Abstract
The hippocampus is composed of various subregions: CA1, CA2, CA3, and the dentate gyrus (DG). Despite the abundant hippocampal research literature, until recently, CA2 received little attention. The development of new genetic and physiological tools allowed recent studies characterizing the unique properties and functional roles of this hippocampal subregion. Despite its small size, the cellular content of CA2 is heterogeneous at the molecular and physiological levels. CA2 has been heavily implicated in social behaviors, including social memory. More generally, the mechanisms by which the hippocampus is involved in memory include the reactivation of neuronal ensembles following experience. This process is coordinated by synchronous network events known as sharp-wave ripples (SWRs). Recent evidence suggests that CA2 plays an important role in the generation of SWRs. The unique connectivity and physiological properties of CA2 pyramidal cells make this region a computational hub at the core of hippocampal information processing. Here, we review recent findings that support the role of CA2 in coordinating hippocampal network dynamics from a systems neuroscience perspective.
Introduction
The basic anatomical organization of the hippocampal formation has been known since the seminal studies of Santiago Ramon y Cajal (Cajal, 1911). The hippocampus has been traditionally conceived as having mainly a feedforward network architecture. Cortical inputs, funneled through the entorhinal cortices, arrive to the dentate gyrus (DG) and from there propagate sequentially through the different subregions of the Cornu Ammonis (CA) to be finally broadcasted to multiple cortical and subcortical brain structures. Rafael Lorente de No continued Cajal’s studies describing the morphological variations of hippocampal pyramidal cells along the transverse axis of the CA. Based on morphological and connectivity differences, he divided the hippocampal field into three subregions: CA1, CA2, and CA3 (Lorente De Nó, 1934). Modern anatomical studies have shown that the hippocampal organization is more complex than a feedforward trisynaptic circuit, highlighting the relevance of numerous feedback loops, local connectivity motifs, and innervation to/from multiple brain areas (Amaral & Witter, 1989; Middleton & McHugh, 2020). Most hippocampal studies had focused on the CA1, CA3, or DG areas; few considered the CA2 region, which was typically regarded as a mere transition zone between CA3 and CA1. This neglection was in part due to the small size of CA2, which difficulted physiological recordings, and in part due to the lack of robust methods to precisely define its borders. Recently, this scenario has changed due to the development of new genetic targeting strategies specific to CA2 pyramidal cells (Farris et al., 2019; Hitti & Siegelbaum, 2014) and the improvement of electrophysiological recordings methods that enabled simultaneous ensemble recordings across hippocampal subregions (Boehringer et al., 2017; Kay et al., 2016; Mankin et al., 2015; Oliva et al., 2016a, 2016b).
Recent studies on in vivo physiological properties and behavioral correlates of CA2 demonstrated that this small subregion has remarkably distinct properties compared to the rest of the hippocampus. The hippocampus has been long known to be a key structure for episodic memory and spatial navigation (Eichenbaum, 2004; O’Keefe & Nadel, 1978). As part of a highly interconnected network, CA2 shares these functional roles, but it also displays unique specializations. Pyramidal cells from every hippocampal subfield fire at specific locations of the environment (‘place cells’), forming a ‘cognitive map’ that supports spatial navigation (O’Keefe & Dostrovsky, 1971; O’Keefe & Nadel, 1978). CA2 firing responses within space are less stable than that of CA1 or CA3, and the CA2 firing fields cover wider regions of space. CA2 firing fields are instead better at discriminating events in time (i.e., CA2 displays firing fields aligned to time elapsed after the trial start, independent of the position of the animal; and they remap after time passes in the same environment) (MacDonald & Tonegawa, 2021; Mankin et al., 2015) or at signaling novelty of both conspecifics and inanimate objects (Chen et al., 2020). CA2 cells also fire during immobility periods (Kay et al., 2016) and responds preferentially to social stimuli (Alexander et al., 2016; Donegan et al., 2020; Oliva et al., 2020). The role of CA2 in social behaviors has been heavily investigated in recent years. CA2 activity is necessary for social memory formation (remembering individuals encountered in a one to one interaction) in both the adult (Leroy et al., 2017; Meira et al., 2018; Oliva et al., 2020) and developing brain (Domínguez et al., 2019; Laham et al., 2021). In addition, CA2 activity increases after a subject mouse is exposed to a novel conspecific during naïve interactions(Alexander et al., 2016; Chen et al., 2020; Donegan et al., 2020) and aggressive encounters (Leroy et al., 2018; Pagani et al., 2015). The correlation patterns of CA2 firing dynamics at the population level also differ for novel versus familiar conspecifics’ representations (Donegan et al., 2020, Boyle et al., 2022). In addition to these functional specializations, a general feature of CA2 is its pivotal role in the orchestration of hippocampal network dynamics. A remarkable example of this is the role of CA2 in triggering sharp-wave ripples (Oliva et al., 2016b), synchronous network patterns fundamental for learning and memory (Buzsáki, 2015; Joo & Frank, 2018). Several previous reviews have extensively discussed the unique genetic and anatomical features of CA2 as well as its role in social memory during physiological and pathological conditions (Chevaleyre & Piskorowski, 2016; Dudek et al., 2016; Middleton & McHugh, 2020). Here we will focus on the role of the CA2 region in the generation and coordination of hippocampal network dynamics. We will discuss recent works that collectively contribute to the emergent picture of CA2 as a privileged computational hub at the core of multiple hippocampal functions.
CA2 pyramidal cells have distinct electrophysiological properties
Pyramidal cells in the CA2 subregion differ from those in the neighboring CA1 and CA3 areas in their gene expression and transcriptomic profiles (Cembrowski et al., 2016; Lein et al., 2007), morphology (Lorente De Nó, 1934; Srinivas et al., 2017; Tamamaki et al., 1988), local and extrinsic connectivity (Cui et al., 2013; Hitti & Siegelbaum, 2014; Kohara et al., 2014), and physiological properties (Chevaleyre & Piskorowski, 2016; Leroy et al., 2017; Sun et al., 2017). In this section, we will review how these particular features of CA2 pyramidal cells contribute to the unique network dynamics of this area.
CA2 was originally distinguished based on morphological characteristics, including the absence of stratum lucidum (an additional layer between the pyramidal and stratum radiatum layers in CA3), larger pyramidal cells somas (Ishizuka et al., 1995; Lorente De Nó, 1934; Sun et al., 2017), and the lack of thorny excrescences (postsynaptic compartment between mossy fibers and targeted dendritic branch) along their dendritic arbor (Srinivas et al., 2017; Tamamaki et al., 1988). CA2 pyramidal cells have larger distal apical dendritic arborizations that branch out densely in the stratum lacunosum moleculare (SLM) compared to CA1 and CA3 pyramidal cells (Chevaleyre & Siegelbaum, 2010; Ishizuka et al., 1995; Sun et al., 2014). From an electrophysiological point of view, CA2 pyramidal cells also differ from their CA1 and CA3 peers. In vitro, CA2 neurons show different membrane dynamics compared to CA1 and CA3, specifically lower input resistance and higher membrane capacitance (Chevaleyre & Siegelbaum, 2010). In addition, CA2 neurons show the least intrinsic excitability in vitro, as measured by the number of action potentials that cells fire during current injections (Sun et al., 2017). On the contrary, CA2 cells in vivo display a higher firing rate than CA1 and CA3 cells (Kay et al., 2016; Oliva et al., 2016a), and have a higher probability to fire bursts of action potentials (Boehringer et al., 2017; Oliva et al., 2016a, 2020), suggesting CA2 has a higher excitability than its neighboring regions. Such a dichotomy of excitability between in vitro and in vivo might be due to endogenous changes in neuromodulators present in vivo, but not in vitro. In addition, CA2 firing rates display a unique modulation by brain state. While other hippocampal pyramidal cells preferentially fire during active locomotion, CA2 pyramidal cells fire more during periods of immobility (Kay et al., 2016; Oliva et al., 2016a, 2020); and while most cells are positively modulated by SWRs, a subset of CA2 cells are strongly inhibited (Kay et al., 2016; Oliva et al., 2020; Valero et al., 2015).
