Abstract
Aims:
Little data is available regarding prognostic implications of Invasive exercise testing in heart failure (HF) with preserved ejection fraction (HFpEF). The present study aimed to investigate whether rest/exercise central hemodynamic abnormalities are associated with adverse clinical outcomes.
Methods and results:
Patients with exertional dyspnea and EF≥50% (n=764) underwent invasive exercise testing and follow-up for HF hospitalization or death. There were 117 patients with events over a median follow-up of 2.7 (IQR 0.5–4.6) years. Among patients with normal resting PAWP (<15mmHg, n=380 [50%]), increased exercise PAWP (≥25mmHg, n= 187 [24%]) was associated with 2.4-fold higher risk of events compared to those with normal exercise PAWP (<25mmHg, n= 193 [25%]) (HR 2.44; 95%CI, 1.11–5.36; p=0.03), while patients with elevated resting PAWP (≥15mmHg, n=384 [50%]) displayed even higher risk compared to HFpEF with normal resting PAWP (HR 2.24; 95%CI, 1.38–3.65; p=0.001). Similar findings were observed for rest/exercise right atrial pressure, and rest/exercise pulmonary artery pressures. Higher peak VO2 was associated with decreased risk of events, and this relationship was solely explained by exercise cardiac output. In a multivariable-adjusted Cox model, each 1 SD increase in exercise PAWP was associated with a 41% greater hazard of events (HR 1.41 [95% CI: 1.13–1.76]; p=0.002), while each 1 SD decrease in exercise CO was associated with a 37% increased risk (HR 0.63 [95% CI: 0.47–0.83]; p=0.001).
Conclusions:
Hemodynamic abnormalities currently used for diagnosis of HFpEF are associated with increased risk for adverse events. Treatments that reduce central pressures while improving cardiac output reserve may offer greatest benefit to improve outcomes in HFpEF.
Keywords: invasive hemodynamics, exercise hemodynamics, heart failure, heart failure with preserved ejection fraction, outcome
Graphical Abstract
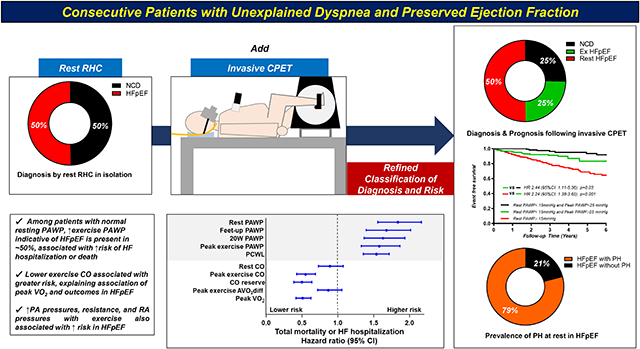
Invasive exercise hemodynamic abnormalities currently used to diagnose or exclude HFpEF was also better for risk stratifications in patients with unexplained dyspnea. AVO2diff, arterial-venous O2 content difference; CO, cardiac output; CPET, cardiopulmonary exercise testing; HFpEF, heart failure with preserved ejection fraction; iCPET, invasive CPET; NCD, non-cardiac dyspnea; PA, pulmonary artery; PAWP, pulmonary artery wedge pressure; PCWL, workload-adjusted PAWP; PH, pulmonary hypertension; RA, right atrial; RHC, right heart catheterization; VO2, oxygen consumption.
INTRODUCTION
Heart failure (HF) with preserved ejection fraction (HFpEF) is characterized by an inability of the heart to perfuse the body without pathological increases in cardiac filling pressures during exertion.1–3 This is manifest by elevations in pulmonary artery (PA) wedge pressure (PAWP) during exercise, often associated with blunted cardiac output (CO) reserve,4–6 impaired peripheral O2 extraction and uptake in muscle (reduced arterial-venous O2 content difference, AVO2 diff),7, 8 and impaired pulmonary vasodilation.5, 6, 9 Collectively, these abnormalities interact to limit aerobic capacity (peak oxygen consumption, VO2) and impair health status in people with HFpEF.4, 10–12
While the pathophysiologic significance of these hemodynamic abnormalities is self-evident, their prognostic implications are less well-understood. Elevated PAWP at rest or exercise is used for diagnosis of HFpEF,2 and increases in PAWP indexed to workload13 and CO14, 15 have been associated with greater risk of adverse events, but the prognostic relevance of individual hemodynamic parameters that become abnormal in HFpEF and are used for clinical diagnosis are not well-described. Reduced peak VO2 is associated with mortality in HFpEF.16, 17 According to the Fick principle, VO2 is equal to the product of central (CO) and peripheral (AVO2 diff) determinants, but no study has yet evaluated which components is most relevant to outcome in HFpEF.
The present study was undertaken to fill these gaps in the literature, assessing the prognostic value of rest and exercise hemodynamic variables currently used in practice to definitively diagnose or exclude HFpEF, in a large series of consecutive patients undergoing invasive hemodynamic exercise testing for the evaluation of unexplained dyspnea.
METHODS
Study Population
Patients undergoing invasive hemodynamic study during maximal effort supine exercise testing with simultaneous expired gas analysis for the evaluation of unexplained dyspnea at the Mayo Clinic, Rochester, MN, between 2006 and 2018 were retrospectively identified. HFpEF case status was defined by the presence of New York Heart Association (NYHA) functional class II-III dyspnea with activity, preserved left ventricular ejection fraction (LVEF) ≥50%, and elevated left heart filling pressures at rest (PAWP≥15mmHg) and/or with exercise (PAWP ≥25mmHg), fulfilling diagnostic criteria from current guidelines.2 Patients with non-cardiac dyspnea (NCD) were defined as those with normal hemodynamics at rest and during exercise, including normal PAWP, mean PAP (mPAP) ≤20 mmHg and pulmonary vascular resistance (PVR) <3 WU at rest, and mPAP ≤30 mmHg or total pulmonary resistance <3 WU during exercise.18
Two-dimensional, M-mode, Doppler, and tissue Doppler echocardiography was performed according to the American Society of Echocardiography guidelines in all patients.19 Those with any history of reduced ejection fraction (<50%), valvular heart disease (greater than moderate left-sided regurgitation, greater than mild stenosis), infiltrative, restrictive, or hypertrophic cardiomyopathy, constrictive pericarditis, primary pulmonary arterial hypertension (non-Group 2), and NYHA class IV were excluded. The study was approved by the Mayo Clinic Institutional Review Board and all participants provided consent for data use through completion of research authorization forms. All the authors had full access to the data and take responsibility for its integrity.
Catheterization Protocol
Patients underwent invasive hemodynamic exercise testing with simultaneous expired gas analysis during supine cycle ergometry to volitional exhaustion, using methods we have previously described.5, 20 Right atrial (RA) pressure (RAP), PAP, and PAWP were measured at end-expiration at rest, and both at end-expiration and as an average of at least 3 respiratory cycles during exercise. For the primary analysis, exercise pressures are averaged over the respiratory cycle. The ratio of PAWP at peak exercise to workload normalized to body weight (PCWL [mmHg/watts/kg]) was calculated as described previously.13 A 4–6 Fr radial arterial cannula was used to measure arterial blood pressure (BP) and obtain arterial blood gas samples throughout the test. Arterial venous O2 difference (AVO2 diff) was directly measured as the difference between systemic arterial and PA O2 contents (=saturation×hemoglobin×1.34). Oxygen consumption (VO2) was measured using expired gas analysis (MedGraphics, St. Paul, MN), with values taken as the mean from 30 seconds preceding arterial and venous blood sampling in each phase. Cardiac output was calculated using the direct Fick method (CO=VO2/AVO2 diff). Pulmonary vascular resistance [PVR=(mean PAP-PAWP)/CO] and PA compliance (PAC=stroke volume/PA pulse pressure) were calculated. Total pulmonary resistance (TPR) was defined by the quotient of mean PAP/CO. While PVR is mainly determined by the resistance of the pulmonary vasculature, TPR is determined by downstream left atrial pressure, as well as PVR, and in many cases, dominated by LV filling pressures.
After baseline data were acquired, hemodynamic assessment and expired gas analysis were performed during exercise, starting at 20 W workload for 5 minutes, and increasing 20 W increments in 2–3 min stages to volitional exhaustion. Increases in PAWP and mean PAP were normalized to changes in CO during exercise (PAWP/CO slope, PAP/CO slope) by subtracting rest data from peak exercise data, with abnormal slopes defined as values >2 mmHg/l/min and >3 mmHg/l/min, respectively, based upon prior studies.14, 15 A few patients (22 [2.9%]) experience reduction in CO with exercise, which would result in a negative slope. For these patients, we substituted +0.1 l/min for the change in CO with exercise for the purposes of outcome analysis, ensuring that these patients would be considered as abnormal, and divided the observed changes in PAWP or PAP by this denominator.
Outcome Assessment
Patient follow-up was initiated on the day of invasive hemodynamic cardiopulmonary exercise testing. Mortality data were ascertained from medical records, death certificates, obituaries, and notices of death in the local newspapers. Data on all deaths were obtained from the State of Minnesota annually. Heart failure hospitalizations were determined from the Mayo Clinic electronic medical record and adjudicated by a single cardiologist (K.O.). Patient follow-up was initiated on the day of cardiac catheterization. Patients were censored at last follow-up contact.
