Abstract
Eating and drinking co-occur and many of the same mechanisms that control one are involved in the control of the other, making it difficult to isolate specific mechanisms for the control of fluid intake. Glucagon-like peptide-1 (GLP-1) is a peptide that seems to be involved in the endogenous control of both ingestive behaviors, but we lack a thorough understanding of how and where GLP-1 is acting to control fluid intake. Vasopressin-deficient Brattleboro rats are a model of hereditary hypothalamic diabetes insipidus that have been used extensively for the study of vasopressin actions in behavior and physiology. Here, we propose that these rats, that eat normally but drink excessively, provide a useful model to dissociate central controls of food and fluid intakes. As an initial step toward establishing this model for these purposes, we focused on GLP-1. Similar to the effect observed after treatment with a GLP-1 receptor (GLP-1R) agonist, the intake difference between wildtype and Brattleboro rats was largely a function in the number of licking bursts, indicating differences in post-ingestive feedback (e.g., satiation). When given central injections of a GLP-1R agonist, the effect on feeding was comparable between wildtype and Brattleboro rats, but the effect of drug on fluid intake was markedly exaggerated in Brattleboro rats. Additionally, Brattleboro rats did not respond to GLP-1R antagonism, whereas wildtype rats did. Taken together, these results suggest that Brattleboro rats exhibit a selective disruption to GLP-1’s control of water intake. Overall, these experiments provide foundational studies of the ingestive behavior of Brattleboro rats and demonstrate the potential to use these rats to disentangle the effects of GLP-1 on food and fluid intakes.
Keywords: Drinking, feeding, thirst, Glucagon-like peptide-1, polydipsia
1. Introduction
Food and fluid intakes are behaviorally and physiologically intertwined, making it difficult to dissociate the mechanisms that control one or the other. Behaviorally, food and fluid intakes have a close temporal relationship. For example, Kissileff [1] found that in a normal rat, feeding occurs in discrete bouts (meals), and that most drinking is termed “prandial,” meaning that it occurs in bouts either before or after meals. This prandial drinking accounts for approximately 70% of water intake in laboratory rats and the amount of drinking is correlated with the amount of food consumed, although the strength of this relationship can be manipulated with changes in temperature, hydration-status, or diet composition [2]. In addition to being behaviorally linked, the underlying physiology of thirst and hunger share neural and chemical elements. For instance, several peptides (e.g., ghrelin and glucagon-like peptide-1) that were identified as relevant for feeding behavior, were subsequently shown to have effects on drinking [3–9].
Glucagon-like peptide-1 (GLP-1) is best known as an incretin hormone that is involved in glycemic control and energy balance [7, 10–12]. Due to its effects on insulin, food intake, and body weight, the GLP-1 system has emerged as an attractive target for treatments of diabetes mellitus and obesity [8, 10, 13]. Treatment with GLP-1 or GLP-1 receptor (GLP-1R) agonists, decreases food intake and body weight [for examples see: 7, 9, 10, 14] and there is strong evidence for a role for endogenous GLP-1 in the control of food intake. Specifically, injection of the GLP-1R antagonist, exendin 9–39 (Ex9), increases food intake and body weight, but only in satiated animals [5, 9, 15–18]. Other manipulations of GLP-1, including viral-mediated knockdown of the precursor to GLP-1 and ablation of GLP-1-producing preproglucagon neurons, also provide strong evidence that endogenous GLP-1 is important for food intake and body weight [19, 20].
Although GLP-1’s role in food intake is well studied, there has been less attention paid to GLP-1 and water intake. Individuals being treated with GLP-1R agonists are prone to reduced water intake [21]. This is particularly concerning because the majority of individuals diagnosed with diabetes mellitus, and therefore potentially treated with GLP-1R agonists, are over the age of 45 and are already at an increased risk for dehydration [22, 23]. Thus, the already common instances of fluid imbalance are potentially exacerbated by a common treatment for diabetes mellitus and obesity.
The control of water intake by GLP-1 appears to occur in a manner that is separable from the role it plays in food intake, and it appears to be exclusively central. For example, the suppression of water intake by GLP-1 occurs at a lower dose than is needed to suppress food intake [8, 9]. The hypodipsic effect of GLP-1 or GLP-1R agonists occurs independent of any effects on food intake [7, 9] and affects water intake stimulated by treatments that do not affect food intake [6]. Additionally, injection of a GLP-1R antagonist has been shown to increase water intake, and water deprivation followed by drinking was associated with hindbrain changes in mRNA that codes for proglucagon, the precursor to GLP-1, without any detectable changes in circulating GLP-1 [5]. Thus, it seems that GLP-1 is not singularly important for the control of food intake, but also plays a role in the control of water intake and may serve as a satiety signal for both ingestive behaviors. Moreover, it appears that the GLP-1 involved in the control of fluid intake is of central origin.
Addressing open questions related to the control of drinking by GLP-1, such as what roles it plays in fluid intake satiety and where in the brain these actions occur, would be aided by a new approach that helps untangle food intake from fluid intake. Here, we propose that the vasopressin-deficient Brattleboro rat may be useful in this respect. Brattleboro rats were discovered in 1961 in Brattleboro, Vermont, and have since provided a useful model of hereditary hypothalamic diabetes insipidus. This condition is characterized with primary polyuria and a secondary polydipsia [24]. The cause of the diabetes insipidus in these rats has been determined to be a single base pair deletion leading to the inability to properly synthesize and fold vasopressin [25]. Due to a lack of vasopressin, these rats cannot retain water, which results in high volume of dilute urine and a rise in body sodium concentration [26]. In spite of abnormally high water intake, food intake by Brattleboro rats is normal [27]. Thus, Brattleboro rats provide an opportunity to use a well-established model that has normal food intake, but abnormal fluid intake, in order to help dissociate the physiological controls of food and fluid intakes. As a first step in testing the utility of this model, we focused on the intake effects of GLP-1 and tested for differences in feeding and drinking effects of GLP-1R ligands in Brattleboro and wildtype rats. In addition to providing novel information about the drinking patterns of Brattleboro rats, and the effect of GLP-1 in this rodent model, these experiments also provide a foundation for future work that may help pinpoint how and where GLP-1 is acting to control drinking, separate from its control of feeding.
2. Materials and Methods
2.1. Animals
Rats were obtained from a breeding colony maintained at the University at Buffalo that was derived from rats from the Rat Resource and Research Center (University of Missouri, Columbia, MO). All rats in the study were produced from pairings of male and female rats that were heterozygous for the Brattleboro mutation. This strategy produced litters of male and female wildtype Long Evans rats (subsequently referred to as wildtype rats), and male and female rats that were homozygous for the Brattleboro mutation (subsequently referred to as Brattleboro rats) and therefore lacked functional vasopressin. Rats that were heterozygous for the Brattleboro mutation were also produced in these litters, but were not used as subjects in the experiments described here. Rats were genotyped between postnatal day (PD) 13–15 with day of birth considered PD 0 (based on the procedure outlined in [28]). On PD 21, rats were weaned into same-sex, same-genotype groups of 2–3 rats per cage. In the few instances when there were not sufficient numbers of rats of the same genotype and sex, rats were weaned into groups of 2–3 with same sex heterozygotes. Rats remained group housed in plastic cages (44 cm × 22.5 cm × 20.5 cm) with corn cob bedding (Envigo, Indianapolis, IN) until after cannula implantation and recovery, at which point rats were single housed in stainless steel wire mesh cages (Unifab, Kalamazoo, MI). Rats were housed in a temperature- and humidity-controlled room with a 12 h light/12 h dark cycle and were given ad libitum access to food and water unless otherwise noted. Before the onset of the experiments described here, rats were used in a separate, unrelated experiment that exposed them to open field, novel object, and social approach tests without any invasive procedures. Body weight was measured at least five days per week throughout the course of all experiments. For plotting body weight by age, we included ages when weight measures were available for all groups (sex/genotype) and at least n=3 within group. This allowed for plotting of most days of the growth curve. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo, and the handling and care of animals was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Genotyping
Ear tissue was collected between PD 13–15, digested, and DNA was extracted using the REDExtract-N-Amp Tissue PCR Kit (SigmaAldrich, St Louis, MO). The single base pair deletion [25] was amplified via PCR. Primer sequences were as follows: GACGAGCTGGGCTGCTTC and CCTCAGTCCCCCACTTAGCC (forward, reverse). The PCR product was digested for 24 h at 37°C with a Bcg1 restriction endonuclease (New England BioLabs, Ipswich, MA) that only cuts the mutant Brattleboro PCR product. After gel electrophoresis using a 2% agarose gel, a single ~95 bp band was used to indicate the presence of the Brattleboro mutation, a single 222 bp band indicated a wildtype genotype, and the presence of both bands classified a rat as heterozygous, as validated previously [28, 29].
2.3. Cannula Implantation and Placement Verification
Rats in all experiments were implanted with a chronic, indwelling cannula aimed at the lateral ventricle (LV). Rats were anesthetized using isoflurane gas (Piramal Critical Care, Bethlehem, PA), secured in a stereotaxic apparatus, and given subcutaneous injections of carprofen (5 mg/kg; Pfizer Animal Health, Parsippany-Troy Hills, NJ) and 0.9% saline (5 mL). After drilling a small burr hole in the skull, a guide cannula (26 gauge; P1 Technologies, Roanoke, VA) was implanted 0.9 mm posterior and 1.4 mm lateral to bregma and 2.8 mm ventral to skull surface. The guide cannula was secured to the skull using bone screws and dental cement. Cannula placement was verified at the conclusion of all experiments by an injection of 1 μl of ink before perfusion or decapitation with gross visualization of ink in the ventricles as an indication of accurate placement. All rats had visible ink in the ventricles.
2.4. Drug Injections and Intake Measures
The GLP-1R agonist exendin 4 (Ex4) and antagonist exendin 9–39 (Ex9) were purchased from Bachem (Torrance, CA). Injections were made with a 33-gauge injection cannula (P1 Technologies, Roanoke, VA) that was fabricated to extend 1.5 mm past the end of the guide cannula. The injection cannula was connected to a 2-μl Hamilton syringe (Ex4) or a 5- or 10-μl Hamilton syringe (Ex9) via flexible PE-50 tubing. For testing, Ex4 injections were given 30 min before lights out and Ex9 injections began 2 h after lights on. Injection cannulae were held in place for ~30 s after each injection. Water bottles and, if applicable, food hoppers were weighed immediately before and after the testing periods. Total food and water intakes were calculated by taking the difference of the pre- and post-test weight measurements. For licking measures, the bottle spouts in wire mesh cages were recessed behind an electrically isolated plate with a 3.175-mm wide opening through which the rat needed to lick in order to reach the spout, thus minimizing non-tongue contact with the spout. All rats were habituated to these cages and bottle arrangement for at least 5 d. A contact lickometer (designed and constructed by the University of Pennsylvania Psychology Electronics Shop) was used to record time-stamped licks. The lickometer interfaced with a computer using an integrated USB digital I/O device (National Instruments, Austin, TX) and data were acquired and processed in a MATLAB (The MathWorks, Natick, MA) software environment.
2.5. Experimental Designs
2.5.1. Experiment 1: Characterization of the ingestive behaviors of wildtype and Brattleboro rats
To evaluate differences in body weights between the sexes and genotypes, averages for the week of age with the greatest number of data points were analyzed (PD 57–63 for Experiments 1–3 with an average of 5.47 measures per rat and PD 113–119 for Experiment 4 with an average of 5.71 measures per rat). To better understand the unperturbed ingestive behavior of these rats, food and water intakes were measured for five consecutive days. Rats (n = 27; 8 wildtype male, 6 wildtype female, 8 Brattleboro male, and 5 Brattleboro female) were used in Experiment 1. All rats underwent surgery on PD 43–49 and testing spanned PD 53–63. Estrous cycle stage was monitored via vaginal cytology [as described in 30, 31–33]. Food hoppers and water bottles were weighed every 24 h and licking for water was monitored using a contact lickometer. In an effort to replicate and extend the previous studies, we analyzed microstructural licking patterns to provide information related to the different drinking behavior by wildtype and Brattleboro rats. As previously described [34, 35], changes in burst number are associated with changes in post-ingestive feedback, whereas changes in the number of licks per burst (burst size) more likely reflect differences in orosensory feedback. For food intake measures, an average of 4.78 days out of 5 days/rat were included in data analysis and for water intake an average of 4.85 days were included. There were two missing data points for the volume of water consumed and, in order for these animals to not be excluded, the missing values were replaced for the repeated measures analyses using the calculated average drop size for the other days for that animal, and this calculated average drop size was multiplied by the number of licks for the day that volume was missing to generate an approximate volume consumed. The number of licks and drinking microstructure across the days were also analyzed.
