Abstract
Objective:
Attention bias modification (ABM) aims to decrease anxiety symptom severity through the reduction of threat-related attention bias (AB). Individual differences in treatment response and poor measurement reliability of AB have called its clinical promise into question. The current study examined whether individual differences in anxiety severity at baseline moderated treatment response, and employed both behavioral and neurophysiological metrics of AB.
Methods:
Participants (N=99) were randomly assigned to four weeks of ABM or placebo control training (PT). Self-reported anxiety symptom severity, and AB metrics and ERPs generated during the dot probe task were collected at baseline (Time 1), one-week post-intervention (Time 5), and at a three-month follow-up (Time 6).
Results:
ABM, relative to PT, reduced ERPs indexing attention discrimination (N170) and increased ERPs indexing salience tracking (P3). Increases in P3 were associated with ABM-related reductions in anxiety. Anxiety severity was reduced following ABM, but only among those with higher baseline anxiety symptom severity.
Conclusions:
ABM effectively reduced symptom severity among those with higher levels of anxiety, and modulated neurophysiological indices of AB.
Significance:
Results provide evidence for attention-relevant ERPs as outcomes of ABM treatment responsivity and suggest that ABM may be most beneficial for those with more severe anxiety symptoms.
Keywords: Attention bias modification, anxiety, event-related potentials, individual differences
Introduction
One third of Americans will suffer from clinical levels of anxiety during their lifetime, making anxiety and stress-related disorders the most common forms of mental illness and among the largest health burdens in society (Kessler et al., 2005). Yet current treatment approaches fall short: rates of quality care using a combination of cognitive behavioral therapy (CBT) and pharmacological treatments are low (Stein et al., 2005), and symptom recurrence remains high with as many as 60% of patients symptomatic after one year (Olatunji et al., 2007; Westen et al., 2004). Thus, there is a pressing public health and scientific need for the development and refinement of interventions for anxiety that are both low-barrier and that potentially target novel mechanisms underlying the emergence and maintenance of anxiety disorders.
One such candidate mechanism is the anxiety-related attention bias (AB), or selective and exaggerated attention towards threat (Bar-Haim et al., 2007; Cisler and Koster, 2010). AB occurs automatically and unconsciously, and acts as an information filter that selects threat-relevant information at the expense of signals indicating positive outcomes or safety. This cognitive bias, if sustained over time, can cause a cascade of cognitive, affective, and biological changes that give rise to and maintain symptoms of anxiety (Bar-Haim et al., 2007; Dennis-Tiwary et al., 2019; MacLeod and Mathews, 1988; MacLeod et al., 1986).
Attention bias modification (ABM) is a computer-based intervention approach that aims to ameliorate AB, with the downstream effect of alleviating anxiety-related symptoms (Beard et al., 2012; Hakamata et al., 2010; Hallion and Ruscio, 2011; MacLeod et al., 2002; Mogoaşe et al., 2014). ABM has received increasing interest as a second-generation, low-barrier cognitive intervention for a range of anxiety disorders, including generalized anxiety disorder and social anxiety disorder (e.g., Amir et al., 2009; Amir et al., 2009; Britton et al., 2014; Klumpp and Amir, 2010), as well as other mental health conditions, including depression (Beevers et al., 2021; Mogoase et al., 2014), and substance use problems (Luehring-Jones et al., 2017). However, initial excitement for ABM has been tempered by a growing number of randomized ABM clinical trials with small effect sizes (e.g., d = 0.2 - 0.5) or null findings (Heeren et al., 2015; Mogoaşe et al., 2014) regarding reductions in anxiety symptoms following training, or comparable effects between active and control training (Enock et al., 2014; McNally et al., 2013; Ollendick et al., 2019; Pergamin-Hight et al., 2016; Pettit et al., 2020).
A barrier to evaluating the efficacy of ABM are challenges in the conceptualization and measurement of AB (Dennis-Tiwary et al., 2019; Kappenman et al., 2014; Waechter et al., 2014). Traditional reaction-time based measures of AB, particularly those derived from the dot probe task, show notoriously poor test-retest and split-half reliability (MacLeod et al., 2019; Price et al., 2018; Schmukle, 2005; Staugaard, 2009). These scores, averaged over dozens or even hundreds of trials obfuscate individual variability in AB over the course of assessment, missing potentially meaningful trial-level variability, thus reducing reliability of measurement (e.g., Zvielli et al., 2016; Egan and Dennis-Tiwary, 2018).
Indeed, AB scores based on more dynamic, trial-level reaction times, tracked over the course of AB assessment are more reliable (Naim et al., 2015; Price et al., 2018; Zvielli et al., 2015, 2016; although for debate see Carlson and Fang, 2020; Kruijt et al., 2016) and are sensitive to anxiety-related individual differences. For example, greater variability in trial-level bias scores (TLBS) predicts greater stress reactivity measured during the Trier Social Stress Test (Egan and Dennis-Tiwary, 2018), and is dampened in response to ABM (Clerkin et al., 2016; Zvielli et al., 2015), suggesting increased regulation of AB in relation to threat stimuli. In addition, increasing trial level bias away from threat coincided with lower anxiety symptoms over time following ABM in one study (Huppert et al., 2018), but the magnitude of TLBS change was not significantly different post vs pre-intervention. In the present study, we build on these prior findings by examining both variability in trial-level bias scores as well as trial-level metrics of bias towards and away from threat.
Another important consideration is that reaction-time-based measures of AB are indirect and downstream measures of neural responses to threat. That is, in comparison to direct measures of neural activity, button-response metrics are more likely to introduce noise due to variation in motor responses. Scalp-recorded event-related potentials (ERPs) address this concern, as well as that of low reliability. ERPs elicited during the dot probe assessment of AB show high test-retest reliability (Kappenman, Farrens, et al., 2014; Kappenman, MacNamara, et al., 2014; Reutter et al., 2017), are temporally sensitive (Banaschewski and Brandeis, 2007), and show high functional specificity. Reviews of ERP indices of threat processing in the context of ABM (e.g., Carlson, 2021; Torrence and Troup, 2018; Wieser and Keil, 2020) suggest that these metrics can be generally divided into two categories: early-latency (e.g., P1 and N170), and later-latency ERPs (N2 and P3), with both types measured during the gold standard AB assessment, the dot probe task (MacLeod and Mathews, 1988; MacLeod et al., 1986). This temporal distinction reflects differential functional sensitivity of ERPs to distinct stages of information processing of threat. Elucidating the specific components of attention implicated in AB and its reduction may inform future clinical intervention targets.
The P1 and N170 are thought to index early attention capture and visual discrimination of visual stimuli, respectively. The P1, peaking around 100 ms in posterior electrodes indexes activity of the extra-striate visual cortex and relatively rapid and automatic shifts in attention (Hillyard and Anllo-Vento, 1998; Luck et al., 1990; Smith et al., 2003). The N170, peaking around 170 ms in posterior scalp electrodes, is a face-sensitive variant of the N1 (Bentin et al., 1996). It is modulated by emotion expression (Hinojosa et al., 2015), with attention to emotional faces appearing to enhance the N170 (Andrzejewski and Carlson, 2020) relative to neutral (Denefrio et al., 2019). Both the P1 and N170 increase in magnitude in response to threat-related stimuli and among those reporting greater anxiety symptom severity (for a review, see Carlson, 2021). The N2 and P3, in contrast, reflect relatively later, more deliberative cognitive processes related to threat processing. The N2, peaking around 250–350 in frontal scalp electrodes has been linked to the maturation and recruitment of cognitive control capacities (Ladouceur et al., 2007; van Veen and Carter, 2002). The posterior P3, peaking around 300–400 ms in posterior electrodes, is amplified with affective salience tracking and strategic orienting of attention (Carretié et al., 2004; Fichtenholtz et al., 2007; Polich, 2007).
ABM directly modifies both early- and later-emerging ERPs measured during the dot probe in adults with elevated trait anxiety (e.g., Bar-Haim et al., 2005; Dennis-Tiwary et al., 2016; Eldar and Bar-Haim, 2010). ABM decreases the magnitude of the P1 and N170 to threat stimuli (O’Toole and Dennis, 2012), and another ABM study documented that increased P1 at baseline predicted greater treatment efficacy (Dennis-Tiwary et al., 2017). In a study with socially anxious individuals, ABM reduced the magnitude of the N170 to disgust faces (representing social threat; Pan et al., 2019). Thus, there is some evidence that ABM reduces both the P1 and the face-sensitive N170, suggesting that training attention away from threat stimuli successfully decreases early-latency indices of attention to threat.
ABM also modulates the magnitude of later-emerging N2 and P3 in trait anxious adults. Eldar and Bar-Haim (2010) reported that ABM relative to placebo resulted in enhanced N2 amplitudes in anxious adults, and in a study using mobile, gamified ABM, males, but not females, showed enhanced N2 following a single session of ABM relative to placebo training (Dennis-Tiwary et al., 2016). The plasticity of the N2 following ABM has been interpreted as reflecting greater recruitment of cognitive control. It is less clear whether and how ABM modulates the posterior P3. One study found that the magnitude of the P3 was reduced following ABM (Eldar and Bar-Haim, 2010), whereas other studies have shown enhanced P3 amplitudes (Suway et al., 2013; Sylvain et al., 2020), including a study in which non-anxious adults were trained to attend to happy rather than angry faces (O’Toole and Dennis, 2012). Importantly, some inconsistencies in prior findings are likely due to differences or variability in sample ranges and degree of anxiety severity (i.e., non-anxious or trait anxious adults). Although these findings are inconclusive, they suggest that ABM can modulate indices of controlled and strategic attention to emotionally salient stimuli.
Despite this growing evidence base, precious little is known about whether and how ERP indices of threat processing during an AB assessment moderate the effects of ABM on anxiety symptom reduction. Some studies suggest that ERPs measured prior to ABM predict clinical outcomes. A study with pregnant women (Dennis-Tiwary et al., 2017), for example, found that those who showed smaller P1 amplitudes to threat prior to a month of mobile ABM training also showed greater decreases in anxiety symptoms relative to placebo training, perhaps indicating that dampened attention capture by threat prior to ABM amplifies treatment responsiveness. Although no studies to date have documented that the degree to which ABM modulates ERP responses to threat mediates the efficacy of ABM, several studies have documented such effects using other metrics of attention bias, including a study showing that ABM-induced changes in attention control correlated with reduced anxiety symptoms in clinically anxious youth (Linetzky et al., 2020) and that reductions in eye-tracking metrics of attention bias fully mediated reductions in depression following ABM for depressive symptoms (Beevers et al., 2021). Since attention-relevant ERPs represent neurophysiological indices of the mechanisms underlying AB, it is crucial to assess whether plasticity of ERPs following ABM mediate its impact on clinical symptom severity.
