Abstract
Although radical nephrectomy (RN) is the most common treatment for kidney cancer, no data on the learning curve for RN are available. In this study we investigated the effect of surgical experience (EXP) on RN outcomes using data for 1184 patients treated with RN for a cT1–3a cN0 cM0 renal mass. EXP was defined as the total number of RNs performed by each surgeon before the patient’s operation. The primary study outcomes were all-cause mortality, clinical progression, Clavien-Dindo grade ≥2 postoperative complications (CD ≥2), and the estimated glomerular filtration rate (eGFR). Secondary outcomes were operative time, estimated blood loss, and length of stay. Multivariable analyses adjusted for case mix revealed no evidence of association between EXP and all-cause mortality (p = 0.7), clinical progression (p = 0.2), CD ≥2 (p = 0.6), or 12-mo eGFR (p = 0.9). Conversely, EXP was associated with shorter operative time (estimate −0.9; p < 0.01). Mortality, cancer control, morbidity, and renal function might not be affected by EXP. The very large cohort examined and the extensive follow-up support the validity of these negative findings.
Patient summary
For patients with kidney cancer undergoing surgical removal of a kidney, those treated by novice surgeons have similar clinical outcomes to those treated by experienced surgeons. Thus, this procedure represents a convenient scenario for surgical training if longer operating theatre time can be planned.
Keywords: Kidney cancer, Surgery, Radical nephrectomy, Surgical experience, Learning curve, Surgical training
Renal cell carcinoma (RCC) is one of the most common solid malignancies and more than 50% of RCC cases are diagnosed with localized disease [1]. In this context, surgery is the cornerstone of management [2].
Although the relationship between patient outcomes and surgical factors such as skill and experience (EXP) has been extensively investigated for nephron-sparing surgery [3], [4], there is no description of the surgical learning curve for radical nephrectomy (RN); this gap is highly relevant, as RN is the most frequent treatment modality for clinically localised RCC [5].
It has been suggested that learning curve studies drive interventions to prevent suboptimal outcomes during a surgeon’s learning phase, such as referral strategies and structured training programs [6], [7], [8]. Therefore, we hypothesised that increasing EXP is associated with better outcomes after RN.
To test this hypothesis, clinical data for 1184 patients diagnosed with a cT1–3a cN0 cM0 renal mass treated with RN between 1987 and 2018 at a single institution were collected (Supplementary Fig. 1) after institutional ethics board approval (protocol RENE 29/08/2007).
For each individual patient, the variable of interest, namely EXP, was defined as the total number of RNs performed by each surgeon before that patient’s operation [4], [9], [10]. Owing to the importance of EXP in our analysis, we excluded cases treated by surgeons who performed fewer than 30 RNs during their entire careers. Given the hypothesis that EXP might impact mortality, cancer control, morbidity, and renal function after surgery, the primary outcomes of the study were all-cause mortality (ACM), defined as death from any cause during follow-up, clinical progression (CP), defined as either local recurrence or systemic progression during follow-up, any Clavien-Dindo (CD) grade ≥2 complication [11], and the 12-mo estimated glomerular filtration rate (eGFR). Secondary outcomes of the study were operative time (OT), estimated blood loss (EBL), and length of stay (LOS).
Statistical analyses and reporting and interpretation of the results were conducted according to established guidelines [12], [13]. Multivariable regression models were used to evaluate the effect of EXP on ACM and CP (Cox), CD ≥2 (logistic), and eGFR, OT, EBL, and LOS (linear). CP was investigated in the subgroup of confirmed RCC cases only. Covariates consisted of age at diagnosis, Charlson comorbidity index, preoperative eGFR, pathological tumour size, tumour grade (grade 1–2 vs grade G–4 vs grade x), pathological T stage (pT1–2 vs pT3–4 vs pTx), pathological N stage (pN0 vs pN1 vs pNx), surgical approach (open vs minimally invasive surgery), and year of surgery. Since the relationship between EXP and each outcome of interest might be nonlinear as result of a learning process, EXP was modelled using restricted cubic splines. In cases of a significant relationship between EXP and an outcome, model-derived coefficients and the local polynomial smoothing method were used to depict actual curves. Sensitivity analyses according to age at surgery or clinical tumour stage were performed. Statistical analysis was performed using the RStudio graphical interface v.0.98 for R software environment v.3.0.2 (http://www.r-project.org) and tests were two-sided, with the significance level set at p < 0.05.
