Abstract
BACKGROUND:
In 2016, the US Food and Drug Administration amended existing regulations to increase access to donated embryos for reproductive use. Current information regarding the characteristics and outcomes of embryo donation cycles could benefit patients and providers during counseling and decision making.
OBJECTIVE:
This study aimed to examine the trends in the utilization of embryo donation, pregnancy rates, and live birth rates per transfer between 2004 and 2019 and to describe the recipients of donated embryos and outcomes of frozen donated embryo transfer cycles during the most recent time period, that is, 2016 to 2019.
STUDY DESIGN:
We conducted a retrospective, population-based cohort study of frozen donated embryo transfer cycles in United States fertility clinics reporting to the National Assisted Reproductive Technology Surveillance System during 2004 to 2019. The trends in the annual number and proportion of frozen donated embryo transfers, pregnancy rates, and live birth rates from 2004 to 2019 were described. During 2016 to 2019, the rates of cycle cancellation, pregnancy, miscarriage, live birth, singleton birth, and good perinatal outcome (delivery ≥37 weeks, birthweight ≥2500 g) of frozen donated embryo transfers were also calculated. Transfer and pregnancy outcomes stratified by oocyte source age at the time of oocyte retrieval were also described.
RESULTS:
From 2004 to 2019, there were 21,060 frozen donated embryo transfers in the United States, resulting in 8457 live births. During this period, the annual number and proportion of frozen donated embryo transfers with respect to all transfers increased, as did the pregnancy rate and live birth rate. Among all initiated cycles during 2016 to 2019, the cancellation rate was 8.2%. Among 8773 transfers with known outcomes, 4685 (53.4%) resulted in pregnancy and 3820 (43.5%) in live birth. Among all pregnancies, 814 (17.4%) resulted in miscarriage. Among all live births, 3223 (84.4%) delivered a singleton, of which 2474 (76.8%) had a good perinatal outcome. The clinical pregnancy rate and live birth rate per frozen donated embryo transfer decreased with increasing age of oocyte source.
CONCLUSION:
The outcomes of embryo donation cycles reported in this national cohort may aid patients and providers when considering the use of donated embryos.
Keywords: donor, embryo donation, in vitro fertilization, live birth, miscarriage, outcome
Introduction
Embryo donation or the transfer of an embryo or embryos to an intended parent with no genetic contribution to the originating oocyte or sperm is an option available for individuals and couples who require the use of donor sperm and/or donor oocytes for successful pregnancy.1 The use of donated embryos may be considered by individuals or couples for various reasons including previous unsuccessful assisted reproductive technology (ART) attempts using autologous gametes, to avoid passing on a genetic condition, or for other reasons not related to fertility.2 Embryo donation is medically less complex and less expensive than in vitro fertilization (IVF) with donor oocytes, because recipients of donated embryos do not incur the cost of ovarian stimulation and oocyte retrieval of oocyte donors; they also do not need to pursue oocyte fertilization and embryo culture before transfer. A cost effectiveness analysis published in 2008 showed that using donated embryos to achieve pregnancy was approximately half as expensive per live delivery compared with IVF with oocyte donation.3
The US Food and Drug Administration (FDA) requirements for donor eligibility determination at the time of embryo creation with regard to embryo donation shifted on August 22, 2016.4 This policy change eliminated the need for an FDA exemption request before transfer of a donated embryo, thereby increasing access to donated embryos for reproductive use.4
We previously published a study describing the national trends and outcomes of donated embryo cycles from 2000 to 2013.5 Given the 2016 policy change by the FDA, in this study, we aim to quantify the trends in utilization and outcomes of frozen donated embryo cycles in the United States using national surveillance data from ART cycles starting in 2004 through 2019. We also aim to characterize clinics that perform embryo donation, describe recipients of donated embryos, and report outcomes among frozen donated embryo transfer cycles during 2016 to 2019.
Materials and Methods
Data for this study were derived from the US National ART Surveillance System (NASS). NASS is a Congressionally-mandated surveillance system that collects information on nearly all (98%) ART cycles performed in the United States.6 In NASS, ART includes fertility treatments in which eggs or embryos are handled in a laboratory. NASS contains cycle-level information on patient characteristics, clinical characteristics of the ART procedure, and pregnancy outcomes.
