Take Home Message
Newer immunotherapy-based regimens are rapidly changing the treatment landscape for patients with metastatic renal cell carcinoma. Our study highlights their rapid uptake in the community setting, which is truly inspiring for this patient population.
Keywords: Metastatic renal cell carcinoma, Immuno-oncology, Overall survival, Time to next treatment, Time on treatment
Abstract
Background
Immuno-oncology (IO) agents and tyrosine kinase inhibitors (TKIs) have revolutionized the treatment paradigm for metastatic renal cell carcinoma (mRCC). Data on real-world usage and outcomes are limited.
Objective
To examine real-world treatment patterns and clinical outcomes for mRCC.
Design, setting, and participants
This retrospective cohort study included 1538 patients with mRCC who received first-line treatment with pembrolizumab + axitinib (P + A; n = 279, 18%), ipilimumab + nivolumab (I + N; n = 618, 40%), or TKI monotherapy (TKIm; cabozantinib, sunitinib, pazopanib, or axitinib; n = 641, 42%) between January 1, 2018 and September 30, 2020 in US Oncology Network/non-network practices.
Outcome measurements and statistical analysis
The relationship with outcomes, time on treatment (ToT), time to next treatment (TTNT), and overall survival (OS) was analyzed using multivariable Cox proportional-hazards models.
Results and limitations
The median age of the cohort was 67 yr (interquartile range 59.5–74.4), 70% were male, 79% had clear cell RCC, and 87% had an intermediate or poor International mRCC Database Consortium risk score. The median ToT was 13.6 for P + A versus 5.8 for I + N versus 3.4 mo for TKIm (p < 0.001) and the median TTNT was 16.4 for P + A versus 8.3 for I + N versus 8.4 mo for TKIm (p < 0.001) . Median OS was not reached for P + A, 27.6 mo for I + N, and 26.9 mo for TKIm (p = 0.237). On adjusted multivariable analysis, treatment with P + A was associated with better ToT (adjusted hazard ratio [aHR] 0.59, 95% confidence interval [CI] 0.47–0.72 vs I + N; 0.37, 95% CI, 0.30–0.45 vs TKIm; p < 0.0001) and better TTNT (aHR 0.61, 95% CI 0.49–0.77 vs I + N; 0.53, 95% CI 0.42–0.67 vs TKIm; p < 0.0001). Limitations include the retrospective design and the limited follow-up for characterization of survival.
Conclusions
We noted substantial uptake of IO-based therapies in the first-line community oncology setting since their approval. In addition, the study provides insights into clinical effectiveness, tolerability, and/or compliance of IO-based therapies.
Patient summary
We examined the use of immunotherapy for patients with metastatic kidney cancer. The findings suggest rapid implementation of these new treatments by oncologists working in the community setting, which is reassuring for patients with this disease.
1. Introduction
Renal cell carcinoma (RCC) is the eighth most common cancer in the USA, affecting more than 580 000 individuals. In 2022, an estimated 79 000 new cases of RCC will be diagnosed with an estimated 13 920 deaths from the disease [1]. The 5-yr RCC survival rates for patients with regional disease (72.3%) or distant disease (15.3%) are still poor [2].
The introduction of tyrosine kinase inhibitors (TKIs) in 2006 [3], [4], [5], [6] and immuno-oncology (IO) agents in 2018 [7], [8], [9] changed the treatment paradigm for metastatic RCC (mRCC). The current National Comprehensive Cancer Network guidelines recommend many first-line (1L) treatments, including IO-IO or IO-TKI combinations and TKI monotherapy [10].
Despite robust clinical trial data for these modern IO- and TKI-based therapies, their effectiveness in the real-world setting is not well reported. Clinical trials have strict inclusion and exclusion criteria that often result in dissimilar baseline characteristics between clinical trial patients and real-world populations, which impacts the external validity of clinical trial results and their generalizability to the real world [11]. A few US real-world studies observed that the ipilimumab + nivolumab combination exhibited clinical efficacy and was well tolerated, but no comparison has been made against other treatments [11], [12], [13]. With the evolving 1L treatment landscape, it is important to understand real-world treatment patterns and sequencing for these modern IO and TKI agents. Here we examined treatment patterns, sequencing, and clinical effectiveness outcomes (time on treatment [ToT], time to next treatment [TTNT], and overall survival [OS]) for patients with mRCC receiving 1L IO- or TKI-based therapy.
2. Patients and methods
2.1. Data source
We used electronic health record (EHR) data maintained by McKesson Specialty Health in the iKnowMed (iKM) database, an oncology-specific EHR system implemented across The US Oncology Network and non-network community oncology practices. The US Oncology Network includes ∼1300 affiliated physicians operating in more than 480 sites of care across the USA and treats approximately 1.2 million cancer patients annually [14]. In addition, approximately 80 non-network clinics have adopted iKM EHRs and provide data for real-world evidence research. Study data were sourced from the structured fields of the iKM EHR database, with supplementary vital status obtained from the Social Security Administration’s Limited Access Death Master File. A waiver for informed consent was obtained from the US Oncology institutional review board for compliance/privacy.
2.2. Study design and population
This retrospective cohort study included adult patients (age ≥18 yr) diagnosed with mRCC who started 1L systemic treatment between January 1, 2018 and September 30, 2020 (study inclusion period) and were followed until December 31, 2020, death, or loss to follow-up, whichever occurred first (study observation period). The date of initiation of 1L systemic treatment was defined as the study index date. Eligible patients were required to have a minimum of two physician visits during study period at a US Oncology Network or non-network practice. Patients enrolled in clinical trials or those who had another primary cancer diagnosis besides mRCC during the study period were excluded.
