Abstract
The hepatitis B virus core antigen (HBcAg) tolerates insertion of foreign epitopes and maintains its ability to self-assemble into virus-like particles (VLPs). We constructed a ΔHBcAg-based VLP vaccine expressing three predicted SARS-CoV-2 B and T cell epitopes and determined its immunogenicity and protective efficacy. The recombinant ΔHBcAg-SARS-CoV-2 protein was expressed in E. coli, purified, and shown to form VLPs. K18-hACE2 transgenic C57BL/6 mice were immunized intramuscularly with ΔHBcAg VLP control (n=15) or ΔHBcAg-SARS-CoV-2 VLP vaccine (n=15). One week after the 2nd booster and prior to virus challenge, five ΔHBcAg-SARS-CoV-2 vaccinated mice were euthanized to evaluate epitope-specific immune responses. There is a statistically significant increase in epitope-specific IgG response, and statistically higher IL-6 and MCP-1 expression levels in ΔHBcAg-SARS-CoV-2 VLP-vaccinated mice compared to ΔHBcAg VLP controls. While not statistically significant, the ΔHBcAg-SARS-CoV-2 VLP mice had numerically more memory CD8+ T-cells, and 3/5 mice also had numerically higher levels of IFN-γ and TNF. After challenge with SARS-CoV-2, ΔHBcAg-SARS-CoV-2 immunized mice had numerically lower viral RNA loads in the lung, and slightly higher survival, but the differences are not statistically significant. These results indicate that the ΔHBcAg-SARS-CoV-2 VLP vaccine elicits epitope-specific humoral and cell-mediated immune responses but they were insufficient against SARS-CoV-2 infection.
Keywords: SARS-CoV-2, Hepatitis B core antigen (HBcAg), virus-like particle (VLP), T cell epitope, B cell epitope, vaccine, humoral immune response, cell-mediated immune response
Introduction
The ongoing COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused unprecedented human suffering worldwide. SARS-CoV-2 in the genus Betacoronavirus of family Coronaviridae1, 2 attaches to host cells via the trimeric spike (S) glycoprotein, which consists of S1 and S2 subunits3, 4. S1 contains the receptor binding domain (RBD), which binds to the human angiotensin converting enzyme 2 (ACE2) receptor and elicits neutralizing antibodies3, 4. The available COVID-19 vaccines are based on the S protein utilizing mRNA, adenoviral-vectored, or protein subunit-based platforms and have demonstrated excellent efficacy5-9 but have short-term protection and require multiple boosters. It is therefore critically important to further identify protective B and T cell epitopes, which will help inform future vaccine designs against not only SARS-CoV-2 variants but other potential emerging coronaviruses as well10.
Epitope-based vaccines consist of immunogenic epitopes and can elicit neutralizing antibodies or a T cell response; the specificity of the epitope-specific neutralizing antibodies can eliminate the possibility of formation of antibodies associated with antibody-dependent enhancement (ADE)11. Numerous in silico studies have predicted B and T cell epitopes in the SARS-CoV-2 S protein12-21, but whether such epitopes are protective against SARS-CoV-2 infection is not well studied. Virus-like particles (VLPs) are nanoparticles that resemble the native virion structure but do not contain viral nucleic acids22, 23. These non-infectious and non-replicating VLPs are an attractive vaccine platform due to their ability to express foreign epitopes and induce both humoral and cell-mediated immune (CMI) responses23-25.
The hepatitis B virus core antigen (HBcAg) is a VLP-forming polymer consisting of 183 amino acid (aa) monomers with an assembly region (aa 1-149, denoted as ΔHBcAg) and a basic C-terminus region (aa 150-183), which can be removed without affecting VLP assembly22, 26-30. The ΔHBcAg VLP contains repeating spikes on the surface of either a T=3 or T=4 capsid, and a major immunodominant region (MIR) at the tips of the spikes between aa 76 and 8122, 28, 30, 31. The ΔHBcAg VLP is stable, and importantly, insertions of foreign epitopes at the MIR, the N- and C- terminus, do not affect VLP self-assembly29, 32-35. Additionally, native ΔHBcAg is highly immunogenic and can induce T cell-dependent and -independent antibody production and activate both T-helper CD4+ and cytotoxic CD8+ T lymphocytes27, 29, 36-40. The immunogenic characteristics of native ΔHBcAg can be conferred to epitopes inserted in the VLP22, 31, 33, 41-44. Therefore, the ΔHBcAg VLP is an attractive platform for expressing SARS-CoV-2 B and T cell epitopes.
In this study we constructed a recombinant ΔHBcAg-based SARS-CoV-2 VLP candidate vaccine expressing three SARS-CoV-2 S1 epitopes and evaluated its immunogenicity and protective efficacy in K18-hACE2 transgenic C57BL/6 mice.
Materials and Methods
Rational design of SARS-CoV-2 VLP vaccine:
We performed an analysis of predicted B and T cell epitopes of SARS-CoV-2 S1 based on bioinformatics and immunogenicity data. Databases utilized included the Immunome Browser and the BepiPred Linear Epitope Prediction tools in the Immune Epitope Database (IEDB). The criteria for selecting optimal B cell epitopes included: (1) epitopes that were predicted to elicit neutralizing antibodies or antibodies that disrupt virus-host cell receptor interactions, (2) epitopes that are not present in the human proteome, and (3) epitopes that are not associated with ADE. The criteria for selecting T cell epitopes included: (1) positive T cell and major histocompatibility complex (MHC) binding assays, (2) the possibility of eliciting strong CMI responses, and (3) epitopes that can bind both MHC I and MHC II.
Construction of ΔHBcAg-SARS-CoV-2 VLP candidate vaccine:
Three selected SARS-CoV-2 epitopes were inserted into the backbone sequence of ΔHBcAg: the B cell epitope (B2) was inserted between an N-terminal 6-Histidine tag (His-6×) and ΔHBcAg aa 1; the dual B and T cell epitope (B/T1) was inserted at the MIR between aa 79 and 80; and the T cell epitope (T2) was placed at the C-terminus after aa 149. Both the ΔHBcAg and ΔHBcAg-SARS-CoV-2 constructs included an N-terminal His-6× to aid in protein purification. The sequences for both constructs were codon optimized for protein expression in E. coli, commercially synthesized and cloned into pET-28a(+) plasmids (GenScript, Piscataway, NJ). Plasmids were transformed into E. coli DH5α cells by heat shock. The inserted sequences were confirmed via restriction enzyme digestion and Sanger sequencing. The verified plasmids were transformed into BL21 (DE3) E. coli cells, cultured in LB broth with kanamycin, pelleted, and stored at −80°C in 20% glycerol.