Individual differences prior to receiving ABM may also moderate its clinical impact. ABM effectively reduces symptoms across a range of anxiety severity, from mild to severe (e.g., for a review see Dennis-Tiwary et al., 2018), and even among those with typical levels of anxiety (e.g., Clarke et al., 2014). However, several studies document that those with greater magnitude AB or anxiety severity respond to ABM with greater symptom reduction following a four-week course of ABM (e.g., O’Toole and Dennis, 2012; Kuckertz et al., 2014). Thus, to advance the personalization of ABM, individual differences in symptom severity must be taken into account to identify those for whom ABM may be most effective (Kapur et al., 2012; Dennis-Tiwary et al., 2018).
The present study was a double-blind, randomized, placebo-controlled trial of ABM in a group of adults with clinically elevated symptoms across a range of anxiety disorders. We examined the effects of ABM on anxiety symptom severity and on multiple metrics of AB, including ERPs, trial-level, and average reaction-time-based assays. A main focus of the study was to further assess whether individual differences in AB and anxiety severity moderated these primary treatment outcomes. We tested the hypothesis that ABM, relative to placebo training, would: (a) reduce anxiety symptom severity, (b) dampen trial-level measures of AB, (c) reduce the magnitude of early-emerging ERP metrics of attention capture (P1 and N170), and (d) increase the magnitude of later-emerging measures of cognitive control and strategic attention (N2 and P3). We further tested whether individual differences in AB and anxiety severity at baseline moderated the impact of ABM on anxiety symptom reduction, and if ABM-induced changes in ERPs mediated the impact of ABM on reduction of anxiety severity. We explored the correspondence between behavioral and neurocognitive temporally-sensitive measures of AB, which is largely unknown. Taken together, this study aimed to advance understanding of mechanisms of action underlying ABM by employing neurocognitive and psychometrically superior measures of AB.
Methods
Participants
One hundred and twenty-six adults (72 Females; 57%) were recruited from a community-based survey center in New York City and selected if they evidenced moderate to severe levels of anxiety or stress (Manxiety = 9.35, SDanxiety = 3.49; Mstress =12.76, SDstress = 3.80) via a phone screen using the Depression, Anxiety, and Stress Scale (DASS-21; Lovibond and Lovibond, 1995; Henry and Crawford, 2005), with scores from at least one of the subscales above the cut-off (6 and above for anxiety and/or 10 or above on stress subscales).
Upon arrival to the lab, the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998) was conducted by a trained lab personnel to assess diagnostic status, comorbidities, and exclusionary criteria were as follows: (a) presence of organic mental disorders, psychotic disorders or diagnosis with psychotic features, substance use disorder and substance dependence (non-alcohol), pervasive developmental disorders, or intellectual disability; (b) high risk for self-harm or violence; (c) concurrent treatment for psychosocial problems; (d) uncorrected vision problems; or (e) physical or motor disability that prevents them from using computer. Use of medication to treat a mental health condition was not an exclusionary criterion unless medication regimen had not been stable for at least 2 months prior to participation.
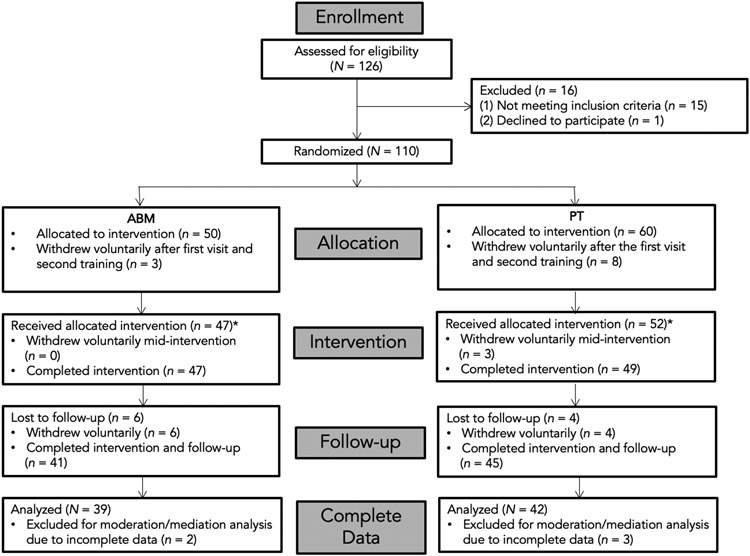
Fifteen participants were excluded due to clinical diagnostic criteria (e.g., past manic episode with psychotic features, substance use disorder, and substance dependence [non-alcohol]) or recent changes in prescription medication use. One person declined to continue to participate. A total of 110 people met criteria and agreed to participate. From 110 participants, 11 individuals withdrew voluntarily after the first visit (see Fig 1 for a participant flow chart).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) Flow Diagram.
* For the Generalized Estimating Equations (GEE) analysis, as it accounts for missing data, we used dataset that includes those who underwent intervention, which is N = 99.
The current study thus included a sample of 99 adults (68 females; 69%) aged 18-41 (Mage = 25.22, SDage = 6.33).1 Among these, 40 individuals met criteria for an anxiety disorder (40.4%). In addition, 27 met criteria for major depressive disorder (27.3%), three for obsessive compulsive disorder (3.0%), three for post-traumatic stress disorder (3.0%), one for alcohol dependence (1.0%), and two for eating disorders (2.0%). Twenty-three had no primary diagnosis (23.2%). Self-reported race/ethnicity of the samples was the following: 51 White (51.5%), 20 Asian (20.2%), 10 Black or African American (10.1%), 1 American Indian (1.0%), and 12 reported more than one race (12.1%). Among these, 20 individuals identified as Hispanic/Latino (20.2%). Five individuals chose not to report their race/ethnicity (5.1%). Participants reported their average annual income including unemployment, public assistance, and disability (M = $62,008.23, SD = $59,373.46; Min-Max = $0 - $400,000).
Participants were randomly assigned to either the ABM (n = 47) or placebo training (PT; n = 52) group. Of the 99 participants, 81 (54 females; Mage = 25.47, SDage = 6.44) attended all visits and completed EEG assessment at Time 6. The remaining participants were lost to three-month follow up (n = 13) or had incomplete interim assessments (n = 5). Participants were compensated a total of $400 if they completed all visits. This study was approved by the Institutional Review Board (IRB) of Hunter College, CUNY (Protocol 20150286) and preregistered on clinicaltrials.gov (Identifier NCT02200003).
Procedure
The study consisted of six visits over the course of four months, with Time 1 being the baseline assessment, Times 2-4 being ABM/PT sessions, Time 5 being the one-week post-intervention follow-up assessment, and Time 6 being the three-month follow-up assessment. At the start of each lab visit, participants were asked whether they were currently under the influence of any drugs or alcohol, and whether they felt rested that day. If participants reported any alterations in their alertness or state of mind that would impede their ability to complete the study procedures, they were asked to reschedule. Participants provided informed consent following a detailed explanation of the study procedures, maintenance of confidentiality, and their right to discontinue participation at any time. Participants were also informed that they would be assigned to either an intervention group or a control group, that neither they nor the experimenter would be aware of their group assignment, and they would learn whether they received the intervention at the conclusion of their participation. To achieve the double-blind design, the ABM and PT groups were assigned numbers which were entered into the computer at the start of each ABM/PT session. Neither the experimenters nor the participants were aware of which numbers corresponded to which group.
Time 1.
After consent procedures, participants were randomly assigned to the ABM or PT condition, and self-report of demographics and stress and anxiety symptoms (DASS-21) were collected. Following the questionnaires, participants were placed in an EEG recording booth, in front of a 17-inch monitor, 65 cm away from it while EEG electrodes were applied. The first attention bias (AB) assessment was completed (i.e., the dot probe task), and during this time EEG was recorded continuously. Upon completion of the AB assessment, EEG was removed. Then, participants reported on anxiety symptoms using the Hamilton Anxiety Scale (HAM-A), and the Mini International Neuropsychiatric Interview (MINI) was administered to the participant by a trained member of the research personnel. The visit lasted approximately three hours.
Time 2.
A week following Time 1, participants completed the second AB assessment (the dot probe task) during a continuous EEG recording. Following EEG removal, participants completed ABM session 1. The visit lasted about 90 minutes.
Times 3 and 4.
One week following Time 2, participants completed two one-hour ABM sessions separated by a week (sessions 2 and 3 ABM). No questionnaires, clinical interviews, or EEG recordings were conducted during this visit. Each visit lasted about 30-60 minutes.
Time 5.
A week after Time 4, participants returned to lab (five weeks after Time 1). Participants completed ABM session 4. Participants completed the third AB assessment during a continuous EEG recording. Following EEG removal, participants reported on anxiety symptoms using the HAM-A, and another MINI was administered to the participant by a trained member of the research personnel. The visit lasted about 90 minutes.
Time 6.
This follow-up visit occurred approximately three months after Time 5. Participants completed the fourth and final AB assessment while EEG was continuously recorded. Participants reported on anxiety symptoms using the HAM-A and a MINI was was conducted. The visit lasted about 90 minutes.
Materials
Screening Measure and Diagnostic Interview
The Depression, Anxiety, and Stress Scale (DASS-21; Henry and Crawford, 2005) is a 21-item survey-formatted questionnaire that assesses the severity of symptoms across depression, anxiety, and stress subscales. Each subscale is consisted of seven items, scored on a scale of 0-3, and with scores ranging from 0 to 21 for each subscale. The measure has shown acceptable internal consistency reliability for all sub-scales (α = .91, .80, and .84 for depression, anxiety, and stress subscales, respectively; Sinclair et al., 2012). We focused on the DASS-21 anxiety subscale for the current study.
The Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998) is a reliable, structured diagnostic interview for current and past DSM-IV diagnoses. One-on-one interviews typically last between 15-30 minutes. In the current study, the MINI was administered at multiple time points (Times 1, 5, and 6) by trained research personnel to identify pre-intervention diagnostic status as well as potential changes in diagnostic status and comorbidities during the study.
