Abstract
Purpose:
In tuberculosis (TB)-endemic areas, lymphadenopathy is frequently due to TB adenitis, but lymphoma and cancers are important differential diagnoses and critical to diagnose at the earliest opportunity. Key obstacles to lymphoma diagnosis include empiric TB treatment and difficulty accessing a biopsy. We report on a specialized clinic utilizing high-yield investigations for patients with lymphadenopathy.
Methods:
This prospective interventional study investigated the utility of a core biopsy and the Xpert MTB/RIF Ultra (Ultra) on fine-needle aspirate (FNA) and tissue in a newly established lymph node biopsy clinic over 4 years. Electronic referral facilitated patient assessment within a week. Hematology fellows without specialist surgical or radiological expertise performed the biopsy on the first visit.
Results:
In 277 patients, including 43% people with HIV, TB was the most frequent diagnosis (34%), followed by lymphoma (27%) and other cancers (17%). Patients were seen a median of 5 days (IQR 2–8.5 days) from referral. Core biopsy provided sufficient tissue for diagnosis in 96% of patients with lymphoma (72/75) and 94% of patients with cancer (44/47). FNA Ultra had a sensitivity of 73.9% (34/46; 95% CI 58.9–85.7), and tissue Ultra 73% (46/63; 95% CI 60.3–83.4). There were 6 false positive Ultra tests, highlighting the value of histology to either support TB or make an alternative diagnosis.
Conclusion:
Core biopsies collected under the conditions described are safe and sensitive and can yield a rapid diagnosis. Combining Ultra and a core biopsy can accurately diagnose TB and cancer. This clinic provides an implementation model for resource-constrained and TB-endemic areas.
Keywords: lymphadenopathy, lymphoma diagnosis, core biopsy, tuberculosis, people with HIV, cancer
Introduction
In tuberculosis (TB)-endemic areas, patients with lymphadenopathy and constitutional symptoms represent a vulnerable group likely to have TB adenitis, lymphoma, or other cancers[1]. People with HIV (PWH) have a markedly elevated risk for both extrapulmonary tuberculosis (EPTB) and lymphoma[2, 3] and the lymph node is the most common site for EPTB involvement[3, 4]. Retrospective observational studies from Africa show that up to 85% of patients with lymphoma are initially misdiagnosed with TB, resulting in delayed diagnosis with devastating clinical effect[5, 6]. Understanding the problem in context requires one to appreciate the sheer size of the TB epidemic, with its attendant physician diagnostic bias toward the disease; the historically poor performance of tests in the paucibacillary EPTB setting, resulting in the practice of ‘empiric/presumptive’ TB therapy; and regional difficulties in accessing specialist surgical services for lymph node biopsies. Urgent and specific regional interventions, including focused clinics utilizing the most sensitive and specific tests, are required to improve the diagnosis of EPTB, cancer, and lymphoma among patients with lymphadenopathy.
Cancer is the leading cause of death among PWH in high-income countries, and lymphoma remains a leading cancer cause of death even among patients treated with antiretroviral therapy (ART)[7, 8]. For PWH, the risk of non-Hodgkin lymphoma is increased 12-fold, and the risk of Hodgkin lymphoma 8-fold, even with ART[9]. Patients in Sub-Saharan Africa are diagnosed with lymphoma at a late stage: 75% are already at an advanced stage of non-Hodgkin lymphoma at diagnosis[10]. Obtaining a representative diagnostic biopsy is critical in diagnosing lymphoma and other cancers. While excision biopsy has been the preferred method in diagnosing lymphoma, it requires surgical skill and access to appropriate facilities. In contrast, a core biopsy taken from the lymph node is a minimally invasive technique capable of identifying the etiology of lymphadenopathy, including in PWH[1]. However, core biopsies are typically performed by specialized radiologists or surgeons, and access to these services may be limited.
Significant advances in molecular TB diagnostic techniques based on real-time polymerase (PRC) chain reactions, for instance the Xpert MTB/Rif assay, have revolutionized the diagnosis of pulmonary TB. In EPTB, however, the sensitivity of the Xpert has been low owing to the disease’s paucibacillary nature[11]. The next-generation Xpert MTB/RIF Ultra (Ultra) has a >5-fold lower limit of detection than the previous Xpert assay (it can detect 16 colony-forming units(cfu)/ml compared to 114cfu/ml detected using Xpert)[12]. Several recent publications have reported its sensitivity for TB adenitis at 68%–82%; and its specificity at 96%–100%[13–15].
We performed an interventional study to assess the diagnostic utility of focused tests on lymph node tissue for TB, lymphoma, other cancers and other conditions causing lymphadenopathy. We introduced two interventions: an electronic rapid-access referral method aiming to get appointments within one week of referral; and a core biopsy or FNA performed by a physician at the first appointment. Patients were seen in the Hematology clinic and those with lymphoma or cancer were directly linked to appropriate care at the same institution. The diagnostic utility of the core biopsy and the TB tests, including the Ultra on FNA and lymph node tissue were assessed over four years.