For this retrospective, population-based cohort analysis, all frozen donated embryo transfers during 2004 to 2019 were selected from NASS. The trends in the annual number of frozen donated embryo transfers, proportion of frozen donated embryo transfers of all embryo transfers, pregnancy rates, and live birth rates per transfer were reported from 2004 to 2019. In addition, trends in the numbers and percentage of clinics performing at least 1 donated embryo transfer among all ART clinics reporting to NASS were examined for this time period.
To provide a more recent description of recipient characteristics and outcomes of frozen donated embryo cycles, we limited our descriptive analyses to cycles that occurred during 2016 to 2019—the most recent years for which data were available. Starting in 2016, NASS required that reporting clinics specify if both patient and donated embryos were transferred in a single cycle. Cycles that reported using both patient and donated embryos (n=149) were excluded when calculating transfer outcomes, given that we were unable to determine if the cycle outcomes were attributed to autologous gametes or donated embryos.
Of all clinics performing at least 1 donated embryo transfer during 2016 to 2019, the mean and median number of donated embryo transfers performed per clinic during this period were calculated. The numbers of clinics from each US Census region (West, South, Northeast, and Midwest) performing donated embryo transfers were also described.7
We investigated the recipient and cycle characteristics and outcomes of frozen donated embryo transfers. These characteristics included gender; age; race and ethnicity; infertility diagnosis; number of embryos transferred; cycle history; number of previous ART cycles; and numbers of previous pregnancies, preterm births (birth at least 20 but <37 completed weeks of gestation), term births (birth that reached 37 weeks gestation), and miscarriages (loss of the entire pregnancy before completion of 20 weeks of gestation or 18 weeks from the date of transfer). To address missing data on recipient race and ethnicity, a multiple imputation procedure based on the sequential regression approach was used to generate 20 imputed datasets that were subsequently analyzed; the estimates were summarized according to Rubin’s rules.8 NASS contains only limited data regarding the donated embryos’ origins, and therefore, information regarding the reason for undergoing ART, outcome of the donor’s ART cycle, or embryo stage at cryopreservation or transfer was not available. When available, we included the age of the individual whose oocytes were used (oocyte source).
We then explored the outcomes of frozen donated embryo transfers. Cycles with unknown treatment and/or pregnancy outcome (N=65) were excluded. We described the rate of cycle cancellation, defined as an ART cycle in which monitoring or endometrial preparation has been carried out with the intent of undergoing embryo transfer that did not occur. We detailed the number and percentage of clinical intrauterine pregnancies (an ultrasound-confirmed gestational sac within the uterus or the documented occurrence of a birth, spontaneous abortion, or induced abortion in cases of missing ultrasound data) and live births among frozen donated embryo transfers and the number and percentage of miscarriages among all resulting pregnancies. Among live birth deliveries, we described the number of singletons, singletons with good perinatal outcome (defined as birthweight ≥2500 grams and at ≥37 weeks gestation), and twin and triplet deliveries. Among cycles where age of oocyte donor at the time of retrieval was available, we calculated these same pregnancy outcomes among the following age groups: <35, 35 to 37, and ≥38 years old.
Statistical analyses were conducted using SAS version 9.4 (SAS Institute) and SUDAAN 11.0.3 (RTI International). Poisson regression with a scale parameter to account for overdispersion in our data was used to examine temporal trends in the annual numbers of frozen donated embryo transfers, pregnancy rates, and live birth rates. Generalized estimating equations were calculated to account for clustering by clinic. For analyses of trends, all P values <.05 were considered statistically significant. NASS includes 98% of all ART cycles in the United States; therefore, confidence intervals were not included in descriptive statistics. Epidemiologic research of NASS data is approved by the institutional review board at the Centers for Disease Control and Prevention.
Results
From 2004 to 2019, there were 21,060 frozen donated embryo transfers in the United States resulting in 8457 live births. During this period, the annual number of donated embryo transfers more than tripled from 666 in 2004 to 2492 in 2019 (P value for trend <.001) (Figure, A). The proportion of donated embryo transfers of all embryo transfers in the time observed also increased significantly (from 0.6% in 2004 to 1.5% in 2019, P<.001). The number of ART clinics that reported at least 1 frozen donated embryo transfer increased from 167 (42.2%) in 2004 to 263 (58.7%) in 2019. The overall pregnancy rate and live birth rate per frozen donated embryo transfer increased significantly between 2004 and 2019 from 40.8% to 54.3% (P<.001) and from 33.3% to 44.8% (P<.001), respectively (Figure, B).
