipitation (IP) of immune complexes was equivalent to laser capture microdissection (LCMD, used to enrich glomeruli from tissue samples) in subtyping MN cases by mass spectrometry, a subset of cases of known antigenic types were examined by both techniques. The same antigen type was identified by protein G IP and LCMD, demonstrating equal sensitivity. This was observed in 3 cases of PLA2R-positive MN, 3 cases of THSD7A-positive MN, 4 cases of EXT1/2-positive MN, 2 cases of NELL1-positive MN, 3 cases of NCAM1-positive MN, 1 case of HTRA1-positive MN, 2 cases of TGFBR3-positive MN, 1 case of SEMA3B-positive MN, 1 case of PCDH7-positive MN, 2 cases of CNTN1-positive MN, and 1 case of NTNG1-positive MN (data not shown). These data were shown previously for PLA2R, THSD7A, EXT1/2, NCAM1, HTRA1, and TGFBR39, 15, 16.As both techniques consistently resulted in the same antigen identification, we did not perform both protein G IP and LCMD for discovery of new putative antigens and Protein G IP was used for all cases.
Protein G Immunoprecipitation Yielded Seven Putative Antigens in MN Biopsies.
Of the 142 quadruple-negative MN biopsies evaluated by MS for discovery, 14 yielded a known minor antigen type, including NCAM1 (n=3), TGFBR3 (n=2), PCDH7 (n=2), NTNG1 (n=1), FAT1 (n=1), SEMA3B (n=2), HTRA1 (n=3), and CNTN1 (n=3). To consider a protein as a potential new target antigen, we looked for outlier proteins in the mass spectrometry data that were enriched in unknown membranous samples as compared with control samples of known antigen types. Seven biopsies showed dominance of a unique protein present within eluted immune complexes, which were evaluated further as potential target antigens, seizure related 6 homolog like 2 (SEZ6L2, n=1), vasorin (VASN, n=1), early endosome antigen 1 (EEA1, n=1), macrophage stimulating 1 (MST1, n=1), natriuretic peptide receptor 3 (NPR3, n=1), ficolin 3 (FCN3, n=1), and cluster of differentiation CD206 (CD206, n=1).
To support each protein as a biomarker in MN, we first examined whether peptides of a putative antigen were uniquely present in the index cases by comparing normalized exclusive intensities with other samples (exclusivity plot in Figure 2A, using VASN as an example). Protein heat maps were then examined to determine whether a protein was increased in these cases compared to controls (Figure 2B, using VASN as an example). At least 3 peptides were identified for each target protein, with peptide heat maps shown in Supplemental Figure 1. Sequence coverage for each target was examined using Scaffold software (Figure 2C, using VASN as an example). Peptide heat maps, exclusivity plots, and protein heat maps demonstrating unique peptides and sequence coverage for SEZ6L2, EEA1, MST1, NPR3, FCN3, and CD206 are shown in Supplemental Figures S1–7, respectively.
Figure 2.
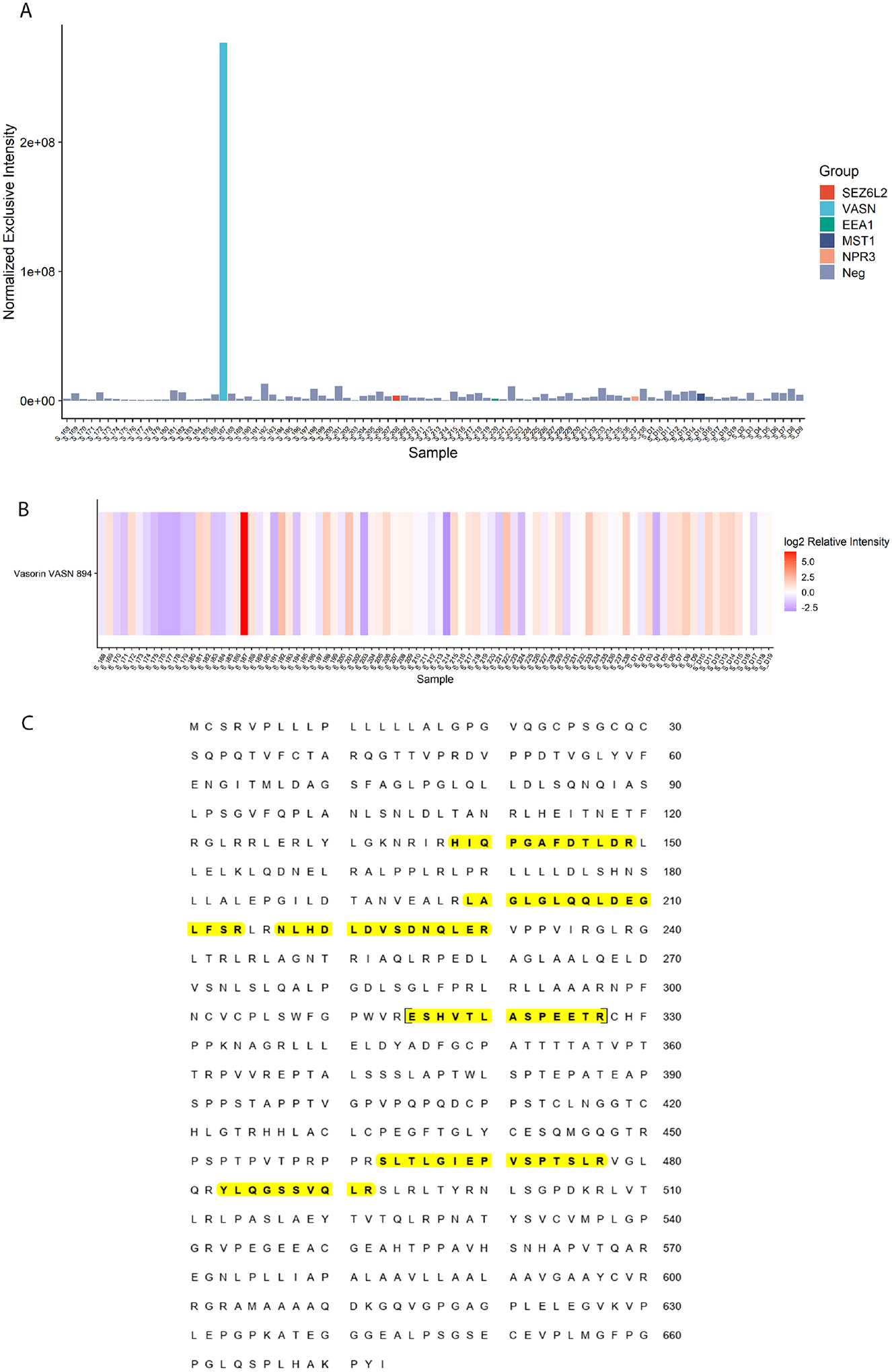
Mass spectrometry data to support identification of a putative antigen in MN, using VASN as an example. A) Scaffold exclusivity plot demonstrating VASN peptides show significantly increased normalized exclusive intensity in the VASN index case compared to other MN samples; B) Protein heat map demonstrating VASN was highly enriched in the index case compared to other MN samples; C) Sequence coverage for VASN (unique peptides highlighted in yellow).
