Abstract
Objective:
Supraventricular arrhythmias are common in adults with Ebstein anomaly (EA). However, there are limited data about prognostic implications of atrial fibrillation (AF) in this population. Accordingly, our aim was to assess the clinical profile and burden of AF in adults with EA, and the relationship between AF and outcomes.
Methods and Results:
Six hundred eighty-two consecutive adults with a median age of 36 (24;49) years from Mayo Clinic, MN, between 2003 and 2020 were included. Sustained episodes of AF, clinical, echocardiographic, rhythm, surgical data were collected. Prevalence of AF at baseline was 18% (126 patients), the first episode occurred at a mean age of 43±17 years. Patients with AF were older, more likely males, had hypertension, renal dysfunction, cardiac devices and more advanced right and left-sided remodeling. During a median follow-up of 156 (81;240) months, 62 patients (11%) developed incident AF. At last encounter, prevalence of AF was 28% (188 patients), of those, 63 (34%) had recurrent AF. HF Hospitalization occurred in 51 patients (7%). AF (hazard ratio 2.32 [1.18; 4.47], p=0.01) was independently associated with HF hospitalization. All-cause death occurred in 53 patients (8%); it was more frequent in those with AF in the univariable analysis, although it did not remain significant in the multivariable analysis.
Conclusions:
AF in EA develops at relatively young ages with one third of the cohort exhibiting a recurrent pattern. Patients with AF had a higher prevalence of comorbidities and worse right and left-sided cardiac remodeling. AF was independently associated with HF hospitalization.
Keywords: Ebstein anomaly, atrial fibrillation, heart failure, left ventricular ejection fraction, tricuspid regurgitation, atrial strain
INTRODUCTION
Atrial arrhythmias (AA) are common in adults with congenital heart disease (CHD), and recent data show a temporal change in patient demographics and arrhythmia types, with atrial fibrillation (AF) now being the predominant arrhythmia in older patients.1–5 Among patients with CHD, Ebstein anomaly (EA) is also characterized by a high lifelong prevalence of AA.6, 7 Although supraventricular tachycardia are the most common arrhythmia at younger ages, acquired AA such as AF, atrial flutter or atrial tachycardia due to adverse cardiac remodeling from chronic volume overload, atriotomy scars, and acquired cardiovascular comorbidities play a more important role as patients age.1, 8 However, most of the existing literature about AA have focused primarily on supraventricular tachycardia and atrial flutter/tachycardia.8–10 As a result, there are limited data about prevalence and prognostic implications of AF in this population.11, 12 Since AF is associated with increased risk of heart failure (HF) and adverse outcomes in other forms of CHD,13–17 delineating the prevalence and prognostic implications of AF in adults with EA would improve risk stratification in this population.
Accordingly, our aim was to assess the clinical profile and burden of AF in adults with EA, and the relationship between AF and outcomes.
METHODS
Study Population
This is a retrospective cohort study of consecutive adults (age ≥18 years) with EA receiving care at Mayo Clinic, MN, between January 1, 2003 and December 31, 2020. The Mayo Clinic Institutional Review Board approved this study (ID#20-007695) and waived informed consent for patients that provided research authorization. Patients and/or the public were not involved in the design, conduct, report or dissemination plans of this research.
Clinical, echocardiographic, electrocardiographic, and laboratory data and surgical and electrophysiology procedural reports were reviewed in all patients. The first clinical evaluation performed in the Adult CHD Clinic on or after January 1, 2003 was considered as the baseline, and clinical indices obtained within the 12 months from this assessment were used to define the baseline clinical characteristics of the cohort. Adverse outcomes were defined as hospitalization for HF and all-cause death.
Assessment of AF
The diagnosis of AF was based on a manual review of medical charts, 12-lead electrocardiograms, Holter monitors, rhythm strips and device interrogation. Only episodes >30 seconds were included. In patients undergoing electrophysiology study, AF was defined as an AA with irregular atrial activation and cycle lengths below 200 milliseconds. AF that was present at the time of, or prior to baseline assessment, was defined as ‘prevalent AF at baseline’, while AF occurring from baseline evaluation to the last follow-up was considered as ‘incident AF’. The sum of ‘prevalent AF at baseline’ and ‘incident AF’ was defined as ‘prevalent AF at last follow-up’. AF was classified according to therapeutic management into rhythm (paroxysmal/persistent AF) and rate (permanent AF) control. Antiarrhythmic therapies were defined as the use of antiarrhythmic drugs (AAD) class I or III, catheter ablation, or antiarrhythmic surgery (right atrial Maze/isthmus ablation and left atrial Maze).
Echocardiographic Assessment
Comprehensive transthoracic echocardiogram was performed according to contemporary guidelines.18–20 Right atrial and left atrial reservoir strain were calculated using speckle tracking strain imaging obtained with Vivid E9 and E95 (General Electric Co, Fairfield, Connecticut) with M5S and M5Sc-D transducers (1.5–4.6 MHz) at frame rate of 40 to 80 Hz. Offline analysis of the exported images was performed with TomTec (TomTec Imaging Systems, Unterschleissheim, Germany). Adequate tracking by the software was verified and retraced if necessary. The rest of structure, function and hemodynamic indices were assessed with standard techniques.18–20
Statistical Analysis
Data were presented as count (%), mean ± standard deviation, or median (interquartile range). Between-group comparisons were performed using unpaired t-test, Wilcoxon rank-sum test, chi-square test and Fisher exact test as appropriate.