Statistical Analysis
Data are presented as mean ± standard deviation (SD), median (interquartile range, IQR) or number (%). Between-group differences were compared by unpaired t-test, Wilcoxon rank-sum test, χ2 or Fisher’s exact test as appropriate. Event rates were compared using Kaplan–Meier curve analysis. Risk of the composite outcome was compared among 3 patient groups defined a priori: (1) NCD (normal rest and exercise hemodynamics, defined above), (2) HFpEF with normal resting PAWP (<15 mmHg), but abnormal exercise PAWP (≥25 mmHg), and (3) HFpEF defined by abnormal resting PAWP (≥15 mmHg). Risk was also compared in HFpEF based upon externally-validated diagnostic cutpoints, including leg raise PAWP≥ or <19 mmHg,21 and by grouping patients with HFpEF by PAWP during exercise above and below the median. Similar comparisons were made using other hemodynamic measures in HFpEF above and below the group median values for other hemodynamic and O2 transport measures, as compared to NCD. To further evaluate prognostic implications for hemodynamic abnormalities in a continuous manner, independent of partition values used to define the study groups (i.e., NCD or HFpEF) univariable and multivariable Cox proportional hazards models were created to assess prognostic relationships independent of relevant baseline group differences including age, sex, body mass index, atrial fibrillation, EF, and estimated glomerular filtration rate. Integrated variables including PCWP/CO slope and mean PAP/CO with non-normal distributions were log-transformed. A two-sided P value of <0.05 was considered statistically significant. All data were analyzed using JMP14.0 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Subject Characteristics
Consistent with multiple prior studies, patients with HFpEF were older, more obese, had more comorbidities, and displayed poorer kidney function and higher N-terminal-pro-B-type natriuretic peptide (NT-proBNP) levels compared to patients with NCD (Table 1). As compared to patients with HFpEF that became apparent only by exercise hemodynamics (Ex HFpEF; resting PAWP<15mmHg and exercise PAWP≥25mmHg), those with HFpEF evident at rest (rest HFpEF, resting PAWP≥15mmHg) were more obese, anemic and had higher NT-proBNP levels. The prevalence of PH (defined by mean PAP>20 mmHg) was 79% among patients with HFpEF overall, 63% among those with rest HFpEF and 16% among those with HFpEF diagnosed only during exercise. While LVEF was similar in all groups, patients with HFpEF displayed greater LV mass and impaired diastolic function with higher E/e’ and larger left atrial volume index, these were more pronounced in patients with rest HFpEF as compared to Ex HFpEF. Subjects with rest HFpEF displayed more impaired RV systolic function as compared to subjects with Ex HFpEF and NCD (Table 1).
Table 1:
Baseline Characteristics
| NCD (n=193) |
Ex HFpEF (n=187) |
Rest HFpEF (n=384) |
P value | |
|---|---|---|---|---|
| Age (years) | 55±14 | 68±10* | 68±11* | <0.0001 |
| Women, n (%) | 112 (58%) | 105 (56%) | 219 (57%) | 0.9 |
| Body mass index (kg/m2) | 27.5±5.3 | 32.1±6.1* | 33.7±7.7*† | <0.0001 |
| Comorbidities, n (%) | ||||
| Coronary disease | 41 (21%) | 74 (40%) | 126 (33%) | 0.0006 |
| Diabetes mellitus | 25 (13%) | 50 (27%) | 102 (27%) | 0.0005 |
| Hypertension | 120 (62%) | 170 (91%) | 364 (95%) | <0.0001 |
| Atrial fibrillation | 11 (6%) | 42 (22%) | 166 (43%) | <0.0001 |
| Medications, n (%) | ||||
| Renin-angiotensin system blocker | 52 (27%) | 72 (39%) | 194 (51%) | <0.0001 |
| Beta-Blocker | 49 (25%) | 89 (48%) | 231 (60%) | <0.0001 |
| Diuretic | 42 (22%) | 90 (48%) | 238 (62%) | <0.0001 |
| Laboratories | ||||
| Hemoglobin (g/dL) | 13.6±1.4 | 13.3±1.5 | 12.9±1.6*† | <0.0001 |
| Estimated GFR (mL/min/1.73m2) | 78±20 | 67±18* | 62±21* | <0.0001 |
| NT-pro BNP (pg/mL) (n=548) | 75 (30, 158) | 146 (64, 485)* | 371 (119, 1208)*† | <0.0001 |
| Echocardiography | ||||
| LV diastolic dimension (mm) | 48±5 | 48±5 | 49±5* | 0.02 |
| LV mass index (g/m2) | 84±18 | 90±21* | 92±23* | 0.0009 |
| LA volume index (mL/m2) | 29±10 | 33±12* | 38±16*† | <0.0001 |
| LVEF (%) | 65±5 | 65±6 | 64±6 | 0.3 |
| E/e’ | 7 (6, 9) | 9 (7, 12)* | 12 (9, 16)*† | <0.0001 |
| TAPSE (mm) | 22±4 | 22±5 | 20±5*† | 0.001 |
| RV s’ (cm/s) | 13±3 | 13±3 | 12±3*† | 0.0005 |
| TR velocity (m/s) | 2.4±0.3 | 2.6±0.3* | 2.8±0.5*† | <0.0001 |
Data are mean ± SD, median (interquartile range), or n (%).E/e’, ratio of early diastolic filling pulsed wave doppler to early diastolic mitral annular tissue doppler velocity; GFR, glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; Ex HFpEF, HFpEF that became evident only during exercise testing; Rest HFpEF, HFpEF that was diagnosed based upon resting hemodynamics alone; LA, left atrial; LV, left ventricular; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; NCD, non-cardiac dyspnea; NT-pro BNP, N-terminal-pro-B-type natriuretic peptide; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation and s’, systolic mitral annular tissue doppler velocity.
P< 0.05 vs NCD.
P< 0.05 vs Ex HFpEF.
Baseline and Exercise Hemodynamics
Patients with HFpEF displayed higher central pressures, with lower CO at rest, where patients with rest HFpEF displayed more elevated central pressure as compared to Ex HFpEF (Table 2). With exercise, as compared to subjects with NCD, patients with HFpEF displayed greater increase in left- and right-side filling pressures and more blunted increased in CO with exercise, resulting in impaired functional capacity, and these changes were more pronounced in patients with rest HFpEF as compared to Ex HFpEF. Abnormalities in hemodynamics at peak exercise were similar at a common matched submaximal objective exercise workload (20W) (Supplemental Table 1).
Table 2:
Invasive hemodynamics at rest and during exercise
| NCD (n=193) |
Ex HFpEF (n=187) |
Rest HFpEF (n=384) |
P value | |
|---|---|---|---|---|
| Rest | ||||
| Vital signs | ||||
| Heart rate (bpm) | 73±13 | 69±11* | 70±12* | 0.002 |
| Systolic BP (mm Hg) | 135±22 | 144±23* | 147±24* | <0.0001 |
| Central pressures | ||||
| RAP (mm Hg) | 5±2 | 7±3* | 12±4*† | <0.0001 |
| Feet-up RAP (mm Hg) | 7±3 | 10±3* | 16±6*† | <0.0001 |
| PA systolic pressure (mm Hg) | 26±6 | 32±7* | 45±14*† | <0.0001 |
| PA mean pressure (mm Hg) | 16±4 | 21±5* | 30±8*† | <0.0001 |
| PAWP (mm Hg) | 9±3 | 11±2* | 20±4*† | <0.0001 |
| Feet-up PAWP (mm Hg) | 12±4 | 16±4* | 23±5*† | <0.0001 |
| Vascular function | ||||
| PVR (WU) | 1.2 (0.9, 1.6) | 1.7 (1.2, 2.5)* | 1.9 (1.1, 3.1)* | <0.0001 |
| PAC (mL/mm Hg) | 5.1±2.0 | 4.2±1.8* | 3.4±1.9*† | <0.0001 |
| PA Ea (mm Hg/mL) | 0.3±0.1 | 0.4±0.1* | 0.6±0.3*† | <0.0001 |
| Oxygen Transport | ||||
| VO2 (mL/min/kg) | 2.8±0.6 | 2.5±0.6* | 2.5±0.6* | <0.0001 |
| A-Vo2 diff (mL/dL) | 4.0±0.8 | 4.4±0.8* | 4.7±1.0*† | <0.0001 |
| CO (L/min) | 5.7±1.6 | 5.3±1.4* | 5.1±1.7* | 0.0006 |
| Peak Exercise | ||||
| Vital signs | ||||
| Heart rate (bpm) | 118±24 | 105±19* | 98±21*† | <0.0001 |
| Systolic BP (mm Hg) | 170±32 | 183±32* | 175±33† | 0.0006 |
| Peak workload (W) | 69±37 | 50±29* | 40±25*† | <0.0001 |
| RER | 1.04±0.13 | 1.02±0.01 | 1.00±0.12* | 0.002 |
| Central pressures | ||||
| RAP (mm Hg) | 7±4 | 15±6* | 22±8*† | <0.0001 |
| PA systolic pressure (mm Hg) | 40±10 | 60±13* | 68±17*† | <0.0001 |
| PA mean pressure (mm Hg) | 25±6 | 41±8* | 47±11*† | <0.0001 |
| PAWP (mm Hg) | 15±5 | 30±5* | 32±7*† | <0.0001 |
| PAWP/CO slope | 1.1 (0.5, 1.9) | 4.4 (3.0, 7.5)* | 3.8 (2.0, 7.4)* | <0.0001 |
| Mean PAP/CO slope | 1.8 (1.1, 2.5) | 4.8 (3.2, 7.8)* | 5.9 (3.0, 10.5)* | <0.0001 |
| PCWL (mmHg/watts/kg) | 17 (11, 28) | 64 (39, 97)* | 90 (54, 137)*† | <0.0001 |
| Vascular function | ||||
| PVR (WU) | 0.9 (0.6, 1.2) | 1.1 (0.6, 1.9)* | 1.7 (1.1, 2.9)*† | <0.0001 |
| PAC (mL/mm Hg) | 4.0±1.6 | 3.1±1.7* | 2.6±1.4*† | <0.0001 |
| PA Ea (mm Hg/mL) | 0.4±0.2 | 0.6±0.2* | 0.8±0.4*† | <0.0001 |
| Oxygen Transport | ||||
| VO2 (mL/min/kg) | 13.5±5.3 | 10.4±3.1* | 8.9±3.2*† | <0.0001 |
| A-VO2 diff (mL/dL) | 9.3±2.0 | 9.8±2.0 | 10.0±2.4* | 0.0009 |
| CO (L/min) | 11.4±3.3 | 9.9±3.5* | 8.5±3.2*† | <0.0001 |
Data are mean ± SD, median (interquartile range), or n (%). AVO2 diff indicates arterial-venous O2 content difference; BP, blood pressure; CO, cardiac output; Ea, elastance; HFpEF, heart failure with preserved ejection fraction; LV, left ventricular; NCD, non-cardiac dyspnea; PA, pulmonary artery; PAC, pulmonary artery compliance; PAWP, pulmonary artery wedge pressure; PCWL, ratio of pulmonary capillary wedge pressure at peak exercise to workload normalized to body weight; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RER, respiratory exchange ratio; RV, right ventricular; and VO2, oxygen consumption volume.