As previously stated, estrous cycle stage was monitored for the duration of this experiment. The following procedure was used to assign days of the cycle to days of intake. Although most female rats have 4-day estrus cycle, a 5-day cycle is not uncommon. When it occurred, a 5th day was treated as an additional day of diestrus 1, consistent with a previous approach [32]. The cycles of Brattleboro rats are more variable [36]. Accordingly, we measured intake for 5 days to be sure every stage of the estrous cycle was captured for every female rat. Data from repeated days of the cycle were averaged to provide a single data point for each of the four stages of the cycle, irrespective of the number of days spent in each cycle stage.
2.5.2. Experiment 2: Effect of central administration of Ex4 on 24-h food and water intakes
To test for an effect of genotype on the intake suppression caused by a GLP-1R agonist, rats underwent surgery PD 46–57 and then we used a counterbalanced, repeated measures design in which rats (n = 28 with equal distribution between sexes and genotypes) received an injection into the LV of vehicle (1 μl 0.9% saline) or Ex4 (0.1 μg) just before dark phase onset (testing spanned PD 59–72). Intakes were measured for the subsequent 24 h and the experiment was repeated 48 h later with rats receiving the other treatment to complete the repeated measures design.
2.5.3. Experiment 3: Effect of central administration of Ex9 on spontaneous water intake
At the conclusion of Experiment 1, all rats in that experiment (n = 27; 8 wildtype male, 6 wildtype female, 8 Brattleboro male, and 5 Brattleboro female) were used to test the effect of a GLP-1R antagonist on drinking in wildtype and Brattleboro rats (PD 61–74). To this end, 2 h after light phase onset, food and water were removed and rats received an injection of vehicle (2 μl 0.9% saline) or Ex9 (100 μg; Bachem, Torrance, CA). Water was returned 30 min after injection and drinking behavior was recorded for 2 h. In this experiment, stage of the estrous cycle was controlled for by testing all female rats during estrus and, as described for Experiment 1, estrous cycle stage was monitored via vaginal cytology. As previously mentioned, the estrous cycle in Brattleboro rats has been shown to be more variable than in Long Evans rats and was subsequently more difficult to track, thus leading to some days when testing should have occurred in Experiments 3 and 4, but did not because cycle stage was unclear [36]. Each day that a female was tested at least 1 same genotype male was also tested. Testing was conducted during the light phase and during vaginal estrus, as defined as the light phase after behavioral estrus and when vaginal cytology showed the presence of cornified vaginal epithelial cells, so that baseline intake would be relatively low. This strategy is similar to that used previously in our laboratory [5, 31]. Treatment condition used a counterbalanced repeated measures design with testing days separated by 4–5 d, depending on female cycle length.
2.5.4. Experiment 4: Effect of central administration of Ex9 on the drinking response to subcutaneous hypertonic saline
As a follow up to Experiment 3 and to address the possibility of a ceiling effect, rats (n = 24; 7 wildtype male, 7 wildtype female, 5 Brattleboro male, and 5 Brattleboro female) were implanted with a cannula aimed at the LV on PD 94–105. After sufficient recovery time, rats were stimulated to drink by injection of hypertonic saline and the effect of Ex9 was tested. All testing was completed between PD 105–137. The same procedure as described for Experiment 3 was used, but immediately after LV injections of Ex9, rats were given a subcutaneous injection of hypertonic saline (2 ml/kg body weight; 1 M NaCl with 0.2% lidocaine). Similar to Experiment 3, female rats were only tested during vaginal estrus and, when possible (9 out of 11 days of testing), a same-genotype male was tested on the same day. Testing was separated by at least one estrus cycle (3 to 5 d), but by up to 4 estrus cycles (22 d), with an average of 7.29 d between testing.
2.6. Data Analysis
All data are presented as means ± SEM and individual data points when possible. Individual data points are included when bars represent one instance in time and are not included when bars represent averages across time. All statistical analyses were performed using Statistica (StatSoft Inc, Tulsa, OK). Effects were considered significant if p ≤ 0.05. Outliers were defined as values ± 2 SD away from the group mean. When the number of licks for a particular day were considered an outlier, that day was excluded from calculation of the average across time for each animal and from analysis of drinking microstructure. Missing values for licks or microstructure values were not replaced. ANOVAs were used as appropriate to examine main effects of sex, genotype, treatment, and/or time, as well as interaction effects. Omnibus ANOVAs were conducted with all variables included (genotype and sex, as well as treatment and time if applicable). Planned analyses included assessing data from wildtype and Brattleboro rats separately when a main effect of genotype was detected. This was to remove the potential for obfuscation because of the vastly different baseline between wildtype and Brattleboro rats [28, 37, 38]. If the omnibus ANOVA detected significant interaction effects, these were further probed using Student-Newman-Keuls post hoc tests. If there was not a main effect of genotype or a significant genotype*treatment interaction effect, the genotypes were not analyzed separately and/or post hoc tests were not conducted. All analyses were conducted with sex as a variable first, but if a main effect of sex was not detected, data were collapsed across sex and re-analyzed. When appropriate, female rats were analyzed separately to test for an effect of cycle stage. Additionally, as previously done in other studies comparing the water intake of Brattleboro rats to other rats, baseline water intake and changes from baseline were taken into account [39, 40].
3. Results
3.1. Experiment 1: Characterization of the body weight and ingestive behaviors of wildtype and Brattleboro rats
3.1.1. Body weight
A summary of daily body weights by age for the rats in Experiments 1–3 are shown in Figure 1A. This summary is for illustration only, and the data were not used for statistical hypothesis testing because of mismatches in available days of data; however, statistical comparisons were made for two specific sets of days (PD 57–63 and PD 113–119; Figures 1B&C) during which more complete data sets were available. At both of these times, wildtype rats weighed more than Brattleboro rats (F1,51 = 23.84, p < 0.001; F1,20 = 30.06, p < 0.001) and male rats weighed more than female rats (F1,51 = 520.42, p < 0.001; F1,20 = 210.38, p < 0.001). From PD 57–63, the sex difference in body weight was apparent even when the genotypes were analyzed separately (Figure 1B; significant main effects highlighted in Figures 1C&D; wildtype, t26 = −17.95, p < 0.001; Brattleboro, t25 = −13.77, p < 0.001). Additionally, during PD 113–119, there was a significant sex*genotype interaction (F1,20 = 10.55, p = 0.004) and post hoc comparisons found that wildtype males weighed more than Brattleboro males, both of which weighed more than female rats of either genotype. There were no genotype differences in body weight of female rats during this time bin. When the genotypes were analyzed separately, the sex difference in each genotype was still apparent (Figure 1E; wildtype, t12 = −11.52, p < 0.001; Brattleboro, t8 = −8.57, p < 0.001).
Figure 1.
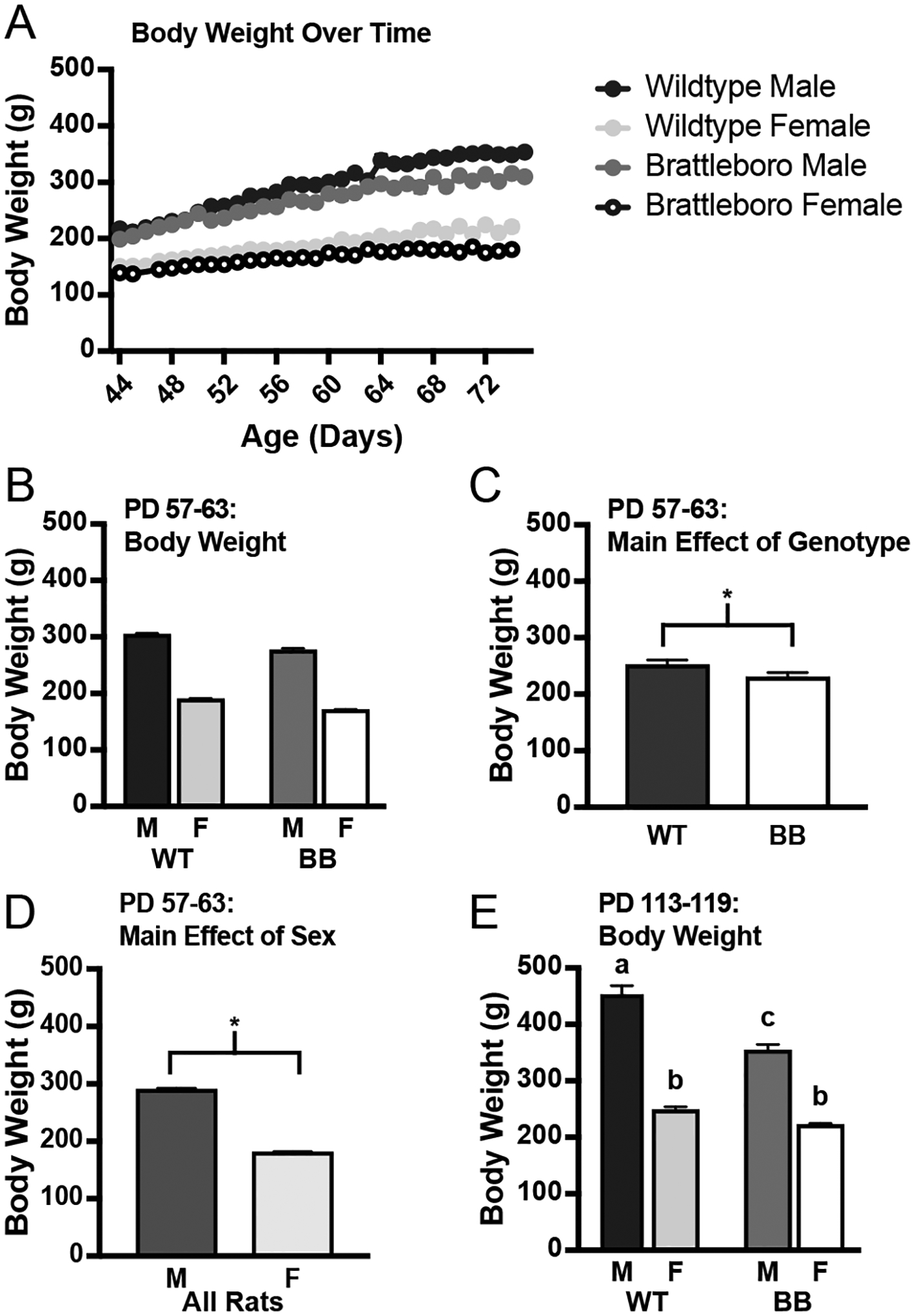
Body weight over time in Brattleboro and wildtype rats. Body weight was measured for the duration of all experiments. A) The development of the differences in body weight in the genotypes for rats in Experiments 1–3 (summary for illustration only). B) Average body weight for each group of rats from Experiments 1–3 from PD 57–63. Wildtype rats weighed more than Brattleboro rats and male rats weighed more than female rats, but there was no statistically significant interaction. C) Significant main effect of genotype for PD 57–63 highlighted; wildtype rats weighed more than Brattleboro rats during this time range. D) Significant main effect of sex for PD 57–63; male rats weighed more than female rats during this time range. E) Average body weight for each group of rats from Experiment 4 from PD 113–119. There was a significant sex*genotype interaction: wildtype male rats weighed more than Brattleboro male rats. Male rats weighed more than the female rats of either genotype, however, there were no genotype differences in female rats. Statistical significance (p ≤ 0.05) is indicated by asterisks or by bars with different letters. Abbreviations used: Male, M; Female, F; Wildtype, WT; Brattleboro, BB.