In terms of local connectivity, CA2 is more similar to CA3 than to CA1 (Figure 1). As the former, it exhibits a high density of recurrent excitatory connections between pyramidal cells (Okamoto & Ikegaya, 2019; Tamamaki et al., 1988). Similar to CA3, CA2 pyramidal cells receive inputs from layer 2 of the entorhinal cortex (Chevaleyre & Siegelbaum, 2010; Cui et al., 2013; Hitti & Siegelbaum, 2014; Kohara et al., 2014) as well as from DG granular cells (Kohara et al., 2014). Analogous to CA1, CA2 pyramidal cells receive projections from the entorhinal cortex (layer 3 in the case of CA1 and layer 2 in the case of CA2) (Rowland et al., 2013). Interestingly, CA2 cells have a larger distal dendritic arborization and branching pattern in the stratum lacunosum moleculare (SLM), where the axons from the entorhinal cortex arrive, and indeed, excitatory inputs from entorhinal cortex are more effective in discharging CA2 action potentials than they are in CA1 or CA3 (Chevaleyre & Siegelbaum, 2010; Srinivas et al., 2017; Sun et al., 2014, 2017). These findings are particularly important as they provide a parallel processing loop, independent of the classical trisynaptic pathway (ECII to CA3 to CA1) between the EC and CA1 (ECII to CA2 to CA1). In addition, given the different anatomical outputs from CA2 compared to CA1, it provides a shorter circuit from the EC to various anatomical targets, including the SUM, LS, and ventral hippocampus. In the CA2 area there is also a higher density of interneurons than in CA1 or CA3, including parvalbumin positive interneurons (PV+), reelin positive interneurons (Reln+), calbindin positive interneurons (CB+), and somatostatin positive interneurons (SOM+). These interneurons locally inhibit CA2 as well as target excitatory cells in CA1 and CA3 (Botcher et al., 2014). In addition, the dendrites of interneurons in the CA2 area tend to extend wider horizontally and show strong spike frequency adaptation, rebound upon stimulus offset, and prominent sag potential (i.e., voltage difference between the peak and steady values in response to hyperpolarizing current stimulus) (Mercer et al., 2007). Although interneurons in the CA2 area target primarily CA2 pyramidal cells, they also project to CA1 and CA3 pyramidal cells and receive inputs from the SUM, EC or DG (Botcher). Importantly, unlike CA1, CA2 pyramidal cells don’t show long-term synaptic potentiation after CA3 input stimulation (Zhao et al., 2007), but they do after EC input stimulation (Chevaleyre & Siegelbaum, 2010; Leroy et al., 2017; Sun et al., 2014). In addition, CA2 pyramidal cells’ potentiation can result from long-term synaptic depression in various types of inhibitory synapses (Leroy et al., 2017; Loisy et al., 2022; Nasrallah et al., 2015).
Figure 1: Hippocampal anatomical connectivity.
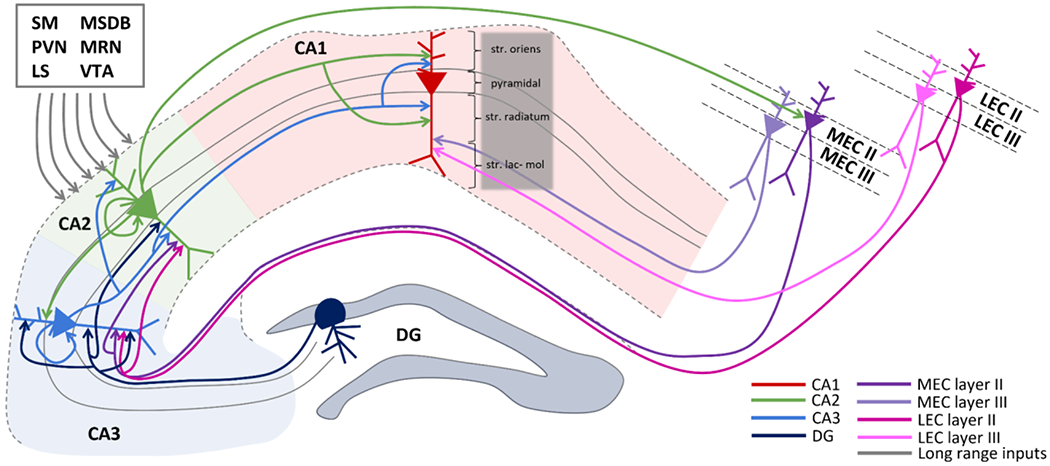
The main inputs and outputs of area CA2 are shown. CA2 receives projections from CA3, DG, layer II in EC (both medial and lateral portions) and send projections to CA1 and back to layer II in MEC. In addition to intrahippocampal and entorhinal cortex connections, long range inputs from supramammillary nucleus (SM), paraventricular nucleus (PVN), lateral septum (LS), medial septum diagonal band (MSDB), median raphe nucleus (MRN), and ventral tegmental area (VTA) are also included.
Taking all these data together, it becomes clear that the CA2 region possesses properties at the molecular, anatomical, and electrophysiological levels that makes it unique within the hippocampus. The combination of pyramidal cells with high firing rates (Kay et al., 2016; Oliva et al., 2016) and dense recurrent connections (Tamamaki et al., 1988, Okamoto & Ikegaya, 2019), together with a high density of interneurons (Botcher et al., 2014), makes this region able to display a large dynamic range of excitatory/inhibitory balance, able to support high levels of synchronous spiking as well as high inhibitory tone. These unique properties at the single cell and micro-circuit levels constitute the mechanistic basis of the distinct network features of CA2, such as oscillations and synchronous population bursts, which have been shown to be key for supporting hippocampal memory functions.
CA2 role in the generation of sharp-wave ripples
One of the prominent features of the hippocampus is its ability to generate synchronous network events, such as SWRs. SWRs are transient bursts (~50-100 ms) of ~150 Hz oscillations (‘ripples’) that can be observed in the pyramidal layer, accompanied by a lower frequency negative deflection (‘sharp-wave’) in the stratum radiatum (Buzsáki, 2015). They coordinate sequential neuronal activity that recapitulates recent experience and have been shown to be fundamental for learning and memory processes (Foster, 2017; Joo & Frank, 2018). Previously, it was shown that most hippocampal pyramidal cells and interneurons are robustly and positively entrained during SWRs. Surprisingly, it was recently reported that about half of CA2 pyramidal cells ramp up prior to SWRs and are later inhibited during SWRs (Figure 2; (Kay et al., 2016; Oliva et al., 2016b; Valero et al., 2015)).
Figure 2: Entrainment of CA1, CA2, and CA3 pyramidal cells by SWRs.
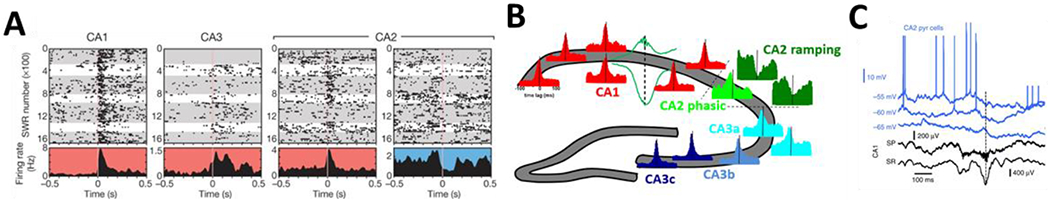
A) Typical SWR responses of CA1, CA3, and CA2 pyramidal cells. Note the strong firing rate increase of CA1, CA3, and one of the CA2 pyramidal cells (red histogram background), while the other CA2 cell ramps up before SWRs and becomes silenced during SWRs (blue histogram background) (Kay et al., 2016). B) SWR responses of hippocampal pyramidal cells along its transverse axis. All neurons exhibit strong phasic entrainment to SWRs except a subset of CA2 pyramidal cells (CA2 ramping, dark green) that became inhibited after an initial ramp up before SWRs (Oliva et al., 2016b). C) Intracellular recordings (blue trace) demonstrated that some CA2 pyramidal cells are silenced during entire SWRs (black trace, extracellular signal in CA1), contrary to most other hippocampal cells (Valero et al., 2015).