FIGURE. Trends in frozen donated embryo transfers and outcomes, 2004–2019.
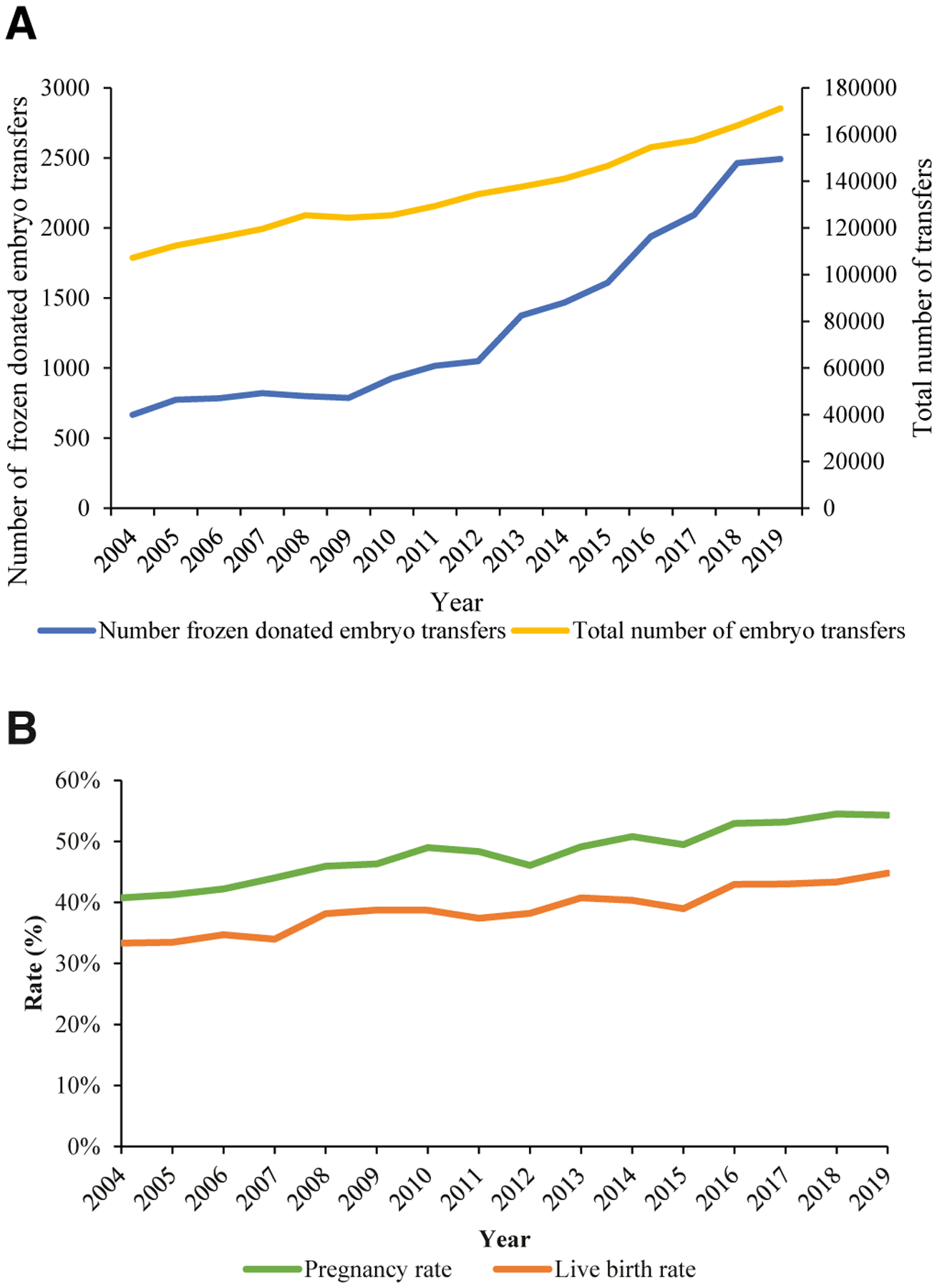
Trends in (A) frozen embryo transfers using donated embryos and total embryo transfers and (B) pregnancy and live birth rates following frozen donated embryo transfer. (P<.001 for trends in proportion of embryo transfers using frozen donated embryos, pregnancy and live birth rates.).