3.1.2. Food and water intakes
Intake of food and water by rats in Experiment 1 was measured for five consecutive days. Analysis of food intake as a function of body weight (Figure 2A) confirmed well-documented sex differences (Figure 2B; F1,23 = 47.31, p < 0.001), but did not find any difference in food intake as a function of genotype (F1,23 = 0.58, p = 0.454). To test for an effect of estrous cycle stage on food and water intakes, we recorded the cycle stage of the female rats and used this information to separate days of intake into the corresponding days of the estrous cycle. Analysis of food intake by cycle stage found no effect of genotype (F1,9 = 3.03, p = 0.116), but detected a main effect of cycle (F3,27 = 2.93, p = 0.05). Post hoc analysis did not reveal any significant differences between the days of the cycle, but an exploratory t-test between diestrus 1, when intake should be highest, and proestrus, which contains the period of behavioral estrus when intake should be lowest, revealed a significant difference (t10 = −2.20, p = 0.026). Because there were significant genotype differences in body weight in rats of this age, and because energy balance is highly dependent on body weight, we analyzed food intake across the estrous cycle as a function of body weight and found the same results (Figure 2C; no effect of genotype, F1,9 = 2.19, p = 0.173; main effect of cycle stage, F3,27 = 4.38, p = 0.012). A post hoc analysis did not reveal any noteworthy differences between the days, but an exploratory t-test between diestrus 1 and proestrus revealed a significant difference (Figure 2D; t10 = −2.88, p = 0.008). Overall, with or without controlling for body weight differences, there was an effect of the estrous cycle on the food intake of both wildtype and Brattleboro rats: intake was suppressed during proestrus relative to diestrus 1, but there were no differences in food intake between the two genotypes.
Figure 2.
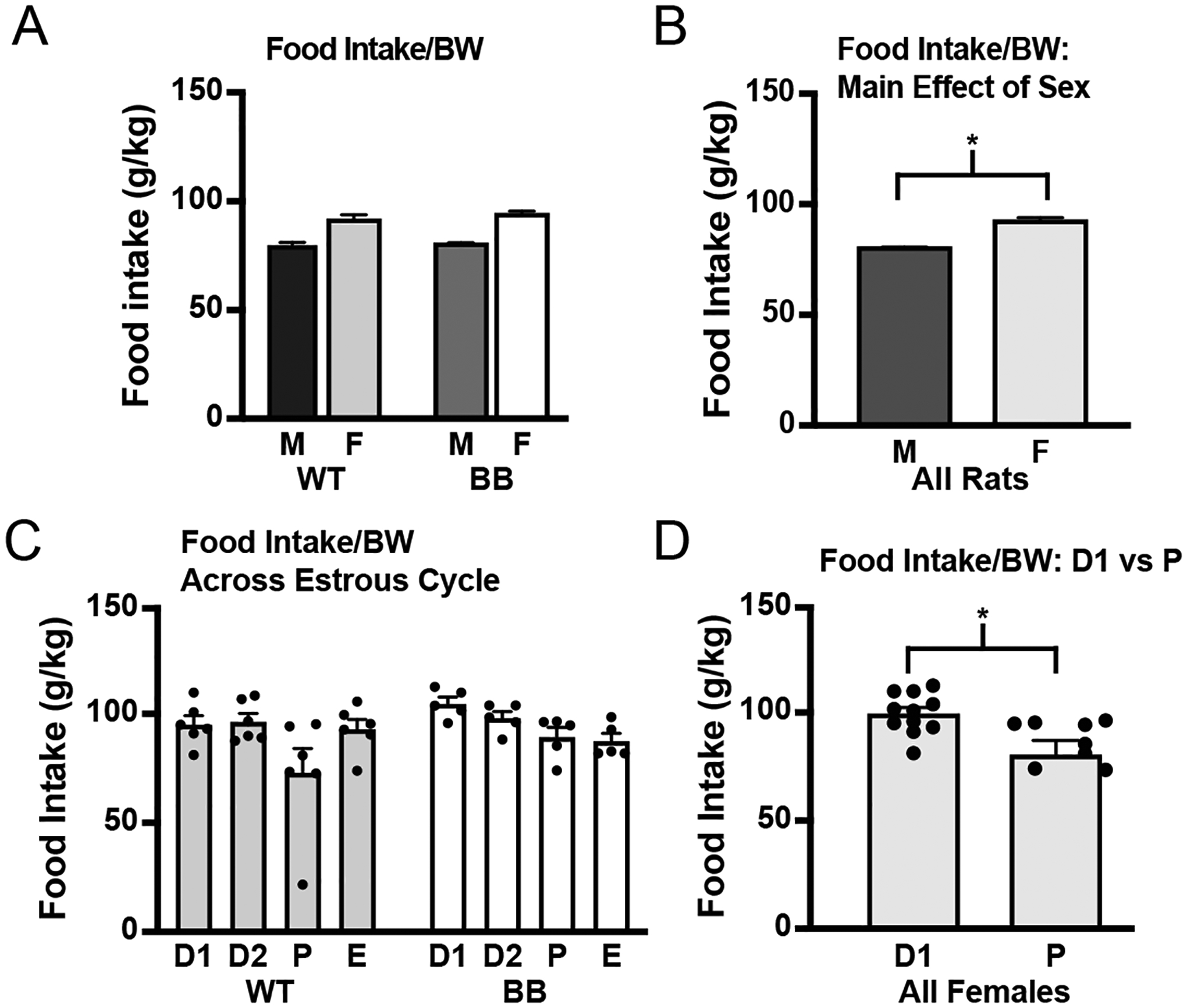
Food intake by wildtype and Brattleboro rats. Food intake was measured for 5 consecutive 24-h periods. A) When differences in body weight were controlled for, same sex wildtype and Brattleboro rats ate similar amounts of food and male rats of both genotypes ate significantly more than female rats of either genotype. B) Significant main effect of sex highlighted. C) When examining food intake across days of the estrous cycle, there were no differences between the genotypes, but there was a main effect of cycle stage, with no comparisons reaching the threshold for significance in the post hoc test. D) Although ANOVA did not reveal differences between the days of the cycle, an exploratory t-test found a significant difference in food intake in all female rats between diestrus 1 and proestrus. Asterisk indicates significant difference, p ≤ 0.05. Abbreviations used: Body weight, BW; Male, M; Female, F; Wildtype, WT; Brattleboro, BB; Diestrus 1, D1; Diestrus 2, D2; Proestrus, P; Estrus, E.
As expected, there were large genotype differences in water intake (Figure 3A). Brattleboro rats drank significantly more than wildtype rats (F1,23 = 220.86, p < 0.001). When the genotypes were analyzed separately, intake appeared to be greater in wildtype male rats than it was in wildtype female rats, but this did not reach statistical significance (F1,12 = 4.03, p = 0.068). There were no sex differences observed in water intake by Brattleboro rats (F1,11 = 0.12, p = 0.734). Thus, as expected, Brattleboro rats drank more than wildtype rats, but without observed sex differences in the amount of water consumed.
Figure 3.
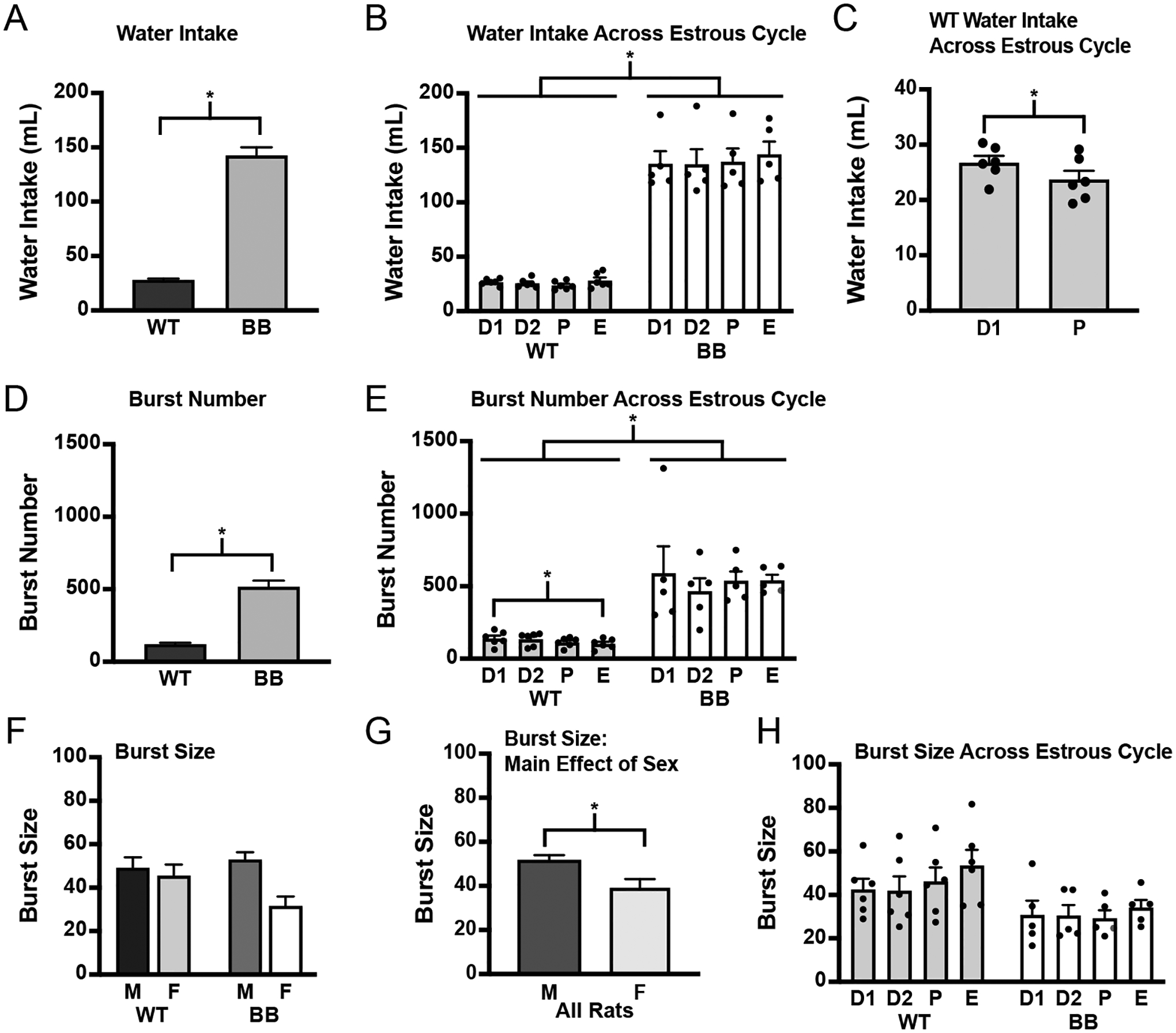
Water intake by wildtype and Brattleboro rats. Water intake was measured for 5 consecutive 24-h periods. A) Brattleboro rats drank significantly more than wildtype rats. B) Water intake across the estrous cycle. There was a main effect of genotype without an effect of cycle stage in wildtype or Brattleboro rats. C) An exploratory t-test between diestrus 1 and proestrus found a significant reduction in water intake during proestrus in wildtype female rats (y axis changed to illustrate the effect), but not in Brattleboro rats (data not shown). D) Brattleboro rats had a significantly higher number of licking bursts that underlie the differences in baseline drinking between wildtype and Brattleboro rats. E) The number of licking bursts is a function of estrous cycle stage, but only in wildtype rats. ANOVA detected a main effect of genotype and a lower number of bursts on the day of estrus compared to the day of diestrus 1 in wildtype rats, but the effect of cycle was not observed in Brattleboro rats. F) There were no differences between the genotypes on burst size. G) Female rats had fewer licks per burst. H) There were no differences in burst size across the estrous cycle between genotypes or in either genotype. Asterisks indicates significant differences, p ≤ 0.05. Abbreviations used: Male, M; Female, F; Wildtype, WT; Brattleboro, BB; Diestrus 1, D1; Diestrus 2, D2; Proestrus, P; Estrus, E.