P< 0.05 vs NCD.
P< 0.05 vs Ex HFpEF.
Risk of Outcome by Categorical Hemodynamic Classifications
Over a median follow-up period of 2.7 years (IQR 0.5–4.6), 117 patients experienced the composite endpoint, including 61 deaths without HF hospitalization (8%) and 56 HF hospitalizations with or without death (7%). Patients with HFpEF and elevated resting PAWP (n=380 [50%]) displayed higher risk of events compared to NCD (n=193 [25%], HR 5.48; 95%CI, 2.75–10.9; p<0.0001) and to patients with normal resting PAWP (n=187 [24%], HR 2.24; 95%CI, 1.38–3.65; p=0.001) (Figure 1). However, patients with normal rest PAWP but elevated PAWP with exercise displayed a 2.4-fold greater risk of events as compared to NCD (HR 2.44; 95%CI, 1.11–5.36; p=0.03). Similarly, greater elevation in PAWP during leg raise, low-level exercise (20W), and peak exercise were all associated with greater risk for adverse events in patients with HFpEF (Figure 1).
Figure 1.
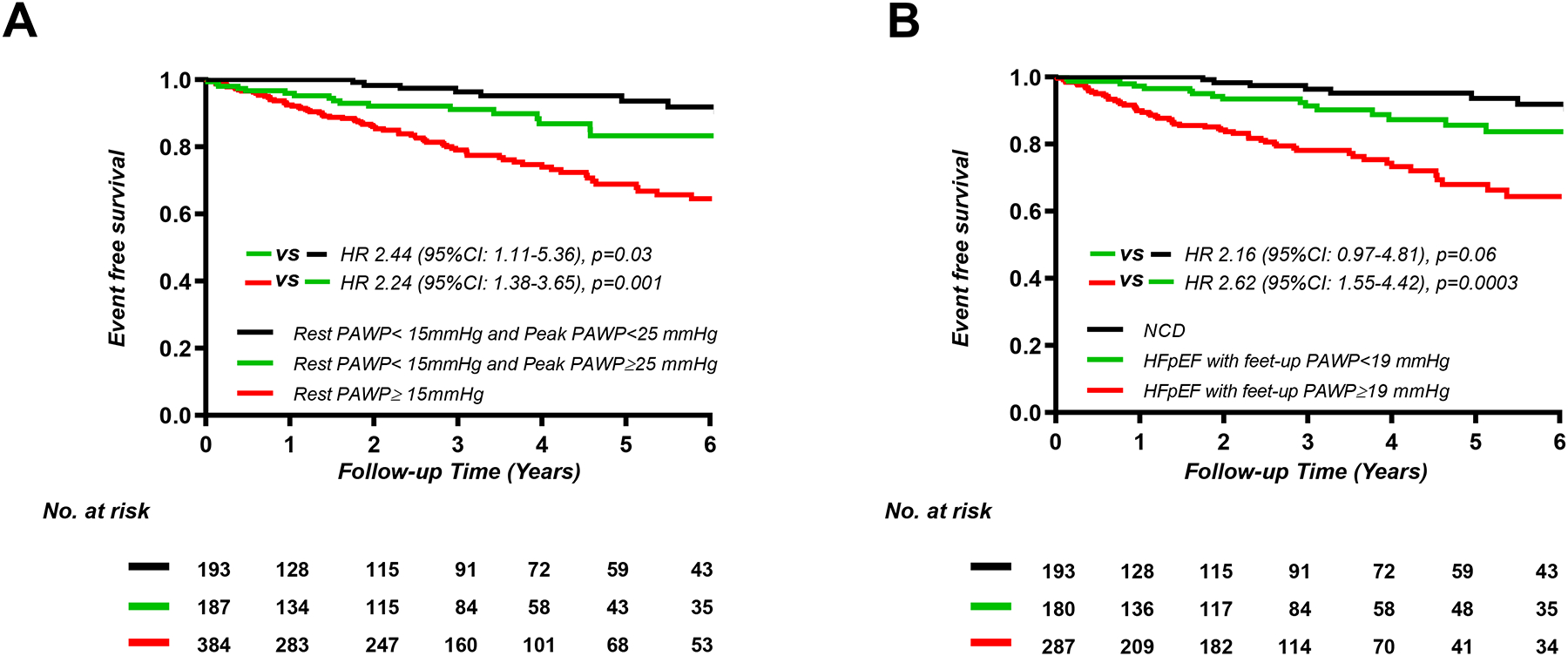
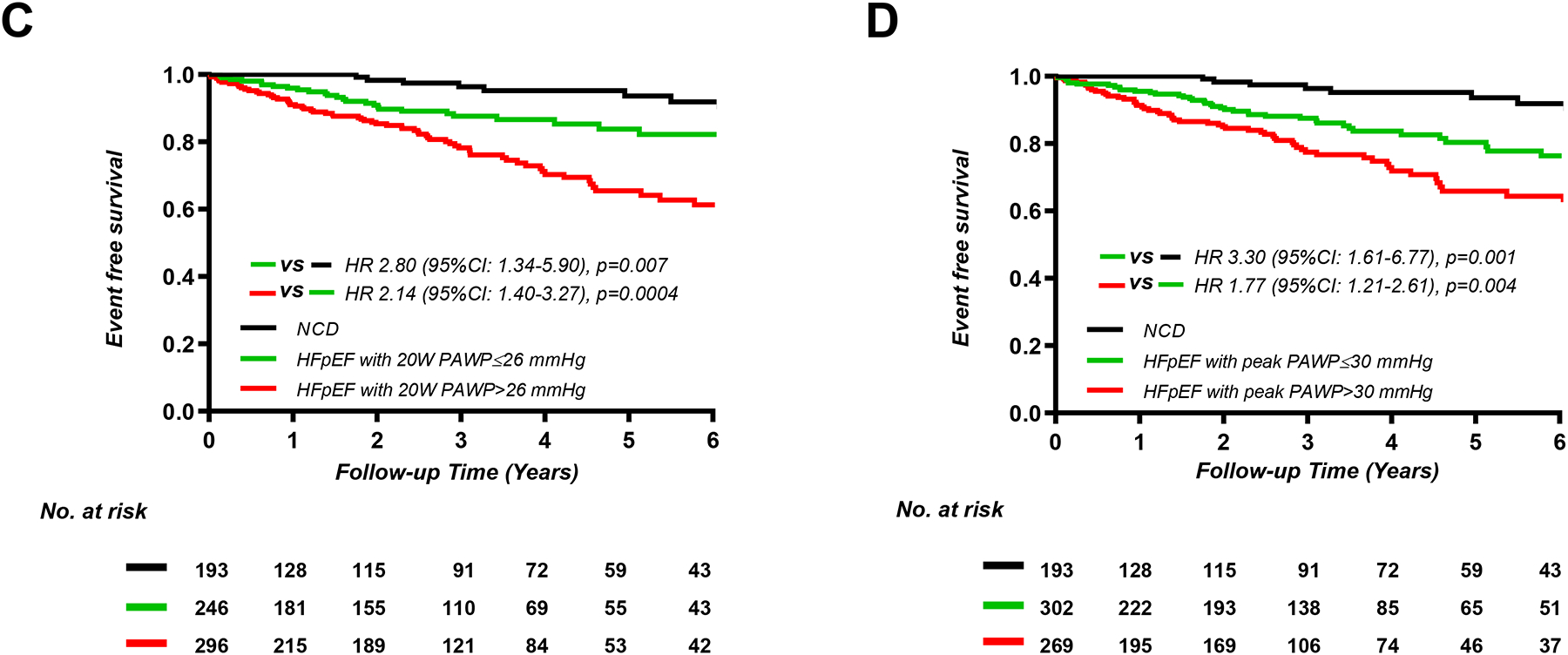
Kaplan-Meier analysis for composite of all-cause death and heart failure hospitalization categorized by current cutoff point of diagnosis or median values of pulmonary artery wedge pressure (PAWP) (A) at rest (n=764), (B) feet-up (n=660), (C) 20W exercise (n=735), and peak exercise (n=764). HFpEF, heart failure with preserved ejection fraction; NCD, non-cardiac dyspnea.
