Abstract
Background & Aims:
There are limited prospective data on patients with type 2 diabetes mellitus (T2DM) specifically enrolled and systematically assessed for advanced fibrosis or cirrhosis due to non-alcoholic fatty liver disease (NAFLD). Therefore, we aimed to evaluate the prevalence of advanced fibrosis and cirrhosis in a prospectively recruited cohort of adults with T2DM.
Methods:
This prospective study enrolled adults aged ≥50 years with T2DM, recruited from primary care or endocrinology clinics. Participants underwent a standardized clinical research visit with MRI-proton density fat fraction (MRI-PDFF), magnetic resonance elastography (MRE), vibration-controlled transient elastography (VCTE) and controlled-attenuation parameter. NAFLD was defined as MRI-PDFF ≥5% after exclusion of other liver diseases. Advanced fibrosis and cirrhosis were defined by established liver stiffness cut-off points on MRE or VCTE if MRE was not available.
Results:
Of 524 patients screened, 501 adults (63% female) with T2DM met eligibility. The mean age and BMI were 64.6 (±8.1) years and 31.4 (±5.9) kg/m2, respectively. The prevalence of NAFLD, advanced fibrosis and cirrhosis was 65%, 14% and 6%, respectively. In multivariable adjusted models, adjusted for age and sex, obesity and insulin use were associated with increased odds of advanced fibrosis (odds ratio 2.50; 95% CI 1.38–4.54; p = 0.003 and odds ratio 2.71; 95% CI 1.33–5.50; p = 0.006, respectively). Among 29 patients with cirrhosis, two were found to have hepatocellular carcinoma and one patient had gallbladder adenocarcinoma.
Conclusion:
Utilizing a uniquely well-phenotyped prospective cohort of patients aged ≥50 years with T2DM, we found that the prevalence of advanced fibrosis was 14% and that of cirrhosis was 6%. These data underscore the high risk of advanced fibrosis/cirrhosis in adults aged ≥50 years with T2DM.
Keywords: nonalcoholic fatty liver disease, diabetes, screening
Introduction
Non-alcoholic fatty liver disease (NAFLD) affects approximately one in four individuals globally,1,2 and is a major cause of liver-related morbidity and mortality, including being the fastest growing cause of hepatocellular carcinoma.3 Obesity, insulin resistance, and type 2 diabetes mellitus (T2DM) are common risk factors for progressive liver disease in NAFLD.4,5 However, current American Association for the Study of Liver Diseases (AASLD) NAFLD practice guidelines6 do not recommend systematic screening, partly because of uncertainties surrounding the true prevalence of advanced fibrosis and cirrhosis in patients with T2DM.
Accurate non-invasive tests are required to define the true prevalence of advanced fibrosis and cirrhosis in at-risk populations. MRI-proton density fat fraction (MRI-PDFF) is the most accurate, quantitative biomarker of liver fat and has significantly higher diagnostic accuracy than the ultrasound-based controlled-attenuation parameter (CAP) across the full spectrum of liver fat.7 Similarly, magnetic resonance elastography (MRE) is the most accurate non-invasive biomarker of liver fibrosis with superior diagnostic accuracy and higher specificity and positive predictive value for liver fibrosis than vibration-controlled transient elastography (VCTE), also known as Fibroscan, a commonly used ultrasound-based method of liver stiffness assessment.7,8 Emerging studies also demonstrate a strong association between higher liver stiffness on MRE and hepatic decompensation and death, further bolstering its value as a standalone test of disease severity in NAFLD.9–12 Together, MRI-PDFF and MRE provide a detailed assessment of liver fat and stiffness, which can be leveraged to accurately diagnose NAFLD, advanced fibrosis and cirrhosis.
A pilot study of 100 prospectively recruited patients with T2DM who underwent MRI-PDFF and MRE demonstrated a prevalence of NAFLD and advanced fibrosis of 65% and 7.1%.13 In addition, there was a clear increase in advanced fibrosis associated with older age, affecting 13.3% of those over 65-years old. However, these preliminary findings require validation before systematic screening can be recommended by practice guidelines. Therefore, we aimed to examine the prevalence of advanced fibrosis and cirrhosis in a prospectively recruited cohort of adults with T2DM.
Materials and methods
Study design
This prospectively enrolled cross-sectional study assessed the prevalence of advanced fibrosis and cirrhosis due to NAFLD in a cohort of patients with T2DM who are currently receiving treatment in the form of insulin, diabetes medications, lifestyle interventions (e.g., diet, physical activity), or any combination thereof. Participants were recruited from primary care and endocrinology clinics in the greater San Diego area. Participants were also recruited through the distribution of educational brochures, ads in local newspapers, local fairs, and social media. This study included 501 patients who underwent a standardized research visit including history, physical exam, laboratory investigation, VCTE, CAP, MRI-PDFF and MRE assessment between 2016 and 2022 at the UCSD NAFLD Research Center.13–17 All patients provided written informed consent prior to enrolling in the study and the study was approved by the UCSD Institutional Review Board.
Inclusion and exclusion criteria
Inclusion criteria included participants aged 50–80 years old with a diagnosis of T2DM according to the American Diabetes Association clinical practice recommendations based on meeting one of the following criteria: diabetes symptoms and plasma glucose ≥200 mg/dl or fasting plasma glucose ≥126 mg/dl or plasma glucose ≥200 mg/dl during two separate 75-g oral glucose tolerance tests or hemoglobin A1c (HbA1c) ≥6.5%. Participants were excluded from the study if they met any of the following criteria: significant alcohol intake (defined as ≥14 drinks/week for men or ≥7 drinks/week for women) within the previous 2-year period or laboratory evidence of liver disease other than NAFLD.