Methods
The clinic was established in November 2017, in the Division of Clinical Haematology at the University of Cape Town (UCT) and Groote Schuur Hospital, a tertiary referral academic center in Cape Town, South Africa. Eligible study participants were >13 years old, inpatients or outpatients, referred with lymphadenopathy of >2 cm diameter located in the cervical, axillary, or inguinal regions. Patients were referred to Groote Schuur from secondary-level hospitals and primary-care day clinics in the referral area. An electronic referral system including a short survey, which could be completed on a mobile phone, were developed and used to collect patient demographic and clinical data on a secure data platform (REDCap). Referral data obtained included patients’ demographic details, HIV status, and the results of any prior TB investigations. The survey questions included their favored diagnosis and how long it would ordinarily take to obtain a lymph node biopsy via their hospital’s lymph node excision clinic. We excluded patients with contraindications to core needle biopsy. Written informed consent was obtained from all participants. The Human Research Ethics Committee of UCT’s Faculty of Health Sciences approved the study.
At enrolment, we recorded symptoms, symptom duration, and prior test results for HIV and TB. We graded performance status according to the ECOG score[16]. We examined the patients for lymphadenopathy and hepatosplenomegaly and performed a chest examination. Blood was taken for a full blood count, lactate dehydrogenase, and an HIV test (in those not known to be HIV-positive). In patients known with HIV, we performed a CD4 count and quantified the viral load for those on ART. In patients newly diagnosed with HIV, we performed a CD4 count at the next visit.
In the first 100 patients, we obtained an FNA on all patients and performed cytology and TB tests on the aspirate. Later, we only obtained an FNA on patients whose lymph node was fluctuant and if >0.5 mL of caseous material was aspirated. We used a 22G or larger bore needle and a 5-mL syringe for the FNA. In such cases, the aspirated material was tested for TB using the Ultra assay, a Ziehl–Neelsen stain, and a TB culture, and the node was not biopsied because of the risk of causing a draining sinus. The Ultra assay and TB culture methods are described elsewhere[13]. We stopped routinely requesting cytology as a pragmatic and cost-saving measure based on preliminary results that showed that the yield from the core biopsy was higher.
Biopsies were taken using an automated biopsy gun (BARD MagnumTM, CR Bard Inc., Covington, GA, USA) with a 14G needle or an automated BARD biopsy needle (14G). The biopsy was performed under handheld ultrasound guidance if the lymph node was not easily palpable. We purchased a basic handheld ultrasound (SONOsite) that could project onto an iPhone screen. From the core biopsy, two or three cores (10mm–15 mm long) were placed in formalin for histology, and one core was cut in two and sent for TB culture and Ultra in 2 mL of 0.9% saline. Patients were monitored for 30minutes post procedure for bleeding or other complications associated with local anaesthetic administration. When these test results were inconclusive, the patient underwent either a repeat core biopsy or an excision biopsy at the treating clinician’s discretion.
Participants were diagnosed according to their histological and or microbiological results. TB was diagnosed if the Ultra test result was positive OR if the culture was positive for M. tuberculosis OR if acid-fast bacillus (AFB) was identified OR if necrotizing granulomas were identified and the patient had proven TB at another site. The Ultra test result was considered false positive if the histology indicated an alternative diagnosis and neither AFBs nor granulomas were present to indicate a dual pathology with TB. Lymphoma was diagnosed according to 2016 WHO classification of lymphoid neoplasms[17]. A qualified anatomical pathologist performed a histological review and immunohistochemistal staining where appropriate (Supplementary methods 1). Where granulomas were identified, a Ziehl–Neelsen stain (for TB) and a periodic acid–Schiff stain (for fungi) were performed. Patients with TB were discharged to the local TB clinic, patients with lymphoma managed by Hematology, and those with other cancers were referred to the appropriate Oncology clinic. We confirmed that patients had been seen at the appropriate referral clinic for lymphoma or cancer by searching clinical records.
Data were entered into a REDCap® database and analyzed using the STATAv14 software package (StataCorp, College Station, Texas, USA). Baseline clinical characteristics were compared using the chi-squared or Fisher’s exact test for categorical variables and the Mann-Whitney U test for continuous variables. Sensitivity and specificity of the TB tests were calculated.