When we tested for an effect of cycle stage on water intake, we found a main effect of genotype (Figure 3B; F1,9 = 106.91, p < 0.001), prompting separate analyses of each genotype. There was no effect of cycle stage on water intake by wildtype rats (F3,15 = 1.39, p = 0.286) or by Brattleboro rats (F3,12 = 1.15, p = 0.367); however, an exploratory t-test revealed a significant difference between diestrus 1 and proestrus, but only in wildtype rats (Figure 3C; wildtype, t5 = −2.76, p = 0.010; Brattleboro, t4 = 0.37, p = 0.635). Consistent with previous reports, there was a suppression of water intake in proestrus by wildtype female rats [41–46], but this was not observed in female Brattleboro rats.
In addition to the volume of water consumed, we also recorded licking behavior with a contact lickometer and examined drinking microstructure across all 5 testing days. There was a significant effect of genotype on the number of bursts of licking. Brattleboro rats had a larger number of licking bursts than wildtype rats (Figure 3D; F1,23 = 90.41, p < 0.001). We did not find any sex differences in burst number, even when the genotypes were analyzed separately (wildtype, F1,12 = 0.06, p = 0.810; Brattleboro, F1,11 = 0.14, p = 0.712). This is suggestive of reduced post-ingestive feedback, or less satiety, in both male and female Brattleboro rats compared to wildtype rats of both sexes. We also analyzed burst number across the estrous cycle. Because there was a main effect of genotype (Figure 3E; F1,9 = 28.01, p < 0.001), we analyzed the genotypes separately and found that there was an effect of cycle stage on burst number in wildtype rats (F3,15 = 3.74, p = 0.034), but not in Brattleboro rats (F3,12 = 0.38, p = 0.767). A post hoc test in wildtype rats revealed that burst number was lower on the day of estrus compared to diestrus 1 (p = 0.049).
Analysis of the number of licks per burst (burst size) did not find a main effect of genotype (Figure 3F; F1,23 = 1.28, p = 0.270), but did reveal a main effect of sex (Figure 3G; F1,23 = 7.90, p = 0.010); female rats had a lower average burst size compared to male rats. Additionally, there appeared to be a genotype*sex interaction, but this did not reach statistical significance (F1,23 = 3.97, p = 0.058) and was, therefore, not explored by a post hoc analysis. These results suggest similar orosensory feedback from water between wildtype and Brattleboro rats, with differences between male and female rats that may be dependent on genotype. When burst size was analyzed across the estrous cycle (Figure 3H), there was not a statistically significant effect of genotype (F1,9 = 4.28, p = 0.069) or cycle stage (F3,27 = 2.31, p = 0.098). Nevertheless, the overall findings point to a difference in post-ingestive feedback being responsible for the intake differences between wildtype and Brattleboro rats.
3.2. Experiment 2: Effect of central administration of Ex4 on 24-h food and water intakes
To test if the GLP-1R agonist, Ex4, suppresses food and water intakes in Brattleboro rats as it has been shown to in other laboratory rats, we injected Ex4 into the LV of rats shortly before the onset of the dark phase and measured food and water intakes for 24 h.
3.2.1. Food intake
Consistent with previous reports [7, 9, 14, 17, 47, 48], Ex4 significantly reduced food intake, but no differences in genotype or sex were found (Figure 4A; no effect of genotype, F1,23 = 2.73, p = 0.112; no effect of sex, F1,23 = 0.65, p = 0.428) when food intake was expressed as a function of body weight. Collapsing the data across sex failed to reveal a statistically significant effect of genotype (F1,25 = 3.03, p = 0.094). The main effect of Ex4 was statistically significant (Figure 4B; F1,25 = 167.02, p < 0.001) without a significant drug*genotype interaction (F1,25 = 2.41, p = 0.133). We also calculated a suppression score by subtracting the intake after Ex4 from the intake after vehicle in the same rat (using intake expressed as a function of body weight). This analysis also did not detect main effects of genotype (F1,23 = 1.39, p = 0.251) or of sex (F1,23 = 2.62, p = 0.12). Collapsing across sex did not reveal a difference between the genotypes (F1,25 =. 1.20, p = 0.284). Thus, it seems clear that the suppression of food intake by Ex4 was the same in male and female wildtype and Brattleboro rats.
Figure 4.
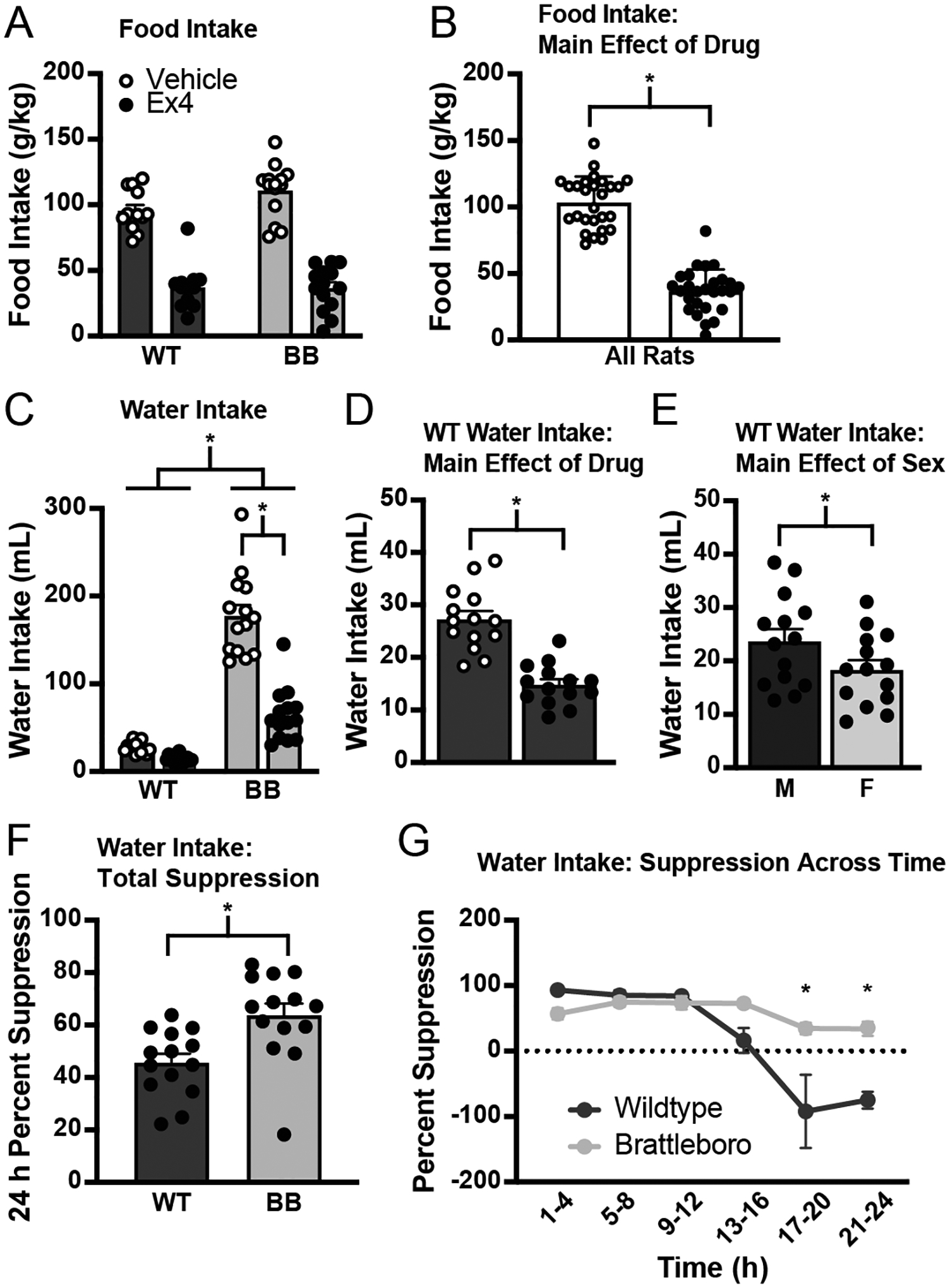
The GLP-1R agonist Ex4, injected into the LV immediately before dark phase onset. A) All groups had an agonist-induced decrease in food intake (normalized by body weight) without any detected genotype or sex differences. The main effect of drug is illustrated in panel B. C) There was a significant drug*genotype interaction on Ex4 drinking with Brattleboro rats showing a larger magnitude of an effect of Ex4 that was statistically significant in the post hoc testing. Main effects of drug and sex in wildtype rats are illustrated in panels D and E, respectively, with y axes changed to illustrate the effects. F) The effect of Ex4 on fluid intake also was analyzed as percent of baseline, further revealing the exaggerated response to Ex4 in Brattleboro rats. G) When data were analyzed as a percent of baseline across time, suppression was equivalent between the two genotypes for the first 16 h of the test, but Brattleboro rats were significantly more suppressed than wildtype rats were during the later hours of the test. Asterisks indicate significant differences, p ≤ 0.05. Abbreviations used: Male, M; Female, F; Wildtype, WT; Brattleboro, BB.
To further test for an effect of Ex4 on food intake, we analyzed data without adjusting for differences in body weight. When differences in body weight were not controlled for, there was a main effect of genotype on the amount of chow consumed (F1,23 = 10.82, p = 0.003), but no significant interactions. When the genotypes were analyzed separately, there was a main effect of sex (wildtype, F1,11 = 28.56, p < 0.001; Brattleboro, F1,12 = 8.48, p < 0.001) and drug (wildtype, F1,11 = 132.89, p < 0.001; Brattleboro, F1,12 = 90.30, p < 0.001) in both genotypes and a significant drug*sex interaction in wildtype rats (F1,11 = 9.21, p = 0.011). When a suppression score was calculated using the aforementioned method, there was no effect of genotype (F1,23 = 0.01, p = 0.940), sex (F1,23 = 3.52, p = 0.073), or a genotype*sex interaction (F1,23 = 3.90, p = 0.060). Thus, even when differences in body weight were not controlled for, the food intake suppression between wildtype and Brattleboro rats was equivalent.
3.2.2. Water intake
Consistent with previous reports, Ex4 also suppressed water intake [5–9]. Similar to the analysis of food intake, we found no effect of sex (F1,24 = 1.69, p = 0.206), but in stark contrast to the food intake analysis, we found a significant main effect of genotype (F1,24 = 186.96, p < 0.001) and a significant drug*genotype interaction (F1,24 = 42.01, p < 0.001) on water intake. Post hoc analyses on the interaction failed to detect a well-documented effect of Ex4 in wildtype rats (male, p = 0.638; female, p = 0.770), but there was a drug-induced suppression of intake in Brattleboro rats (male, p < 0.001, female, p < 0.001). When sex was removed as a variable in the analysis, the main and interaction effects persisted (main effect of genotype, F1,26 = 186.92, p < 0.001; main effect of drug, F1,26 = 66.39, p < 0.001; significant drug*genotype interaction, F1,26 = 42.66, p < 0.001), with post hoc analyses showing an effect of Ex4 in Brattleboro rats (p < 0.001), but not in wildtype rats (p = 0.264). The lack of a well-documented effect of Ex4 on fluid intake in wildtype rats was likely a function of the largely different baseline intakes of the two genotypes because when wildtype rats were examined separately, there was a main effect of drug (Figure 4D; F1,12 = 79.75, p < 0.001) and a main effect of sex (Figure 4E; F1,12 = 8.67, p = 0.012), without a significant sex*drug interaction (F1,12 = 1.50, p = 0.245). When analyzed separately, Brattleboro rats did not show a sex difference in intake (no effect of sex: F1,12 = 0.88, p = 0.366). We, therefore, collapsed the data across sex and found a significant suppression of water intake associated with Ex4 administration (F1,13 = 54.33, p < 0.001) in Brattleboro rats, but with what appeared to be a greater magnitude of suppression. To test the hypothesis that the magnitude of suppression was genotype-dependent, we analyzed the suppression generated by Ex4 as a percent of baseline intake (Figure 4F). When examining intake over the entire 24-h test, there was a significant difference in the percent suppression between the two genotypes (F1,24 = 11.08, p = 0.003). There was no effect of sex on the percent suppression in either genotype when they were analyzed separately (wildtype, F1,12 = 0.01, p = 0.924; Brattleboro, F1,12 = 3.46, p = 0.087). To gain a better understanding of when this suppression was occurring across the 24-h test, we analyzed percent suppression across time and found that the difference in water intake suppression between the genotypes was driven largely by an effect that persisted into the later hours of the test (h 17–24) in Brattleboro rats, with no difference in percent suppression between wildtype and Brattleboro rats during the earlier hours of the test (Figure 4G). Specifically, there was a main effect of genotype (F1,24 = 13.05, p = 0.001), a main effect of time (F5,120 = 13.52, p < 0.001), and a significant time*genotype interaction (F5,120 = 6.20, p < 0.001), but no effect of sex (F1,24 = 2.09, p = 0.161). When sex was removed as a variable from the analysis, the main and interaction effects were still statistically significant (main effect of genotype, F1,26 = 12.99, p = 0.001; main effect of time, F5,130 = 14.45, p < 0.001; time*genotype interaction, F5,130 = 6.63, p < 0.001). A post hoc test revealed significant differences from h 17–20 (p < 0.001) and h 21–24 (p < 0.001). Thus, it seems that Brattleboro rats are suppressed more and for longer than their wildtype counterparts. Indeed, as shown in Figure 4G, the effect of Ex4 wanes faster in wildtype rats, but persists in Brattleboro rats well into the last 12 h of the test.
