Abstract
Background.
Potassium channels play an important role in the basal tone and dilation of cerebral resistance arterioles in response to many stimuli. However, the effect of prenatal alcohol exposure (PAE) on specific potassium channel function remains unknown. The first goal of this study was to determine the influence of PAE on reactivity of cerebral arterioles to activation of KATP and BK channels. Our second goal was to determine whether oxidative stress contributed to potassium channel dysfunction of cerebral arterioles following PAE.
Methods.
We fed Sprague-Dawley dams a liquid diet with or without alcohol (3% ethanol) for the duration of their pregnancy (21-23 days). We examined in vivo responses of cerebral arterioles in control and PAE male and female offspring (14-16 weeks after birth) to activators of potassium channels, (Iloprost (BK channels) and pinacidil (KATP channels)), before and following inhibition of oxidative stress with apocynin.
Results.
We found that PAE impaired dilation of cerebral arterioles in response to activation of potassium channels with iloprost and pinacidil, and this impairment was similar in male and female rats. In addition, treatment with apocynin reversed the impaired vasodilation to iloprost and pinacidil in PAE rats to levels observed in control rats. This effect of apocynin also was similar in male and female rats.
Conclusions.
PAE induces dysfunction in the ability of specific potassium channels to dilate cerebral arterioles which appears to be mediated by an increase in oxidative stress. We suggest that these alterations in potassium channel function may contribute to the pathogenesis of cerebral vascular abnormalities and/or behavioral/cognitive deficits observed in fetal alcohol spectrum disorders.
Keywords: Brain, Pial arterioles, Potassium channels, Cerebral blood flow, Iloprost and Pinacidil
Introduction
Individuals exposed to alcohol prenatally manifest a group of conditions referred to as fetal alcohol spectrum disorders (FASDs). FASDs are life-long disorders that may affect as many as 1% to 5% of children (May et al., 2018). Prenatal alcohol exposure (PAE) has been shown to produce growth abnormalities and damage to the heart, vasculature and the brain (Jones et al., 1973; Riley et al., 2011; Daft et al., 1986; Bukiya and Dopico, 2018; Nakhoul et al., 2017; Ramadoss and Magness, 2012). PAE also promotes cognitive decline, behavioral disorders, dementia, and seizures that can manifest in early childhood and persist into adulthood (Guerri et al., 2009; Graham et al., 2013; Ware et al., 2013). We have suggested that abnormalities of the brain observed during PAE may be related to alterations in reactivity of cerebral arterioles in response to activation of important vasodilator mechanisms, which in turn would affect the regulation of cerebral blood flow during changes in metabolic demand (neurovascular coupling). In support of this concept, we have shown that PAE impairs reactivity of cerebral arterioles to eNOS- and nNOS-dependent agonists by mechanisms that appear to involve suppression of neuroinflammation and/or oxidative stress (Cananzi and Mayhan, 2019b; Cananzi and Mayhan, 2019a; Saha et al., 2021).
ATP-sensitive potassium channels (KATP) and calcium-activated potassium channels (BK) have been shown to be important in regulating basal tone and changes in diameter of cerebral arteries/arterioles, and hence cerebral blood flow, in response to many stimuli (Nnorom et al., 2014; Philip and Armstead, 2004; Lindauer et al., 2003; Brayden and Nelson, 1992; Sobey et al., 1998; Horiuchi et al., 2001; Faraci and Heistad, 1998; Faraci et al., 1994b). In addition, it appears that reactivity of cerebral arteries/arterioles in response to activation of these potassium channels is altered during a variety of disease states (Dong et al., 2009; Sun et al., 2008; Erdos et al., 2004a; Mayhan et al., 2004; Mayhan and Faraci, 1993; Faraci et al., 1994b). Unfortunately, there is a lack of information regarding the influence of PAE on responses of cerebral arterioles to activation of potassium channels. Thus, the first goal of the present study was to examine in vivo responses of pial arterioles to activation of KATP and BK channels in male and female rats exposed to prenatal alcohol. The second goal of this study was to determine the role of oxidative stress in impaired responses of cerebral arterioles to activation of KATP and BK channels during PAE.
Materials and Methods
All procedures are in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of South Dakota Sanford School of Medicine.
Experimental diets.
Virgin adult male and female Sprague-Dawley rats were allowed to mate. Dams were then housed singly and assigned randomly to groups that were fed a control diet (0% alcohol) or a 3% alcohol diet, as we have described previously (Zhao et al., 2010; Mayhan, 1992). The control diet contained 1.0 kcal/ml of which 35% are derived from fat, 47% from carbohydrates, and 18% from protein. The 3% alcohol diet contained 1.0 kcal/ml of which 35% are derived from fat, 18% from protein, 29% from carbohydrates and 18% from alcohol. The diets were prepared daily and the total daily volume of diet fed to the control animals was based upon the daily consumption of diet by the alcohol animals. We have shown that the alcohol diet results in a blood alcohol concentration (BAC) between 0.06% and 0.07%, respectively (Cananzi and Mayhan, 2019b). The liquid diets fed to the dams were replaced by a normal chow/water diet within a day of the birth of the pups. Rats were cross-fostered and were weaned at 3 weeks of age and placed in cages with those of the same sex. Both male and female rats were used for these experiments.
Measurement of cerebral vascular reactivity.
The rats were prepared for studies at 14-16 weeks of age and were obtained from multiple different litters. On the day of the experiment, the rats were anesthetized with thiobutabarbital sodium (Inactin, 100 mg/kg IP) and a tracheotomy was performed. The rats were ventilated mechanically with room air and supplemental oxygen. A femoral artery was cannulated for the measurement of arterial blood pressure and to obtain a blood sample for the determination of blood gases.