Results
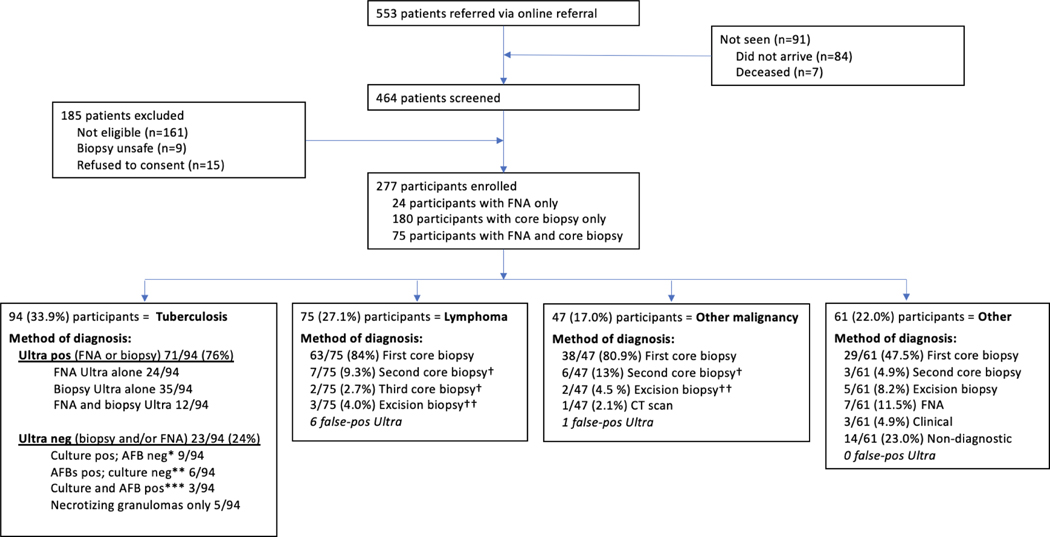
Between November 2017 and June 2022, 553 patients were referred for lymph node biopsy, 462 of whom arrived at the clinic and were screened (Figure 1). Seven patients died within a week of referral, before attending the clinic. We excluded 185 patients who did not meet the enrollment criteria (Figure 1); the main reasons for exclusion were that the lymph node was < 2 cm (84/185, 45%), or it was not an enlarged lymph node but another mass (e.g., sebaceous cyst, enlarged thyroid) (39/185, 21%). We enrolled 277 patients who underwent investigations for lymphadenopathy – their baseline characteristics by final diagnoses are shown in Table 1. Doctors referred patients to the clinic from within the same tertiary level/academic Groote Schuur hospital (42%); day clinics (44%); or secondary-level hospitals (10%). Referring clinicians revealed that their next diagnostic step would have been an FNA for cytology in 40% of cases and an excision biopsy in 40% of cases. Most referring clinicians (49%) said it would take them longer than two weeks to access a lymph node biopsy, while 19% said they were unsure how long it would take. Nine (12%) patients diagnosed with lymphoma had been presumptively started on TB therapy before referral for biopsy. Twenty-three (24%) patients diagnosed with TB were on TB therapy at the time of the biopsy. The median time between a referring clinician’s online referral and biopsy was five days (IQR 2–8.5).
Figure 1. Consort diagram showing enrolment, final diagnosis, and methods used to make the diagnosis.
*with necrotizing granulomas in 7/9; **with necrotizing granulomas in 6/6; ***with necrotizing granulomas in 3/3
†Diagnoses made on second or third core: Hodgkin lymphoma (n=6), Diffuse-large B-cell lymphoma (n=2), Follicular lymphoma (n=1), Squamous cell carcinoma (n=3), Kaposi sarcoma (n=1), Adenocarcinoma (n=1), Nasopharyngeal carcinoma (n=1)
†† Diagnoses made on excision biopsy: Diffuse-large B-cell lymphoma (n=3), Squamous cell carcinoma (n=2)
Table 1.
Baseline characteristics and final diagnoses of enrolled patients.
| Total (n=277) | Lymphoma (n=75) | Tuberculosis adenitis (n=94) | Other malignancy (n=47) | Other diagnosis* (n=61) | |
|---|---|---|---|---|---|
|
| |||||
| Sex | |||||
| Males | 133 (48.0) | 45 (60.0) | 33 (35.1) | 27 (57.5) | 28 (45.9) |
| Females | 144 (52.0) | 30 (40.0) | 61 (64.9) | 20 (42.6) | 33 (54.1) |
| Age (median [IQR]) | 39.0 (29.4–49.5) | 41.8 (28.5–50.2) | 31.9 (26.9–40.0) | 58.3 (45.8–65.3) | 37.2 (30.3–49.1) |
| HIV status | |||||
| Positive | 120 (43.3) | 39 (52.0) | 50 (53.2) | 8 (17.0) | 23 (37.7) |
| Negative | 157 (56.7) | 36 (48.0) | 44 (46.8) | 39 (83.0) | 38 (62.3) |
| Performance Score (n=275) | |||||
| ECOG 0 | 140 (51.0) | 25 (33.8) | 56 (60.2) | 14 (29.8) | 45 (73.8) |
| ECOG 1 | 77 (28.0) | 25 (33.8) | 25 (26.9) | 18 (38.3) | 9 (14.8) |
| ECOG 2 | 24 (8.7) | 9 (12.2) | 6 (6.5) | 5 (10.6) | 4 (6.6) |
| ECOG 3 | 28 (10.2) | 13 (17.6) | 6 (6.5) | 7 (14.9) | 2 (3.3) |
| ECOG 4 | 6 (2.2) | 2 (2.7) | 0 | 3 (6.4) | 1 (1.6) |
| Patient Type | |||||
| Inpatient | 52 (18.8) | 22 (29.3) | 11 (11.7) | 12 (25.5) | 7 (11.5) |
| Outpatient | 225 (81.2) | 53 (70.7) | 83 (88.3) | 35 (74.5) | 54 (88.5) |
| Previous tuberculosis (n=275) | 64 (23.3) | 16 (21.3) | 28 (30.1) | 11 (23.9) | 9 (14.8) |
| On tuberculosis treatment | 39 (14.1) | 9 (12.0) | 23 (24.5) | 2 (4.3) | 5 (8.2) |
| Consistency of LN (n=273) | |||||
| Firm | 236 (86.5) | 73 (97.3) | 71 (77.2) | 41 (89.1) | 51 (85.0) |
| Fluctuant | 26 (9.5) | 0 | 15 (16.3) | 2 (4.4) | 9 (15.0) |