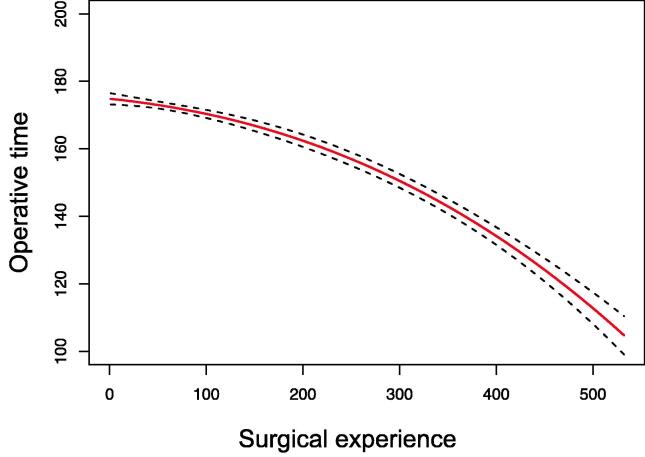
The median EXP was 60 procedures (Supplementary Table 1). After median follow-up of 81 mo, 5-yr rates of ACM and CP were 13% and 12%, respectively. The rate of CD≥2 was 14%. The median 12-mo eGFR was 56 ml/min/1.73 m2. Multivariable analyses adjusted for case mix (Table 1 and Supplementary Table 2) revealed that the associations between EXP and ACM (p = 0.7), CP (p = 0.2), CD ≥2 complications (p = 0.6), 12-mo eGFR (p = 0.9), EBL (p = 0.4), and LOS (p = 0.7) were not statistically significant. Conversely, EXP was associated with shorter OT (p < 0.01; Fig. 1). Sensitivity analyses according to patient age, clinical stage, and year of surgery confirmed these findings (all p > 0.05 on interaction tests).
Table 1.
Multivariable models predicting all-cause mortality, clinical progression, Clavien-Dindo grade ≥2 complications, and 12-mo eGFR after radical nephrectomy among 1184 patients diagnosed with a cT1–3a cN0 cM0 renal mass
| Predictor | Cox regression analysis |
Logistic regression analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| All-cause mortality |
Clinical progression a |
CD grade≥ complications |
12-mo eGFR |
|||||
| HR (95% CI) | p value | HR (95% CI) | p value | OR (95% CI) | p value | EST (95% CI) | p value | |
| Surgical experience | 1.00 (0.99–1.00) | 0.3 | 0.99 (0.99–1.00) | 0.3 | 0.99 (0.99–1.00) | 0.1 | 0.002 (−0.01 to 0.01) | 0.9 |
| Age at diagnosis | 1.05 (1.04–1.07) | <0.001 | 1.02 (1.00–1.03) | 0.01 | 1.01 (1.00–1.03) | 0.2 | −0.5 (−0.7 to −0.3) | <0.001 |
| CCI | 1.3 (1.2–1.4) | <0.001 | 1.03 (0.9–1.2) | 0.5 | 1.2 (1.1–1.4) | <0.001 | 1.6 (−0.2 to 3.4) | 0.08 |
| Preoperative eGFR | 0.99 (0.99–1.00) | 0.5 | 1.0 (0.99–1.01) | 0.9 | 0.99 (0.98–1.00) | 0.7 | 0.3 (0.2 to 0.4) | <0.001 |
| Pathologic size | 1.1(1.05–1.13) | <0.001 | 1.1 (1.1–1.2) | <0.001 | 0.9 (0.9–1.02) | 0.2 | −0.7 (−1.5 to −0.08) | 0.08 |
| Tumour stage | ||||||||
| pT1–2 | Reference | – | Reference | – | Reference | – | Reference | – |
| pT3–T4 | 1.4 (1.01–1.8) | 0.04 | 2.5 (1.8–3.5) | <0.001 | 1.2 (0.8–1.8) | 0.3 | 1.6 (−3.1 to 6.4) | 0.5 |
| pTx | 0.4 (0.1–1.7) | 0.2 | – | – | 2.3 (0.2–55) | 0.5 | 6.9 (−23 to 37) | 0.6 |
| Tumour grade | ||||||||
| Grade 1–2 | Reference | – | Reference | – | Reference | – | Reference | – |
| Grade 3–4 | 1.7 (1.3–2.3) | <0.001 | 2.4 (1.7–3.3) | <0.001 | 1.3 (0.9–1.9) | 0.2 | −1.6 (−6.6 to 3.4) | 0.5 |
| Grade x | 1.9 (0.5–8.0) | 0.3 | 0.6 (0.1–2.3) | 0.5 | 0.5 (0.02–4.6) | 0.8 | 0.4(−27 to 28) | 0.9 |
| Nodal stage | ||||||||
| pN0 | Reference | – | Reference | – | Reference | – | Reference | – |