Expression and purification of ΔHBcAg and ΔHBcA-SARS-CoV-2 proteins:
BL21 (DE3) E. coli cells containing plasmids were grown as previously described44. Once the OD600 reached 0.6-0.8, the cells were induced with 1.0 mM isopropyl-β-D-thiogalactopyranoside (IPTG, Sigma Aldrich) and incubated for an additional 4 hours at 28°C and then pelleted by centrifugation. Cells were resuspended and sonicated in lysis buffer and centrifuged as previously described45. The ΔHBcAg protein was detected in the soluble fraction by SDS-PAGE and therefore this lysis supernatant was collected for protein purification. The ΔHBcAg-SARS-CoV-2 protein was present in the insoluble fraction and therefore an additional step in solubilization buffer (20mM Na2HPO4, 50mM NaCl, 2M Urea, 0.9% Sarkosyl, pH 7.0) was performed overnight. The samples were then centrifuged and the supernatant was collected for protein purification. Purification included anion exchange chromatography followed by immobilized metal affinity chromatography (IMAC). IMAC samples underwent step-wise dialysis to decrease sarkosyl concentration to 0% and ultracentrifugal filtration to remove unassembled protein as described previously44. Samples were stored in 1× PBS (without Ca2+ and Mg2+) at −80°C until use. Purified ΔHBcAg and recombinant ΔHBcAg-SARS-CoV-2 VLPs were tested for endotoxin levels with Pierce Chromogenic Endotoxin Quant Kit (Thermo Scientific) following the manufacturer’s protocol.
Transmission electron microscopy (TEM) to evaluate VLP assembly of purified ΔHBcAg and ΔHBcAg-SARS-CoV-2 proteins:
A formvar carbon coated copper grid was incubated in 1% aqueous Alcian blue for 5 minutes and washed with deionized water. Protein samples (25μL) were applied to the grid for 45 seconds and excess sample was removed with filter paper. Next, 3% phosphotungstic acid (25μL; pH 7.03) was applied to the grid for 45 seconds, and excess was removed with filter paper. The grids were examined for VLPs by TEM (JEOL JEM 1400, Peabody, MA) and images captured at 300,000× magnification.
SDS-PAGE and Western blot analyses to characterize VLP proteins:
The purified VLP samples were separated by SDS-polyacrylamide gel electrophoresis using Mini-PROTEAN TGX precast gels (Bio-Rad, Hercules, CA). Gels were stained with Bio-safe Coomassie stain and imaged on a ChemiDoc Imaging System (Bio-Rad). To confirm the authenticity of the proteins, the separated proteins were transferred from gel to polyvinylidene difluoride (PVDF) membranes. The PVDF was blocked for 2 hours with Odyssey TBS Blocking Buffer (LI-COR Biosciences, Lincoln, NE, USA), and Western blot analysis was performed as previously described46. The primary antibody was a 6×-His Tag Monoclonal Antibody (1:2000, Invitrogen) and the secondary antibody, IRDye 800CW donkey anti-mouse IgG (1:5000, LI-COR Biosciences).
Formulation of VLP vaccines:
ΔHBcAg control and ΔHBcAg+SARS-CoV-2 VLP vaccines formulations included: 20μg protein in 25μL PBS (without Ca2+ or Mg2+), and 25μL AddaVax squalene-based oil-in-water adjuvant (InvivoGen, San Diego, CA).
Experimental design for immunogenicity and challenge study in K18-hACE2 C57BL/6 mice:
All animal procedures were approved by the Virginia Tech Institutional Animal Care and Use Committee (protocol no. 20-232). A total of 30 mice of at least 6 weeks old (19 males, 11 females; bred at Virginia Tech using breeding pair from the Jackson Lab) were divided into a ΔHBcAg-SARS-CoV-2 VLP vaccine group and ΔHBcAg VLP control group (n=15/group). After one week acclimation, mice were immunized intramuscularly with 20 μg of adjuvant formulated ΔHBcAg-SARS-CoV-2 VLP vaccine or ΔHBcAg VLP control. Twenty-one days post-prime immunization (DPI21), each mouse received a booster dose of 20 μg, and at DPI42, a 2nd booster dose of 40 μg. Blood was collected via the retroorbital sinus just prior to each immunization. One week after the 2nd booster (DPI49), five mice in each group were euthanized to evaluate humoral and CMI parameters.
Three weeks after the 2nd booster (DPI63), the remaining 10 mice from each group were moved to ABSL-3 containment, and each mouse was challenged intranasally with SARS-CoV-2/human/USA-WA1/2020 (5x104 PFU/mouse in 20 uL). Five mice from each group were euthanized on day 3 post-challenge (DPC3), and the remaining mice at the end of study (DPC8). Subjects were monitored daily post-challenge and scored on a scale of 1-4 for clinical signs (daily weight loss, quality of pelage, activity level and responsiveness). Animals graded at Stage 4 were humanely euthanized. A panel of tissues (lung, brain, kidney, spleen) were collected at each necropsy.
ELISA to detect SARS-CoV-2 S1 epitope-specific antibodies:
Epitope-specific IgG antibody titers in sera of immunized mice were measured via ELISA, essentially as described47. All sera were heat-inactivated at 56°C for 30 minutes. Ninety-six well microtiter plates were initially coated with 10 μg/mL B/T1 epitope peptide. Overnight incubation at 4°C with a secondary antibody consisting of goat anti-mouse IgG, Fcγ fragment specific, peroxidase-conjugated, polyclonal antibody) (Jackson ImmunoResearch, West Grove, PA). Plates were developed with Sigmafast o-Phenylenediamine dihydrochloride (OPD) for 30 minutes at room temperature (Sigma-Aldrich, St Louis, MO). The reaction was stopped with 0.15 M oxalic acid dihydrate and optical density measured at 492 nm.
Plaque Reduction Neutralization Test (PRNT) to detect SARS-CoV-2 neutralizing antibodies:
Vero E6 cells were seeded on a 12-well plate in Dulbecco’s minimal essential medium (DMEM; Gibco-Thermo Fisher, Waltham, MA, USA). Serially diluted serum samples were incubated with SARS-CoV-2 (USA-WA1/2020; 30-40 PFU) and added to the pre-seeded 12-well plates, incubated, and fixed as previously described48. Plates were stained with 0.2% crystal violet and plaques were counted manually.