In addition to analyses of volume consumed, drinking microstructure was examined. Consistent with the results of Experiment 1, Brattleboro rats had a greater number of bursts than wildtype rats in both the first and second halves of the test (Figure 5A; h 0–12, F1,20 = 38.53, p < 0.001; Figure 5D; h 13–24, F1,23 = 60.30, p = 0.001). Because there were no significant interactions in the first half of the test, the genotypes were analyzed separately. There was an effect of drug in the first half of the test in wildtype rats (Figure 5B; t27 = −3.52, p < 0.001), but no effect of sex (F1,10 = 1.59, p = 0.235). This was different from Brattleboro rats, the analysis of which found there was no effect of Ex4 in Brattleboro rats in the first half of the test (F1,10 = 0.53, p = 0.482), although there was a sex difference in the number of bursts (Figure 5C; F1,10 = 5.99, p = 0.034). In the second half of the test, there was a significant drug*genotype*sex interaction (Figure 5D; F1,23 = 12.83, p = 0.002). A post hoc analysis was conducted and found that Ex4 treatment was associated with a greater number of bursts in Brattleboro rats, but only in female rats (Brattleboro male, p = 0.429; Brattleboro female, p < 0.001). For wildtype rats, post hoc analysis found no effect of drug in either sex (wildtype male, p = 0.529; wildtype female, p = 0.340). When the genotypes were analyzed separately, there was no effect of sex in either genotype (wildtype, F1,12 = 0.00, p = 0.971; Brattleboro, F1,11 = 2.97, p = 0.112); however, when collapsed across sex there was a main effect of drug in both genotypes (Figure 5E; wildtype, F1,13 = 19.01, p < 0.001; Figure 5F; Brattleboro, F1,12 = 8.31, p = 0.014), although the effect of drug on burst number in Brattleboro rats seems to have been driven by changes in female rats.
Figure 5.
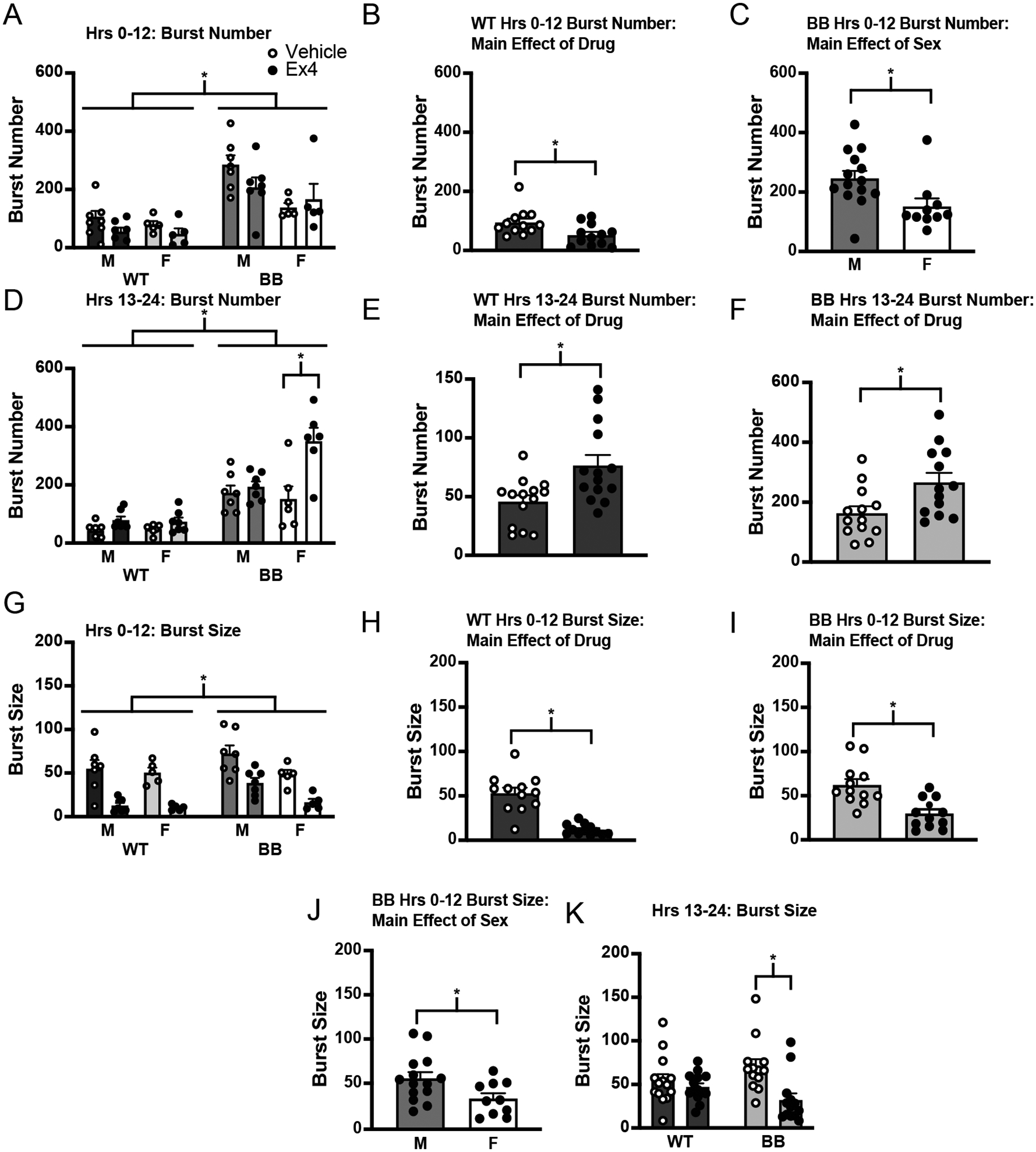
Drinking microstructure across the Ex4 intake measure. A) Brattleboro rats had a greater number of licking bursts across the first 12 hours of the test. B) In wildtype rats, there was a main effect of drug in the first 12 hours, with Ex4 causing a decrease in the number of bursts. C) In Brattleboro rats, there was no effect of drug on burst number in the first 12 hours, but there was a main effect of sex (highlighted here). D) In the second 12 hours of the test, there was a significant drug*genotype*sex interaction. Brattleboro rats had a greater number of licking bursts. Brattleboro female rats had a greater number of bursts after Ex4 treatment. E) When the genotypes were analyzed separately, there was a main effect of drug on burst number in wildtype rats, with more licking bursts in the Ex4-treated group in the second half of the test (y-axis changed to illustrate effect). F) When the genotypes were analyzed separately, there was a main effect of drug on burst number in Brattleboro rats, with more licking bursts in the Ex4-treated group in the second half of the test. This effect seemed to be driven by changes in Brattleboro female rats. G) Analyses of burst size in the first half of the test found that Brattleboro rats had more licks per burst than wildtype rats. H) When analyzed separately, Ex4-treatment was associated with a decrease in the number of licks per burst in wildtype rats. I) The same was true for Brattleboro rats—Ex4-treatment was associated with a decrease in the number of licks per burst. J) There was a significant main effect of sex on burst size in Brattleboro rats. K) There was a significant drug*genotype interaction on burst size in the last 12 hours of the test. Post hoc test showed a main effect of drug in Brattleboro rats that was not present in wildtype rats. Asterisks indicate significant differences, p ≤ 0.05. Abbreviations used: Male, M; Female, F; Wildtype, WT; Brattleboro, BB.
Analysis of the average number of licks per burst found a significant difference between wildtype and Brattleboro rats, with a main effect of genotype in the first 12 h of the test (Figure 5G; F1,20 = 5.05, p = 0.036) and a significant drug*genotype interaction in the second 12 h (Figure 5K; F1,23 = 24.08, p < 0.001). When the genotypes were analyzed separately to test for an effect of Ex4, we found main effects of drug (Figure 5H; t23 = −5.34, p < 0.001), but not sex (F1,12 = 0.13, p = 0.724), in the first 12 h of the test in wildtype rats and an effect of both drug (Figure 5I; F1,10 = 22.90, p < 0.001) and sex (Figure 5J; F1,10 = 9.24, p = 0.012) in Brattleboro rats. When examining the last 12 h of the test, there was not a main effect of genotype (F1,23 = 0.00, p = 0.963) or of sex (F1,23 = 3.53, p = 0.073), but there was a significant drug*genotype interaction (F1,23 = 24.08, p < 0.001). Post hoc analysis showed a main effect of drug in Brattleboro rats (male, p < 0.001; female, p < 0.001) but not wildtype rats (male, p = 0.497; female, p = 0.983) When data were collapsed across sex there was a significant effect of drug (Figure 5H; F1,25 = 52.74, p < 0.001) and a significant drug*genotype interaction (F1,25 = 24.51, p < 0.001), with a post hoc test revealing a significant decrease in burst size in Brattleboro rats given Ex4 compared to Brattleboro rats given vehicle (p < 0.001), but not wildtype rats (p = 0.115).
3.3. Experiment 3: Effect of central administration of Ex9 on spontaneous water intake
To test for genotype differences in a role for endogenous GLP-1 in fluid intake, the GLP-1R antagonist Ex9 was administered shortly after the onset of the light phase and water intake was measured for the subsequent 2 h (without food available). An omnibus ANOVA found a main effect of genotype (Figure 6A; F1,21 = 190.14, p < 0.001). When each genotype was analyzed separately, there was a significant increase in water intake in wildtype rats given Ex9, but only in males (Figure 6B; significant drug*sex interaction, F1,10 = 15.25, p = 0.003; post hoc test male, p = 0.012, female, p = 0.149). There was no difference in the amount of water consumed in any other group (Brattleboro rats, F1,12 = 0.05, p = 0.830).
Figure 6.