In all studies, a craniotomy was prepared over the left parietal cortex to visualize the cerebral microcirculation. The microcirculation was suffused with a bicarbonate buffer (2 ml/min) that was bubbled continuously with 95% nitrogen and 5% carbon dioxide. The temperature of the suffusate was maintained at 37 ± 1°C. The cranial window was connected via a three-way valve to an infusion pump, which allowed for the infusion of agonists/antagonists into the suffusate. This method maintained a constant temperature, pH, pCO2, and pO2 of the suffusate during the infusion of drugs. Arterial blood gases were monitored and maintained within normal limits throughout the experimental period. The diameter of arterioles was measured using a video image-shearing device before and at 1-minute intervals for 5 minutes during the application of agonists. Baseline diameter of cerebral arterioles returned to control levels (before application of agonists) within 2-3 minutes after application of agonists was stopped.
Responses of cerebral arterioles in male and female rats from control and alcohol groups were examined during superfusion of agonists that produce dilation via activation of KATP channels (iloprost; 1 and 10 μM) and BK channels (pinacidil; 10 and 100 μM). To determine the influence of oxidative stress on potassium channel-dependent dilation of cerebral arterioles in adult male and female control rats and in PAE rats, we examined responses to iloprost and pinacidil before and during superfusion with apocynin (1.0 μM). Although controversial as to whether apocynin specifically inhibits NADPH oxidase (Heumuller et al., 2008), previous studies have shown that apocynin is efficacious for inhibition of oxidative stress (Cananzi and Mayhan, 2019a; Sun et al., 2006; Picchi et al., 2006). Thirty minutes after starting the suffusion with apocynin and continuing for the duration of the experiment, we again examined responses of cerebral arterioles to the agonists.
In separate studies, we examined responses of cerebral arterioles to iloprost or pinacidil while treatment with iberiotoxin (0.1 μM) or glibenclamide (10 μM) to inhibit BK or KATP channels, respectively. In these studies, we initially examined responses to iloprost (n=5) or pinacidil (n=4), started a continuous superfusion of iberiotoxin or glibenclamide over the cranial window, and then 30 minutes later again examined responses of cerebral arterioles to iloprost or pinacidil.
Statistics.
