Colorectal cancer (CRC) is the third most common cancer worldwide.1,2 Screening reduces CRC incidence and mortality through the removal of precancerous lesions and early detection of cancer.2 Although colonoscopy is a high-sensitivity screening method, cancers are sometimes diagnosed after a CRC-negative colonoscopy, a colonoscopy in which cancer was not detected. These post-colonoscopy colorectal cancers (PCCRC) can lead to substantial morbidity and mortality,3 and identifying their etiologies can inform interventions to improve colonoscopy effectiveness.
The World Endoscopy Organization (WEO) recently published a consensus statement to classify PCCRCs into their most plausible explanations (Figure 1).3 Few studies have utilized this methodology and all were European, single-center studies with modest sample sizes (i.e., 47–107 PCCRC cases).4–6 To our knowledge, no studies have used the WEO methodology to investigate PCCRCs occurring within large, diverse community-based populations in the United States.
Figure 1.
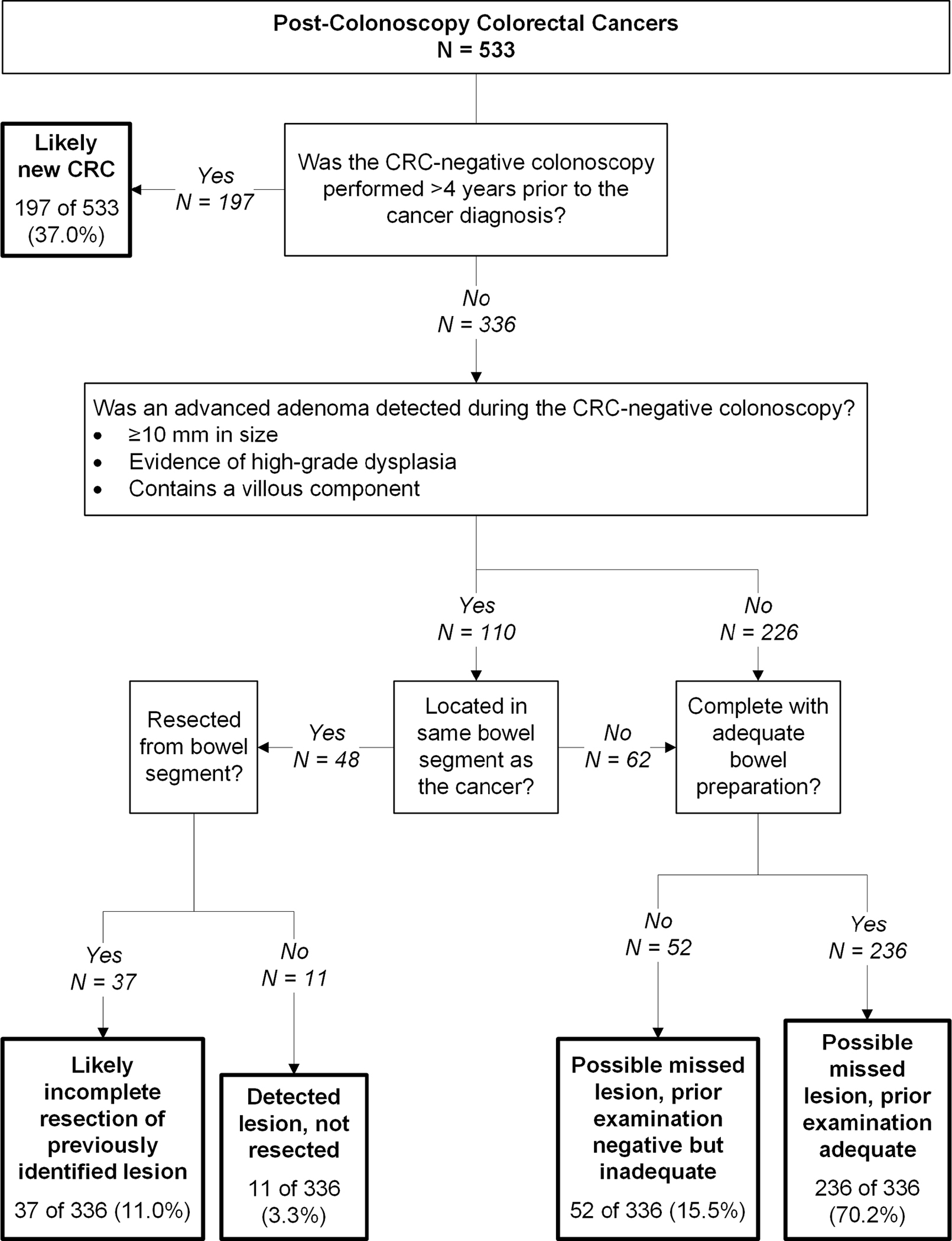
Root cause analysis: most plausible explanations for post-colonoscopy colorectal cancers using World Endoscopy Organization methods
To address this knowledge gap, we randomly selected 523 of 1,497 CRC-negative colonoscopies performed by board-certified gastroenterologists in 2006 through June 30, 2018, among Kaiser Permanente Northern California (KPNC) health plan members, that were followed by a CRC diagnosis 6 months to 10 years later (Supplementary Methods). These 523 procedures were associated with 533 PCCRC cases. Patient and endoscopist characteristics are shown in Supplementary Table 1.