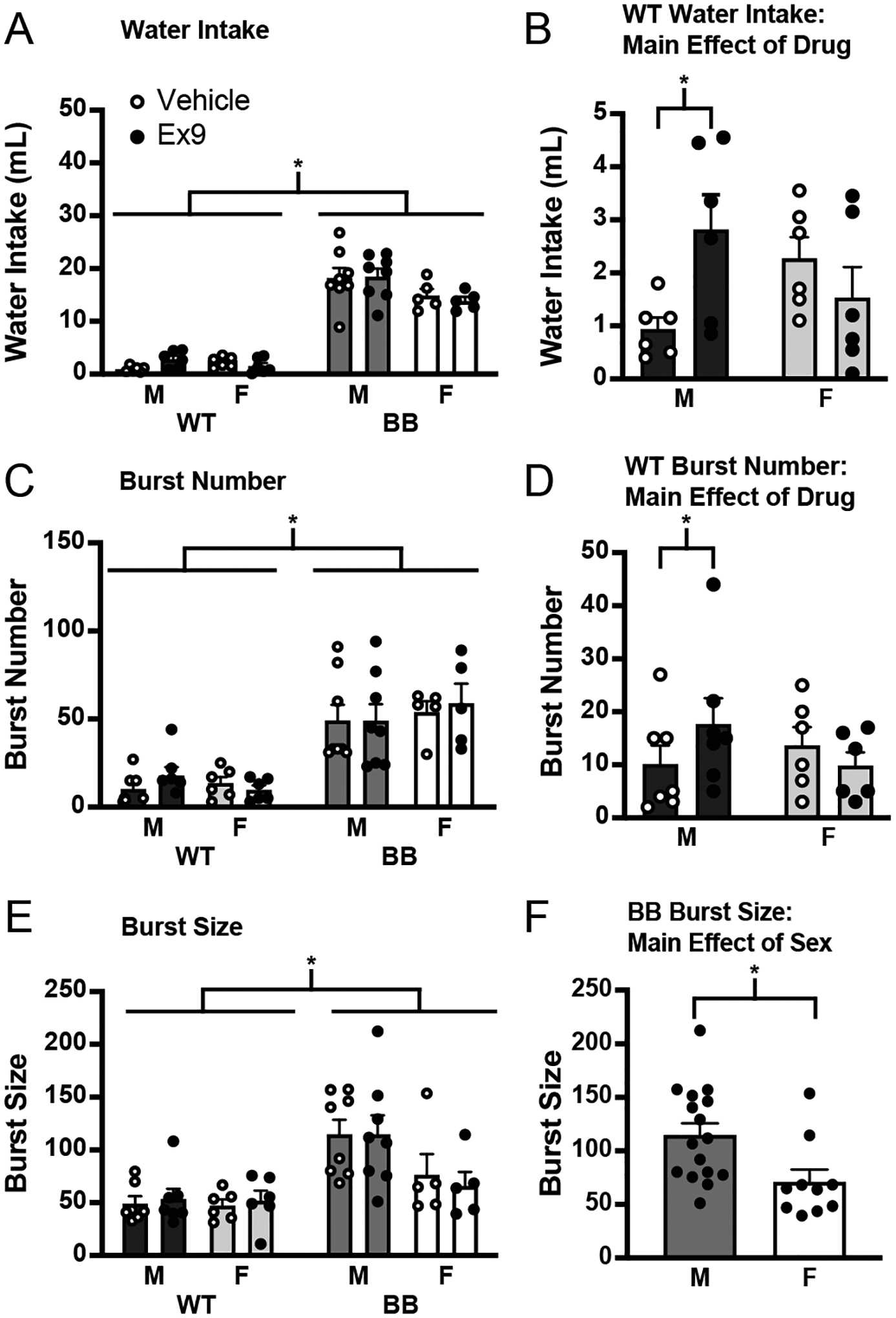
The GLP-1R antagonist, administered during the light phase via an indwelling cannula aimed at the LV. A) There was a main effect of genotype on water intake after Ex9. B) When not otherwise stimulated to drink, an injection of Ex9 caused an increase in drinking, but only in male wildtype rats (y-axis changed to illustrate effect). C) There was a significant effect of genotype on burst number during the Ex9 test. D) There was a significant increase in burst number in wildtype male rats given Ex9 (y-axis changed to illustrate effect). E) There was a significant difference between the size of the licking bursts between genotypes, but there were no changes in burst size in response to Ex9 injection. F) There was a main effect of sex on burst size in Brattleboro rats. Asterisk indicates significant difference, p ≤ 0.05. Abbreviations used: Male, M; Female, F; Wildtype, WT; Brattleboro, BB.
Drinking microstructure was examined for the duration of the test and this analysis found a significant difference in burst number between the genotypes (Figure 6C; F1,22 = 42.63, p < 0.001). When the genotypes were analyzed separately, there was a significant drug*sex interaction in wildtype rats (F1,11 = 10.13, p = 0.009) with a post hoc test revealing a significant Ex9-associated increase in burst number in wildtype male rats (p = 0.031), but no change was observed in wildtype female rats (p = 0.323). In Brattleboro rats, there was no effect of sex (F1,11 = 0.42, p = 0.530) or Ex9 on burst number (F1,12 = 0.07, p = 0.799). Similar to Experiment 2, there was a difference in burst size between the genotypes (F1,22 = 19.34, p < 0.001) with Brattleboro rats having a significantly higher burst size than wildtype rats. When the genotypes were analyzed separately, there was no effect of sex (F1,11 = 0.06, p = 0.812) or drug (F1,12 = 0.25, p = 0.624) in wildtype rats. In Brattleboro rats, there was a main effect of sex (F1,11 = 5.79, p = 0.004), but no effect of drug (F1,11 = 0.11, p = 0.748) on burst size. Thus, in wildtype male rats there was an increase in water intake after injection of Ex9 that was driven largely by an increase in burst number.
3.4. Experiment 4: Effect of central administration of Ex9 on the drinking response to subcutaneous hypertonic saline
To address the possibility of a ceiling effect preventing the observation of an Ex9-induced increase in water intake by Brattleboro rats in Experiment 3, we repeated the experiment, but rats were stimulated to drink by an injection of hypertonic saline immediately after injection of either vehicle or the GLP-1R antagonist. Because the experiments were conducted separately, the results from Experiments 3 and 4 were not directly/statistically compared, but the results of this experiment suggest that increased intake is possible in Brattleboro rats, reducing the likelihood that the lack of an effect of Ex9 observed in Experiment 3 was because of a ceiling effect. Analysis of the data using an omnibus ANOVA found a significant effect of genotype in the amount of water consumed after the injection of Ex9 in rats given hypertonic saline (Figure 7A; F1,20 = 898.13, p < 0.001). When wildtype rats were analyzed separately, there was a main effect of sex (Figure 7B; F1,12 = 14.80, p = 0.002) and of drug (Figure 7C; F1,12 = 6.09, p = 0.030), but there was no drug*sex interaction (F1,12 = 1.81, p = 0.204). Because the interaction did not reach the threshold for significance, a post hoc analysis was not conducted, but the effect of drug seems to be driven by the difference in intake in wildtype male rats. In Brattleboro rats, however, there was no effect of sex (F1,8 = 1.42, p = 0.267) or drug (F1,9 = 0.07, p = 0.791) on water intake.
Figure 7.
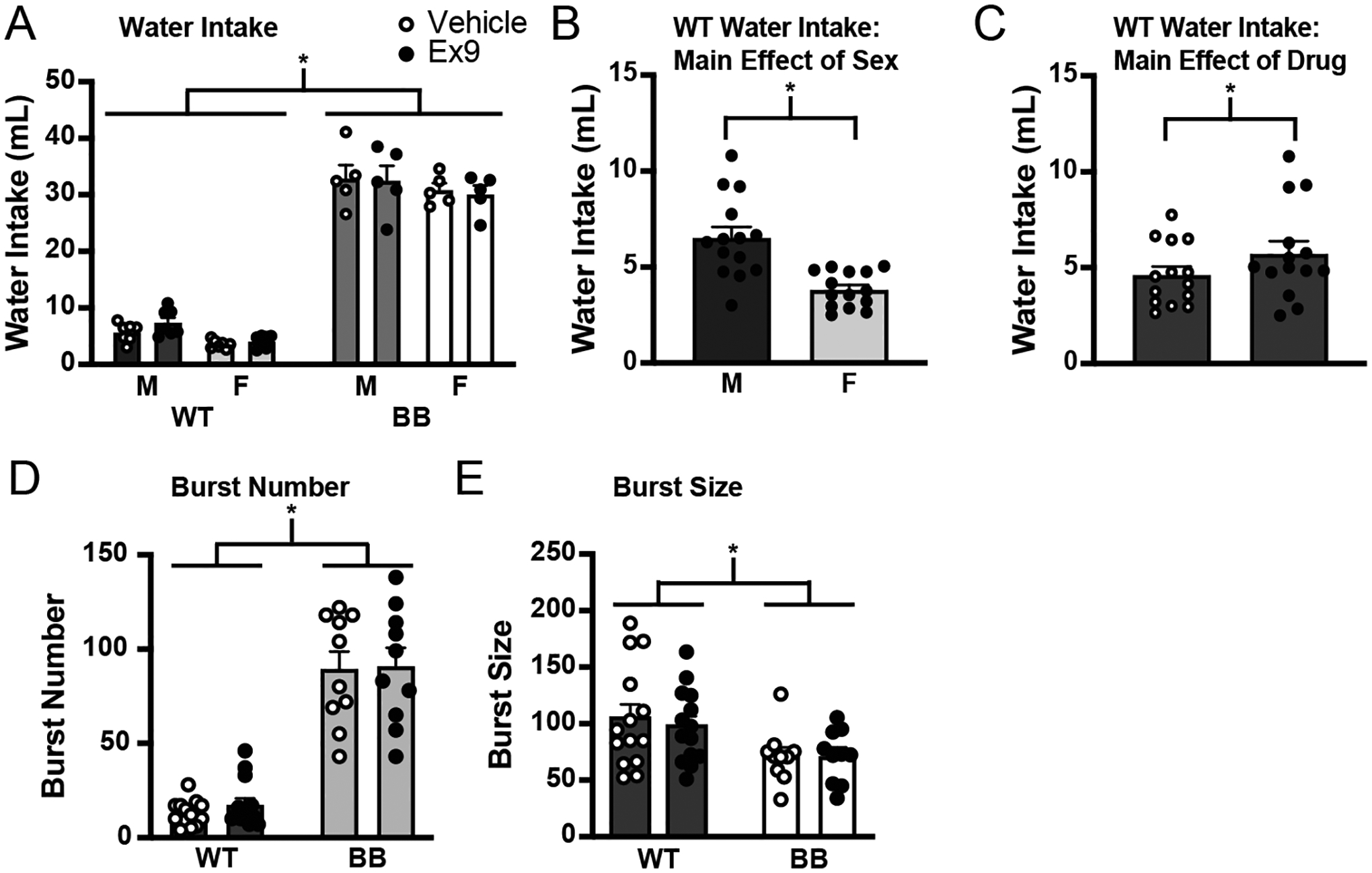
The GLP-1R antagonist, as well as a subcutaneous injection of hypertonic saline to further stimulate drinking, administered during lights on via an indwelling cannula aimed at the LV. A) There was a main effect of genotype on drinking. B) When the genotypes were analyzed separately, there was a significant difference in the amount of water consumed between male and female wildtype rats (y-axis changed to illustrate effect). C). There was a main effect of Ex9 on drinking in wildtype rats. This effect seemed to be driven by male wildtype rats (y-axis changed to illustrate effect). D) There was a main effect of genotype on burst number, but there were no drug-induced differences in burst number in any group. E) There was a main effect of genotype on burst size, but there were no drug-induced differences in burst size in any group. Asterisks indicate significant differences, p ≤ 0.05. Abbreviations used: Male, M; Female, F; Wildtype, WT; Brattleboro, BB.
Analysis of the drinking microstructure for the duration of the test found that Brattleboro rats had a significantly greater number of licking bursts than wildtype rats (Figure 7D; F1,20 = 111.99, p < 0.001). Although there was a significant drug*genotype*sex interaction (F1,20 = 4.69, p = 0.04), post hoc tests did not find noteworthy differences. When the genotypes were analyzed separately, there was no effect of sex or drug in wildtype rats (sex, F1,12 = 2.76, p = 0.123; drug, F1,13 = 3.40, p = 0.088) or in Brattleboro rats (sex, F1,8 = 0.46, p = 0.518; drug, F1,9 = 0.02, p = 0.902). Similar to the analysis of burst number, we found a significant difference in burst size between the two genotypes (Figure 7E; F1,20 = 6.37, p = 0.020). When the genotypes were analyzed separately there was no effect of sex (wildtype, F1,12 = 0.59, p = 0.458; Brattleboro, F1,8 = 1.63, p = 0.237) or drug (wildtype rats, F1,13 = 0.40, p = 0.536; Brattleboro rats, F1,9 = 0.00, p = 0.999) in either genotype.
4. Discussion
These experiments suggest that the Brattleboro rat can serve as a fruitful model for the disassociation of fluid and food intakes, especially with respect to control by GLP-1. The experiments show, for the first time, that Brattleboro rats were hypersensitive to GLP-1R agonist treatment. It is especially striking that this hypersensitivity affected water intake, but not food intake, because of the critical need for water intake by Brattleboro rats. Moreover, Brattleboro rats had no hyperdipsia after GLP-1R antagonism. Collectively, these data suggest there are differences in GLP-1 production, GLP-1 release, or GLP-1R expression between wildtype and Brattleboro rats. Identifying these differences may help determine which parts of the GLP-1 system are specific to fluid intake without having any direct control of food intake. Elucidating these differences will help us gain a better understanding of the roles played by GLP-1 in the control of ingestive behaviors.