2.3. Treatments and outcomes
Patient characteristics were assessed during a period of 60 d before the index date, which included age, sex, race, smoking status, body mass index (BMI), Eastern Cooperative Oncology Group (ECOG) performance status (PS), and International Metastatic RCC Database Consortium (IMDC) risk score. Since direct information on IMDC risk status was not available for all patients, information on six prognostic factors needed to assign IMDC risk category was collected at the index date. According to the number of risk factors they had, patients were classified as having favorable (0 factors), intermediate (1 or 2 factors), or poor risk (3–6 factors). Patients with information missing for one or more factors were classified considering the number of available factors meeting or not meeting the criteria and the number of missing factors (Supplementary Table 1). In addition, the reason for treatment discontinuation was not available as structured data and was not captured.
The 1L treatments considered for inclusion were pembrolizumab + axitinib, ipilimumab + nivolumab, and TKI monotherapy (pazopanib, cabozantinib, sunitinib, axitinib). Individual components of combination regimens had to be started within 28 d of each other. Five patients had started a regimen of avelumab + axitinib as 1L treatment and were not included in the study because of the small sample size of this group.
Treatment sequences were considered according to the absolute order of treatment regimens. The start and stop dates for individual medications (of the regimen) were used to capture treatment order. A change in treatment (or line of therapy) was considered when a new oral treatment started with at least two consecutive records, or a new intravenous (IV) treatment started with at least one record of IV infusion. We examined clinical effectiveness outcomes in terms of time on treatment (ToT), time to next treatment (TTNT), and overall survival (OS). ToT is defined as the time between the index date and one of the following: date of initiation of second-line (2L) therapy, date of death while on 1L therapy, or date of last administration if there was a gap of ≥120 d between last administration date for 1L therapy and last known activity date in the data set or the study end date, whichever occurred first. If none of these criteria were met, patients were censored at last administration date for 1L therapy [15]. TTNT was defined as the time between the index date and initiation of the next treatment or death, whichever occurred first. If none of these criteria were met, patients were censored at the last visit date or the study end date [11], [16]. OS was defined as the time from the index date to date of death. Patients who did not have a record of death during the follow-up period were censored at the last visit date or the study end date, whichever occurred first.
2.4. Statistical analysis
Patient characteristics were summarized for the overall cohort and for the treatment groups. Continuous variables are reported as the median and interquartile range (IQR), and categorical variables as the frequency and percentage. Differences in continuous and categorical variables were assessed using Student’s t test and a χ2 test, respectively. We used the Kaplan-Meier method with a log-rank test to analyze all time-to-event outcomes. Unadjusted and adjusted Cox proportional-hazard models were constructed to assess the association of 1L treatment with clinical outcomes. We controlled for patient age, sex, race, smoking status, BMI, ECOG PS, and IMDC risk score in adjusted regression analyses. These variables were selected on the basis of their statistical significance and/or clinical importance. Similar analyses were also performed for subgroups of patients with clear cell histology and intermediate/poor IMDC risk score. All statistical analyses were performed using SAS v9.4 with an a priori significance level of p < 0.05.