Anxiety Symptoms
The Hamilton Anxiety Scale (HAM-A; Hamilton, 1959) was administered at all assessment points at the end of each MINI interview by a trained research personnel. The HAM-A is a clinician rating of anxiety symptom severity, consisting of 14 items describing symptoms such as tension, anxious mood, fears, somatic complaints, and behavior during interview. Items are rated on a scale from 0 (“not present”) to 4 (“severe”). The sum of the value on each item ranges from 0 to 56. On the measure, scores 8-14 indicated mild anxiety, scores 15-23 indicated moderate anxiety, and scores greater than 24 indicated severe anxiety. The measure shows high internal consistency (α = .77-.92; Maier et al., 1988).
AB Assessment and ABM
The Dot Probe.
The dot probe task (MacLeod and Mathews, 1988; MacLeod et al., 1986) was administered at multiple time points across the visits (Times 1, 5, and 6) to derive behavioral and ERP measures of AB. The task followed parameters as suggested by Tel-Aviv University/National Institute of Mental Health protocol. Stimuli for the task were consisted of photos of 20 different individuals (10 males, 10 females) from the NimStim stimulus set (Tottenham et al., 2009) with one of the female photos taken from the Matsumoto and Ekman (1989) set. Stimuli were presented using E-Prime version 2.0 (Schneider, Eschman, and Zuccolotto, 2002).
On each trial, two facial stimuli were presented, face pairs with either angry-neutral or neural-neutral of the same individual. The pictures were presented above and below a fixation cross 14 mm apart. The task included total of 120 trials [80 threat (angry faces and neutral faces; ‘TN’ trials) and 40 non-threat (both neutral faces; ‘NN’ Trials)]. Each trial consisted of the following: 1) 500 ms fixation period, 2) 500 ms face-pair cue which then disappears, 3) target probe (an arrow) in the location in which one of the faces had appeared (with the probe staying on the screen until a response with the left or right mouse button to indicate the direction in which the probe is pointing, and 4) 500 ms inter-trial interval. Participants were instructed to respond as quickly and as accurately as possible to indicate the direction of the arrow. Probes were equally likely to appear on the top or the bottom of the fixation cross where the angry or neutral face cues were presented and pointing to either direction.
Quantification of AB.
The dot probe task was used to measure AB, and the scores were generated from each dot probe assessment at each time point (Times 1, 5, and 6). Incorrect responses of the dot probe trials were excluded from processing and analyses. In addition, all responses faster than −3 SD and slower than +3 SD from an individual’s mean were removed. Lastly, the accuracy rate of all participants was 85% or above.
Mean AB was calculated to examine three metrics of AB: a) threat bias, reflecting overall attention capture by threat. The measure is calculated as the average RTs for neutral in TN trials minus RTs for angry in TN trials; b) vigilance, reflecting more automatic and bottom-up attention to threat. This measure is computed as the average RTs for neutral in the NN trials minus the average RTs for threat in TN trials; and c) disengagement, reflecting difficulty in the effortful disengagement or top-down inhibition of attention to threat. This is calculated as the average RTs for neutral in TN trial minus RTs for neutral in the NN trials.
Trial-Level Bias Scores (TLBS).
In addition to mean AB scores, which tend to have low split-half reliability and obfuscate individual variability in AB throughout the assessments, TLBS were quantified by taking the TN-Threat and TN-Neutral trial pairs that are temporally contiguous for each of the three AB assessments, separately (e.g., Egan and Dennis-Tiwary, 2018). Trial pairs were defined as unique sets of TN-Neutral and the next closest TN-Threat trial (and vice versa), within a maximum of 5 trials apart (before or after). Within each pair of matched trials, trial-level bias scores (TLBS) were computed by subtracting RTs for TN-Neutral minus RTs for TN-Threat, consistent with the mean AB score previously mentioned, but done on the level of each trial pair.
From the set of TLBS generated for each participant, four measures were calculated: Mean positive (average of all TLBS greater than zero), mean negative (average of all TLBS less than zero), peak difference (highest positive TLBS minus lowest negative TLBS), and variability. The variability score was generated as the sum of the distance between each sequential TLBS divided by the number of pairs, providing a measure of the “length” of the plotted TLBS line. This indicates the higher the value of the variability sum, the greater the variability toward and away from threat over the course of assessment.
ABM or Control Placebo Training (PT).
Participants were randomly assigned to either complete four sessions of ABM or PT (each week for a month). ABM systematically trains attention away from threat using a modified version of dot probe [i.e., the probe always replaces the neutral face cue (100 % contingency for TN-Neutral Trials)]. In contrast, PT has an equal likelihood of the probe replacing either the threat or the neutral cue so that the probe would replace the neutral face cue for only half of the times (50% TN-Neutral Trials and 50% TN-Threat Trials, in which the probe replaces angry face). Like the dot probe task, described above, each trial consisted of a 1) 500 ms fixation period, 2) 500 ms face-pair cue which then disappears, 3) target probe (an arrow) in the location in which one of the faces had appeared (with the probe staying on the screen until a response is made to indicate the direction of the arrow), and 4) 500 ms inter-trial interval. Each training session consisted of four blocks of 160 trials (120 threat-neutral training trials and 40 neutral baseline pair trials). A break was offered to the participants every 40 trials during each training block. If accuracy rate fell below 70% a warning was presented with the break slide. Each training session lasted 5-8 minutes. Participants completed one session per week for four consecutive weeks. Reaction times to the probe on each trial was recorded during the training.
Electrophysiological Recording and Data Reduction
A Biosemi system (BioSemi; Amsterdam, NL) was used to continuously record EEG activity during the dot probe task. Electrodes (i.e., 64 Ag/AgCl scalp electrodes) were fixed into an elasticized nylon cap and arranged according to the International 10/20 system. Electro-oculogram (EOG) signals from electrodes were used to monitor eye movements, via an electrode placed 1 cm above and below the left eye (for vertical eye movements) and 1 cm on the outer edge of each eye (for horizontal eye movements). To improve the signal-to-noise ratio, preamplification of the EEG signal was done at each electrode (with a sampling rate of 512 Hz). The voltage from each of the 64 electrodes was referenced online with respect to the common mode sense active electrode and driven right leg electrode during EEG acquisition, which produces a non-differential channel. Brain Vision Analyzer (Version 2.2, GmbH; Munich, DE) was used to process and prepare the acquired EEG data. All offline data were re-referenced to the average of the scalp and filtered with high (0.1 Hz) and low (30 Hz) pass frequencies.
Stimulus-locked EEG data to faces (event-related potentials; ERPs) were segmented into epochs from 200 ms before stimulus presentation to 500 ms after stimulus onset. Each epoch included a 200 ms baseline correction (−200 ms to 0 ms before the onset of stimulus). Following ocular (Gratton, Coles, and Donchin, 1983) and baseline corrections, artifacts were identified and removed from further analyses using the following criteria: 1) greater voltage steps than 50 μV, 2) changes within a segment greater than 300 μV, and 3) lower activity than .5 μV per 100 ms.
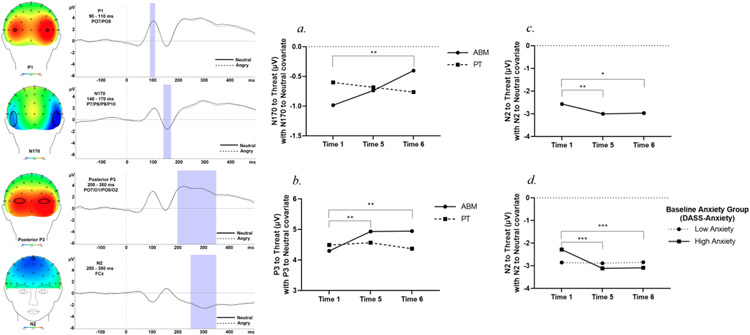
For ERP components, electrodes were selected by visual inspection of the topographical distribution of the data from first AB assessment, and the grand average was calculated across all stimulus conditions and participants. Artifact-free EEG trials were used to generate ERPs for each participant separately for TNNT (TN and NT trials combined) and NN trials: The P1 was calculated as the average amplitude between 90 and 110 ms at PO7 and PO8. The N170 was calculated as the average amplitude between 140 ms and 170 ms at P7, P8, P9, and P10. The N2 was calculated as the average amplitude between 250 ms and 350 ms at FCz. Lastly, the P3 was calculated as the average amplitude between 200 ms and 350 ms at PO7, PO8, O1, and O2. See Figure 2 for grand-average waveforms.
Figure 2.
Topographic maps and waveforms of the analyzed, entire sample averaged across both ABM and PT for P1, N170, N2, and P3 are presented (Left). (a.) N170 to threat faces significantly decreased (became less negative) from T1 to T6 for the ABM group. (b.) For the ABM group, P3 to threat faces significantly increased from T1 to T5, and from T1 to T6. No significant changes over time were detected for the PT group. (c.) N2 to threat faces significantly increased (became more negative over time). (d.) In the high baseline anxiety group, N2 significantly increased from T1 to T5 and T1 to T6 (Right).
Note. *Difference is significant at the .05 level, **Difference is significant at the .01 level. ***Difference is significant at the < .001 level. T1 = Time 1, T5 = Time 5, T6 = Time 6, ABM = attention bias modification training, PT = placebo control training, TLBS = trial-level bias score, DASS = Depression Anxiety Stress Scales.
Analytic approach
The goal of the present study was to test the hypothesis that ABM, relative to control training, would be associated with reduced anxiety symptom severity, dampened trial-level measures of AB, reduced magnitude of early-emerging ERP metrics of attention capture (P1 and N170), and increased magnitude of later-emerging measures of cognitive control and strategic attention (N2 and P3). We examined whether these effects were moderated by individual differences in high vs. low levels of anxiety severity at baseline, based on a median split (Med. = 5.00; low: Manxiety = 2.31, SDanxiety = 1.70; high: Manxiety = 9.81, SDanxiety = 3.47) for DASS-21 Anxiety scores at T1. These low and high baseline anxiety groups were confirmed to be significantly different regarding T1 DASS-21 Anxiety (p < .001) and T1 DASS-21 Stress (low: Mstress = 6.57, SDstress = 3.79; high: Mstress = 13.06, SDstress = 4.23, p < .001).