There were 369 unique clinics in the United States that reported at least 1 frozen donated embryo transfer during 2016 to 2019. During this 4-year period, the mean number of frozen donated embryo transfers per clinic was 24.5, and the median was 9.0. In addition, 146 (39.6%) clinics performed 5 or fewer total frozen donated embryo transfers, and 50 (13.6%) clinics performed 50 or more total frozen donated embryo transfers over this time period. When examining the geographic distribution of frozen donated embryo transfers during this period, 1 clinic from Puerto Rico was excluded.7 Of the remaining clinics, 125 (40.0%) were located in the South, and 103 (30.0%), 71 (19.3%), and 69 (18.8%) were located in the West, Northeast, and Midwest, respectively.
Of all frozen donated embryo cycles performed during 2016 to 2019 in this national cohort, 27.8% had missing data on age of oocyte source. Among the 6380 donated embryo transfers where the age of oocyte source was reported, 3746 (58.7%) embryos originated from patients who were <35 years of age at the time of oocyte retrieval (Table 1). In contrast, donated embryo recipients tended to be older: 61.1% (n=5404) were 38 years or older, and 30.8% (n=2719) were ages 43 years or older (Table 1). Most (n=6776; 76.7%) recipients identified as non-Hispanic White, and the most common infertility diagnosis listed was diminished ovarian reserve (n=4527; 51.2%), followed by male factor infertility (n=2345; 26.5%). Regarding recipient cycle history, 42.5% (n=3707) of recipients reported at least 1 previous ART cycle and no previous births. In addition, 34.9% (n=3039) of recipients reported no previous ART cycles.
TABLE 1.
Recipient and cycle characteristics of frozen donated embryo transfers, 2016–2019
| Donated embryo transfer cycles | |
|---|---|
| N (%) | |
| Total | 8838 |
| Age of oocyte source (y) | |
| <35 | 3746 (42.4) |
| 35–37 | 1325 (15.0) |
| 38–40 | 586 (6.6) |
| 41–42 | 244 (2.8) |
| ≥43 | 479 (5.4) |
| Missinga | 2458 (27.8) |
| Recipient gender | |
| Female | 8718 (98.6) |
| Male | 120 (1.4) |
| Recipient age (y) | |
| <35 | 2152 (24.3) |
| 35–37 | 1282 (14.5) |
| 38–40 | 1494 (16.9) |
| 41–42 | 1191 (13.5) |
| ≥43 | 2719 (30.8) |
| Recipient race/ethnicityb | |
| Non-Hispanic White | 6776 (76.7) |
| Non-Hispanic Asian | 885 (10.0) |
| Hispanic | 556 (6.3) |
| Non-Hispanic Black | 520 (5.9) |
| Two or more races | 54 (0.6) |
| Non-Hispanic Native Hawaiian or Pacific Islander | 24 (0.3) |
| Non-Hispanic American Indian or Alaska Native | 22 (0.2) |
| Recipient infertility diagnosisc,d | |
| Diminished ovarian reserve | 4527 (51.2) |
| Male factor | 2345 (26.5) |
| Ovulatory dysfunction | 1603 (18.1) |
| Unexplained | 708 (8.0) |
| Endometriosis | 560 (6.3) |
| Tubal factor | 538 (6.1) |
| Uterine factor | 419 (4.7) |
| Other | 1896 (21.5) |
| Recipient cycle historyd | |
| First ART, no previous birth | 2380 (27.3) |
| First ART, ≥1 previous births | 659 (7.6) |
| ≥1 previous ART, no previous birth | 3707 (42.5) |
| ≥1 previous ART, ≥1 previous births | 1972 (22.6) |
| Recipient number of previous ART cyclesd,e | |
| 0 | 3039 (34.9) |
| 1 | 1945 (22.3) |
| 2+ | 3734 (42.8) |
| Recipient number of previous pregnanciesd | |
| 0 | 3609 (41.4) |
| 1 | 2210 (25.3) |
| 2+ | 2899 (33.3) |
| Recipient number of previous miscarriagesd | |
| 0 | 5804 (66.6) |
| 1 | 1797 (20.6) |
| 2+ | 1117 (12.8) |
| Recipient number of previous preterm birthsd,f | |
| 0 | 8349 (95.8) |
| 1 | 342 (3.9) |
| 2+ | 27 (0.3) |
| Recipient number of previous term birthsd,g | |
| 0 | 6325 (72.6) |
| 1 | 1683 (19.3) |
| 2+ | 710 (8.1) |
| Number of embryos transferred per cycle | |
| 1 | 4865 (55.0) |
| 2 | 3573 (40.4) |
| 3 | 381 (4.3) |
| >3 | 19 (0.2) |
ART, assisted reproductive technology.