For all new target antigens, peptides showed orders of magnitude higher intensity in the index cases (on a log2 scale) compared to control cases. FCN3 and CD206 were identified from a cohort of 59 cases (Figure 1A) and the remaining biomarkers were discovered from a larger cohort of 364 cases (Figure 1B).
Biomarkers Show High Specificity Compared to Controls of Known Antigen Type.
Mass spectrometry peptide abundance for each biomarker was compared to controls. SEZ6L2, VASN, EEA1, VASN, and MST1 peptide abundance was compared to a total of 265 controls, including 96 PLA2R+ MN, 60 THSD7A+ MN, 57 NELL1+ MN, and 52 EXT1/2-positive MN. FCN3 and CD206 were discovered from a separate cohort with 13 control samples (4 PLA2R+ MN, 3 THSD7A+ MN, 4 EXT1/2+ MN, and 2 NELL1-positive MN). Plots comparing peptide abundance among index cases and controls are shown in Figure 3.
Figure 3. Biomarker protein abundances in MN cases of known antigen type.
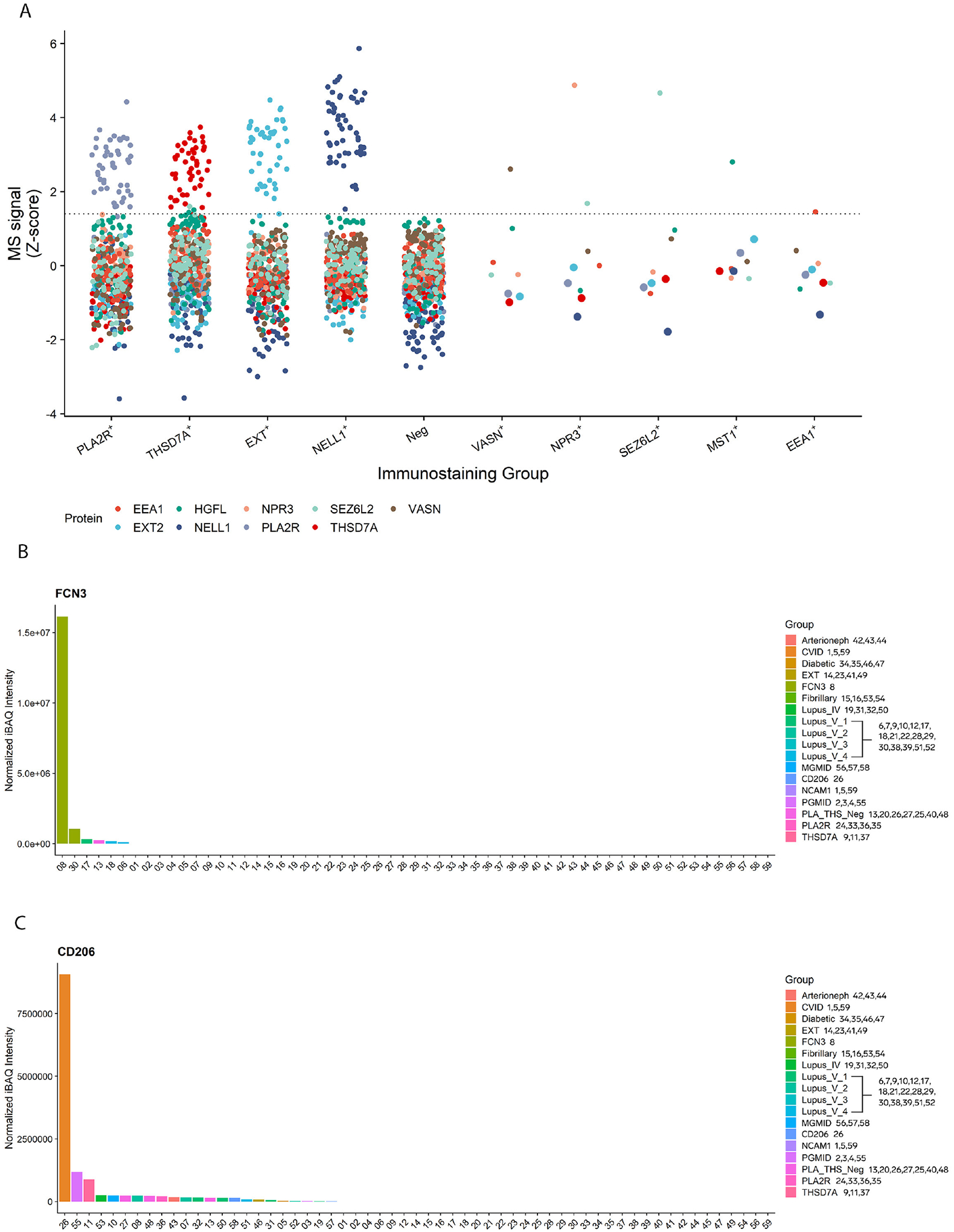
A) Mass spectrometry data from 279 MN cases are shown and are grouped together based on immunostaining results. The abundances of the indicated proteins are plotted as Z-scores. Abundances of the index biomarker proteins are relatively low in MN cases of known antigen type (PLA2R+, THSD7A+, EXT+, NELL1+), and abundances of canonical antigens are low in the index cases. Peptides of the known antigen types are highlighted with an increased size within the dot plots.
B) Protein abundance of FCN3 in index case and controls.
C) Protein abundance of CD206 in index case and controls.
Biomarker Candidates Co-localize with IgG in Glomerular Immune Deposits.
Paraffin immunofluorescence identified strongly positive granular capillary loop staining within glomeruli for each of the seven protein candidates identified by MS. Confocal microscopy demonstrated that each of the target proteins co-localized with IgG along the glomerular capillary loops. There was no co-localization for any of the targets with IgG in control PLA2R-positive MN cases. Images of co-localization of antigen candidates with IgG immune deposits are shown in Figure 4 and lack of co-localization within negative controls in Supplemental Figure S8.
Figure 4.