To do so, we utilized generalized estimating equations (GEE) to examine changes in each of these dependent variables over the full course of the study [T1 (baseline), T5 (immediate follow-up), and T6 (three-month follow-up)] in relation to intervention group (ABM vs PT), anxiety severity at baseline, and their interaction. GEE allows for the addition of repeated measures to a generalized linear model, and accounts for missing values that are missing completely at random (MCAR). Little’s MCAR test was conducted and confirmed that data was MCAR [χ2 (227) = 70.51, p = .999]. A series of linear models were conducted as follows. Predictors were Time (T1, T5, T6), Group (ABM, PT), and baseline anxiety group (high anxiety, low anxiety) was entered as a moderator. Outcomes were mean AB scores (threat bias, vigilance, disengagement), TLBS (mean positive, mean negative, peak difference, variability), anxiety symptoms measured via HAM-A, and ERPs to threat (P1, N170, N2, P3), with separate models for each dependent variable (12 models total). For models with ERPs as outcomes, the corresponding ERP to the baseline condition (NN) was entered as a covariate. Significant interactions were probed using pairwise comparison follow-up tests with Bonferroni’s correction applied to account for multiple comparisons. Bonferroni-adjusted criterion for follow-up comparisons were as follows: main effects (p < .017), 2-way interactions (p < .008), 3-way interactions (p < .004).
Based on the GEE analysis, variables for mediation analysis were chosen to test whether the change in the ERPs (N170, N2, and P3 T1-T5 or T1-T6 change scores) mediated the association between Group and Anxiety (measured via HAM-A), while considering the role of baseline anxiety severity (DASS at T1). All analyses were conducted using SPSS PROCESS Model 4 (Version 3.5; Hayes, 2017). Path a referred to a regression predicting Group and ERPs; path b, to a relationship between ERPs and Anxiety as an outcome after controlling for the predictor; and lastly path c’ referred to the relationship between Group and Anxiety as an outcome after controlling for the mediator. The confidence interval for the indirect effect (ab) was a bootstrapped confidence interval based on 5,000 samples.
Results
Descriptive statistics.
Descriptive statistics for age, AB metrics, ERPs, and anxiety at Time 1 (baseline) are presented in Table 1. The ABM and PT groups did not significantly differ at baseline for all of the variables presented in Table 1 (p’s > .05). Groups also did not differ in terms of sex distribution (ABM: 32 females, 15 males; PT: 36 females, 16 males; χ2 = .015, p = .902) or regarding anxiety disorder diagnosis presence or absence (ABM: 24 anxiety disorder present, 23 absent; PT: 28 present, 24 absent; χ2 = .077, p = .782). Table 2 presents correlations among baseline measures. Correlations among the three indices of attention and processing of threat (mean AB scores, TLBS scores, and ERPs) tended to tightly cluster together, suggesting significant method variance. Moreover, behavioral indices of AB were rarely significantly correlated with ERPs (with the exception of small-magnitude correlations between the N170 and two TLBS measures). Interestingly, vigilance was negatively correlated with mean positive, peak difference, and variability TLBS scores, but positively correlated with mean negative AB. Finally, no measures of AB were significantly correlated with anxiety (HAM-A or DASS-A).
Table 1.
Descriptive Statistics for Age, AB metrics, ERPs, and Anxiety Symptoms at Baseline (T1).
| ABM (N = 47) | PT (N = 52) | |||
|---|---|---|---|---|
| Variable | M | SD | M | SD |
| Age | 24.20 | 5.31 | 26.16 | 7.07 |
| Threat Bias | 1.81 | 23.81 | .23 | 25.52 |
| Vigilance | .27 | 22.57 | −2.95 | 27.42 |
| Disengagement | −.46 | 25.17 | 2.43 | 25.54 |
| TLBS-Mean Pos | 95.58 | 38.43 | 100.60 | 39.02 |
| TLBS-Mean Neg | −91.89 | 35.89 | −102.45 | 45.41 |
| TLBS-Peak Diff | 686.53 | 311.36 | 689.52 | 314.77 |
| TLBS-Variability | 1.86 | .64 | 1.95 | .76 |
| P1 | 3.65 | 2.16 | 2.81 | 2.23 |
| N170 | −1.57 | 2.15 | −1.54 | 2.74 |
| N2 | −2.15 | 1.74 | −2.24 | 1.63 |
| P3 | 3.13 | 2.28 | 3.43 | 2.47 |
| DASS-Anxiety | 6.67 | 4.20 | 5.35 | 4.95 |
| HAM-A | 18.99 | 7.64 | 17.63 | 10.42 |
Note. AB = attention bias, ERPs = event-related potentials, T1 = Time 1, ABM = attention bias modification training, PT = placebo control training, TLBS = trial-level bias score, DASS = Depression Anxiety Stress Scales, HAM-A = Hamilton Anxiety Rating Scale, Mean Pos = Mean Positive, Mean Neg = Mean Negative, Peak Diff = Peak Positive minus Peak Negative.
Table 2.
Correlation Matrix for Measures at Baseline
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.Threat Bias | - | .44** | .48** | .23* | .24* | .016 | −.031 | .056 | .19 | .13 | −.096 | −.095 | .079 |
| 2. Vigilance | - | −.54** | −.31** | .44** | −.35** | −.37** | 15 | .096 | .12 | −.089 | −.050 | .10 | |
| 3. Disengage | - | .44** | −.12 | .25* | .23* | −.12 | .13 | −.056 | .011 | −.025 | −.065 | ||
| 4. Mean Pos | - | −.74** | .82** | .86** | −.10 | −.048 | .068 | −.026 | −.052 | .050 | |||
| 5. Mean Neg | - | −.77** | −.89** | .19 | .21* | −.051 | .044 | .060 | .078 | ||||
| 6. Peak Diff | - | .83** | −.072 | −.17 | .097 | −.007 | −.11 | −.015 | |||||
| 7. Variability | - | −.15 | −.20* | .13 | −.093 | −.031 | .027 | ||||||
| 8. P1 | - | .14 | −.18 | .38** | .13 | .20 | |||||||
| 9. N170 | - | −.085 | .15 | .098 | .048 | ||||||||
| 10. N2 | - | −.60** | .068 | .073 | |||||||||
| 11. P3 | - | .77 | .77 | ||||||||||
| 12. DASS-A | - | .74** | |||||||||||
| 13. HAM-A | - |
Note.
Correlation is significant at the .05 level (2-tailed)
Correlation is significant at the .01 level (2-tailed). Mean Pos = Mean Positive, Mean Neg = Mean Negative, Peak Diff = Peak Positive minus Peak Negative, DASS = Depression Anxiety Stress Scales, HAM-A = Hamilton Anxiety Rating Scale.
Split-half reliability of AB scores
Split-half reliability was examined for the dot probe task by creating mean RTs by experimental condition (neutral probes in TN trials, angry probes in TN trials, neutral probes in NN trials) and mean AB scores (threat bias, vigilance, and disengagement), separately for even and odd trial for every session of dot probe task. In addition, split-half reliability of trial-level bias scores (TLBS) was computed separately for the first half and second half of each session. This approach was used, rather than the even/odd approach, to preserve the continuity of trial presentation inherent to TLBS. To quantify reliability of these AB measures, Pearson correlations were conducted between even and odd trials used to generate mean RT and mean AB scores, and between the first and second half of trials for TLBS scores.
Although mean RTs for all four dot probes administered were highly significantly correlated between even and odd trials (rs > .81, all ps < .001), mean AB scores showed non-significant split-half reliability (all p’s > .08) with the exception of one mean AB score generated from the Time 1 dot probe (disengagement; r = .24, p = .008). The overall non-significant split-half reliability for mean AB measures is consistent with the previous literature (Kappenman et al., 2014; Rodebaugh et al., 2016; Schmukle, 2005). In contrast, split-half reliability for the four TLBS metrics (mean positive, mean negative, peak difference, and variability) were significant at Time 1 (rs > .28, all ps < .01), Time 5 (rs > .40, all ps < .0001), and Time 6 (rs > .32, all ps < .0001).
Reliable Change Index
A reliable change index (RCI) was calculated for HAM-A anxiety scores between Time 1 (baseline) and the three-month follow-up at Time 6 based on the guidelines from Jacobson and Truax (1991). RCI for each participant was calculated as the change in scores from pre to post intervention (ABM or PT) divided by the standard error of the difference calculated based on the standard deviation of the current sample.
Fifty out of 86 participants (58.2%) demonstrated a significant reliable decrease in HAM-A scores from Time 1 to Time 6. Of the 50 participants, 14 met criteria for clinical significance (i.e., post-treatment HAM-A scores ≤ 7). In the ABM group, 23 out of 41 participants (56.10%) demonstrated a significant reliable decrease in HAM-A scores from Time 1 to Time 6. Of the 23 participants, 4 met criteria for clinical significance (i.e., post-treatment HAM-A scores ≤ 7). In the control group, 27 out of 45 participants (60.0%) demonstrated a significant reliable decrease in HAM-A scores from Time 1 to Time 6. Of the 27 participants, 10 met criteria for clinical significance (i.e., post-treatment HAM-A scores ≤ 7).
Effects of ABM on Anxiety Symptom Severity
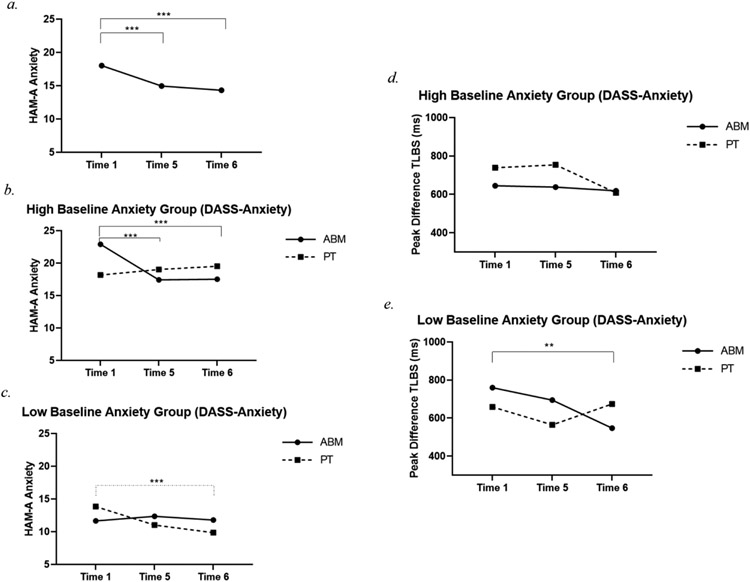
A significant main effect of Time [χ2 (2) = 26.38, p < .001; Figure 3] indicated that anxiety symptom severity (HAM-A) decreased from T1 to T5 (Mdiff = −3.06, SE = 0.84, p < .001) and T1 to T6 (Mdiff = −3.69, SE = 0.74, p < .001). A main effect of Anxiety [χ2 (1) = 34.72, p < .001] showed that, as would be expected, anxiety symptom severity was greater in the high vs low baseline anxiety group (DASS - Anxiety groups: Mdiff = 8.03, SE = 1.36, p < .001). Finally, a significant Time * Group * Anxiety interaction [χ2 (5) = 13.27, p = .021; Figure 3] showed that, among those highly anxious at baseline who received ABM, anxiety symptom severity decreased from T1 to T5 (Mdiff = −5.47, SE = 1.21, p < .001) and from T1 to T6 (Mdiff = −5.38, SE = 1.28, p < .001). Among those with low anxiety at baseline, anxiety symptom severity significantly decreased from T1 to T6 but only in the PT group (Mdiff = −3.99, SE = 1.12, p < .001).