Missing included for all characteristics with >5% missing values; for remainder of characteristics <1% missing;
Recipient race/ethnicity are derived from multiple imputed analyses;
Diagnoses are not mutually exclusive; sum is greater than 100%, as cycle may be associated with more than 1 diagnosis;
Reproductive history variables do not include 120 male patients;
Number of previous ART cycles includes both oocyte retrievals and embryo transfers;
A preterm birth is a birth at ≥20 but <37 completed weeks’ gestation and includes both live births and stillbirths;
A term birth is a birth at ≥37 weeks’ gestation and includes both live birth and stillbirths.
Among all initiated frozen donated embryo transfer cycles during 2016 to 2019 in the United States, 802 (8.2%) were cancelled. Among frozen donated embryo transfers for which a transfer was performed, the most common number of embryos transferred was 1 (n=4865; 55.0%), followed by 2 (n=3573; 40.4%). Three or more embryos were transferred in 4.5% (n=400) of frozen donated embryo transfers. Among 8773 frozen donated embryo transfers with known outcomes, 4685 (53.4%) resulted in an intrauterine pregnancy and 3820 (43.5%) in live birth (Table 2). Among intrauterine pregnancies, 814 (17.4%) resulted in miscarriage. Among all pregnancies resulting in live births, 3223 (84.4%) were singleton, 571 (15.1%) were twin, and 20 (0.5%) were triplet deliveries. Of singleton live birth deliveries, 2474 (76.6%) had a good perinatal outcome.
TABLE 2.
Outcomes of frozen donor embryo transfers, 2016–2019
| All ages | <35 | 35–37 | 38+ | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| Among transfersa | 8773 (100) | 3172 (100) | 1315 (100) | 1301 (100) |
| Intrauterine pregnancy | 4685 (53.4) | 2020 (54.4) | 690 (52.5) | 635 (48.8) |
| Live birth (≥ 20 wk) | 3820 (43.5) | 1646 (44.3) | 550 (41.8) | 507 (39.0) |
| Among pregnancies | ||||
| Miscarriage (<20 wk) | 814 (17.4) | 350 (17.3) | 132 (19.1) | 123 (19.4) |
| Among live births | ||||
| Singleton | 3223 (84.4) | 1375 (83.5) | 459 (83.5) | 438 (86.4) |
| Twin | 577 (15.1) | 271 (16.5)b | 91 (16.5)b | 69 (13.6)b |
| Triplet | 20 (0.5) | |||
| Among singleton live births | ||||
| Good perinatal outcomec | 2474 (76.8) | 1056 (76.8) | 343 (74.7) | 338 (77.2) |
Sixty-five transfer cycles were excluded with unknown pregnancy and/or birth outcomes;
To protect patient confidentiality owing to the small numbers of triplet births, twin and triplet births are combined when reported by age;
Infants with birthweight ≥2500 g and ≥37 weeks gestation.
Transfers where age of oocyte source was younger than 35 years resulted in a pregnancy rate of 54.4% and live birth rate of 44.3% (Table 2). An oocyte source age between 35 and 37 years resulted in a pregnancy rate of 52.5% and live birth rate of 41.8%. Finally, oocyte source age 38 years and older resulted in a pregnancy rate of 48.8% and live birth rate of 39.0% (Table 2). In these 3 age groups, the miscarriage rates per clinical pregnancy were 17.3%, 19.1%, and 19.4%, respectively (Table 2).
Comment
Principal findings
From 2004 to 2019, the overall number of frozen donated embryo transfers and proportion of all transfers that used frozen donated embryos increased in the United States, as did the pregnancy rate and live birth rate per frozen donated embryo transfer. The small increase in the proportion of frozen donated embryo transfers of all embryo transfers from 0.6% in 2004 to 1.5% in 2019 highlights that donated embryos are still used in only a few transfer cycles in the United States. The most common reason for ART among recipients of frozen donated embryo transfers during 2016 to 2019 was diminished ovarian reserve (51.2%), and 42.5% of donated embryo recipients reported at least 1 previous ART cycle and no previous births. Among live births after frozen donated embryo transfers, most (84.4%) were singleton deliveries.