3. Results
We identified 1538 patients with mRCC, of whom 18% (n = 279) received pembrolizumab + axitinib, 42% (n = 641) received ipilimumab + nivolumab, and 40% (n = 618) received TKI monotherapy. In the TKI monotherapy cohort, 33% (n = 203), 32% (n = 197), 22% (n = 137), and 13% (n = 81) received pazopanib, cabozantinib, sunitinib, and axitinib monotherapy, respectively.
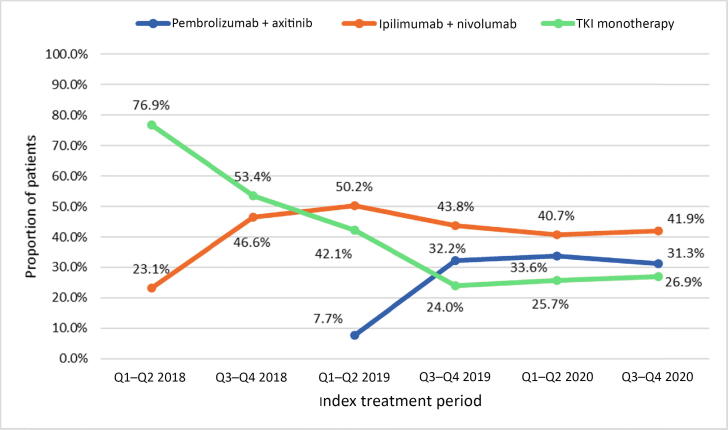
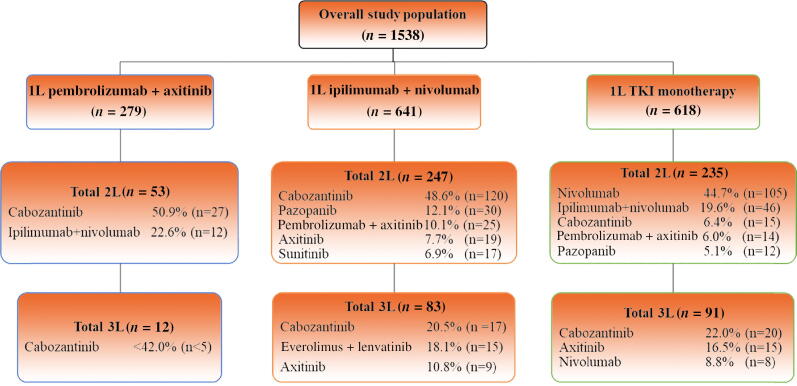
Table 1 lists the patient characteristics for the full study cohort and the treatment groups. For the full study cohort, the median age at mRCC diagnosis was 67 yr (IQR 60–74), 70% (n = 1076) were male, 70% (n = 1081) were White, 6% (n = 92) were African American, and 38% (n = 587) had BMI ≥30 kg/m2. Clear cell carcinoma was the most common histology (n = 1208, 79%) and 87% of the patients (n = 1338) had an intermediate/poor IMDC risk score. The IMDC risk score was missing for 55 patients (3%). The ipilimumab + nivolumab cohort tended to be slightly younger (median age 65.8 vs 67.8 vs 68.6 yr) and had a higher proportion of patients with intermediate/poor IMDC risk (93.6% vs 85.7% vs 80.7%) in comparison to the pembrolizumab + axitinib and TKI monotherapy cohorts. Median follow-up was 7.2 mo (IQR 4.2–11.8) for the pembrolizumab + axitinib group, 8.5 mo (IQR 3.5–15.7) for the ipilimumab + nivolumab group, and 7.8 mo (IQR 2.0–18.0) for the TKI monotherapy group. We observed an increases in the use of pembrolizumab + axitinib (8% in quarter 1 [Q1] and Q2 2019 to 31% in Q3 2020) and ipilimumab + nivolumab (23% in Q1 and Q2 2018 to 42% in Q3 2020) following their approval by the US Food and Drug Administration (FDA; pembrolizumab + axitinib in April 2019; ipilimumab + nivolumab in April 2018; Fig. 1). Overall, 35% (n = 535) of the patients received 2L treatment including 19% (n = 53) of the pembrolizumab + axitinib group, 39% (n = 247) of the ipilimumab + nivolumab group, and 38% (n = 235) of the TKI monotherapy group. The most common 2L therapies were cabozantinib (51%, n = 27) and ipilimumab + nivolumab (23%, n = 12) for the pembrolizumab + axitinib cohort; cabozantinib (49%, n = 120), pazopanib (12%, n = 30), and pembrolizumab + axitinib (10%, n = 25) for the ipilimumab + nivolumab cohort; and nivolumab (45%, n = 105), ipilimumab + nivolumab (20%, n = 46), and cabozantinib (6%, n = 15) for the TKI monotherapy cohort. Overall, only 13% (n = 186) of patients subsequently received 3L therapy, for which cabozantinib was the most common 3L drug among all three cohorts (Fig. 2).
Table 1.
Demographic and clinical characteristics of patients with metastatic renal cell carcinoma starting first-line systemic treatment
| Parameter | Overall study | Treatment group |
|||
|---|---|---|---|---|---|
| population | Pembro + Axi | Ipi + Nivo | TKIm | p value | |
| (n = 1538) | (n = 279) | (n = 641) | (n = 618) | ||
| Median age, yr (IQR) | 67.1 (59.5–74.4) | 67.8 (60.6–76.0) | 65.8 (57.8–72.1) | 68.6 (60.9–75.6) | <0.0001 |
| Age category, n (%) | 0.0073 | ||||
| 18–49 yr | 114 (7.4) | 63 (9.8) | 15 (5.4) | 36 (5.8) | |
| 50–64 yr | 543 (35.3) | 238 (37.1) | 101 (36.2) | 204 (33.0) | |
| ≥65 yr | 881 (57.3) | 340 (53.0) | 163 (58.4) | 378 (61.2) | |
| Sex, n (%) | 0.0237 | ||||
| Female | 462 (30.0) | 87 (31.2) | 169 (26.4) | 206 (33.3) | |
| Male | 1076 (70.0) | 192 (68.8) | 472 (73.6) | 412 (66.7) | |