Survival analysis was performed with the Kaplan-Meier method. Multivariable Cox regression models were created to analyze the association between prevalent AF at baseline and cardiovascular adverse events. Patients with incident AF were considered as part of the ‘non-AF’ cohort. The time-to-event was defined as the interval from/prior to the baseline assessment to the occurrence of the adverse event or the end of the study period in patients without its occurrence. Due to the low number of outcomes and to avoid model overfitting, the covariates included in the model were determined a priori based on prior evidence5, 12, 21–23 and comprised clinical parameters and echocardiographic indices characterizing the function of the 4 cardiac chambers and tricuspid regurgitation assessed at baseline. Variables with a p-value <0.05 on univariable analysis were included in the multivariable model. This was followed by stepwise backwards selection of covariates in the multivariable model. Sensitivity analyses were performed by repeating the multivariable models after exclusion of patients with incident AF without significant changes in the results. These patients were therefore kept in the analyses to increase the robustness of the models. Due to the large overall sample size, to missing data of the covariates from the multivariable models comprising ≤10% of the observations, and to the large size of the multivariable models, missing data were treated by deletion (complete case analysis). Statistical analysis was conducted with JMP for SAS V. 14.1.0; p values <0.05 were considered statistically significant.
RESULTS
The cohort comprised 682 patients, median age was 36 (24; 49) years, 41% were males, and 174 (26%) had prior repair of EA (tricuspid valve repair or replacement). Of the 682 patients, 126 patients (18%) had prevalent AF at baseline, and Table 1 shows a comparison of the baseline characteristics of patients with versus without prevalent AF at baseline. Compared to patients without prior history of AF, those with prevalent AF were older, more likely to be males, had a higher prevalence of hypertension, renal dysfunction, cardiac implantable electronic devices, and more advanced chamber remodeling and dysfunction.
Table 1:
Demographic and Clinical Features
| Variables | N | All (682) | N | AF (126) | N | No AF (556) | p |
|---|---|---|---|---|---|---|---|
| Demographics and comorbidities | 682 | 126 | 556 | ||||
|
| |||||||
| Sex, male | 279 (41%) | 67 (53%) | 212 (38%) | 0.002 | |||
| Age at first visit, years | 36 (24; 49) | 36 (24; 49) | 33 (22; 46) | <0.0001 | |||
| Hypertension | 104 (15%) | 33 (26%) | 71 (13%) | 0.0004 | |||
| Dyslipidemia | 111 (16%) | 28 (22%) | 83 (15%) | 0.05 | |||
| Diabetes mellitus | 24 (4%) | 5 (4%) | 19 (3%) | 0.8 | |||
| Obesity | 126 (19%) | 29 (23%) | 97 (17%) | 0.2 | |||
| Coronary artery disease | 26 (4%) | 8 (6%) | 18 (3%) | 0.1 | |||
| Previous stroke | 38 (6%) | 9 (7%) | 29 (5%) | 0.4 | |||
| Creatinine, mg/dL | 0.98±0.24 | 1.0±0.24 | 0.97±0.24 | 0.009 | |||
| Associated congenital defects | 682 | 126 | 556 | ||||
|
| |||||||
| Atrial septal defect | 353 (52%) | 65 (52%) | 288 (52%) | >0.9 | |||
| Ventricular septal defect | 40 (6%) | 8 (6%) | 32 (6%) | 0.8 | |||
| Pulmonary stenosis | 21 (3%) | 2 (2%) | 19 (3%) | 0.4 | |||
| Prior cardiac procedures | 682 | 126 | 556 | ||||
|
| |||||||
| Cardiac surgery | 174 (26%) | 31 (25%) | 143 (26%) | 0.8 | |||
| Tricuspid valve repair | 114 (20%) | 24 (19%) | 90 (16%) | 0.4 | |||
| Tricuspid valve replacement | 60 (10%) | 7 (6%) | 53 (10%) | 0.1 | |||
| - Bioprosthesis | 58 (9%) | 7 (6%) | 51 (9%) | 0.2 | |||
