Summary
Desmoplastic melanoma is a subtype of melanoma characterised by amelanotic fusiform melanocytes dispersed in a collagenous stroma. Cell-poor and fibrous stroma-rich ‘pure’ variants have been distinguished from ‘mixed’ variants with areas of higher cell density and/or less desmoplastic stroma. This distinction is relevant because patients whose tumours display a pure phenotype have a lower risk for regional lymph node metastasis and distant recurrence. However, little is known about interobserver agreement among pathologists in the subclassification of desmoplastic melanoma. To address this issue, we conducted a study in which eleven dermatopathologists independently evaluated whole slide scanned images of excisions from 30 desmoplastic melanomas. The participating pathologists were asked to classify the tumours as pure or mixed. They were also asked to record the presence or absence of neurotropism and angiotropism. We found substantial interobserver agreement between the 11 dermatopathologists in the classification of tumours as pure versus mixed desmoplastic melanoma (kappa=0.64; p<0.0001). There was fair agreement between the 11 dermatopathologists in the evaluation of presence versus absence of neurotropism (kappa=0.26; p<0.0001), and slight agreement in the assessment of angiotropism (kappa=0.13; p<0.0001). The level of concordance in the subclassification of desmoplastic melanomas is encouraging for the acceptance of this prognostic parameter in the real-world practice of melanoma pathology.
Keywords: Desmoplastic, melanoma, pure, mixed, neurotropism, angiotropism
INTRODUCTION
Desmoplastic melanoma (DM) is a rare subtype of melanoma that typically affects chronically sun-damaged skin of elderly individuals and is histopathologically characterised by the association of usually amelanotic spindle tumour cells with an abundant fibrous matrix,1-6
DM has been further subclassified into a stroma-rich and cell-poor ‘pure’ variant, in which the entire tumour, or the overwhelming majority of it (typically ≥90%), is characterised by isolated tumour cells at low cell density dispersed in abundant fibrous stroma, and a ‘mixed’ variant in which there are other areas of higher cell density with less or no significant desmoplasia.1,3,7 This subclassification has clinical relevance. Previous studies found shorter time to recurrence and disease-free survival as well as higher incidence of nodal and distant metastasis for mixed desmoplastic melanomas when compared against pure desmoplastic melanomas.2,4,8-10
Given that the assessment of histological features in desmoplastic melanomas may have implications for patient care and prognosis, we sought to evaluate the level of interobserver agreement amongst dermatopathologists who reviewed a set of 30 desmoplastic melanomas.
MATERIALS AND METHODS
Cases and participants
The study was conducted under the IRB-approved protocol 17-70. Digital links to whole slide scanned images (WSI) of a representative H&E section of 30 anonymised cases of DM were sent to 12 different dermatopathologists using the PathPresenter platform. Of a total of 12 dermatopathologists invited to participate, 11 completed their assignment (CL, RB, LMD, PG, LL, JLM, RAS, BW, IY, AZ, and KJB). Each pathologist independently evaluated the study cases. There were no time limits for review of each case and/or each of the features to be assessed.
Case classification criteria
Upon acceptance of participation, each pathologist was provided with written criteria for tumour classification as pure versus mixed desmoplastic melanoma, presence or absence of neurotropism, and presence or absence of angiotropism, as detailed below.
Pure versus mixed desmoplastic melanoma
A desmoplastic melanoma was defined as ‘pure’ if the melanoma was characterised by isolated tumour cells dispersed at low tumour cell density in a fibrous matrix throughout the tumour, with absence of compact fascicles, nests or sheets of solid tumour cell aggregates lacking intervening collagenous stroma. A desmoplastic melanoma was classified as ‘mixed’ if the tumour cell density was not uniformly low, but some areas within the tumour contained compact fascicles or nodules or tumour cells were present in close proximity to each other with minimal or no intervening fibrous stroma.
Neurotropism
Neurotropism was defined according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (8th ed) and College of American Pathologists (CAP) reporting guidelines as the presence of melanoma cells abutting nerve sheaths usually circumferentially (perineural invasion) or within nerves (intraneural invasion). Occasionally, the tumour itself may form neuroid structures (termed ‘neural transformation’) and this is also regarded as neurotropism. Neurotropism is best identified at the periphery of the tumour; the presence of melanoma cells around nerves in the main tumour mass caused by ‘entrapment’ of nerves in the expanding tumour does not represent neurotropism.11,12
Angiotropism
Angiotropism was defined as melanoma cells cuffing the external surfaces of either capillary or venular blood vessels or lymphatic channels without the presence of tumour within the vascular lumina.13,14
Statistical analysis
Fleiss’ kappa statistic was calculated to measure agreement for more than two observers on binary ratings. Data were analysed using the Magree macro in SAS software (version 9.4; SAS, USA). The Landis and Koch scale was used to qualify degrees of agreement.