The traditional model posits that SWRs are initially generated in CA3 (Buzsáki, 2015). The synchronous activation of CA3 pyramidal cells, sustained by reciprocal excitatory connections (Ishizuka et al., 1995), produces a large depolarizing volley in CA1 apical dendrites (the ‘sharp-wave’). In turn, this excitatory drive induces high frequency ‘ripple’ oscillations in CA1 that rely on the precisely timed interactions between pyramidal cells and local interneurons (Gan et al., 2017; Stark et al., 2014; Ylinen et al., 1995). Using large scale silicon probe recordings that enable simultaneous recordings of SWRs in all hippocampal subregions, it was shown that CA2 cells tend to fire prior to SWR onset (Figure 3). A subset of CA2 pyramidal cells displayed a ramping activity 20-30 ms before SWR onset (‘ramping cells’) and were rapidly inhibited afterwards. They were followed by a phasic response from another subset of CA2 pyramidal cells (‘phasic cells’) right at the onset of the SWR, which then propagated to CA3. Finally, the input from CA3 to CA1 produced the classical SWR pattern, with the oscillation in the pyramidal layer and the depolarization in the stratum radiatum. This sequence of activity suggests that CA2 has a key role in triggering SWRs, likely facilitated by its dense excitatory recurrent connections and abundant parvalbumin-expressing basket cells. However, it has been suggested that several mechanisms could support the generation of SWRs.
Figure 3: Synchronous activity in CA2 precedes SWR.
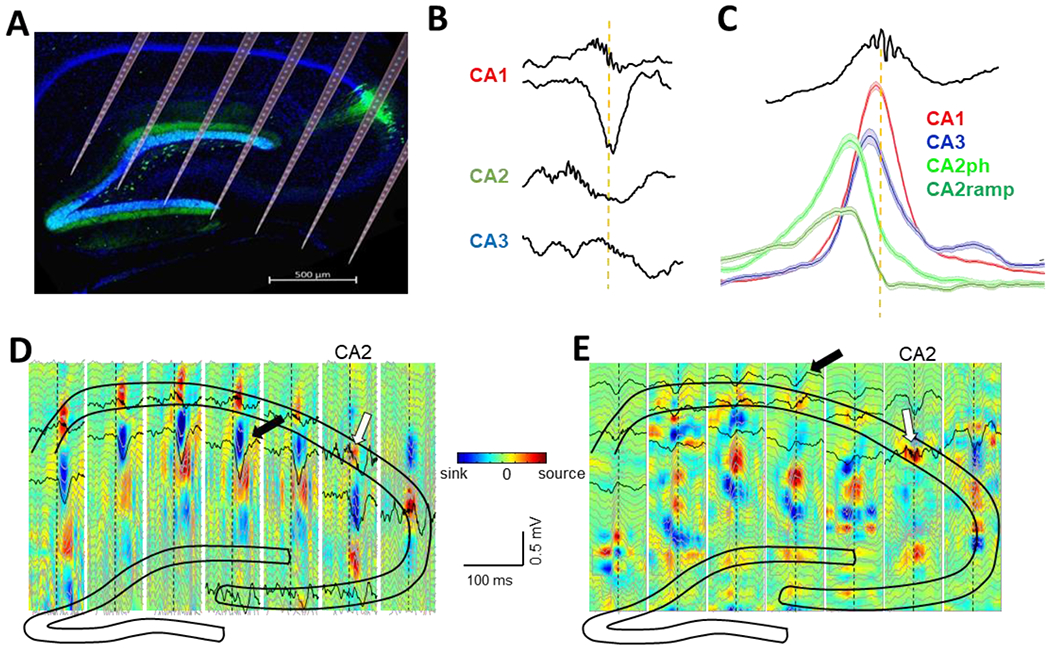
A) Large scale silicon probes enabled simultaneous recordings of all hippocampal subfields. CA2 can be identified by immunolabeling against specific proteins such PCP4 (green in the figure; Oliva et al., 2016b). B) Ripple oscillations and sharp-waves occur simultaneously in CA1 (top LFP traces) and coincide with CA3 ripples. Ripple activity starts in CA2 ~20 ms before CA1 and CA3. C) Activation of pyramidal cells from different regions along the hippocampal transverse axis during SWRs. CA2 ramping cells (dark green) discharge right before SWR onset and become silenced afterwards. CA2 phasic cells (light green) follow next and after them activity propagates through CA3 (blue) and finally CA1 (red) (Oliva et al., 2016b). D) Most SWRs occur simultaneously across the CA1 region and are accompanied by current ‘sinks’ (black arrow) in the stratum radiatum reflecting the synchronous depolarization from CA3 axons. CA2 ripples (white arrow) precede the onset of activity in CA1 (Oliva et al., 2016b). E) A minority of SWRs are triggered by direct inputs from CA2 to CA1 basal dendrites. They are characterized by a negative deflection in stratum oriens (black arrow) and the absence of a sharp-wave in stratum radiatum (Oliva et al., 2016b).
An important determinant for SWR generation is brain state. SWRs occur both during awake behavior, when animals are immobile or engaged in ‘consummatory’ tasks, or during non-REM sleep (Buzsáki, 2015; Joo & Frank, 2018). CA2 cells were shown to be active prior to a SWR’s occurrence in CA1 were more likely to contribute prior to a CA1 SWR during awake immobility rather than non-REM sleep (Oliva et al., 2016b). This is likely related to CA2 firing patterns identified specifically during immobility (Kay et al., 2016).
Importantly, some SWRs were shown to directly travel from CA2 to CA1 without involving CA3 (Figure 3; (Oliva et al., 2016b)). While axons from both CA2 and CA3 innervate apical and basal CA1 dendrites, they have opposite target regions. CA3 innervation is denser in the apical dendrites (stratum radiatum) and CA2 in the basal ones (stratum oriens). As a consequence of this, the sharp-wave generated by CA3 inputs is recorded as a negative deflection in the stratum radiatum, accompanied by a current ‘sink’ (reflecting inward flow of positive ions into the cell). On the other hand, the signature of SWRs propagating directly from CA2 to CA1 is a negative wave and associated sink in the stratum oriens, and a positive wave and current ‘source’ in the radiatum (reflecting passive outward flow of positive ions (Figure 3)).
The observation that both CA2 and CA3 can trigger SWRs that propagate to the main output of the hippocampus (CA1), suggests that they may have different spiking ‘content’ and, potentially, distinct functional correlates. CA2 and CA3 preferentially target different CA1 pyramidal cell subpopulations, anatomically segregated along its radial axis. CA3 is biased to innervate pyramidal cells in the CA1 ‘superficial’ sublayer while CA2 inputs those in the ‘deep’ sublayer (Kohara et al., 2014; Meira et al., 2018). Interestingly, a minority of deep CA1 cells also show negative modulation during SWRs, similar to many CA2 cells (Kay et al., 2016; Oliva et al., 2016b; Valero et al., 2015). In addition, CA1 deep and superficial cells preferentially encode different types of information during behavior (Danielson et al., 2016; Sharif et al., 2021). This evidence supports the hypothesis that SWRs initiated in CA2 or CA3 engage segregated downstream circuits and encode different types of information.
One of the functional specializations that CA2-initiated SWRs may serve is the encoding and consolidation of social memories. SWRs have been shown to coordinate the sequential reactivation of cells that encoded recent experiences (Diba & Buzsáki, 2007; Foster & Wilson, 2006; Karlsson & Frank, 2009). Memory ‘replay’ during SWRs had been investigated almost exclusively in the context of spatial experiences (Diba & Buzsáki, 2007; Foster & Wilson, 2006; Karlsson & Frank, 2009). A recent study investigated the role of SWRs in mice performing a social memory task (Oliva et al., 2020). In this work, it was found that silencing CA2 activity during long periods of time (~30s every two minutes) during sleep, decreased the number of SWRs, which resulted in social memory deficits. Furthermore, artificially generating SWRs using previously defined optogenetic methods (Fernández-Ruiz et al., 2019; Stark et al., 2015) selectively from CA2 (using Amigo2-Cre animals, which express Cre in CA2 pyramidal cells) during post-experience sleep, was sufficient for extending social memory recall in mice (Oliva et al., 2020). In an analogous experiment, SWRs were triggered from the CA3 region (using Grik-4 animals, which express Cre in CA3 pyramidal cells), but showed no impact on social memory (Oliva et al., 2020). These results were not observed when mice, instead of learning to discriminate between other conspecifics, explored novel inanimate objects. This work supports the idea that the extensively studied hippocampal mechanisms of spatial learning and memory can be generalized to other cognitive domains, reflecting the emerging view of the hippocampus as a general-purpose memory structure rather than being exclusively specialized in spatial coding (Behrens et al., 2018).