We report the percentage change in diameter of cerebral arterioles during the application of agonists. Analysis of variance (ANOVA) with Fisher’s least significant difference (LSD) was used to compare responses of cerebral arterioles to the agonists, baseline diameter of cerebral arterioles, blood pressure and body weight in male and female control and PAE rats. A paired t-test was used to compare responses before and after treatment with iberiotoxin or glibenclamide. Values are mean±SEM. A p-value of 0.05 or less was considered to be significant.
Results
Functional responses of pial arterioles.
Baseline diameter of cerebral arterioles was similar in all groups of rats (43±2 μm in male control rats, 42±4 μm in male PAE rats, 45±2 μm in control female rats and 48±2 μm in female PAE rats (means±SE; p>0.05)). Mean arterial pressure was similar in groups of rats (128±6 mmHg in male control rats, 131±5 mmHg in male PAE rats, 121±6 mmHg in control female rats and 120±8 mmHg in female PAE rats (means±SE; p>0.05)). However, body weight was greater in the groups of male rats (378±12 grams in male control rats and 394±22 grams in male PAE rats) versus female rats (241±8 grams in female control rats and 237±6 grams in female PAE rats (means±SE; p<0.05 male versus female).
Application of iloprost (Figure 1) and pinacidil (Figure 2) produced dose-related dilation of cerebral arterioles by a similar magnitude in control rats of both sexes. In contrast, the magnitude of dilation in PAE male and female rats in response to iloprost (Figure 1) and pinacidil (Figure 2) was less than that observed in their respective controls.
Figure 1.
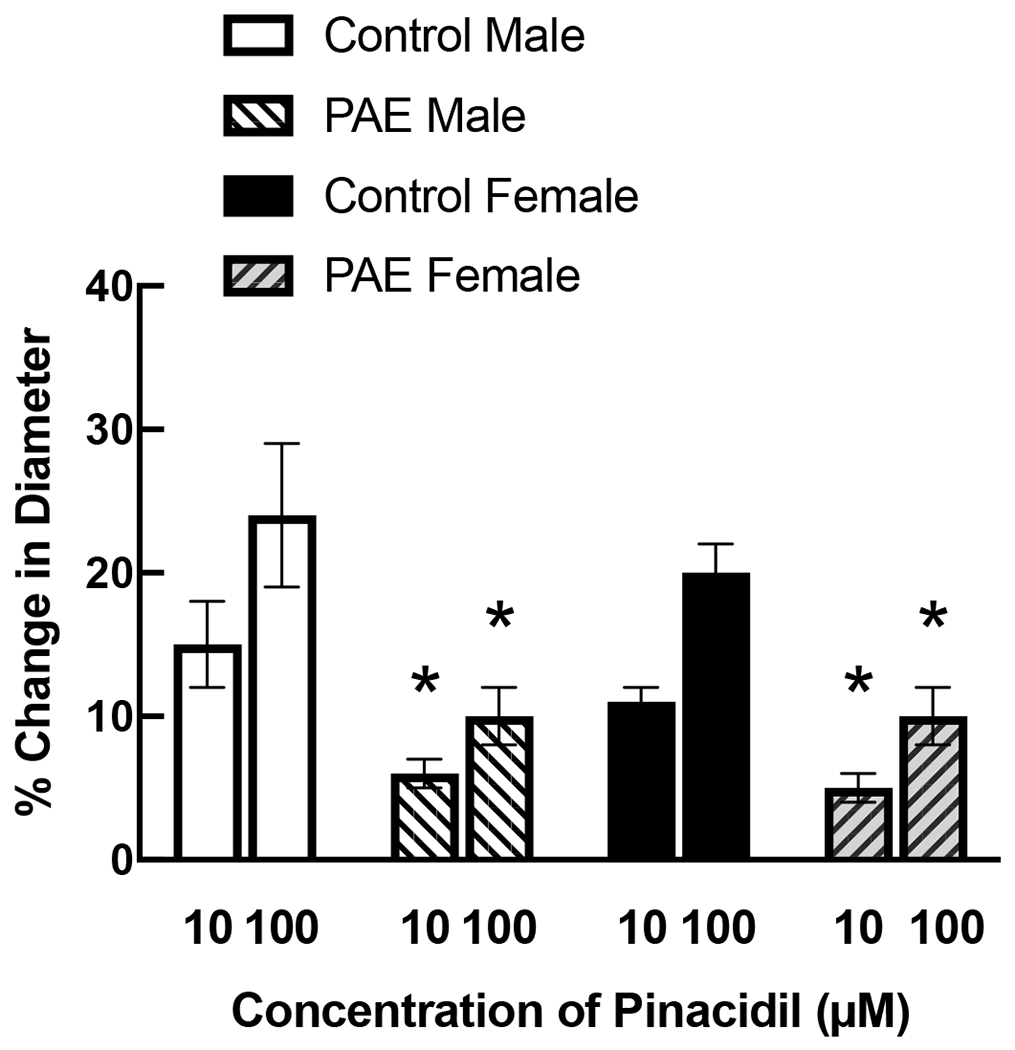
Responses of cerebral arterioles to activation of KATP channels with pinacidil in male and female control and PAE rats. Values are means±SE. * p < 0.05 versus response in control male or control female.
Figure 2.
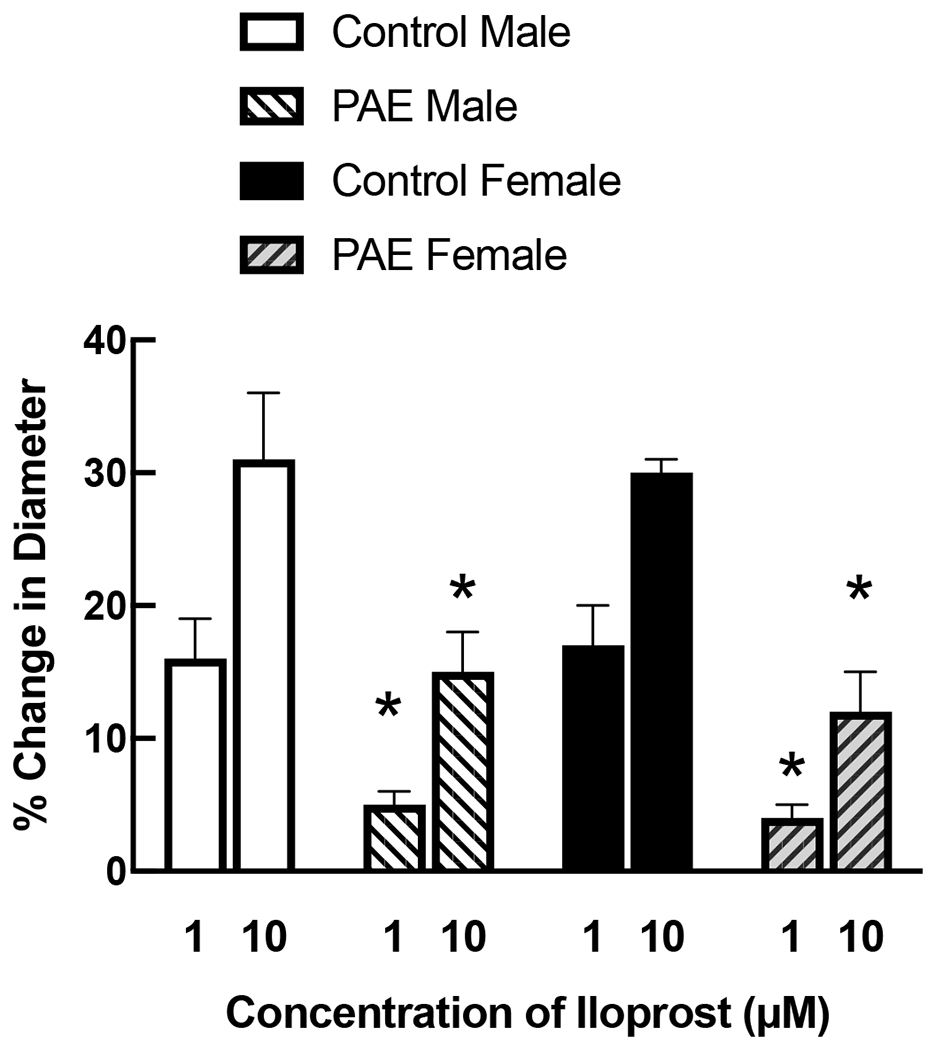
Responses of cerebral arterioles to activation of BK channels with iloprost in male and female control and PAE rats. Values are means±SE. * p < 0.05 versus response in control male or control female.
To determine the role of activation of potassium channels by iloprost and pinacidil, we examined responses of cerebral arterioles to iloprost in the presence of iberiotoxin (n=5) and pinacidil in the presence of glibenclamide (n=4). We found that dilation of cerebral arterioles to iloprost (0.1 and 1 μM) was 18±2% and 30±1%, respectively prior to superfusion with iberiotoxin (0.01 μM) and 5±1% and 17±1%, respectively during superfusion with iberiotoxin (p<0.05 versus response before iberiotoxin). In addition, we found that dilation of cerebral arterioles to pinacidil (10 and 100 μM) was 12±1% and 20±0.5%, respectively prior to superfusion with glibenclamide (10 μM) and 4±1% and 12±1%, respectively during superfusion with glibenclamide (p<0.05 versus response before glibenclamide).