Findings from the current study showing the Brattleboro rats’ hypersensitivity to the fluid intake suppressive effects of the GLP-1R agonist with normal food intake suppression adds to this growing body of literature on the separability of GLP-1’s control of food and fluid intakes. Previous studies have shown that maintenance on a high fat diet alters the feeding effects of Ex4 [49], but has no effect on Ex4-induced suppression of fluid intake [50]. Additionally, food intake was associated with changes in plasma GLP-1 levels that were not observed after drinking, but either eating or drinking caused changes in central proglucagon mRNA [5], suggesting that feeding-related GLP-1 has both central and peripheral effects, whereas drinking-related GLP-1 is exclusively central. Collectively, these studies are consistent with other reports separating peripheral and central GLP-1 systems [50, 51] and additionally show that the feeding and drinking effects of GLP-1 are separable. This is an important finding because GLP-1 has been shown to reduce a wide variety of motivated behaviors, but whether it does so selectively or not, and if it does so through different sites of action, remains unknown.
The model described here has the potential to reframe and advance our understanding of fluid intake satiation. Indeed, satiation of fluid intake is poorly understood. This may be because the field has, for too long, attempted to fit fluid intake satiation into the framework that describes food intake satiation. If there are considerable differences in these forms of satiation, however, the erroneous application of the feeding-related framework to drinking could prevent a better understanding of fluid intake satiation. When evaluating licking patterns during intake of caloric substances, the classic view is that licking microstructure can be dichotomized into oral and post-oral feedback, which are related, respectively, to burst size and number [34, 35]. Because these differences in licking microstructure also map onto differences in taste and satiety, this framework places the oral cavity in the taste (hedonics) category and post-oral systems in the satiety category. But more recent advances in the study of fluid intake, along with often overlooked earlier findings, provide evidence that fluid intake satiation is both oral and post-oral, making us reconsider what now appears to be a false dichotomy in our earlier conceptualization [52–56]. This is important because there is growing evidence that water itself is the satiating factor [57]. Indeed, the act of drinking causes rapid suppression of dehydration-induced neural activity in the hypothalamus [58], and human studies have shown that sensation of thirst and desire to drink is quenched by drinking long before the water has a chance to enter the bloodstream and reverse the deficit that caused the thirst in the first place [59]. If water in the mouth quenches thirst, how does the Brattleboro rat drink copious amounts of water without that water causing satiation? The answer to this question could provide important information about the underlying mechanism of fluid intake satiety because it seems plausible to conclude that Brattleboro rats a) lack the system that water acts on to cause satiation, b) have a system that is relatively insensitive to water, or c) are somehow able to override the satiety signals from water that normally terminate intake. Accordingly, studies already underway in the laboratory that evaluate differences in the brains of Brattleboro and wildtype rats could provide a path toward discovering the key neural circuits that underlie fluid intake satiety.
In addition to the studies that pharmacologically stimulated GLP-1R, we also tested the effect of GLP-1R antagonism. The results of these experiments will help guide future studies aimed at elucidating differences between wildtype and Brattleboro rats. Our lab found that male Sprague Dawley rats drank more water after injection of the GLP-1R antagonist, Ex9, and that the effect was primarily a function of burst number [5]. The current study replicated this finding in wildtype male rats, and extended the results to include a test of Brattleboro rats and female wildtype rats. Interestingly, the antagonist-induced increase in drinking that was found in male rats was not present in female wildtype rats or Brattleboro rats of either sex. This might suggest that GLP-1R is less responsive or expressed at lower levels, but it is difficult to reconcile this with the exaggerated response to the GLP-1R agonist in these rats. This might suggest differences in GLP-1 production or release between male and female, and between wildtype and Brattleboro rats. Indeed, GLP-1 producing and GLP-1R expressing neurons have been mapped in the male [60, 61] and female [12, 61] rat brain, but a thorough comparison between the two has not been reported. The present findings demonstrate the need for a systematic study testing for sex differences in the GLP-1 system, and indicate that a thorough comparison of Brattleboro and wildtype rats could be particularly helpful in understanding which, if any, sex differences contribute to the observed behavioral differences.
When considering possible sex differences in the response to GLP-1, it is important to include the role of the estrous cycle. Indeed, estradiol has a well-documented effect on ingestive behaviors [41]. Female Brattleboro rats have a more variable cycle [36], but we are not aware of any studies directly measuring estradiol levels across the cycle in Brattleboro rats. In the present studies, we found that food intake was a function of the estrous cycle in both wildtype and Brattleboro rats. Interestingly, we did not find any estrous cycle-related changes in fluid intake in Brattleboro rats. This makes it tempting to speculate that fluid intake is more sensitive than food intake to cycle perturbations. Indeed, food intake cyclicity was found to be intact in Brattleboro rats, even with the documented disruption in the estrous cycle, but the disruption in the estrous cycle was apparently sufficient to prevent cyclic changes in fluid intake. Thus, these rats could be a helpful tool to provide further insight into estradiol’s role in the control of water intake.
The relationship between the estrous cycle and intake is an important factor when considering sex differences in ingestive behaviors. In this respect, it is noteworthy that previous studies found an interaction between GLP-1 and estradiol in food intake. Specifically, rats given GLP-1 and estradiol ate less than rats given GLP-1 alone, and the effect of GLP-1 lasted longer in estradiol-treated rats than is typically seen in male rats [62]. Although the present studies did not manipulate estradiol, we made a direct comparison between male and female rats and found a main effect of sex on food intake in both wildtype and Brattleboro rats. Further analysis of the data, however, suggests that body weight may be more directly responsible for the effect because when food intake was normalized by body weight, the sex differences were no longer detected by the ANOVA. Whether or not body weight was responsible for the differences observed in Maske et al. [62] remains an empirical question, but the authors explicitly noted the difference in body weight between the oil- and estradiol-treated groups. This effect of estradiol on body weight is well documented in female rats [43, 63–69]. Accordingly, additional studies to tease apart the roles of estradiol and of body weight on food intake suppression by GLP-1 in both laboratory animal and human subjects are needed.
In spite of decades of work characterizing the Brattleboro rat, the present experiments extend this work and force the reconsideration of our current understanding of these rats. Specifically, the prevailing view is that Brattleboro rats have normal thirst responses, and that the observed polydipsia is entirely secondary to the polyuria [40]. Given the difference in the response to GLP-1R agonist treatment in these rats, it calls other results into question. Brattleboro rats, for instance, have elevated plasma renin and angiotensin II, and relatively low levels of aldosterone and corticosterone [70]. Although it was previously believed that this was entirely a consequence of the polyuria, it may reflect longer-term adaptations in these rats that allow them to continue to drink in spite of an abundance of what would otherwise be satiating water intake. Moreover, the reported differences in responses to dipsogenic treatments [40] may reflect adaptations that would impact thirst responses even if the polyuria were resolved. Although this possibility presents an exciting research direction, it is purely speculative at this point. Indeed, the present studies did not measure urine output. This limitation prevents us from drawing firm conclusions about any more direct effects on diuresis (or antidiuresis) on the observed changes in fluid intake in Brattleboro rats. Similarly, the studies do not directly address or rule out any interactions with vasopressin, which is absent in Brattleboro rats, on the effect of Ex4. We find these potential interactions to be an unlikely explanation for the observed differences in wildtype and Brattleboro rats because of the direction of the difference. Interactions between GLP-1 and vasopressin have been identified. Central injections of GLP-1 increase plasma vasopressin [9, 71] (but peripheral injections have the opposite effect [72]). There is colocalization of GLP-1R with vasopressin mRNA in the paraventricular and supraoptic nuclei of the hypothalamus [72], and GLP-1 immunoreactive terminals have been observed in the paraventricular hypothalamus, but very few of these terminals were found to be in apposition to vasopressinergic neurons [73]. These interactions appear inconsistent with the results observed in the present study and in other studies of GLP-1 and drinking. For instance, GLP-1R agonists decrease fluid intake when injected centrally or peripherally, but, as stated above, central and peripheral injections of GLP-1 have opposite effects on peripheral vasopressin. This seems to rule out an effect of peripheral vasopressin on the effects of GLP-1R agonists. Central vasopressin may not have the same response to GLP-1 as does peripheral vasopressin, but central vasopressin does not acutely affect fluid intake [74]. Moreover, any effect of vasopressin in the periphery would likely be antidipsogenic, secondary to the antidiuresis causing retention of fluid and suppression of thirst. In the absence of this, one would predict more drinking, not less, after GLP-1 treatment in Brattleboro rats. This is not what was observed in the present studies. Accordingly, we are more confident that the observed results are because of a hypersensitivity to the GLP-1R agonist, and not directly because of the lack of vasopressin.
In summary, the present report describes foundational work on Brattleboro rats and the response to GLP-1 in this rodent model. These findings provide a novel path toward elucidating central mechanisms that control food and fluid intakes. Separating how GLP-1 controls various functions is instrumental in furthering our understanding of these basic life processes, as well as refining current and discovering new pharmacological interventions.
Highlights.
GLP-1R agonist caused equal suppression of eating in wildtype and Brattleboro rats
Brattleboro rats were hyperresponsive to the drinking suppression by GLP-1R agonist
There were sex and genotype differences in GLP-1R antagonist response
Female Brattleboro rats had wildtype-like cycle-associated changes in food intake
Estrous cycle had no effect on fluid intake in Brattleboro rats
Acknowledgments
Dr. K. Linnea Volcko, Quinn Carroll, Gaayathri Varavenkataraman, and Sydney David provided valuable technical assistance. This work was supported by the National Institutes of Health awards R01DK107500 (DD) and R01DK133818 (DD) and by a grant from the National Science Foundation (IOS-1754878) to MJP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: The authors have no competing interests and no conflicts nothing to disclose.