The evidence discussed above suggests that functional specializations of different hippocampal subfields can influence the content of memory reactivation during SWRs. However, SWRs are not restricted to specific subregions but are a global phenomenon that engage the whole hippocampus, and the synchronous output from CA1 triggers the reactivation of neuronal ensembles in multiple downstream areas (Ji & Wilson, 2007; Pennartz et al., 2004; Peyrache et al., 2009; Tang et al., 2017, 2017). Thus, a complementary possibility is that CA2 and CA3 inputs play different roles in the organization of SWR-associated neuronal sequences that are later distributed to downstream targets. Silencing of CA3 strongly reduces the number of SWRs (Davoudi & Foster, 2019; Yamamoto & Tonegawa, 2017), suggesting that this is the main excitatory input that directly drives ripple generation in CA1. Acute CA2 silencing also reduced SWR occurrence and power, although in smaller magnitude than CA3 silencing (He et al., 2021; Oliva et al., 2020). Conversely, chronic or extended periods of CA2 silencing results in more SWRs (Alexander et al., 2018; Boehringer et al., 2017). CA2 silencing had little effect on CA1 or CA3 firing rates but it strongly affected the temporal precision of assembly activation during replay events, as well as their sequential structure (He et al., 2021). Optogenetic stimulation of hippocampal pyramidal cells in CA1 (Fernández-Ruiz et al., 2019; Stark et al., 2015), CA2, or CA3 subregions (Oliva et al., 2020) induce artificial SWRs with properties and associated neuronal sequences that closely resemble those of spontaneous ones. These experiments suggest that local interactions in every subregion are sufficient to generate at least some of the observed sequential dynamics during SWRs. Interestingly, in addition to local activity, SWRs generated in CA2 entrained both CA3 and CA1 neuronal responses, while SWRs generated in CA3 entrained mainly CA1 activity. Intriguingly, optogenetic silencing of the entorhinal cortex also decreases SWR rate detected in the hippocampus (Yamamoto & Tonegawa, 2017). In parallel, entorhinal activity has been shown to precede and follow SWRs differentially during awake vs sleep states, respectively (Oliva et al., 2018; Yamamoto & Tonegawa, 2017). Because CA2 pyramidal cells strongly respond to cortical inputs from the medial (MEC, (Chevaleyre & Siegelbaum, 2010; Srinivas et al., 2017; Sun et al., 2014, 2017) and lateral (LEC, (Lopez-Rojas et al., 2022)) entorhinal cortices, it is plausible that entorhinal inputs play an important role in triggering SWRs, specifically in CA2. Future work is needed to elucidate how inputs from CA3 and CA2 interact with local dynamics in CA1 to precisely organize replay sequences and how inputs from the entorhinal cortices affect the coordination of local ensemble dynamics in the distinct subregions.
Theta, gamma and neuromodulatory control of CA2 network dynamics
In addition to SWRs, other types of coordinated population activity are prominent in the hippocampus. During active locomotion and REM sleep, hippocampal activity is dominated by the theta rhythm, 6-10 Hz oscillations that entrain both pyramidal cells and interneurons and have been shown to be important for memory encoding and retrieval (Buzsáki, 2002, p. 200; Colgin, 2013; Hasselmo, 2006). As most other hippocampal neurons, CA2 pyramidal cells fire phase locked to theta oscillations (Oliva et al., 2016a). The precise timing of spiking within the theta cycle is determined by the relative strength of inputs that arrive at different theta phases (Fernández-Ruiz et al., 2017; Navas-Olive et al., 2020). The two main excitatory inputs that control CA2 firing come from CA3 (at the descending theta phase) and layer 2 of the entorhinal cortex (at the theta trough), while inhibition is maximal at the ascending theta phase (Fernández-Ruiz et al., 2017; Oliva et al., 2016a). Because of this, the firing probability of CA2 pyramidal cells is greatest at the theta trough (Fernandez-Lamo et al., 2019; Oliva et al., 2016a). However, the timing of spiking during theta is dynamically modulated as a function of behavior. As an animal runs, the phase of a place cell’s spikes systematically shifts along its place field, a phenomenon known as ‘phase precession’ (O’Keefe & Recce, 1993) which is considered a hallmark of temporal coding. Similar to CA1 and CA3, CA2 place cells also exhibit phase precession, but their phase range is greatly reduced compared to that of CA1 place cells (Oliva et al., 2016a). This spike phase shift can be explained by the shifting strength of CA3 and entorhinal inputs within the place field, whose relative strengths determine the output phase of target neurons (Fernández-Ruiz et al., 2017). Since CA3 and ECII inputs peak at closer theta phases than CA3 and ECIII (in CA1), there is a resultant reduced phase precession range for CA2. Other important contributors to the theta firing dynamics of CA2 could be the inputs from the supramammillary nucleus (SUM) or medial septum diagonal band (MSDB), both important theta pacemakers. Local photostimulation of SUM axons reduces the spike timing variability of CA2 pyramidal cells (Chen et al., 2020), and photostimulation of ChAT+ neurons in the MSDB increases the firing of CA2 neurons via disinhibition (Pimpinella et al., 2021; Robert et al., 2020). At the population level, phase precessing place cells with overlapping fields organize as compressed ‘theta sequences’ (ordered firing of a subgroup of pyramidal cells within a theta cycle) that represent past, current, and future position within each theta cycle (Dragoi & Buzsáki, 2006; Foster & Wilson, 2007; Skaggs et al., 1996). Theta sequences have been proposed to contribute to navigation and planning (Gupta et al., 2012; Tang et al., 2021). Future work is needed to elucidate the representational role of temporal coding (MacDonald & Tonegawa, 2021) and theta sequences in CA2, as well as how they are coordinated across hippocampal subregions.
Within theta cycles, hippocampal assemblies are temporally synchronized at ‘gamma’ frequency (~30-100 Hz; (Harris et al., 2003)). Different theta-nested gamma oscillations generated by afferent inputs have been characterized, specifically in the CA1 and DG regions (Colgin et al., 2009; Fernández-Ruiz et al., 2017, 2021; Lasztóczi & Klausberger, 2016; Lopes-dos-Santos et al., 2018; Schomburg et al., 2014). While such detailed characterization is still missing for CA2, several studies have analyzed the broad gamma modulation of CA2 firing. CA2 pyramidal cells display phase locking to a wide range of frequencies in the gamma band (Alexander et al., 2018; Fernandez-Lamo et al., 2019), and CA2 silencing for long periods of time preferentially affected the low gamma band component in CA1 (Alexander et al., 2018). Interestingly, theta modulation strength increases across CA1 towards the border with CA2, while gamma modulation displays an opposite trend (Fernandez-Lamo et al., 2019). Further research is needed to elucidate the mechanisms by which CA2 excitatory and inhibitory inputs contribute to the spatio-temporal profile of gamma oscillations and how these are modulated by behavioral demands and brain state.
The distinct anatomical connectivity of CA2 can be a major contributor to the generation of network patterns in this area. Various subcortical neuromodulatory areas send projections into the CA2 area, including the SUM (Cui et al., 2013; Hitti & Siegelbaum, 2014; Kohara et al., 2014; Middleton & McHugh, 2020), medial septum diagonal band (MSDB), paraventricular nucleus (PVN), median raphe nucleus (MRN) (Cui et al., 2013; Hitti & Siegelbaum, 2014; Middleton & McHugh, 2020), and ventral tegmental area (VTA) (Takeuchi et al., 2016). Importantly, neuromodulatory inputs to the hippocampus change as a function of behavioral and brain state and strongly influence its network dynamics (Hasselmo, 2006). For instance, cholinergic tone is higher during locomotion and REM sleep, and is tightly coupled to the occurrence of theta oscillations (Hasselmo, 1999). On the other hand, SWRs coincide with the lowest level of acetylcholine in the hippocampus (Zhang et al., 2021), supporting the hypothesis that a permissive neuromodulatory tone is required to trigger these events. In addition, CA2 is specifically enriched in receptors for other neuromodulators and peptides, such as oxytocin and vasopressin. Activation of both local oxytocin receptors and vasopressin receptors in CA2 induce burst firing in pyramidal cells and enhance their feedforward output onto CA1 (Eyring et al., 2020; Tirko et al., 2018). Oxytocin receptors have been proposed to enhance the saliency of social information (Froemke & Young, 2021) while vasopressin 1b receptors are necessary for affective behavior (Bielsky & Young, 2004). It is therefore plausible that both oxytocin and vasopressin receptors help coordinate the CA2 ensemble dynamics necessary for encoding features of a social episode (Alexander et al., 2016; Donegan et al., 2020; Oliva et al., 2020) within different social contexts (Leroy et al., 2017; Meira et al., 2018).