To determine the role of oxidative stress in impaired responses of cerebral arterioles to activation of potassium channels in PAE male and female rats, we applied apocynin (1.0 μM) to the cerebral microcirculation for 30 minutes and continued for the duration of the experiment. Topical application of apocynin did not affect baseline diameter of cerebral arterioles in either control rats (males: 43±2 μm prior to versus 45±4 μm during apocynin, p > 0.05; females: 45±2 μm prior to versus 46±3 μm during apocynin, p > 0.05) or in PAE rats (males: 42±4 μm prior to versus 42±3 μm during apocynin, p > 0.05; females: 49±3 μm prior to versus 47±3 μm during apocynin, p > 0.05).
Treatment with apocynin did not alter responses of cerebral arterioles to iloprost (Figure 3) or pinacidil (Figure 4) in either control male or control female rats. In contrast, treatment with apocynin increased responses of cerebral arterioles to iloprost (Figure 3) and pinacidil (Figure 4) in male and female PAE rats towards that observed in their respective controls.
Figure 3.
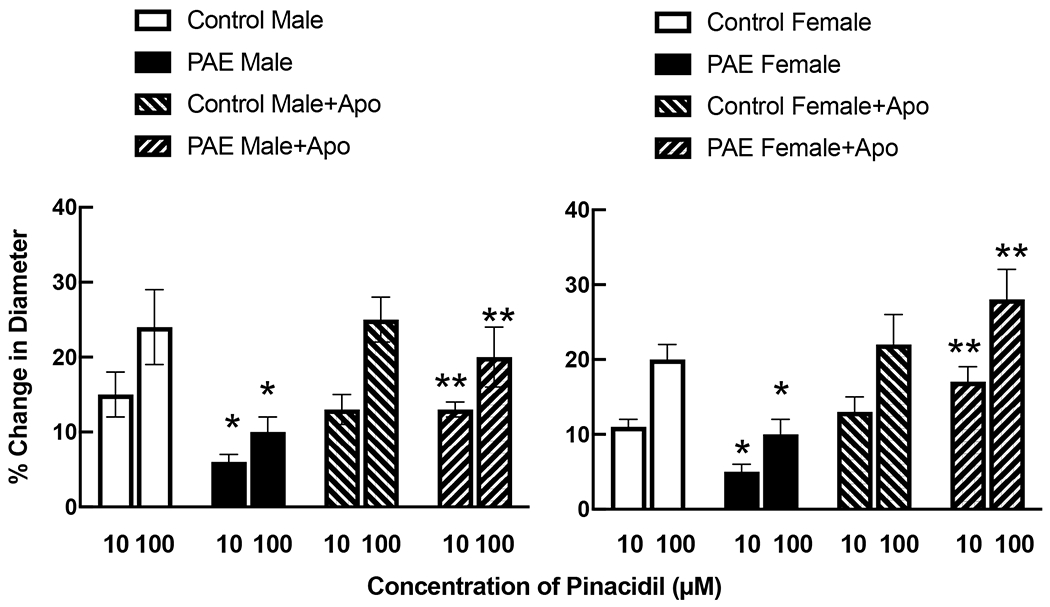
Responses of cerebral arterioles to activation of KATP channels with pinacidil in male and female control and PAE rats before and following treatment with apocynin (100 μM). Values are means±SE. * p < 0.05 versus response in control male or control female. ** p < 0.05 versus response prior to treatment with apocynin. Control data for male and female rats is the same as that reported in Figure 1.
Figure 4.
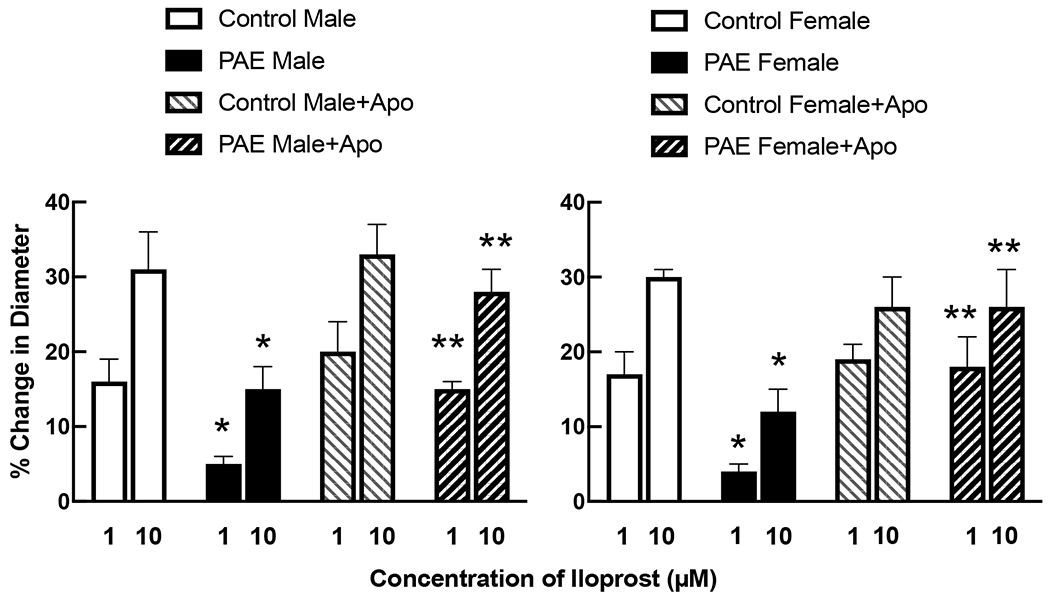
Responses of cerebral arterioles to activation of BK channels with iloprost in male and female control and PAE rats before and following treatment with apocynin (100 μM). Values are means±SE. * p < 0.05 versus response in control male or control female. ** p < 0.05 versus response prior to treatment with apocynin. Control data for male and female rats is the same as that reported in Figure 2.