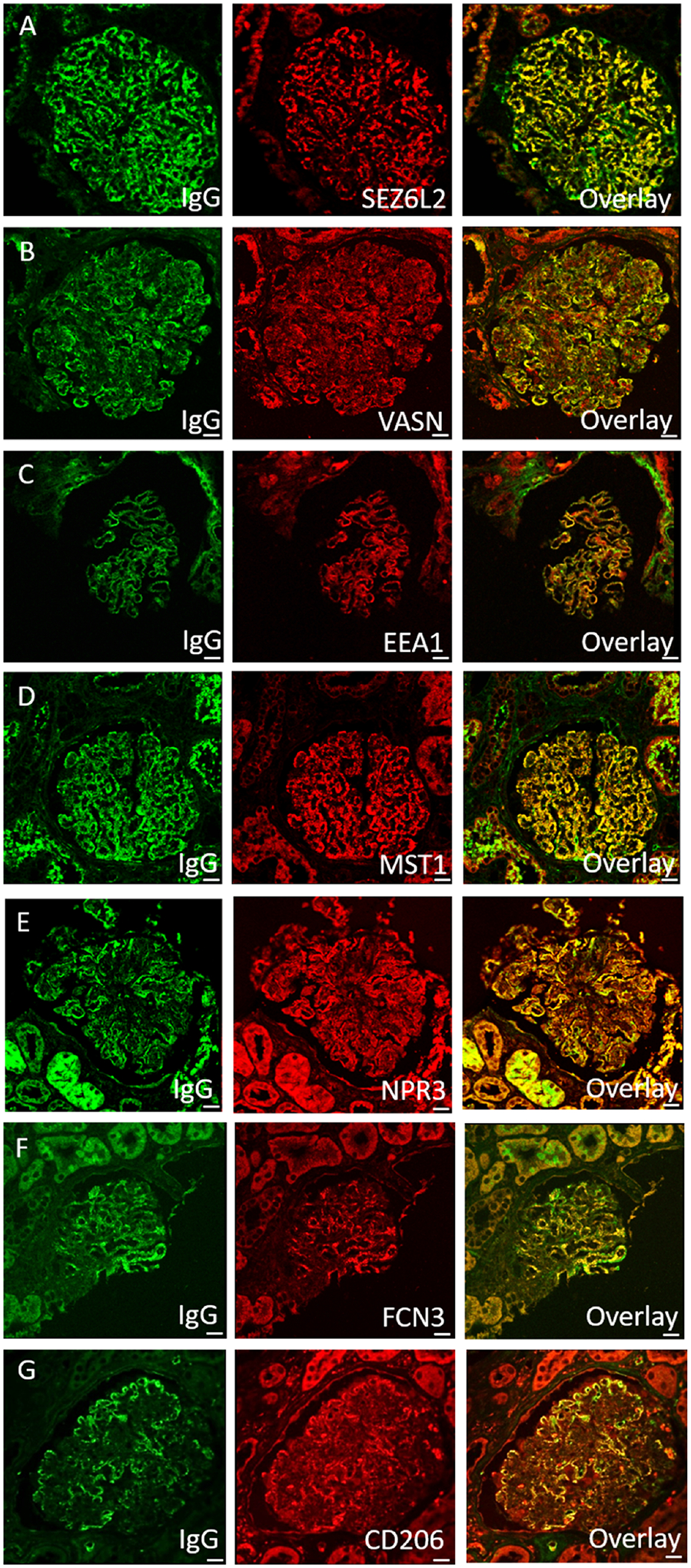
Co-localization of antigen candidates with IgG immune deposits. A) SEZ6L2; B) VASN; C) EEA1; D) MST1; E) NPR3; F) FCN3; G) CD206.
All control cases stained to further investigate the specificity of paraffin immunofluorescence were negative. A total of fifteen control cases were stained for each putative antigen, including 3 PLA2R, 3 THSD7A, 3 EXT1/2, 3 NELL1, and 3 diabetic nephropathy (negative control) (Supplemental Figure S9, with representative positive cases in Supplemental Figure S10).
Consecutive Series of Patients Determined Frequency and Characteristics of MN and MLN Positive for the Identified Putative antigens.
Consecutive series of 165 cases of PLA2R-negative ‘idiopathic’ MN and 142 cases of MLN were stained by paraffin immunofluorescence for each biomarker to investigate the frequency in disease. In addition, these cases were stained for major known antigens, including THSD7A, EXT1/2, and NELL1 in ‘idiopathic’ MN and NCAM1, EXT1/2, and TGFBR3 in MLN cases.
In ‘idiopathic’ MN cases, SEZ6L2 was positive in 1/165 (0.6%), VASN in 7/165 (4.2%), EEA1 in 6/165 (3.6%), MST1 in 5/165 (3.0%), FCN3 in 1/165 (0.6%), and no additional cases of NPR3 or CD206-positivity were identified.. Two cases were positive for two antigens (1.2%), including one dual MST1/VASN case and one EXT1/EEA1 dual positive case (Figure 1C).
For MLN, VASN was positive in 4/142 11.9). No cases of NPR3, SEZL6L2, or CD206 were identified in MLN cases (Figure 1D). Two dual positive cases were identified (1.6%), including one EXT1/VASN-positive case and one EEA1/NCAM1 positive case. The frequency of dual positivity in this cohort is similar to those described previously for other MN antigens, where approximately 1% of ‘idiopathic’ MN cases were reported to be PLA2R and THSD7A dual positive and 2% demonstrated dual PLA2R and EXT1/2 positivity35, 36. Clinical, laboratory, and histopathologic features of patients identified for these biomarkers are shown in Supplemental Table S1.
IgG Subclass Distribution of Antibodies Directed to Candidate Antigens.
IgG subclass staining was performed in cases with sufficient tissue available for analysis (n=17). These included 1 SEZ6L2-positive case, 2 FCN3-positive cases, 6 EEA1-positive cases, 4 VASN-positive cases, and 4 MST1 positive cases (of which one was concurrently positive for VASN). Overall, FCN3, VASN, and MST1 biopsies were IgG1-predominant (Supplemental Table S2). SEZ6L2 had a typical pattern of ‘idiopathic’ MN with IgG4 predominance. EEA1 positive biopsies had variable IgG subclass staining patterns, although the majority of cases tested were from MLN biopsies (Supplemental Table S2).
Discussion.
We identified seven novel putative antigens in MN through tissue-based proteomics. All candidates were discovered by protein G immunoprecipitation, a technique that was instrumental to our discovery of other antigenic targets in autoimmune kidney diseases, including NCAM115, TGFBR316, HTRA19, and CUBN/AMN37. Confirmation of target antigens was performed through co-localization of each putative antigen with IgG within the subepithelial immune deposits. Immunostaining case series of MN biopsies was performed to determine antigenic frequencies. This analysis revealed that three of the newly identified antigens were very rare (<1% of cases), including CD206, SEZ6L2, and NPR3, whereas four of the antigens FCN3, VASN, EEA1, and MST1 were more common. Two of these more common antigens, FCN3 and EEA1, had an increased frequency in patients with MLN (2.8% and 4.9%, respectively), proportions similar to other known antigens. These discoveries reduce our knowledge gap in MLN, where now more than half of the inciting autoantigens are known (19% EXT1/2, 8.5% NCAM1, 7.7% TGFBR3, 2.8% FCN3, 4.9% EEA1, 1.4% MST1, and 2.8% VASN; Figure 5 and Table 1). In addition, these putative antigens comprise 11.9% of PLA2R-negative idiopathic MN cases. As only 29.3% of our ‘idiopathic’ MN cases are PLA2R-negative, these new biomarkers account for only 3.5% of idiopathic MN cases (Figure 5 and Table 1).
