Abstract
Trauma-informed beliefs often decrease during posttraumatic stress disorder (PTSD) treatment. This may also extend to anxiety sensitivity (AS), defined as a fear of anxiety-related sensations and beliefs that anxiety is dangerous and/or intolerable. However, little is known about how AS changes during exposure-based and psychopharmacological PTSD treatments. Further, high AS may be a risk factor for diminished PTSD symptom improvement and increased treatment dropout. To better understand how AS impacts and is impacted by PTSD treatment, we conducted a secondary analysis of a randomized clinical trial with a sample of 223 veterans (87.0% male, 57.5% White) with PTSD from four U.S. sites. Veterans were randomized to receive prolonged exposure (PE) plus placebo (n = 74), sertraline plus enhanced medication management (n = 74), or PE plus sertraline (n = 75). Veterans answered questions about PTSD symptoms and AS at baseline and 6-, 12-, 24-, 36-, and 52-week follow-ups. High baseline AS was related to high levels of PTSD severity at 24 weeks across all conditions, β = .244, p = .013, but did not predict dropout from exposure-based, β = .077, p = .374, or psychopharmacological therapy, β = .009, p = .893. AS also significantly decreased across all three treatment arms, with no between-group differences; these reductions were maintained at the 52-week follow-up. These findings suggest that high AS is a risk factor for attenuated PTSD treatment response but also provide evidence that AS can be improved by both PE and an enhanced psychopharmacological intervention for PTSD.
Anxiety sensitivity (AS) is defined as a fear of anxiety-related sensations and the belief that anxiety is dangerous or intolerable. AS often compounds functional impairment in anxiety-spectrum disorders as part of a negative feedback loop wherein maladaptive cognitions about anxiety and fearful interpretations of anxiety beget more anxiety (Reiss et al., 1986). Although AS is most often discussed in relation to mental health conditions such as panic and generalized anxiety disorders (Naragon-Gainey, 2010), research also suggests that AS and posttraumatic stress disorder (PTSD) are strongly correlated in a reciprocal association (Marshall et al., 2010). This may be because AS causes individuals to avoid anxiety-provoking situations, such as trauma cues, thereby impeding trauma processing and reinforcing beliefs about the dangerousness of anxiety symptoms. Indeed, AS may lead to avoidance, which may ultimately diminish the effectiveness of treatment and increase PTSD symptom severity (Naifeh et al., 2012). Given these associations, understanding the role that AS plays in PTSD-focused treatment could provide valuable clinical information such that clinicians might tailor treatment recommendations based on pretreatment AS.
Existing psychotherapies for PTSD may incidentally reduce AS. Gutner and colleagues (2013) found that AS decreased from pre- to posttreatment after each of three trauma-focused treatments, including standard cognitive processing therapy (CPT; Resick et al., 2016), CPT with only cognitive components, and written trauma accounts only. This may be because confronting trauma-related memories in psychotherapy causes patients to experience previously avoided symptoms of anxiety and ultimately teaches them that these anxiety experiences are safer and more tolerable than they once believed (i.e., a change in fear cognitions). Interestingly, participants who were randomized to write trauma accounts experienced the largest decrease in AS among all three groups (Gutner et al., 2013). This finding suggests that exposure-based approaches are especially effective in decreasing AS, which may lead to downstream effects on PTSD. This supposition is supported by studies of interoceptive exposure for PTSD, which includes exercises aimed at simulating distressing physical sensations for patients. One such study randomized patients to either an interoceptive exposure condition or a health education control group (Allen et al., 2015). Individuals in the interoceptive exposure condition had larger reductions in PTSD symptoms than those in the control group, and this treatment effect was mediated through reductions in global and social AS concerns. Short and colleagues (2017) reported observed results, finding that cognitive AS concerns mediated the association between interoceptive exposure and PTSD symptoms. Thus, exposure-based techniques can be effective in reducing AS, which, in turn, can lead to reductions in PTSD symptoms.
One of the most empirically supported exposure-based treatments for PTSD is prolonged exposure (PE). PE helps patients extinguish fear responses and challenges their maladaptive beliefs through in vivo and imaginal exposures (Foa et al., 2019; Hamblen et al., 2019; Powers et al., 2010). Little is known about how an individual’s perception of anxiety symptoms may change throughout PE; however, facing anxiety-provoking experiences and seeing that the resulting symptoms are safe and tolerable may allow patients to extinguish AS responses and learn that anxiety is less consequential than they previously believed.
Selective serotonin reuptake inhibitors (SSRIs), such as sertraline, have also been shown to effectively reduce symptoms of PTSD (Brady et al., 2000; Davidson et al., 2001; Brady & Clary, 2003). Additional studies suggest that AS may also be improved by both anxiolytics and antidepressants. For example, Simon and colleagues (2004) found that AS decreased similarly over three pharmacotherapies for panic disorder, including paroxetine, paroxetine plus sustained clonazepam, and paroxetine plus brief clonazepam. Although sertraline is effective in reducing symptoms of PTSD, and although similar SSRIs have been shown to reduce AS for patients with other mental health disorders, it is still unclear if sertraline may influence AS among those with PTSD.
Given the established efficacy of PE and sertraline in the treatment of PTSD, it is not unreasonable to hypothesize that there may be an additive effect of combining PE with sertraline. For example, Rothbaum and colleagues (2006) found that individuals who received both sertraline and PE had larger reductions in PTSD symptoms than those who received sertraline alone. However, Rauch and colleagues (2019) found that there were no differences in the rate of improvement for PTSD severity between individuals randomized to PE plus placebo, PE plus sertraline, and sertraline plus enhanced medication management (EMM). Thus, mixed findings on sertraline and PE warrant a deeper examination into other possible outcomes of interest, such as AS.