| Race, n (%) | 0.1388 | ||||
| White or Caucasian | 1081 (70.3) | 184 (66.0) | 470 (73.3) | 427 (69.1) | |
| Black or African American | 92 (6.0) | 18 (6.5) | 39 (6.1) | 35 (5.7) | |
| Other | 365 (23.7) | 77 (27.6) | 132 (20.6) | 156 (25.2) | |
| Tobacco use, n (%) | 0.9072 | ||||
| No history of tobacco use | 475 (30.9) | 85 (30.5) | 201 (31.4) | 189 (30.6) | |
| Former tobacco use | 469 (30.5) | 90 (32.3) | 197 (30.7) | 182 (29.5) | |
| Current tobacco use | 154 (10.0) | 31 (11.1) | 68 (10.6) | 55 (8.9) | |
| Not documented | 440 (28.6) | 73 (26.2) | 175 (27.3) | 192 (31.1) | |
| BMI category, n (%) | <0.0001 | ||||
| Underweight/normal (<24.9 kg/m2)a | 405 (26.3) | 66 (23.7) | 175 (27.3) | 164 (26.5) | |
| Overweight (25–29.9 kg/m2) | 492 (32.0) | 97 (34.8) | 218 (34.0) | 177 (28.6) | |
| Obese (≥30 kg/m2) | 587 (38.2) | 111 (39.8) | 238 (37.1) | 238 (38.5) | |
| Not documented | 54 (3.5) | 5 (1.8) | 10 (1.6) | 39 (6.3) | |
| ECOG PS at index date, n (%) | 0.0293 | ||||
| 0–1 | 782 (50.8) | 142 (50.9) | 354 (55.2) | 286 (46.3) | |
| ≥2 | 232 (15.1) | 38 (13.6) | 90 (14.0) | 104 (16.8) | |
| Not documented | 524 (34.1) | 99 (35.5) | 197 (30.7) | 228 (36.9) | |
| Histology, n (%) | <0.0001 | ||||
| Clear cell carcinoma | 1208 (78.5) | 239 (85.7) | 524 (81.7) | 445 (72.0) | |
| Non–clear cell carcinoma | 166 (10.8) | 20 (7.2) | 58 (9.0) | 88 (14.2) | |
| Not documented | 164 (10.7) | 20 (7.2) | 59 (9.2) | 85 (13.8) | |
| IMDC risk score, n (%) | <0.0001 | ||||
| Favorable/intermediate | 150 (9.7) | 33 (11.8) | 34 (5.3) | 83 (13.4) | |
| Intermediate/poor | 1338 (87.0) | 239 (85.7) | 600 (93.6) | 499 (80.7) | |
| Unknown | 50 (3.3) | 7 (2.5) | 7 (1.1) | 36 (5.8) | |
| Median follow-up, mo (IQR) | 8.0 (3.2–15.6) | 7.2 (4.2–11.8) | 8.5 (3.5–15.7) | 7.8 (2.0–18.0) | 0.1308 |
Axi = axitinib; BMI = body mass index; ECOG PS = Eastern Cooperative Oncology Group performance status; IMDC = International Metastatic Renal Cell Carcinoma Database Consortium; Ipi = ipilimumab; IQR = interquartile range; Nivo = nivolumab; Pembro = pembrolizumab; TKIm = tyrosine kinase inhibitor monotherapy.
Since the sample size for one of the categories was <5, we combined underweight and normal weight.
Fig. 1.
Trends in first-line systemic treatment use for patients with metastatic renal cell carcinoma. Q = quarter; TKI = tyrosine kinase inhibitor.
Fig. 2.
Treatment patterns for patients with metastatic renal cell carcinoma starting first-line (1L) systemic treatment. 2L = second-line; 3L = third-line; TKI = tyrosine kinase inhibitor.
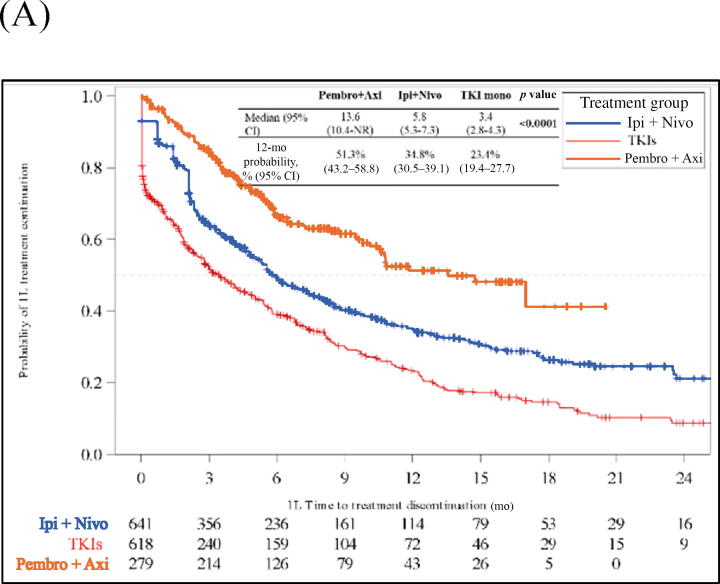
Median ToT was 13.6 mo (95% CI 10.4–not reached [NR]) for the pembrolizumab + axitinib group, 5.8 mo (95% CI 5.3–7.3) for the ipilimumab + nivolumab group, and 3.4 mo (95% CI 2.8–4.3) for the TKI monotherapy group. At 12 mo, 51% (95% CI 43–59%), 35% (95% CI 31–39%), and 23% (95% CI 19–28%) of patients in the pembrolizumab + axitinib, ipilimumab + nivolumab, and TKI monotherapy cohorts, respectively, were still on 1L treatment (Fig. 3A). On adjusted multivariable analysis, pembrolizumab + axitinib (adjusted hazard ratio [aHR] 0.37, 95% CI 0.30–0.45) and ipilimumab + nivolumab (aHR 0.63, 95% CI 0.54–0.73) were associated with longer ToT in comparison to TKI monotherapy (p < 0.001; Table 2 and Supplementary Table 2). In comparison to ipilimumab + nivolumab, pembrolizumab + axitinib was associated with longer ToT (aHR 0.59, 95% CI 0.47–0.72; p < 0.001) and TKI monotherapy was associated with shorter ToT (aHR 1.59, 95% CI 1.38–1.84; p < 0.001; Table 2 and Supplementary Table 3). Similar results were observed in all three subgroup analyses (Supplementary Figs. 1–3 and Supplementary Tables 4–6).
