Abstract
Objective:
Hypertension is a critical cause of cardiovascular disease, and women with HIV have a higher prevalence of hypertension compared to women without HIV. The relationship between hypertension and mortality has not been well characterized in women with treated HIV. Here, we estimate the effect of hypertension on one-year risk of all-cause mortality among women with HIV on antiretroviral therapy (ART) in the United States.
Design:
An analysis of multicenter, observational cohort data from the Women’s Interagency HIV Study (WIHS) collected between 1995 and 2019.
Methods:
We included women with HIV who reported ever using ART. We used parametric g-computation to estimate the effect of hypertension (systolic blood pressure ≥140 [mmHg], diastolic blood pressure ≥90, or use of hypertensive medication) on all-cause mortality within one year of a WIHS visit.
Results:
Among 2,929 unique women, we included 57,034 visits with a median age of 45 (interquartile range: 39, 52). Women had hypertension at 34.5% of visits, and 641 deaths occurred within one year of a study visit. Comparing women at visits with hypertension to women at visits without hypertension, the standardized one-year risk ratio (RR) for mortality was 1.16 (95% confidence interval [CI]: 1.01, 1.33). The risk ratios were higher in Hispanic (RR: 1.23, 95% CI: 0.86, 1.77) and non-Hispanic Black women (RR: 1.19, 95% CI: 1.04, 1.37) and lower in non-Hispanic white women (RR: 0.93, 95% CI: 0.58, 1.48).
Conclusion:
Among women with treated HIV, those with hypertension, compared to those without, had an increased one-year risk of all-cause mortality.
Keywords: HIV, hypertension, mortality, epidemiology, antiretroviral therapy, chronic comorbidities, cohort study
Introduction
People with HIV are surviving longer due to the efficacy and uptake of antiretroviral therapy (ART).[1–3] Aging of people with HIV has resulted in a larger burden of chronic conditions and comorbidities in this population.[4] As deaths from AIDS-related causes have declined in the era of modern ART, the leading causes of death have shifted to chronic conditions such as cardiovascular disease.[3,5–7] HIV has been recognized as a risk factor for cardiovascular disease, as people with HIV are at higher risk than people without HIV for cardiovascular disease and events.[8–12] Moreover, women with HIV have a higher burden of non-AIDS comorbidities than men with HIV.[13,14] The impact of cardiovascular disease and chronic conditions on women with HIV, compared to men with HIV, is less well understood due to under-representation of women in HIV research and lack of sex-stratified analyses.[15–17]
Hypertension is a leading cause of myocardial infarction, sudden cardiac death, and stroke, [18–20] and is more common in women with HIV than HIV seronegative women. In 2011-2014, the estimated prevalence of hypertension among women with HIV was 46.3%[21] while the prevalence among HIV seronegative women was 28.1%.[22] The higher prevalence in women with HIV may be due to immune activation, altered hormone production, and health behaviors.[16] The effect of hypertension on all-cause mortality is not well characterized among women with HIV.[23]
Among women with HIV, disparities exist in the prevalence of hypertension. Black women with HIV have a higher prevalence of hypertension than white women with HIV,[24] likely due to social determinants of health, exposure to structural racism, and poorer access to healthcare.[25] Women with HIV with insurance coverage interruptions have an increased risk of loss of hypertensive control.[26] Yet, few studies have assessed whether these disparities influence the relationship between hypertension and health outcomes, including mortality.
We estimated the effect of hypertension on one-year risk of all-cause mortality among women with HIV in the Women’s Interagency HIV Study (WIHS, now the MACS/WIHS Combined Cohort Study, MWCCS), and whether the estimated effect varied by racial-ethnic categories, age, and health insurance.
Methods
Data.
We analyzed data from the WIHS.[27] Since starting recruitment in 1994, the WIHS expanded from 6 to 10 sites including Brooklyn, NY; the Bronx/Manhattan, NY, Washington, DC; Chicago, IL; San Francisco, CA; Los Angeles, CA; Chapel Hill, NC; Atlanta, GA; Birmingham, AL/Jackson, MS; and Miami, FL. Additional recruitment waves occurred in 2001-02, 2011-12, and 2013-15. Eligibility criteria remained similar throughout the study. WIHS enrollees participated in study visits approximately every 6 months that consisted of laboratory testing, clinical evaluation, and structured interviews. Social, behavioral, clinical, and medication use information was collected at each visit. All participants provided informed consent, and each study site’s institutional review board approved study protocols.
Eligible participants for this study had 1) HIV infection at WIHS enrollment or seroconverted (n=21) before the one-year follow-up period and 2) reported ever receiving triple combination ART before the one-year follow-up period. Upon reported use of ART, women contributed attended visits thereafter. We excluded 90 women who did not have visits after reported use of triple combination ART.
Exposure definition.
At each visit, women were categorized as hypertensive if they had at least one of the following: 1) systolic blood pressure (SBP) ≥140 (millimeters of mercury, mmHg), 2) diastolic blood pressure (DBP) ≥90 (mmHg), or 3) reported use of hypertensive medication over the past 6 months. Guidelines recommend the threshold for hypertension be ≥130 SBP or ≥80 DBP, though pharmacological treatment is typically administered at ≥140 SBP or ≥90 DBP.[28]
Prior to 2001, blood pressure was measured and recorded once at each visit. Between 2001 to 2004, blood pressure was measured twice per visit. The higher measurement was recorded if SBP or DBP measurements differed by more than 80 and 45 mmHg, respectively; otherwise, the second measurement was recorded. Starting in 2004, three blood pressure measurements were taken at each visit, and the average of the second and third measurement was recorded. Study staff collected blood pressure from the seated participant’s right arm at one-minute intervals by an oscillometric automated blood pressure monitor (DinaMap ProCare Series; GE Medical Systems, Chicago, IL). Between each measurement, study staff raised the participant’s arm for five seconds.[29]
Outcome definition.
The outcome was all-cause mortality within one year of a WIHS visit. The one year follow up period employed the 6-month WIHS visit interval and allows hypertensive status to vary over follow up. Dates of death were determined by matching to the National Death Index, reviewing death certificates and medical records, and contacting next of kin following participants’ missed visits.[30] Women who died within one year of their visit were classified as experiencing the outcome.