Isolation of mononuclear cells (MNC) from spleen to assess cell-mediated immune responses:
One week after the 2nd booster and prior to virus challenge, five mice in each group were humanely euthanized. At necropsy, spleen from each mouse was collected and immediately placed in 5 mL sterile RPMI media (GIBCO) plus 5% FBS. Splenic tissue was processed through a 70μm strainer to remove stromal tissue, and the remaining cells were resuspended in the same media and centrifuged (450 g, 5 minutes, 10°C). The pellet was resuspended in 5 mL 1× RBC Lysis Buffer (Invitrogen, Carlsbad, CA) to lyse erythrocytes. The reaction was neutralized with 10 mL RPMI/5% FBS media and centrifuged again, and the cell pellet was resuspended in 5 mL RPMI/5% FBS. After a cell count was obtained, cells were again pelleted and then resuspended in Complete Mouse Medium (RPMI, L Glutamine, 10% FBS, GIBCO).
Flow cytometry to determine SARS-CoV-2 S1 epitope-specific T-cells:
The frequency of activated, effector and memory, SARS-CoV-2 S1 epitope-specific, CD4+ and CD8+ T-cells from the MNCs in spleen were determined by flow cytometry. The three study epitope peptides (B2, B/T1, T2) were pooled together. In a 96-well, U-bottom plate, 0.3 x 106 cells / well were stimulated with 1 μg of the SARS-CoV-2 S1 pooled peptides. Positive control splenocytes were stimulated with anti-CD3 + anti-CD28 cocktail (~0.15 μg each), and negative control cells were not stimulated. The cells were incubated at 37°C, 5% CO2, for 72 hrs and then stained with APC-Cy7 hamster anti-mouse CD3e (1 μL; Clone 145-2C11, Cat# 561042), PerCP-Cy 5.5 rat anti-mouse CD4 (1 μL; RM4-5, 561115), FITC rat anti-mouse CD8a (1 μL; 53-6.7, 561966), PE-Cy7 rat anti-mouse CD25 (1 μL; PC61, 561780), PE rat anti-mouse CD44 (1 μL, IM7, 561860), and APC rat anti-mouse CD62L (1 μL; MEL-14, 561919) per the manufacture’s protocol. The samples were acquired using BD FACSARIA (BD Biosciences) and analyzed with FlowJo (BD Biosciences).
Cytometric Bead Array to characterize cytokine responses to SARS-CoV-2 vaccination:
The cytokine (IL-6, IL-10, MCP-1, IFN-γ, TNF, and IL-12p70) responses to SARS-CoV-2 vaccination were assessed on the supernatant collected from the stimulated splenocytes as described above using Cytometric Bead Array-Mouse inflammation kit (Cat# 552364, BD Biosciences). Results were compared to mouse inflammation standards per manufacturer’s instructions.
RNA extraction and RT-qPCR for quantification of SARS-CoV-2 RNA loads in lung tissues:
The homogenized lung tissue suspension (20% V/V) from each mouse was used for extracting total RNAs using TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH, USA), according to manufacturer’s instructions. The extracted RNAs were resuspended in 30 μL water (RNAse and DNAse-free) and quantified by a one-step qPCR protocol per manufacturer’s instructions (SensiFAST™ Probe No-ROX One-Step Kit, Thomas Scientific, Swedesboro, NJ, USA). The PCR assay for quantifying SARS-CoV-2 RNA utilizes the N1 primer/probe set from a 2019-nCoV RUO kit (IDT), which targets the nucleocapsid gene as originally developed by the CDC49. Ten-fold serial dilutions of SARS-CoV-2 RNA standards ranging from 101 – 104 were used for the quantification of viral RNA loads (copy number) by a standard curve analysis. The detection limit of the assay was 10 genomic copies per reaction.
Histopathologic examination:
Lung tissues from necropsy were routinely processed in 10% formalin for examination by a board-certified pathologist that was blinded to subject identification and treatment group. Severity of histological lung lesions was scored on a scale of 0-5: 0 (normal), 1 (minimal), 2 (mild), 3 (moderate), 4 (severe, focal), and 5 (severe, multifocal or diffuse) and type of inflammation observed was recorded.
Statistical analysis:
The Kruskal-Wallis rank sum test was used to compare results from CBA, flow cytometry, lung lesion scores, and viral RNA load in the lung, between the ΔHBcAg control and ΔHBcAg-SARS-CoV-2 vaccine groups. Repeated measures ANOVA was used to analyze changes in weight post-challenge and SARS-CoV-2 epitope-specific IgG antibody titers in serum. Survival time was modeled via Kaplan-Meier curves and group differences were analyzed using the log-rank test. P-values < 0.05 were considered statistically significant. All analyses were performed utilizing SAS OnDemand for Academics and graphics created in JMP Pro.
Results
Three selected SARS-CoV-2 S1 B and T cell epitopes were predicted to be immunogenic:
Based on the established selection criteria, we identified three potential epitopes, including a dual B and T cell epitope (B/T1), a B cell epitope (B2), and a T cell epitope (T2). The B/T1 (371SASFSTFKCYGVSPTKLNDL390) is a dual B and T cell epitope located distal to the RBD that is well conserved between SARS-CoV and SARS-CoV-2 and is predicted to elicit a strong antibody response; furthermore, the core sequence of this epitope is not present in the human proteome12-14, 20. The majority of this B/T1 epitope was also predicted to be a dominant Type I MHC T cell epitope by the Immunome Browser (IEDB). The B2 epitope (562FQQFGRDIA DTTDAVRDPQT581) is located downstream of the receptor binding motif (RBM) of the RBD and predicted to be a B cell epitope by the BepiPred Linear Epitope Prediction tool (IEDB). This B2 epitope shares similarities with a region of the SARS-CoV S protein that produces neutralizing antibodies against SARS-CoV17, 21. The T2 epitope (440NLDSKVGGNYNYLYRLFRKSN460) is within the RBD, and majority of its sequence (448NYNYLYRLFR457) is predicted to be a dominant class II T cell epitope 15, 16, 18, 19.
ΔHBcAg and ΔHBcAg-SARS-CoV-2 proteins were successfully expressed in E. coli, recovered with high purity, and authenticated by Western blot analysis.
Successful insertions of the identified B and T cell epitopes and the His-6× tag into the ΔHBcAg backbone sequence cloned in pET-28a(+) plasmid (Fig. 1) were confirmed by Sanger sequencing and restriction enzyme digestion. SDS-PAGE showed bands of the expected size of the expressed proteins, thereby confirming successful transformation of each recombinant plasmid and subsequent IPTG-induced expression in BL21 (DE3) E. coli cells. A two-step purification process yielded a highly pure product in both the ΔHBcAg (Fig. 2A) and ΔHBcAg-SARS-CoV-2 (Fig. 2B) proteins. The identity of each expressed protein was confirmed by Western blot analysis with anti-His-6× antibody (Fig. 2C). Endotoxin levels of both purified antigens were found to be <1 EU/mL. We also showed that both the ΔHBcAg (Fig. 3A) and ΔHbcAg-SARS-CoV-2 (Fig. 3B) proteins self-assembled into VLPs of approximately 30-35 nm in diameter by TEM. The results indicated that the three selected B and T cell epitopes were successfully incorporated into the ΔHBcAg, expressed, purified at high concentration with high purity, and formed VLPs.