Although PE, sertraline, and/or their combination may theoretically decrease AS over the course of treatment, high AS may also be a risk factor for attenuated PE treatment outcomes by interfering with effective engagement in exposure-based elements of treatment. Prior research suggests that AS is a risk factor for slower PTSD improvement, poorer PTSD outcomes (Zandberg et al., 2016), and higher PE treatment dropout (Bealleau, 2017). Additionally, higher AS is associated with lower positive beliefs about the efficacy of PE (Zoellner et al., 2009), which may lead patients to engage less with treatment and respond more poorly to the intervention (Taylor, 2003).
High AS may also be a risk factor for attenuated treatment outcomes in pharmacotherapies. Olatunji and colleagues (2007) found that anxiety symptoms decreased after a 6-week open trial of fluoxetine in a small group of patients with generalized anxiety disorder and that there was a trend wherein baseline physical and social AS concerns were associated with slower improvement in anxiety during treatment.
The current study examined the impact of AS on three treatment approaches for PTSD: PE plus placebo, sertraline plus EMM, and PE plus sertraline. We hypothesized that high AS at baseline would predict high posttreatment PTSD symptom severity and treatment dropout. We also hypothesized that AS would decrease in all conditions over the course of treatment and at each follow-up assessment. Given evidence that both sertraline and PE are associated with reductions in PTSD symptoms and anxiety, we hypothesized that the largest reductions in AS would be observed in the PE plus sertraline condition.
METHOD
Participants
Participant demographic characteristics can be found in Table 1 and are summarized in greater detail in the primary outcome publication from this project (Rauch et al., 2019). Veterans (N = 223) were recruited from four sites across the United States (i.e., Ann Arbor, Michigan; Boston, Massachusetts; Charleston, South Carolina; and San Diego, California). The average participant age was 34.2 years (SD = 8.2). All veterans served in support of recent conflicts in Iraq and Afghanistan. Additional inclusion criteria were based on the PTSD criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV; American Psychiatric Association, 1994) and included a Clinician-Administered PTSD Scale for DSM-IV (CAPS-IV; Weathers et al., 1994) score of 50 or higher and exposure to a combat-related Criterion A traumatic event. Participants were excluded if they endorsed imminent risk of suicide, active psychosis, substance dependence over the past 8 weeks, a medical illness that would result in imminent hospitalization, concurrent use of antidepressants or antipsychotics, and/or serious cognitive impairment. In total, 223 veterans were randomized to receive either sertraline plus EMM (n = 74), PE plus placebo (n = 74), or PE plus sertraline (n = 75).
TABLE 1.
Sample demographic characteristics, by treatment arm
| All treatment arms (N = 223) |
PE + placebo (n = 74) |
Sertraline + EMM (n = 74) |
PE + sertraline (n = 75) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | % | M | SD | n | % | M | SD | n | % | M | SD | n | % | M | SD |
| Male gender | 194 | 87.0 | 65 | 87.8 | 69 | 93.2 | 60 | 80.0 | ||||||||
| Race | ||||||||||||||||
| White | 129 | 57.8 | 40 | 54.1 | 45 | 60.8 | 44 | 58.7 | ||||||||
| Black | 65 | 29.1 | 22 | 29.7 | 20 | 27.0 | 23 | 30.7 | ||||||||
| Other | 29 | 13.0 | 12 | 16.2 | 9 | 12.2 | 8 | 10.7 | ||||||||
| Hispanic/Latino ethnicity | 31 | 13.9 | 7 | 9.5 | 13 | 17.6 | 11 | 14.7 | ||||||||
| Marital statusa | ||||||||||||||||
| Married/remarried | 114 | 51.4 | 37 | 50.0 | 42 | 57.5 | 35 | 46.7 | ||||||||
| Separated/divorced | 58 | 26.1 | 23 | 31.1 | 11 | 15.1 | 24 | 32.0 | ||||||||
| Never married | 50 | 22.5 | 14 | 18.9 | 20 | 24.7 | 16 | 21.3 | ||||||||
| Years of education | 13.6 | 2.3 | 14.1 | 2.3 | 13.0 | 2.6 | 13.8 | 2.0 | ||||||||
| Number of deployments | 2.6 | 3.0 | 2.3 | 1.3 | 3.1 | 4.8 | 2.4 | 1.6 | ||||||||
| Baseline CAPS-IV score | 74.6 | 17.8 | 79.2 | 16.0 | 72.1 | 17.67 | 72.5 | 18.8 | ||||||||
Note: PE = prolonged exposure; EMM = enhanced medication management; CAPS-IV = Clinician-Administered PTSD Scale for DSM-IV.
One veteran in the sertraline + EMM condition had unknown marital status.
Procedure
Institutional review board approval was obtained from each recruiting site. Veterans were primarily assessed for eligibility via the CAPS-IV (Blake et al., 1995) and the Mini International Neuropsychiatric Interview for DSM-IV (MINI; Sheehan et al., 1998). Eligible veterans were randomized by site to one of the three treatment conditions. Randomization was stratified by site using varying block sizes within each site. Trained study assessors were blinded to treatment condition and medication, and sessions were checked for fidelity throughout the course of the study. Participants were compensated at a rate of $50 (USD) per assessment, which occurred at baseline and 6-, 12-, 24-, and 52-week follow-up appointments. Additional details about the study design and procedures have been published elsewhere (Rauch et al., 2018). This study was preregistered at ClinicalTrials.gov, although the specific set of secondary analyses presented in this manuscript was not included in the preregistration.