Results in the context of what is known
Despite adjustments to FDA requirements in 2016 that eliminated the need for an FDA exemption request before transfer of donated embryos for embryos created without donor eligibility determination, our data do not reveal a corresponding change in trends for donated embryo transfers.4 Donated embryo transfer cycles have continued to increase rapidly since the early 2010s. However, if changes to clinical practice were delayed, the effects of this change may not have been observed within the time frame examined.
When comparing this study with the previous study on embryo donation trends in the United States by Kawwass et al, several differences in practice patterns and outcomes are observed.5 The rate of single embryo transfer increased from 14.4% during 2007 to 2013 to 55.0% during 2016 to 2019, with a concomitant increase in singleton live births from 74.5% to 84.4% of all live births. This change is consistent with changes in clinical practice such as increasing use of frozen embryos and expanded recommendations that outline a broader approach to single embryo transfer.9
Clinical implications
The overall live birth rate from frozen donated embryo cycles also increased from 38.2% to 43.5% between the 2 periods, which may reflect improvements in ART, changes to clinical practice and procedures, and differences in donated embryo quality and recipient characteristics.5,10 Notably, the overall live birth rate in this cohort of donated embryo transfers is similar to the reported live birth rate per transfer for patients in the United States <35 years old at time of retrieval using autologous oocytes (41.4% in 2018).11
The rate of intrauterine pregnancy and live birth per transfer decreased with increasing age of oocyte source. This supports the known age-related decline in fertility driven by the increase in meiotic nondisjunction of the oocyte and resulting aneuploidy seen with increasing age of the oocyte source.12 Conversely, the live birth rate per transfer of 43.5% in a cohort where 75.7% of recipients were over 35 years old supports that embryo donation is a way to overcome the age-related decline in fertility and IVF success rates.12
Our results also show that in the United States, outcomes for frozen donated embryo cycles are similar to those for cycles using donor oocytes. Compared with the live birth rate of 43.5% in this cohort of donated embryo transfers, 49.4% of transfers of embryos from fresh donor oocytes and 39.1% of transfers from frozen donor oocytes resulted in live birth in the United States in 2018.11 Although embryo donation has been shown to be simpler and more cost-effective than oocyte donation,3 having a genetic relationship with the transferred embryo may be a consideration, and oocyte donation allows for use of a partner’s sperm.13
Strengths and limitations
This study is limited by the lack of data about individuals and cycles from which donated embryos were created. Previous studies have reported that patients with unused embryos derived from donor oocytes are more likely to donate to other individuals than those with embryos from autologous oocytes. However, we were unable to determine which embryos were donated after donor oocyte fertilization in this cohort.14,15 As embryo donation cycles in NASS are currently not linked to their cycles of origin, information on donors’ infertility diagnoses (if applicable), age of sperm source, initial treatment outcomes, use of slow freezing vs embryo vitrification, or the use of preimplantation genetic testing was not available.
We observed that most patients undergoing embryo donation cycles in the study period were of an older reproductive age and had diminished ovarian reserve and at least 1 previous ART attempt. However, for 1 in every 5 recipients, “other” was reported among their infertility diagnoses, and 34.9% had no previous ART cycles. Owing to the limitations of surveillance data, we were unable to fully categorize patients who are accessing embryo donation in the United States, specifically with regard to reason for pursuing ART. Data collection that includes information for indications beyond infertility may enhance descriptive analyses of ART cycles using donated embryos, which may be particularly informative to couples pursuing ART to avoid passing on genetic conditions; unpartnered patients; or lesbian, gay, bisexual, and transgender patients and couples.2 These data describe the transfer and pregnancy outcomes of donated embryo transfers, including the rates of good perinatal outcomes. However, the rates of pregnancy complications and information regarding neonatal developmental outcomes after donated embryo transfer are beyond the scope of this study and require further investigation.
To our knowledge, this is the only comprehensive study so far that describes the trends and recipient and cycle characteristics and outcomes for frozen donated embryo cycles in the United States that includes data after 2013. This study therefore reflects the most recent technology used in assisted reproduction and clinical practice patterns. Moreover, it includes all clinics reporting to a national surveillance system, making the results both generalizable and relevant for providers and patients considering using donated embryos.