RESULTS
There was substantial interobserver agreement between all 11 participants in the classification of the set of 30 tumours as pure versus mixed desmoplastic melanoma (kappa=0.64; p<0.0001). A subgroup of five dermatopathologists had excellent agreement with each other on subtype (kappa=0.84; p<0.0001). There was fair agreement between the 11 pathologists in the evaluation of presence versus absence of neurotropism (kappa=0.26; p<0.0001), and slight agreement in the assessment of angiotropism (kappa=0.13; p<0.0001).
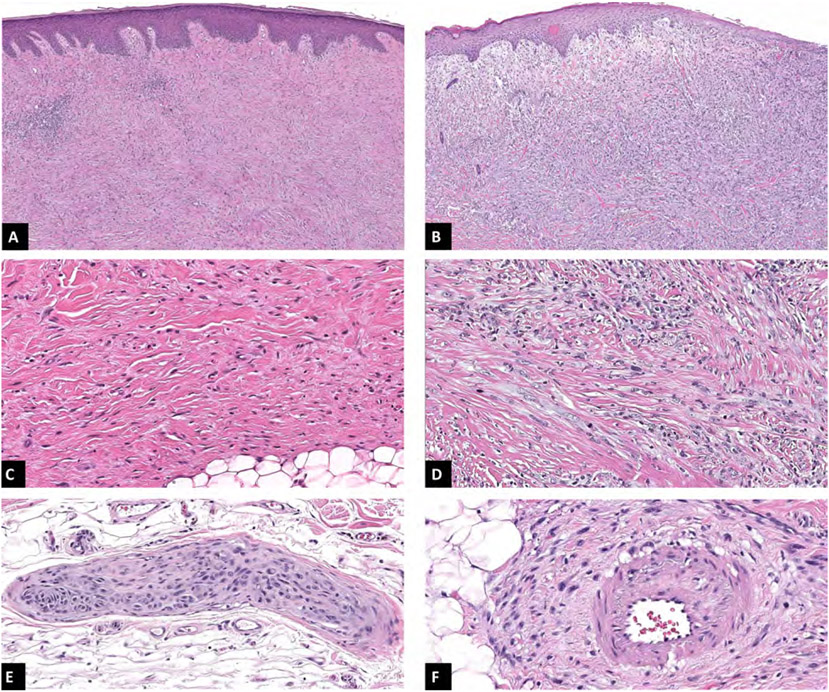
For 13 of the total 30 cases (43.3%) there was perfect agreement among all 11 observers on tumour subtype; of these, seven were categorised as mixed and six as pure desmoplastic melanomas. Perfect agreement regarding neurotropism was achieved in five cases (16.7%) in which all observers reported presence of this feature. In five cases (16.7%) there was perfect agreement on the evaluation of angiotropism, with three cases on which this feature was observed. In three of the 30 cases (10%) there was perfect agreement for all features evaluated: one of them corresponding to a pure desmoplastic melanoma with presence of both neurotropism and angiotropism, and the other two cases corresponding to mixed desmoplastic melanomas with neurotropism, one of them with and the other without angiotropism. Figure 1 illustrates examples of each variable obtained from cases in which perfect agreement among observers was achieved for the depicted feature.
Fig. 1.
(A,C) Pure desmoplastic melanoma showing low cellularity and abundant fibrotic stroma with uniform low-grade cytomorphology. (B,D) Mixed desmoplastic melanoma with high cellularity present in the superficial aspect of the tumour where an epithelioid cell component is present showing frequent mitotic figures. (E) Neurotropism: perineural and intraneural invasion. (F) Angiotropism: tumour cells in close association to vascular wall without frank invasion into the vessel lumen.