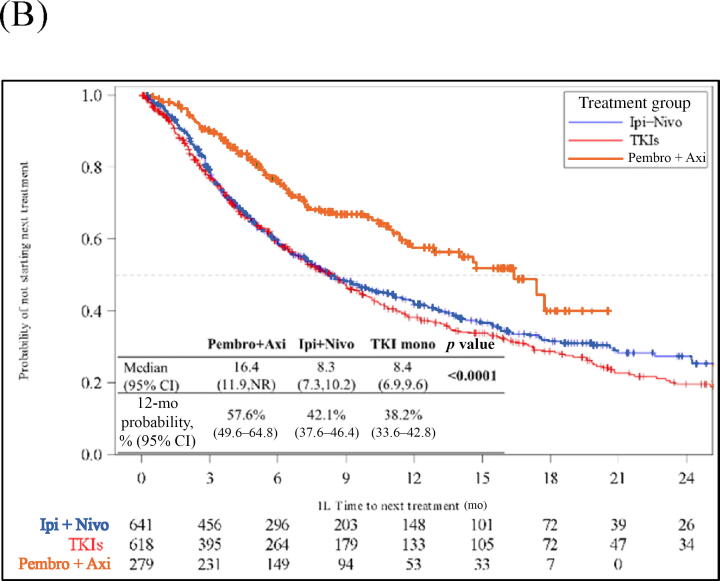
Fig. 3.
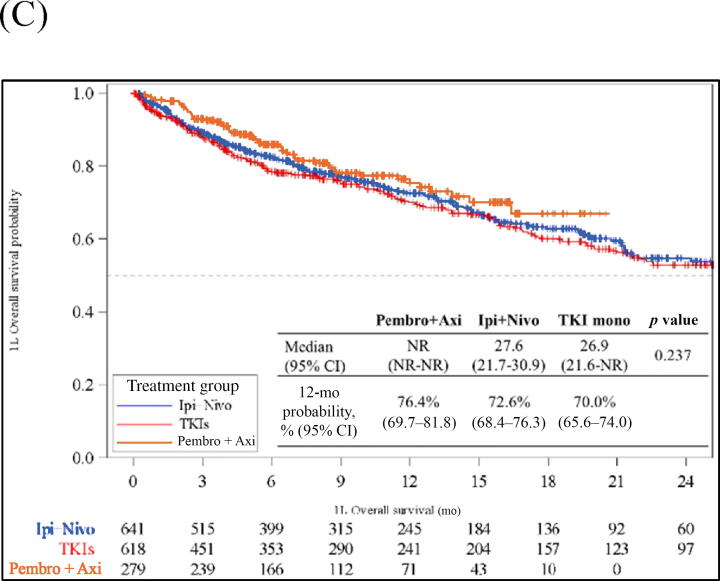
Kaplan‐Meier analysis of clinical outcomes for patients with metastatic renal cell carcinoma starting 1L systemic treatments. (A) Time on treatment. (B) Time to next treatment. (C) Overall survival. 1L = first-line; Axi = axitinib; CI = confidence interval; Ipi = ipilimumab; Nivo = nivolumab; NR = not reached; Pembro = pembrolizumab; TKI = tyrosine kinase inhibitor.
Table 2.
Association of first-line treatments with clinical outcomes according to unadjusted and adjusted Cox regression models
| Clinical outcome | Pembrolizumab + axitinib |
Ipilimumab + nivolumab |
TKI monotherapy |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Time on treatment | ||||||
| Unadjusted HR | 0.36 (0.30–0.45) | <0.0001 | 0.65 (0.56–0.74) | <0.0001 | Reference | |
| Adjusted HRa | 0.37 (0.30–0.45) | <0.0001 | 0.63 (0.54–0.73) | <0.0001 | Reference | |
| Unadjusted HR | 0.56 (0.46–0.70) | <0.0001 | Reference | 1.55 (1.35–1.78) | <0.0001 | |
| Adjusted HRa | 0.59 (0.47–0.72) | <0.0001 | Reference | 1.59 (1.38–1.84) | <0.0001 | |
| TTNT | ||||||
| Unadjusted HR | 0.53 (0.42–0.66) | <0.0001 | 0.90 (0.78–1.05) | 0.1711 | Reference | |
| Adjusted HRa | 0.53 (0.42–0.67) | <0.0001 | 0.86 (0.74–1.01) | 0.0739 | Reference | |
| Unadjusted HR | 0.59 (0.47–0.74) | <0.0001 | Reference | 1.11 (0.96–1.29) | 0.1711 | |
| Adjusted HRa | 0.61 (0.49–0.77) | <0.0001 | Reference | 1.16 (0.99–1.36) | 0.0739 | |
| Overall survival | ||||||
| Unadjusted HR | 0.77 (0.57–1.04) | 0.0896 | 0.96 (0.78–1.17) | 0.6735 | Reference | |
| Adjusted HRa | 0.79 (0.58–1.08) | 0.1371 | 0.94 (0.76–1.17) | 0.6096 | Reference | |
| Unadjusted HR | 0.81 (0.60–1.08) | 0.1511 | Reference | 1.04 (0.86–1.28) | 0.6735 | |
| Adjusted HRa | 0.84 (0.62–1.13) | 0.2454 | Reference | 1.06 (0.85–1.32) | 0.6096 | |
CI = confidence interval; HR = hazard ratio; TKI = tyrosine kinase inhibitor; TTNT = time to next treatment.
Adjusted analysis accounted for age, sex, race, tobacco use, body mass index, Eastern Cooperative Oncology Group performance status, histology, and International Metastatic Renal Cell Carcinoma Database Consortium risk score.