Figure 1. Schematic diagram of ΔHBcAg control and ΔHBcAg-SARS-CoV-2 VLP vaccine designs.

Each construct is composed of the hepatitis B core antigen absent the C-terminal aa 150-183 (ΔHBcAg), with an inserted N-terminal poly-histidine tag (His-6×) to allow for protein purification by immobilized metal affinity chromatography (IMAC). (A) ΔHBcAg control construct with a molecular weight of 19 kDa for the expected protein. (B) SARS-CoV-2 VLP vaccine construct containing the ΔHBcAg backbone with insertions of a 20-aa SARS-CoV-2 B cell epitope (B2) at the N-terminal region, a 21-aa dual B and T cell epitope (B/T1) at the major immunodominant region (MIR) between aa 79 and 80, and a 21-aa T cell epitope (T2) at the C-terminus. The molecular weight of the expected recombinant protein is 26 kDa.
Figure 2. Expression and purification of recombinant ΔHBcAg control and ΔHBcAg-SARS-CoV-2 vaccine proteins.
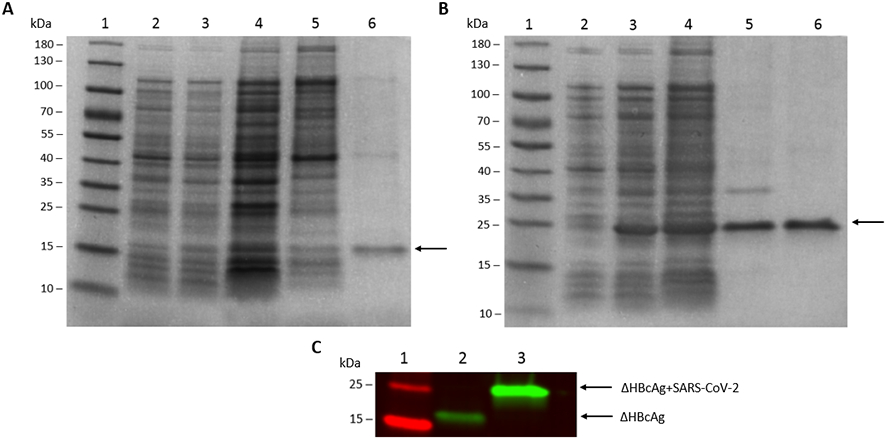
A two-step protein purification process yielded a high purity protein for both the ΔHBcAg control and ΔHBcAg-SARS-CoV-2 vaccine antigens. (A) ΔHBcAg control, showing an SDS-PAGE of different protein fractions during the purification process. Lane 2: uninduced E. coli; Lane 3: IPTG-induced E. coli; Lane 4: cell lysate; Lane 5: purified ΔHBcAg control protein after anion exchange chromatography (AEC); Lane 6: purified ΔHBcAg control protein following immobilized metal affinity chromatography (IMAC). An arrow indicates the ΔHBcAg control protein with an expected molecular weight of 19 kDa. (B) ΔHBcAg-SARS-CoV-2 vaccine antigen, showing an SDS-PAGE of protein fractions during the purification process; lane labels are the same as described for control in (A). An arrow indicates the ΔHBcAg-SARS-CoV-2 protein with an expected molecular weight of 26 kDa. (C) Western blot analysis with anti-His-6 tag antibody of ΔHBcAg control protein (lane 2) and ΔHBcAg-SARS-CoV-2 vaccine protein (lane 3) following AEC and IMAC. Lane 1: protein marker in kDa (PageRuler prestained protein ladder, ThermoFisher Scientific, Cat #26616).
Figure 3. Transmission electron microscopy (TEM) images of purified ΔHBcAg control (A) and ΔHBcAg-SARS-CoV-2 vaccine (B) VLPs.

The purified protein samples were processed with negative staining with 3% phosphotungstic acid (PTA) at pH 7.03. Images taken at 300,000× magnification; scale bar = 200 nm.
ΔHBcAg-SARS-CoV-2 VLP vaccine stimulated an epitope-specific IgG antibody response:
The experimental design for the animal study included an initial immunization followed by two boosters, and three different necropsy time points (Fig. 4). Mice that received the ΔHBcAg-SARS-CoV-2 VLP vaccine had a significant increase in B/T1 epitope-specific IgG antibodies at DPI42 (p=0.045) and DPI49 (p=0.045) compared to baseline (Fig. 5A). The ΔHBcAg-SARS-CoV-2 vaccinated mice also had significantly higher overall trends of B/T1 epitope-specific IgG titer compared to ΔHBcAg controls (p=0.035). Detection of B/T1 epitope-specific IgG response in ΔHBcAg-SARS-CoV-2 vaccinated mice is supportive of surface presentation of the B/T1 epitope. ELISA for IgG antibody response to the B2 epitope did not detect B2 epitope-specific IgG antibody response in either group.
Figure 4. Experimental design for immunogenicity and challenge study in K18-hACE2 transgenic C57BL/6 mice.

The animals were each vaccinated with 20 μg of either a ΔHBcAg VLP control plus adjuvant (control group; n=15), or ΔHBcAg-SARS-CoV-2 VLP vaccine plus adjuvant (vaccine group; n=15). A booster was administered on DPI21 (20 μg) and again on DPI42 (40 μg). One week after the 2nd booster (DPI49), 5 mice from each group were necropsied, and spleen from each animal was harvested to evaluate cell-mediated immune parameters via flow cytometry and cytokine assay. Three weeks after the 2nd booster, mice were moved to ABSL-3 animal facilities and subsequently each challenged with an intranasal administration of infectious SARS-CoV-2 (5x104 PFU/mouse). Five mice from each group were necropsied at DPC3 and DPC8, respectively. DPI: Days post-inoculation; DPC: Days post-challenge.
Figure 5. Humoral and cell-mediated immune responses one week after 2nd booster vaccination at 49 days post-immunization in ΔHBcAg-SARS-CoV-2 VLP vaccinated mice (n=5) and ΔHBcAg VLP control mice (n=5).