Figure 3.
(a.) Anxiety symptom severity decreased over time in the sample as a whole. (b.) Anxiety decreased from T1 to T6, and from T5 to T6 among the high baseline anxiety group who received ABM. (c.) Among those with low baseline anxiety, anxiety decreased from T1 to T6 in the PT group. (d., e.) Peak difference TLBS significantly decreased over time, particularly for those starting with low baseline anxiety who received ABM.
Note. *Difference is significant at the .05 level, **Difference is significant at the .01 level. ***Difference is significant at the < .001 level. T1 = Time 1, T5 = Time 5, T6 = Time 6, ABM = attention bias modification training, PT = placebo control training, TLBS = trial-level bias score, DASS = Depression Anxiety Stress Scales, HAM-A = Hamilton Anxiety Rating Scale.
Effects of ABM on AB and ERP Indices
For mean bias scores, hypotheses were not supported. No significant main effects or interactions emerged (p’s > .09). However, significant changes in TLBS over time were detected. A main effect of Time [χ2 (2) = 9.35, p = .009], and subsequent pairwise comparisons, showed that TLBS variability significantly decreased from T1 to T6 (Mdiff = −.20, SE = .08, p = .011). Similarly, a main effect of Time [χ2 (2) = 17.87, p < .001] showed that mean positive TLBS significantly decreased from T1 to T6 (Mdiff = −15.32, SE = 4.45, p = .001) and T5 to T6 (Mdiff = −11.23, SE = 3.95, p = .004). No significant effects emerged for mean negative (p’s > .11) TLBS.
There was a significant main effect of Time [χ2 (2) = 6.37, p = .041] such that magnitude of peak difference TLBS (peak positive minus peak negative) significantly decreased from T1 to T6 (Mdiff = −88.59, SE = 36.71, p = .016). Further, a significant 3-way interaction [Time * Group * Anxiety: χ2 (3) = 9.85, p = .020; Figure 2] indicated that, among the ABM group, those who had low baseline anxiety showed a dampening of extreme positive and negative AB from T1 to T6 (Mdiff = −212.84, SE = 71.79, p = .003).
No significant changes in TLBS were detected for the PT group, nor for those starting with high baseline anxiety in either the ABM or PT group.
P1 to faces.
There were no significant interactions for Time * Group (p = .96), Time * Anxiety (p = .75), or Time * Group * Anxiety (p = .38). There was a marginal main effect of Group [χ2 (1) = 3.40, p = .065; Mdiff = 0.37, SE = 0.20, p = .065] indicating greater P1 in the ABM versus PT group, at the level of a trend.
N170 to faces.
Among the ABM group, N170 to threat decreased (became significantly less negative) from T1 to T6 [Time * Group: χ2 (2) = 6.47, p = .039; Mdiff = 0.58, SE = 0.22, p = .008; Figure 2], while there was no significant change over time for the PT group. However, the three-way interaction (Time * Group * Anxiety) was not significant (p = .94).
N2 to faces.
A significant Time * Anxiety interaction [χ2 (2) = 10.94, p = .004; Figure 2] and main effect of Time [χ2 (2) = 11.06, p = .004; Figure 2] indicated that N2 significantly increased (became more negative) from T1 to T5 (Mdiff = −0.43, SE = 0.15, p = .003) and T1 to T6 (Mdiff = −0.40, SE = 0.16, p = .013)], and this change was driven by those with high baseline anxiety [T1 to T5: (Mdiff = −0.83, SE = 0.21, p < .001); T1 to T6: (Mdiff = −0.81, SE = 0.21, p < .001)]. The three-way interaction was not significant (p = .34).
P3 to faces.
A Time * Group interaction [χ2 (2) = 6.80, p = .033; Figure 2] showed those who received ABM showed a significant increase in P3 to threat from T1 to T5 (Mdiff = 0.64, SE = 0.22, p = .004) and from T1 to T6 (Mdiff = 0.65, SE = 0.24, p = .008). There was no significant change over time for the PT group. The three-way interaction was not significant (p = .81).
Associations between Treatment Effects and Anxiety Symptom Severity
We next explored whether changes in continuous variables impacted by ABM as indicated by GEE analyses (i.e., AB and ERP metrics of threat processing), mediated ABM’s impact on symptom severity. First, we conducted correlations between the change in anxiety symptom severity (HAM-A) and in those DVs that were significantly predicted in the GEE analyses - ABs (variability and peak difference) and ERPs (N170, N2, and P3) from T1-T5 and T1-T6. Change in P3 from T1 to T5 was significantly correlated with reduction in anxiety symptom severity, r(78) = −.23, p = .044, such that as P3 increased in magnitude post-ABM, anxiety decreased. There was also a significant correlation between change in N170 from T1-T5 and reduction in anxiety symptom severity by T6, r(78) = −.23, p = .039, such that as N170 decreased in magnitude post-ABM, anxiety also decreased. Changes from pretreatment to post-treatment in attention bias from T1 to T5 or T1 to T6 did not correlate with symptoms change, all ps > .05.
Based on the GEE analysis, variables for mediation analysis were chosen to test whether the change in the ERPs mediated the association between Group and Anxiety, while considering the role of baseline anxiety severity. Across the models, the path coefficient (b) of the total effects of X on Y ranged from −1.71 to 2.00, the path coefficient (b) of the direct effects of X on Y ranged from −1.77 to 2.09, and the path coefficient (b) of the indirect effects of X on Y ranged from −.38 to .072. None of these mediation analyses reached significance (ps > .05).2
Discussion
Research on mechanisms underlying psychological treatments has steadily shifted away from a one-size fits all approach, where a specific therapy targets a specific disease, towards process-based approaches focusing on theory-driven moderators and mediators of treatment effects, with an emphasis on individual differences in treatment fit and responsiveness (Hofmann and Hayes, 2019). The current RCT of ABM adds to this body of research, with a particular focus on two methodological and conceptual considerations: (1) we examined individual differences in treatment fit by testing whether anxiety severity prior to treatment onset moderated treatment effects, and (2) we included ERP-indices of AB given their superior reliability and sensitivity as metrics of treatment-related affective-cognitive processes.
We found that ABM, relative to PT, was associated with reduced anxiety symptoms, but only among those reporting higher baseline anxiety severity. Those receiving ABM with low baseline anxiety severity instead showed a dampening of extreme positive and negative AB, as measured by trial-level bias scores. Regardless of baseline anxiety severity, ABM was further associated with reduced N170 and increased P3 at post-intervention. Yet, ABM and PT also yielded similar intervention effects: regardless of group, AB variability dampened over time, and the magnitude of the N2 increased, suggesting constraining of neurocognitive processing of threat and modulation of cognitive control recruitment, respectively. Moreover, modulation of the P3 was associated with ABM-related changes in anxiety: Increased P3 over the course of intervention was correlated with reduced anxiety symptoms, with a trend showing that this effect was significant only among those receiving ABM. Changes in behavioral and ERP metrics failed to mediate treatment effects on anxiety severity. Taken together, results shed light on individual differences in response to ABM, highlight the importance of identifying treatment-relevant biobehavioral signatures of changes in threat processing induced by ABM, and suggest the need to reconsider the impact and significance of ABM “placebo” training methods.
Prior research has similarly demonstrated that ABM may be most effective for those with higher symptom severity prior to intervention in anxiety (e.g., Bo et al., 2021), and similarly in depression (e.g., Baert et al., 2010). This pattern can be interpreted in several ways. First, it may indicate floor effects – it is more difficult to reduce anxiety severity when participants start with lower severity levels. Yet, another possibility is that ABM does not engage and effectively retrain AB unless anxiety severity is significantly elevated because only clinically meaningful anxiety is thought to be correlated with significant disruptions in threat detection and processing underlying AB. In this study, there were no significant correlations between anxiety severity and any metrics of AB (behavioral or ERP) at baseline, suggesting that there is not a linear association between greater anxiety and greater attention bias towards threat. Indeed, in a study of child ABM, participants who were diagnosed with anxiety disorders were pre-selected only if they showed an RT facilitation to threat faces in the dot probe task of at least 8 ms (Eldar et al., 2012). Half of the participants did not show even these subtle signs of AB, suggesting that there is not a one-to-one correspondence between anxiety severity and AB towards threat measured via RT-based metrics.
This highlights an emerging issue in research on AB – anxious individuals appear to evidence both a bias towards and away from threat, and these associations may differ depending on anxiety symptom type – distress- versus fear-related (Dennis-Tiwary et al., 2019; Waters et al., 2014). From this perspective, it will be crucial for future research to test whether directionality in baseline AB, and associations with anxiety subtypes, impact the efficacy of ABM. Indeed, one study found that anxious individuals with a greater bias towards threat respond more robustly to ABM (Kuckertz et al., 2014). This may in part explain the past decade of mixed and null ABM effects, dampening early enthusiasm for this novel therapeutic approach (e.g., Emmelkamp, 2012). Given growing awareness of the unreliability of AB metrics based on RTs, and given the variety of ERP metrics potentially implicated in ABM (Carlson, 2021), a fruitful next step will be to classify anxious individuals based on whether they evidence disruptions or adaptive functioning of target ERP metrics of threat processing that suggest increased attention capture versus controlled avoidance of threat (Dennis-Tiwary et al., 2017). In addition, we focused on the DASS-21 anxiety subscale as the indicator of baseline anxiety symptom severity, which emphasizes the fear-related symptom types, while the DASS-21 stress subscale also evidenced a considerable baseline variance across high and low anxiety groups (low: Mstress = 6.57, SDstress = 3.79; high: Mstress = 13.06, SDstress = 4.23), which may more directly capture distress-related symptomatology. Future work should examine whether these variations in anxiety and stress symptoms at baseline, independently or together, predict responses to ABM.