Figure 5.
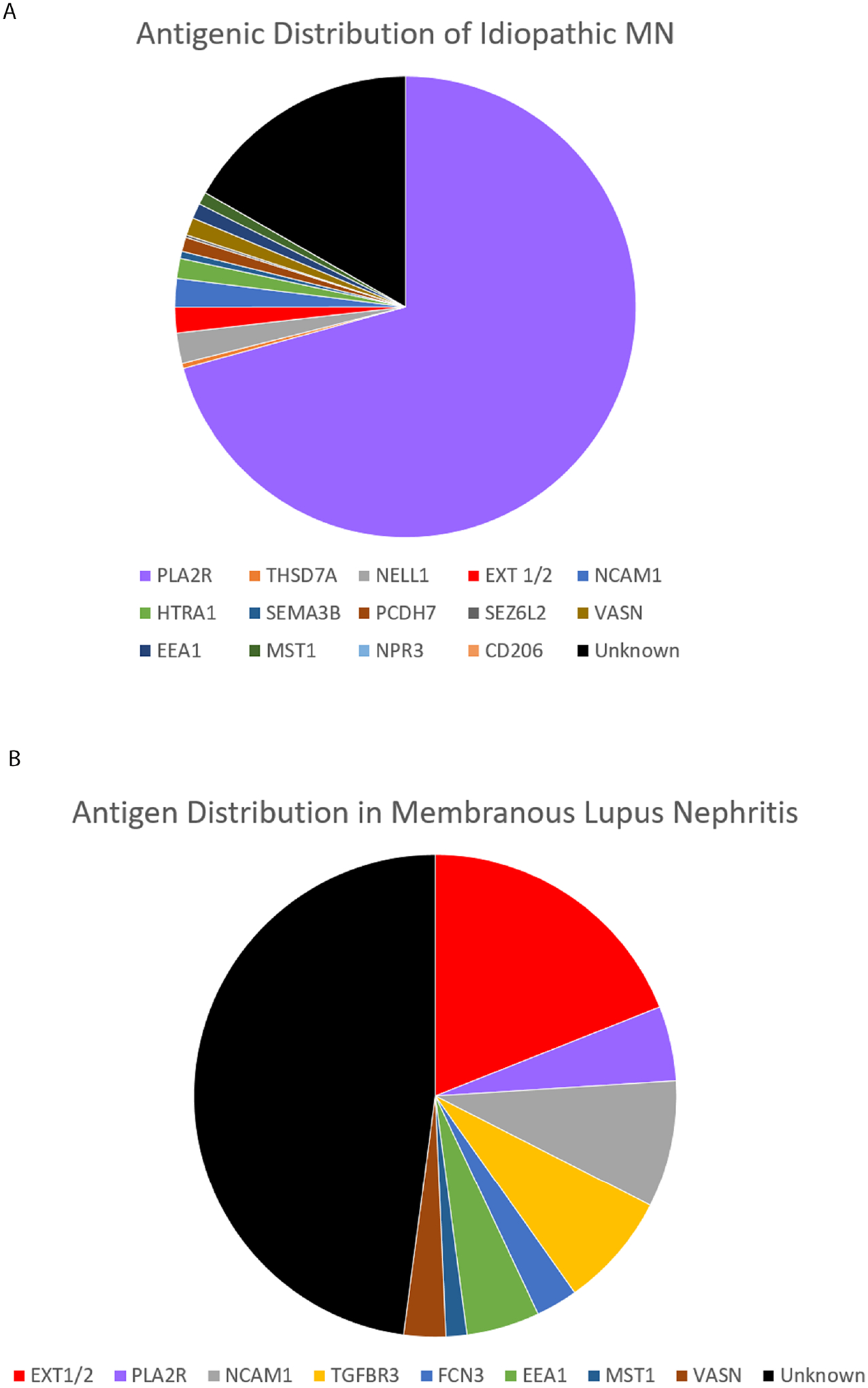
Frequency of autoantigens in A) ‘Idiopathic’ MN; and B) Membranous lupus nephritis. Frequencies for NCAM1, HTRA1, SEMA3B, and PCDH7 are those previously reported and were not examined within this study.
Table 1.
Characteristics of candidate biomarkers in MN. Protein characteristics are provided, including cellular localization, protein function, molecular weight, expression within podocytes, and antibodies used for detection. Frequencies of each biomarker in PLA2R-negative MN and MLN are included. While antibodies against these target proteins were not examined in this study, three showed evidence of circulating antibodies in other disease states and/or reported in autoimmune disease. Presence or absence of each protein in podocytes was examined using the human protein and transcriptomic atlases38–40.
| Characteristic | SEZ6L2 | VASN | EEA1 | MST1 | NPR3 | FCN3 | CD206 |
|---|---|---|---|---|---|---|---|
| Expanded name | Seizure related 6 homolog like 2 | Vasorin | Early endosome antigen 1 | Macrophage stimulating 1 | Natriuretic peptide receptor 3 | Ficolin 3 | Cluster of differentiation 206 |
| Frequency - PLA2R neg MN | 0.6% | 4.2% | 3.6% | 3.6% | <1% | 0.6% | <1% |
| Frequency - MLN | 0% | 2.8% | 4.9% | 2.1% | 0% | 2.8% | 0% |
| Function | Synapse maturation | TGF beta antagonism | Endosomal trafficking | Innate and adaptive immunity | Regulation of vascular tone | Innate immune response | Innate immune response |
| Localization | Plasma membrane, endoplasmic reticulum | Plasma membrane, mitochondria, exosomes | Plasma membrane, Endosomes | Cytoplasm, extracellular/secreted | Plasma membrane | Cytoplasm, endosomes, extracellular, secreted | Plasma membrane |
| Protein characteristics | Single pass type 1 membrane protein | Surface glycoprotein | Endosomal tethering to Rab GTPases | Protein kinase (MAP4K) | Atrial natriuretic peptide receptor | Lectin | Lectin |
| Molecular weight (kDa) | 98 | 72 | 162 | 60 | 60 | 33 | 166 |
| Podocyte expression? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Circulating antibodies? | Cerebellar ataxia | None | Cutaneous lupus | None | None | Systemic lupus | None |
| Autoimmunity | Promotes | Unknown | Promotes | Promotes | Unknown | Promotes | Protective |
| Antibody for detection | Invitrogen PA5-64172 | Invitrogen PA5-98236 | Invitrogen PA5-29013 | Invitrogen PA5-42762 | Invitrogen PA5-85282 | Invitrogen PA5-71727 | Invitrogen PA5-82136 |
Newly identified protein candidates may serve as primary podocyte antigens or have ‘secondary’ deposition within glomeruli. All of these proteins are known to be expressed in podocytes (Table 1)38–40. Interestingly, the majority of these proteins are also expressed within the central nervous system, including NPR339, SEZ6L241, EEA142, VASN43, MST144, and CD20645. Similarly, many known MN antigens shared by both the central nervous system and podocytes, including NELL146, NCAM147, CNTN114, THSD7A48, HTRA149, PCDH750, and SEMA3B51.