| Matted | 11 (4.0) | 2 (2.7) | 6 (6.5) | 3 (6.5) | 0 |
| Symptoms (n=275) | |||||
| Cough | 74 (26.9) | 22 (29.7) | 24 (25.8) | 19 (40.4) | 9 (14.8) |
| Weight loss | 133 (48.4) | 40 (54.1) | 53 (57.0) | 27 (57.5) | 13 (21.3) |
| Night sweats | 89 (32.7) | 34 (46.0) | 28 (30.4) | 13 (28.9) | 14 (23.0) |
| Location of lymph nodes | |||||
| Unilateral | 189 (68.2) | 38 (50.7) | 68 (72.3) | 36 (76.6) | 47 (77.1) |
| Bilateral | 88 (31.8) | 37 (49.3) | 26 (27.7) | 11 (23.4) | 14 (23.0) |
| Lymph node FNA/biopsy site | |||||
| Neck | 242 (87.4) | 59 (78.7) | 89 (94.7) | 42 (89.4) | 52 (85.3) |
| Axilla | 28 (10.1) | 14 (18.7) | 4 (4.3) | 3 (6.4) | 7 (11.5) |
| Inguinal | 7 (2.5) | 2 (2.7) | 1 (1.1) | 2 (4.3) | 2 (3.3) |
| Blood results (median [IQR]) | |||||
| LDH (units/L) (n=233) | 263 (216–333) | 303 (239–482) | 251 (191–299) | 268.5 (221.5–353.5) | 247 (215–306) |
| Hb (g/dL) (n=270) | 12.0 (9.6–13.4) | 10.6 (8.9–12.9) | 11.6 (9.6–12.7) | 12.9 (11.3–13.9) | 13.0 (11.6–13.9) |
| WCC (cells × 10^9) (n=271) | 7.3 (5.0–9.9) | 8.0 (4.9–11.6) | 6.2 (4.5–8.0) | 8.2 (5.7–10.1) | 8.0 (6.3–10.2) |
| Lymphocytes (cells × 10^9) (n=249) | 1.7 (1.0–2.3) | 1.5 (0.8–2.4) | 1.72 (0.9–2.1) | 1.9 (1.4–2.4) | 1.9 (1.4–3.1) |
Data are n/N; % (95% CI). ECOG = Eastern Cooperative Oncology Group.
Oncology Group. LN=Lymph node. LDH=Lactate dehydrogenase. Hb=Haemoglobin. WCC=White cell count. IQR=Interquartile range.
Other: reactive (n=15), salivary gland tumor (n=12), bacterial adenitis (n=5), sarcoidosis (n=4), lipoma (n=2), histoplasmosis (n=1), sinus histiocytosis (n=1), granulomatous inflammation unspecified (n=2), fibroadipose tissue (n=1), cyst (n=2), previous TB with scar tissue (n=1), fat necrosis (n=1), no diagnosis (n=14)
Most patients were ambulatory and came as outpatients (81%). One hundred and twenty patients (43%) were HIV positive, with a median CD4 count of 215 cells/mm3 (IQR 101–360), 81/120 HIV-positive patients (68%) were on ART, and 38 (32%) were virally suppressed (i.e., had a viral load less than 40 copies/mL). Patients were categorized into four diagnostic groups, based on investigation results: TB (94/277, 34%), lymphoma (75/277, 27%), disseminated malignancy (47/277, 17%) or other (61/277, 22%) (Table 1). In PWH, a higher proportion had TB (42% vs. 28%, p=0.02) and lymphoma (33% vs. 23%, p=0.08), and a lower proportion had another malignancy (7% vs. 25%, p<0.001). No patients had a dual diagnosis of TB plus malignancy on the lymph node biopsy. Figure 1 shows a consort diagram and test performance to reach the final diagnosis.
There were several differences among the groups by the diagnostic outcome. Patients with disseminated malignancy were older (median age 58 years) than those with lymphoma (42 years, p<0.001) or TB (32 years, p<0.001). Patients with any cancer were more likely to have a poor performance score with an ECOG score of 2 or higher in 32% (39/121) vs. 12% (19/154), p <0.001 (Table 1). All three groups reported a high proportion of cough and constitutional symptoms (fever, weight loss, night sweats). Interestingly, cough was most frequently reported in the patients with disseminated malignancy (40%), followed by lymphoma (30%), and then TB (26%). Lymph node enlargement was unilateral in most cases but was more likely to be bilateral in the lymphoma than in the other groups combined (49% vs. 25%, p<0.001). The median Hb was lower in patients with lymphoma than in other groups (10.6 g/dl vs. 12.2 g/dl, p<0.001), and median lactate dehydrogenase higher (303 IU/L vs. 251.1 IU/L, normal range 105–333IU/L).