The current study also documented that ABM modulated only the TLBS metrics of AB. However, the pattern of this change was unexpected, with ABM resulting in a dampened magnitude of the difference between positive and negative TLBS, but only among those who had low anxiety levels pre-intervention. It is generally assumed that individuals evidencing higher anxiety severity will show more extreme AB scores. But, given that anxiety severity and AB metrics were not significantly correlated in this study, it is possible that individuals less debilitated by anxiety evidence lower threat reactivity and are therefore more responsive to the dampening effect of ABM on extreme positive and negative AB (Zvielli et al., 2015; Egan and Dennis, 2018).
ABM also modulated ERP indices regardless of anxiety severity. In the ABM group only, post-intervention N170 decreased (became less negative) and P3 increased. The amplitudes of ERPs typically decrease with repeated stimulus exposure (Kotchoubey et al. 1997; Segalowitz et al. 2001; Carretie et al. 2003), suggesting that ABM facilitates this dampening of relatively early stimulus detection among anxious individuals, who might be less likely to habituate over the course of exposures. The P3 finding further suggests that ABM also might strengthen recruitment of relatively later, more deliberative attention selection and control resources, which are thought to be disrupted in those with elevated anxiety. These AB-related processes are important to consider, particularly, as documented in the present study, behavioral and ERP metrics are rarely significantly inter-correlated and thus likely capture distinct processes underlying AB. Taken together, these ERP findings show that a four-week course of ABM stimulates significant neural and behavioral plasticity in how individuals attend to, detect, and process threat (Shoji and Skrandies, 2006; Tanaka and Pierce, 2009). Future investigations of mechanistic targets of ABM should build on these ERP findings, as well as examine other metrics of neural functioning relevant to cognitive functioning (e.g., neural oscillations, coherence) to better understand how and for whom ABM may be beneficial.
ABM and PT also showed comparable clinical effects: regardless of intervention group, AB variability dampened over time, and the magnitude of the N2 increased from pre to post intervention, suggesting constraining of extreme AB and modulation of cognitive control recruitment. The increase in N2 magnitude may reflect general amplifying of N2 during tasks in which there is repeated stimulus presentation (Kotchoubey et al. 1997). But it is also important to consider that the placebo performed as well – or even, as suggested by the RIC analyses slightly better – than the ABM condition. Indeed, the term PT is somewhat misleading because 50% of trials administered during PT are active training trials, in which, like ABM, attention is systematically directed away from threat (leading others to term it attention control training, e.g., Pettit et al., 2020). That not only makes this conventional PT condition extremely stringent, but may reveal individual differences in the number of training trials needed to affect clinical change. Indeed, some research suggests that excessive training trials might lead to a plateau of benefits, or even decrements in clinical efficacy (Price et al., 2017). Future research should include a greater number of “dosage” studies, modeled after pharmaceutical trials, to understand the optimal number of training trials and how that number might vary across individuals, improving the potential for personalization. Considering additional comparison conditions, such as forms of attention control training, will also be helpful in pursuit of optimizing treatment fit (e.g., Linetzky et al., 2020).
Findings further suggest that ABM-inducted changes in the P3 was associated with ABM-related changes in anxiety: increased P3 over the course of the clinical trial was associated with reduced anxiety symptoms, with a trend showing that this effect was significant only among those receiving ABM. While this suggests that bolstering effortful, cognitive control processes during the detection and processing of threat measuring during the dot probe task may be a key process underlying beneficial effects of ABM on anxiety symptoms, mediation analyses did not yield significant findings. This indicates that neurocognitive changes indexed by the P3 may not be necessary for ABM to be efficacious. Yet, because the present study was somewhat underpowered for mediation analyses, future research should continue to directly test the causal impact of strengthened recruitment of cognitive control, versus dampening of initial attentional vigilance or avoidance (Dennis-Tiwary et al., 2019; Heeren and McNally, 2016).
Several limitations to the present study should be noted. AB was only measured during the dot probe task, a cognitive assay that has received extensive critique surrounding its suitability for measuring multiple components of threat-related processing disruptions, its reliability, and its psychometric appropriateness (MacLeod et al., 2019; Price et al., 2018; Schmukle, 2005; Staugaard, 2009). We mitigated this psychometric limitation by using trial-level metrics of AB, which in this study, as in others, show adequate split-half reliability. Notably, our findings that mean AB metrics showed poor split-half reliability and subsequently yielded null results highlights the need to move beyond these traditional mean measures of quantifying behavioral AB.
Another limitation of the dot probe relevant to the present study is that it may selectively reflect the rapid and automatic allocation of spatial attention to emotionally salient stimuli and be less suitable for measured a range of later, more deliberative cognitive control processes. The inclusion of additional and multiple AB assays, including eye tracking and tasks assessing the generalizability of training effects, is a crucial direction for future research (Carlson, 2021). Another goal of future research, noted above, is more careful consideration of heterogeneity of AB (Dennis-Tiwary et al., 2019; Waters et al., 2014).
Taken together, the present study adds to the growing body of research on evidence-based, cognitive treatment approaches with an emphasis on process-oriented analyses and consideration of treatment-relevant individual differences, thus strengthening our ability to develop and refine highly accessible and personalized therapies for clinically elevated anxiety.
Manuscript Highlights.
This RCT aims to reduce anxiety symptom severity via a 4-week attention bias modification (ABM) vs placebo control training.
We examined treatment effects on attention bias (AB) via ERPs and the moderating role of baseline anxiety severity.
ABM reduced symptom severity among those with higher anxiety and modulated ERP indices of AB.
Acknowledgements
The research was made possible by the following funding source granted to Tracy Dennis-Tiwary: National Institute of Health (5SC1MH104907).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Authors have no conflict of interest.
For the main analysis (GEE), we used a dataset that includes those who underwent intervention (N = 99) as GEE method accounts for missing data. Additional analyses (e.g., regression analyses) were carried out with those who attended all visits and completed EEG at Time 6, n = 81.
We also conducted a version of these analyses with age as a covariate as there was a significant difference between age across ABM and PT groups. The non-significant pattern of results remained unchanged.
References
- Amir N, Beard C, Burns M, & Bomyea J (2009). Attention modification program in individuals with generalized anxiety disorder. J. Abnorm. Psychol, 118(1), 28–33. 10.1037/a0012589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Beard C, Taylor CT, Klumpp H, Elias J, Burns M, & Chen X (2009). Attention training in individuals with generalized social phobia: A randomized controlled trial. J. Consult. Clin. Psychol, 77(5), 961–973. 10.1037/a0016685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejewski JA, & Carlson JM (2020). Electrocortical responses associated with attention bias to fearful facial expressions and auditory distress signals. Int. J. Psychophysiol, 151, 94–102. 10.1016/j.ijpsycho.2020.02.014 [DOI] [PubMed] [Google Scholar]
- Banaschewski T, & Brandeis D (2007). Annotation: what electrical brain activity tells us about brain function that other techniques cannot tell us - a child psychiatric perspective. J. Child. Psychol. Psychiatry, 48(5), 415–435. 10.1111/j.1469-7610.2006.01681.x [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, & Glickman S (2005). Attentional bias in anxiety: a behavioral and ERP study. Brain. Cogn, 59(1), 11–22. 10.1016/j.bandc.2005.03.005 [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, & van IJzendoorn MH (2007). Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol. Bull, 133(1), 1–24. 10.1037/0033-2909.133.1.1 [DOI] [PubMed] [Google Scholar]
- Baert S, De Raedt R, Schacht R, & Koster EH (2010). Attentional bias training in depression: therapeutic effects depend on depression severity. J. Behav. Ther. Exp. Psychiatr, 41(3), 265–274. 10.1016/j.jbtep.2010.02.004 [DOI] [PubMed] [Google Scholar]
- Beard C, Sawyer AT, & Hofmann SG (2012). Efficacy of attention bias modification using threat and appetitive stimuli: a meta-analytic review. Behav. Ther, 43(4), 724–740. 10.1016/j.beth.2012.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, Hsu KJ, Schnyer DM, Smits J, & Shumake J (2021). Change in negative attention bias mediates the association between attention bias modification training and depression symptom improvement. J. Consult. Clin. Psychol, 89(10), 816–829. 10.1037/ccp0000683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, & McCarthy G (1996). Electrophysiological studies of face perception in humans. J. Cogn. Neurosci, 8(6), 551–565. 10.1162/jocn.1996.8.6.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo R, Kraft B, Jonassen R, Harmer CJ, Hilland E, Stiles TC, et al. (2021). Symptom severity moderates the outcome of attention bias modification for depression: An exploratory study. J. Psychiatr. Res, 138, 528–534. [DOI] [PubMed] [Google Scholar]
- Britton WB, Lepp NE, Niles HF, Rocha T, Fisher NE, & Gold JS (2014). A randomized controlled pilot trial of classroom-based mindfulness meditation compared to an active control condition in sixth-grade children. J. Sch. Psychol, 52(3), 263–278. 10.1016/j.jsp.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM (2021). A Systematic Review of Event-Related Potentials as Outcome Measure of Attention Bias Modification. Psychophysiology, 56(6). [DOI] [PubMed] [Google Scholar]