Patients with HFpEF and increasing PA and RA pressures at rest, low-level exercise, and peak exercise displayed progressively greater risk of adverse events, with both HFpEF groups displaying higher risk that patients with NCD (Figure 2, Supplemental Figures 1 and 2). Patients with worsening PAC compliance and PVR at rest and during exercise were also found to display higher risk for adverse events (Supplemental Figures 3 and 4).
Figure 2.
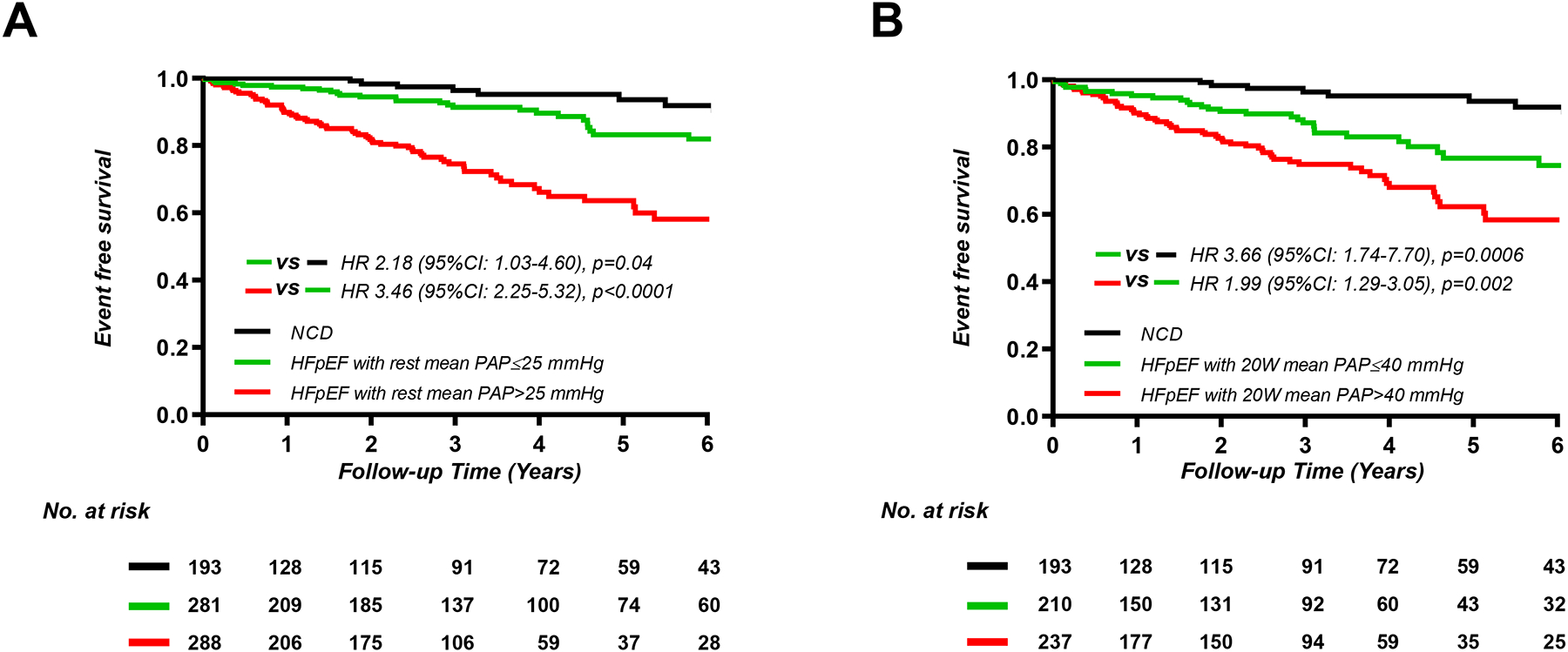
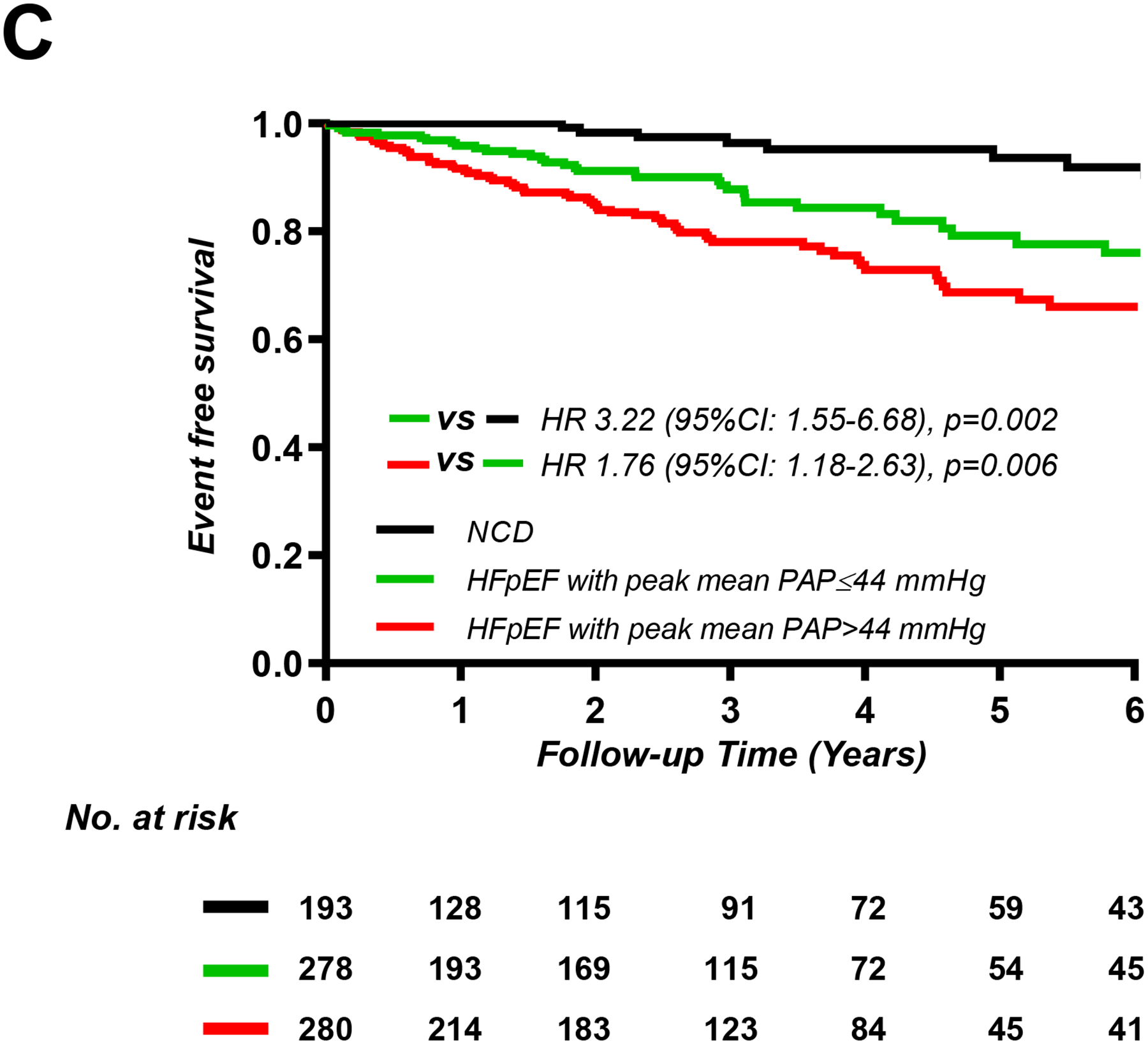
Kaplan-Meier analysis for composite of all-cause death and heart failure hospitalization categorized by median values of mean pulmonary artery pressure (PAP) (A) at rest (n=762), (B) 20W exercise (n=640), and peak exercise (n=751).