Of the 533 PCCRC cases, 197 (37.0%) were diagnosed more than 4 years after colonoscopy (Figure 1). Per WEO guidelines, these were classified as likely new CRC; however, in 25 of 197 cases (12.7%), an advanced adenoma was detected at their prior colonoscopy, with 9 (4.6%) detected in the same bowel segment as the subsequently diagnosed cancer; 30 of 197 cases (15.2%) had a low-risk adenoma detected at their prior colonoscopy, with 6 (3.0%) detected in the same bowel segment as the subsequently diagnosed cancer. For 13 of 197 cases (6.7%), the prior colonoscopy was incomplete or had an inadequate bowel preparation.
For comparisons between studies with different lengths of follow-up, we calculated percentages for the other most plausible explanation categories based on the remaining 336 of 533 cases (63.0%) diagnosed within 4 years of the CRC-negative colonoscopy; of these, 48 had an advanced adenoma detected in the same bowel segment as the diagnosed cancer, 11 of 336 (3.3%) were unresected and classified as detected lesion, not resected, and 37 of 336 (11.0%) were classified as likely incomplete resection of previously identified lesion. Among 288 cases without an advanced adenoma detected in the same bowel segment as the diagnosed cancer (Figure 1), 236 of 336 (70.2%) followed a complete examination with adequate bowel preparation and were classified as possible missed lesion, prior examination adequate and 52 of 336 (15.5%) were classified as possible missed lesion, prior examination negative but inadequate.
Our findings are in agreement with two prior studies which reported possible missed lesion, prior examination adequate as the most common plausible explanation for PCCRCs diagnosed within 4 years of the CRC-negative colonoscopy.4,6 A third study reported possible missed lesion, prior examination negative but inadequate as the most common plausible explanation; however, this discrepancy is likely explained by their definition of adequate examination which required photo documentation of the cecum, ileocecal valve, or terminal ileum.5 Regardless, all four studies identified the broader category of possible missed lesion as the most frequent plausible explanation for PCCRCs occurring within 4 years of a CRC-negative colonoscopy.4–6
An important colonoscopy quality metric for ensuring adequate inspection of the colon is physician adenoma detection rate (ADR), the proportion of screening colonoscopies in which at least one adenoma is detected. Physician ADR is strongly inversely associated with patient risk of PCCRC.7,8 The US Multi-Society Task Force recommends ADR benchmarks of ≥25% for men and women combined, though data consistently suggest substantial additional potential benefit with higher ADRs.8 Several interventions have been reported to help improve inspection of the colon and have resulted in higher ADRs and lower adenoma miss rates, including colon retroflexion in the right colon, water exchange, extending withdrawal time, distal attachment devices, chromoendoscopy, feedback, training, and artificial intelligence.9 Incomplete lesion resection also represents a modifiable risk factor for PCCRC in our data. This finding is consistent with a report from England5 and reflects the importance of optimizing polypectomy technique and ensuring complete resection of lesions. In a prospective study from the United States, 10.1% of polyps were found to be incompletely resected.10 In addition, rates of incomplete resection varied by physician,10 demonstrating another dimension of colonoscopy quality that needs further attention. However, unlike ADR, the feasibility of measuring polypectomy completeness through resection margin biopsies and/or histological examination of en bloc resection specimens is challenging to implement due to the additional time, labor, and costs required. Thus, further research is needed on resection training and ascertaining competency along with developing pragmatic approaches or artificial intelligence to measuring polypectomy completeness.
Given temporal changes in physician ADRs, we also evaluated two time periods (2006–2011 vs. 2012–2018). The latter period had higher ADRs among the physicians whose patients experienced a PCCRC (26.7% vs. 22.1%, respectively) and the ratio of likely missed lesions to likely incompletely or not resected lesions among PCCRCs diagnosed within 4 years was higher in the latter period (7.4/1 vs 4.6/1, respectively) (Supplementary Table 2). Thus, these findings suggest that even with temporal increases in physician ADRs, missed and incompletely/not resected lesions should remain targets for quality improvement.
In conclusion, using the WEO3 methodology for PCCRC classification, sampling within a large and demographically diverse population-based setting in the United States, and with a sample size that was many-fold larger than prior studies, we found nearly 40% of PCCRCs were classified as likely new cancers and missed lesions despite a prior adequate examination was the most common plausible explanation for PCCRCs.
Supplementary Material
Funding:
This study was conducted within the National Cancer Institute-funded Population-based Research to Optimize the Screening Process II (PROSPR II) consortium (UM1 CA222035), which conducts multisite, coordinated, transdisciplinary research to evaluate and improve cancer-screening processes.
Footnotes
Conflict of Interest Statement: All authors declare that there is no conflict of interest.
Study Materials: Data and analytic methods will not be made available due to the brevity of the research letter format.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bray F, et al. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Dekker E, et al. Lancet 2019;394:1467–1480. [DOI] [PubMed] [Google Scholar]
- 3.Rutter M, Beintaris I, et al. Gastroenterology 2018;155:909–925.e3. [DOI] [PubMed] [Google Scholar]
- 4.Beaton D, et al. Endoscopy 2022;54:270–277. [DOI] [PubMed] [Google Scholar]
- 5.Anderson R, et al. Gastroenterology 2020;158:1287–1299.e2. [DOI] [PubMed] [Google Scholar]
- 6.Aerts R, et al. Acta Gastroenterol Belg 2021;84:401–405. [DOI] [PubMed] [Google Scholar]
- 7.Kaminski MF, et al. N Engl J Med 2010;362:1795–1803. [DOI] [PubMed] [Google Scholar]
- 8.Schottinger JE, Jensen CD, et al. JAMA 2022;327:2114–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam AY, et al. Gastrointest Endosc Clin N Am 2022;32:329–349. [DOI] [PubMed] [Google Scholar]
- 10.Pohl H, et al. Gastroenterology 2013;144.24083342 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