Most antigenic targets of MN and MLN discovered thus far were discovered by MS-based protein identification. For the majority of these targets, circulating antibodies have been identified in patient serum, providing future potential for non-invasive monitoring of the disease. For PLA2R-positive MN, autoantibody titers correlate with disease activity, fall prior to remission, and increase in disease occurrence, making serologic studies effective in clinical management for monitoring of the disease, as well as primary diagnosis. For three of the new antigens, FCN3, EEA1, and SEZ6L2, circulating antibodies have already been identified, although these proteins were not previously described to be MN antigens52, 53. Autoantibodies against FCN3 (also known as the Hakata antigen) have been identified in patients with Systemic Lupus Erythematosus54, 55, correlate with the SLE disease activity index, as well as titers of anti-dsDNA and anti-C1q antibodies52. Antibodies against EEA1 are seen in sera from patients with subacute cutaneous lupus erythematosus53. Anti-SEZ6L2 antibodies have been identified in patients with cerebellar ataxia and have not been previously implicated with proteinuria or kidney disease56. Further investigations will elucidate whether antibodies against these other antigen types can be identified within patient serum for potential development of serologic assays.
Due to the large number of antigens identified, it is becoming impractical to type PLA2R-negative MN or MLN cases through immunostaining techniques. A multiplex approach is needed for subtyping of this disease. One possibility is through MS analysis, which is sensitive and specific for the detection of the antigen type and has been used for discovery of almost all known MN antigens. Of note, MS has already been instrumental in typing amyloidosis, as it is impractical to type non-AL amyloid by immunohistochemistry since >30 types exist57, 58. Serologic testing is another possibility for antigenic sub-typing if circulating antibodies are able to be identified in all types of MN and MLN. Development of multiplexed serologic assays may enable non-invasive monitoring of disease. If serologic tests are highly sensitive and specific, they may enable primary diagnosis without a kidney biopsy, of which is supported for PLA2R-positive MN in the KDIGO 2021 glomerular disease guidelines59. This approach, however, could have low sensitivity as serologic remission precedes clinical remission and some patients may be seronegative at the time of kidney biopsy. While not examined within our study, examining for circulating antibodies against these putative antigens is needed to enable serologic assay development.
Limitations.
Although staining of consecutive case series was performed to determine the frequency of each antigen in idiopathic MN and MLN, the number of identified cases for any individual antigen is low, and therefore, it is largely unknown whether unique clinical and/or pathologic associations exist.
In this retrospective study, we did not have serum samples available from the MN patients, for which would require prospective collection. Therefore, seroreactivity studies were not performed, without which we cannot definitely conclude that all of the identified putative antigens are true autoantigens. However, an antigen-antibody interaction is suspected for each of these seven targets, as these proteins immunoprecipitate with IgG and co-localize with IgG within glomerular immune deposits.
Conclusions.
Seven novel probable antigens were identified in MN through protein G immunoprecipitation of biopsy tissue. Further work is required to examine for circulating antibodies and to determine whether unique histopathologic characteristics or disease associations exist. We hope that this work will encourage further investigation of these targets in MN.
Supplementary Material
Supplemental Figure S1. Peptide heat maps of novel MN antigen candidates, demonstrating enrichment of unique antigen peptides in the index cases and not in other MN samples. A) SEZ6L2; B) VASN; C) EEA1; D) MST1; E) NPR3; F) FCN3; G) CD206. Heat maps from data collected by DIA (A-E) and DDA (F, G) are shown. Instances of missing values are colored white, and are more frequent in DDA data due to stochastic sampling.
Supplemental Figure S2. Mass spectrometry data supporting SEZ6L2 as a putative antigen in MN. A) Scaffold exclusivity plot demonstrating SEZ6L2 peptides show significantly increased normalized exclusive intensity in the SEZ6L2 index case compared to other MN samples; B) Protein heat map demonstrating SEZ6L2 was highly enriched in the index case compared to other MN samples; C) Sequence coverage for SEZ6L2 (unique peptides highlighted in yellow).
Supplemental Figure S3. Mass spectrometry data supporting EEA1 as a putative antigen in MN. A) Scaffold exclusivity plot demonstrating EEA1 peptides show significantly increased normalized exclusive intensity in the EEA1 index case compared to other MN samples; B) Protein heat map demonstrating EEA1 was highly enriched in the index case compared to other MN samples; C) Sequence coverage for EEA1 (unique peptides highlighted in yellow).
Supplemental Figure S4. Mass spectrometry data supporting MST1 as a putative antigen in MN. A) Scaffold exclusivity plot demonstrating MST1 peptides show significantly increased normalized exclusive intensity in the MST1 index case compared to other MN samples; B) Protein heat map demonstrating MST1 was highly enriched in the index case compared to other MN samples; C) Sequence coverage for MST1 (unique peptides highlighted in yellow). This candidate biomarker was identified from a mass spectrometry run of only 19 samples and therefore, shows comparison to fewer cases.
Supplemental Figure S5. Mass spectrometry data supporting NPR3 as a putative antigen in MN. A) Scaffold exclusivity plot demonstrating NPR3 peptides show significantly increased normalized exclusive intensity in the NPR3 index case compared to other MN samples; B) Protein heat map demonstrating NRP3 was highly enriched in the index case compared to other MN samples; C) Sequence coverage for NPR3 (unique peptides highlighted in yellow).
Supplemental Figure S6. Mass spectrometry data supporting FCN3 as a putative antigen in MN. A) Scaffold exclusivity plot demonstrating FCN3 peptides show significantly increased normalized exclusive intensity in the FCN3 index case compared to other MN samples; B) Protein heat map demonstrating FCN3 was highly enriched in the index case compared to other MN samples; C) Sequence coverage for FCN3 (unique peptides highlighted in orange).