Discussion
In the present study we characterized in vivo reactivity of cerebral arterioles in response to activation of potassium channels (KATP or BK channels) in rats exposed to alcohol prenatally. There are two major findings from the present study. First, PAE impairs responses of cerebral arterioles in male and female rats to activation of KATP and BK channels. Second, impaired dilation of cerebral arterioles to activation of potassium channels in male and female rats exposed to prenatal alcohol appears to be related to an increase in oxidative stress. We suggest that our findings provide important information regarding PAE-induced dysfunction of cerebral blood vessels in response to stimuli that may activate critical potassium channels.
Influence of KATP channels on cerebral arterioles.
KATP channels have been identified in vascular smooth muscle, including that of cerebral arteries and arterioles (Nelson et al., 1990; Standen et al., 1989; Nelson and Quayle, 1995; Standen et al., 1989). Many studies have reported that activation of potassium channels in response to a variety of agonists, including pinacidil, induce hyperpolarization and dilation of cerebral arteries/arterioles (Faraci and Heistad, 1994; Mayhan and Faraci, 1993; Faraci and Sobey, 1998; Wahl et al., 1994; Ksoll et al., 1991). Other studies have shown that physiological stimuli (hypercapnia, hypotension, hypoxia) dilate cerebral arteries and arterioles, in part, via activation of KATP channels (Nnorom et al., 2014; Lindauer et al., 2003; Santa et al., 2003; Armstead, 1999a; Taguchi et al., 1994; Faraci et al., 1994a). In addition, many studies have shown that dilation of cerebral arterioles in response to activation of KATP channels is altered during a variety of disease states and following cerebral ischemia-reperfusion injury (Matsumoto et al., 2004; Erdos et al., 2004a; Erdos et al., 2002; Bari et al., 1996; Pieper et al., 1997; Mayhan and Faraci, 1993). Thus, KATP channels are active in cerebral arterioles, account for dilation of cerebral arterioles in response to various agonists/physiological stimuli and are impaired during many disease states. In the present study examined dilation of cerebral arterioles in response to activation of KATP channels using pinacidil. To determine the role of pinacidil on KATP channels, we examined responses of arterioles before and after treatment with glibenclamide, an inhibitor of KATP channels. We found that glibenclamide significantly reduced, but did not abolish, dilation of cerebral arterioles to pinacidil. This suggests that dilation of cerebral arterioles to pinacidil is related to activation of KATP channels. The cellular pathway responsible for the residual dilation to pinacidil is not clear from the present study.
The present study is the first to our knowledge to examine the functional role of KATP channels in dilation of cerebral arterioles in male and female rats exposed to alcohol prenatally. We found that responses of cerebral arterioles to activation of KATP channels with pinacidil were qualitatively similar in male and female rats. While PAE significantly impaired responses of cerebral arterioles to pinacidil, this again did not differ between males and females. Thus, sex does not appear to influence responses of cerebral arterioles to activation of KATP channels. This is similar to that reported by others regarding responses of cerebral arterioles to activation of KATP channels (Chrissobolis and Sobey, 2004). Further, PAE-induced impairment to this important dilator pathway is not affected by either in utero sexual differentiation or postnatal maturation.
Influence of BK channels on cerebral arterioles.
BK channels are activated by an increase in intracellular calcium and by depolarization of vascular smooth muscle (Faraci and Sobey, 1998; Faraci and Heistad, 1998). Investigators have shown that BK channels can influence basal tone of large and small cerebral arteries/arterioles (Fujii et al., 1991; Sobey and Faraci, 1997; Horiuchi et al., 2001; Gokina et al., 1996). In addition, many studies have shown that activation of BK channels plays a significant role in dilation of cerebral blood vessels in response to various agonists/physiological stimuli (Nnorom et al., 2014; Leffler et al., 2011; Vincent et al., 2005; Gebremedhin et al., 1994; Armstead, 1999a). Further, similar to KATP channels, disease states can alter responses of cerebral arterioles to activation of BK channels (Erdos et al., 2002; Vetri et al., 2017; Dong et al., 2009).
In the present study, we used iloprost to examine the effects of activation of BK channels on the reactivity of cerebral arterioles in control and PAE male and female rats. Although iloprost has been shown to be a prostacyclin analogue (Wahl et al., 1989), several studies have used iloprost to specifically activate BK channels in cerebral arteries (Erdos et al., 2004b; Erdos et al., 2004a). In the present study, we found that treatment with iberiotoxin significantly reduced, but did not abolish, dilation of cerebral arterioles in response to iloprost. This suggests that dilation of cerebral arterioles to iloprost is related to activation of BK channels. Similar to our findings with pinacidil, we found that responses of cerebral arterioles to activation of BK channels with iloprost were similar in both sexes. In addition, impaired responses of cerebral arterioles to iloprost following PAE were similar in magnitude in male and female rats. Thus, as for KATP channels, sex does not appear to influence responses of cerebral arterioles to activation of BK channels, and PAE produces comparable impairment in BK channel regulation of cerebral arteriolar dilation in each sex.