- Carretié L, Hinojosa JA, Martín-Loeches M, Mercado F, & Tapia M (2004). Automatic attention to emotional stimuli: neural correlates. Hum. Brain. Mapp, 22(4), 290–299. 10.1002/hbm.20037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié L, Hinojosa JA, & Mercado F (2003). Cerebral patterns of attentional habituation to emotional visual stimuli. Psychophysiology, 40(3), 381–388. 10.1111/1469-8986.00041 [DOI] [PubMed] [Google Scholar]
- Carretié L, Mercado F, Hinojosa JA, Martín-Loeches M, & Sotillo M (2004). Valence-related vigilance biases in anxiety studied through event-related potentials. J. Affect. Disord, 78(2), 119–130. 10.1016/s0165-0327(02)00242-2 [DOI] [PubMed] [Google Scholar]
- Carretié L, Tapia M, Mercado F, Albert J, López-Martin S, & de la Serna JM (2004). Voltage-based versus factor score-based source localization analyses of electrophysiological brain activity: a comparison. Brain Topogr., 17(2), 109–115. 10.1007/s10548-004-1008-1 [DOI] [PubMed] [Google Scholar]
- Chen YP, Ehlers A, Clark DM, & Mansell W (2002). Patients with generalized social phobia direct their attention away from faces. Behav. Res. Ther, 40(6), 677–687. 10.1016/s0005-7967(01)00086-9 [DOI] [PubMed] [Google Scholar]
- Cisler JM, & Koster EH (2010). Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clin. Psychol. Rev, 30(2), 203–216. 10.1016/j.cpr.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PJ, Browning M, Hammond G, Notebaert L, & MacLeod C (2014). The causal role of the dorsolateral prefrontal cortex in the modification of attentional bias: evidence from transcranial direct current stimulation. Biol. Psychiatry, 76(12), 946–952. 10.1016/j.biopsych.2014.03.003 [DOI] [PubMed] [Google Scholar]
- Clerkin EM, Magee JC, Wells TT, Beard C, and Barnett NP (2016). Randomized controlled trial of attention bias modification in a racially diverse, socially anxious, alcohol dependent sample. Behav. Res. Ther, 87, 58–69. 10.1016/j.brat.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denefrio S, Myruski S, Mennin D, & Dennis-Tiwary TA (2019). When neutral is not neutral: Neurophysiological evidence for reduced discrimination between aversive and non-aversive information in Generalized Anxiety Disorder, Motiv. Emot, 43(2), 325–338. 10.1007/s11031-018-9732-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis-Tiwary TA, Denefrio S, & Gelber S (2017). Salutary effects of an attention bias modification mobile application on biobehavioral measures of stress and anxiety during pregnancy. Biol. Psychol, 127, 148–156. 10.1016/j.biopsycho.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis-Tiwary TA, Egan LJ, Babkirk S, & Denefrio S (2016). For whom the bell tolls: Neurocognitive individual differences in the acute stress-reduction effects of an attention bias modification game for anxiety. Behav. Res. Ther, 77, 105–117. 10.1016/j.brat.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, & O'Toole L (2014). Mental Health on the Go: Effects of a gamified attention bias modification mobile application in trait anxious adults. Clin. Psychol. Sci, 2(5), 576–590. 10.1177/2167702614522228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis-Tiwary TA, Roy AK, Denefrio S, & Myruski S (2019). Heterogeneity of the Anxiety-related attention bias: A review and working model for future research. Clin. Psychol. Sci, 7(5), 879–899. 10.1177/2167702619838474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan LJ, Dennis-Tiwary TA (2018). Dynamic measures of anxiety-related threat bias: Links to stress reactivity. Motiv Emot 42, 546–554. 10.1007/s11031-018-9674-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar S, & Bar-Haim Y (2010). Neural plasticity in response to attention training in anxiety. Psychol. Med, 40(4), 667–677. 10.1017/S0033291709990766 [DOI] [PubMed] [Google Scholar]
- Eldar S, Apter A, Lotan D, Edgar KP, Naim R, Fox NA, Pine DS, and Bar-Haim Y (2012). Attention bias modification treatment for pediatric anxiety disorders: a randomized controlled trial. Am. J. Psychiatry, 169(2), 213–220. 10.1176/appi.ajp.2011.11060886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmelkamp PM (2012). Attention bias modification: the Emperor's new suit? BMC Med., 10, 63. 10.1186/1741-7015-10-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enock PM, Hofmann SG, & McNally RJ (2014). Attention bias modification training via smartphone to reduce social anxiety: A randomized, controlled multi-session experiment. Cognit. Ther. Res, 38(2), 200–216. 10.1007/s10608-014-9606-z [DOI] [Google Scholar]
- Fichtenholtz HM, Hopfinger JB, Graham R, Detwiler JM, & LaBar KS (2007). Happy and fearful emotion in cues and targets modulate event-related potential indices of gaze-directed attentional orienting. Soc. Cogn. Affect. Neurosci, 2(4), 323–333. 10.1093/scan/nsm026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald A, Rawdon C, & Dooley B (2016). A randomized controlled trial of attention bias modification training for socially anxious adolescents. Behav. Res. Ther, 84, 1–8. 10.1016/j.brat.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol, 55(4), 468–484. 10.1016/0013-4694(83)90135-9 [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, Ernst M, & Pine DS (2010). Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biol. Psychiatry, 68(11), 982–990. 10.1016/j.biopsych.2010.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallion LS, & Ruscio AM (2011). A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychol. Bull, 137(6), 940–958. 10.1037/a0024355 [DOI] [PubMed] [Google Scholar]
- Hamilton M (1959). The assessment of anxiety states by rating. Br. J. Med. Psychol, 32(1), 50–55. 10.1111/j.2044-8341.1959.tb00467.x [DOI] [PubMed] [Google Scholar]
- Hayes AF (2017). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford publications. [Google Scholar]
- Hayes AF, & Scharkow M (2013). The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: does method really matter? Psychol. Sci, 24(10), 1918–1927. 10.1177/0956797613480187 [DOI] [PubMed] [Google Scholar]
- Heeren A, and McNally RJ (2016). An integrative network approach to social anxiety disorder: The complex dynamic interplay among attentional bias for threat, attentional control, and symptoms. J. Anxiety. Disord, 42, 95–104. 10.1016/j.janxdis.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Heeren A, Mogoaşe C, Philippot P, and McNally RJ (2015). Attention bias modification for social anxiety: a systematic review and meta-analysis. Clin. Psychol. Rev, 40, 76–90. 10.1016/j.cpr.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Henry JD, & Crawford JR (2005). The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol, 44(Pt 2), 227–239. 10.1348/014466505X29657 [DOI] [PubMed] [Google Scholar]
- Hillyard SA, and Anllo-Vento L (1998). Event-related brain potentials in the study of visual selective attention. Proc. Natl. Acad. Sci. U.S.A, 95(3), 781–787. 10.1073/pnas.95.3.781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa JA, Mercado F, and Carretié L (2015). N170 sensitivity to facial expression: A meta-analysis. Neurosci. Biobehav. Rev, 55, 498–509. 10.1016/j.neubiorev.2015.06.002 [DOI] [PubMed] [Google Scholar]
- Hofmann SG, and Hayes SC (2019). The future of intervention science: Process-based therapy. Clin. Psychol. Sci, 7(1), 37–50. 10.1177/2167702618772296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert JD, Kivity Y, Cohen L, Strauss AY, Elizur Y, and Weiss M (2018). A pilot randomized clinical trial of cognitive behavioral therapy versus attentional bias modification for social anxiety disorder: An examination of outcomes and theory-based mechanisms. J. Anxiety. Disord, 59, 1–9. 10.1016/j.janxdis.2018.08.002 [DOI] [PubMed] [Google Scholar]
- Jacobson NS, and Truax P (1991). Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J. Consult. Clin. Psychol, 59(1), 12–19. 10.1037//0022-006x.59.1.12 [DOI] [PubMed] [Google Scholar]
- Kappenman ES, Farrens JL, Luck SJ, and Proudfit GH (2014). Behavioral and ERP measures of attentional bias to threat in the dot-probe task: poor reliability and lack of correlation with anxiety. Front. Psychol, 5, 1368. 10.3389/fpsyg.2014.01368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappenman ES, MacNamara A, and Proudfit GH (2015). Electrocortical evidence for rapid allocation of attention to threat in the dot-probe task. Soc. Cogn. Affect. Neurosci, 10(4), 577–583. 10.1093/scan/nsu098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Phillips AG, and Insel TR (2012). Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol. Psychiatry, 17(12), 1174–1179. 10.1038/mp.2012.105 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, and Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry, 62(6), 593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, and Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry, 62(6), 617–627. 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, and Amir N (2010). Preliminary study of attention training to threat and neutral faces on anxious reactivity to a social stressor in social anxiety. Cognit. Ther. Res, 34(3), 263–271. 10.1007/s10608-009-9251-0 [DOI] [Google Scholar]
- Kotchoubey B, Schneider D, Uhlmann C, Schleichert H, and Birbaumer N (1997). Beyond habituation: long-term repetition effects on visual event-related potentials in epileptic patients. Electroencephalogr. Clin. Neurophysiol, 103(4), 450–456. 10.1016/s0013-4694(97)00026-6 [DOI] [PubMed] [Google Scholar]
- Kruijt AW, Field AP, and Fox E (2016). Capturing dynamics of biased attention: Are new attention variability measures the way forward? PloS One, 11(11), e0166600. 10.1371/journal.pone.0166600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuckertz JM, Gildebrant E, Liliequist B, Karlström P, Väppling C, Bodlund O, Stenlund T, Hofmann SG, Andersson G, Amir N, and Carlbring P (2014). Moderation and mediation of the effect of attention training in social anxiety disorder. Behav. Res. Ther, 53, 30–40. 10.1016/j.brat.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, and Carter CS (2007). Development of action monitoring through adolescence into adulthood: ERP and source localization. Dev. Sci, 10(6), 874–891. 10.1111/j.1467-7687.2007.00639.x [DOI] [PubMed] [Google Scholar]
- Linetzky M, Kahn M, Lazarov A, Pine DS, and Bar-Haim Y (2020). Gaze-contingent music reward therapy for clinically anxious 7- to 10-Year-olds: An open multiple baseline feasibility study. J. Clin. Child Adolesc. Psychol, 49(5), 618–625. 10.1080/15374416.2019.1573685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond PF, and Lovibond SH (1995). The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther, 33(3), 335–343. 10.1016/0005-7967(94)00075-u [DOI] [PubMed] [Google Scholar]