Median TTNT was 16.4 mo (95% CI 11.9–NR) for pembrolizumab + axitinib, 8.3 mo (95% CI 6.9–9.6) for ipilimumab + nivolumab, and 8.4 mo (95% CI 6.9–9.6); p < 0.001) for TKI monotherapy. At 12 mo, 58% (95% CI 50–65%), 42% (95% CI 38–46%), and 38% (95% CI 34–43%) of patients in the pembrolizumab + axitinib, ipilimumab + nivolumab, and TKI monotherapy cohorts, respectively, were alive or had not started 2L treatment (Fig. 3B). On adjusted multivariable analysis, pembrolizumab + axitinib was associated with longer TTNT (aHR 0.53, 95% CI 0.42–0.67; p < 0.001) and ipilimumab + nivolumab was associated with similar TTNT (aHR 0.87, 95% CI 0.74–1.01; p = 0.074) in comparison to TKI monotherapy (Table 2 and Supplementary Table 2). On multivariable analysis with ipilimumab + nivolumab as the reference group, pembrolizumab + axitinib was associated with longer TTNT (aHR 0.61, 95% CI 0.49–0.77; p < 0.001) and TKI monotherapy was associated with similar TTNT (aHR 1.16, 95% CI 0.99–1.36; p = 0.074; Table 2 and Supplementary Table 3). Pembrolizumab + axitinib and ipilimumab + nivolumab were associated with longer TTNT in comparison to TKI monotherapy across three subgroups: patients with intermediate/poor IMDC risk score (Supplementary Fig. 1 and Supplementary Table 4), patients with clear cell histology (Supplementary Fig. 2 and Supplementary Table 5, and patients with both clear cell histology and intermediate/poor IMDC risk score (Supplementary Fig. 3 and Supplementary Table 6).
Median OS was not reached for pembrolizumab + axitinib, 27.6 mo (95% CI 21.7–30.9) for ipilimumab + nivolumab, and 26.9 mo (95% CI 21.7–30.9) for TKI monotherapy. No difference in OS was noted between the three study cohorts. At 12 mo, 76% (95% CI 70–82%), 73% (95% CI 68–76%), and 70% (95% CI 66–74%) of patients in the pembrolizumab + axitinib, ipilimumab + nivolumab, and TKI monotherapy cohorts, respectively, were still alive (Fig. 3C). On adjusted multivariable analysis, no significant difference in OS was noted for pembrolizumab + axitinib (aHR 0.79, 95% CI 0.58–1.08; p = 0.1371) or ipilimumab + nivolumab (aHR 0.95, 95% CI 0.76–1.17; p = 0.610) in comparison to TKI monotherapy (Table 2 and Supplementary Table 2). In comparison to ipilimumab + nivolumab, there was no significant difference in OS for pembrolizumab + axitinib (aHR 0.84, 95% CI 0.62–1.13; p = 0.245) or TKI monotherapy (aHR 1.06, 95% CI 0.85–1.32; p = 0.610; Table 2 and Supplementary Table 3). Similar results were observed in subgroup analyses, except the pembrolizumab + axitinib cohort had longer survival in comparison to the TKI monotherapy cohort in the subgroup of patients with intermediate/poor IMDC risk score (aHR 0.72, 95% CI 0.52–0.99; p = 0.045; Supplementary Figs. 1–3 and Supplementary Tables 4–6).
4. Discussion
IO + IO and IO + TKI combination therapies have revolutionized management of mRCC. This is the largest study to provide insights into real-world treatment patterns, sequencing, and clinical outcomes for these therapies in the community oncology setting in the USA. Our study mostly included White males older than 65 yr. Approximately 30% of the study population were not White, and only 6% were African American. Despite being one of the largest studies capturing data from more than 1300 physicians providing care in more than 480 centers, our study highlights the disparity in access to and utilization of these therapies.
Consistent with the study by Zakharia et al. [17], we observed a marked increase in the uptake of both pembrolizumab + axitinib and ipilimumab + nivolumab combinations since their respective FDA approval. In our study, consistent with the literature [18], [19], [20], [21], 2L therapies were guided by 1L therapies: patients received TKI monotherapy after IO-based 1L treatment, and received an IO-based therapy after TKI-based 1L treatment. Cabozantinib was the most common 2L therapy among patients receiving IO-based 1L therapy, and nivolumab was the most common 2L therapy among patients receiving 1L TKI monotherapy. We also observed many instances of off-label use of IO-based therapies in patients with non–clear cell histology, use of 2L TKI therapies in the post-IO setting, and use of another IO-based therapy after receiving IO-based 1L therapy. These patterns of use suggest rapid advancement of the 1L treatment landscape for mRCC and a lack of data for the advanced 2L setting and beyond.
This is the first real-world study to compare 1L treatments in terms of ToT and TTNT, which have been suggested as effectiveness endpoints in real-world studies owing to their moderate to high correlation with progression-free survival (PFS) [22], [23], [24], [25]. In our study, the pembrolizumab + axitinib combination provided the longest ToT and TTNT, followed by the ipilimumab + nivolumab combination. In comparison to TKI monotherapy, pembrolizumab + axitinib and ipilimumab + nivolumab were associated with reductions in the risk of treatment discontinuation (ToT) of 63% and 37%, and reductions in the risk of subsequent treatment (TTNT) of 47% and 14%, respectively. Furthermore, in comparison to ipilimumab + nivolumab, pembrolizumab + axitinib reduced the risk of treatment discontinuation (ToT) by 41% and the risk of subsequent treatment initiation (TTNT) by 39%. Our findings are consistent with systematic reviews and network meta-analyses of clinical trials on 1L systemic treatment in advanced RCC that noted a robust treatment response in terms of PFS with pembrolizumab + axitinib in comparison to ipilimumab + nivolumab and sunitinib monotherapy [26], [27].