The core biopsy was safe and well-tolerated, with no complications (no excess bleeding, no patients returned with infective complications). The first-attempt core biopsy diagnosed lymphoma in 63/75 (84%), the second-attempt biopsy in 7/75 (9%), a third-attempt biopsy in 2/75 (3%), and an excision biopsy in 3/75 (4%) (Figure 1). In keeping with local epidemiological data, the most common types of lymphoma were Hodgkin lymphoma (HL) (40%) and Diffuse large B-cell lymphoma (DLBCL) (35%) (Table 2). Immunohistochemical staining was performed on all tissues for diagnosis and subtyping, and the diagnosis was considered actionable for treatment in 72/75 (96%) cases. Three cases were unresolved diagnostic dilemmas (Table 2). There was insufficient tissue to subtype Hodgkin lymphoma in 18 of the 30 cases or to grade follicular lymphoma (n=3), but this did not influence treatment. Newly diagnosed lymphoma patients were referred to the hematology clinic; 72/75 (96%) attended follow-up care or were seen by the hematology inpatient service (2 died as in-patients soon after diagnosis), 4 patients did not attend a follow-up visit and could not be contacted.
Table 2.
Lymphoma type and subtype
| Lymphoma type | Total (n=75) |
|---|---|
|
| |
| Hodgkin lymphoma (n=30) | |
| Nodular-lymphocyte predominant | 1 |
| Classical | |
| Nodular sclerosing | 9 |
| Mixed cellularity | 1 |
| Lymphocyte rich | 1 |
| Unable to subtype | 18 |
| Diffuse large B-cell lymphoma (n=26) | |
| Activated B-cell | 8 |
| Germinal centre | 7 |
| EBV-associated | 2 |
| Unable to subtype | 9 |
| Other B-cell lymphomas (n=9) | |
| Follicular lymphoma | 3 |
| Burkitt lymphoma | 3 |
| Primary effusion lymphoma | 1 |
| Primary mediastinal B-cell lymphoma | 1 |
| Small lymphocytic lymphoma | 1 |
| T-cell lymphomas (n=3) | |
| T-lymphoblastic lymphoma | 1 |
| Peripheral T-cell lymphoma | 1 |
| Anaplastic large cell lymphoma | 1 |
| Other (n=8) | |
| HHV-8-associated Castleman’s | 4 |
| Lymphoma unclassified* | 3 |
EBV = Epstein-Barr Virus. HHV = Human Herpes Virus.
Plasmablastic lymphoma vs Castleman’s disease (died before an excision biopsy could be performed); atypical lymphoid proliferation (declined excision biopsy); and Hodgkin lymphoma vs anaplastic large-cell lymphoma (could not be contacted for a repeat biopsy).
In patients with other cancers, sufficient tissue was obtained at the first-attempt core biopsy to detect disseminated malignancy in 81% of patients. A second-attempt biopsy was sufficient in 8.5% of patients. The types of cancers diagnosed were squamous cell (n=15); adenocarcinoma (n=13); Kaposi sarcoma (n=5); and other (n=14). Where applicable, patients underwent further investigations at the oncology clinic to determine the primary site of cancer, and 43/47 (91%) attended follow-up care.
The Ultra was most often used to diagnose TB and detected in 71/94 patients; the diagnostic performance of the TB tests is shown in Table 3. The Ultra test result was false-positive in seven cases. In 5/7 of these false-positive cases, the Ultra test result was reported as ‘trace-positive’; in 2/7 it was low-positive. None of the false positive tests showed granulomas on histology, and the combined Ultra plus granulomas on histology showed 100% specificity for TB. Cultures were not a useful initial diagnostic test because of the long turnaround time (up to 6 weeks) but helped confirm TB diagnosis and drug sensitivity.
Table 3.
TB diagnostic test performance
| Tuberculosis test | Sensitivity | Specificity |
|---|---|---|
| AFB on FNA (n=63) | 8/28; 28.6% (13.2–48.7) | 35/35; 100% (90.0–100) |
| FNA culture (n=28) | 6/20; 30.0% (12.0–54.3) | 8/8; 100% (63.1–100) |
| AFB on tissue (n=212) | 26/69; 37.7% (26.3–50.2) | 143/143; 100% (97.5–100) |
| Tissue culture (n=175) | 24/55; 43.5% (30.3–57.7) | 120/120; 100% (97.0–100) |
| Necrotizing granulomas on histology (n=213) | 67/69; 97.1% (89.9–99.6) | 142/144; 98.6% (95.1–99.8) |
| Ultra on FNA (n=93) | 34/46; 73.9% (58.9–85.7) | 46/47; 98.0 (88.7–100) |
| Ultra on tissue (n=186) | 46/63; 73.0% (60.3–83.4) | 116/123; 94.3% (88.6–97.7) |
AFB = Acid-fast bacilli. FNA = fine-needle aspirate.