Supplemental Figure S7. Mass spectrometry data supporting CD206 as a putative antigen in MN. A) Scaffold exclusivity plot demonstrating CD206 peptides show significantly increased normalized exclusive intensity in the CD206 index case compared to other MN samples; B) Protein heat map demonstrating CD206 was highly enriched in the index case compared to other MN samples; C) Sequence coverage for CD206 (unique peptides highlighted in orange).
Supplemental Figure S8. Confocal microscopy images demonstrating a lack of co-localization of each putative antigen within IgG immune deposits in PLA2R-positive MN control cases. A) SEZ6L2; B) VASN; C) EEA1; D) MST1; E) NPR3; F) FCN3; G) CD206.
Supplemental Figure S9. Paraffin immunofluorescence staining of PLA2R, THSD7A, EXT1/2, NELL1, and diabetic nephropathy biopsies as negative controls for each candidate antigen. A) SEZ6L2; B) VASN; C) EEA1; D) MST1; E) NPR3; F) FCN3; G) CD206.
Supplemental Figure S10. Representative paraffin immunofluorescence images for the putative antigens.
Supplemental Table S1. Clinical and pathologic characteristics of MN patients positive for new target antigens.
Abbreviations: ANA, antinuclear antibodies; APLS, anti-phospholipid antibody syndrome; BPH, benign prostatic hypertrophy; COPD, chronic obstructive pulmonary disease; Cr, creatinine; DJD, degenerative joint disease/osteoarthritis; DM, diabetes mellitus; DVT, deep venous thrombosis; F, female; GERD, gastroesophageal reflux disease; HTN, hypertension; ITP, immune thrombocytopenic purpura; IUFD, intrauterine fetal demise; M, male; MGUS, monoclonal gammopathy of undetermined significance; NR, nephrotic range; N/A, not applicable/not available; NSAIDs, non-steroidal anti-inflammatory drugs; prot, proteinuria; PRES, posterior reversible encephalopathy syndrome; PE, pulmonary embolism; RA, rheumatoid arthritis; RVT, renal vein thrombosis; SLE, systemic lupus erythematosus; subendo, subendothelial deposits; TBM, tubular basement membrane; TIA, transient ischemic attack; WNL, within normal limits
Supplemental Table S2. IgG subclass staining of biopsies positive for candidate antigens.
Acknowledgements.
We thank Sudhir Joshi for expert technical assistance. This work was supported by the National Institutes of Health grant number MD014110 to CPL, DJK, and JA and grant number 1R41DK130702 awarded to AS, TC, and CL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures.
All of the authors declared no competing interests.
An abstract containing a subset of this data will be presented in abstract form at the American Society of Nephrology 2022 annual scientific meeting.
Data availability.
The mass spectrometry proteomics data was submitted to the ProteomeXchange Consortium, via the PRIDE partner repository, with the dataset identifiers PXD035853 and PXD035854.
References.
- 1.Schieppati A, Mosconi L, Perna A, et al. Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med 1993; 329: 85–89. [DOI] [PubMed] [Google Scholar]
- 2.Lai WL, Yeh TH, Chen PM, et al. Membranous nephropathy: a review on the pathogenesis, diagnosis, and treatment. J Formos Med Assoc 2015; 114: 102–111. [DOI] [PubMed] [Google Scholar]
- 3.Nazareth TA, Kariburyo F, Kirkemo A, et al. Patients with Idiopathic Membranous Nephropathy: A Real-World Clinical and Economic Analysis of U.S. Claims Data. J Manag Care Spec Pharm 2019; 25: 1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck LH, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009; 361: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomas NM, Beck LH Jr., Meyer-Schwesinger C, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 2014; 371: 2277–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debiec H, Guigonis V, Mougenot B, et al. Antenatal Membranous Glomerulonephritis due to anti-neutral Endopeptidase antibodies. N Engl J Med 2002; 346: 2053–2060. [DOI] [PubMed] [Google Scholar]
- 7.Sethi S, Madden BJ, Debiec H, et al. Exostosin 1/Exostosin 2-Associated Membranous Nephropathy. J Am Soc Nephrol 2019; 30: 1123–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sethi S, Debiec H, Madden B, et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int 2020; 97: 163–174. [DOI] [PubMed] [Google Scholar]
- 9.Al-Rabadi LF, Caza T, Trivin-Avillach C, et al. Serine Protease HTRA1 as a Novel Target Antigen in Primary Membranous Nephropathy. J Am Soc Nephrol 2021; 32: 1666–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sethi S, Debiec H, Madden B, et al. Semaphorin 3B-associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int 2020; 98 (5): 1253–1264. [DOI] [PubMed] [Google Scholar]
- 11.Sethi S, Madden B, Debiec H, et al. Protocadherin 7-Associated Membranous Nephropathy. J Am Soc Nephrol 2021; 32: 1249–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sethi S, Madden B, Casal Moura M, et al. Hematopoietic Stem Cell Transplant-Membranous Nephropathy Is Associated with Protocadherin FAT1. J Am Soc Nephrol 2022; 33: 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinhard L, Machalitza M, Wiech T, et al. Netrin G1 is a Novel Target Antigen in Primary Membranous Nephropathy. J Am Soc Nephrol 2022; 33 (10): 1823–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Quintrec M, Teisseyre M, Bec N, et al. Contactin-1 is a novel target antigen in membranous nephropathy associated with chronic inflammatory demyelinating polyneuropathy. Kidney Int 2021; 100: 1240–1249. [DOI] [PubMed] [Google Scholar]
- 15.Caza TN, Hassen SI, Kuperman M, et al. Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis. Kidney Int 2021; 100: 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caza TN, Hassen SI, Kenan DJ, et al. Transforming growth factor beta receptor 3 (TGFBR3)-associated membranous nephropathy. Kidney360 2021; 2 (8): 1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sethi S, Madden B, Fervenza FC, et al. Neuron-derived neurotrophic factor (NDNF) is a novel protein associated with membranous nephropathy (MN). American Society of Nephrology annual scientific meeting 2022: TH-PO453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sethi S, Casal Moura M, Madden B, et al. NSAID-associated membranous nephropathy (MN) is associated with PCSK6. American Society of Nephrology annual scientific meeting 2022: TH-PO454. [Google Scholar]
- 19.Ronco P, Plaisier E, Debiec H. Advances in Membranous Nephropathy. J Clin Med 2021; 10 (4): 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bobart SA, Tehranian S, Sethi S, et al. A Target Antigen-Based Approach to the Classification of Membranous Nephropathy. Mayo Clin Proc 2021; 96: 577–591. [DOI] [PubMed] [Google Scholar]