Taken together, we suggest that the functional significance of impaired reactivity in response to KATP and BK channels by PAE may be realized by contemplating the regulation of cerebral vascular diameter, and hence cerebral blood flow, during many physiological stimuli. First, acute increases in intravascular pressure will depolarize the smooth muscle of cerebral arteries/arterioles which then leads to vasoconstriction. This myogenic response is governed by voltage-gated calcium channels that allow calcium to enter vascular smooth muscle. Increases in intracellular calcium also activate BK channels to produce relaxation of vascular smooth muscle and thus dilation of cerebral arteries/arterioles. Thus, activation of BK channels during increases in pressure may assist in maintaining appropriate vasoconstrictor tone. Given that responses of cerebral arterioles to activation of BK channels are impaired by PAE, we suggest that this may have important implications for the regulation of cerebral blood flow during changes in blood pressure. Second, studies have shown that dilation of cerebral arterioles/changes in cerebral blood flow in response to hypoxia, hypercapnia and cerebral ischemia may be related, in part, to activation of KATP and BK channels. Given that these vasodilator pathways are altered by PAE, we suggest that the regulation of blood flow during changes in metabolic demand may be impaired in individuals exposed to prenatal alcohol. This concept is supported by a previous study (Bake et al., 2017) that found PAE adult mice exhibited a long-term loss of cerebral blood flow following occlusion of the middle cerebral artery which was associated with a decrease in post-stroke recovery. Thus, impaired responses of cerebral vessels to activation of KATP and BK channels may have important implications for the regulation of cerebral blood flow in PAE adults, especially during situations of increased metabolic demand.
Role of oxidative stress.
We and others have shown that PAE increases oxidative stress in many organ systems, including the brain (Saha et al., 2021; Cananzi and Mayhan, 2019a; Cananzi and Mayhan, 2019b). We have shown that this increase in oxidative stress accounted for impaired responses of cerebral arterioles to eNOS- and nNOS-dependent agonists (Saha et al., 2021; Cananzi and Mayhan, 2019a; Cananzi and Mayhan, 2019b). Although no studies to our knowledge have examined the role of an increase in oxidative stress in impaired potassium channel function following PAE, there are studies that have suggested that oxidative stress contributes to impaired K+ channel function in large cerebral and coronary arteries (Erdos et al., 2004a; Armstead, 1999b; Armstead, 2001; Li et al., 2004; Liu and Gutterman, 2002). The mechanism by which an increase is oxidative stress influences potassium channel function remains unknown. It has been suggested that an increase in oxidative stress may inactivate calcium channels, leading to an increase in intracellular calcium, which might reduce the open state probability of potassium channels (#19981} and thus impair potassium channel function. In the present study, we found that acute treatment of the cerebral microcirculation with apocynin reversed impaired responses to iloprost and pinacidil in male and female PAE rats. In contrast, treatment with apocynin did not alter responses of cerebral arterioles in male or female control rats. While many studies have suggested that apocynin acts via inhibition of NADPH oxidase, this is not a universal finding (Heumuller et al., 2008). Thus, the precise cellular mechanisms by which apocynin is inhibiting the influence of oxidative stress on cerebral vascular function cannot be determined in this study.
In summary, we found that responses of cerebral arterioles to activation of KATP and BK channels are similar in male and female control rats. In addition, we found that PAE impaired responses of cerebral arterioles to activation of KATP and BK channels to a similar degree in both sexes. Finally, we found that inhibition of oxidative stress with apocynin could reverse impaired reactivity in male and female PAE rats, but apocynin did not affect responses in control rats. We suggest that our findings have important implications for the pathogenesis of behavior/cognitive dysfunction observed in adolescent and adult subjects exposed to alcohol prenatally.
Sources of Support:
These studies were supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (1 R01 AA027206-01) and funds from the Sanford School of Medicine at the University of South Dakota.
References
- Armstead WM (1999a) Hypotension dilates pial arteries by Katp and Kca channel activation. Brain Research. 816: 158–164. [DOI] [PubMed] [Google Scholar]
- Armstead WM (1999b) Superoxide generation links protein kinase C activation to impaired ATP-sensitive K+ channel function after brain injury. Stroke. 30: 153–159. [DOI] [PubMed] [Google Scholar]
- Armstead WM (2001) Vasopressin induced cyclooxygenase dependent superoxide generation contributes to K+ channel function impairment after brain injury. Brain Research. 910: 19–28. [DOI] [PubMed] [Google Scholar]
- Bake S, Gardner R, Tingling JD, Miranda RC, Sohrabji F (2017) Fetal Alcohol Exposure Alters Blood Flow and Neurological Responses to Transient Cerebral Ischemia in Adult Mice. Alcohol Clin Exp Res. 41: 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari F, Louis TM, Meng W, Busija DW (1996) Global ischemia impairs ATP-sensitive K+ channel function in cerebral arterioles in piglets. Stroke. 27: 1874–1881. [DOI] [PubMed] [Google Scholar]
- Brayden JE, Nelson MT (1992) Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 256: 532–535. [DOI] [PubMed] [Google Scholar]