The local circuit dynamics of the CA2 area, combined with the intrinsic properties of its cells and the region’s rich anatomical connectivity, make CA2 a privileged functional hub that could coordinate network events across hippocampal subregions in a brain-state dependent manner. Further investigations will elucidate the relation between the cortical and subcortical inputs into CA2 and their impact in the broad hippocampal dynamics, as well as how these inputs contribute to the diversity of cellular ensemble responses observed in CA2.
CA2 in epilepsy: hyperexcitability and resilience
The role of the hippocampus in epilepsy has been extensively studied as it is one of the most common epileptic loci in humans (Schwartzkroin, 1994). The mechanisms of epileptogenesis involve a dysregulation of the excitatory-inhibitory balance that enables the propagation of hypersynchronous excitatory activity through a network with no (or low) inhibition (Traub & Wong, 1982). In slices, stimulation of the fornix in the absence of inhibition triggers synchronized bursts of action potentials that originate in the CA2/CA3a border (Wong & Traub, 1983). The high excitability of CA2 pyramidal cells and their recurrent connectivity suggest that this region could contribute to the generation of epileptic activity. Indeed, in a mouse model of temporal lobe epilepsy (TLE) there was an increase in CA2 excitability that contributed to seizure generation (Whitebirch et al., 2022). Another feature of epilepsy is the interictal discharge (ID), a large but brief (~30 ms) synchronous population event that occurs between seizures (Wadman et al., 1983). Typically, IDs are evoked by a large depolarization from the Schaffer collaterals, presenting a negative deflection in the stratum radiatum and a positive wave in the pyramidal layer when detected in CA1 (Wadman et al., 1983), resembling the classical SWR events triggered by CA3 input (Buzsáki, 2015; Oliva et al., 2016b). Other less common types of IDs are evoked by a large depolarization of the basal dendrites in CA1, manifested as a negative deflection above the pyramidal layer and a positive wave in the stratum radiatum (Wadman et al., 1983), similar to the SWRs that are generated in CA2 and directly propagate to CA1 (Oliva et al., 2016b). This suggests that IDs can be generated by both CA3 and CA2 population discharges. In fact, recordings of CA2 neurons in human hippocampal slices show interictal-like event generation in this region (Wittner et al., 2009). Also, inhibitory circuits are altered in the CA2 region in the epileptic brain, showed by a decrease in the number of PV+ interneurons (Andrioli et al., 2007) or by an inhomogeneous distribution of the branching pattern of interneurons (Arellano et al., 2004). In addition to a lack of inhibition (Williamson & Spencer, 1994), electron microscopy revealed that there are more frequent synapses between excitatory cells in epileptic tissue (Wittner et al., 2009). Furthermore, hippocampal slices from patients with TLE show progressive neuronal death of CA1 and CA3 pyramidal cells, but not CA2, subiculum, or granular cells in the dentate gyrus, which are resistant to this defeat (Blümcke et al., 2013; Steve et al., 2014). Recent evidence suggests that sprouting of mossy fibers (i.e., the axons of dentate granule cells) into CA2 underlies this resilience (Freiman et al., 2021; Häussler et al., 2016). These results suggest that the high excitability of CA2 in vivo makes it especially prone to generating epileptic activity. At the same time, the intrinsic properties of CA2, which allow these cells to maintain high firing rates for long periods of time (Kay et al., 2016; Oliva et al., 2016b), could make these cells more resilient to epileptic activity. Altogether, this makes the CA2 region a potential therapeutic target of special interest in epilepsy. Combining optogenetic tools with electrophysiological recordings and targeted closed-loop manipulations could help reveal the role of this region in the generation of epileptogenic activity and seizure development.
Conclusions
In recent years, new results have highlighted the role of CA2 in social behaviors, facilitated by the help of novel transgenics and large-scale physiological tools. However, recent results have also described a more general role of CA2 in coordinating hippocampal network dynamics during learning and memory. In addition to the unique molecular content of CA2 pyramidal cells, recent data suggest additional physiological heterogeneity compared to cells in other hippocampal subfields. Future research will clarify how these two axes of variability, molecular and physiological features, contribute to the particular functional roles of this area. Further experiments combining behavioral assays, in vivo recordings, optogenetics or chemogenetics manipulations, and anatomical and molecular profiling will help bridge the gap on how the unique CA2 area coordinates hippocampal network events such as sharp-wave ripples, theta, or gamma oscillations during different behaviors. The profuse anatomical connectivity of CA2 makes this region uniquely suited to coordinate information processing across distributed brain circuits, a key aspect to understanding how the brain generates higher order cognitive functions.
Acknowledgements
This work was supported by BBRF NARSAD Award, NIH grant 4R00MH122582, BBRF NARSAD Award (AO), Sloan Fellowship, Whitehall Research Grant and Klingenstein-Simons Fellowship, NIH grant 4R00MH120343 (AFR) and National Science Foundation Graduate Research Fellowship grant DGE–2139899 (LAK). The authors thank Heath L Robinson for helpful comments on the manuscript.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Alexander GM, Brown LY, Farris S, Lustberg D, Pantazis C, Gloss B, Plummer NW, Jensen P, & Dudek SM (2018). CA2 neuronal activity controls hippocampal low gamma and ripple oscillations. ELife, 7, e38052. 10.7554/eLife.38052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Farris S, Pirone JR, Zheng C, Colgin LL, & Dudek SM (2016). Social and novel contexts modify hippocampal CA2 representations of space. Nature Communications, 7(1), 10300. 10.1038/ncomms10300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, & Witter MP (1989). The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience, 31(3), 571–591. 10.1016/0306-4522(89)90424-7 [DOI] [PubMed] [Google Scholar]
- Andrioli A, Alonso-Nanclares L, Arellano JI, & DeFelipe J (2007). Quantitative analysis of parvalbumin-immunoreactive cells in the human epileptic hippocampus. Neuroscience, 149(1), 131–143. 10.1016/j.neuroscience.2007.07.029 [DOI] [PubMed] [Google Scholar]
- Arellano JI, Muñoz A, Ballesteros‐Yáñez I, Sola RG, & DeFelipe J (2004). Histopathology and reorganization of chandelier cells in the human epileptic sclerotic hippocampus. Brain, 127(1), 45–64. 10.1093/brain/awh004 [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Muller TH, Whittington JCR, Mark S, Baram AB, Stachenfeld KL, & Kurth-Nelson Z (2018). What Is a Cognitive Map? Organizing Knowledge for Flexible Behavior. Neuron, 100(2), 490–509. 10.1016/j.neuron.2018.10.002 [DOI] [PubMed] [Google Scholar]
- Bielsky IF, & Young LJ (2004). Oxytocin, vasopressin, and social recognition in mammals. Peptides, 25(9), 1565–1574. 10.1016/j.peptides.2004.05.019 [DOI] [PubMed] [Google Scholar]
- Blümcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A, Bernasconi N, Bien CG, Cendes F, Coras R, Cross JH, Jacques TS, Kahane P, Mathern GW, Miyata H, Moshé SL, Oz B, Özkara Ç, Perucca E, … Spreafico R (2013). International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: A Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia, 54(7), 1315–1329. 10.1111/epi.12220 [DOI] [PubMed] [Google Scholar]
- Boehringer R, Polygalov D, Huang AJY, Middleton SJ, Robert V, Wintzer ME, Piskorowski RA, Chevaleyre V, & McHugh TJ (2017). Chronic Loss of CA2 Transmission Leads to Hippocampal Hyperexcitability. Neuron, 94(3), 642–655.e9. 10.1016/j.neuron.2017.04.014 [DOI] [PubMed] [Google Scholar]
- Botcher NA, Falck JE, Thomson AM, & Mercer A (2014). Distribution of interneurons in the CA2 region of the rat hippocampus. Frontiers in Neuroanatomy, 8. https://www.frontiersin.org/article/10.3389/fnana.2014.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle L, Posani L, Irfan S, Siegelbaum SA, & Fusi S (2022). The geometry of hippocampal CA2 representations enables abstract coding of social familiarity and identity (p. 2022.01.24.477361). bioRxiv. 10.1101/2022.01.24.477361 [DOI] [Google Scholar]
- Buzsáki G (2002). Theta Oscillations in the Hippocampus. Neuron, 33(3), 325–340. 10.1016/S0896-6273(02)00586-X [DOI] [PubMed] [Google Scholar]
- Buzsáki G (2015). Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus, 25(10), 1073–1188. 10.1002/hipo.22488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal S. R. y. (1911). Histologie du système nerveux de l’homme & des vertébrés: Cervelet, cerveau moyen, rétine, couche optique, corps strié, écorce cérébrale générale & régionale, grand sympathique. A. Maloine.