References
- [1].Kissileff HR Food-associated drinking in the rat. J Comp Physiol Psychol. 1969,67:284–300. [DOI] [PubMed] [Google Scholar]
- [2].Fitzsimons TJ, Le Magnen J Eating as a regulatory control of drinking in the rat. J Comp Physiol Psychol. 1969,67:273–83. [DOI] [PubMed] [Google Scholar]
- [3].Mietlicki EG, Daniels D Ghrelin reduces hypertonic saline intake in a variety of natriorexigenic conditions. Exp Physiol. 2011,96:1072–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mietlicki EG, Nowak EL, Daniels D The effect of ghrelin on water intake during dipsogenic conditions. Physiol Behav. 2009,96:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McKay NJ, Galante DL, Daniels D Endogenous glucagon-like Peptide-1 reduces drinking behavior and is differentially engaged by water and food intakes in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014,34:16417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McKay NJ, Daniels D Glucagon-like Peptide-1 receptor agonist administration suppresses both water and saline intake in rats. Journal of neuroendocrinology. 2013,25:929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McKay NJ, Kanoski SE, Hayes MR, Daniels D Glucagon-like peptide-1 receptor agonists suppress water intake independent of effects on food intake. Am J Physiol Regul Integr Comp Physiol. 2011,301:R1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Navarro M, Rodriquez de Fonseca F, Alvarez E, Chowen JA, Zueco JA, Gomez R, et al. Colocalization of glucagon-like peptide-1 (GLP-1) receptors, glucose transporter GLUT-2, and glucokinase mRNAs in rat hypothalamic cells: evidence for a role of GLP-1 receptor agonists as an inhibitory signal for food and water intake. Journal of neurochemistry. 1996,67:1982–91. [DOI] [PubMed] [Google Scholar]
- [9].Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, et al. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. The American journal of physiology. 1996,271:R848–56. [DOI] [PubMed] [Google Scholar]
- [10].Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity (Silver Spring). 2011,19:1342–9. [DOI] [PubMed] [Google Scholar]
- [11].Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997,77:257–70. [DOI] [PubMed] [Google Scholar]
- [12].Merchenthaler I, Lane M, Shughrue P Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999,403:261–80. [DOI] [PubMed] [Google Scholar]
- [13].Mietlicki-Baase EG, McGrath LE, Koch-Laskowski K, Krawczyk J, Pham T, Lhamo R, et al. Hindbrain DPP-IV inhibition improves glycemic control and promotes negative energy balance. Physiol Behav. 2017,173:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang T, Edwards GL, Baile CA Glucagon-like peptide-1 (7–36) amide administered into the third cerebroventricle inhibits water intake in rats. Proc Soc Exp Biol Med. 1998,219:85–91. [DOI] [PubMed] [Google Scholar]
- [15].Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 2011,13:320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V Peptides that regulate food intake: glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2003,284:R1427–35. [DOI] [PubMed] [Google Scholar]
- [17].Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996,379:69–72. [DOI] [PubMed] [Google Scholar]
- [18].Williams DL, Baskin DG, Schwartz MW Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009,150:1680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Barrera JG, Jones KR, Herman JP, D’Alessio DA, Woods SC, Seeley RJ Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011,31:3904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Holt MK, Richards JE, Cook DR, Brierley DI, Williams DL, Reimann F, et al. Preproglucagon Neurons in the Nucleus of the Solitary Tract Are the Main Source of Brain GLP-1, Mediate Stress-Induced Hypophagia, and Limit Unusually Large Intakes of Food. Diabetes. 2019,68:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gutzwiller JP, Hruz P, Huber AR, Hamel C, Zehnder C, Drewe J, et al. Glucagon-like peptide-1 is involved in sodium and water homeostasis in humans. Digestion. 2006,73:142–50. [DOI] [PubMed] [Google Scholar]
- [22].Hawkins RC Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta. 2003,337:169–72. [DOI] [PubMed] [Google Scholar]
- [23].National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States. In: U.S. Department of Health and Human Services CfDCaP, editor. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2017. [Google Scholar]
- [24].Laycock JF Review: the Brattleboro rat with hereditary hypothalamic diabetes insipidus as an ideal experimental model. Lab Anim. 1976,10:261–70. [DOI] [PubMed] [Google Scholar]
- [25].Schmale H, Richter D Single base deletion in the vasopressin gene is the cause of diabetes insipidus in Brattleboro rats. Nature. 1984,308:705–9. [DOI] [PubMed] [Google Scholar]
- [26].Saul GB 2nd, Garrity EB, Benirschke K, Valtin H Inherited hypothalamic diabetes insipidus in the Brattleboro strain of rats. J Hered. 1968,59:113–7. [DOI] [PubMed] [Google Scholar]
- [27].Burlet A, Desor D, Max JP, Nicolas JP, Krafft B, Burlet C Ingestive behaviors of the rat deficient in vasopressin synthesis (Brattleboro strain). Effect of chronic treatment by dDAVP. Physiol Behav. 1990,48:813–9. [DOI] [PubMed] [Google Scholar]
- [28].Paul MJ, Peters NV, Holder MK, Kim AM, Whylings J, Terranova JI, et al. Atypical Social Development in Vasopressin-Deficient Brattleboro Rats. eNeuro. 2016,3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schatz KC, Martin CD, Ishiwari K, George AM, Richards JB, Paul MJ Mutation in the vasopressin gene eliminates the sex difference in social reinforcement in adolescent rats. Physiol Behav. 2019,206:125–33. [DOI] [PubMed] [Google Scholar]
- [30].Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005,146:1650–73. [DOI] [PubMed] [Google Scholar]
- [31].Santollo J, Torregrossa AM, Daniels D Sex differences in the drinking response to angiotensin II (AngII): Effect of body weight. Horm Behav. 2017,93:128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Asarian L, Geary N Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol. 2013,305:R1215–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Santollo J, Eckel LA The orexigenic effect of melanin-concentrating hormone (MCH) is influenced by sex and stage of the estrous cycle. Physiol Behav. 2008,93:842–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Davis JD The microstructure of ingestive behavior. Ann N Y Acad Sci. 1989,575:106–21. [DOI] [PubMed] [Google Scholar]
- [35].Davis JD, Smith GP, Singh B A microstructural analysis of the control of water and isotonic saline ingestion by postingestional stimulation. Physiol Behav. 1999,66:543–8. [DOI] [PubMed] [Google Scholar]
- [36].Boer K, Boer GJ, Swaab DF Reproduction in Brattleboro rats with diabetes insipidus. J Reprod Fertil. 1981,61:273–80. [DOI] [PubMed] [Google Scholar]
- [37].Feifel D, Mexal S, Melendez G, Liu PY, Goldenberg JR, Shilling PD The brattleboro rat displays a natural deficit in social discrimination that is restored by clozapine and a neurotensin analog. Neuropsychopharmacology. 2009,34:2011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fodor A, Kovacs KB, Balazsfi D, Klausz B, Pinter O, Demeter K, et al. Depressive- and anxiety-like behaviors and stress-related neuronal activation in vasopressin-deficient female Brattleboro rats. Physiol Behav. 2016,158:100–11. [DOI] [PubMed] [Google Scholar]
- [39].Murphy HM, Wideman CH Self-starvation and activity stress in Brattleboro rats. Physiological Psychology. 1983,11:209–13. [Google Scholar]
- [40].Fuller LM, Fitzsimons JT Thirst in Brattleboro rats. The American journal of physiology. 1988,255:R217–25. [DOI] [PubMed] [Google Scholar]
- [41].Asarian L, Geary N Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006,361:1251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Drewett RF Oestrous and dioestrous components of the ovarian inhibition on hunger in the rat. Anim Behav. 1973,21:772–80. [DOI] [PubMed] [Google Scholar]
- [43].Blaustein JD, Wade GN Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976,17:201–8. [DOI] [PubMed] [Google Scholar]
- [44].Eckel LA, Houpt TA, Geary N Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav. 2000,70:397–405. [DOI] [PubMed] [Google Scholar]
- [45].Findlay AL, Fitzsimons JT, Kucharczyk J Dependence of spontaneous and angiotensin-induced drinking in the rat upon the oestrous cycle and ovarian hormones. J Endocrinol. 1979,82:215–25. [DOI] [PubMed] [Google Scholar]
- [46].Czaja JA, Butera PC, McCaffrey TA Independent effects of estradiol on water and food intake. Behav Neurosci. 1983,97:210–20. [DOI] [PubMed] [Google Scholar]
- [47].Hayes MR, De Jonghe BC, Kanoski SE Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav. 2010,100:503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Meeran K, O’Shea D, Edwards CM, Turton MD, Heath MM, Gunn I, et al. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7–36) amide or exendin-(9–39) alters body weight in the rat. Endocrinology. 1999,140:244–50. [DOI] [PubMed] [Google Scholar]
- [49].Williams DL, Hyvarinen N, Lilly N, Kay K, Dossat A, Parise E, et al. Maintenance on a high-fat diet impairs the anorexic response to glucagon-like-peptide-1 receptor activation. Physiol Behav. 2011,103:557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Volcko KL, Carroll QE, Brakey DJ, Daniels D High-fat diet alters fluid intake without reducing sensitivity to glucagon-like peptide-1 receptor agonist effects. Physiol Behav. 2020,221:112910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Brierley DI, Holt MK, Singh A, de Araujo A, McDougle M, Vergara M, et al. Central and peripheral GLP-1 systems independently suppress eating. Nat Metab. 2021,3:258–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hall WG A remote stomach clamp to evaluate oral and gastric controls of drinking in the rat. Physiol Behav. 1973,11:897–901. [DOI] [PubMed] [Google Scholar]
- [53].Ichiki T, Wang T, Kennedy A, Pool AH, Ebisu H, Anderson DJ, et al. Sensory representation and detection mechanisms of gut osmolality change. Nature. 2022,602:468–74. [DOI] [PubMed] [Google Scholar]
- [54].Miller NE, Sampliner RI, Woodrow P Thirst-reducing effects of water by stomach fistula vs. water by mouth measured by both a consummatory and an instrumental response. J Comp Physiol Psychol. 1957,50:1–5. [DOI] [PubMed] [Google Scholar]
- [55].Zimmerman CA, Huey EL, Ahn JS, Beutler LR, Tan CL, Kosar S, et al. A gut-to-brain signal of fluid osmolarity controls thirst satiation. Nature. 2019,568:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zimmerman CA, Lin YC, Leib DE, Guo L, Huey EL, Daly GE, et al. Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature. 2016,537:680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Augustine V, Gokce SK, Lee S, Wang B, Davidson TJ, Reimann F, et al. Hierarchical neural architecture underlying thirst regulation. Nature. 2018,555:204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mandelblat-Cerf Y, Kim A, Burgess CR, Subramanian S, Tannous BA, Lowell BB, et al. Bidirectional Anticipation of Future Osmotic Challenges by Vasopressin Neurons. Neuron. 2017,93:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rolls BJ, Wood RJ, Rolls ET, Lind H, Lind W, Ledingham JG Thirst following water deprivation in humans. The American journal of physiology. 1980,239:R476–82. [DOI] [PubMed] [Google Scholar]
- [60].Gu G, Roland B, Tomaselli K, Dolman CS, Lowe C, Heilig JS Glucagon-like peptide-1 in the rat brain: distribution of expression and functional implication. J Comp Neurol. 2013,521:2235–61. [DOI] [PubMed] [Google Scholar]
- [61].Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J Comp Neurol. 1988,271:519–32. [DOI] [PubMed] [Google Scholar]
- [62].Maske CB, Jackson CM, Terrill SJ, Eckel LA, Williams DL Estradiol modulates the anorexic response to central glucagon-like peptide 1. Horm Behav. 2017,93:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Santollo J, Edwards AA, Howell JA, Myers KE Bidirectional effects of estradiol on the control of water intake in female rats. Horm Behav. 2021,133:104996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].McElroy JF, Wade GN Short- and long-term effects of ovariectomy on food intake, body weight, carcass composition, and brown adipose tissue in rats. Physiol Behav. 1987,39:361–5. [DOI] [PubMed] [Google Scholar]
- [65].Mook DG, Kenney NJ, Roberts S, Nussbaum AI, Rodier WI Ovarian-adrenal interactions in regulation of body weight by female rats. J Comp Physiol Psychol. 1972,81:198–211. [DOI] [PubMed] [Google Scholar]
- [66].Tarttelin MF, Gorski RA Variations in food and water intake in the normal and acyclic female rat. Physiol Behav. 1971,7:847–52. [DOI] [PubMed] [Google Scholar]
- [67].Varma M, Chai JK, Meguid MM, Laviano A, Gleason JR, Yang ZJ, et al. Effect of estradiol and progesterone on daily rhythm in food intake and feeding patterns in Fischer rats. Physiol Behav. 1999,68:99–107. [DOI] [PubMed] [Google Scholar]
- [68].Witte MM, Resuehr D, Chandler AR, Mehle AK, Overton JM Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen Comp Endocrinol. 2010,166:520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wade GN Some effects of ovarian hormones on food intake and body weight in female rats. J Comp Physiol Psychol. 1975,88:183–93. [DOI] [PubMed] [Google Scholar]
- [70].Sokol HW, Zimmerman EA The hormonal status of the Brattleboro rat. Ann N Y Acad Sci. 1982,394:535–48. [DOI] [PubMed] [Google Scholar]
- [71].Larsen PJ, Tang-Christensen M, Jessop DS Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997,138:4445–55. [DOI] [PubMed] [Google Scholar]
- [72].Zueco JA, Esquifino AI, Chowen JA, Alvarez E, Castrillon PO, Blazquez E Coexpression of glucagon-like peptide-1 (GLP-1) receptor, vasopressin, and oxytocin mRNAs in neurons of the rat hypothalamic supraoptic and paraventricular nuclei: effect of GLP-1(7–36)amide on vasopressin and oxytocin release. Journal of neurochemistry. 1999,72:10–6. [DOI] [PubMed] [Google Scholar]
- [73].Tauchi M, Zhang R, D’Alessio DA, Stern JE, Herman JP Distribution of glucagon-like peptide-1 immunoreactivity in the hypothalamic paraventricular and supraoptic nuclei. J Chem Neuroanat. 2008,36:144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Epstein AN, Fitzsimons JT, Rolls BJ Drinking induced by injection of angiotensin into the rain of the rat. J Physiol. 1970,210:457–74. [DOI] [PMC free article] [PubMed] [Google Scholar]