| - Mechanical prosthesis | 2 (0.3%) | 0 | 2 (0.4%) | >0.9 | |||
| CIED | 57 (8%) | 18 (14%) | 39 (7%) | 0.01 | |||
| Echocardiography | |||||||
|
| |||||||
| RA volume index, mL/m2 | 609 | 56 (42; 81) | 113 | 77 (54; 133) | 496 | 52 (42; 73) | <0.0001 |
| RA reservoir strain*, % | 604 | 29±14 | 111 | 21±12 | 493 | 31±14 | <0.0001 |
| RA pressure, mmHg | 654 | 5 (5; 10) | 119 | 10 (5; 15) | 535 | 5 (5; 10) | <0.0001 |
| ≥Moderate tricuspid regurgitation | 673 | 495 (74%) | 124 | 90 (73%) | 549 | 405 (74%) | 0.8 |
| TR velocity, m/s | 584 | 2.5±0.9 | 95 | 2.4±0.3 | 489 | 2.5±1.0 | 0.8 |
| RV end-diastolic area, cm2 | 610 | 45±17 | 116 | 50±19 | 494 | 44±16 | 0.002 |
| Fractional area shortening, % | 610 | 31±9 | 116 | 29±9 | 494 | 32±9 | 0.01 |
| RV systolic pressure, mmHg | 576 | 33±8 | 94 | 34±8 | 482 | 32±7 | 0.04 |
| LA volume index, mL/m2 | 638 | 25±12 | 117 | 32±18 | 521 | 23±9 | <0.0001 |
| LA reservoir strain*, % | 607 | 32±12 | 111 | 27±12 | 496 | 33±11 | <0.0001 |
| Mitral valve E-wave, m/s | 627 | 0.7±0.3 | 109 | 0.8±0.4 | 518 | 0.7±0.2 | 0.08 |
| Medial E/e’ ratio | 504 | 9±6 | 84 | 11±8 | 420 | 9±5 | 0.04 |
| ≥ moderate mitral regurgitation | 482 | 21 (4%) | 101 | 11 (11%) | 381 | 10 (3%) | 0.001 |
| LV end-diastolic diameter, mm | 670 | 43±6 | 123 | 44±8 | 547 | 43±6 | 0.1 |
| LV end-systolic diameter, mm | 660 | 28±6 | 120 | 29±7 | 540 | 28±5 | 0.04 |
| LV ejection fraction, % | 676 | 58±8 | 124 | 56±10 | 552 | 59±7 | 0.01 |
AF: atrial fibrillation; CIED: Cardiac Implantable Electronic Device; LA: left atrial; LV: left ventricle; RA: right atrial; RV: right ventricle; TAPSE: tricuspid annular plane systolic excursion; TR: tricuspid regurgitation.
Strain was modeled as absolute values.
Of the 126 patients with prevalent AF at baseline, the mean age at the first episode was 43±17 years, 57 (45%) received AAD (25 [20%] class I AAD, 32 [25%] class III AAD), while 38 (30%) underwent direct current cardioversion for episode termination. At the time of baseline evaluation, 40 patients (32%) were on AAD (20 [16%] class I, 20 [16%] class III), 1 (0.8%) had had percutaneous catheter ablation exclusively for AF (pulmonary vein isolation [PVI] of all 4 veins), 4 (3%) right atrial Maze, with no patient having had a left atrial Maze procedure.
Sixty-two patients (11%) developed incident AF during a median follow-up time of 156 (81; 240) months (Figure 1A). At last encounter, 188 patients (28%) had at least one episode of AF. The first episode of AF of these 188 patients occurred at a mean age of 45±17 years. At last follow-up, 39 patients (21%) were on AAD (7 [4%] class I, 32 [17%] class III). Nine patients (5%) had had percutaneous catheter ablation, 2 of them underwent 2 procedures each. One patient (11%) had exclusively catheter ablation for AF (second procedure, right-sided PVI), 3 (33%) underwent AF (PVI of all 4 veins) and typical atrial flutter ablation, 3 (33%) AF (PVI of all 4 veins), typical and atypical right atrial flutter ablation, 1 (11%) AF (second procedure, left-sided PVI) and atypical right atrial flutter, and the last one (11%) AF (PVI of all 4 veins) and atypical left atrial flutter ablation. Surgical ablation was performed in 89 patients (47%): 68 (36%) right atrial, 1 (0.1%) left atrial and 20 (11%) biatrial Maze. Maximal number of left atrial Maze procedures per patient was 1, while 3 (2%) and 1 (0.5%) had 2 and 3 right atrial Maze interventions, respectively.
Figure 1.
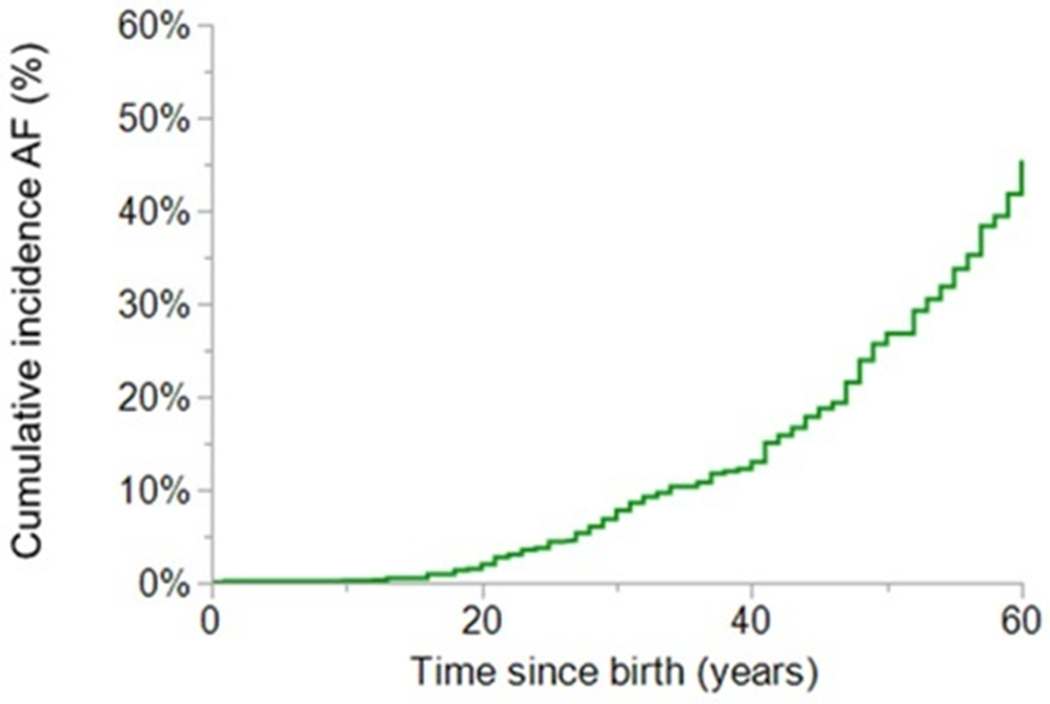
Kaplan-Meier curve depicting lifetime cumulative incidence of atrial fibrillation from birth. AF: atrial fibrillation.