Covariates.
We constructed a causal diagram to identify potential confounders.[31,32] To ensure that confounders temporally preceded hypertension, we used values taken at the preceding visit for select variables that may affect hypertension with delay. Figure 1 illustrates measurement timing of analysis variables with Vt (center) as the visit of interest. We specified the following continuous covariates as restricted quadratic splines with knots placed at the 5th, 35th, 65th, and 95th percentiles:[33] age, body mass index (kg/m2), previous visit CD4 cell count (cells/mm3), and previous visit kidney function (estimated glomerular filtration rate).[34]
Figure 1:
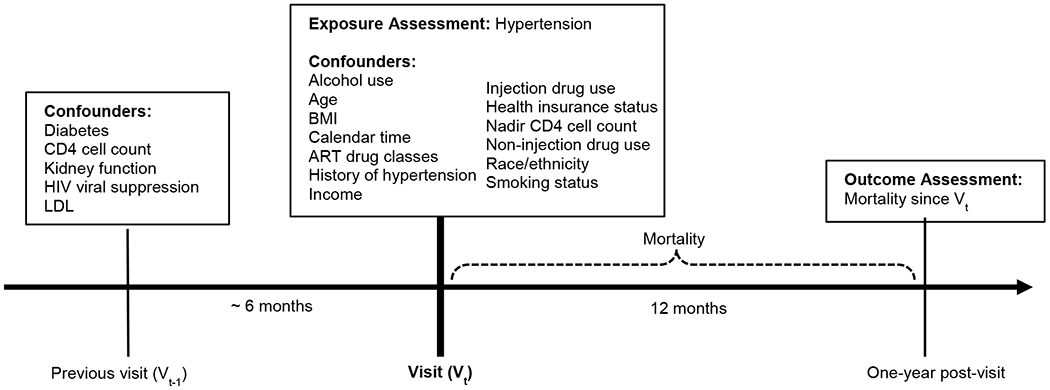
Measurement timing of key variables in the relationship between hypertension and mortality in the WIHS
Vt (center) is the visit of interest. Values for diabetes, CD4 cell count, kidney function, HIV viral suppression, and high LDL are from the previously attended visit (Vt-1) which was approximately six months prior to Vt. Values used from Vt includes alcohol use, age, BMI, ART drug classes, history of hypertension, income, injection drug use, health insurance status, nadir CD4 cell count, non-injection drug use, race/ethnicity, and smoking status. The outcome, mortality, is measured one year after Vt. BMI, body mass index; ART, antiretroviral therapy; LDL, low-density lipoprotein.
We used binary indicator variables for categorical covariates: alcohol use in past 6 months (abstainer, 0-7 drinks per week, >7-12 drinks per week, >12 drinks per week); annual household income (<$6,000, $6,001-$12,000, >$12,000-$18,000, >$18,000); ART drug classes used in past 6 months (nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, protease inhibitors, entry inhibitors, and integrase inhibitors); health insurance (insured or uninsured); self-reported mutually exclusive racial-ethnic category (Hispanic Black, non-Hispanic Black, Hispanic white, non-Hispanic white, Hispanic other, or non-Hispanic other); history of hypertension (hypertension at any of previous four visits); smoking status (never, former, current); self-reported use of non-injection drugs (e.g., marijuana, hallucinogens, PCP, club drugs, methamphetamines) within past six months; self-reported injection of any illicit drug within past six months; diabetes at previous visit (when non-pregnant, self-reported anti-diabetic medication, two fasting glucose measurements ≥126 mg/dL, or concurrent measurements of hemoglobin A1c ≥6.5% and fasting glucose ≥126 mg/dL, or not); nadir CD4 cell count (<200 cells/mm3, or not); previous visit low-density lipoprotein (LDL, >130 mg/dL or not); and previous visit HIV viral suppression (<80 copies/mL, or not). For 21 visits where HIV viral load was undetectable using tests with a lower limit of detection >80 copies/mL, we assumed viral load was <80 copies/mL. We adjusted for calendar time (WIHS visit) to account for secular trends in hypertension and mortality not captured by other covariates.
Missing data.
We used Markov chain Monte Carlo multivariate normal multiple imputation to impute missing values.[35,36] We imputed 30 datasets. While we had complete data on mortality, blood pressure measurement was missing at 7.8% of visits. The alternative hypertension definition (controlled or uncontrolled) had more missing data (17.4%), due to additional missingness on medication use. Most variables had less than 10% of missing data across visits; however, LDL was missing at 30.4% of visits due to annual measurement of cholesterol in the WIHS. Using published guidance,[37] our imputation model included hypertension, mortality, covariates, and interaction terms included in analysis models.[36]
Statistical analysis.
We estimated marginal risks, risk differences (RDs), and risk ratios (RRs) using parametric g-computation, a model-based form of standardization.[38–41] Parametric g-computation removes measured confounding by standardizing the study sample with hypertension and study sample without hypertension to the covariate distributions of the combined study sample. Under certain necessary assumptions (see Discussion),[38] estimated risks can represent a causal effect.[39]
We used the following steps, adapted from Snowden et al.,[39] to obtain estimates using parametric g-computation. First, we copied each visit twice to create a g-computation analysis dataset that contained the original visit (with observed exposure and outcome), a visit copy with exposure assigned to “hypertensive” and a missing mortality outcome, and another copy with exposure assigned to “not hypertensive” and a missing mortality outcome. All visits (original and copied) maintained observed covariate values. We fit a logistic regression model, with covariates identified from our causal diagram, on the combined copied and observed data. Using this model, we predicted the probability of all-cause mortality within one year for visit copies with assigned hypertension exposure. We averaged predicted probabilities among those assigned to “hypertensive,” and then those assigned to “not hypertensive,” to obtain estimated marginal one-year risks of all-cause mortality under each level of hypertension. Those marginal risks were used to estimate RDs and RRs. We evaluated effect modification by calculating contrasts within strata of age (<40, 40-<55, or ≥55 years), racial-ethnic category (non-Hispanic Black, non-Hispanic White, or Hispanic; “other” race not assessed due to small cell counts), and health insurance coverage (insured or uninsured).