In the PE plus placebo condition, veterans were scheduled for 13 sessions of PE, each lasting 90 min. Sessions were led by a VA-trained PE therapist (i.e., master’s level or above) and involved all manualized components, including psychoeducation, imaginal exposures, in vivo exposures, and processing traumatic memories. All sessions were expected to be completed within 24 weeks. Participants who were randomized to receive PE and medication management were also prescribed a placebo medication that was encapsulated to blind participants and providers. In addition, veterans in this condition received brief medication management that lasted roughly 15 min in length for eight nonconsecutive weeks.
In the sertraline plus EMM condition, veterans were prescribed a sertraline dosage that gradually increased from 50 to 200 mg per day, with the last dosage increase at Week 10. Medication continued until week 24, which was considered the end of treatment. All participants were blinded to the medication condition, and all medication was encapsulated to ensure blinding. EMM lasted roughly 30 min for eight nonconsecutive weeks. The extended time in EMM for individuals randomized to sertraline plus EMM helped balance the contact time when compared to PE and included additional psychoeducation and active listening.
Veterans randomized to the PE plus sertraline condition received all components of PE along with the same sertraline dosage as previously described. Individuals in this condition also received brief medication management, similar to the PE plus placebo condition.
Measures
Anxiety sensitivity
The Anxiety Sensitivity Index (ASI-16; Reiss et al., 1986) is a 16-item questionnaire that is used to assess concerns respondents have about their anxiety symptoms (e.g., “It scares me when my heart beats rapidly”). Respondents answer questions using a scale between 0 (very little) and 4 (very much). A total score is computed by adding all 16 responses, resulting in a range of 0 to 64, with higher scores indicating higher AS. Psychometric research on the ASI-16 suggests that it has strong reliability and validity (Reiss et al., 1986). In the present sample, Cronbach’s alpha for the total ASI-16 scale was .93 at baseline.
PTSD symptoms and diagnosis
The CAPS-IV (Blake et al., 1995; Weathers et al., 1994) was used to assess PTSD severity and diagnosis. Trained interviewers assess symptom frequency and intensity over the last 30 days indexed to a specific event that is considered the “worst or currently most distressing” by the patient. Frequency and intensity scores were used to create an overall PTSD severity score and determine diagnostic status. The CAPS-IV has demonstrated strong interrater reliability and convergent validity (Weathers et al., 2001). For this study, the CAPS-IV was used as both a screening measure and an outcome measure. In the present sample, Cronbach’s alpha for the CAPS-IV total severity score was .92 at baseline.
Treatment dropout
Two dichotomous variables were calculated to assess treatment dropout. The first assessed dropout from PE among participants who were scheduled for either the PE plus sertraline or PE plus placebo condition. A second variable assessed dropout from the medication portion of all three conditions after the first session was scheduled (i.e., representing dropout from sertraline or placebo).
Data analysis
All variables were checked for skewness and kurtosis using recommendations by Tabachnick and Fidell (2013). Outliers were addressed by changing z scores greater than 3 to one value larger than the next, nonoutlying score. All analyses were performed using SPSS (Version 23).
To determine if high baseline AS was a risk factor for attenuated PTSD outcomes, we conducted a hierarchical multiple regression with baseline AS as the independent variable and 24-week CAPS-IV score as the outcome. In the first block, we controlled for baseline CAPS-IV score, and two dummy-coded variables for treatment arm (PE plus sertraline vs. other arms; PE plus placebo vs. other arms) were included in the second block. Due to significant missing data on the 24-week CAPS-IV variable, all analyses using this variable were imputed using five iterations. Two logistic regression analyses were also used to determine if baseline AS was associated with treatment dropout for medication and exposure-based treatments.
To determine changes in AS over six time points (i.e., baseline and 6-, 12-, 24-, 36-, and 52-week follow-up) and between three treatment arms (PE plus placebo vs. sertraline plus EMM vs. PE plus sertraline), we conducted a mixed-models repeated-measures analysis in SPSS. To this end, we used an autoregressive covariance structure for estimating the variation across repeated measures using a maximum likelihood estimate. Pairwise comparisons were based on estimated marginal means. All analyses used an alpha of .05.
This trial was originally powered to detect differences in PTSD severity between the three treatment arms. However, an a priori power analysis was conducted using AS data from a sample of Cambodian refugees with PTSD who received either sertraline alone or sertraline plus cognitive behavioral therapy that included exposure-based elements (Otto et al., 2003; the results suggest that the current analysis may have been adequately powered to detect differences on AS.
RESULTS
PTSD symptom change, treatment dropout, and AS
High baseline AS was related to 24-week PTSD severity, pooled β = .244, p = .013, after accounting for treatment arm and baseline PTSD severity. Baseline PTSD severity contributed significant variance in the model, pooled β = .369, p < .001, although treatment arm did not, pooled β = .001, p = .669. The model explained 19.5% of the total variance, F(4, 218) = 13.25, p < .001. In contrast to the stated hypotheses, baseline AS was not related to treatment dropout for veterans receiving medication, β = .009, p = .893, or exposure-based treatments, β = .077, p = .374, in the logistic regression models.
Change in AS over the course of treatment
Estimated marginal means for AS scores, stratified by assessment point and treatment arm, are presented in Table 2. Data on AS for each treatment arm are also displayed in Figure 1. A Type III test for fixed effects indicated a main effect for time, F(5, 459.52) = 18.97, p < .001, suggesting that all groups had a reduction in AS over the course of treatment. There was no main effect for treatment arm, F(2, 223.12) = 1.39, p = .252, and the interaction between time and treatment arm was not significant, F(10, 458.93) = 1.28, p = .240, suggesting that all treatments resulted in a similar decrease in AS.