Sixty-three (34%) of the patients with AF at last follow-up had recurrent AF (Figure 1B). Compared to those with a single episode, patients with recurrent AF were more likely to receive AAD (45 [71%] versus 46 [37%], p<0.0001) and direct current cardioversion for episode termination (37 [59%] versus 40 [32%], p=0.0004). Percutaneous catheter ablation was also more common among those with recurrent AF (8 [13%] versus 1 [1%], p=0.0008). Treatment with AAD at last follow-up and surgical ablation did not differ according to presence of recurrent AF.
Overall, rhythm control was the management approach for AF in 159 patients (85%), while 29 (15%) received rate control. From the latter group, 10 (34%) underwent directly rate control after the first AF episode, while in the remaining 19 (66%) transition from rhythm to rate control occurred after a mean time of 8±4 years.
From the 188 patients with AF at last follow-up, 94 (50%) also had had at least one episode of atrial flutter and/or atrial tachycardia, 38 (20%) atrioventricular reciprocating tachycardia and 4 (2%) atrioventricular nodal reentrant tachycardia during the study period. There were 52 (47%) episodes of atrial flutter and/or atrial tachycardia preceding AF, while 58 (53%) occurred in patients with prior history of AF. All but one (3%) episode of atrioventricular reciprocating tachycardia and the 4 (100%) of atrioventricular nodal reentrant tachycardia preceded AF development.
Risk of Adverse Outcomes According to Prevalence of AF
Hospitalization for HF occurred in 51 patients (7%). Hospitalization for HF stratified by prevalence of AF at baseline is depicted in Figure 2A. AF was associated with higher risk of admission for HF. Multivariable Cox proportional analysis is presented in Table 2. AF at baseline (hazard ratio [HR] 2.32 [1.18; 4.47], p=0.01), right atrial reservoir strain (HR 0.95 [0.92; 0.99], p=0.006) and left ventricular ejection fraction (HR 0.96 [0.93; 0.99], p=0.04) were independently associated with admission for HF while male sex was protective (HR 0.45 [0.22; 0.91], p=0.03).
Figure 2. Kaplan-Meier curves showing freedom from adverse events according to presence (blue) or absence (red) of atrial fibrillation.
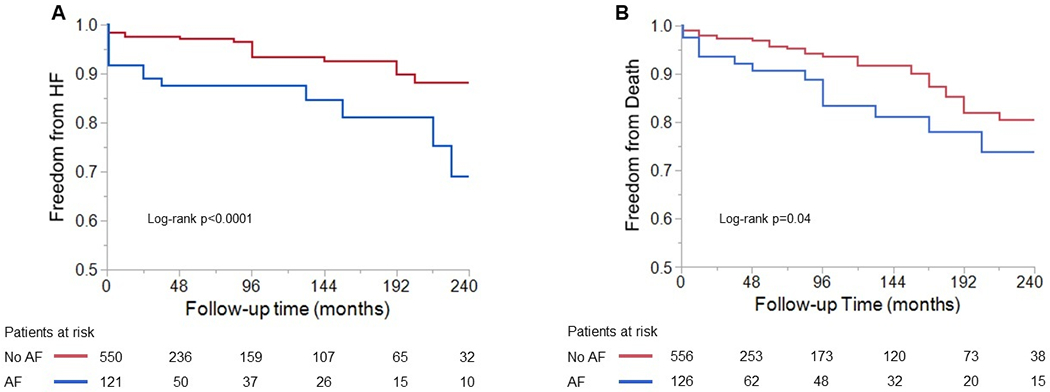
(A) Freedom from heart failure hospitalization according to prevalence of atrial fibrillation at baseline. (B) Freedom from all-cause death according to prevalence of atrial fibrillation at baseline. AF: atrial fibrillation; HF: heart failure.
Table 2:
Multivariable Cox Model of Risk Factors Associated with Heart Failure.