We checked for adequate parametric g-computation model specification by comparing main effects, marginal estimates of the RD and RR to those from an inverse-probability-of-treatment-weighted (IPTW) model[41,42] (steps in Supplementary Digital Content). Upon confirming similar estimates from g-computation and the IPTW model, we used AIC to select interaction terms for a more flexible parametric g-computation model. We included interaction terms between hypertension and 1) CD4 cell count, 2) suppression of HIV viral load, 3) racial-ethnic category, 4) health insurance, and 5) continuous age.
We calculated Wald 95% confidence intervals (CIs) by employing nonparametric bootstrap methods.[43] Bootstrapping must accommodate lack of independence between observations due to multiple visits per woman; thus, bootstrap samples selected (with replacement) unique women from the study sample.[43] If a woman was selected for the bootstrap sample, she contributed all eligible visits to the sample. Each bootstrap sample applied the steps outlined for g-computation (and IPTW) to obtain risk estimates for that sample.[40,44] After repeating this process 200 times, we obtained the standard deviation of the 200 risks and contrast estimates, which served as the estimated standard errors for Wald 95% CIs.
Sensitivity analyses.
We performed sensitivity analyses using an alternative definition of hypertension, as mortality may differ by controlled or uncontrolled hypertension.[45,46] The alternative exposure definition used three hypertension categories. Normotensive women had no indications of hypertension; women with controlled hypertension had SBP <140, DBP <90, and reported hypertensive medication use; and women with uncontrolled hypertension had SBP ≥140 or DBP ≥90 with or without reported use of hypertensive medication. We performed an additional sensitivity analysis for mortality events that occurred within 18 months of the last attended visit, which included 23 additional mortality events. Further, we restricted the analysis to 2000-2019 to evaluate whether results were robust to potential increases in ART efficacy during later years of the study.
Results
The analysis sample contained 2,929 unique women and 57,034 study visits from 1995-2019, with 42.7% of visits occurring from 2010 to 2019. The median number of visits contributed by each woman was 16 (IQR: 10, 32). The median age at analysis baseline was 39 years (IQR: 33, 46) while the median age over all visits was 45 (IQR: 39, 52; range: 18-89) (Table 1). The median age at visits with hypertension (50, IQR: 44, 56) was higher than at visits without (42, IQR: 36, 49). Most visits were attended by non-Hispanic Black women (59.4%), followed by Hispanic women (23.7%) and non-Hispanic white women (13.6%). Women reported having insurance coverage at 86.7% of visits. Current smoking (40.9% vs. 39.8%) and heavy alcohol use (>7 drinks per week) (7.9% vs 7.7%) were similar at hypertensive and normotensive visits. Obesity (50.2% vs. 34.0%) and diabetes (24.5% vs. 7.7%) were more common at hypertensive visits (Table 1).
Table 1:
Characteristics at analysis baseline and at study visits among women with HIV in the Women’s Interagency HIV Study, by hypertension status, 1995-2019
| Total women | Women with hypertension at baseline | Women with no hypertension at baseline | Total visits | Visits with hypertension | Visits with no hypertension | |
|---|---|---|---|---|---|---|
|
| ||||||
| (n=2,929) | (n=780) | (n=2,039) | (n=57,034) | (n=19,674) | (n=32,895) | |
|
|
||||||
| Year of visit | ||||||
| 1995-1999 | 1,050 (35.9) | 210 (26.9) | 817 (40.1) | 4,186 (7.3) | 835 (4.2) | 3,225 (9.8) |
| 2000-2009 | 1,006 (34.3) | 187 (24.0) | 754 (37.0) | 28,493 (50.0) | 7,957 (40.4) | 18,096 (55.0) |
| 2010-2019 | 873 (29.8) | 383 (49.1) | 468 (22.9) | 24,355 (42.7) | 10,882 (55.3) | 11,574 (35.2) |
| Age in years, median (IQR) | 39 (33, 46) | 45 (39, 52) | 37 (32, 43) | 45 (39, 52) | 50 (44, 56) | 42 (36, 49) |
| <40 years | 1,505 (51.4) | 207 (26.5) | 1,245 (61.1) | 15,914 (27.9) | 2,370 (12.0) | 12,503 (38.0) |
| 40 to <55 years | 1,239 (42.3) | 452 (57.9) | 740 (36.3) | 31,222 (54.7) | 11,315 (57.5) | 17,389 (52.9) |
| ≥55 years | 185 (6.3) | 121 (15.5) | 54 (2.6) | 9,898 (17.4) | 5,989 (30.4) | 3,003 (9.1) |
| Race and Ethnicity | ||||||
| Non-Hispanic Black | 1,827 (62.4) | 612 (78.5) | 1,153 (56.5) | 33,900 (59.4) | 14,137 (71.9) | 17,537 (53.3) |
| Non-Hispanic white | 379 (12.9) | 68 (8.7) | 294 (14.4) | 7,734 (13.6) | 1,949 (9.9) | 4,787 (14.6) |
| Hispanic | 637 (21.7) | 87 (11.2) | 522 (25.6) | 13,512 (23.7) | 3,058 (15.5) | 9,397 (28.6) |