The aspirate was sent for cytology in 51 cases. Cytology was reported as ‘unsatisfactory for analysis’ in 22/51 cases. Of the 11 patients with lymphoma who underwent FNAC, only one was reported as a lymphoma (two reported as ‘atypical’, three as ‘normal’, and five as ‘unsatisfactory for analysis’). In 5 of the 16 patients with TB, the cytology was reported as consistent with TB (granulomas, caseous necrosis). Cytology was most sensitive for cancers other than lymphoma, where it detected malignancy in 70% (7/10).
DISCUSSION
We report the results of an electronic referral system and focused diagnostic procedures in a rapid-access lymph node biopsy clinic where roughly half of the patients were HIV positive. The etiology of lymphadenopathy was predominantly TB, lymphoma, and other disseminated cancers. The online referral system ensured that patients were seen rapidly in the clinic after referral. Using a combination of high-yield investigations, we could accurately diagnose our patients, and 94% (115/122) of patients with lymphoma and other cancers were linked to appropriate follow-up care. The core biopsy was safe and had a high yield for lymphoma and other cancers. The Ultra had a high yield for TB both on FNA and tissue, but false positive tests were a concern. The core biopsy could be safely and competently performed at the bedside by a healthcare practitioner without specialist surgical or radiological training.
The age, number of HIV-positive patients, presence of constitutional symptoms, location of lymph nodes, and basic blood test results were similar for the TB and lymphoma groups. Lymph node examinations did not help to distinguish between the two unless the lymph node was fluctuant, which occurred more frequently in the TB group. The false-positive Ultra tests indicate the need to interpret ‘trace positive’ results with caution and highlight the need to obtain tissue and request histology simultaneously with the TB Ultra test, both to identify supporting features of TB (granulomas or AFBs) or identify an alternative histological diagnosis that might have catastrophic consequences if missed. In our cohort, 6 patients with false positive Ultra results had lymphoma, and 1 had other cancer, they had no other TB-positive tests and did not develop TB during chemotherapy. Prior TB infection is the primary reason for false positive Xpert MTB/RIF TB tests in a TB-endemic setting; this has been highlighted in other studies, especially when using the Ultra, where higher sensitivity has come at the expense of a decrease in specificity when compared to the Xpert MTB/RIF[12, 18].
Our easy online referral system provided important clinical information that previous time-consuming telephone referrals had not. We advertised the clinic and interim findings at the one-year mark as an awareness campaign, highlighting the importance of biopsies for patients with lymphadenopathy and the dangers associated with presumptive TB therapy. In the study referral questionnaire, 5% of clinicians said they would have started empiric tuberculosis treatment if the biopsy clinic was unavailable; this is lower than historical data from the same institution where published data showed that at the time of HL diagnosis, 33% (72/219) of patients overall, and 72% PWH (46/64), were on TB treatment. In only 10%, the diagnosis of TB was proven (21/219)[19]. The questionnaire result of 5% is likely low due to a combination of the Hawthorn effect and selection bias (the clinicians choosing to obtain a biopsy are those less likely to start empiric TB treatment). We have previously reported the diagnostic interval (time from first health visit to diagnostic biopsy) in patients with lymphoma from our hospital[20]. Our clinic appears to have reduced this interval from 48 days (IQR 21–116 days) in the historical cohort, to 27 days (IQR 9–74 days) (p=0.016) in our cohort. However, recall bias inherent in the questionnaire method and the differences between the cohorts limit the strength of this finding.
Patient attrition between visits is an important problem in our context; each visit is a critical opportunity to make a diagnosis. In a recent South-African publication, the linkage to care following an FNAC suspicious for lymphoma was abysmal: of 45 PWH with cytology suggestive of lymphoma, only 24 (53%) returned for a biopsy confirming the diagnosis. Among the patients lost to follow-up at the one-year timepoint, 57% had died, 29% were untraceable, and only 14% were confirmed alive[21]. We stopped performing cytology early in our study due to poor diagnostic yield for lymphoma, even though the aspirate came from the same site as a biopsy (i.e., confirming representative tissue), and best-practice guidelines were followed. This may be partially attributed to a lack of dedicated cytology service, and ancillary tests such as flow cytometry and immunohistochemistry were not performed on the aspirate. However, it might also because we had histology as a reference, which is uncommon among studies reporting the sensitivity of FNAC. In a meta-analysis of 42 studies (1989–2012) that reported the sensitivity of FNAC to detect lymphoma, only a single paper included a centralized review of specimens where the FNA and the excisional biopsy results were independently correlated [22]. This paper reported only 12% diagnostic accuracy for lymphoma (and 29% when immunophenotyping was included)[23]; we have previously reported similar sensitivity in patients who were diagnosed with lymphoma at our institution (63/90 (11%) of FNACs were ‘suggestive’ for lymphoma)[20]. These data emphasize the need to maximize the opportunity for a diagnostic intervention with a high yield at the earliest opportunity. In a setting where lymphomas are common, economic and other obstacles may limit the patient’s ability to return for follow-up, and FNAC is not supported by ancillary techniques such as flow cytometry; we have presented implementation data demonstrating feasibility for a core biopsy to replace the FNA for cytology as the first investigation for lymphadenopathy.