(A) ΔHBcAg-SARS-CoV-2 VLP vaccinated mice had significantly higher serum levels of epitope-specific IgG antibodies against the B/T1 epitope compared to the ΔHBcAg control mice (p=0.035), and significantly higher IgG antibody levels at DPI42 (p=0.045) and DPI49 (p=0.045) compared to baseline levels. (B) Spleen was harvested at necropsy and splenocytes were isolated and subjected to stimulation with: (1) pooled peptides of the 3 SARS-CoV-2 epitopes included in the VLP vaccine, (2) CD28 (positive control), and (3) no stimulation (negative control). Results are shown only for the cells stimulated with the specific SARS-CoV-2 epitopes and expressed as percentage of CD3+ cells. Cells stimulated with specific SARS-CoV-2 peptides showed numerically higher frequencies in CD4+ or CD8+ cell responses, but not statistically different, between the ΔHBcAg-SARS-CoV-2 VLP vaccinated mice and control mice. (C) Supernatant from the stimulated cells in (B) was collected for cytometric bead assay to evaluate a panel of cytokine concentrations. Cytokines are presented in two graphs because of differences in the scale of mean concentrations. The ΔHBcAg-SARS-CoV-2 VLP vaccinated mice stimulated with a pool of three SARS-CoV-2 peptides had significantly higher levels of IL-6 (p=0.029) and MCP-1 (p=0.031) compared to controls. IFN-γ production was subjectively higher in 3 of the 5 ΔHBcAg-SARS-CoV-2 VLP vaccinated mice; however, the other 2 vaccinated mice were comparable to controls and when comparing means, the two groups were not significantly different. DPI: Days post-inoculation.
ΔHBcAg-SARS-CoV-2 VLP vaccine supports B cell proliferation and IgG production and initiates a Th1 cell-mediated immune response:
MNCs isolated from ΔHBcAg-SARS-CoV-2 VLP vaccinated mice that were stimulated with the study SARS-CoV-2 S1 peptides had numerically higher frequencies of central memory CD8+ T-cells (CD44+CD62L+) compared to ΔHBcAg controls, though this difference was not statistically significant (Fig. 5B). The number of naïve (CD44-CD62+), effector memory (CD44+CD62L-) and activated T-cells (CD25+) for both CD4+ and CD8+ T cells were similar between the two groups. Levels of IL-6 (p=0.029) and MCP-1 (p=0.033) in the supernatant of MNCs stimulated with study-specific SARS-CoV-2 S1 peptides were significantly higher in the ΔHBcAg-SARS-CoV-2 VLP vaccinated mice compared to that from ΔHBcAg controls (Fig. 5C). The levels of IFN-γ and TNF were subjectively higher in ΔHBcAg+SARS-CoV-2 VLP mice; however, 2 of the 5 ΔHBcAg-SARS-CoV-2 VLP animals did not respond after stimulation, which led to insufficient statistical power to determine a difference.
ΔHBcAg-SARS-CoV-2 VLP vaccinated mice had numerically lower viral RNA loads in lung after challenge with SARS-CoV-2:
Ten mice from each group were challenged intranasally with SARS-CoV-2, and the infected mice were monitored for up to 8 DPC. On DPC3, five mice in each group were necropsied. Three mice exhibited severe disease following virus challenge (1 control mouse at DPC5; 1 control and 1 vaccinated mouse each at DPC6) and were humanely euthanized; the remaining mice were euthanized on DPC8. ΔHBcAg-SARS-CoV-2 VLP vaccinated mice had numerically lower viral RNA loads in the lung at DPC3 and DPC8 (more noticeable difference at DPC3) compared to ΔHBcAg controls, though this difference was not statistically significant (Fig. 6).
Figure 6. Viral RNA loads in lung tissues of mice after challenge with SARS-CoV-2.

The viral loads are estimated by a SARS-CoV-2-specific RT-qPCR from homogenized lung tissues of each animal collected at each necropsy. The viral loads in the lung from the ΔHBcAg-SARS-CoV-2 VLP vaccinated mice are numerically lower than that in the ΔHBcAg control mice at both DPC3 and DPC8 (particularly at DPC3), although the differences are not statistically significant. The detection limit of the assay was 10 genomic copies per reaction.
ΔHBcAg-SARS-CoV-2 VLP vaccine confers only limited protection against virus challenge:
No difference in weight loss was noted prior to DPC3. In animals necropsied after DPC3 (Fig. 7A), both ΔHBcAg-SARS-CoV-2 VLP vaccine (n=5) and ΔHBcAg VLP control (n=5) animals lost a significant percentage of body weight compared to the day of challenge (p<0.01); however, there was no difference in weight loss between the two groups. Survival rates after SARS-CoV-2-challenge past DPC3 were compared between ΔHBcAg-SARS-CoV-2 VLP-vaccinated and ΔHBcAg VLP control mice. There was a slightly higher survival probability of ΔHBcAg-SARS-CoV-2 VLP mice, but this difference was not statistically significant (p=0.22) (Fig. 7B). Histological lesion scoring of lungs was similar between vaccinated and control animals. Despite the detection of significant levels of B/T1 IgG antibody response in ΔHBcAg-SARS-CoV-2 VLP mice, there was no significant difference in neutralizing antibodies between the two groups.
Figure 7. Weight loss and survival probability in ΔHBcAg-SARS-CoV-2 VLP vaccinated or ΔHBcAg control mice challenged with SARS-CoV-2 that survived at least 3 days post-challenge (DPC).

(A) In animals necropsied after DPC3, both ΔHBcAg-SARS-CoV-2 VLP vaccinated (n=5) and ΔHBcAg VLP control (n=5) animals lost a significant percentage of body weight compared to the day of challenge (p<0.01); however there was no statistical difference in weight loss between the two groups at the end of the study. (B) There was a slightly higher survival probability in the ΔHBcAg-SARS-CoV-2 VLP vaccinated mice than in the ΔHBcAg control mice but there was no statistical difference between the two groups.
Discussion
Emerging coronaviruses like SARS-CoV-2 will likely continue to be a threat to global public health and ongoing research is necessary to identify conserved immunogenic B and T cell epitopes that can be incorporated into novel vaccines. Rational design of VLP vaccines can potentially produce a universal vaccine when conserved epitopes are included, and a vaccine with an enhanced safety profile by excluding epitopes associated with ADE11. The HBcAg-based VLP is an ideal candidate as it is highly immunogenic and can self-assemble into a stable VLP even with multiple insertions of foreign antigens27, 30, 35, 36.