Our study did not reveal any difference in survival between 1L therapies, most likely because of the short follow-up duration. In addition, one-third of patients in the TKI monotherapy or IO-IO cohorts received cabozantinib- or nivolumab-based 2L therapy, which may have influenced OS [28]. We plan to continue following patients closely and to provide subsequent updates in a future paper. Our study findings are consistent with two real-world studies that found no difference in OS between pembrolizumab + axitinib and ipilimumab + nivolumab [29], [30].
Our study has some limitations. The retrospective design is prone to selection bias arising from how the study participants were selected or followed, which could have affected the apparent association between exposure and outcomes. In addition, even though we adjusted for key variables, there may be unobservable differences in treatment cohorts not accounted for in the adjusted analysis, biasing the results. iKM EHR data are not collected for research purposes but for clinical practice reasons. This may have impeded standardization of the data collection methods and physician reporting. Services and procedures provided outside of physician offices (eg, hospitalizations) are not captured by the database. Oral therapies were recorded as prescribed through iKM, but fulfillment of those prescriptions was not observable. Reasons for discontinuation are not collected in the structured data fields and there may be unobservable differences in treatment cohorts not accounted for in the adjusted analyses, biasing the results. The study also has limited follow-up of 8 mo for the overall cohort; longer follow-up for assessing any OS benefit is warranted.
5. Conclusions
We noted a substantial increase in the uptake of IO-based combination 1L treatments in the community oncology setting since their regulatory approval. Choice of 2L treatment was guided by the 1L treatment received, with most patients receiving a TKI if they received IO-based 1L therapy, and IO-based therapy if they received 1L TKI treatment. IO-based therapies yielded better clinical outcomes in comparison to TKI monotherapy. Longer follow-up is needed to understand oncological outcomes according to treatment sequences.
Author contributions: Neil J. Shah had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Shinde, Singhal, Sura, Shah.
Acquisition of data: Shi.
Analysis and interpretation of data: Shah, Sura, Shinde, Shi, Singhal, Robert, Vogelzang, Perini, Motzer.
Drafting of the manuscript: Sura, Shinde.
Critical revision of the manuscript for important intellectual content: Shah, Sura, Shinde, Singhal.
Statistical analysis: Shi.
Obtaining funding: Shinde.
Administrative, technical, or material support: None.
Supervision: None.
Other: None.
Financial disclosures: Neil J. Shah certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Neil J. Shah has a consulting/advisory role with Merck Sharp & Dohme LLC, Exelixis, Aravive and has received research funding from Aravive. Sneha Sura is an employee of Ontada. Reshma Shinde is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and own stock in Merck & Co., Inc., Rahway, NJ, USA. Junxin Shi is an employee of Ontada. Puneet Singhal is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and own stock in Merck & Co., Inc., Rahway, NJ, USA. Nicholas J. Robert is an employee of and owns stock in Ontada; has a consulting/advisory role with New Century Health, Bristol-Myers Squibb, Boehringer Ingelheim, and Advi; and has received honoraria from Roche and Bristol-Myers Squibb. Nicholas J. Vogelzang has a consulting/advisory role with Pfizer, Bayer, Genentech/Roche, AstraZeneca, Caris Life Sciences, Tolero Pharmaceuticals, Merck, Astellas Pharma, Boehringer Ingelheim, Corvus Pharmaceuticals, Modra Pharmaceuticals, Clovis Oncology, Janssen Oncology, Eisai, and Myovant Sciences; participates in speaker bureaus for Bayer, Sanofi, Genentech/Roche, Bristol-Myers Squibb, Seattle Genetics/Astellas, Clovis Oncology, AVEO, Myovant Sciences, and AstraZeneca; has received travel and accommodation expenses from Genentech/Roche, US Oncology, Pfizer, Bayer/Onyx, Exelixis, AstraZeneca/MedImmune, and Sanofi/Aventis; has provided expert testimony for Novartis; holds stock and other ownership interests in Caris Life Sciences; has received honoraria from Pfizer, Novartis, and Merck; and has received research funding from US Oncology, Endocyte, Merck, and Suzhou Kintor Pharmaceuticals. Rodolfo F. Perini is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and own stock in Merck & Co., Inc., Rahway, NJ, USA. Robert J. Motzer has a consulting/advisory role for Novartis, Eisai, Exelixis, Merck, Genentech/Roche, Incyte, Lilly, Pfizer, AstraZeneca, EMD Serono, and Calithera Biosciences; has received travel and accommodation expenses from Bristol-Myers Squibb; and has received research funding from Pfizer, Bristol-Myers Squibb, Eisai, Novartis, Genentech/Roche, Exelixis, and Merck.
Funding/Support and role of the sponsor: This work is supported by Merck & Co., Inc., Rahway, NJ, United States of America. The sponsor played a role in the design and conduct of the study; analysis and interpretation of the data; and preparation, review, and approval of the manuscript.
Associate Editor: M. Carmen Mir
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2022.12.015.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.American Cancer Society. Key statistics about kidney cancer. https://www.cancer.org/cancer/kidney-cancer/about/key-statistics.html.
- 2.Surveillance, Epidemiology and End Results Program. Cancer stat facts: kidney and renal pelvis cancer. https://seer.cancer.gov/statfacts/html/kidrp.html.
- 3.Sternberg C.N., Davis I.D., Mardiak J., et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 4.Motzer R.J., Hutson T.E., Tomczak P., et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 5.Rini B.I., Escudier B., Tomczak P., et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 6.Choueiri T.K., Escudier B., Powles T., et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1814–1823. doi: 10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rini B.I., Plimack E.R., Stus V., et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 8.Motzer R.J., Tannir N.M., McDermott D.F., et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motzer R.J., Penkov K., Haanen J., et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. Kidney cancer version 4.2022. Plymouth meeting, PA: NCCN; 2021.