DISCUSSION
Several studies have confirmed that when desmoplastic melanomas are subdivided into ‘pure’ versus ‘mixed’ variants, there are associated significant differences in rates of local recurrence, time to recurrence, positive sentinel lymph node, distant metastasis, and melanoma-specific survival. 1,2,4,8-10,15 Accordingly, the current guidelines of CAP for the reporting of cutaneous melanoma recommend the distinction between these subtypes of desmoplastic melanoma.11,16-18
While reports of similar results from different groups suggest that the distinction of pure from mixed desmoplastic melanoma is reproducible, there are limited data on measured interobserver agreement between different pathologists. A prior small study published as a meeting abstract in 2012 reported only moderate concordance (kappa=0.47).19 The limited agreement was probably in part due to the fact that criteria were not discussed or shared with the study participants. Now that the concept of pure versus mixed desmoplastic melanoma has been accepted at many centres, we decided to revisit the issue of interobserver agreement and conduct a study on a larger number of cases involving more pathologists. Interobserver agreement was analysed on 30 cases between 11 dermatopathologists. This time, definitions of parameters were shared in writing with each participant prior to scoring. The main interest was in the assessment of pure versus mixed desmoplastic melanomas, but we also tested interobserver variability on the presence versus absence of neurotropism and angiotropism.
We found substantial agreement (kappa=0.64) in the classification of the 30 tumours as pure versus mixed desmoplastic melanomas. This result is encouraging, considering the relatively high number of observers (11) practising at 10 different institutions. It is of interest that among the 11 participating dermatopathologists five had excellent interobserver agreement (kappa=0.84), indicating that improvement of agreement beyond substantial is possible.
As desmoplastic melanomas may be associated with neurotropism and occasionally also angiotropism, both of which have been suggested to be relevant for risk of recurrence, we decided to also assess interobserver agreement for these parameters. Assessment for angiotropism or neurotropism showed only slight (kappa=0.13) and fair (kappa=0.26) interobserver concordance, respectively, with only five of 30 evaluated cases (16.7%) in which all observers agreed on the presence or absence of either feature. While our findings on interobserver agreement on neurotropism and angiotropism in the context of desmoplastic melanomas should not be extrapolated to other melanoma types, they warrant some caution regarding the reliability of pathology reports commenting on the presence or absence of these parameters. The definition of neurotropism in melanoma according to the AJCC Cancer Staging Manual and the CAP reporting guidelines states that this feature is best identified at the periphery of tumours and should be distinguished from nerve ‘entrapment’, however it does not specifically address bona fide perineural and intraneural invasion that are occasionally found within the bulk of the tumour. It is possible that this could contribute to variable interpretations of what constitutes sufficient evidence of neurotropism and in turn impact agreement among observers. It is of interest that a similarly low level of interobserver concordance was previously found for perineural invasion in desmoplastic melanoma.19 On the other hand, a prior report on interobserver concordance for angiotropism found good agreement amongst the three study participants.20 More work is needed to determine the best definition of these terms and how to make their application more reliable. It also needs to be said that the assessment of angiotropism and neurotropism is particularly challenging in desmoplastic melanomas because of the difficulty in distinguishing tumour cells from non-neoplastic perineural, perivascular, and stromal spindle cells without immunohistochemical stains, which were not made available to observers in this study.
Lastly, a significant limitation of our study was the use of whole slide scanned images (WSI). While equivalency between WSI and glass slides diagnoses has been well documented for pathologists experienced in reading high quality digital scans,21 it is possible that some pathologists were less comfortable reading digital scans, which may have contributed to discordant assessments of neurotropism or angiotropism.
CONCLUSION
In summary, our study documents substantial agreement amongst 11 pathologists for the distinction between ‘pure’ versus ‘mixed’ desmoplastic melanomas on WSI of 30 different tumours. We found limited agreement in the assessment of neurotropism or angiotropism. Good interobserver reproducibility is an important factor in the value that a given histopathological feature can provide as a tool for reliable clinical prediction. Our results document that the level of interobserver agreement in the evaluation of desmoplastic melanoma subtypes is strong enough to support recommending the use of this subclassification in pathology reports for its clinical relevance for prognosis.
Acknowledgements:
The authors wish to thank Yesenia Gonzalez and Stephanie Regalado for their help retrieving and scanning archival material.