- Bukiya AN, Dopico AM (2018) Fetal Cerebral Circulation as Target of Maternal Alcohol Consumption. Alcohol Clin Exp Res. 42: 1006–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cananzi SG, Mayhan WG (2019a) In utero exposure to alcohol alters reactivity of cerebral arterioles. J Cereb Blood Flow Metab. 39: 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cananzi SG, Mayhan WG (2019b) In Utero Exposure to Alcohol Impairs Reactivity of Cerebral Arterioles and Increases Susceptibility of the Brain to Damage Following Ischemia/Reperfusion in Adulthood. Alcohol Clin Exp Res. 43: 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrissobolis S, Sobey CG (2004) Influence of gender on K+-induced cerebral vasodilatation. Stroke. 35: 747–752. [DOI] [PubMed] [Google Scholar]
- Daft PA, Johnston MC, Sulik KK (1986) Abnormal heart and great vessel development following acute ethanol exposure in mice. Teratology. 33: 93–104. [DOI] [PubMed] [Google Scholar]
- Dong L, Xie MJ, Zhang P, Ji LL, Liu WC, Dong MQ, Gao F (2009) Rotenone partially reverses decreased BK Ca currents in cerebral artery smooth muscle cells from streptozotocin-induced diabetic mice. Clin Exp Pharmacol Physiol. 36: e57–64. [DOI] [PubMed] [Google Scholar]
- Erdos B, Miller AW, Busija DW (2002) Alterations in KATP and KCa channel function in cerebral arteries of insulin-resistant rats. Am J Physiol Heart Circ Physiol. 283: H2472–7. [DOI] [PubMed] [Google Scholar]
- Erdos B, Simandle SA, Snipes JA, Miller AW, Busija DW (2004a) Potassium channel dysfunction in cerebral arteries of insulin-resistant rats is mediated by reactive oxygen species. Stroke. 35: 964–969. [DOI] [PubMed] [Google Scholar]
- Erdos B, Snipes JA, Miller AW, Busija DW (2004b) Cerebrovascular dysfunction in zucker obese rats is mediated by oxidative stress and protein kinase C. Diabetes. 53: 1352–1359. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Sobey CG (1998) Role of potassium channels in regulation of cerebral vascular tone. J Cereb Blood Flow Metab. 18: 1047–1063. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Breese KR, Heistad DD (1994a) Cerebral vasodilation during hypercapnia: role of glibenclamide sensitive potassium channels and nitric oxide. Stroke. 25: 1679–1683. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD (1994) Responses of cerebral arterioles to N-methyl-D-aspartate and activation of ATP-sensitive potassium channels in old rats. Brain Research. 654: 349–351. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD (1998) Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiological Reviews. 78: 53–97. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Orgren K, Heistad DD (1994b) Impaired relaxation of the carotid artery during activation of ATP-sensitive potassium channels in atherosclerotic monkeys. Stroke. 25: 178–182. [DOI] [PubMed] [Google Scholar]
- Fujii K, Heistad DD, Faraci FM (1991) Flow-mediated dilatation of the basilar artery in vivo. Circulation Research. 69: 697–705. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Bonnet P, Greene AS, England SK, Rusch NJ, Lombard JH, Harder DR (1994) Hypoxia increases the activity of Ca2+-sensitive K+ channels in cat cerebral arterial muscle cell membranes. Pflugers Archives. 428: 621–630. [DOI] [PubMed] [Google Scholar]
- Gokina NI, Wellman TD, Bevan RD, Walters CL, Penar PL, Bevan JA (1996) Role of Ca(2+)-activated K+ channels in the regulation of membrane potential and tone of smooth muscle in human pial arteries. Circ Res. 79: 881–886. [DOI] [PubMed] [Google Scholar]
- Graham DM, Crocker N, Deweese BN, Roesch SC, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Jones KL, Riley EP, Mattson SN (2013) Prenatal alcohol exposure, attention-deficit/hyperactivity disorder, and sluggish cognitive tempo. Alcohol Clin Exp Res. 37 Suppl 1: E338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP (2009) Foetal Alcohol Spectrum Disorders and alterations in brain and behaviour. Alcohol Alcohol. 44: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP (2008) Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 51: 211–217. [DOI] [PubMed] [Google Scholar]
- Horiuchi T, Dietrich HH, Tsugane S, Dacey RG (2001) Role of potassium channels in regulation of brain arteriolar tone. Comparison of cerebrum versus brain stem. Stroke. 32: 218–224. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth P (1973) Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1: 1267–1271. [DOI] [PubMed] [Google Scholar]
- Ksoll E, Parsons AA, Mackert JRL, Schilling L, Wahl M (1991) Analysis of cromakalim-, pinacidil-, and nicorandil-induced relaxation of the 5-hydroxytryptamine precontracted rat isolated basilar artery. Archives of Pharmacology. 343: 377–383. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Parfenova H, Jaggar JH (2011) Carbon monoxide as an endogenous vascular modulator. Am J Physiol Heart Circ Physiol. 301: H1–H11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Gutterman DD, Rusch NJ, Bubolz A, Liu Y (2004) Nitration and functional loss of voltage-gated K+ channels in rat coronary microvessels exposed to high glucose. Diabetes. 53: 2436–2442. [DOI] [PubMed] [Google Scholar]
- Lindauer U, Vogt J, Schuh-Hofer S, Dreier JP, Dirnagl U (2003) Cerebrovascular vasodilation to extraluminal acidosis occurs via combined activation of ATP-sensitive and Ca2+-activated potassium channels. J Cereb Blood Flow Metab. 23: 1227–1238. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gutterman DD (2002) Oxidative stress and potassium channel function. Clin Exp Pharmacol Physiol. 29: 305–311. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Yoshiyama S, Wakabayashi K, Kobayashi T, Kamata K (2004) Effect of chronic insulin on cromakalim-induced relaxation in established streptozotocin-diabetic rat basilar artery. Eur J Pharmacol. 504: 129–137. [DOI] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Vaux K, Jewett T, Elliott AJ, Kable JA, Akshoomoff N, Falk D, Arroyo JA, Hereld D, Riley EP, Charness ME, Coles CD, Warren KR, Jones KL, Hoyme HE (2018) Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. JAMA. 319: 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhan WG, Mayhan JF, Sun H, Patel KP (2004) In vivo properties of potassium channels in cerebral blood vessels during diabetes mellitus. Microcirculation. 11: 605–613. [DOI] [PubMed] [Google Scholar]