- 11.Gan C.L., Stukalin I., Meyers D.E., et al. Outcomes of patients with solid tumour malignancies treated with first-line immuno-oncology agents who do not meet eligibility criteria for clinical trials. Eur J Cancer. 2021;151:115–125. doi: 10.1016/j.ejca.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Doshi G.K., Robert N.J., Chen L., et al. Real-world outcomes in patients with metastatic renal cell carcinoma treated with first-line nivolumab plus ipilimumab. J Clin Oncol. 2021;39:305. [Google Scholar]
- 13.Brown L.C., Desai K., Wei W., et al. Clinical outcomes in patients with metastatic renal cell carcinoma and brain metastasis treated with ipilimumab and nivolumab. J Immunother Cancer. 2021;9:e003281. doi: 10.1136/jitc-2021-003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The US Oncology Network. https://usoncology.com/.
- 15.Friends of Cancer Research. Considerations for use of real-world evidence in oncology. https://friendsofcancerresearch.org/wp-content/uploads/Use_of_Real-World_Evidence_in_Oncology_0-1.pdf.
- 16.Martin T., Krishnan A., Yong K., et al. Comparative effectiveness of ciltacabtagene autoleucel in CARTITUDE-1 versus physician’s choice of therapy in the Flatiron Health multiple myeloma cohort registry for the treatment of patients with relapsed or refractory multiple myeloma. EJHaem. 2022;3:97–108. doi: 10.1002/jha2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zakharia Y., Zanotti G., Liu F.X., Levin R.J., Meche A. 34P Patient characteristics and treatment patterns in advanced renal cell carcinoma (aRCC): following introduction of new therapies. Ann Oncol. 2020;31:S1429–S1430. [Google Scholar]
- 18.Shaw T., Lee H., Figlin R. Second-line therapies in the changing landscape of first-line therapies for metastatic clear cell renal cell cancer. Oncology. 2021;35:306–310. doi: 10.46883/ONC.2021.3506.0306. [DOI] [PubMed] [Google Scholar]
- 19.George S., Faccone J., Huo S., et al. Real-world treatment patterns and sequencing for metastatic renal cell carcinoma (mRCC): results from the Flatiron database. J Clin Oncol. 2021;39:286. [Google Scholar]
- 20.Geynisman D.M., Faccone J., Zhang Y., et al. Treatment sequence after first-line nivolumab plus ipilimumab or sunitinib monotherapy in patients with metastatic renal cell carcinoma (mRCC) using real-world data. J Clin Oncol. 2021;39:288. [Google Scholar]
- 21.Zakharia Y., Thomaidou D., Li B., et al. Real-world treatment outcomes of first-line axitinib plus pembrolizumab in patients with advanced renal cell carcinoma in the United States. J Clin Oncol. 2022;40:314. doi: 10.3389/fonc.2022.861189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker B., Boyd M., Aguilar K., et al. Comparisons of real-world time-to-event end points in oncology research. JCO Clin Cancer Informatics. 2021;5:45–46. doi: 10.1200/CCI.20.00125. [DOI] [PubMed] [Google Scholar]
- 23.Friends of Cancer Research. Establishing a framework to evaluate real-world endpoints. https://www.focr.org/sites/default/files/pdf/RWE_FINAL%207.6.18.pdf.
- 24.Gong Y., Kehl K., Oxnard G., Khozin S., Mishra-Kalyani P., Blumenthal G. Time to treatment discontinuation (TTD) as a pragmatic endpoint in metastatic non-small cell lung cancer (mNSCLC): a pooled analysis of 8 trials. J Clin Oncol. 2018;36:9064. [Google Scholar]
- 25.Blumenthal G.M., Gong Y., Kehl K., et al. Analysis of time-to-treatment discontinuation of targeted therapy, immunotherapy, and chemotherapy in clinical trials of patients with non-small-cell lung cancer. Ann Oncol. 2019;30:830–838. doi: 10.1093/annonc/mdz060. [DOI] [PubMed] [Google Scholar]
- 26.Mori K., Mostafaei H., Miura N., et al. Systemic therapy for metastatic renal cell carcinoma in the first-line setting: a systematic review and network meta-analysis. Cancer Immunol Immunother. 2021;70:265–273. doi: 10.1007/s00262-020-02684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elaidi R., Phan L., Borchiellini D., et al. Comparative efficacy of first-line immune-based combination therapies in metastatic renal cell carcinoma: a systematic review and network meta-analysis. Cancers. 2020;12:1673. doi: 10.3390/cancers12061673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choueiri T.K., Hessel C., Halabi S., et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): progression-free survival by independent review and overall survival update. Eur J Cancer. 2018;94:115–125. doi: 10.1016/j.ejca.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gan C.L., Dudani S., Wells J.C., et al. Outcomes of first-line (1L) immuno-oncology (IO) combination therapies in metastatic renal cell carcinoma (mRCC): results from the International mRCC Database Consortium (IMDC) J Clin Oncol. 2021;39:276. [Google Scholar]
- 30.Zarrabi KK, Handorf E, Miron B, et al. Comparative effectiveness of front-line ipilimumab and nivolumab or axitinib and pembrolizumab in metastatic clear cell renal cell carcinoma. Oncologist. In press. 10.1093/oncolo/oyac195. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.