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p | HR (95% CI) | p |
| Age, per year | 1.04 (1.02; 1.05) | <0.0001 | - | - |
| Atrial fibrillation at baseline | 3.32 (1.90; 5.77) | <0.0001 | 2.32 (1.18; 4.57) | 0.01 |
| Creatinine, per mg/dL | 1.67 (0.51; 4.44) | 0.35 | - | - |
| Fractional area shortening, per % | 0.94 (0.92; 0.97) | 0.0001 | - | - |
| Left atrial reservoir strain*, per % | 0.95 (0.92; 0.97) | 0.0002 | - | - |
| Left ventricle ejection fraction, per % | 0.94 (0.91; 0.97) | <0.0001 | 0.96 (0.93; 0.99) | 0.01 |
| ≥ moderate tricuspid regurgitation | 0.97 (0.52; 1.78) | 0.91 | - | - |
| Right atrial reservoir strain*, per % | 0.93 (0.90; 0.96) | <0.0001 | 0.95 (0.92; 0.99) | 0.006 |
| Sex, male | 0.70 (0.39; 1.26) | 0.23 | 0.45 (0.22; 0.91) | 0.03 |
CI: confidence interval; HR: hazard ratio.
Strain was modeled as absolute values.
At the end of the study period, 53 patients (8%) died at a median follow-up time of 84 months (12; 168), and this occurred more frequently in those with AF at baseline (Figure 2B). Multivariable Cox proportional analyses are presented in Table 3. Although prevalence of AF was associated with higher likelihood of all-cause mortality in the univariable analysis, it did not remain significant after adjusting for covariates. Older age at presentation (HR 1.03 [1.01; 1.05], p=0.009), male sex (HR 2.10 [1.06; 4.17], p=0.03), left atrial reservoir strain (HR 0.93 [0.90; 0.97], p=0.0002) and presence of ≥moderate tricuspid regurgitation (HR 2.82 [1.22; 6.53], p=0.02) were independent predictors of all-cause death.
Table 3:
Multivariable Cox Model of Risk Factors Associated with All-Cause Death.
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p | HR (95% CI) | p |
| Age, per year | 1.05 (1.03; 1.07) | <0.0001 | 1.03 (1.01; 1.05) | 0.009 |
| Atrial fibrillation at baseline | 1.82 (1.02; 3.24) | 0.04 | - | - |
| Creatinine, per mg/dL | 4.70 (1.86; 9.95) | 0.0003 | - | - |
| Fractional area shortening, per % | 0.96 (0.93; 0.99) | 0.04 | - | - |
| Left atrial reservoir strain*, per % | 0.93 (0.89; 0.96) | <0.0001 | 0.93 (0.90; 0.97) | 0.0002 |
| Left ventricle ejection fraction, per % | 0.96 (0.93; 0.99) | 0.004 | - | - |
| ≥ moderate tricuspid regurgitation | 2.01 (1.07; 3.79) | 0.03 | 2.82 (1.22; 6.53) | 0.02 |
| Right atrial reservoir strain*, per % | 0.96 (0.93; 0.99) | 0.02 | - | - |
| Sex, male | 1.73 (1.01; 2.98) | 0.045 | 2.10 (1.06; 4.17) | 0.03 |
CI: confidence interval; HR: hazard ratio.
Strain was modeled as absolute values.
DISCUSSION
In this study we assessed the prevalence and prognostic implications of AF in adults with EA, and the main findings are (Figure 3): (1) AF was common in adults with EA (lifetime prevalence 28%), the initial onset occurred at a relatively young age (43 years), and one-third of the patients had recurrence of AF. (2) Patients with AF had more comorbidities and worse cardiac remodeling as compared to patients without AF. (3) AF was an independent risk factor for HF hospitalization but not all-cause mortality.
Figure 3. Prognostic implications of atrial fibrillation in adults with Ebstein anomaly.
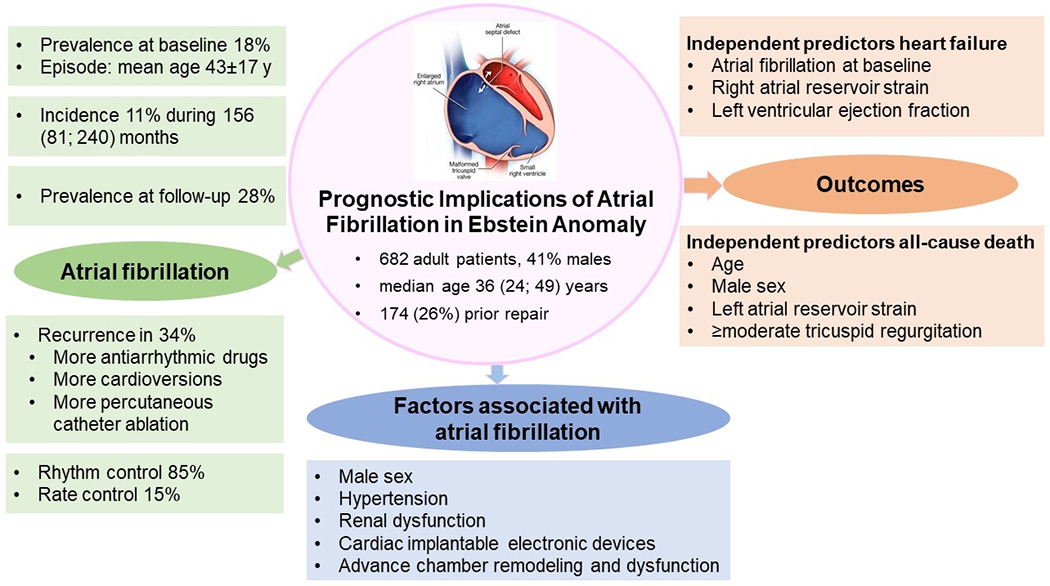
In this retrospective single center cohort study of consecutive adults with Ebstein anomaly, prevalence of atrial fibrillation was high despite the relatively young age of the cohort, and antiarrhythmic treatment for episode termination and catheter ablation were more common in patients with recurrent episodes. Atrial fibrillation was associated with more comorbidities and worse cardiac remodeling. Prevalence of atrial fibrillation at baseline was an independent risk factor for heart failure hospitalization.