- 21.Caza TN, Al-Rabadi LF Jr. LLB. How times have changed! A cornucopia of antigens for membranous nephropathy. Frontiers in Immunology 2021; 12: 800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bech AP, Hofstra JM, Brenchley PE, et al. Association of anti-PLA2R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. CJASN 2014; 9: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai H, Zhang H, He Y. Diagnostic accuracy of PLA2R autoantibodies and glomerular staining for the differentiation of idiopathic and secondary membranous nephropathy: an updated meta-analysis. Sci Rep 2015; 5: 8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerry MJ, Vanhille P, Ronco P, et al. Serum anti-PLA2R antibodies may be present before clinical manifestations of membranous nephropathy. Kidney Int 2016; 89: 1399. [DOI] [PubMed] [Google Scholar]
- 25.Kanigicherla D, Gummadova J, McKenzie EA, et al. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int 2013; 83: 940–948. [DOI] [PubMed] [Google Scholar]
- 26.Radice A, Pieruzzi F, Trezzi B, et al. Diagnostic specificity of autoantibodies to M-type phospholipase A2 receptor (PLA2R) in differentiating idiopathic membranous nephropathy (IMN) from secondary forms and other glomerular diseases. J Nephrol 2018; 31: 271–278. [DOI] [PubMed] [Google Scholar]
- 27.Ravindran A, Casal Moura M, Fervenza FC, et al. In Patients with Membranous Lupus Nephritis, Exostosin-Positivity and Exostosin-Negativity Represent Two Different Phenotypes. J Am Soc Nephrol 2021; 32 (3): 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saïdi M, Brochériou I, Estève E, et al. The Exostosin Immunohistochemical Status Differentiates Lupus Membranous Nephropathy Subsets With Different Outcomes. Kidney Int Rep 2021; 6: 1977–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spain RI, Andeen NK, Gibson PC, et al. Lipoic acid supplementation associated with neural epidermal growth factor-like 1 (NELL1)-associated membranous nephropathy. Kidney Int 2021; 100 (6): 1208–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caza TN, Larsen CP. Lipoic acid in neural epidermal growth factor-like 1-associated membranous nephropathy: more than a coincidence? Kidney Int 2022; 101: 418–419. [DOI] [PubMed] [Google Scholar]
- 31.Pathak N, Gunasekaran I, Ambriose M, et al. Nell1 as Target Antigen for Mercury Related Membranous Nephropathy: A Case Report. Indian J Nephrol 2022: E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurien AA, Jansi Prema KS, Walker P, et al. Traditional indigenous medicines are an etiologic consideration for NELL1-positive membranous nephropathy. Kidney Int 2022. [DOI] [PubMed] [Google Scholar]
- 33.Caza TN, Hassen SI, Dvanajscak Z, et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int 2021; 99: 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Searle BC, Pino LK, Egertson JD, et al. Chromatogram libraries improve peptide detection and quantification by data independent acquisition mass spectrometry. Nat Commun 2018; 9: 5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsen CP, Cossey LN, Beck LH. THSD7A staining of membranous glomerulopathy in clinical practice reveals cases with dual autoantibody positivity. Mod Pathol 2016; 29: 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwakura T, Ema C, Sato T, et al. Primary Membranous Nephropathy With Enhanced Staining of Exostosin 1/Exostosin 2 in the Glomeruli: A Report of 2 Cases. Kidney Med 2021; 3: 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morelle J, Caza T, Debiec H, et al. Cubilin and amnionless protein are novel target antigens in anti-brush border antibody disease. Kidney Int 2022: 101 (5): 1063–1068. [DOI] [PubMed] [Google Scholar]
- 38.Rinschen MM, Gödel M, Grahammer F, et al. A Multi-layered Quantitative In Vivo Expression Atlas of the Podocyte Unravels Kidney Disease Candidate Genes. Cell Rep 2018; 23: 2495–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uhlen M, Fagerberg L, Hallstrom BM, et al. Proteomics. Tissue-based map of the human proteome. Science 2015; 347: 1260419. [DOI] [PubMed] [Google Scholar]
- 40.Wu H, Malone AF, Donnelly EL, et al. Single-Cell Transcriptomics of a Human Kidney Allograft Biopsy Specimen Defines a Diverse Inflammatory Response. J Am Soc Nephrol 2018; 29: 2069–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pigoni M, Wanngren J, Kuhn PH, et al. Seizure protein 6 and its homolog seizure 6-like protein are physiological substrates of BACE1 in neurons. Mol Neurodegener 2016; 11: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson JM, de Hoop M, Zorzi N, et al. EEA1, a tethering protein of the early sorting endosome, shows a polarized distribution in hippocampal neurons, epithelial cells, and fibroblasts. Mol Biol Cell 2000; 11: 2657–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Söllner C, Wright GJ. A cell surface interaction network of neural leucine-rich repeat receptors. Genome Biol 2009; 10: R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao L, Chen D, Hu P, et al. The c-Abl-MST1 signaling pathway mediates oxidative stress-induced neuronal cell death. J Neurosci 2011; 31: 9611–9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohgidani M, Kato TA, Haraguchi Y, et al. Microglial CD206 Gene Has Potential as a State Marker of Bipolar Disorder. Front Immunol 2016; 7: 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desai J, Shannon ME, Johnson MD, et al. Nell1-deficient mice have reduced expression of extracellular matrix proteins causing cranial and vertebral defects. Hum Mol Genet 2006; 15: 1329–1341. [DOI] [PubMed] [Google Scholar]
- 47.Nomura T, Yabe T, Rosenthal ES, et al. PSA-NCAM distinguishes reactive astrocytes in 6-OHDA-lesioned substantia nigra from those in the striatal terminal fields. J Neurosci Res 2000; 61: 588–596. [DOI] [PubMed] [Google Scholar]
- 48.Ho AM, Coombes BJ, Nguyen TTL, et al. Mood-Stabilizing Antiepileptic Treatment Response in Bipolar Disorder: A Genome-Wide Association Study. Clin Pharmacol Ther 2020; 108: 1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Launay S, Maubert E, Lebeurrier N, et al. HtrA1-dependent proteolysis of TGF-beta controls both neuronal maturation and developmental survival. Cell Death Differ 2008; 15: 1408–1416. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Kerrisk Campbell M, Tom I, et al. PCDH7 interacts with GluN1 and regulates dendritic spine morphology and synaptic function. Sci Rep 2020; 10: 10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pasterkamp RJ, Giger RJ. Semaphorin function in neural plasticity and disease. Curr Opin Neurobiol 2009; 19: 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plawecki M, Lheritier E, Clavarino G, et al. Association between the Presence of Autoantibodies Targeting Ficolin-3 and Active Nephritis in Patients with Systemic Lupus Erythematosus. PLoS One 2016; 11: e0160879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waite RL, Sentry JW, Stenmark H, et al. Autoantibodies to a novel early endosome antigen 1. Clin Immunol Immunopathol 1998; 86: 81–87. [DOI] [PubMed] [Google Scholar]