HFpEF, heart failure with preserved ejection fraction; NCD, non-cardiac dyspnea
Patients with HFpEF and peak VO2 above the group median (>8.9 ml/min/kg, n=264) displayed higher risk of events than patients with NCD (HR 2.40; 95% CI, 1.13–5.08; p=0.03), and patients with HFpEF and peak VO2 below the group median (≤8.9 ml/min/kg, n=263) displayed incrementally higher risk than HFpEF patients with peak VO2 above the median (HR 2.61; 95% CI, 1.68–4.07; p<0.0001). There was no statistically significant association between resting CO (HR 0.80; 95% CI, 0.54–1.17; p=0.3) or resting AVO2 diff (HR 0.88; 95% CI, 0.60–1.29; p=0.5) and risk of events in patients with HFpEF (Figure 3, Supplemental Figure 5). There was no association between exercise AVO2 diff and event rates in patients with HFpEF (HR 1.37; 95% CI, 0.92–2.06; p=0.1) (Supplemental Figure 5), but lower cardiac output during exercise in HFpEF was associated with progressively greater risk (HR 2.35; 95% CI, 1.50–3.68; p=0.0002) (Figure 3).
Figure 3.
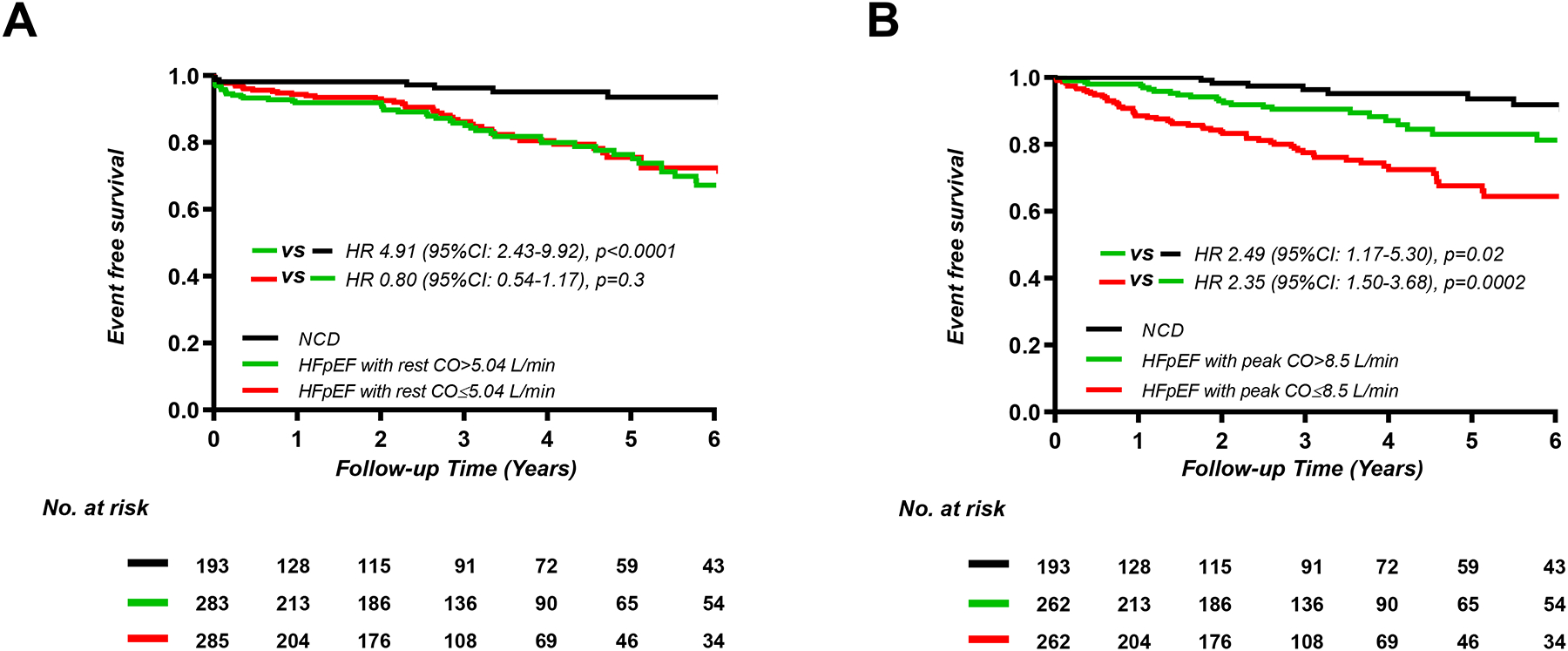
Kaplan-Meier analysis for composite of all-cause death and heart failure hospitalization categorized by median values of cardiac output (CO) (A) at rest (n=761), (B) peak exercise (n=717).
HFpEF, heart failure with preserved ejection fraction NCD, non-cardiac dyspnea.
Abnormal PAWP/CO slope (>2mmHg/l/min) and mPAP/CO slope (>3 mmHg/l/min) defined using established cutpoints did not differentiate risk among patients with HFpEF (HR 1.28; 95% CI, 0.72–2.25; p=0.4, and HR 1.23; 95% CI, 0.73–2.08; p=0.4, respectively) (Supplemental Figure 6). However, comparing groups according to median observed values in the HFpEF group revealed a trend for higher incidence of composite events with PAWP/CO slope >4.2 mmHg/l/min (HR 1.43; 95% CI, 0.94–2.15; p=0.09) and higher risk in those with mean PAP/CO slope >5.5 mmHg/l/min (HR 1.75; 95% CI, 1.15–2.67; p=0.009; Supplemental Figure 6).
Risk of Outcome by Continuous Hemodynamic Measures
When examined as continuous measures rather than by predefined groups, increasing PAWP, PA pressure, RA pressure, and PVR were all associated with increased risk of events, as was increasing PCWL, lower PA compliance and lower peak VO2 (Table 3, Figure 4). In multivariable linear regression analyses, peak VO2 was related to both exercise CO (β=0.94 per 1 l/min, p<0.0001) and exercise AVO2 diff (β=0.56 per 1 ml/dl, p<0.0001). However, only exercise CO was associated with risk of adverse events (HR 0.55; 95% CI, 0.43–0.69; p<0.0001), as there was no association of risk and exercise AVO2 diff (HR 0.87; 95% CI, 0.72–1.06; p=0.2) (Table 3, Figure 4). In an univariable Cox model, there were statistically significant associations between continuous values of log peak PAWP/CO slope, log peak mean PAP/CO slope and risk of events (HR 1.40; 95% CI, 1.21–1.60; p<0.0001 and HR 1.39; 95% CI, 1.20–1.61; p<0.0001, respectively) (Table 3, Figure 4).
Table 3:
Univariable and multivariable models for death and HF hospitalization
| Univariable Model | Multivariable Model* | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Rest PAWP | 1.84 (1.56–2.17) | <0.0001 | 1.50 (1.22–1.83) | <0.0001 |
| Feet-up PAWP | 1.68 (1.40–2.02) | <0.0001 | 1.28 (1.01–1.62) | 0.04 |
| 20W PAWP | 1.63 (1.37–1.94) | <0.0001 | 1.32 (1.07–1.63) | 0.01 |
| Peak exercise PAWP | 1.58 (1.33–1.87) | <0.0001 | 1.30 (1.05–1.62) | 0.02 |
| PCWL | 1.54 (1.36–1.72) | <0.0001 | 1.66 (1.34–2.05) | <0.0001 |
| Rest systolic PAP | 1.64 (1.43–1.87) | <0.0001 | 1.29 (1.09–1.54) | 0.005 |
| Peak exercise systolic PAP | 1.62 (1.36–1.92) | <0.0001 | 1.26 (1.01–1.58) | 0.04 |
| Rest mean PAP | 1.78 (1.54–2.04) | <0.0001 | 1.45 (1.21–1.74) | <0.0001 |
| 20W mean PAP | 1.81 (1.52–2.16) | <0.0001 | 1.44 (1.16–1.79) | 0.0009 |
| Peak exercise mean PAP | 1.70 (1.42–2.04) | <0.0001 | 1.33 (1.06–1.66) | 0.01 |
| Rest RAP | 1.69 (1.46–1.95) | <0.0001 | 1.43 (1.19–1.72) | 0.0002 |
| Feet-up RAP | 1.56 (1.30–1.86) | <0.0001 | 1.29 (1.02–1.60) | 0.03 |
| 20W RAP | 1.66 (1.41–1.94) | <0.0001 | 1.34 (1.08–1.64) | 0.008 |
| Peak exercise RAP | 1.64 (1.39–1.93) | <0.0001 | 1.37 (1.10–1.70) | 0.005 |
| Rest PVR | 1.22 (1.12–1.31) | <0.0001 | 1.18 (1.0004–1.38) | 0.0495 |
| Peak exercise PVR | 1.15 (1.06–1.23) | 0.0001 | 1.10 (0.93–1.30) | 0.3 |
| Rest PAC | 0.51 (0.39–0.66) | <0.0001 | 0.72 (0.53–0.96) | 0.03 |
| Peak exercise PAC | 0.56 (0.42–0.73) | <0.0001 | 0.77 (0.58–1.04) | 0.09 |
| Rest CO | 0.89 (0.73–1.08) | 0.3 | - | - |
| Peak exercise CO | 0.55 (0.43–0.69) | <0.0001 | 0.67 (0.51–0.89) | 0.007 |
| CO reserve | 0.50 (0.39–0.64) | <0.0001 | 0.65 (0.50–0.85) | 0.002 |
| Peak exercise AVO2 difference | 0.87 (0.72–1.06) | 0.2 | - | - |
| Peak VO2 | 0.51 (0.42–0.63) | <0.0001 | 0.53 (0.38–0.73) | 0.0001 |
| Log 20W PAWP/CO slope | 0.10 (0.97–1.32) | 0.1 | ||
| Log 20W Mean PAP/CO slope | 0.12 (0.57–1.31) | 0.1 | ||
| Log PAWP/CO slope | )1.40 (1.21–1.60) | <0.0001 | 1.20 (1.02–1.41) | −0.03 |
| Log Mean PAP/CO slope | 1.39 (1.20–1.61) | <0.0001 | −1.16 (0.98–1.37) | −0.08 |
| 20W PAWP/CO slope >2 mmHg/L/min | 1.91 (1.06–3.47) | 0.03 | 1.45 (0.78–2.71) | 0.2 |
| 20W Mean PAP/CO slope >3 mmHg/L/min | 2.04 (1.10–3.78) | 0.02 | 1.46 (0.79–2.72) | 0.2 |
| PAWP/CO slope >2 mmHg/L/min | 1.98 (1.23–3.21) | 0.005 | 1.16 (0.68–1.99) | 0.6 |
| Mean PAP/CO slope >3 mmHg/L/min | 2.03 (1.27–3.23) | 0.003 | 1.22 (0.74–2.00) | 0.4 |
| PAWP/CO slope >4.2 mmHg/L/min** | 1.86 (1.26–2.75) | 0.002 | 1.25 (0.83–1.90) | 0.3 |
| Mean PAP/CO slope >5.5 mmHg/L/min** | 2.42 (1.63–3.61) | <0.0001 | 1.39 (0.91–2.13) | 0.1 |
Hazard ratios (HR) shown are for a 1 standard deviation change in each hemodynamic parameter, unless otherwise indicated.