TABLE 2.
Estimated marginal means and standard errors for anxiety sensitivity
| All Arms | PE + placebo | Sertraline + EMM | PE + sertraline | |||||
|---|---|---|---|---|---|---|---|---|
| Time point | M | SE | M | SE | M | SE | M | SE |
| Baseline | 24.52 | .90 | 25.05 | 1.55 | 24.32 | 1.53 | 24.20 | 1.59 |
| 6 weeks | 22.48 | .95 | 24.06 | 1.69 | 20.71 | 1.56 | 22.67 | 1.66 |
| 12 weeks | 19.19 | .98 | 19.32 | 1.75 | 18.37 | 1.62 | 19.88 | 1.71 |
| 24 weeks | 18.24 | 1.00 | 20.05 | 1.80 | 15.08 | 1.65 | 19.59 | 1.72 |
| 36 weeks | 17.78 | 1.01 | 18.35 | 1.83 | 15.32 | 1.66 | 19.68 | 1.76 |
| 52 weeks | 17.05 | 1.02 | 18.68 | 1.84 | 14.19 | 1.71 | 18.28 | 1.75 |
Note: PE = prolonged exposure; EMM = enhanced medication management.
FIGURE 1.
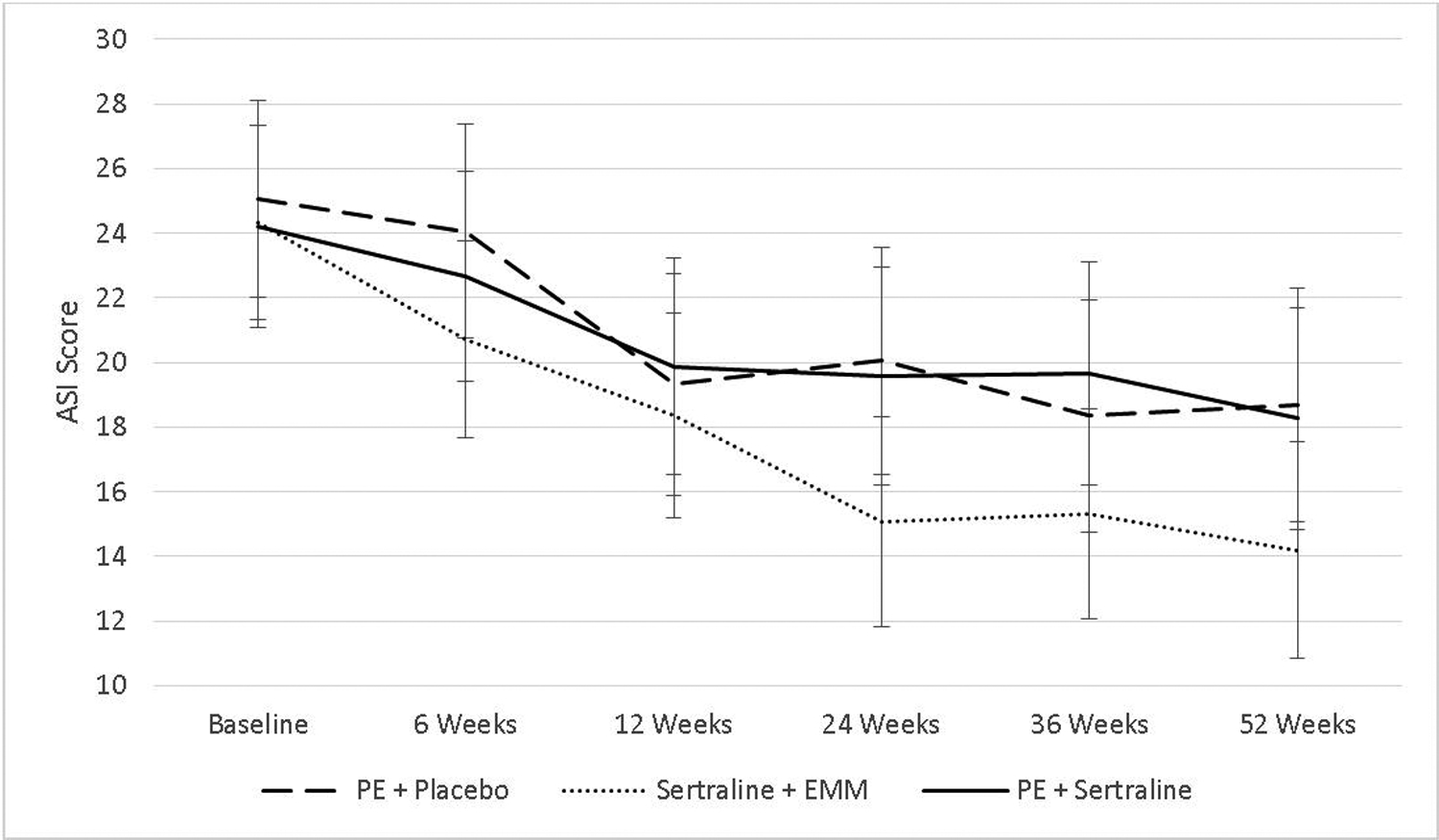
Change in anxiety sensitivity over time and between the three treatment approaches
Note: Data used model-based mean estimates that account for missing data. Error bars represent 95% confidence intervals.