| Other | 86 (2.9) | 13 (1.7) | 70 (3.4) | 1888 (3.3) | 530 (2.7) | 1,174 (3.6) |
| Annual household income | ||||||
| <$6,000 | 610 (24.2) | 172 (24.4) | 427 (24.0) | 7,797 (16.4) | 2,565 (14.6) | 4,975 (17.3) |
| $6,001–$12,000 | 980 (38.9) | 283 (40.1) | 677 (38.1) | 18,944 (39.7) | 7,824 (44.6) | 10,560 (36.8) |
| $12,001–$18,000 | 418 (16.6) | 114 (16.2) | 299 (16.8) | 7,707 (16.2) | 2,872 (16.4) | 4,606 (16.1) |
| >$18,000 | 513 (20.3) | 136 (19.3) | 373 (21.0) | 13,223 (27.7) | 4,268 (24.3) | 8,548 (29.8) |
| Missing | 408 | 75 | 263 | 9363 | 2,145 | 4,206 |
| Health insurance coverage | ||||||
| Insured | 2,292 (78.4) | 620 (79.6) | 1,587 (78.0) | 46,988 (86.7) | 16,478 (90.6) | 26,744 (84.3) |
| Uninsured | 632 (21.6) | 159 (20.4) | 448 (22.0) | 7216 (13.3) | 1,710 (9.4) | 4,963 (15.7) |
| Missing | 5 | 1 | 4 | 2,830 | 1,486 | 1,188 |
| CD4 cell count, cells/mm3, median (IQR) | 408 (245, 610) | 435 (282, 655) | 397 (236, 592) | 495 (301, 716) | 539 (334, 776) | 472 (286, 682) |
| Missing | 282 | 50 | 201 | 5261 | 1,304 | 2,561 |
| HIV viral load <80 copies/mL | 695 (25.5) | 307 (40.8) | 371 (19.7) | 22,394 (41.0) | 9,772 (51.0) | 10,776 (34.0) |
| Missing | 202 | 27 | 151 | 2,429 | 502 | 1,208 |
| BMI (kg/m2), median (IQR) | 27.8 (23.9, 33.4) | 31 (25.5, 38.6) | 26.8 (23.4, 31.6) | 28.1 (24.0, 33.5) | 30 (25.0, 36.1) | 27.1 (23.6, 32.0) |
| ≥30 kg/m2 | 1,082 (38.7) | 424 (55.0) | 649 (32.3) | 21,064 (40.1) | 9,737 (50.2) | 11,081 (34.0) |
| Missing | 130 | 9 | 30 | 4455 | 262 | 286 |
| eGFR (ml/min/1.73 m2), median (IQR) | 102.6 (85.2, 118.4) | 95.7 (76.4, 112.0) | 105.8 (89.3, 120.2) | 96.7 (78.8, 112.9) | 87.9 (69.6, 106.4) | 101.7 (85.0, 116.0) |
| Missing | 689 | 150 | 516 | 7,140 | 1,671 | 4,145 |
| LDL cholesterol >130 mg/dL | 264 (19.0) | 93 (20.4) | 162 (18.1) | 7,189 (18.1) | 2,751 (18.3) | 4,131 (18.0) |
| Missing | 1,543 | 325 | 1,144 | 17,320 | 4,612 | 9,970 |
| Diabetes | 162 (5.5) | 103 (13.2) | 54 (2.6) | 7,961 (14.0) | 4,820 (24.5) | 2,525 (7.7) |
| Smoking status | ||||||
| Current | 1,334 (46.6) | 375 (48.1) | 932 (45.9) | 21,726 (40.1) | 8,030 (40.9) | 13,055 (39.8) |
| Former | 547 (19.1) | 159 (20.4) | 378 (18.6) | 15,415 (28.4) | 6,196 (31.6) | 8,642 (26.4) |
| Never | 980 (34.3) | 245 (31.5) | 721 (35.5) | 17,045 (31.5) | 5,408 (27.5) | 11,090 (33.8) |
| Missing | 68 | 0 | 8 | 2,848 | 40 | 108 |
| Alcohol use >7 drinks per week | 272 (9.6) | 97 (12.5) | 168 (8.3) | 4,162 (7.7) | 1,549 (7.9) | 2,504 (7.7) |
| Missing | 89 | 6 | 22 | 3164 | 143 | 244 |
Observed data (before multiple imputation) are shown. Data are presented as column n (%) unless otherwise noted. Categories without a row for missing did not have any missing values. Hypertension status was missing for 110 women at baseline and at 4,465 visits; therefore “total” columns may not be the sum of hypertension and no hypertension. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or reported use of any hypertensive medication
BMI, body mass index; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LDL, low-density lipoprotein.
The prevalence of hypertension was 26.6% at analysis baseline, and women were hypertensive at 34.5% of all visits (Table 1). Non-Hispanic Black women had hypertension at 41.7% of visits. Non-Hispanic white women and Hispanic women had hypertension at 25.2% and 22.6% of visits, respectively. Among women ≤40, 40- <55, and ≥55 years of age, the proportions of visits with hypertension were 14.9%, 36.2%, and 60.5%, respectively. Among visits with non-missing insurance data, more women had hypertension at visits with reported health insurance (38.1%) than without health insurance (25.6%).
Within one year of a visit, 641 women experienced death from any cause. The crude RR comparing the one-year risk of death among women at visits with hypertension to those without was 1.63 (95% CI: 1.48, 1.80), and the crude RD was 1.0% (95% CI: 0.7%, 1.2%).
Main analysis.
The covariate standardized one-year RR and RD estimated from the parametric g-computation main effects model were similar to the IPTW estimates, indicating adequate model specification for the parametric g-computation model (data not shown). Upon including interaction terms in the parametric g-computation model, the estimated one-year risks of mortality for women with hypertension and without hypertension at their visit, respectively, were 2.0% (95% CI: 1.8%, 2.3%) and 1.8% (95% CI: 1.6%, 1.9%). The RR comparing women with hypertension at their visit to women without was 1.16 (95% CI: 1.01, 1.33), and the RD was 0.3% (95% CI: 0.0%, 0.6%) (Table 2).