CI indicates confidence interval; CO, cardiac output; HR, hazard ratio; PA, pulmonary artery; PAC, pulmonary artery compliance; PAP, pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PCWL, ratio of pulmonary capillary wedge pressure at peak exercise to workload normalized to body weight; RAP, right atrial pressure; VO2, oxygen consumption volume; and CO reserve = peak CO – rest CO.
Multivariable Model was adjusted by age, gender, body mass index, prevalence of atrial fibrillation, left ventricular ejection fraction, hemoglobin and estimated glomerular filtration rate.
Cutoff value was calculated from each of the median value in participants with HFpEF.
Figure 4.
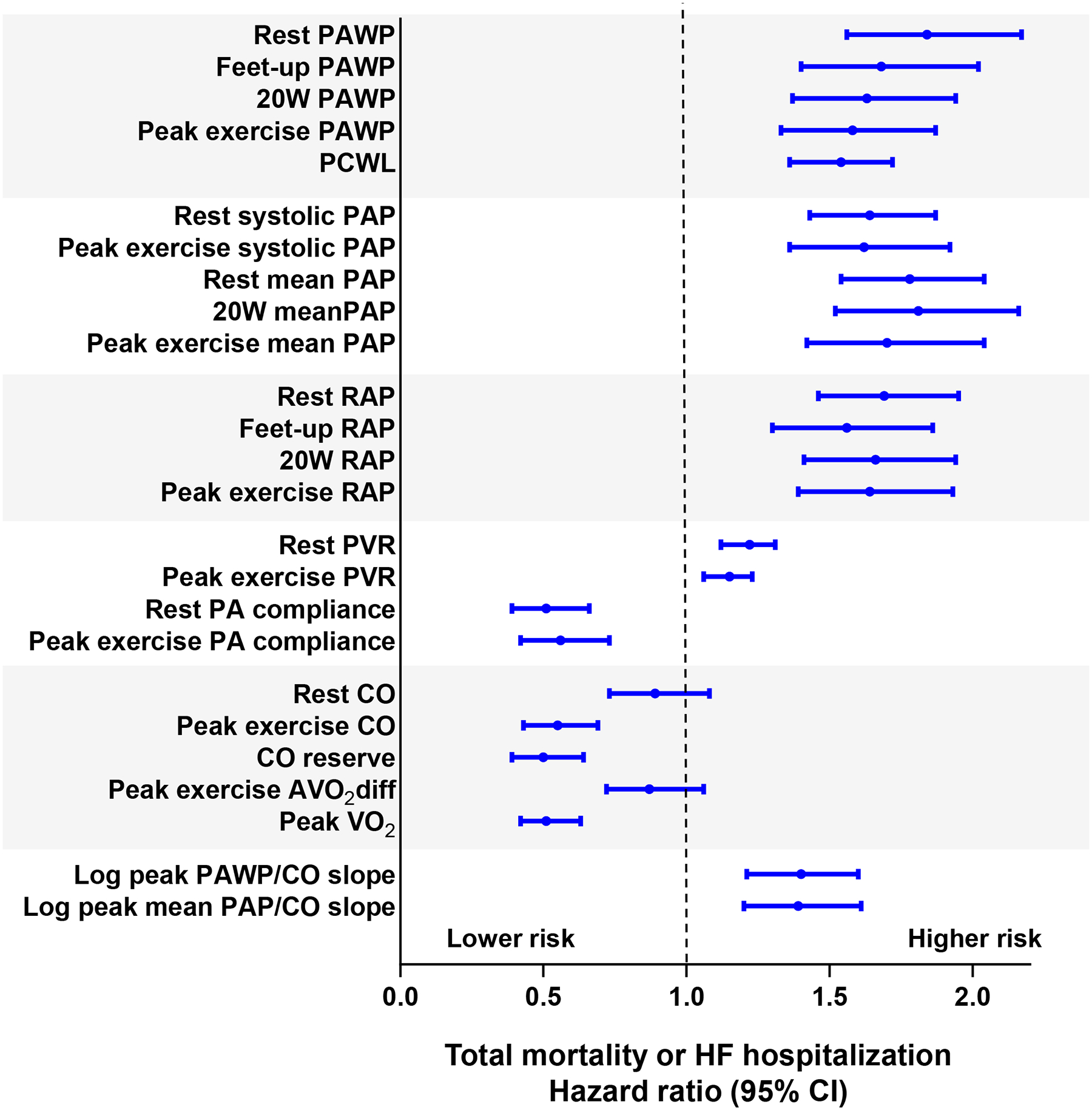
Univariable Cox models were used to determine the hazard ratio (each 1 SD increase) for the heart failure hospitalization or death. AVO2 diff indicates arterial-venous O2 content difference; CO, cardiac output; PA, pulmonary artery; PAC, pulmonary artery compliance; PAP, pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PCWL, ratio of pulmonary capillary wedge pressure at peak exercise to workload normalized to body weight; PVR, pulmonary vascular resistance; RAP, right atrial pressure and VO2, oxygen consumption volume.
In a multivariable Cox model, each 1 SD increase in PAWP, PCWL, RA pressure, mean and systolic PAP, resting PVR, and log peak PCWP/CO slope, and each 1 SD decrease in rest PA compliance and peak exercise CO remained significantly associated with risk for adverse events after adjustment for age, sex, body mass index, atrial fibrillation, LVEF, hemoglobin and estimated glomerular filtration rate (Table 3).
Most associations between hemodynamics and risk of adverse outcome were consistently observed using pressures during exercise measured at end-expiration rather than an average of respiratory cycles (Supplemental Table 2). In a multivariable-adjusted Cox model, log mean PAP/CO slope measured at end-expiration was significantly associated with risk for adverse events (HR 1.18; 95% CI, 1.005–1.38; p=0.04), while the same variable using the average of respiratory cycles was no longer statistically significant (HR 1.16; 95% CI, 0.98–1.37; p=0.08).
DISCUSSION
The present study provides new insights into the prognostic implications of invasive exercise hemodynamic abnormalities currently used to diagnose or exclude HFpEF, from a large consecutive series of individuals with unexplained dyspnea (Graphical Abstract). The data reveal greater risk for adverse events as hemodynamic abnormalities at rest and with exercise worsen. Patients with elevated PAWP at rest display the greatest risk, but importantly, the presence of elevated PAWP during exercise or leg-rise is shown to be associated with increased risk of adverse events among patients with normal resting PAWP. In addition, we also provide a comprehensive analysis of all other hemodynamic data that are available from rest/exercise invasive testing, most particularly, cardiac output and AVO2 difference reserve, as well as pulmonary vascular function and right heart filling pressures. Importantly, aerobic capacity, measured as peak VO2, was correlated with both exercise CO and AVO2diff, but only exercise CO was associated with adverse events, emphasizing the importance of CO reserve in HFpEF. These data further validate the importance of invasive exercise hemodynamics, expanding the clinical significance from diagnosis to include risk stratification. These data further emphasize the importance of limitations in CO reserve, in addition to elevated central hemodynamics as key treatment targets to improve clinical outcomes in HFpEF.