We first rationally selected three B and T cell epitopes that were predicted to be immunogenic and incorporated them into three unique sites of the ΔHBcAg backbone to produce a recombinant ΔHBcAg-SARS-CoV-2 VLP vaccine (Fig. 1). We showed that the recombinant ΔHBcAg-SARS-CoV-2 vaccine and ΔHBcAg control proteins were expressed and purified from E. coli self-assembled into VLPs (Fig. 3). We found that K18-hACE2 transgenic C57BL/6 mice that were vaccinated with ΔHBcAg-SARS-CoV-2 VLP vaccine developed an IgG antibody response specific to the dual B and T cell epitope (B/T1) inserted in the MIR of ΔHBcAg (Fig. 5A). However, an IgG antibody response to the second B cell epitope (B2) inserted at the N-terminus was not detected. The MIR site is the preferred site for insertion of foreign sequences, which are expressed and displayed on tips of surface spikes of VLPs. Therefore, the detection of B/T1 epitope-specific antibody in vaccinated mice is expected. However, the B2 epitope was inserted into the N-terminus, and it was reported that foreign epitopes presented at this site were not readily accessible from the surface22, which likely explains the lack of detectable B2-specific IgG antibody response. Addition of a linker sequence prior to the B2 epitope in future studies may help display epitopes inserted at the N-terminus on the surface to elicit the desired humoral immune response22. Nevertheless, we demonstrated that the dual B and T cell epitope B/T1 expressed in ΔHBcAg-SARS-CoV-2 VLP elicited epitope-specific humoral immune response.
The ΔHBcAg VLPs elicit both CD4+ and CD8+ T cell activation (Fig. 5B), and the T cell epitopes included in the vaccine were predicted to bind both MHC I and II and stimulate a memory response in both CD4 and CD8 T cells15, 16, 18, 19. Our results showed that there was some evidence of CD8 memory T cell activation, with numerically increased levels in ΔHBcAg-SARS-CoV-2 VLP mice compared to the ΔHBcAg controls, although this did not achieve statistical significance. However, there was evidence of T cell activation in the ΔHBcAg-SARS-CoV-2 VLP mice, with statistically higher levels of IL-6 and MCP-1 production, as well as numerically higher levels of Th1-associated cytokines IFN-γ and TNF in 3 out of 5 ΔHBcAg-SARS-CoV-2 VLP mice (Fig. 5C). As stated above, it is high likely that the dual B and T cell epitope B/T1 inserted in the MIR was correctly presented on the surface of our VLP vaccine. A previous study reported that inserted antigens at the C-terminus, where the T2 epitope was inserted in this study, had a low immunogenicity22. Flow cytometry and cytokine analyses were based on cells stimulated by a pooled sample of the three study SARS-CoV-2 S1 epitope peptides, therefore, it is not possible to determine the individual contribution of the two individual T cell epitopes (B/T1 vs T2) on the CMI response. Regardless, we showed that the ΔHBcAg-SARS-CoV-2 VLP vaccinated mice did develop a specific T cell immune response.
Despite evidence of induction of antigen-specific B and T cell immune responses in vaccinated mice, we did not detect neutralizing antibodies. We did find that there were numerically lower viral loads, particularly at DPC3, in the lung (Fig. 6) and slightly lower overall mortality compared to ΔHBcAg control (Fig. 7B), but these differences were not statistically significant. The PCR assay used in this study, which targets the nucleocapsid gene, does not distinguish between genomic and subgenomic RNA (sgRNA) but is a sensitive method of virus detection because of the relative abundance of nucleocapsid sgRNA following SARS-CoV-2 infection49. In this study, unvaccinated mice experienced a lower mortality than expected following virus challenge50. We administrated a moderate challenge dose of SARS-CoV-2 (5x104 PFU/mouse) in an attempt to delay mortality and allow for evaluation of vaccine-induced response to infection at multiple time points. Also, in this study the animals were challenged with a smaller volume of inoculum (20 uL) compared to previous studies (up to 50 uL)50. It is possible that an increased challenge dose and a higher inoculum volume will facilitate more severe viral infection and mortality. Additionally, it is possible that this study (n=10 per group for virus challenge) did not carry enough power to detect a difference between these two groups, and the use of larger number of animals per group in the future may provide a more definitive conclusion. We used an intramuscular route of immunization of either 20 μg (first two doses) or 40 μg (2nd booster). Further optimization studies of the SARS-CoV-2 VLP vaccines, including identifying optimal route and dose of vaccination, testing different adjuvants, and identifying additional SARS-CoV-2 immunogenic epitopes, may bolster immunity and elicit better protection
In summary, we successfully incorporated three immunogenic B and T cell epitopes of SARS-CoV-2 S into ΔHBcAg, expressed and purified a recombinant ΔHBcAg-SARS-CoV-2 protein that self-assembles into VLPs. We further demonstrated that K18-hACE2 mice vaccinated with the ΔHBcAg-SARS-CoV-2 VLP vaccine elicited epitope-specific humoral and CMI responses. However, the ΔHBcAg-SARS-CoV-2 VLP vaccine conferred only limited protection against SARS-CoV-2 infection, as there were numerically lower viral loads in the lung and lower overall mortality in vaccinated mice.
Acknowledgments
This work is funded internally by Virginia-Maryland College of Veterinary Medicine at Virginia Tech, and by grants from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI153433 to AJA. AJA is partially supported by a USDA National Institute of Food and Agriculture, Hatch award VA-160103.
We would like to acknowledge Dr. Nathalie del Pilar Becerra Mora and Sandy Hancock for their assistance with obtaining TEM images.
Footnotes
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that influenced the work reported herein.
Data Availability
Study data are available upon request.