Table 2:
Estimates of marginal, counterfactual risks and contrasts of hypertension on one-year risk of all-cause mortality, Women’s Interagency HIV Study (WIHS), 1995-2019
| Risk, no hypertension | Risk, hypertension | RD (hypertension vs. no hypertension) | RR (hypertension vs. no hypertension) | |||||
|---|---|---|---|---|---|---|---|---|
| Overall | 1.8% | (1.6%, 1.9%) | 2.0% | (1.8%, 2.3%) | 0.3% | (0.0%, 0.6%) | 1.16 | (1.01, 1.33) |
| Race and Ethnicity | ||||||||
| Non-Hispanic Black | 2.0% | (1.7%, 2.2%) | 2.3% | (2.0%, 2.6%) | 0.4% | (0.1%, 0.7%) | 1.19 | (1.04, 1.37) |
| Non-Hispanic white | 1.5% | (1.1%, 1.9%) | 1.4% | (0.9%, 1.9%) | −0.1% | (−0.8%, 0.6%) | 0.93 | (0.58, 1.48) |
| Hispanic | 1.5% | (1.2%, 1.7%) | 1.8% | (1.2%, 2.4%) | 0.3% | (−0.3%, 1.0%) | 1.23 | (0.86, 1.77) |
| Age | ||||||||
| <40 years | 1.1% | (1.0%, 1.3%) | 1.9% | (1.3%, 2.4%) | 0.7% | (0.1%, 1.3%) | 1.64 | (1.19, 2.26) |
| 40-<55 years | 1.9% | (1.8%, 2.1%) | 1.9% | (1.2%, 2.4%) | −0.1% | (−0.4%, 0.3%) | 0.97 | (0.83, 1.14) |
| ≥55 years | 2.2% | (1.7%, 2.7%) | 2.8% | (2.5%, 3.1%) | 0.6% | (−0.1%, 1.3%) | 1.28 | (0.96, 1.71) |
| Health insurance coverage | ||||||||
| Insured | 1.9% | (1.7%, 2.1%) | 2.2% | (1.9%, 2.5%) | 0.3% | (0.0%, 0.6%) | 1.17 | (1.03, 1.34) |
| Uninsured | 0.8% | (0.6%, 1.0%) | 0.8% | (0.5%, 1.1%) | 0.0% | (−0.4%, 0.3%) | 0.97 | (0.60, 1.56) |
| HIV viral load | ||||||||
| <80 copies/mL | 1.1% | (0.9%, 1.3%) | 1.4% | (1.2%, 1.6%) | 0.3% | (0.0%, 0.6%) | 1.30 | (1.03, 1.63) |
| ≥80 copies/mL | 2.2% | (2.0%, 2.4%) | 2.5% | (2.1%, 2.9%) | 0.3% | (−0.2%, 0.7%) | 1.11 | (0.94, 1.32) |
Estimates presented as risk % (95% CI). RRs and RDs may differ from presented marginal risks due to post-calculation rounding. Adjusted model included continuous variables as restricted quadratic splines and categorical covariates as indicator variables. Estimates adjusted for age (continuous), alcohol use in past 6 months (abstainer, 0-7 drinks per week, >7-12 drinks per week, >12 drinks per week), annual household income (<$6,000, $6,001-$12,000, $12,001-$18,000, >$18,000), BMI (continuous), race/ethnicity (Hispanic Black, non-Hispanic Black, Hispanic white, non-Hispanic white, Hispanic other, or non-Hispanic other), ART drug classes used since last visit (nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, protease inhibitors, entry inhibitors, and integrase inhibitors), health insurance coverage (insured or uninsured), history of hypertension (hypertension at any previous 4 visits), smoking status (current, former, never), diabetes status (binary), self-reported injection drug use (binary), self-reported non-injection drug use (binary), CD4 cell count (continuous), nadir CD4 cell count <200 cells/mm3 (binary), HIV viral load <80 copies/mL (binary), LDL cholesterol >130 mg/dL (binary), and eGFR (continuous).
ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LDL, low-density lipoprotein; RD, risk difference; RR, risk ratio.
Analysis of health disparities.
RDs and RRs modestly varied by racial-ethnic categories, age, and health insurance coverage (Table 2). Hispanic women with hypertension at their visit had 1.23 (95% CI: 0.86, 1.77) times the one-year risk of all-cause mortality than Hispanic women without hypertension. The RR for non-Hispanic Black women was 1.19 (95% CI: 1.04, 1.37). Among non-Hispanic white women, those with hypertension at their visit had 0.93 (95% CI: 0.58, 1.48) times the risk for all-cause mortality than those without hypertension. The RRs and RDs did not linearly increase or decrease with age (Table 2). The estimated RRs and RDs comparing women with hypertension to those without was greatest among women who were ≤40 years of age at their visit (RR: 1.64, 95% CI: 1.19, 2.26).
Insurance status did not modify the estimates of hypertension and one-year risk of all-cause mortality. At visits where women reported no health insurance, the RR was 0.97 (95% CI: 0.60, 1.56), which was slightly lower than visits with health insurance (RR: 1.17, 95% CI: 1.03, 1.34).
Sensitivity analyses.
Using the alternate exposure definition for hypertension, 69.8% of women were normotensive, 21.4% had controlled hypertension, and 8.8% had uncontrolled hypertension. The RR comparing the risk of all-cause mortality for controlled hypertension to the risk for no hypertension was 1.22 (95% CI: 0.89, 1.66) (Table S1, Supplementary Digital Content). The RR comparing the mortality risk for uncontrolled hypertension to the risk for no hypertension was 1.13 (95% CI: 0.83, 1.54) (Table S2, Supplementary Digital Content). We saw no qualitative differences in our estimates when extending the risk window from 12 to 18 months (Table S3, Supplementary Digital Content). Restricting the analysis to the years 2000-2019 yielded qualitatively similar results as the main analysis (data not shown).
Discussion
We assessed the relationship between hypertension and one-year risk of all-cause mortality among women with HIV with a reported history of triple combination ART in the WIHS. Women with hypertension, compared to those without, had an increased one-year risk of death among women with treated HIV. The RDs and RRs varied slightly by age and racial-ethnic category. While variations were modest, they were consistent with current knowledge about disparities in the prevalence, incidence, and control of hypertension, as well as the incidence of cardiovascular events.[13,24,47–50]
The higher RRs for non-Hispanic Black and Hispanic women reflect racial-ethnic disparities in the relationship between hypertension and mortality. Results may help clinicians target subgroups of women with treated HIV who may benefit from prioritizing hypertensive treatment. This disparity aligns with racial disparities that exist in the prevalence of hypertension and incidence of cardiovascular events.[24,48] It is important to note racial-ethnic categories do not fully capture exposure to racism and structural racism, which are known to negatively impact health, including cardiovascular health.[25,51]
The prevalence of hypertension at analysis baseline (26.6%) and included visits (34.5%) were lower than a recent estimate among people with HIV engaged in care (42.4%),[21] but were within the range of prevalence estimates among people with HIV globally.[47] Further, we observed disparities in the prevalence of hypertension: Non-Hispanic Black women were hypertensive at 41.7% of their visits, compared to 25.2% of non-Hispanic white women and 22.6% of Hispanic women. Older women were more likely than younger women to have hypertension at their visit. The proportion of current smoking did not substantially differ by hypertensive status, a phenomenon that has been previously observed in HIV cohorts.[52] However, women who were never smokers were slightly more likely to be normotensive at their visit.