| pN1 | 1.9 (1.01–3.5) | 0.04 | 2.4 (1.3–4.6) | <0.01 | 1.5 (0.5–4.1) | 0.4 | −2.2 (−6.8 to 2.4) | 0.3 |
| pNx | 1.1 (0.8–1.4) | 0.5 | 0.8 (0.5–1.1) | 0.2 | 1.2 (0.8–1.7) | 0.4 | −0.5(−20 to 19) | 0.9 |
| Surgical approach | ||||||||
| Open surgery | Reference | – | Reference | – | Reference | – | Reference | – |
| MIS | 0.8 (0.4–1.6) | 0.6 | 0.8 (0.4–1.6) | 0.6 | 0.4 (0.2–0.7) | <0.001 | 3.4 (−2.1 to 8.8) | 0.2 |
| Year of surgery | 0.95 (0.93–0.97) | <0.001 | 0.98 (0.96–1.01) | 0.3 | 1.05 (1.02–1.07) | <0.001 | −0.2 (−0.6 to 0.2) | 0.2 |
CCI = Charlson comorbidity index, eGFR = estimated glomerular filtration rate; CD = Clavien-Dindo; HR = hazard ratio; OR = odds ratio; EST = estimate; CI = confidence interval; MIS = minimally invasive surgery.
Clinical progression was investigated in a subcohort of 1097 patients with confirmed renal cell carcinoma at final pathology.
Fig. 1.
Surgical learning curve for radical nephrectomy: effect of increasing experience on operative time. The estimate is adjusted for age at surgery, gender, Charlson comorbidity index, pathological tumour size, pathological T stage, tumour grade, pathological N stage, surgical approach, and year of surgery. The red line denotes the probability and black dotted lines indicate the 95% confidence intervals.
The study hypothesis was that patients diagnosed with kidney cancer who opt for RN have better clinical outcomes if treated by experienced surgeons, in line with the concept that surgical results are highly dependent on human factors such as individual skills and previous background. The analysis of a very large population of RCC patients treated with RN did not provide any evidence of a relationship between EXP and clinical outcomes. Specifically, the risks of long-term mortality, cancer progression, perioperative morbidity, and renal function impairment are virtually the same for expert surgeons and novice surgeons. Hence, our observations reject this hypothesis and are in contrast to other studies demonstrating better outcomes after increased EXP in urology. For example, a patient diagnosed with prostate cancer treated by an experienced surgeon has a lower risk of positive surgical margins [14], biochemical recurrence [9], [10], and poor functional outcomes [15], [16] after radical prostatectomy. Similarly, in the case of urethroplasty, the higher the EXP of the treating physician, the lower the risk of repeat surgery [17]. In the context of renal surgery, perioperative complications and ischaemia time in patients undergoing nephron-sparing surgery fluctuate significantly according to the background and ability of the surgeon [3], [4].