References
- 1.Cui J, Li F and Shi Z-L, Origin and evolution of pathogenic coronaviruses. Nature Reviews Microbiology, 2019. 17(3): p. 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, Penzar D, Perlman S, Poon LLM, Samborskiy DV, Sidorov IA, Sola I, Ziebuhr J and V. Coronaviridae Study Group of the International Committee on Taxonomy of, The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology, 2020. 5(4): p. 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C and Pöhlmann S, SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell, 2020. 181(2): p. 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.V’kovski P, Kratzel A, Steiner S, Stalder H and Thiel V, Coronavirus biology and replication: implications for SARS-CoV-2. Nature Reviews Microbiology, 2021. 19(3): p. 155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, Himansu S, Schäfer A, Ziwawo CT, DiPiazza AT, Dinnon KH, Elbashir SM, Shaw CA, Woods A, Fritch EJ, Martinez DR, Bock KW, Minai M, Nagata BM, Hutchinson GB, Wu K, Henry C, Bahl K, Garcia-Dominguez D, Ma L, Renzi I, Kong W-P, Schmidt SD, Wang L, Zhang Y, Phung E, Chang LA, Loomis RJ, Altaras NE, Narayanan E, Metkar M, Presnyak V, Liu C, Louder MK, Shi W, Leung K, Yang ES, West A, Gully KL, Stevens LJ, Wang N, Wrapp D, Doria-Rose NA, Stewart-Jones G, Bennett H, Alvarado GS, Nason MC, Ruckwardt TJ, McLellan JS, Denison MR, Chappell JD, Moore IN, Morabito KM, Mascola JR, Baric RS, Carfi A and Graham BS, SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature, 2020. 586(7830): p. 567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi P-Y, Türeci Ö, Tompkins KR, Lyke KE, Raabe V, Dormitzer PR, Jansen KU, Şahin U and Gruber WC, Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. New England Journal of Medicine, 2020. 383(25): p. 2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, Offergeld K, Scheper G, Taylor KL, Robb ML, Treanor J, Barouch DH, Stoddard J, Ryser MF, Marovich MA, Neuzil KM, Corey L, Cauwenberghs N, Tanner T, Hardt K, Ruiz-Guiñazú J, Le Gars M, Schuitemaker H, Van Hoof J, Struyf F and Douoguih M, Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. New England Journal of Medicine, 2021. 384(23): p. 2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Shen Q and Chang H, Vaccines for COVID-19: A Systematic Review of Immunogenicity, Current Development, and Future Prospects. Frontiers in Immunology, 2022. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, Chadwick DR, Clark R, Cosgrove C, Galloway J, Goodman AL, Heer A, Higham A, Iyengar S, Jamal A, Jeanes C, Kalra PA, Kyriakidou C, McAuley DF, Meyrick A, Minassian AM, Minton J, Moore P, Munsoor I, Nicholls H, Osanlou O, Packham J, Pretswell CH, San Francisco Ramos A, Saralaya D, Sheridan RP, Smith R, Soiza RL, Swift PA, Thomson EC, Turner J, Viljoen ME, Albert G, Cho I, Dubovsky F, Glenn G, Rivers J, Robertson A, Smith K and Toback S, Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. New England Journal of Medicine, 2021. 385(13): p. 1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu B, Zeng LP, Yang XL, Ge XY, Zhang W, Li B, Xie JZ, Shen XR, Zhang YZ, Wang N, Luo DS, Zheng XS, Wang MN, Daszak P, Wang LF, Cui J and Shi ZL, Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog, 2017. 13(11): p. e1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oscherwitz J, The promise and challenge of epitope-focused vaccines. Hum Vaccin Immunother, 2016. 12(8): p. 2113–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed SF, Quadeer AA and McKay MR, Preliminary Identification of Potential Vaccine Targets for the COVID-19 Coronavirus (SARS-CoV-2) Based on SARS-CoV Immunological Studies. Viruses, 2020. 12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baruah V and Bose S, Immunoinformatics-aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019-nCoV. J Med Virol, 2020. 92(5): p. 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharya M, Sharma AR, Patra P, Ghosh P, Sharma G, Patra BC, Lee SS and Chakraborty C, Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): Immunoinformatics approach. J Med Virol, 2020. 92(6): p. 618–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Channappanavar R, Fett C, Zhao J, Meyerholz DK and Perlman S, Virus-Specific Memory CD8 T Cells Provide Substantial Protection from Lethal Severe Acute Respiratory Syndrome Coronavirus Infection. Journal of Virology, 2014. 88(19): p. 11034–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grifoni A, Sidney J, Zhang Y, Scheuermann RH, Peters B and Sette A, A Sequence Homology and Bioinformatic Approach Can Predict Candidate Targets for Immune Responses to SARS-CoV-2. Cell Host Microbe, 2020. 27(4): p. 671–680.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua RH, Wang YF, Bu ZG, Zhou YJ, Ge JY, Wang XJ and Tong GZ, Identification and antigenic epitope mapping of immunodominant region amino residues 510 to 672 on the spike protein of the severe acute respiratory syndrome coronavirus. DNA Cell Biol, 2005. 24(8): p. 503–9. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, Cao Y, Du J, Bu X, Ma R and Wu C, Priming with SARS CoV S DNA and boosting with SARS CoV S epitopes specific for CD4+ and CD8+ T cells promote cellular immune responses. Vaccine, 2007. 25(39-40): p. 6981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li CK, Wu H, Yan H, Ma S, Wang L, Zhang M, Tang X, Temperton NJ, Weiss RA, Brenchley JM, Douek DC, Mongkolsapaya J, Tran BH, Lin CL, Screaton GR, Hou JL, McMichael AJ and Xu XN, T cell responses to whole SARS coronavirus in humans. J Immunol, 2008. 181(8): p. 5490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucchese G, Epitopes for a 2019-nCoV vaccine. Cellular & Molecular Immunology, 2020. 17(5): p. 539–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou T, Wang H, Luo D, Rowe T, Wang Z, Hogan RJ, Qiu S, Bunzel RJ, Huang G, Mishra V, Voss TG, Kimberly R and Luo M, An exposed domain in the severe acute respiratory syndrome coronavirus spike protein induces neutralizing antibodies. J Virol, 2004. 78(13): p. 7217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schödel F, Moriarty AM, Peterson DL, Zheng JA, Hughes JL, Will H, Leturcq DJ, McGee JS and Milich DR, The position of heterologous epitopes inserted in hepatitis B virus core particles determines their immunogenicity. J Virol, 1992. 66(1): p. 106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nooraei S, Bahrulolum H, Hoseini ZS, Katalani C, Hajizade A, Easton AJ and Ahmadian G, Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. Journal of Nanobiotechnology, 2021. 19(1): p. 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohsen MO, Gomes AC, Vogel M and Bachmann MF, Interaction of Viral Capsid-Derived Virus-Like Particles (VLPs) with the Innate Immune System. Vaccines (Basel), 2018. 6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohsen MO and Bachmann MF, Virus-like particle vaccinology, from bench to bedside. Cellular & Molecular Immunology, 2022. 19(9): p. 993–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen BJ and Richmond JE, Electron microscopy of hepatitis B core antigen synthesized in E. coli. Nature, 1982. 296(5858): p. 677–9. [DOI] [PubMed] [Google Scholar]