- Cembrowski MS, Wang L, Sugino K, Shields BC, & Spruston N (2016). Hipposeq: A comprehensive RNA-seq database of gene expression in hippocampal principal neurons. ELife, 5, e14997. 10.7554/eLife.14997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, He L, Huang AJY, Boehringer R, Robert V, Wintzer ME, Polygalov D, Weitemier AZ, Tao Y, Gu M, Middleton SJ, Namiki K, Hama H, Therreau L, Chevaleyre V, Hioki H, Miyawaki A, Piskorowski RA, & McHugh TJ (2020). A hypothalamic novelty signal modulates hippocampal memory. Nature, 586(7828), 270–274. 10.1038/s41586-020-2771-1 [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, & Piskorowski RA (2016). Hippocampal Area CA2: An Overlooked but Promising Therapeutic Target. Trends in Molecular Medicine, 22(8), 645–655. 10.1016/j.molmed.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, & Siegelbaum SA (2010). Strong CA2 Pyramidal Neuron Synapses Define a Powerful Disynaptic Cortico-Hippocampal Loop. Neuron, 66(4), 560–572. 10.1016/j.neuron.2010.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL (2013). Mechanisms and Functions of Theta Rhythms. Annual Review of Neuroscience, 36(1), 295–312. 10.1146/annurev-neuro-062012-170330 [DOI] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser M-B, & Moser EI (2009). Frequency of gamma oscillations routes flow of information in the hippocampus. Nature, 462(7271), 353–357. 10.1038/nature08573 [DOI] [PubMed] [Google Scholar]
- Cui Z, Gerfen CR, & Young WS 3rd (2013). Hypothalamic and other connections with dorsal CA2 area of the mouse hippocampus. Journal of Comparative Neurology, 521(8), 1844–1866. 10.1002/cne.23263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson NB, Zaremba JD, Kaifosh P, Bowler J, Ladow M, & Losonczy A (2016). Sublayer-Specific Coding Dynamics during Spatial Navigation and Learning in Hippocampal Area CA1. Neuron, 91(3), 652–665. 10.1016/j.neuron.2016.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoudi H, & Foster DJ (2019). Acute silencing of hippocampal CA3 reveals a dominant role in place field responses. Nature Neuroscience, 22(3), 337–342. 10.1038/s41593-018-0321-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diba K, & Buzsáki G (2007). Forward and reverse hippocampal place-cell sequences during ripples. Nature Neuroscience, 10(10), 1241–1242. 10.1038/nn1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez S, Rey CC, Therreau L, Fanton A, Massotte D, Verret L, Piskorowski RA, & Chevaleyre V (2019). Maturation of PNN and ErbB4 Signaling in Area CA2 during Adolescence Underlies the Emergence of PV Interneuron Plasticity and Social Memory. Cell Reports, 29(5), 1099–1112.e4. 10.1016/j.celrep.2019.09.044 [DOI] [PubMed] [Google Scholar]
- Donegan ML, Stefanini F, Meira T, Gordon JA, Fusi S, & Siegelbaum SA (2020). Coding of social novelty in the hippocampal CA2 region and its disruption and rescue in a 22q11.2 microdeletion mouse model. Nature Neuroscience, 23(11), 1365–1375. 10.1038/s41593-020-00720-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, & Buzsáki G (2006). Temporal Encoding of Place Sequences by Hippocampal Cell Assemblies. Neuron, 50(1), 145–157. 10.1016/j.neuron.2006.02.023 [DOI] [PubMed] [Google Scholar]
- Dudek SM, Alexander GM, & Farris S (2016). Rediscovering area CA2: Unique properties and functions. Nature Reviews Neuroscience, 17(2), 89–102. 10.1038/nrn.2015.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2004). Hippocampus: Cognitive Processes and Neural Representations that Underlie Declarative Memory. Neuron, 44(1), 109–120. 10.1016/j.neuron.2004.08.028 [DOI] [PubMed] [Google Scholar]
- Eyring KW, Liu J, König GM, Hidema S, Nishimori K, Kostenis E, & Tsien RW (2020). Oxytocin signals via Gi and Gq to drive persistent CA2 pyramidal cell firing and strengthen CA3-CA1 neurotransmission (p. 2020.05.07.082727). bioRxiv. 10.1101/2020.05.07.082727 [DOI] [Google Scholar]
- Farris S, Ward JM, Carstens KE, Samadi M, Wang Y, & Dudek SM (2019). Hippocampal Subregions Express Distinct Dendritic Transcriptomes that Reveal Differences in Mitochondrial Function in CA2. Cell Reports, 29(2), 522–539.e6. 10.1016/j.celrep.2019.08.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lamo I, Gomez-Dominguez D, Sanchez-Aguilera A, Oliva A, Morales AV, Valero M, Cid E, Berenyi A, & Menendez de la Prida L (2019). Proximodistal Organization of the CA2 Hippocampal Area. Cell Reports, 26(7), 1734–1746.e6. 10.1016/j.celrep.2019.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz A, Oliva A, Fermino de Oliveira E, Rocha-Almeida F, Tingley D, & Buzsáki G (2019). Long-duration hippocampal sharp wave ripples improve memory. Science, 364(6445), 1082–1086. 10.1126/science.aax0758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz A, Oliva A, Nagy GA, Maurer AP, Berényi A, & Buzsáki G (2017). Entorhinal-CA3 Dual-Input Control of Spike Timing in the Hippocampus by Theta-Gamma Coupling. Neuron, 93(5), 1213–1226.e5. 10.1016/j.neuron.2017.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz A, Oliva A, Soula M, Rocha-Almeida F, Nagy GA, Martin-Vazquez G, & Buzsáki G (2021). Gamma rhythm communication between entorhinal cortex and dentate gyrus neuronal assemblies. Science, 372(6537), eabf3119. 10.1126/science.abf3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ (2017). Replay Comes of Age. Annual Review of Neuroscience, 40(1), 581–602. 10.1146/annurev-neuro-072116-031538 [DOI] [PubMed] [Google Scholar]
- Foster DJ, & Wilson MA (2006). Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature, 440(7084), 680–683. 10.1038/nature04587 [DOI] [PubMed] [Google Scholar]
- Foster DJ, & Wilson MA (2007). Hippocampal theta sequences. Hippocampus, 17(11), 1093–1099. 10.1002/hipo.20345 [DOI] [PubMed] [Google Scholar]
- Freiman TM, Häussler U, Zentner J, Doostkam S, Beck J, Scheiwe C, Brandt A, Haas CA, & Puhahn-Schmeiser B (2021). Mossy fiber sprouting into the hippocampal region CA2 in patients with temporal lobe epilepsy. Hippocampus, 31(6), 580–592. 10.1002/hipo.23323 [DOI] [PubMed] [Google Scholar]
- Froemke R, & Young L (2021). Oxytocin, Neural Plasticity, and Social Behavior. Annual Review of Neuroscience, 44. 10.1146/annurev-neuro-102320-102847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan J, Weng S, Pernía-Andrade AJ, Csicsvari J, & Jonas P (2017). Phase-Locked Inhibition, but Not Excitation, Underlies Hippocampal Ripple Oscillations in Awake Mice In Vivo. Neuron, 93(2), 308–314. 10.1016/j.neuron.2016.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AS, van der Meer MAA, Touretzky DS, & Redish AD (2012). Segmentation of spatial experience by hippocampal theta sequences. Nature Neuroscience, 15(7), 1032–1039. 10.1038/nn.3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Csicsvari J, Hirase H, Dragoi G, & Buzsáki G (2003). Organization of cell assemblies in the hippocampus. Nature, 424(6948), 552–556. 10.1038/nature01834 [DOI] [PubMed] [Google Scholar]
- Hasselmo ME (1999). Neuromodulation: Acetylcholine and memory consolidation. Trends in Cognitive Sciences, 3(9), 351–359. 10.1016/S1364-6613(99)01365-0 [DOI] [PubMed] [Google Scholar]
- Hasselmo ME (2006). The role of acetylcholine in learning and memory. Current Opinion in Neurobiology, 16(6), 710–715. 10.1016/j.conb.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussler U, Rinas K, Kilias A, Egert U, & Haas CA (2016). Mossy fiber sprouting and pyramidal cell dispersion in the hippocampal CA2 region in a mouse model of temporal lobe epilepsy. Hippocampus, 26(5), 577–588. 10.1002/hipo.22543 [DOI] [PubMed] [Google Scholar]
- He H, Boehringer R, Huang AJY, Overton ETN, Polygalov D, Okanoya K, & McHugh TJ (2021). CA2 inhibition reduces the precision of hippocampal assembly reactivation. Neuron, 109(22), 3674–3687.e7. 10.1016/j.neuron.2021.08.034 [DOI] [PubMed] [Google Scholar]