In the sertraline plus EMM condition, pairwise comparisons revealed significant decreases in AS starting at 6 weeks (baseline to 6 weeks: ΔM = 3.61), p = .017, with improvements present at 52 weeks (baseline to 52 weeks: ΔM = 10.13), p < .001. In the PE plus placebo condition, significant decreases in AS started at Week 12 (baseline to 12 weeks: ΔM = 5.73), p = .002, with improvements present at 52 weeks (baseline to 52 weeks: ΔM = 6.37), p = .002. In the PE plus sertraline condition, significant decreases in AS started at 12 weeks (baseline to 12 weeks: ΔM = 4.33) p = .030, with improvements present at 52 weeks (baseline to 52 weeks: ΔM = 5.92), p = .002.
DISCUSSION
The findings demonstrate a positive association between baseline AS and posttreatment PTSD severity, supporting the hypothesis that baseline AS is a risk factor for attenuated PTSD treatment response to PE, sertraline, and their combination. These findings mirror those reported by Zandberg and colleagues (2016), who observed that AS was a risk factor for poor PE response among a sample of adults with PTSD and concurrent substance dependence, and run somewhat counter to Bluiett and colleagues (2013), who found individuals with higher baseline AS were slightly more likely to have a reliable change in distress during imaginal exposure. We extend these findings by also showing that treatment type did not contribute meaningful variance in this association. Thus, AS also appears to be a risk factor for attenuated treatment outcomes in treatments involving sertraline with EMM as well as the combination of PE and sertraline.
For participants who received PE, unstudied mediating variables may be responsible for this association. For example, individuals with higher AS may be more reluctant to engage in PE assignments, which would ultimately have downstream effects on PTSD symptom improvement. Some aspect of AS also appears to negatively impact the effectiveness of medication on posttreatment PTSD severity. One logical explanation may be that higher AS inhibits the likelihood of incidental exposure to feared stimuli as anxiety levels decrease in response to medication to a relatively larger extent compared with lower AS.
In addition to assessing posttreatment PTSD severity, we also examined how baseline AS may impact treatment dropout. Contrary to our hypotheses, the findings revealed that AS was not associated with dropout from either the medication components of treatment or PE. This finding is promising for clinicians who may be hesitant to initiate PE with a client who has a strong fear of experiencing anxiety and suggests that both PE and medication can be tolerated by patients with varying degrees of anxiety-related fear.
We also hypothesized that AS would decrease over time and across the three treatment conditions, with the largest decreases occurring in the PE plus sertraline condition. The results from this analysis partially support this hypothesis. Although we observed reductions in AS over the course of treatment in all three conditions, there were no differences across treatment conditions. These findings align with previous research showing decreases in AS across three CPT-based PTSD treatment approaches (Gutner et al., 2013). This suggests that both exposure-based psychotherapy, when combined with a pill, and sertraline, when combined with 30-min medication management, may similarly improve beliefs about the dangerousness of anxiety symptoms. It may also suggest that PE and sertraline reduce approach fear either by homework directive or reduced anxiety, thus facilitating incidental exposure. For PE, it is likely that having veterans spend time in anxiety-provoking situations teaches them that anxiety can be tolerated. For sertraline, an antidepressant with ant-anxiety properties, it may be that a decrease in anxiety or depression-related negative thinking is responsible for decreases in AS. This is in line with previous research on anxiety and depression showing that symptoms of both are associated with AS (Taylor et al., 1996).
Interestingly, veterans randomized to receive sertraline plus EMM had a quicker reduction in AS, with significant changes occurring as early as 6 weeks, compared with those in either of the two PE conditions, where significant changes in AS were not noted until the 12-week assessment. This may imply that combined treatment may impede change over monotherapy. As there was no standalone PE condition, it is unclear if concurrent exposure therapy may temporarily impact the effect of sertraline on AS or whether adding a placebo to PE reduced the effect size of PE (Rauch et al., 2022). Additional research on this topic is needed to better understand this finding.
Because AS was associated with attenuated PTSD outcomes across treatments but also improved during PTSD treatments, it may be beneficial for therapists to consider addressing AS behaviors and beliefs early in therapy and within effective protocols. To achieve this, clinicians may wish to assess AS prior to therapy and directly target AS with psychoeducation. This may include brief interventions to reduce AS, which have been shown to be effective in alleviating AS from pre- to posttreatment (Fitzgerald et al., 2021) and may help patients benefit more from PTSD treatment, during which further decreases in AS can occur. Additionally, interoceptive exposure may be integrated throughout PE as part of the in vivo exposure hierarchy as an effective strategy for reducing anxiety sensitivity. Interoceptive exposure integrated into PE may be especially effective because both approaches utilize similar principles. Integrating components of these interventions directly into PE as opposed to prefacing treatment with brief interventions should be considered so as not to delay the benefits of PE (Wiedeman et al., 2020). More research is needed to understand who might benefit from such an integrated AS intervention. Until then, clinicians can consider using ASI-16 scores greater than 20, or ASI-3 scores greater than 23, as potential intervention screeners (Allan et al., 2014; Smits et al., 2016).
The study had a number of strengths. First, we included measurements of AS at multiple time points from baseline to 52 weeks (i.e., 28 weeks posttreatment), which allowed us to take a more fine-grained analytic approach. Second, the use of multiple sites increases the external validity of the study. However, several limitations should also be noted. For example, our study did not include a standalone PE, standalone placebo, or waitlist-only condition; thus, it is not possible to know if reductions in AS are the result of time or an intervention effect. Additionally, this study did not use the more recent version of the CAPS (Weathers et al., 2018) or the ASI (Taylor et al., 2007). Finally, our use of EMM to balance contact time is not generalizable to other sertraline or placebo studies.