Access to health care, partially reflected by health insurance coverage, is associated with improved management of chronic diseases, such as hypertension,[51] but loss of health insurance can increase the risk of loss of hypertension control.[26] Health insurance status in the WIHS cohort is mostly stable, as most do not experience insurance interruptions, though change of insurance type is fairly common.[26,53] Estimated RRs and RDs were slightly higher among insured women than uninsured women, though confidence intervals were wide. This seemingly paradoxical finding is common in mortality studies among people with HIV as people with severe disease or risk of death are more likely to be insured.[54,55] Further, less than 10% of visits were attended by women without insurance, resulting in underpowered stratified analyses.
People with controlled hypertension have improved mortality and cardiovascular disease outcomes compared to those who have uncontrolled hypertension.[45,46] However, our sensitivity analysis using the controlled, uncontrolled, and no hypertension definition revealed minimal differences in the risk contrasts for visits with controlled and uncontrolled hypertension, compared to visits without hypertension. The estimates for visits with controlled and uncontrolled hypertension agreed with the binary definition of hypertension in the main analysis, though confidence intervals were less precise due to decreased sample sizes in the uncontrolled and controlled hypertension groups.
Hypertension increases the risk for adverse health outcomes, leading to decreased quality of life or chronic illnesses, not only cardiovascular events or deaths.[56] While the WIHS cohort ascertains cause of death among participants using death certificates and the NDI, determining primary cause of death is challenging and often misclassified on death certificates.[57] The most common cause of death in the WIHS was AIDS until 2002 and non-AIDS causes thereafter, which included non-AIDS malignancies and liver- and cardiovascular-related deaths.[30,58] Our time-restricted sensitivity analysis indicated our results were not sensitive to including visits prior to the modern ART era, likely because most study visits occurred after 1999 and analysis inclusion criteria required at least triple combination ART.
We applied a novel method (i.e., parametric g-computation) to answer our research question; but this analysis presents some limitations. Parametric g-computation estimates the causal RR and RD assuming no uncontrolled confounding, causal consistency of the exposure, no measurement error, and correct model specification.[38,39,42] Blood pressure, a continuous measure, was used to create a binary hypertension variable. Within levels of the binary exposure, blood pressure has varying levels of severity, as very high and very low blood pressure have a strong relationship with mortality.[28,59] Nevertheless, this threshold aligns with clinical practice guidelines for pharmacologic treatment of hypertension.[28] Despite including several covariates in models to account for confounding, unobserved confounding remains a threat in observational research. Possible unmeasured confounders include diet, exercise, and other lifestyle factors; these data were not collected routinely during follow-up.[45,46] Lastly, results may not be generalizable to all ART-treated women with HIV in the United States. Most women in the WIHS were engaged in HIV care and regularly attended study visits; therefore, they may be different than women with HIV not engaged in care. Despite limitations, this analysis builds upon previous research[6,21,24,26] and presents an application of parametric g-computation to estimate the effect of hypertension on one-year risk of mortality, rather than estimating conditional, associational measures from ordinary regression models.
The small increase in the one-year RD for all-cause mortality among women with hypertension, compared to women without, was not surprising given the short risk period. However, the increase in the one-year risk of death in the RR is notable. While we did not evaluate an intervention for hypertension (e.g., blood pressure lowering medication), evidence supports efficacy of lifestyle modification and pharmaceuticals to lower blood pressure.[28] The largest estimated RRs and RDs for mortality comparing women with hypertension to women without were among non-Hispanic Black women, Hispanic women, and women <40 years of age. Clinicians providing care to women of color with HIV or young women with HIV may want to prioritize reducing blood pressure through lifestyle modification or hypertensive medication. More attention is needed to understand disparities in the relationship between hypertension and mortality.
Women with HIV on ART had hypertension at over a third of their visits. Given the high prevalence in this population, this analysis underscores how even a moderate relative increase in risk of death can be clinically important. While hypertension may not have been the direct cause of death for women with treated HIV, improvements in population health can result from intervening on a condition with a modest to moderate association that is highly prevalent in the population, rather than a rare exposure with a strong association.[60] Thus, hypertension is a promising target for intervention to improve population health for women with treated HIV in the United States.
Supplementary Material
Acknowledgements
CRediT author contributions. L.M.S.: conceptualization, methodology, software, formal analysis, writing- original draft. D.W.: conceptualization, methodology, writing- review and editing, supervision, funding acquisition. A.E.: methodology, software, resources, data curation, writing- review and editing, supervision. T.L.B.: methodology, software, writing- review and editing. S.R.C.: writing- review and editing. C.R.: project administration, resources, writing- review and editing. T.B., I.O., D.K.P., S.K., D.L.W., G.D., M.C., P.C.T., T.T., K.A: investigation, resources, writing- review and editing, funding acquisition. A.A.A.: conceptualization, investigation, resources, writing- review and editing, supervision, funding acquisition. All authors revised the manuscript and gave final approval.
This research was supported by the National Institutes of Health Office of Research and Women’s Health (ORWH) through an Administrative Supplement for Research on the Health of Women of Understudied, Underrepresented and Underreported Populations (U01HL146194-01S1).
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS), now the MACS/WIHS Combined Cohort Study (MWCCS). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01- HL146242; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), P30-AI-050409 (Atlanta CFAR), P30-AI-073961 (Miami CFAR), P30-AI-050410 (UNC CFAR), P30-AI-027767 (UAB CFAR), and P30-MH-116867 (Miami CHARM). L. Sadinski was supported in part by T32-AI070114.