- 27.Milich DR, McLachlan A, Thornton GB and Hughes JL, Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature, 1987. 329(6139): p. 547–9. [DOI] [PubMed] [Google Scholar]
- 28.Crowther RA, Kiselev NA, Böttcher B, Berriman JA, Borisova GP, Ose V and Pumpens P, Three-dimensional structure of hepatitis B virus core particles determined by electron cryomicroscopy. Cell, 1994. 77(6): p. 943–50. [DOI] [PubMed] [Google Scholar]
- 29.Schödel F, Peterson D and Milich D, Hepatitis B virus core and e antigen: immune recognition and use as a vaccine carrier moiety. Intervirology, 1996. 39(1-2): p. 104–10. [DOI] [PubMed] [Google Scholar]
- 30.Böttcher B, Wynne SA and Crowther RA, Determination of the fold of the core protein of hepatitis B virus by electron cryomicroscopy. Nature, 1997. 386(6620): p. 88–91. [DOI] [PubMed] [Google Scholar]
- 31.Schödel F, Peterson D, Hughes J, Wirtz R and Milich D, Hybrid hepatitis B virus core antigen as a vaccine carrier moiety: I. presentation of foreign epitopes. J Biotechnol, 1996. 44(1-3): p. 91–6. [DOI] [PubMed] [Google Scholar]
- 32.Karpenko LI, Ivanisenko VA, Pika IA, Chikaev NA, Eroshkin AM, Veremeiko TA and Ilyichev AA, Insertion of foreign epitopes in HBcAg: how to make the chimeric particle assemble. Amino Acids, 2000. 18(4): p. 329–37. [DOI] [PubMed] [Google Scholar]
- 33.Pumpens P and Grens E, HBV Core Particles as a Carrier for B Cell/T Cell Epitopes. Intervirology, 2001. 44(2-3): p. 98–114. [DOI] [PubMed] [Google Scholar]
- 34.Böttcher B, Vogel M, Ploss M and Nassal M, High plasticity of the hepatitis B virus capsid revealed by conformational stress. J Mol Biol, 2006. 356(3): p. 812–22. [DOI] [PubMed] [Google Scholar]
- 35.Schumacher J, Bacic T, Staritzbichler R, Daneschdar M, Klamp T, Arnold P, Jägle S, Türeci Ö, Markl J and Sahin U, Enhanced stability of a chimeric hepatitis B core antigen virus-like-particle (HBcAg-VLP) by a C-terminal linker-hexahistidine-peptide. J Nanobiotechnology, 2018. 16(1): p. 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milich DR, McLachlan A, Moriarty A and Thornton GB, Immune response to hepatitis B virus core antigen (HBcAg): localization of T cell recognition sites within HBcAg/HBeAg. The Journal of Immunology, 1987. 139(4): p. 1223–1231. [PubMed] [Google Scholar]
- 37.Milich DR, T- and B-cell recognition of hepatitis B viral antigens. Immunology Today, 1988. 9(12): p. 380–386. [DOI] [PubMed] [Google Scholar]
- 38.Milich DR, McLachlan A, Stahl S, Wingfield P, Thornton GB, Hughes JL and Jones JE, Comparative immunogenicity of hepatitis B virus core and E antigens. The Journal of Immunology, 1988. 141(10): p. 3617–3624. [PubMed] [Google Scholar]
- 39.Milich DR, Chen M, Schödel F, Peterson DL, Jones JE and Hughes JL, Role of B cells in antigen presentation of the hepatitis B core. Proc Natl Acad Sci U S A, 1997. 94(26): p. 14648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belnap DM, Watts NR, Conway JF, Cheng N, Stahl SJ, Wingfield PT and Steven AC, Diversity of core antigen epitopes of hepatitis B virus. Proceedings of the National Academy of Sciences, 2003. 100(19): p. 10884–10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borisova G, Arya B, Dislers A, Borschukova O, Tsibinogin V, Skrastina D, Eldarov MA, Pumpens P, Skryabin KG and Grens E, Hybrid hepatitis B virus nucleocapsid bearing an immunodominant region from hepatitis B virus surface antigen. J Virol, 1993. 67(6): p. 3696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murthy AM, Ni Y, Meng X and Zhang C, Production and evaluation of virus-like particles displaying immunogenic epitopes of porcine reproductive and respiratory syndrome virus (PRRSV). Int J Mol Sci, 2015. 16(4): p. 8382–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang M, Lai H, Sun H and Chen Q, Virus-like particles that display Zika virus envelope protein domain III induce potent neutralizing immune responses in mice. Scientific Reports, 2017. 7(1): p. 7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu Y, Clark-Deener S, Gillam F, Heffron CL, Tian D, Sooryanarain H, LeRoith T, Zoghby J, Henshaw M, Waldrop S, Pittman J, Meng XJ and Zhang C, Virus-like particle vaccine with B-cell epitope from porcine epidemic diarrhea virus (PEDV) incorporated into hepatitis B virus core capsid provides clinical alleviation against PEDV in neonatal piglets through lactogenic immunity. Vaccine, 2020. 38(33): p. 5212–5218. [DOI] [PubMed] [Google Scholar]
- 45.Gillam F, Zhang J and Zhang C, Hepatitis B core antigen based novel vaccine against porcine epidemic diarrhea virus. Journal of Virological Methods, 2018. 253: p. 61–69. [DOI] [PubMed] [Google Scholar]
- 46.Wang B, Tian D, Sooryanarain H, Mahsoub HM, Heffron CL, Hassebroek AM and Meng X-J, Two mutations in the ORF1 of genotype 1 hepatitis E virus enhance virus replication and may associate with fulminant hepatic failure. Proceedings of the National Academy of Sciences, 2022. 119(34): p. e2207503119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maeda D, Tian D, Yu H, Dar N, Rajasekaran V, Meng S, Mahsoub HM, Sooryanarain H, Wang B, Heffron CL, Hassebroek A, LeRoith T, Meng XJ and Zeichner SL, Killed whole-genome reduced-bacteria surface-expressed coronavirus fusion peptide vaccines protect against disease in a porcine model. Proc Natl Acad Sci U S A, 2021. 118(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perera RA, Mok CK, Tsang OT, Lv H, Ko RL, Wu NC, Yuan M, Leung WS, Chan JM, Chik TS, Choi CY, Leung K, Chan KH, Chan KC, Li KC, Wu JT, Wilson IA, Monto AS, Poon LL and Peiris M, Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Euro Surveill, 2020. 25(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu X, Wang L, Sakthivel SK, Whitaker B, Murray J, Kamili S, Lynch B, Malapati L, Burke SA, Harcourt J, Tamin A, Thornburg NJ, Villanueva JM and Lindstrom S, US CDC Real-Time Reverse Transcription PCR Panel for Detection of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis, 2020. 26(8): p. 1654–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oladunni FS, Park J-G, Pino PA, Gonzalez O, Akhter A, Allué-Guardia A, Olmo-Fontánez A, Gautam S, Garcia-Vilanova A, Ye C, Chiem K, Headley C, Dwivedi V, Parodi LM, Alfson KJ, Staples HM, Schami A, Garcia JI, Whigham A, Platt RN, Gazi M, Martinez J, Chuba C, Earley S, Rodriguez OH, Mdaki SD, Kavelish KN, Escalona R, Hallam CRA, Christie C, Patterson JL, Anderson TJC, Carrion R, Dick EJ, Hall-Ursone S, Schlesinger LS, Alvarez X, Kaushal D, Giavedoni LD, Turner J, Martinez-Sobrido L and Torrelles JB, Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nature Communications, 2020. 11(1): p. 6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Study data are available upon request.