However, a similar learning effect does not apply to RN, for which the impact of EXP on patient outcomes was irrelevant. This discrepancy might be explained by the technical differences that distinguish nephron-sparing surgery from RN: the latter does not involve parenchymal resection, opening of the collecting system, reconstructive suturing, or time-sensitive parameters such as warm ischaemia time. As a consequence, RN can be regarded as a less challenging surgical procedure than nephron-sparing surgery.
The clinical implications of these observations are clear: during surgical training it is mandatory to protect patients from suboptimal outcomes resulting from the learning process. In this light, RN should be regarded as an ideal scenario for clinical training in urological surgery. This important notion corroborates many other training strategies aimed at maximal reduction of any detrimental effects on patient outcome caused by the learning process, such as emphasis on preclinical training [18], modular configuration of the curriculum [6], and structured training programmes [7], [8].
Despite no evidence of superior results after extensive EXP in terms of mortality, cancer control, morbidity, and renal function, increasing EXP was associated with shorter OT. In this regard, it is important to remember that although the relevance of this finding for RN candidates might be marginal, operating theatre occupation affects daily surgical planning and health care expenditure [8].
Our study is not devoid of limitations, such as the observational noncomparative design. The inclusion of cases treated over a wide time span is both a weakness, since indications for RN or nephron-sparing surgery were different and nephrometry scores were not available, and an important strength, since a key element in learning curve analysis is the inclusion of any single patient treated by the surgeons in the study cohort. Notably, the sensitivity analyses did not provide evidence of such a confounding effect. Notwithstanding these limitations, the study has multiple important strengths, including the large study population, the long follow-up, the consideration of hard clinical endpoints, and the inclusion of very experienced surgeons, all of which are noteworthy with respect to the negative findings recorded.
In conclusion, patients undergoing RN performed by novice surgeons have similar clinical outcomes to those for patients treated by experienced surgeons. This finding highlights the status of RN as a convenient setting for clinical training in urological surgery, provided that longer operating theatre time is planned.
Author contributions: Alessandro Larcher had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Larcher, Capitanio.
Acquisition of data: Cei, Belladelli, Rosiello, Bravi, Fallara, Basile, Lucianò, Briganti, Salonia, Bertini, Montorsi.
Analysis and interpretation of data: Larcher, Rosiello, Capitanio.
Drafting of the manuscript: Larcher, Rosiello.
Critical revision of the manuscript for important intellectual content: Cei, Belladelli, Bravi, Fallara, Basile, Lucianò, Karakiewicz, Mottrie, Breda, Briganti, Salonia, Bertini, Montorsi, Capitanio.
Statistical analysis: Larcher, Rosiello.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Briganti, Salonia, Montorsi.
Other: None.
Financial disclosures: Alessandro Larcher certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: Research activity in the field of kidney cancer at the Urological Research Institute, IRCCS Ospedale San Raffaele, is supported by an unrestricted grant from Recordati. The kidney cancer data set of the Urological Research Institute is managed by Cristina Carenzi and Daniela Canibus.