- Luck SJ, Heinze HJ, Mangun GR, and Hillyard SA (1990). Visual event-related potentials index focused attention within bilateral stimulus arrays. II. Functional dissociation of P1 and N1 components. Electroencephalogr. Clin. Neurophysiol, 75(6), 528–542. 10.1016/0013-4694(90)90139-b [DOI] [PubMed] [Google Scholar]
- Luehring-Jones P, Louis C, Dennis-Tiwary TA, and Erblich J (2017). A Single session of attentional bias modification reduces alcohol craving and implicit measures of alcohol bias in young adult drinkers. Alcohol. Clin. Exp. Res, 41(12), 2207–2216. 10.1111/acer.13520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C, Grafton B, and Notebaert L (2019). Anxiety-Linked Attentional Bias: Is It Reliable? Annu. Rev. Clin. Psychol, 15, 529–554. 10.1146/annurev-clinpsy-050718-095505 [DOI] [PubMed] [Google Scholar]
- MacLeod C, and Mathews A (1988). Anxiety and the allocation of attention to threat. Q. J. Exp. Psychol. A, 40(4-A), 653–670. 10.1080/14640748808402292 [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, and Tata P (1986). Attentional bias in emotional disorders. J. Abnorm. Psychol, 95(1), 15–20. 10.1037/0021-843X.95.1.15 [DOI] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, and Holker L (2002). Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. J. Abnorm. Psychol, 111(1), 107–123. [PubMed] [Google Scholar]
- Maier W, Buller R, Philipp M, and Heuser I (1988). The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J. Affect. Disord, 14(1), 61–68. 10.1016/0165-0327(88)90072-9 [DOI] [PubMed] [Google Scholar]
- Mansell W, Clark DM, Ehlers A, and Chen Y-P (1999). Social anxiety and attention away from emotional faces. Cogn. Emot, 13(6), 673–690. 10.1080/026999399379032 [DOI] [Google Scholar]
- Matsumoto D, and Ekman P (1989). American-Japanese cultural differences in intensity ratings of facial expressions of emotion. Motiv. Emot, 13(2), 143–157. 10.1007/BF00992959 [DOI] [Google Scholar]
- McNally RJ, Enock PM, Tsai C, and Tousian M (2013). Attention bias modification for reducing speech anxiety. Behav. Res. Ther, 51(12), 882–888. 10.1016/j.brat.2013.10.001 [DOI] [PubMed] [Google Scholar]
- Mogoaşe C, David D, and Koster EH (2014). Clinical efficacy of attentional bias modification procedures: an updated meta-analysis. J. Clin. Psychol, 70(12), 1133–1157. 10.1002/jclp.22081 [DOI] [PubMed] [Google Scholar]
- Morales S, Pérez-Edgar KE, and Buss KA (2015). Attention biases towards and away from threat mark the relation between early dysregulated fear and the later emergence of social withdrawal. J. Abnorm. Child. Psychol, 43(6), 1067–1078. 10.1007/s10802-014-9963-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim R, Abend R, Wald I, Eldar S, Levi O, Fruchter E, Ginat K, Halpern P, Sipos ML, Adler AB, Bliese PD, Quartana PJ, Pine DS, and Bar-Haim Y (2015). Threat-Related Attention Bias Variability and Posttraumatic Stress. Am. J. Psychiatry, 172(12), 1242–1250. 10.1176/appi.ajp.2015.14121579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole L, and Dennis TA (2012). Attention training and the threat bias: an ERP study. Brain Cogn., 78(1), 63–73. 10.1016/j.bandc.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olatunji BO, Cisler JM, and Tolin DF (2007). Quality of life in the anxiety disorders: a meta-analytic review. Clin. Psychol. Rev, 27(5), 572–581. 10.1016/j.cpr.2007.01.015 [DOI] [PubMed] [Google Scholar]
- Ollendick TH, White SW, Richey J, Kim-Spoon J, Ryan SM, Wieckowski AT, Coffman MC, Elias R, Strege MV, Capriola-Hall NN, and Smith M (2019). Attention bias modification treatment for adolescents with social anxiety disorder. Behav. Ther, 50(1), 126–139. 10.1016/j.beth.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan DN, Wang Y, Lei Z, Wang Y, and Li X (2019). The altered early components and the decisive later process underlying attention bias modification in social anxiety: evidence from event-related potentials. Soc. Cogn. Affect. Neurosci, 14(12), 1307–1316. 10.1093/scan/nsz098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergamin-Hight L, Pine DS, Fox NA, and Bar-Haim Y (2016). Attention bias modification for youth with social anxiety disorder. J. Child. Psychol. Psychiatry, 57(11), 1317–1325. 10.1111/jcpp.12599 [DOI] [PubMed] [Google Scholar]
- Pettit NN, MacKenzie EL, Ridgway JP, Pursell K, Ash D, Patel B, and Pho MT (2020). Obesity is associated with increased risk for mortality among hospitalized patients with COVID-19. Obesity (Silver Spring), 28(10), 1806–1810. 10.1002/oby.22941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J (2007). Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol, 118(10), 2128–2148. 10.1016/j.clinph.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Kuckertz JM, Amir N, Bar-Haim Y, Carlbring P, and Wallace ML (2017). Less is more: Patient-level meta-analysis reveals paradoxical dose-response effects of a computer-based social anxiety intervention targeting attentional bias. Depress. Anxiety, 34(12), 1106–1115. 10.1002/da.22634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Brown V, and Siegle GJ (2019). Computational modeling applied to the dot-probe task yields improved reliability and mechanistic insights. Biol. Psychiatry, 85(7), 606–612. 10.1016/j.biopsych.2018.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutter M, Hewig J, Wieser MJ, and Osinsky R (2017). The N2pc component reliably captures attentional bias in social anxiety. Psychophysiology, 54(4), 519–527. 10.1111/psyp.12809 [DOI] [PubMed] [Google Scholar]
- Rodebaugh TL, Scullin RB, Langer JK, Dixon DJ, Huppert JD, Bernstein A, Zvielli A, and Lenze EJ (2016). Unreliability as a threat to understanding psychopathology: The cautionary tale of attentional bias. J. Abnorm. Psychol, 125(6), 840–851. 10.1037/abn0000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosa D (1980). A critique of cross-lagged correlation. Psychol. Bull, 88(2), 245–258. 10.1037/0033-2909.88.2.245 [DOI] [Google Scholar]
- Schmukle SC (2005). Unreliability of the dot probe task. Eur. J. Pers,19(7), 595–605. 10.1002/per.554 [DOI] [Google Scholar]
- Schneider W, Eschman A, and Zuccolotto A (2002). E-Prime Reference Guide. Pittsburg, PA: Psychology Software Tools. [Google Scholar]
- Segalowitz SJ, Wintink AJ, and Cudmore LJ (2001). P3 topographical change with task familiarization and task complexity. Cogn. Brain Res, 12(3), 451–457. 10.1016/s0926-6410(01)00082-9 [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, and Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry, 59 Suppl 20, 22–57. [PubMed] [Google Scholar]
- Shoji H, and Skrandies W (2006). ERP topography and human perceptual learning in the peripheral visual field. Int. J. Psychophysiol, 61(2), 179–187. 10.1016/j.ijpsycho.2005.09.007 [DOI] [PubMed] [Google Scholar]
- Sinclair SJ, Siefert CJ, Slavin-Mulford JM, Stein MB, Renna M, and Blais MA (2012). Psychometric evaluation and normative data for the depression, anxiety, and stress scales-21 (DASS-21) in a nonclinical sample of US adults. Eval. Health. Prof, 35(3), 259–279. [DOI] [PubMed] [Google Scholar]
- Smith NK, Cacioppo JT, Larsen JT, and Chartrand TL (2003). May I have your attention, please: electrocortical responses to positive and negative stimuli. Neuropsychologia, 41(2), 171–183. 10.1016/s0028-3932(02)00147-1 [DOI] [PubMed] [Google Scholar]
- Staugaard SR (2009). Reliability of two versions of the dot-probe task using photographic faces. Psychol. Sci. Q, 51(3), 339–350 [Google Scholar]
- Stein MB, Roy-Byrne PP, Craske MG, Bystritsky A, Sullivan G Pyne JM, Katon W, Sherbourne CD (2005) Functional impact and health utility of anxiety disorders in primary care outpatients. Med. Care, 43(12), 1164–1170. doi: 10.1097/01.mlr.0000185750.18119.fd [DOI] [PubMed] [Google Scholar]
- Suway JG, White LK, Vanderwert RE, Bar-Haim Y, Pine DS, and Fox NA (2013). Modification of threat-processing in non-anxious individuals: a preliminary behavioral and ERP study. J. Behav. Ther. Exp. Psychiatry, 44(3), 285–292. 10.1016/j.jbtep.2012.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvain R, Gilbertson H, and Carlson JM (2020). Single session positive attention bias modification training enhances reward-related electrocortical responses in females. Int. J. Psychophysiol, 156, 10–17. 10.1016/j.ijpsycho.2020.07.002 [DOI] [PubMed] [Google Scholar]
- Tanaka JW, and Pierce LJ (2009). The neural plasticity of other-race face recognition. Cogn. Affect. Behav. Neurosci, 9(1), 122–131. 10.3758/CABN.9.1.122 [DOI] [PubMed] [Google Scholar]
- Torrence RD, and Troup LJ (2018). Event-related potentials of attentional bias toward faces in the dot-probe task: A systematic review. Psychophysiology, 55(6), e13051. 10.1111/psyp.13051 [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, and Nelson C (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res., 168(3), 242–249. 10.1016/j.psychres.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter S, Nelson AL, Wright C, Hyatt A, and Oakman J (2014). Measuring attentional bias to threat: Reliability of dot probe and eye movement indices. Cognit. Ther. Res, 38(3), 313–333. 10.1007/s10608-013-9588-2 [DOI] [Google Scholar]
- Waters AM, Bradley BP, and Mogg K (2014). Biased attention to threat in pediatric anxiety disorders (generalized anxiety disorder, social phobia, specific phobia, separation anxiety disorder) as a function of 'distress' versus 'fear' diagnostic categorization. Psychol. Med, 44(3), 607–616. 10.1017/S0033291713000779 [DOI] [PubMed] [Google Scholar]
- Westen D, Novotny CM, and Thompson-Brenner H (2004). The empirical status of empirically supported psychotherapies: assumptions, findings, and reporting in controlled clinical trials. Psychol. Bull, 130(4), 631–663. 10.1037/0033-2909.130.4.631 [DOI] [PubMed] [Google Scholar]
- Wieser MJ, and Keil A (2020). Attentional threat biases and their role in anxiety: A neurophysiological perspective. Int. J. Psychophysiol, 153, 148–158. 10.1016/j.ijpsycho.2020.05.004 [DOI] [PubMed] [Google Scholar]
- Van Veen V, and Carter CS (2002). The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol. Behav, 77(4-5), 477–482. 10.1016/s0031-9384(02)00930-7 [DOI] [PubMed] [Google Scholar]
- Van Veen V, and Carter CS (2002). The timing of action-monitoring processes in the anterior cingulate cortex. J. Cogn. Neurosci, 14(4), 593–602. 10.1162/08989290260045837 [DOI] [PubMed] [Google Scholar]
- Zvielli Bernstein, A., and Koster EHW (2015). Temporal dynamics of attentional bias. Clin. Psychol. Sci, 3(5), 772–788. 10.1177/2167702614551572 [DOI] [Google Scholar]
- Zvielli A, Vrijsen JN, Koster EH, and Bernstein A (2016). Attentional bias temporal dynamics in remitted depression. J. Abnorm. Psychol, 125(6), 768–776. 10.1037/abn0000190 [DOI] [PubMed] [Google Scholar]