Prognostic Influence of Left Heart Filling Pressures
Hemodynamic abnormalities represent the physiologic force linking alterations in myocardial structure, function, and loading conditions to symptoms, functional impairment, and organ damage in HF.22 In patients with HFpEF, increases in PAWP and PA pressure during exercise lead to development of lung congestion that acutely promotes dyspnea,11, 23 chronically alters pulmonary vascular structure and function,24–26 and eventually leads to development of clinically overt right-sided HF.27, 28 In addition to congestive abnormalities related to high filling pressures, impairments in O2 delivery to the tissues are also common in HFpEF, related to limitations in CO reserve, anemia, and abnormalities in pulmonary and skeletal muscle gas diffusion.4, 12, 25 For these reasons, invasive exercise testing has emerged as the gold standard method to diagnose or exclude HFpEF,1–3 and has further been proposed as a key method to help phenotype patients based upon physiologic responses to stress.12
Despite this established value for diagnosis and pathophysiologic phenotyping, there is less data available regarding the prognostic implications of exercise hemodynamics in HFpEF. Dorfs et al. first reported that elevation in PAWP indexed to workload during exercise was associated with increased risk of all-cause mortality.13 While this observation has supported the importance of exercise PAWP elevation, it has remained unclear if the finding was driven more by PAWP elevation (numerator), or by the denominator of peak workload achieved during exercise, especially as it is well established that exercise capacity is also a powerful predictor of mortality.16, 17, 29 Two separate studies demonstrated a that PAWP/CO slope>2 during upright exercise was associated with greater risk of cardiovascular events.14, 15 As with the Dorfs study, it was again unclear whether the authors’ finding was related to pathologic PAWP increase (numerator), blunted CO increase (denominator), or some combination of both.14 The present study now resolves these questions. Importantly, among patients with normal PAWP at rest, those with abnormal increases in PAWP during exercise to currently-endorsed cutpoints (≥25 mmHg) displayed 2.4-fold greater risk for the combined endpoint of HF hospitalization or death compared to those without exertional PAWP elevation, providing further evidence to the diagnostic cutpoints for exercise PAWP in consensus guidelines.2
Aerobic Capacity, O2 Transport, and Outcome
Peak VO2 is well known to predict risk of adverse outcome in HFpEF, just as in HFrEF.16, 17, 29 According to the Fick principle, VO2 is equal to the product of CO and AVO2diff. While both components are known to correlate with peak VO2 in HFpEF,4, 8, 12 to our knowledge, no study has yet reported on the prognostic impact of these individual determinants of VO2 in this patient population. While resting PAWP and PA pressures were associated with outcome, we found that patients with HFpEF and low resting CO had similar risk of events as HFpEF patients with normal resting CO (Figure 3A). Conversely, impairments in CO reserve (i.e. the ability to augment CO with exercise) were strongly predictive of adverse outcome, similar to prior observations in HFrEF.30, 31 Patients with HFpEF are well-known to display abnormalities in myocardial stress reserve with exercise, often despite apparently normal resting values,32–34 explaining this limitation, and by confirming the prognostic impact of this reserve limitation, the current data reinforce the clinical significance for impairments in CO reserve in HFpEF.
In contrast to exercise CO, there was no association between AVO2diff at rest or exercise and risk for adverse outcomes. Multiple (though not all) studies have shown that the ability to augment AVO2 difference with exercise is blunted in patients with HFpEF, contributing to impairments in exercise capacity.7, 8, 12 In the present study, we observed highly significant associations with peak VO2, but no association between exercise AVO2 difference. However, not all patients were able to attain an RER at peak exercise indicating a true “maximal” workload. This may result in lower AVO2 difference as compared to what would be expected at higher RER, which could influence the prognostic value of peak AVO2 difference. It should also be remembered that AVO2 difference increases as CO decreases, because there is prolonged capillary transit time to allow for O2 diffusion in the tissues, functioning as an “auto-correcting” adaptation to tissue hypoperfusion.4, 12 It is also important to consider that relationships with HF hospitalizations or death are not equivalent to relationships with exercise capacity, quality of life, or health status, and peripheral impairments are well-known to play important roles in these aspects of the HFpEF syndrome.7, 8, 12
Integrated Measures
Applying partition values previously shown to be associated with risk during upright exercise, mean PAP/CO slope (>3 mmHg/l/min)15 and PAWP/CO slope (>2 mmHg/l/min)14 were both associated with increased risk of events in univariate analysis in the overall group (i.e. including both NCD and HFpEF), but this was not maintained in the multivariable Cox analysis, except for mean PAP/CO using end-expiratory pressure.14, 15 However, these slopes were determined based upon peak exercise and resting data alone, rather than through linear regression of multipoint pressure and flow measures throughout the totality of exercise, and this methodologic difference may explain some of the discrepant findings.
Other reasons for the different findings between the present study and the prior two studies14, 15 are not clear, but may relate in part to differences in body position during exercise, and the method of deriving the pressure-flow slopes as well as differences in patient characteristics. The prior studies included patients who were much younger, less obese, and that displayed much lower comorbidity burden than in the present study, and also included a more heterogenous sample, including patients with other disease states such as precapillary pulmonary hypertension.14, 15 In this study, the pressure-flow slopes derived relied on subtraction of peak from baseline values. This is quite distinct from the multiple matched PCWP and CO measurements used in these two studies, which may have less variability through use of repeated measures.
A potentially even more relevant difference is body position. The earlier studies were performed upright, whereas exercise was performed in the supine position in the present study. Pressures are lower at rest, and pulmonary vascular pressures increase in a more linear fashion during exercise when upright, but in the supine position, venous return is higher at rest and maximized earlier during exercise, such that pressures are higher at rest, rise more dramatically at lower workloads, and then tend to plateau, resulting in a curvilinear relationship.35 While the absolute change of CO during exercise was similar in the present and the prior studies,15 the increase in pulmonary vascular pressures was greater in the present study with supine exercise. This suggests that a higher cutpoint for PAWP/CO and mean PAP/CO slope may be necessary for supine exercise, and this is supported by the stronger associations with outcome using higher cutpoints (>4.2 mmHg/L/min, >5.5 mmHg/L/min) in the present analysis. However, even using these higher cutpoints, risk stratification using these integrated measures was not superior to using individual pressures or exercise CO measures and did not remain independently associated with outcome in the multivariable Cox modeling, with the exception of end-expiratory mean PAP/CO slope. This reinforces the importance of considering body position in the interpretation of invasive exercise hemodynamic data for both clinical and research purposes.
Limitations
Individuals participating in this study were referred for invasive testing at a tertiary referral center, which may introduce bias. The observational nature of the data does not provide ability to discern causality in the associations with outcome. The NCD population was not truly normal in that they had prevalent comorbidities and by the fact that they were referred to invasive exercise stress testing for evaluation of exertional dyspnea, but this would only be expected to bias the results toward the null, as events would be expected to be less common in a cohort of completely healthy volunteers. Deaths and HF hospitalizations were identified and adjudicated through exhaustive chart review. While this captures events occurring within the Mayo Health system and other health systems that using the common EPIC electronic health record platform, it is possible that some events were not captured. While this may decrease our estimate for the annual event rate, this limitation applies equally to HFpEF and NCD patients, resulting in no bias regarding risk stratification. Many of the hemodynamic indices evaluated in this study may also be estimated noninvasively using echocardiography and magnetic resonance imaging in place of invasive testing, which may also have prognostic importance, but the variability and accuracy is less robust for these methods compared to invasive exercise testing. Pulmonary vascular pressure-flow relationships were estimated by subtracting resting pressures and CO from corresponding values at peak exercise, rather than by applying linear regression to multiple datapoints throughout all exercise stages as shown in the previous studies.14, 15 While values estimated in this way would differ from regression-based estimates, this method better aligns with the analysis that is performed in clinical laboratories were multipoint linear regression is not used in reporting. Finally, as typically observed in practice, not all patients (cases and controls) were able to attain an RER>1.05 at peak exercise. This may result in lower CO and AVO2 difference as compared to what would be expected at higher RER, which could influence the associations between CO and AVO2 difference with outcomes.
Conclusions
Increases in PAWP at rest and during exercise to levels currently used for diagnostic evaluation of HFpEF are associated with increased risk of adverse events among patients with exertional dyspnea and preserved EF. Similar associations with adverse outcome exist for additional hemodynamic variables, including RA and PA pressure, and PA compliance at rest and during exercise. While resting CO does not discriminate risk in patients with HFpEF, limited ability to augment CO with exercise is independently associated with adverse outcomes and appears to principally explain the association between peak VO2 and outcomes in HFpEF. These data provide new insight into the prognostic implications for hemodynamic abnormalities observed in patients with HFpEF and support their importance as therapeutic targets to improve outcome.
Supplementary Material
Acknowledgments
The authors thank the staff of the Mayo Clinic Earl Wood Catheterization Laboratory and the patients who agreed to participate in research, allowing for this study to be completed.