The results of this study suggest that although AS may be a risk factor for attenuated PTSD treatment response, it can also be improved over the course of exposure-based psychotherapy and SSRI-based pharmacotherapy for PTSD. Our novel examination of AS in PE is especially important because clinicians may hold inaccurate beliefs about using PE to treat PTSD despite evidence demonstrating its efficacy (Meyer et al., 2014). For example, clinicians may wrongfully assume that individuals with high levels of AS may be unable to benefit from exposure-based treatment approaches or that exposure will lead to dropout among those with high AS (Deacon et al., 2013). This study suggests that neither is true. Prescribers may also benefit from knowing that sertraline can be effective in reducing AS, a question that has not received any attention in the literature to our knowledge.
Future research should examine how PE may reduce AS when compared to a control group to increase confidence in the present findings. Additionally, further analyses should examine ASI subscales to understand which beliefs are driving the effects we observed. Finally, researchers should test whether integrating content on AS within evidence-based treatment protocols for PTSD adds additional benefit to the effects seen here.
Acknowledgments
This material is the result of work supported by resources from and the use of facilities at Massachusetts General Hospital, the Veterans Affairs (VA) Ann Arbor Healthcare System, Ralph H. Johnson VA Medical Center, and the VA San Diego Healthcare System. Funding for this work was made possible by the U.S. Department of Defense through the U.S. Army Medical Research and Materiel Command (W81XWH-11-1-0073; principal investigator [PI]: Rauch). Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH; UL1TR000433). This work was also supported by the National Institutes of Health (T32 AA013525).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other funding sources. The funding sources had no involvement other than financial support. The views expressed in this article are solely those of the authors and do not reflect an endorsement by or the official policy of the VA or the U.S. Government.
In the past 12 months, Sonya B. Norman has received research support from the U.S. Department of Defense (DoD), Patient-Centered Outcomes Research Institute (PCORI), NIH, and royalties from Elsevier Press. Peter W. Tuerk receives support from the National Institute of Mental Health (NIH), Joint Warfighters Medical Research Program; the Australian National Centre of Excellence in Posttraumatic Mental Health, Virtually Better, and royalties from Springer International Publishing. Murray B. Stein has in the past 3 years been a consultant for Actelion, Alkermes, Aptinyx, Bionomics, EpiVario, GW Pharma, Janssen, and Oxeia Biopharmaceuticals. He reports stock options in Oxeia Biopharmaceuticals and EpiVario. Naomi M. Simon received grant research from the NIH, DoD, American Foundation for Suicide Prevention, PCORI, and Vanda Pharmaceuticals; support from Cohen Veterans Network; personal fees from Praxis Therapeutics, Genomind, Bionomics Limited, Cerevel, and Engrail Therapeutics Inc; fees and royalties from Wiley, Wolters Kluwer, and American Psychiatric Association Publishing; and spousal stock from G1 Therapeutics and Zentalis (outside of submitted work). Sheila A. M. Rauch receives support from Wounded Warrior Project, the VA, the NIH, the Hearst Foundation, and the Woodruff Foundation, and royalties from Oxford University Press and American Psychological Association Press.
Footnotes
OPEN PRACTICES STATEMENT
This trial was preregistered at ClinicalTrials.gov (number: NCT01524133). Neither data nor the materials have been made available on a permanent third-party archive. Requests for data used in this manuscript should be sent to the principal investigator of the study at sheila.a.m.rauch@emory.edu.
REFERENCES
- Allan NP, Raines AM, Capron DW, Norr AM, Zvolensky MJ, & Schmidt NB (2014). Identification of anxiety sensitivity classes and clinical cut-scores in a sample of adult smokers: Results from a factor mixture model. Journal of Anxiety Disorders, 28(7), 696–703. 10.1016/j.janxdis.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan NP, Short NA, Albanese BJ, Keough ME, & Schmidt NB (2015). Direct and mediating effects of an anxiety sensitivity intervention on posttraumatic stress disorder symptoms in trauma-exposed individuals. Cognitive Behaviour Therapy, 44(6), 512–524. 10.1080/16506073.2015.1075227 [DOI] [PubMed] [Google Scholar]
- Allgulander C, Dahl AA, Austin C, Morris PL, Sogaard JA, Fayyad R, Kutcher SP, & Clary CM (2004). Efficacy of sertraline in a 12-week trial for generalized anxiety disorder. American Journal of Psychiatry, 161(9), 1642–1649. 10.1176/appi.ajp.161.9.1642 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.).