AA is one of the leading causes for hospital admission in adults with CHD, including patients with EA.6, 7 The relative prevalence of intra-atrial reentrant tachycardias and AF varies depending on the CHD complexity and the demographic characteristics of the study cohort.2, 4 14 With an increase in the long-term survival of adults with CHD, AF is now the predominant AA in patients >50 years of age.1, 5 Adults with EA are at risk for AA but there are limited data about prevalence and prognostic implications of AF in this population.4 Our results show that 18% of the patients had a history of AF when they presented to our institution, and the prevalence of AF was as high as 28% at last follow-up. Studies conducted in older CHD patients (mean age >60 years) reported an AF prevalence of 18% similar to our study,2 while studies in younger CHD patients (mean age 42 years) have reported a much lower AF prevalence of 8.3%.14 We postulate that the higher prevalence and earlier age of onset in our study may be related to certain anatomic substrates for AF unique to the EA population; furthermore, referral bias cannot be excluded since our data come from a center with large experience in surgical management of EA. Patients with EA have chronic right heart volume overload due to tricuspid regurgitation, and this in turn leads to right atrial remodeling. Furthermore, some of the patients have atrial scars from prior surgery. Collectively, these factors, coupled with multiple comorbidities, provide substrates for the initiation and propagation of AF as observed in our study.13, 24 While it is intuitive that adverse right-sided parameters will drive AF in this population, our results show that also metrics reflecting worse left-sided remodeling, hemodynamics, systolic and diastolic function were associated with a higher prevalence of AF. A higher proportion of patients with hypertension and significant mitral regurgitation among those with AF could have potentially contributed to these observations. The relevance of the left-sided chambers has been previously reported in the setting of survival assessment in tetralogy of Fallot, another mainly ‘right-sided’ CHD.25 These novel findings in adults with EA, therefore, underscore the importance of systematic evaluation of the left-sided chambers, despite EA initially being a predominantly ‘right-sided’ pathology.
Cardiac implantable electronic devices, the majority of which were pacemakers, were associated with baseline AF. Sinus node dysfunction is frequently encountered in EA; together with AF they represent different manifestations of underlying atrial pathology.26 Additionally, these devices could have allowed a more readily identification of AF.
Another relevant finding of our study was that one third of the cohort had recurrence of AF. These patients more commonly required antiarrhythmic treatment or direct current cardioversion for episode termination, suggesting a more resistant underlying arrhythmic substrate. Almost 50% of the cohort underwent Maze ablation, without differences according to arrhythmia burden. This reflects a common institutional practice of performing right atrial MAZE at the time of tricuspid valve surgery. We have previously reported on lower arrhythmia burden following surgical MAZE in EA.10 Percutaneous catheter ablation was performed in only a few patients and its efficacy in EA remains to be determined.
An important observation of the study was that AF was independently associated with the risk of HF hospitalization. Previous data from the CHD population have documented an increased risk of HF among those with AF;5, 13 our results reproduce these findings specifically for adults with EA, and show that this association was independent of other predictors of HF. The interplay between arrhythmogenesis and HF development in CHD is complex and incompletely understood. Rapid heart rates, irregular beats and loss of atrioventricular synchrony reduce ventricular filling time, enhance systolic and diastolic dysfunction, elevate filling pressures and precipitate HF. Structural and electrical remodeling from HF in the per se structurally and hemodynamically abnormal hearts in turn increases the risk of AF recurrence.13, 17 Furthermore, additional structural abnormalities such as valve regurgitation may be common risk factors for both AF and HF. Lastly, AF may also represent a sign of deteriorating hemodynamics.27 Therefore, it is of utmost importance to identify potential reversible causes, and to develop management strategies that interrupt this vicious circle before it becomes irreversible.
All-cause death occurred more frequently in patients with AF, but it did not remain an independent predictor after adjusting for other covariates. Although some studies have reported an association between AF and mortality in adults with CHD,14–16 other studies focused exclusively on patients with EA did not observe this association similar to our study.7 23 28 29 This might suggest that risk of death in EA derives from a complex interaction of several factors, among which AF might play a less significant role.
Other independent prognostic markers for HF and death in our cohort were related to worse chamber remodeling and older age; highlighting that a more advanced disease and the ageing process results in higher vulnerability for adverse events.23 Indeed, impaired quantitative right heart atrio-ventricular deformation and left atrial function in EA have been associated with HF severity,24 while postoperative improvement in atrial function predicted postoperative increase in aerobic capacity.30 Female sex was independently associated with HF hospitalization while it was protective for all-cause death. We do not have a clear explanation for these findings, and the possibility of collinearity cannot be excluded.