- 54.Yae Y, Inaba S, Sato H, et al. Isolation and characterization of a thermolabile beta-2 macroglycoprotein (‘thermolabile substance’ or ‘Hakata antigen’) detected by precipitating (auto) antibody in sera of patients with systemic lupus erythematosus. Biochim Biophys Acta 1991; 1078: 369–376. [DOI] [PubMed] [Google Scholar]
- 55.Andersen T, Munthe-Fog L, Garred P, et al. Serum levels of ficolin-3 (Hakata antigen) in patients with systemic lupus erythematosus. J Rheumatol 2009; 36: 757–759. [DOI] [PubMed] [Google Scholar]
- 56.Landa J, Guasp M, Petit-Pedrol M, et al. Seizure-related 6 homolog like 2 autoimmunity: Neurologic syndrome and antibody effects. Neurol Neuroimmunol Neuroinflamm 2021; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sethi S, Vrana JA, Theis JD, et al. Laser microdissection and mass spectrometry-based proteomics aids the diagnosis and typing of renal amyloidosis. Kidney Int 2012; 82: 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yaguchi H, Yabe I, Takahashi H, et al. Anti-Sez6l2 antibody detected in a patient with immune-mediated cerebellar ataxia inhibits complex formation of GluR1 and Sez6l2. J Neurol 2018; 265: 962–965. [DOI] [PubMed] [Google Scholar]
- 59.KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int 2021; 100: S1–S276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Peptide heat maps of novel MN antigen candidates, demonstrating enrichment of unique antigen peptides in the index cases and not in other MN samples. A) SEZ6L2; B) VASN; C) EEA1; D) MST1; E) NPR3; F) FCN3; G) CD206. Heat maps from data collected by DIA (A-E) and DDA (F, G) are shown. Instances of missing values are colored white, and are more frequent in DDA data due to stochastic sampling.
Supplemental Figure S2. Mass spectrometry data supporting SEZ6L2 as a putative antigen in MN. A) Scaffold exclusivity plot demonstrating SEZ6L2 peptides show significantly increased normalized exclusive intensity in the SEZ6L2 index case compared to other MN samples; B) Protein heat map demonstrating SEZ6L2 was highly enriched in the index case compared to other MN samples; C) Sequence coverage for SEZ6L2 (unique peptides highlighted in yellow).
Supplemental Figure S3. Mass spectrometry data supporting EEA1 as a putative antigen in MN. A) Scaffold exclusivity plot demonstrating EEA1 peptides show significantly increased normalized exclusive intensity in the EEA1 index case compared to other MN samples; B) Protein heat map demonstrating EEA1 was highly enriched in the index case compared to other MN samples; C) Sequence coverage for EEA1 (unique peptides highlighted in yellow).
Supplemental Figure S4. Mass spectrometry data supporting MST1 as a putative antigen in MN. A) Scaffold exclusivity plot demonstrating MST1 peptides show significantly increased normalized exclusive intensity in the MST1 index case compared to other MN samples; B) Protein heat map demonstrating MST1 was highly enriched in the index case compared to other MN samples; C) Sequence coverage for MST1 (unique peptides highlighted in yellow). This candidate biomarker was identified from a mass spectrometry run of only 19 samples and therefore, shows comparison to fewer cases.
Supplemental Figure S5. Mass spectrometry data supporting NPR3 as a putative antigen in MN. A) Scaffold exclusivity plot demonstrating NPR3 peptides show significantly increased normalized exclusive intensity in the NPR3 index case compared to other MN samples; B) Protein heat map demonstrating NRP3 was highly enriched in the index case compared to other MN samples; C) Sequence coverage for NPR3 (unique peptides highlighted in yellow).
Supplemental Figure S6. Mass spectrometry data supporting FCN3 as a putative antigen in MN. A) Scaffold exclusivity plot demonstrating FCN3 peptides show significantly increased normalized exclusive intensity in the FCN3 index case compared to other MN samples; B) Protein heat map demonstrating FCN3 was highly enriched in the index case compared to other MN samples; C) Sequence coverage for FCN3 (unique peptides highlighted in orange).
Supplemental Figure S7. Mass spectrometry data supporting CD206 as a putative antigen in MN. A) Scaffold exclusivity plot demonstrating CD206 peptides show significantly increased normalized exclusive intensity in the CD206 index case compared to other MN samples; B) Protein heat map demonstrating CD206 was highly enriched in the index case compared to other MN samples; C) Sequence coverage for CD206 (unique peptides highlighted in orange).
Supplemental Figure S8. Confocal microscopy images demonstrating a lack of co-localization of each putative antigen within IgG immune deposits in PLA2R-positive MN control cases. A) SEZ6L2; B) VASN; C) EEA1; D) MST1; E) NPR3; F) FCN3; G) CD206.
Supplemental Figure S9. Paraffin immunofluorescence staining of PLA2R, THSD7A, EXT1/2, NELL1, and diabetic nephropathy biopsies as negative controls for each candidate antigen. A) SEZ6L2; B) VASN; C) EEA1; D) MST1; E) NPR3; F) FCN3; G) CD206.
Supplemental Figure S10. Representative paraffin immunofluorescence images for the putative antigens.
Supplemental Table S1. Clinical and pathologic characteristics of MN patients positive for new target antigens.
Abbreviations: ANA, antinuclear antibodies; APLS, anti-phospholipid antibody syndrome; BPH, benign prostatic hypertrophy; COPD, chronic obstructive pulmonary disease; Cr, creatinine; DJD, degenerative joint disease/osteoarthritis; DM, diabetes mellitus; DVT, deep venous thrombosis; F, female; GERD, gastroesophageal reflux disease; HTN, hypertension; ITP, immune thrombocytopenic purpura; IUFD, intrauterine fetal demise; M, male; MGUS, monoclonal gammopathy of undetermined significance; NR, nephrotic range; N/A, not applicable/not available; NSAIDs, non-steroidal anti-inflammatory drugs; prot, proteinuria; PRES, posterior reversible encephalopathy syndrome; PE, pulmonary embolism; RA, rheumatoid arthritis; RVT, renal vein thrombosis; SLE, systemic lupus erythematosus; subendo, subendothelial deposits; TBM, tubular basement membrane; TIA, transient ischemic attack; WNL, within normal limits
Supplemental Table S2. IgG subclass staining of biopsies positive for candidate antigens.
Data Availability Statement
The mass spectrometry proteomics data was submitted to the ProteomeXchange Consortium, via the PRIDE partner repository, with the dataset identifiers PXD035853 and PXD035854.