There are some limitations to our study. Our clinic only admits patients with lymph nodes larger than 2 cm in diameter because these nodes are accessible for core biopsy by a physician without surgical or radiological expertise. Smaller lymph nodes, however, can also be pathological. Because the larger lymph nodes are easier to palpate, we frequently did not use the handheld ultrasound, and physicians typically preferred to biopsy ‘blind’. Biopsies could be taken from smaller lymph nodes with ultrasound guidance by physicians with more experience and training. Another limitation is that we did not include patients with difficult-to-access lymphadenopathy (mediastinal or abdominal). These patients may have an even higher risk of lymphoma/cancer being misdiagnosed as TB, especially as the radiological findings can be similar (splenic hypodense lesions, tree-in-bud appearance on lung parenchyma). In these cases, we recommend specialist consultation at a tertiary institute where specialised tests such as flow cytometry of FNAs or bone marrow biopsies can be used for diagnosis or specialized surgical services are available to access nodes in difficult sites. Improved molecular tests and biomarkers from peripheral blood or urine are urgently needed in TB-endemic areas, and exciting advances in the use of cell-free DNA or liquid biopsy may improve diagnosis in these patients[24].
Further research efforts will be directed at training healthcare professionals in outlying clinics to perform biopsies; and setting up biopsy clinics at the primary and secondary care levels. The interventions reported in this study were carried out within the diagnostic interval; another critical intervention within this interval is health education for primary care providers. We were surprised by the high number of referrals of patients with lumps, not due to lymphadenopathy (sebaceous cysts, enlarged thyroid, and submandibular salivary glands). This, along with the inappropriate use of repeated FNA for cytology[20], and the previously reported high rates of empiric TB therapy for patients with lymphadenopathy, highlights the need for improvements in medical education on a diagnostic approach to lymphadenopathy.
In conclusion, we report a physician-led biopsy clinic with focused bedside tests that are a safe, feasible, and effective way to assess lymphadenopathy. This approach to evaluating lymphadenopathy could be applied in referral clinics, at the primary-care level, or within HIV-care clinics. The indistinguishability of patients in terms of symptoms and examination findings and the high attrition rate described among this vulnerable patient group highlights the importance of combining high-yield tests with maximum diagnostic potential at the earliest opportunity.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this manuscript was supported by the Fogarty International Center of the National Institutes of Health [D43-TW010345, D43-TW010543, K43TW011986]; the National Research Foundation Thuthuka grant [TTK14052267787]; the Cancer Association of South Africa (CANSA). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and bears final responsibility for the decision to submit for publication.
Footnotes
Conflict of Interest: None of the authors have a conflict of interest.
List of when the study has been presented in part
We have published two papers from a smaller group within this cohort with a different focus to this paper. We previously published a paper on the diagnostic accuracy of the Xpert Ultra to detect tuberculosis from lymph node tissue in BMC Infectious Diseases, 2020. We then published a short report highlighting how infectious disease epidemics overshadow cancer diagnosis during COVID and highlighted the interventions we implemented in our lymph node biopsy clinic that could be used during the COVID epidemic, Leukemia & Lymphoma, 2020.
Consent
All patients included in the study signed an informed consent form. The Human Research Ethics Committee of the University of Cape Town’s Faculty of Health Sciences approved the study (HREC 647/2017).