Future Directions and Clinical Implications
Our results show that adult patients with EA and AF, with ancillary contribution of other adverse imaging and clinical parameters, are at higher risk for complications. Specifically, AF was independently associated with HF hospitalization. The pathophysiology of HF in EA is multifactorial and derives from complex interactions between the structurally and hemodynamically abnormal left and right chambers.27 Furthermore, the role of the left heart in HF development is expected to augment due to increased survival and accordingly higher prevalence of comorbidities. Our results provide useful clinical tools to help cardiologists involved in the management of adults with EA identify high-risk patients that might benefit from more aggressive treatment. An important question remains as to whether these “aggressive treatments” including HF medications and antiarrhythmic treatment impact clinical outcomes in this population.
Limitations
We report data from a referral center with large experience in management of EA with risk of referral bias. This study has the inherent limitations of a retrospective design. Treatment of missing data by deletion may result in loss of information, however, we do not expect this to have changed our results, since they were considered as missing at random data and represented a very low proportion of observations from a large cohort. Although the size of the cohort was large, the rate of adverse events was low, which could have reduced the statistical power. We found a lower-than-expected recurrence rate of AF, which could be related to limited follow-up time. Nevertheless, to our knowledge, this is the first study to date specifically addressing the clinical profile and outcomes of a large cohort of adults with EA and AF.
CONCLUSIONS
AF in EA develops at relatively young ages with one third of the cohort exhibiting a recurrent pattern. Patients with AF had a higher prevalence of comorbidities and worse right and left chamber remodeling, and targeted antiarrhythmic management was more common in those with recurrent episodes of AF. AF was independently associated with HF hospitalization. Further studies addressing the role of HF and antiarrhythmic treatment to interrupt the vicious cycle of arrhythmogenesis and HF and ultimately improve clinical outcomes are warranted.
Key messages.
What is already known on this topic
Prevalence of atrial arrhythmia in Ebstein anomaly is very high, however, there is limited data about the prevalence and prognostic implications of atrial fibrillation in this population.
Atrial fibrillation is associated with increased risk of heart failure and adverse outcomes in other forms of congenital heart disease, therefore, assessing the prevalence and prognostic implications of atrial fibrillation in adults with Ebstein anomaly could improve risk stratification in this population.
What this study adds
In a cohort of adult patients with Ebstein anomaly, prevalence of atrial fibrillation was high, and the initial onset occurred at a relatively young age compared to other populations.
Echocardiographic metrics related to right but also to left-sided remodeling reflecting worse hemodynamics, systolic and diastolic function were associated with a higher prevalence of atrial fibrillation.
Atrial fibrillation was associated with hospitalization for heart failure independent of other markers of adverse outcomes.
How this study might affect research, practice or policy
This study provides useful clinical and echocardiographic tools for identification of patients with Ebstein anomaly at highest risk for complications that might benefit from more aggressive management.
The association of the left-sided chambers with a higher arrhythmic risk underscores the importance of routine evaluation of the left-sided chambers in this population, despite Ebstein anomaly initially being a predominantly ‘right-sided’ disease.
Acknowledgements:
We thank the Spanish Society of Cardiology for providing Dr. Martin de Miguel with a research fellow scholarship for her stay at Mayo Clinic, Minnesota.
Funding Statement:
Dr. Egbe is supported by National Heart, Lung, and Blood Institute (NHLBI) grants (R01 HL158517 and K23 HL141448).
Footnotes
Declaration of Interest: The authors declare no competing financial interests.
REFERENCES
- 1.Labombarda F, Hamilton R, Shohoudi A, et al. Increasing Prevalence of Atrial Fibrillation and Permanent Atrial Arrhythmias in Congenital Heart Disease. J Am Coll Cardiol 2017;70(7):857–865. [DOI] [PubMed] [Google Scholar]
- 2.LOOMBA RS, BUELOW MW, AGGARWAL S, ARORA RR, KOVACH J, GINDE S. Arrhythmias in Adults with Congenital Heart Disease: What Are Risk Factors for Specific Arrhythmias? Pacing and Clinical Electrophysiology 2017;40(4):353–361. [DOI] [PubMed] [Google Scholar]
- 3.Teuwen CP, Ramdjan TT, Götte M, et al. Time Course of Atrial Fibrillation in Patients With Congenital Heart Defects. Circ Arrhythm Electrophysiol 2015;8(5):1065–72. [DOI] [PubMed] [Google Scholar]