- Mayhan WG (1992) Responses of cerebral arterioles during chronic alcohol exposure. American Journal of Physiology. 262: H787–H791. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Faraci FM (1993) Responses of cerebral arterioles in diabetic rats to activation of ATP-sensitive potassium channels. American Journal of Physiology. 265: H152–H157. [DOI] [PubMed] [Google Scholar]
- Nakhoul MR, Seif KE, Haddad N, Haddad GE (2017) Fetal Alcohol Exposure: The Common Toll. J Alcohol Drug Depend. 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Patlak JB, Worley JF, Standen NB (1990) Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 259: C3–18. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM (1995) Physiological roles and properties of potassium channels in arterial smooth muscle. American Journal of Physiology. 268: C799–C822. [DOI] [PubMed] [Google Scholar]
- Nnorom CC, Davis C, Fedinec AL, Howell K, Jaggar JH, Parfenova H, Pourcyrous M, Leffler CW (2014) Contributions of KATP and KCa channels to cerebral arteriolar dilation to hypercapnia in neonatal brain. Physiol Rep. 2: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip S, Armstead WM (2004) NMDA dilates pial arteries by KATP and Kca channel activation. Brain Res Bull. 63: 127–131. [DOI] [PubMed] [Google Scholar]
- Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C (2006) Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. 99: 69–77. [DOI] [PubMed] [Google Scholar]
- Pieper GM, Langenstroer P, Siebeneich W (1997) Diabetic-induced endothelial dysfunction in rat aorta: role of hydroxyl radicals. Cardiovascular Research. 34: 145–156. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR (2012) Vascular effects of maternal alcohol consumption. Am J Physiol Heart Circ Physiol. 303: H414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR (2011) Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. 21: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha PS, Kim Sawtelle KR, Bamberg BN, Arrick DM, Watt MJ, Scholl JL, Zheng H, Mayhan WG (2021) Rosiglitazone restores nitric oxide synthase-dependent reactivity of cerebral arterioles in rats exposed to prenatal alcohol. Alcohol Clin Exp Res. 45: 1359–1369. [DOI] [PubMed] [Google Scholar]
- Santa N, Kitazono T, Ago T, Ooboshi H, Kamouchi M, Wakisaka M, Ibayashi S, Iida M (2003) ATP-sensitive potassium channels mediate dilatation of basilar artery in response to intracellular acidification in vivo. Stroke. 34: 1276–1280. [DOI] [PubMed] [Google Scholar]
- Sobey CG, Faraci FM (1997) Effect of nitric oxide and potassium channel agonists and inhibitors on basilar artery diameter. American Journal of Physiology. 272: H256–H262. [DOI] [PubMed] [Google Scholar]
- Sobey CG, Heistad DD, Faraci FM (1998) Potassium channels mediate dilatation of cerebral arterioles in response to arachidonate. American Journal of Physiology. 275: H1606–H1612. [DOI] [PubMed] [Google Scholar]
- Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT (1989) Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 245: 177–180. [DOI] [PubMed] [Google Scholar]
- Sun H, Zhao H, Sharpe GM, Arrick DM, Mayhan WG (2008) Influence of chronic alcohol consumption on inward rectifier potassium channels in cerebral arterioles. Microvasc Res. 75: 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Zheng H, Molacek E, Fang Q, Patel KP, Mayhan WG (2006) Role of NAD(P)H oxidase in alcohol-induced impairment of endothelial nitric oxide synthase-dependent dilation of cerebral arterioles. Stroke. 37: 495–500. [DOI] [PubMed] [Google Scholar]
- Taguchi H, Heistad DD, Kitazono T, Faraci FM (1994) ATP-sensitive K+ channels mediate dilatation of cerebral arterioles during hypoxia. Circulation Research. 74: 1005–1008. [DOI] [PubMed] [Google Scholar]
- Vetri F, Qi M, Xu H, Oberholzer J, Paisansathan C (2017) Impairment of neurovascular coupling in Type 1 Diabetes Mellitus in rats is prevented by pancreatic islet transplantation and reversed by a semi-selective PKC inhibitor. Brain Res. 1655: 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JM, Kwan YW, Chan SL, Perrin-Sarrado C, Atkinson J, Chillon JM (2005) Constrictor and dilator effects of angiotensin II on cerebral arterioles. Stroke. 36: 2691–2695. [DOI] [PubMed] [Google Scholar]
- Wahl M, Parsons AA, Schilling L (1994) Dilating effect of perivascularly applied potassium channel openers cromakalim and pinacidil in rat and cat pial arteries in situ. Cardiovascular Research. 28: 1803–1807. [DOI] [PubMed] [Google Scholar]
- Wahl M, Schilling L, Whalley ET (1989) Cerebrovascular effects of prostanoids: in situ studies in pial arteries of the cat. Archives of Pharmacology. 340: 314–320. [DOI] [PubMed] [Google Scholar]
- Ware AL, O’Brien JW, Crocker N, Deweese BN, Roesch SC, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Jones KL, Riley EP, Mattson SN (2013) The effects of prenatal alcohol exposure and attention-deficit/hyperactivity disorder on psychopathology and behavior. Alcohol Clin Exp Res. 37: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Mayhan WG, Arrick DM, Xiong W, Sun H (2010) Alcohol-induced exacerbation of ischemic brain injury: Role of NAD(P)H oxidase. Alcohol Clin Exp Res. 34: 1948–1955. [DOI] [PubMed] [Google Scholar]