- Hitti FL, & Siegelbaum SA (2014). The hippocampal CA2 region is essential for social memory. Nature, 508(7494), 88–92. 10.1038/nature13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N, Cowan WM, & Amaral DG (1995). A quantitative analysis of the dendritic organization of pyramidal cells in the rat hippocampus. Journal of Comparative Neurology, 362(1), 17–45. 10.1002/cne.903620103 [DOI] [PubMed] [Google Scholar]
- Ji D, & Wilson MA (2007). Coordinated memory replay in the visual cortex and hippocampus during sleep. Nature Neuroscience, 10(1), 100–107. 10.1038/nn1825 [DOI] [PubMed] [Google Scholar]
- Joo HR, & Frank LM (2018). The hippocampal sharp wave–ripple in memory retrieval for immediate use and consolidation. Nature Reviews Neuroscience, 19(12), 744–757. 10.1038/s41583-018-0077-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson MP, & Frank LM (2009). Awake replay of remote experiences in the hippocampus. Nature Neuroscience, 12(7), 913–918. 10.1038/nn.2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay K, Sosa M, Chung JE, Karlsson MP, Larkin MC, & Frank LM (2016). A hippocampal network for spatial coding during immobility and sleep. Nature, 531(7593), 185–190. 10.1038/nature17144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara K, Pignatelli M, Rivest AJ, Jung H-Y, Kitamura T, Suh J, Frank D, Kajikawa K, Mise N, Obata Y, Wickersham IR, & Tonegawa S (2014). Cell type–specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nature Neuroscience, 17(2), 269–279. 10.1038/nn.3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laham BJ, Diethorn EJ, & Gould E (2021). Newborn mice form lasting CA2-dependent memories of their mothers. Cell Reports, 34(4), 108668. 10.1016/j.celrep.2020.108668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasztóczi B, & Klausberger T (2016). Hippocampal Place Cells Couple to Three Different Gamma Oscillations during Place Field Traversal. Neuron, 91(1), 34–40. 10.1016/j.neuron.2016.05.036 [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen T-M, Chi Chin M, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, … Jones AR (2007). Genome-wide atlas of gene expression in the adult mouse brain. Nature, 445(7124), 168–176. 10.1038/nature05453 [DOI] [PubMed] [Google Scholar]
- Leroy F, Brann DH, Meira T, & Siegelbaum SA (2017). Input-Timing-Dependent Plasticity in the Hippocampal CA2 Region and Its Potential Role in Social Memory. Neuron, 95(5), 1089–1102.e5. 10.1016/j.neuron.2017.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy F, Park J, Asok A, Brann DH, Meira T, Boyle LM, Buss EW, Kandel ER, & Siegelbaum SA (2018). A circuit from hippocampal CA2 to lateral septum disinhibits social aggression. Nature, 564(7735), 213–218. 10.1038/s41586-018-0772-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loisy M, Bouisset G, Lopez S, Muller M, Spitsyn A, Duval J, Piskorowski RA, Verret L, & Chevaleyre V (2022). Sequential inhibitory plasticities in hippocampal area CA2 and social memory formation. Neuron, 110(17), 2854–2866.e4. 10.1016/j.neuron.2022.06.013 [DOI] [PubMed] [Google Scholar]
- Lopes-dos-Santos V, van de Ven GM, Morley A, Trouche S, Campo-Urriza N, & Dupret D (2018). Parsing Hippocampal Theta Oscillations by Nested Spectral Components during Spatial Exploration and Memory-Guided Behavior. Neuron, 100(4), 940–952.e7. 10.1016/j.neuron.2018.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rojas J, de Solis CA, Leroy F, Kandel ER, & Siegelbaum SA (2022). A direct lateral entorhinal cortex to hippocampal CA2 circuit conveys social information required for social memory. Neuron, 110(9), 1559–1572.e4. 10.1016/j.neuron.2022.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente De Nó R (1934). Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. Journal Für Psychologie Und Neurologie, 46, 113–177. [Google Scholar]
- MacDonald CJ, & Tonegawa S (2021). Crucial role for CA2 inputs in the sequential organization of CA1 time cells supporting memory. Proceedings of the National Academy of Sciences, 118(3), e2020698118. 10.1073/pnas.2020698118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin EA, Diehl GW, Sparks FT, Leutgeb S, & Leutgeb JK (2015). Hippocampal CA2 Activity Patterns Change over Time to a Larger Extent than between Spatial Contexts. Neuron, 85(1), 190–201. 10.1016/j.neuron.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meira T, Leroy F, Buss EW, Oliva A, Park J, & Siegelbaum SA (2018). A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nature Communications, 9(1), 4163. 10.1038/s41467-018-06501-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer A, Trigg HL, & Thomson AM (2007). Characterization of Neurons in the CA2 Subfield of the Adult Rat Hippocampus. Journal of Neuroscience, 27(27), 7329–7338. 10.1523/JNEUROSCI.1829-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton SJ, & McHugh TJ (2020). CA2: A Highly Connected Intrahippocampal Relay. Annual Review of Neuroscience, 43(1), 55–72. 10.1146/annurev-neuro-080719-100343 [DOI] [PubMed] [Google Scholar]
- Nasrallah K, Piskorowski RA, & Chevaleyre V (2015). Inhibitory Plasticity Permits the Recruitment of CA2 Pyramidal Neurons by CA3. ENeuro, 2(4), ENEURO.0049–15.2015. 10.1523/ENEURO.0049-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Olive A, Valero M, Jurado-Parras T, de Salas-Quiroga A, Averkin RG, Gambino G, Cid E, & de la Prida LM (2020). Multimodal determinants of phase-locked dynamics across deep-superficial hippocampal sublayers during theta oscillations. Nature Communications, 11(1), 2217. 10.1038/s41467-020-15840-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, & Ikegaya Y (2019). Recurrent connections between CA2 pyramidal cells. Hippocampus, 29(4), 305–312. 10.1002/hipo.23064 [DOI] [PubMed] [Google Scholar]
- O’Keefe J, & Dostrovsky J (1971). The hippocampus as a spatial map: Preliminary evidence from unit activity in the freely-moving rat. Brain Research, 34, 171–175. 10.1016/0006-8993(71)90358-1 [DOI] [PubMed] [Google Scholar]
- O’Keefe J, & Nadel L (1978). The hippocampus as a cognitive map. Clarendon Press; Oxford University Press. [Google Scholar]
- O’Keefe J, & Recce ML (1993). Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus, 3(3), 317–330. 10.1002/hipo.450030307 [DOI] [PubMed] [Google Scholar]
- Oliva A, Fernández-Ruiz A, Buzsáki G, & Berényi A (2016a). Spatial coding and physiological properties of hippocampal neurons in the Cornu Ammonis subregions. Hippocampus, 26(12), 1593–1607. 10.1002/hipo.22659 [DOI] [PubMed] [Google Scholar]
- Oliva A, Fernández-Ruiz A, Buzsáki G, & Berényi A (2016b). Role of Hippocampal CA2 Region in Triggering Sharp-Wave Ripples. Neuron, 91(6), 1342–1355. 10.1016/j.neuron.2016.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A, Fernández-Ruiz A, Fermino de Oliveira E, & Buzsáki G (2018). Origin of Gamma Frequency Power during Hippocampal Sharp-Wave Ripples. Cell Reports, 25(7), 1693–1700.e4. 10.1016/j.celrep.2018.10.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A, Fernández-Ruiz A, Leroy F, & Siegelbaum SA (2020). Hippocampal CA2 sharp-wave ripples reactivate and promote social memory. Nature, 587(7833), 264–269. 10.1038/s41586-020-2758-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani JH, Zhao M, Cui Z, Williams Avram SK, Caruana DA, Dudek SM, & Young WS (2015). Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Molecular Psychiatry, 20(4), 490–499. 10.1038/mp.2014.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CMA, Lee E, Verheul J, Lipa P, Barnes CA, & McNaughton BL (2004). The Ventral Striatum in Off-Line Processing: Ensemble Reactivation during Sleep and Modulation by Hippocampal Ripples. Journal of Neuroscience, 24(29), 6446–6456. 10.1523/JNEUROSCI.0575-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrache A, Khamassi M, Benchenane K, Wiener SI, & Battaglia FP (2009). Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nature Neuroscience, 12(7), 919–926. 10.1038/nn.2337 [DOI] [PubMed] [Google Scholar]