- 4.de Miguel IM, Ávila P. Atrial Fibrillation in Congenital Heart Disease. Eur Cardiol 2021;16:e06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldmann V, Laredo M, Abadir S, Mondésert B, Khairy P. Atrial fibrillation in adults with congenital heart disease. Int J Cardiol 2019;287:148–154. [DOI] [PubMed] [Google Scholar]
- 6.Holst KA, Connolly HM, Dearani JA. Ebstein’s Anomaly. Methodist Debakey Cardiovasc J 2019;15(2):138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celermajer DS, Bull C, Till JA, et al. Ebstein’s anomaly: presentation and outcome from fetus to adult. J Am Coll Cardiol 1994;23(1):170–6. [DOI] [PubMed] [Google Scholar]
- 8.Delhaas T, Sarvaas GJ, Rijlaarsdam ME, et al. A multicenter, long-term study on arrhythmias in children with Ebstein anomaly. Pediatr Cardiol 2010;31(2):229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roten L, Lukac P, N DEG, et al. Catheter ablation of arrhythmias in ebstein’s anomaly: a multicenter study. J Cardiovasc Electrophysiol 2011;22(12):1391–6. [DOI] [PubMed] [Google Scholar]
- 10.Stulak JM, Sharma V, Cannon BC, Ammash N, Schaff HV, Dearani JA. Optimal surgical ablation of atrial tachyarrhythmias during correction of Ebstein anomaly. Ann Thorac Surg 2015;99(5):1700–5; discussion 1705. [DOI] [PubMed] [Google Scholar]
- 11.Kim YG, Kim SJ, Nam GB. Radiofrequency catheter ablation for drug-refractory paroxysmal atrial fibrillation in a patient with Ebstein’s anomaly. HeartRhythm Case Rep 2017;3(1):109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentles TL, Calder AL, Clarkson PM, Neutze JM. Predictors of long-term survival with Ebstein’s anomaly of the tricuspid valve. Am J Cardiol 1992;69(4):377–81. [DOI] [PubMed] [Google Scholar]
- 13.Escudero C, Khairy P, Sanatani S. Electrophysiologic considerations in congenital heart disease and their relationship to heart failure. Can J Cardiol 2013;29(7):821–9. [DOI] [PubMed] [Google Scholar]
- 14.Mandalenakis Z, Rosengren A, Lappas G, et al. Atrial Fibrillation Burden in Young Patients With Congenital Heart Disease. Circulation 2018;137(9):928–937. [DOI] [PubMed] [Google Scholar]
- 15.Bouchardy J, Therrien J, Pilote L, et al. Atrial arrhythmias in adults with congenital heart disease. Circulation 2009;120(17):1679–86. [DOI] [PubMed] [Google Scholar]
- 16.Yap SC, Harris L, Chauhan VS, Oechslin EN, Silversides CK. Identifying high risk in adults with congenital heart disease and atrial arrhythmias. Am J Cardiol 2011;108(5):723–8. [DOI] [PubMed] [Google Scholar]
- 17.Bessière F, Mondésert B, Chaix M-A, Khairy P. Arrhythmias in adults with congenital heart disease and heart failure. Heart Rhythm O2 2021;2(6, Part B):744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28(1):1–39.e14. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019;32(1):1–64. [DOI] [PubMed] [Google Scholar]
- 20.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23(7):685–713; quiz 786-8. [DOI] [PubMed] [Google Scholar]
- 21.Brown ML, Dearani JA, Danielson GK, et al. The outcomes of operations for 539 patients with Ebstein anomaly. J Thorac Cardiovasc Surg 2008;135(5):1120–36, 1136.e1–7. [DOI] [PubMed] [Google Scholar]
- 22.Egbe AC, Miranda WR, Dearani JA, Connolly HM. Hemodynamics and Clinical Implications of Occult Left Ventricular Dysfunction in Adults Undergoing Ebstein Anomaly Repair. Circ Cardiovasc Imaging 2021;14(2):e011739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckerström F, Eriksson P, Dellborg M, et al. Mortality burden in patients born with Ebstein’s anomaly: a 40-year nationwide cohort study. Eur Heart J Qual Care Clin Outcomes 2021;7(3):312–319. [DOI] [PubMed] [Google Scholar]
- 24.Steinmetz M, Broder M, Hösch O, et al. Atrio-ventricular deformation and heart failure in Ebstein’s Anomaly - A cardiovascular magnetic resonance study. Int J Cardiol 2018;257:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghonim S, Gatzoulis MA, Ernst S, et al. Predicting Survival in Repaired Tetralogy of Fallot: A Lesion-Specific and Personalized Approach. JACC Cardiovasc Imaging 2022;15(2):257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silka MJ, Manwill JR, Kron J, McAnulty JH. Bradycardia-mediated tachyarrhythmias in congenital heart disease and responses to chronic pacing at physiologic rates. Am J Cardiol 1990;65(7):488–93. [DOI] [PubMed] [Google Scholar]
- 27.Schultz K, Haeffele CL. Heart failure in the adult Ebstein patient. Heart Fail Rev 2020;25(4):623–632. [DOI] [PubMed] [Google Scholar]
- 28.Attenhofer Jost CH, Tan NY, Hassan A, et al. Sudden death in patients with Ebstein anomaly. Eur Heart J 2018;39(21):1970–1977a. [DOI] [PubMed] [Google Scholar]
- 29.Geerdink LM, Delhaas T, Helbing WA, et al. Paediatric Ebstein’s anomaly: how clinical presentation predicts mortality. Arch Dis Child 2018;103(9):859–863. [DOI] [PubMed] [Google Scholar]
- 30.Egbe A, Miranda W, Connolly H, Dearani J. Haemodynamic determinants of improved aerobic capacity after tricuspid valve surgery in Ebstein anomaly. Heart 2021;107(14):1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]