Conflicts of interest and sources of funding:
Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. RAS is supported by a National Health and Medical Research Council of Australia (NHMRC) Practitioner Fellowship (APP1141295). RAS has received fees for professional services from MetaOptima Technology Inc, F. Hoffmann-La Roche Ltd, Evaxion, Provectus Biopharmaceuticals Australia, Qbiotics, Novartis, Merck Sharp & Dohme, NeraCare, AMGEN Inc, Bristol-Myers Squibb, Myriad Genetics, GlaxoSmithKline. The remaining authors have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Busam KJ, Mujumdar U, Hummer AJ, et al. Cutaneous desmoplastic melanoma: reappraisal of morphologic heterogeneity and prognostic factors. Am J Surg Pathol 2004; 28:1518–25. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins WG, Busam KJ, Ben-Porat L, et al. Desmoplastic melanoma: a pathologically and clinically distinct form of cutaneous melanoma. Ann Surg Oncol 2005; 12:207–13. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy SW, Scolyer RA, Palmer AA. Desmoplastic melanoma: a diagnostic trap for the unwary. Pathology 2004; 36: 445–51. [DOI] [PubMed] [Google Scholar]
- 4.Murali R, Shaw HM, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer 2010; 116: 4130–8. [DOI] [PubMed] [Google Scholar]
- 5.Wiesner T, Kiuru M, Scott SN, et al. NF1 mutations are common in desmoplastic melanoma. Am J Surg Pathol 2015; 39: 1357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol 2013; 68: 825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elder DE, Bastian BC, Cree IA, et al. The 2018 World Health Organization classification of cutaneous, mucosal, and uveal melanoma: detailed analysis of 9 distinct subtypes defined by their evolutionary pathway. Arch Pathol Lab Med 2020; 144:500–22. [DOI] [PubMed] [Google Scholar]
- 8.George E, McClain SE, Slingluff CL, et al. Subclassification of desmoplastic melanoma: pure and mixed variants have significantly different capacities for lymph node metastasis. J Cutan Pathol 2009; 36: 425–32. [DOI] [PubMed] [Google Scholar]
- 9.Laeijendecker AE, El Sharouni MA, Sigurdsson V, et al. Desmoplastic melanoma: the role of pure and mixed subtype in sentinel lymph node biopsy and survival. Cancer Med 2020; 9: 671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawlik TM, Ross MI, Prieto VG, et al. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer 2006; 106: 900–6. [DOI] [PubMed] [Google Scholar]
- 11.Shon W, Frishberg DP, Gershenwald JE, et al. Protocol for the Examination of Excision Specimens From Patients With Melanoma of the Skin. Version: 4.3.0.2 Nov 2021; cited Oct 2022. Northfield, IL: College of American Pathologists, 2021. https://documents.cap.org/protocols/Skin.Melanoma_4.3.0.2.REL_CAPCP.pdf [Google Scholar]
- 12.Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. New York: Springer, 2017. [Google Scholar]
- 13.Barnhill R, Dy K, Lugassy C. Angiotropism in cutaneous melanoma: a prognostic factor strongly predicting risk for metastasis. J Invest Dermatol 2002; 119: 705–6. [DOI] [PubMed] [Google Scholar]
- 14.Lugassy C, Kleinman HK, Vermeulen PB, et al. Angiotropism, pericytic mimicry and extravascular migratory metastasis: an embryogenesis-derived program of tumour spread. Angiogenesis 2020; 23: 27–41. [DOI] [PubMed] [Google Scholar]
- 15.Howard MD, Wee E, Wolfe R, et al. Differences between pure desmoplastic melanoma and superficial spreading melanoma in terms of survival, distribution and other clinicopathologic features. J Eur Acad Dermatol Venereol 2019; 33: 1899–906. [DOI] [PubMed] [Google Scholar]
- 16.Hughes TM, Williams GJ, Gyorki DE, et al. Desmoplastic melanoma: a review of its pathology and clinical behaviour, and of management recommendations in published guidelines. J Eur Acad Dermatol Venereol 2021; 35: 1290–8. [DOI] [PubMed] [Google Scholar]
- 17.Nicolson NG, Han D. Desmoplastic melanoma. J Surg Oncol 2019; 119: 208–15. [DOI] [PubMed] [Google Scholar]
- 18.Wood BA. Desmoplastic melanoma: recent advances and persisting challenges. Pathology 2013; 45: 453–63. [DOI] [PubMed] [Google Scholar]
- 19.Murali R, Riedel ER, Busam KJ, et al. Interobserver agreement of assessment of desmoplasia and neurotropism in melanoma. 101st Annual Meeting of the United States and Canadian Academy of Pathology (USCAP). Vancouver: Nature Publishing Group, 2012: 128–9A. [Google Scholar]
- 20.Barnhill RL, Busam KJ, From L, et al. Inter-observer concordance for the recognition of angiotropism in human melanoma. Pigment Cell Melanoma Res 2011; 24: 582–3. [DOI] [PubMed] [Google Scholar]
- 21.Hanna MG, Reuter VE, Ardon O, et al. Validation of a digital pathology system including remote review during the COVID-19 pandemic. Mod Pathol 2020; 33: 2115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]