- Belleau EL, Chin EG, Wanklyn SG, Zambrano-Vazquez L, Schumacher JA, & Coffey SF (2017). Pre-treatment predictors of dropout from prolonged exposure therapy in patients with chronic posttraumatic stress disorder and comorbid substance use disorders. Behaviour Research and Therapy, 91, 43–50. 10.1016/j.brat.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, & Keane TM (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8(1), 75–90. 10.1007/bf02105408 [DOI] [PubMed] [Google Scholar]
- Brady KT, & Clary CM (2003). Affective and anxiety comorbidity in post-traumatic stress disorder treatment trials of sertraline. Comprehensive Psychiatry, 44(5), 360–369. 10.1016/S0010-440X(03)00111-1 [DOI] [PubMed] [Google Scholar]
- Brady KT, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, & Farfel GM (2000). Efficacy and safety of sertraline treatment of posttraumatic stress disorder: A randomized controlled trial. JAMA, 283(14), 1837–1844. 10.1001/jama.283.14.1837 [DOI] [PubMed] [Google Scholar]
- Davidson JR, Rothbaum BO, van der Kolk BA, Sikes CR, & Farfel GM (2001). Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Archives of General Psychiatry, 58(5), 485–492. 10.1001/archpsyc.58.5.485 [DOI] [PubMed] [Google Scholar]
- Deacon BJ, Farrell NR, Kemp JJ, Dixon LJ, Sy JT, Zhang AR, & McGrath PB (2013). Assessing therapist reservations about exposure therapy for anxiety disorders: The Therapist Beliefs about Exposure Scale. Journal of Anxiety Disorders, 27(8), 772–780. 10.1016/j.janxdis.2013.04.006 [DOI] [PubMed] [Google Scholar]
- Fitzgerald HE, Hoyt DL, Kredlow MA, Smits JAJ, Schmidt NB, Edmondson D, & Otto MW (2021). Anxiety sensitivity as a malleable mechanistic target for prevention interventions: A meta-analysis of the efficacy of brief treatment interventions. Clinical Psychology: Science and Practice, 28(4), 323–337. 10.1037/cps0000038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Hembree EA, Rothbaum BO, & M. RSA (2019). Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences. Oxford University Press. [Google Scholar]
- Friedman MJ, Marmar CR, Baker DG, Sikes CR, & Farfel GM (2007). Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. The Journal of Clinical Psychiatry, 68(5), 711–720. 10.4088/jcp.v68n0508 [DOI] [PubMed] [Google Scholar]
- Frye LA, & Spates CR (2012). Prolonged exposure, mindfulness, and emotion regulation for the treatment of PTSD. Clinical Case Studies, 11(3), 184–200. 10.1177/1534650112446850 [DOI] [Google Scholar]
- Gutner CA, Nillni YI, Suvak M, Wiltsey-Stirman S, & Resick PA (2013). Longitudinal course of anxiety sensitivity and PTSD symptoms in cognitive-behavioral therapies for PTSD. Journal of Anxiety Disorders, 27(7), 728–734. 10.1016/j.janxdis.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblen JL, Norman SB, Sonis JH, Phelps AJ, Bisson JI, Nunes VD, Megnin-Viggars O, Forbes D, Riggs DS, & Schnurr PP (2019). A guide to guidelines for the treatment of posttraumatic stress disorder in adults: An update. Psychotherapy, 56(3), 359–373. 10.1037/pst0000231 [DOI] [PubMed] [Google Scholar]
- Kumpula MJ, Pentel KZ, Foa EB, LeBlanc NJ, Bui E, McSweeney LB, Knowles K, Bosley H, Simon NM, & Rauch SA (2017). Temporal sequencing of change in posttraumatic cognitions and PTSD symptom reduction during prolonged exposure therapy. Behavior Therapy, 48(2), 156–165. 10.1016/j.beth.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Marshall GN, Miles JN, & Stewart SH (2010). Anxiety sensitivity and PTSD symptom severity are reciprocally related: Evidence from a longitudinal study of physical trauma survivors. Journal of Abnormal Psychology, 119(1), 143–150. 10.1037/a0018009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JM, Farrell NR, Kemp JJ, Blakey SM, & Deacon BJ (2014). Why do clinicians exclude anxious clients from exposure therapy? Behaviour Research and Therapy, 54, 49–53. 10.1016/j.brat.2014.01.004 [DOI] [PubMed] [Google Scholar]
- Naifeh JA, Tull MT, & Gratz KL (2012). Anxiety sensitivity, emotional avoidance, and PTSD symptom severity among crack/cocaine dependent patients in residential treatment. Cognitive Therapy and Research, 36(3), 247–257. 10.1007/s10608-010-93378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naragon-Gainey K (2010). Meta-analysis of the relations of anxiety sensitivity to the depressive and anxiety disorders. Psychological Bulletin, 136(1), 128–150. 10.1037/a0018055 [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Feldman G, Smits JA, Christian KM, Zalta AK, Pollack MH, & Simon NM (2008). Examination of the decline in symptoms of anxiety and depression in generalized anxiety disorder: Impact of anxiety sensitivity on response to pharmacotherapy. Depression and Anxiety, 25(2), 167–171. 10.1002/da.20283 [DOI] [PubMed] [Google Scholar]
- Olatunji BO, & Wolitzky-Taylor KB (2009). Anxiety sensitivity and the anxiety disorders: A meta-analytic review and synthesis. Psychological Bulletin, 135(6), 974–999. 10.1037/a0017428 [DOI] [PubMed] [Google Scholar]
- Otto MW, Hinton D Korbly NB, Chea A, Ba P, Gershuny BS, & Pollack MH (2003). Treatment of pharmacotherapy-refractory posttraumatic stress disorder among Cambodian refugees: A pilot study of combination treatment with cognitive-behavior therapy vs sertraline alone. Behaviour Research and Therapy, 41, 1271–1276. 10.1016/S0005-7967(03)00032-9 [DOI] [PubMed] [Google Scholar]
- Pohl RB, Wolkow RM, & Clary CM (1998). Sertraline in the treatment of panic disorder: A double-blind multicenter trial. American Journal of Psychiatry, 155(9), 1189–1195. 10.1176/ajp.155.9.1189 [DOI] [PubMed] [Google Scholar]
- Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, & Foa EB (2010). A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clinical Psychology Review, 30(6), 635–641. 10.1016/j.cpr.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Rauch SA, Kim HM, Powell C, Tuerk PW, Simon NM, Acierno R, Allard CB, Norman SB, Venners MR, Rothbaum BO, Stein MB, Porter K, Martis B, King AP, Liberzon I, Phan KL, & Hoge CW (2019). Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: A randomized clinical trial. JAMA Psychiatry, 76(2), 117–126. 10.1001/jamapsychiatry.2018.3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SAM, Kim HM, Venners MR, Porter KE, Norman SB, Simon NM, Rothbaum BO, Tuerk PW, Acierno RE, Bui E, Powell C, Smith ER, Goetter E, & McSweeney LB (2022). Change in posttraumatic stress disorder–related thoughts during treatment: Do thoughts drive change when pills are involved? Journal of Traumatic Stress, 35(2), 496–507. 10.1002/jts.22762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SA, Simon NM, Kim HM, Acierno R, King AP, Norman SB, Venners MR, Porter K, Phan KL, Tuerk PW, Allard C, Liberzon I, Rothbaum BO, Martis B, Stein MB, & Hoge CW (2018). Integrating biological treatment mechanisms into randomized clinical trials: Design of PROGrESS (PROlonGed ExpoSure and Sertraline Trial). Contemporary Clinical Trials, 64, 128–138. 10.1016/j.cct.2017.10.013 [DOI] [PubMed] [Google Scholar]
- Resick PA, Monson CM, & Chard KM (2016). Cognitive processing therapy for PTSD: A comprehensive manual. Guilford Publications. [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, & McNally RJ (1986). Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour Research and Therapy, 24(1), 1–8. 10.1016/0005-7967(86)90143-9 [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Cahill SP, Foa EB, Davidson JR, Compton J, Connor KM, Astin MC, & Hahn CG (2006). Augmentation of sertraline with prolonged exposure in the treatment of posttraumatic stress disorder. Journal of Traumatic Stress, 19(5), 625–638. 10.1002/jts.20170 [DOI] [PubMed] [Google Scholar]
- Schneier FR, Neria Y, Pavlicova M, Hembree E, Suh EJ, Amsel L, & Marshall RD (2012). Combined prolonged exposure therapy and paroxetine for PTSD related to the World Trade Center attack: A randomized controlled trial. American Journal of Psychiatry, 169(1), 80–88. 10.1176/appi.ajp.2011.11020321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59(20), 22–33. [PubMed] [Google Scholar]
- Simon NM, Otto MW, Smits JA, Nicolaou DC, Reese HE, & Pollack MH (2004). Changes in anxiety sensitivity with pharmacotherapy for panic disorder. Journal of Psychiatric Research, 38(5), 491–495. 10.1016/j.jpsychires.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Short NA, Boffa JW, Norr AM, Albanese BJ, Allan NP, & Schmidt NB (2017). Randomized clinical trial investigating the effects of an anxiety sensitivity intervention on posttraumatic stress symptoms: A replication and extension. Journal of Traumatic Stress, 30(3), 296–303. 10.1002/jts.22194 [DOI] [PubMed] [Google Scholar]
- Smits JA, Zvolensky MJ, Davis ML, Rosenfield D, Marcus BH, Church TS, Powers MB, Frierson GM, Otto MW, Hopkins LB, Brown RA & Baird SO (2016). The efficacy of vigorous-intensity exercise as an aid to smoking cessation in adults with high anxiety sensitivity: A randomized controlled trial. Psychosomatic Medicine, 78(3), 354–364. 10.1097/FPSY.0000000000000264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, & Fidell LS (2018). Using multivariate statistics (7th ed.). Pearson. [Google Scholar]
- Taylor S (2003). Outcome predictors for three PTSD treatments: Exposure therapy, EMDR, and relaxation training. Journal of Cognitive Psychotherapy, 17(2), 149–162. 10.1891/jcop.17.2.149.57432 [DOI] [Google Scholar]
- Taylor S, Koch WJ, Woody S, & McLean P (1996). Anxiety sensitivity and depression: How are they related? Journal of Abnormal Psychology, 105(3), 474–479. 10.1037/0021-843X.105.3.474 [DOI] [PubMed] [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, Abramowitz JS, Holaway RM, Sandin B, Stewart SH, Coles M, Eng W, Daly ES, Arrindell WA, Bouvard M, & Cardenas SJ (2007). Robust dimensions of anxiety sensitivity: Development and initial validation of the Anxiety Sensitivity Index-3. Psychological Assessment, 19(2), 176–188. 10.1037/F1040-3590.19.2.176 [DOI] [PubMed] [Google Scholar]
- Wiedeman LD, Hannan SM, Maieritsch KP, Robinson C, & Bartoszek G (2020). Treatment choice among veterans with PTSD symptoms and substance-related problems: Examining the role of preparatory treatments in trauma-focused therapy. Psychological Services, 17(4), 405–413. 10.1037/ser0000313 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, & Davidson JR (2001). Clinician-Administered PTSD Scale: A review of the first ten years of research. Depression and Anxiety, 13(3), 132–156. 10.1002/da.1029 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, Keane TM, & Marx BP (2018). The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military Veterans. Psychological Assessment, 30(3), 383–395. 10.1037/pas0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandberg LJ, Rosenfield D, McLean CP, Powers MB, Asnaani A, & Foa EB (2016). Concurrent treatment of posttraumatic stress disorder and alcohol dependence: Predictors and moderators of outcome. Journal of Consulting and Clinical Psychology, 84(1), 43–56. 10.1037/ccp0000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoellner LA, Feeny NC, & Bittinger JN (2009). What you believe is what you want: Modeling PTSD-related treatment preferences for sertraline or prolonged exposure. Journal of Behavior Therapy and Experimental Psychiatry, 40(3), 455–467. 10.1016/j.jbtep.2009.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]