- Pimpinella D, Mastrorilli V, Giorgi C, Coemans S, Lecca S, Lalive AL, Ostermann H, Fuchs EC, Monyer H, Mele A, Cherubini E, & Griguoli M (2021). Septal cholinergic input to CA2 hippocampal region controls social novelty discrimination via nicotinic receptor-mediated disinhibition. ELife, 10, e65580. 10.7554/eLife.65580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert V, Therreau L, Davatolhagh MF, Bernardo-Garcia FJ, Clements KN, Chevaleyre V, & Piskorowski RA (2020). The mechanisms shaping CA2 pyramidal neuron action potential bursting induced by muscarinic acetylcholine receptor activation. Journal of General Physiology, 152(4). 10.1085/jgp.201912462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland DC, Weible AP, Wickersham IR, Wu H, Mayford M, Witter MP, & Kentros CG (2013). Transgenically Targeted Rabies Virus Demonstrates a Major Monosynaptic Projection from Hippocampal Area CA2 to Medial Entorhinal Layer II Neurons. Journal of Neuroscience, 33(37), 14889–14898. 10.1523/JNEUROSCI.1046-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg EW, Fernández-Ruiz A, Mizuseki K, Berényi A, Anastassiou CA, Koch C, & Buzsáki G (2014). Theta Phase Segregation of Input-Specific Gamma Patterns in Entorhinal-Hippocampal Networks. Neuron, 84(2), 470–485. 10.1016/j.neuron.2014.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin PA (1994). Role of the hippocampus in epilepsy. Hippocampus, 4(3), 239–242. 10.1002/hipo.450040302 [DOI] [PubMed] [Google Scholar]
- Sharif F, Tayebi B, Buzsáki G, Royer S, & Fernandez-Ruiz A (2021). Subcircuits of Deep and Superficial CA1 Place Cells Support Efficient Spatial Coding across Heterogeneous Environments. Neuron, 109(2), 363–376.e6. 10.1016/j.neuron.2020.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, & Barnes CA (1996). Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus, 6(2), 149–172. [DOI] [PubMed] [Google Scholar]
- Srinivas KV, Buss EW, Sun Q, Santoro B, Takahashi H, Nicholson DA, & Siegelbaum SA (2017). The Dendrites of CA2 and CA1 Pyramidal Neurons Differentially Regulate Information Flow in the Cortico-Hippocampal Circuit. The Journal of Neuroscience, 37(12), 3276–3293. 10.1523/JNEUROSCI.2219-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, Roux L, Eichler R, & Buzsáki G (2015). Local generation of multineuronal spike sequences in the hippocampal CA1 region. Proceedings of the National Academy of Sciences, 112(33), 10521–10526. 10.1073/pnas.1508785112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, Roux L, Eichler R, Senzai Y, Royer S, & Buzsáki G (2014). Pyramidal Cell-Interneuron Interactions Underlie Hippocampal Ripple Oscillations. Neuron, 83(2), 467–480. 10.1016/j.neuron.2014.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steve TA, Jirsch JD, & Gross DW (2014). Quantification of subfield pathology in hippocampal sclerosis: A systematic review and meta-analysis | Elsevier Enhanced Reader. 10.1016/j.eplepsyres.2014.07.003 [DOI] [PubMed] [Google Scholar]
- Sun Q, Sotayo A, Cazzulino AS, Snyder AM, Denny CA, & Siegelbaum SA (2017). Proximodistal Heterogeneity of Hippocampal CA3 Pyramidal Neuron Intrinsic Properties, Connectivity, and Reactivation during Memory Recall. Neuron, 95(3), 656–672.e3. 10.1016/j.neuron.2017.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Srinivas KV, Sotayo A, & Siegelbaum SA (2014). Dendritic Na+ spikes enable cortical input to drive action potential output from hippocampal CA2 pyramidal neurons. ELife, 3, e04551. 10.7554/eLife.04551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, Smith CC, Fernández G, Deisseroth K, Greene RW, & Morris RGM (2016). Locus coeruleus and dopaminergic consolidation of everyday memory. Nature, 537(7620), 357–362. 10.1038/nature19325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Abe K, & Nojyo Y (1988). Three-dimensional analysis of the whole axonal arbors originating from single CA2 pyramidal neurons in the rat hippocampus with the aid of a computer graphic technique. Brain Research, 452(1), 255–272. 10.1016/0006-8993(88)90030-3 [DOI] [PubMed] [Google Scholar]
- Tang W, Shin JD, Frank LM, & Jadhav SP (2017). Hippocampal-Prefrontal Reactivation during Learning Is Stronger in Awake Compared with Sleep States. Journal of Neuroscience, 37(49), 11789–11805. 10.1523/JNEUROSCI.2291-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Shin JD, & Jadhav SP (2021). Multiple time-scales of decision-making in the hippocampus and prefrontal cortex. ELife, 10, e66227. 10.7554/eLife.66227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirko NN, Eyring KW, Carcea I, Mitre M, Chao MV, Froemke RC, & Tsien RW (2018). Oxytocin Transforms Firing Mode of CA2 Hippocampal Neurons. Neuron, 100(3), 593–608.e3. 10.1016/j.neuron.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, & Wong RKS (1982). Cellular Mechanism of Neuronal Synchronization in Epilepsy. Science, 216(4547), 745–747. 10.1126/science.7079735 [DOI] [PubMed] [Google Scholar]
- Valero M, Cid E, Averkin RG, Aguilar J, Sanchez-Aguilera A, Viney TJ, Gomez-Dominguez D, Bellistri E, & de la Prida LM (2015). Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp-wave ripples. Nature Neuroscience, 18(9), 1281–1290. 10.1038/nn.4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadman WJ, Lopes Da Silva FH, & Leung L-WS (1983). Two types of interictal transients of reversed polarity in rat hippocampus during kindling. Electroencephalography and Clinical Neurophysiology, 55(3), 314–319. 10.1016/0013-4694(83)90209-2 [DOI] [PubMed] [Google Scholar]
- Whitebirch AC, LaFrancois JJ, Jain S, Leary P, Santoro B, Siegelbaum SA, & Scharfman HE (2022). Enhanced excitability of the hippocampal CA2 region and its contribution to seizure generation in a mouse model of temporal lobe epilepsy (p. 2022.02.02.478736). bioRxiv. 10.1101/2022.02.02.478736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A, & Spencer DD (1994). Electrophysiological characterization of CA2 pyramidal cells from epileptic humans. Hippocampus, 4(2), 226–237. 10.1002/hipo.450040213 [DOI] [PubMed] [Google Scholar]
- Wittner L, Huberfeld G, Clémenceau S, Erőss L, Dezamis E, Entz L, Ulbert I, Baulac M, Freund TF, Maglóczky Z, & Miles R (2009). The epileptic human hippocampal cornu ammonis 2 region generates spontaneous interictal-like activity in vitro. Brain, 132(11), 3032–3046. 10.1093/brain/awp238 [DOI] [PubMed] [Google Scholar]
- Wong RK, & Traub RD (1983). Synchronized burst discharge in disinhibited hippocampal slice. I. Initiation in CA2-CA3 region. Journal of Neurophysiology, 49(2), 442–458. 10.1152/jn.1983.49.2.442 [DOI] [PubMed] [Google Scholar]
- Yamamoto J, & Tonegawa S (2017). Direct Medial Entorhinal Cortex Input to Hippocampal CA1 Is Crucial for Extended Quiet Awake Replay. Neuron, 96(1), 217–227.e4. 10.1016/j.neuron.2017.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, & Buzsaki G (1995). Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: Network and intracellular mechanisms. Journal of Neuroscience, 15(1), 30–46. 10.1523/JNEUROSCI.15-01-00030.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cao L, Varga V, Jing M, Karadas M, Li Y, & Buzsáki G (2021). Cholinergic suppression of hippocampal sharp-wave ripples impairs working memory. Proceedings of the National Academy of Sciences, 118(15), e2016432118. 10.1073/pnas.2016432118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Choi Y-S, Obrietan K, & Dudek SM (2007). Synaptic Plasticity (and the Lack Thereof) in Hippocampal CA2 Neurons. Journal of Neuroscience, 27(44), 12025–12032. 10.1523/JNEUROSCI.4094-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]