Associate Editor: M. Carmen Mir
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2022.12.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Capitanio U., Bensalah K., Bex A., et al. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75:74–84. doi: 10.1016/j.eururo.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ljungberg B., Albiges L., Abu-Ghanem Y., et al. European Association of Urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75:799–810. doi: 10.1016/j.eururo.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Dagenais J., Bertolo R., Garisto J., et al. Variability in partial nephrectomy outcomes: does your surgeon matter? Eur Urol. 2019;75:628–634. doi: 10.1016/j.eururo.2018.10.046. [DOI] [PubMed] [Google Scholar]
- 4.Larcher A., Muttin F., Peyronnet B., et al. The learning curve for robot-assisted partial nephrectomy: impact of surgical experience on perioperative outcomes. Eur Urol. 2019;75:253–256. doi: 10.1016/j.eururo.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 5.Stewart-Merrill S.B., Thompson R.H., Boorjian S.A., et al. Oncologic surveillance after surgical resection for renal cell carcinoma: a novel risk-based approach. J Clin Oncol. 2015;33:4151–4157. doi: 10.1200/jco.2015.61.8009. [DOI] [PubMed] [Google Scholar]
- 6.Stolzenburg J.-U., Rabenalt R., Do M., Horn L.C., Liatsikos E.N. Modular training for residents with no prior experience with open pelvic surgery in endoscopic extraperitoneal radical prostatectomy. Eur Urol. 2006;49:491–500. doi: 10.1016/j.eururo.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Volpe A., Ahmed K., Dasgupta P., et al. Pilot validation study of the European Association of Urology robotic training curriculum. Eur Urol. 2015;68:292–299. doi: 10.1016/j.eururo.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Larcher A., De Naeyer G., Turri F., et al. The ERUS curriculum for robot-assisted partial nephrectomy: structure definition and pilot clinical validation. Eur Urol. 2019;75:1023–1031. doi: 10.1016/j.eururo.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Vickers A.J., Bianco F.J., Serio A.M., et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007;99:1171–1177. doi: 10.1093/jnci/djm060. [DOI] [PubMed] [Google Scholar]
- 10.Vickers A.J., Savage C.J., Hruza M., et al. The surgical learning curve for laparoscopic radical prostatectomy: a retrospective cohort study. Lancet Oncol. 2009;10:475–480. doi: 10.1016/S1470-2045(09)70079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clavien P.A., Barkun J., de Oliveira M.L., et al. The Clavien-Dindo classification of surgical complications. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 12.Vickers A.J., Sjoberg D.D. Guidelines for reporting of statistics in European Urology. Eur Urol. 2015;67:181–187. doi: 10.1016/j.eururo.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Assel M., Sjoberg D.D., Catto J.W.F., Vickers A.J. Innovations in statistical review at European Urology. Eur Urol. 2019;75:1–2. doi: 10.1016/j.eururo.2018.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bravi C.A., Tin A., Vertosick E., et al. The impact of experience on the risk of surgical margins and biochemical recurrence after robot-assisted radical prostatectomy: a learning curve study. J Urol. 2019;202:108–113. doi: 10.1097/JU.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson J.E., Egger S., Böhm M., et al. Superior quality of life and improved surgical margins are achievable with robotic radical prostatectomy after a long learning curve: a prospective single-surgeon study of 1552 consecutive cases. Eur Urol. 2014;65:521–531. doi: 10.1016/j.eururo.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Thompson J.E., Egger S., Böhm M., et al. Superior biochemical recurrence and long-term quality-of-life outcomes are achievable with robotic radical prostatectomy after a long learning curve—updated analysis of a prospective single-surgeon cohort of 2206 consecutive cases. Eur Urol. 2018;73:664–671. doi: 10.1016/j.eururo.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Fossati N., Barbagli G., Larcher A., et al. The surgical learning curve for one-stage anterior urethroplasty: a prospective single-surgeon study. Eur Urol. 2016;69:686–690. doi: 10.1016/j.eururo.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 18.Larcher A., Turri F., Bianchi L., et al. Virtual reality validation of the ERUS simulation-based training programmes: results from a high-volume training centre for robot-assisted surgery. Eur Urol. 2019;75:885–887. doi: 10.1016/j.eururo.2019.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.