Conclusions
The outcomes of embryo donation cycles reported in this national cohort may aid patients and providers when considering the use of donated embryos. Outcomes can be better characterized with more robust data collection on individuals whose oocyte(s) were used in donated embryos and indications for use beyond infertility.
AJOG at a Glance.
Why was this study conducted?
This study aimed to describe the trends in the utilization and outcomes of frozen donated embryo cycles in the United States.
Key findings
From 2004 to 2019, the annual number of frozen donated embryo transfers, pregnancy rate, and live birth rate per transfer increased. During 2016 to 2019, the clinical pregnancy rate, live birth rate per transfer, and miscarriage rate per pregnancy was 53.4%, 43.5%, and 17.4%, respectively, for all frozen donated embryo transfers.
What does this add to what is known?
The most recent outcomes of frozen donated embryo cycles reported in the national cohort considered in this study may aid patients and providers when considering the use of donated embryos.
Acknowledgments
Support for this research was provided by Open Philanthropy through a grant to the CDC Foundation, which was responsible for the salary and benefits for C.E.D.
Footnotes
The authors report no conflict of interest.
This study was presented at the 70th annual meeting of the Pacific Coast Reproductive Societies, Indian Wells, CA, March 23–27, 2022.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the CDC Foundation.
References
- 1.Ethics Committee of the American Society for Reproductive Medicine. Electronic address: ASRM@asrm.org, Ethics Committee of the American Society for Reproductive Medicine. Defining embryo donation: an Ethics Committee opinion. Fertil Steril 2016;106:56–8. [DOI] [PubMed] [Google Scholar]
- 2.Lee J, Yap C. Embryo donation: a review. Acta Obstet Gynecol Scand 2003;82:991–6. [DOI] [PubMed] [Google Scholar]
- 3.Finger R, Sommerfelt C, Freeman M, Wilson CK, Wade A, Daly D. A cost-effectiveness comparison of embryo donation with oocyte donation. Fertil Steril 2010;93:379–81. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration, HHS HHS. Revisions to exceptions applicable to certain human cells, tissues, and cellular and tissue-based products. Final rule. Fed Regist 2016;81:40512–8. [PubMed] [Google Scholar]
- 5.Kawwass JF, Crawford S, Hipp HS, et al. Embryo donation: national trends and outcomes, 2000 through 2013. Am J Obstet Gynecol 2016;215:747–e1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sunderam S, Kissin DM, Zhang Y, et al. Assisted reproductive technology surveillance - United States, 2017. MMWR Surveill Summ 2020;69:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.2010 Census Regions and Divisions of the United States. Available at: https://www.census.gov/geographies/reference-maps/2010/geo/2010-census-regions-and-divisions-of-the-united-states.html. Accessed November 1, 2021, 2022
- 8.Barnard J, Rubin D. Small-sample degrees of freedom with multiple imputation. Biometrika 1999;86:948–55. [Google Scholar]
- 9.Practice Committee of the American Society for Reproductive Medicine. Electronic address: ASRM@asrm.org, Practice Committee of the Society for Assisted Reproductive Technologies. Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril 2021;116:651–4. [DOI] [PubMed] [Google Scholar]
- 10.Niederberger C, Pellicer A, Cohen J, et al. Forty years of IVF. Fertil Steril 2018;110:185–324.e5. [DOI] [PubMed] [Google Scholar]
- 11.Society for Assisted Reproductive Technology. Final national summary report for 2018. 2018. Available at: https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2018. Accessed November 1, 2021.
- 12.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril 2014;101:633–4. [DOI] [PubMed] [Google Scholar]
- 13.Goedeke S, Daniels K, Thorpe M, Du Preez E. Building extended families through embryo donation: the experiences of donors and recipients. Hum Reprod 2015;30:2340–50. [DOI] [PubMed] [Google Scholar]
- 14.Hill GA, Freeman MR. Embryo disposition: choices made by patients and donor oocyte recipients. Fertil Steril 2011;95:940–3. [DOI] [PubMed] [Google Scholar]
- 15.Bruno C, Dudkiewicz-Sibony C, Berthaut I, et al. Survey of 243 ART patients having made a final disposition decision about their surplus cryopreserved embryos: the crucial role of symbolic embryo representation. Hum Reprod 2016;31:1508–14. [DOI] [PubMed] [Google Scholar]