The authors thank Rachael K. Ross for her helpful guidance on data analysis. The authors gratefully acknowledge the contributions of the study participants and dedication of the staff at the MWCCS sites. Access to individual-level data from the MACS/WIHS Combined Cohort Study Data (MWCCS) may be obtained upon review and approval of a MWCCS concept sheet. Links and instructions for online concept sheet submission are on the study website. The authors have no conflicts of interest to declare. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Conflicts of interest:
PCT’s institution has received grants from Merck to support her research and Gilead for the conduct of sponsor-initiated clinical trials. AAA has received consulting fees from Merck and Gilead. Her institution has received funding from Merck and Gilead for her research. All other authors have none to declare.
References
- 1.May MT, Gompels M, Delpech V, Porter K, Orkin C, Kegg S, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS 2014; 28:1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trickey A, May MT, Vehreschild JJ, Obel N, Gill MJ, Crane HM, et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017; 4:e349–e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wada N, Jacobson LP, Cohen M, French A, Phair J, Muñoz A. Practice of Epidemiology Cause-Specific Life Expectancies After 35 Years of Age for Human Immunodeficiency Syndrome-Infected and Human Immunodeficiency Syndrome-Negative Individuals Followed Simultaneously in Long-term Cohort Studies, 1984-2008. Am J Epidemiol 2013; 177:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallant J, Hsue PY, Shreay S, Meyer N. Comorbidities Among US Patients With Prevalent HIV Infection-A Trend Analysis. J Infect Dis 2017; 216:1525–1533. [DOI] [PubMed] [Google Scholar]
- 5.Farahani M, Mulinder H, Farahani A, Marlink R. Prevalence and distribution of non-AIDS causes of death among HIV-infected individuals receiving antiretroviral therapy: a systematic review and meta-analysis. Int J STD AIDS 2017; 28:636–650. [DOI] [PubMed] [Google Scholar]
- 6.Feinstein MJ, Bahiru E, Achenbach C, Longenecker CT, Hsue P, So-Armah K, et al. Patterns of Cardiovascular Mortality for HIV-Infected Adults in the United Stated: 1990 to 2013. Am J Cardiol 2016; 117:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breger TL, Edwards JK, Cole SR, Saag M, Rebeiro PF, Moore RD, et al. Estimating a Set of Mortality Risk Functions with Multiple Contributing Causes of Death. Epidemiology 2020; 31:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsue PY, Waters DD. Time to Recognize HIV Infection as a Major Cardiovascular Risk Factor. Circulation 2018; 138:1113–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Islam F, Wu J, Jansson J, Wilson D. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med 2012; 13:453–468. [DOI] [PubMed] [Google Scholar]
- 10.Shah ASV, Stelzle D, Ken Lee K, Beck EJ, Alam S, Clifford S, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV systematic review and meta-analysis. Circulation 2018; 138:1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso A, Barnes AE, Guest JL, Shah A, Shao IY, Marconi V. HIV Infection and Incidence of Cardiovascular Diseases: An Analysis of a Large Healthcare Database. J Am Heart Assoc 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living with HIV: A Scientific Statement from the American Heart Association. Circulation 2019; 140:e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins LF, Sheth AN, Mehta CC, Naggie S, Golub ET, Anastos K, et al. The Prevalence and Burden of Non-AIDS Comorbidities Among Women Living With or at Risk for Human Immunodeficiency Virus Infection in the United States. Clin Infect Dis 2021; 72:1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palella FJ, Hart R, Armon C, Tedaldi E, Yangco B, Novak R, et al. Non-AIDS comorbidity burden differs by sex, race, and insurance type in aging adults in HIV care. AIDS 2019; 33:2327–2335. [DOI] [PubMed] [Google Scholar]
- 15.Grewe ME, Ma Y, Gilbertson A, Rennie S, Tucker JD. Women in HIV cure research: multilevel interventions to improve sex equity in recruitment. J Virus Erad 2016; 2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone L, Looby SE, Zanni MV. Cardiovascular Disease Risk among Women Living with HIV in North America & Europe. Curr Opin HIV AIDS 2017; 12:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi M, Smeaton LM, Vernon C, Scully EP, Gianella S, Poongulali S, et al. Low rate of sex-specific analyses in presentations at the Conference of Retroviruses and Opportunistic Infections (CROI) meeting, 2018: Room to improve. J Acquir Immune Defic Syndr 2019; 81:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gakidou E, Afshin A, Alemu Abajobir A, Hassen Abate K, Abbafati C, Abbas KM, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390:1345–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjeldsen SE. Hypertension and cardiovascular risk: General aspects. Pharmacol Res 2018; 129:95–99. [DOI] [PubMed] [Google Scholar]
- 20.Pan H, Hibino M, Kobeissi E, Aune D. Blood pressure, hypertension and the risk of sudden cardiac death: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol 2020; 35:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olaiya O, Weiser J, Zhou W, Patel P, Bradley H. Hypertension among persons living with HIV in medical care in the united states-medical monitoring project, 2013–2014. Open Forum Infect Dis 2018; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon SS, Fryar CD, Carroll MDH. Hypertension prevalence and control among adults: United States, 2011–2014. Hyattsville, MD: National Center for Health Statistics; 2015. [Google Scholar]
- 23.Curno MJ, Rossi S, Hodges-Mameletzis I, Johnston R, Price MA, Heidari S. A Systematic Review of the Inclusion (or Exclusion) of Women in HIV Research. J Acquir Immune Defic Syndr 2016; 71:181–188. [DOI] [PubMed] [Google Scholar]
- 24.Burkholder GA, Tamhane AR, Safford MM, Muntner PM, Willig AL, Willig JH, et al. Racial disparities in the prevalence and control of hypertension among a cohort of HIV-infected patients in the southeastern United States. PLoS One 2018; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Churchwell K, Elkind MSV, Benjamin RM, Carson AP, Chang EK, Lawrence W, et al. Call to Action: Structural Racism as a Fundamental Driver of Health Disparities: A Presidential Advisory from the American Heart Association. Circulation 2020; 142:E454–E468. [DOI] [PubMed] [Google Scholar]
- 26.Edmonds A, Ludema C, Eron JJ, Cole SR, Adedimeji AA, Cohen MH, et al. Effects of Health Insurance Interruption on Loss of Hypertension Control in Women With and Women Without HIV. J Women’s Heal 2017; 26:1292–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adimora AA, Ramirez C, Benning L, Greenblatt RM, Kempf M-C, Tien PC, et al. Cohort Profile: The Women’s Interagency HIV Study (WIHS). Int J Epidemiol 2018; 47:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary. Hypertension 2017; 71. [DOI] [PubMed] [Google Scholar]
- 29.Women’s Interagency HIV Study. Visit 50 manual of operations. Version: October 1, 2016. Available at: https://statepi.jhsph.edu/wihs/admin/moo/ (accessed 19 Aug 2020).