Sources of Funding
B.A.B. is supported by R01 HL128526 and U01 HL160226 from the NHLBI and W81XWH2210245 from the United States Department of Defense. H.S. is supported by a research fellowship from the Uehara Memorial Foundation, Japan. K.O. is supported by Japan Heart Foundation / Bayer Yakuhin Research Grant Abroad and the JSPS Overseas Research Fellowships from the Japan Society for the Promotion of Science. F.H.V. is supported by a Fellowship of the Belgian American Educational Foundation (B.A.E.F.) and by the Special Research Fund (BOF) of Hasselt University (BOF19PD04).
Disclosures
Dr. Borlaug receives research support from the National Institutes of Health (NIH) and the United States Department of Defense, as well as research grant funding from AstraZeneca, Axon, GlaxoSmithKline, Medtronic, Mesoblast, Novo Nordisk, and Tenax Therapeutics. Dr. Borlaug has served as a consultant for Actelion, Amgen, Aria, Axon Therapies, BD, Boehringer Ingelheim, Cytokinetics, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, NGM, NXT, and VADovations, and is named inventor (US Patent no. 10,307,179) for the tools and approach for a minimally invasive pericardial modification procedure to treat heart failure.
References
- 1.Pfeffer MA, Shah AM and Borlaug BA. Heart Failure With Preserved Ejection Fraction In Perspective. Circ Res. 2019;124:1598–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske-Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P and Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40:3297–3317. [DOI] [PubMed] [Google Scholar]
- 3.Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020;17:559–573. [DOI] [PubMed] [Google Scholar]
- 4.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD and Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borlaug BA, Kane GC, Melenovsky V and Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37:3293–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos M, Opotowsky AR, Shah AM, Tracy J, Waxman AB and Systrom DM. Central cardiac limit to aerobic capacity in patients with exertional pulmonary venous hypertension: implications for heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS and Lewis GD. Mechanisms of Exercise Intolerance in Heart Failure with Preserved Ejection Fraction: The Role of Abnormal Peripheral Oxygen Extraction. Circ Heart Fail. 2015;8:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM and Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorter TM, Obokata M, Reddy YNV, Melenovsky V and Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J. 2018;39:2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy YNV, Olson TP, Obokata M, Melenovsky V and Borlaug BA. Hemodynamic Correlates and Diagnostic Role of Cardiopulmonary Exercise Testing in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2018;6:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obokata M, Olson TP, Reddy YNV, Melenovsky V, Kane GC and Borlaug BA. Haemodynamics, dyspnea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J. 2018;39:2810–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houstis NE, Eisman AS, Pappagianopoulos PP, Wooster L, Bailey CS, Wagner PD and Lewis GD. Exercise Intolerance in Heart Failure With Preserved Ejection Fraction: Diagnosing and Ranking Its Causes Using Personalized O2 Pathway Analysis. Circulation. 2018;137:148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorfs S, Zeh W, Hochholzer W, Jander N, Kienzle RP, Pieske B and Neumann FJ. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3103–12. [DOI] [PubMed] [Google Scholar]
- 14.Eisman AS, Shah RV, Dhakal BP, Pappagianopoulos PP, Wooster L, Bailey C, Cunningham TF, Hardin KM, Baggish AL, Ho JE, Malhotra R and Lewis GD. Pulmonary Capillary Wedge Pressure Patterns During Exercise Predict Exercise Capacity and Incident Heart Failure. Circ Heart Fail. 2018;11:e004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho JE, Zern EK, Lau ES, Wooster L, Bailey CS, Cunningham T, Eisman AS, Hardin KM, Farrell R, Sbarbaro JA, Schoenike MW, Houstis NE, Baggish AL, Shah RV, Nayor M, Malhotra R and Lewis GD. Exercise Pulmonary Hypertension Predicts Clinical Outcomes in Patients With Dyspnea on Effort. J Am Coll Cardiol. 2020;75:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guazzi M, Myers J and Arena R. Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. J Am Coll Cardiol. 2005;46:1883–90. [DOI] [PubMed] [Google Scholar]
- 17.Nadruz W Jr., West E, Sengelov M, Santos M, Groarke JD, Forman DE, Claggett B, Skali H and Shah AM. Prognostic Value of Cardiopulmonary Exercise Testing in Heart Failure With Reduced, Midrange, and Preserved Ejection Fraction. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovacs G, Herve P, Barbera JA, Chaouat A, Chemla D, Condliffe R, Garcia G, Grunig E, Howard L, Humbert M, Lau E, Laveneziana P, Lewis GD, Naeije R, Peacock A, Rosenkranz S, Saggar R, Ulrich S, Vizza D, Vonk Noordegraaf A and Olschewski H. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J. 2017;50. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W and Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 20.Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V and Borlaug BA. Role of Diastolic Stress Testing in the Evaluation for Heart Failure With Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation. 2017;135:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Bovenkamp AA, Wijkstra N, Oosterveer FPT, Vonk Noordegraaf A, Bogaard HJ, van Rossum AC, De Man FS, Borlaug BA and Handoko ML. The Value of Passive Leg Raise During Right Heart Catheterization in Diagnosing Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2022;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verbrugge FH, Guazzi M, Testani JM and Borlaug BA. Altered Hemodynamics and End-Organ Damage in Heart Failure: Impact on the Lung and Kidney. Circulation. 2020;142:998–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy YNV, Obokata M, Wiley B, Koepp KE, Jorgenson CC, Egbe A, Melenovsky V, Carter RE and Borlaug BA. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J. 2019;40 3721–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fayyaz AU, Edwards WD, Maleszewski JJ, Konik EA, DuBrock HM, Borlaug BA, Frantz RP, Jenkins SM and Redfield MM. Global Pulmonary Vascular Remodeling in Pulmonary Hypertension Associated with Heart Failure and Preserved or Reduced Ejection Fraction. Circulation. 2018;137:1796–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olson TP, Johnson BD and Borlaug BA. Impaired Pulmonary Diffusion in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016;4:490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain CC, Tschirren J, Reddy YNV, Melenovsky V, Redfield M and Borlaug BA. Subclinical Pulmonary Congestion and Abnormal Hemodynamics in Heart Failure With Preserved Ejection Fraction. JACC Cardiovasc Imaging. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melenovsky V, Hwang SJ, Lin G, Redfield MM and Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obokata M, Reddy YNV, Melenovsky V, Pislaru S and Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J. 2019;40:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guazzi M, Bandera F, Ozemek C, Systrom D and Arena R. Cardiopulmonary Exercise Testing: What Is its Value? J Am Coll Cardiol. 2017;70:1618–1636. [DOI] [PubMed] [Google Scholar]
- 30.Chomsky DB, Lang CC, Rayos GH, Shyr Y, Yeoh TK, Pierson RN 3rd, Davis SF and Wilson JR. Hemodynamic exercise testing. A valuable tool in the selection of cardiac transplantation candidates. Circulation 1996;94:3176–83. [DOI] [PubMed] [Google Scholar]
- 31.Lang CC, Agostoni P and Mancini DM. Prognostic significance and measurement of exercise-derived hemodynamic variables in patients with heart failure. J Card Fail. 2007;13:672–9. [DOI] [PubMed] [Google Scholar]
- 32.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD and Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A and Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009;54:402–9. [DOI] [PubMed] [Google Scholar]
- 34.Burrage MK, Hundertmark M, Valkovic L, Watson WD, Rayner J, Sabharwal N, Ferreira VM, Neubauer S, Miller JJ, Rider OJ and Lewis AJM. Energetic Basis for Exercise-Induced Pulmonary Congestion in Heart Failure With Preserved Ejection Fraction. Circulation. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasler ED, Muller-Mottet S, Furian M, Saxer S, Huber LC, Maggiorini M, Speich R, Bloch KE and Ulrich S. Pressure-Flow During Exercise Catheterization Predicts Survival in Pulmonary Hypertension. Chest. 2016;150:57–67. [DOI] [PubMed] [Google Scholar]
- 36.Vanderpool RR, Saul M, Nouraie M, Gladwin MT and Simon MA. Association Between Hemodynamic Markers of Pulmonary Hypertension and Outcomes in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Naamani N, Preston IR, Paulus JK, Hill NS and Roberts KE. Pulmonary Arterial Capacitance Is an Important Predictor of Mortality in Heart Failure With a Preserved Ejection Fraction. JACC Heart Fail. 2015;3:467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang W, Oliveira RKF, Lei H, Systrom DM and Waxman AB. Pulmonary Vascular Resistance During Exercise Predicts Long-Term Outcomes in Heart Failure With Preserved Ejection Fraction. J Card Fail. 2017;24:169–176. [DOI] [PubMed] [Google Scholar]
- 39.Malhotra R, Dhakal BP, Eisman AS, Pappagianopoulos PP, Dress A, Weiner RB, Baggish AL, Semigran MJ and Lewis GD. Pulmonary Vascular Distensibility Predicts Pulmonary Hypertension Severity, Exercise Capacity, and Survival in Heart Failure. Circ Heart Fail. 2016;9:pii: e003011. doi: 10.1161/CIRCHEARTFAILURE.115.003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reddy YNV, Obokata M, Koepp KE, Egbe AC, Wiley B and Borlaug BA. The beta-Adrenergic Agonist Albuterol Improves Pulmonary Vascular Reserve in Heart Failure With Preserved Ejection Fraction. Circ Res. 2019;124:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