References:
- 1.Antel K, Louw VJ, Maartens G, Oosthuizen J, Verburgh E: Diagnosing lymphoma in the shadow of an epidemic: lessons learned from the diagnostic challenges posed by the dual tuberculosis and HIV epidemics. Leukemia & lymphoma 2020, 61(14):3417–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simard EP, Engels EA: Cancer as a cause of death among people with AIDS in the United States. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2010, 51(8):957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma SK, Mohan A: Extrapulmonary tuberculosis. Indian J Med Res 2004, 120(4):316–353. [PubMed] [Google Scholar]
- 4.Van Rie A, Page-Shipp L, Mellet K, Scott L, Mkhwnazi M, Jong E, Omar T, Beylis N, Stevens W, Sanne I et al. : Diagnostic accuracy and effectiveness of the Xpert MTB/RIF assay for the diagnosis of HIV-associated lymph node tuberculosis. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology 2013, 32(11):1409–1415. [DOI] [PubMed] [Google Scholar]
- 5.Buyego P, Nakiyingi L, Ddungu H, Walimbwa S, Nalwanga D, Reynolds SJ, Parkes-Ratanshi R: Possible misdiagnosis of HIV associated lymphoma as tuberculosis among patients attending Uganda Cancer Institute. AIDS Res Ther 2017, 14(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puvaneswaran B, Shoba B: Misdiagnosis of tuberculosis in patients with lymphoma. S Afr Med J 2012, 103(1):32–33. [DOI] [PubMed] [Google Scholar]
- 7.Achenbach CJ, Cole SR, Kitahata MM, Casper C, Willig JH, Mugavero MJ, Saag MS: Mortality after cancer diagnosis in HIV-infected individuals treated with antiretroviral therapy. AIDS (London, England) 2011, 25(5):691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnet F, Burty C, Lewden C, Costagliola D, May T, Bouteloup V, Rosenthal E, Jougla E, Cacoub P, Salmon D et al. : Changes in Cancer Mortality among HIV-Infected Patients: The Mortalité 2005 Survey. Clinical Infectious Diseases 2009, 48(5):633–639. [DOI] [PubMed] [Google Scholar]
- 9.Yarchoan R, Uldrick TS: HIV-Associated Cancers and Related Diseases. New England Journal of Medicine 2018, 378(11):1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mezger NCS, Feuchtner J, Griesel M, Hämmerl L, Seraphin TP, Zietsman A, Péko JF, Tadesse F, Buziba NG, Wabinga H et al. : Clinical presentation and diagnosis of adult patients with non-Hodgkin lymphoma in Sub-Saharan Africa. British journal of haematology 2020, 190(2):209–221. [DOI] [PubMed] [Google Scholar]
- 11.Moure R, Martin R, Alcaide F: Effectiveness of an integrated real-time PCR method for detection of the Mycobacterium tuberculosis complex in smear-negative extrapulmonary samples in an area of low tuberculosis prevalence. J Clin Microbiol 2012, 50(2):513–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B, Hall SL, Chakravorty S, Cirillo DM, Tukvadze N et al. : Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. The Lancet Infectious Diseases 2018, 18(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antel K, Oosthuizen J, Malherbe F, Louw VJ, Nicol MP, Maartens G, Verburgh E: Diagnostic accuracy of the Xpert MTB/Rif Ultra for tuberculosis adenitis. BMC infectious diseases 2020, 20(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christopher DJ, Coelho V, Ebby GS, Shankar D, Gupta R, Thangakunam B: Incremental yield of Xpert((R)) MTB/RIF Ultra over Xpert((R)) MTB/RIF in the diagnosis of extrapulmonary TB. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease 2021, 25(11):939–944. [DOI] [PubMed] [Google Scholar]
- 15.Yu X, Zhang T, Kong Y, Wang F, Dong L, Han M, Huang H: Xpert MTB/RIF Ultra outperformed the Xpert assay in tuberculosis lymphadenitis diagnosis: a prospective head-to-head cohort study. Int J Infect Dis 2022. Sep;122:741–746. [DOI] [PubMed] [Google Scholar]
- 16.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP: Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982, 5(6):649–655. [PubMed] [Google Scholar]
- 17.WHO classification of tumours of haematopoietic and lymphoid tissues te, 4th edn. Lyon, France: International Agency for Research on Cancer; 2017. [Google Scholar]
- 18.Kohli M, Schiller I, Dendukuri N, Yao M, Dheda K, Denkinger CM, Schumacher SG, Steingart KR: Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2021. Jan 15;1(1):CD012768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swart L, Novitzky N, Mohamed Z, Opie J: Hodgkin lymphoma at Groote Schuur Hospital, South Africa: the effect of HIV and bone marrow infiltration. Annals of hematology 2019, 98(2):381–389. [DOI] [PubMed] [Google Scholar]
- 20.Antel K, Levetan C, Mohamed Z, Louw VJ, Oosthuizen J, Maartens G, Verburgh E: The determinants and impact of diagnostic delay in lymphoma in a TB and HIV endemic setting. BMC Cancer. 2019. Apr 25;19(1):384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogt SL, Maloma L, Xian RR, Ambinder RF, Philip V, Patel M, Martinson NA, Omar T: Significance of lymph node fine needle aspiration for the diagnosis of HIV-associated lymphoma in a low-resource setting. AIDS (London, England) 2022, 36(10):1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frederiksen JK, Sharma M, Casulo C, Burack WR: Systematic Review of the Effectiveness of Fine-Needle Aspiration and/or Core Needle Biopsy for Subclassifying Lymphoma. Archives of pathology & laboratory medicine 2015, 139(2):245–251. [DOI] [PubMed] [Google Scholar]
- 23.Hehn ST, Grogan TM, Miller TP: Utility of fine-needle aspiration as a diagnostic technique in lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2004, 22(15):3046–3052. [DOI] [PubMed] [Google Scholar]
- 24.Vogt SL, Patel M, Lakha A, Philip V, Omar T, Ashmore P, Pather S, Haley LM, Zheng G, Stone J et al. : Feasibility of Cell-Free DNA Collection and Clonal Immunoglobulin Sequencing in South African Patients With HIV-Associated Lymphoma. JCO Global Oncology 2021(7):611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.