- 30.Cohen MH, French AL, Benning L, Kovacs A, Anastos K, Young M, et al. Causes of Death among Women with Human Immunodeficiency Virus Infection in the Era of Combination Antiretroviral Therapy. Am J Med 2002; 113:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenland S, Pearl J, Robins JM. Causal Diagrams for Epidemiologic Research. Epidemiology 1999; 10:37–48. [PubMed] [Google Scholar]
- 32.Westreich D Epidemiology by Design. New York: Oxford University Press; 2019. [Google Scholar]
- 33.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ, et al. Splines for trend analysis and continuous confounder control. Epidemiology 2011; 22:874–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berglund P, Heeringa S. Multiple Imputation of Missing Data Using SAS®. Cary, NC: SAS Insitute; 2014. [Google Scholar]
- 36.Allison PD. Missing Data. SAGE Publications Inc.; 2002. [Google Scholar]
- 37.Harel O, Mitchell EM, Perkins NJ, Cole SR, Tchetgen Tchetgen EJ, Sun B, et al. Multiple Imputation for Incomplete Data in Epidemiologic Studies. Am J Epidemiol 2018; 187:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Heal 2006; 60:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snowden JM, Rose S, Mortimer KM. Practice of Epidemiology Implementation of G-Computation on a Simulated Data Set: Demonstration of a Causal Inference Technique. Am J Epidemiol 2011; 173:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahern J, Hubbard A, Galea S. Practice of Epidemiology Estimating the Effects of Potential Public Health Interventions on Population Disease Burden: A Step-by-Step Illustration of Causal Inference Methods. Am J Epidemiol 2009; 169:1140–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernán MA, Robins JM. Causal Inference: What If. 1st ed. Boca Raton: Chapman & Hall/CRC; 2020. https://www.hsph.harvard.edu/miguel-hernan/causal-inference-book/ (accessed 5 Mar2021). [Google Scholar]
- 42.Cole SR, Hernán MA. Constructing Inverse Probability Weights for Marginal Structural Models. Am J Epidemiol 2008; 168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang FL. Using Cluster Bootstrapping to Analyze Nested Data With a Few Clusters. Educ Psychol Meas 2018; 78:297–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Efron B, Tibshirani RJ. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 45.Gu Q, Dillon CF, Burt VL, Gillum RF. Association of hypertension treatment and control with all-cause and cardiovascular disease mortality among US adults with hypertension. Am J Hypertens 2010; 23:38–45. [DOI] [PubMed] [Google Scholar]
- 46.Zhou D, Xi B, Zhao M, Wang L, Veeranki SP. Uncontrolled hypertension increases risk of all-cause and cardiovascular disease mortality in US adults: the NHANES III Linked Mortality Study OPEN. Sci Rep 2018; 8:9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta-analysis. J Am Soc Hypertens 2017; 11:530–540. [DOI] [PubMed] [Google Scholar]
- 48.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, et al. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation 2017; 136:e393–e423. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, et al. Status of cardiovascular disease and stroke in hispanics/latinos in the united states: A science advisory from the american heart association. Circulation 2014; 130:593–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Birabaharan M, Strunk A, Martin TCS. Burden of Hypertension, Diabetes, Cardiovascular Disease, and Lung Disease Among Women Living With Human Immunodeficiency Virus (HIV) in the United States. Clin Infect Dis 2021; 73:169–170. [DOI] [PubMed] [Google Scholar]
- 51.Havranek EP, Mujahid MS, Barr DA, Blair IV., Cohen MS, Cruz-Flores S, et al. Social determinants of risk and outcomes for cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2015; 132:873–898. [DOI] [PubMed] [Google Scholar]
- 52.Hatleberg CI, Ryom L, d’Arminio Monforte A, Fontas E, Reiss P, Kirk O, et al. Association between exposure to antiretroviral drugs and the incidence of hypertension in HIV-positive persons: the D:A:D Study. HIV Med 2018; 19:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edmonds A, Belenky N, Adedimeji AA, Cohen MH, Wingood G, Fischl MA, et al. Impacts of Medicaid Expansion on Health Insurance and Coverage Transitions among Women with or at Risk for HIV in the United States. Womens Health Issues 2022; 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lancaster T, Intrator O, Intrator O. Panel data with survival: Hospitalization of hiv-positive patients. J Am Stat Assoc 1998; 93:46–53. [Google Scholar]
- 55.Goldman DP, Bhattacharya J, Mccaffrey DF, Duan N, Leibowitz AA, Joyce GF, et al. Effect of Insurance on Mortality in an HIV-Positive Population in Care. J Am Stat Assoc 2001; 96:883–894. [Google Scholar]
- 56.Kokubo Y, Iwashima Y. Higher Blood Pressure as a Risk Factor for Diseases Other Than Stroke and Ischemic Heart Disease. Hypertension 2015; 66:254–259. [DOI] [PubMed] [Google Scholar]
- 57.Goldacre MJ. Cause-specific mortality: understanding uncertain tips of the disease iceberg. J Epidemiol Community Health 1993; 47:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.French AL, Gawel SH, Hershow R, Benning L, Hessol NA, Levine AM, et al. Trends in mortality and causes of death among women with HIV in the United States: A 10-year study. J Acquir Immune Defic Syndr 2009; 51:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet 2014; 383:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rose G Sick Individuals and Sick Populations. Int J Epidemiol 1985; 14:32–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