Clinical assessment and laboratory tests
All patients underwent a standardized clinical evaluation including a detailed history and a physical examination, which included vital signs, height, weight, and anthropometric measurements, performed by a trained clinical investigator. BMI was defined as the body weight (in kilograms) divided by height (in meters) squared. Alcohol consumption was documented outside of clinical visits and confirmed in the research clinic using the AUDIT (Alcohol Use Disorders Identifications Test) and the Skinner questionnaire. Other causes of liver disease were systematically ruled out based on history and laboratory tests. Patients underwent the following biochemical tests: glucose, albumin, HbA1c, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, alkaline phosphatase, fasting lipid panel, platelets, insulin, international normalized ratio (INR). FIB-418 and NAFLD fibrosis score19 were calculated as described previously. Participants were instructed to fast for a minimum of 8 h prior to collection of laboratory tests.
MRI
Participants underwent a non-contrast MR (magnetic resonance) exam with liver fat quantification and liver stiffness assessment using MRI-PDFF and MRE. Imaging was performed at the UCSD MR3T Research Laboratory using a 3 T research scanner (GE Signa EXCITE HDxt; GE Healthcare, Waukesha, WI). Liver stiffness data was obtained using 2D MRE at 60 Hz. Acquired MR images were interpreted by a radiologist who was blinded to clinical and laboratory data.
Vibration-controlled transient elastography
CAP for the detection of liver fat, and VCTE for the quantification of liver stiffness were obtained using FibroScan® (Echosens).20 All exams were performed by an experienced technician after a minimum fast of 4 h as recommended. During patient breath holding, a minimum of 10 repeated valid measurements, assessed automatically by the FibroScan® system, were performed. All participants were first scanned using the M probe (3.5 MHz). If indicated upon initial assessment, participants were re-scanned using the XL probe (2.5 MHz).
Liver biopsy assessment
A liver biopsy was offered to study participants with elevated ALT (≥30 U/L), elevated liver fat (MRI-PDFF ≥5%) with elevated liver stiffness (VCTE ≥6.9 kPa or MRE ≥2.65 kPa) or MRE ≥3.63 regardless of MRI-PDFF or high liver fat (MRI-PDFF ≥10%). Liver biopsy assessment was performed by an experienced central hepato-pathologist and the presence of NAFLD, nonalcoholic steatohepatitis (NASH) and fibrosis stage were assessed using the NASH Clinical Research Network histologic scoring system as previously described.21
Outcome measures
Primary outcome
Advanced fibrosis was defined as MRE ≥3.63 kPa or, if MRE was not available, as VCTE ≥8.8 kPa based upon previously published data.7
Secondary outcomes
NAFLD was defined as MRI-PDFF ≥5% or, if MRI-PDFF was not available, as CAP ≥288 dB/m among individuals who consume little or no alcohol without any secondary cause for hepatic steatosis or other causes of liver disease. Cirrhosis was defined as MRE ≥4.67 kPa22 or, if MRE was not available, as VCTE ≥15 kPa (high specificity cut-off point).23 Hepatobiliary malignancy was defined clinically and on histology, or by AASLD practice guidelines for cases of hepatocellular carcinoma24 requiring treatment during follow-up and diagnosed after the research visit.
Statistical analysis
For patient characteristics, a t test was performed on continuous variables presented as mean (SD), Wilcoxon rank sum was performed on those presented as median (IQR). Chi-square or Fisher’s exact test were performed as appropriate for all categorical variables. Unadjusted logistic regression was used to assess for the association between advanced fibrosis and age, sex, ethnicity, obesity, duration of T2DM, insulin use and the presence of the metabolic syndrome defined by the Joint Societies 2009 criteria.25 Variables with a significant association (p <0.05) were assessed in a multivariable model adjusted for age and sex. Effect modification by obesity was evaluated for the primary and secondary outcomes.
Sample size was estimated to compare the screening strategies using MRE and VCTE. The estimated prevalence of advanced fibrosis will range between 7.5 to 13%, as detected by MRE, vs. 5 to 8.5%, as detected by VCTE, considering an effect size of 1.5. These estimates are based on published data on the prevalence of advanced fibrosis in the T2DM population from a published pilot study13 and the US population (National Health and Nutrition Examination Survey 2011–201426). Using a McNemar normal approximation test, we present a conservative scenario that assumes the prevalence of advanced fibrosis would be 7.5% by MRE and 5% by VCTE. Using the desired set of classified proportions, we would need a sample size of 488 individuals to undergo paired assessment by both MRE and VCTE for a power of at least 80%, with a two-sided alpha of 0.05.
All statistical analyses were performed using SAS 9.4 (SAS Institute), and a p value less than 0.05 was considered statistically significant.
Results
Characteristics of the study population
Of 524 patients who were screened for the study, 501 met eligibility criteria (Fig. 1). Of the included participants, 98% (n = 493) had a valid liver stiffness measurement on MRE or VCTE. Participants had a mean age of 64.4 (±8.1) years and were predominantly female (63%). The mean BMI was 31.4 (±5.9) kg/m2. The median (IQR) HbA1c and HOMA-IR were 6.8% (1.6) and 4.8 (5.5), respectively. The most prescribed pharmacotherapy was metformin, which was used by 63% of the study population, while 72.4% reported being on at least one medication for the treatment of T2DM. GLP-1 (glucagon-like-peptide-1) agonists, SGLT2 (sodium-glucose transport protein 2) inhibitors and insulin were reportedly used by 8%, 5.2% and 12.4% of patients, respectively (Table S1).
Fig. 1. Derivation of study cohort.
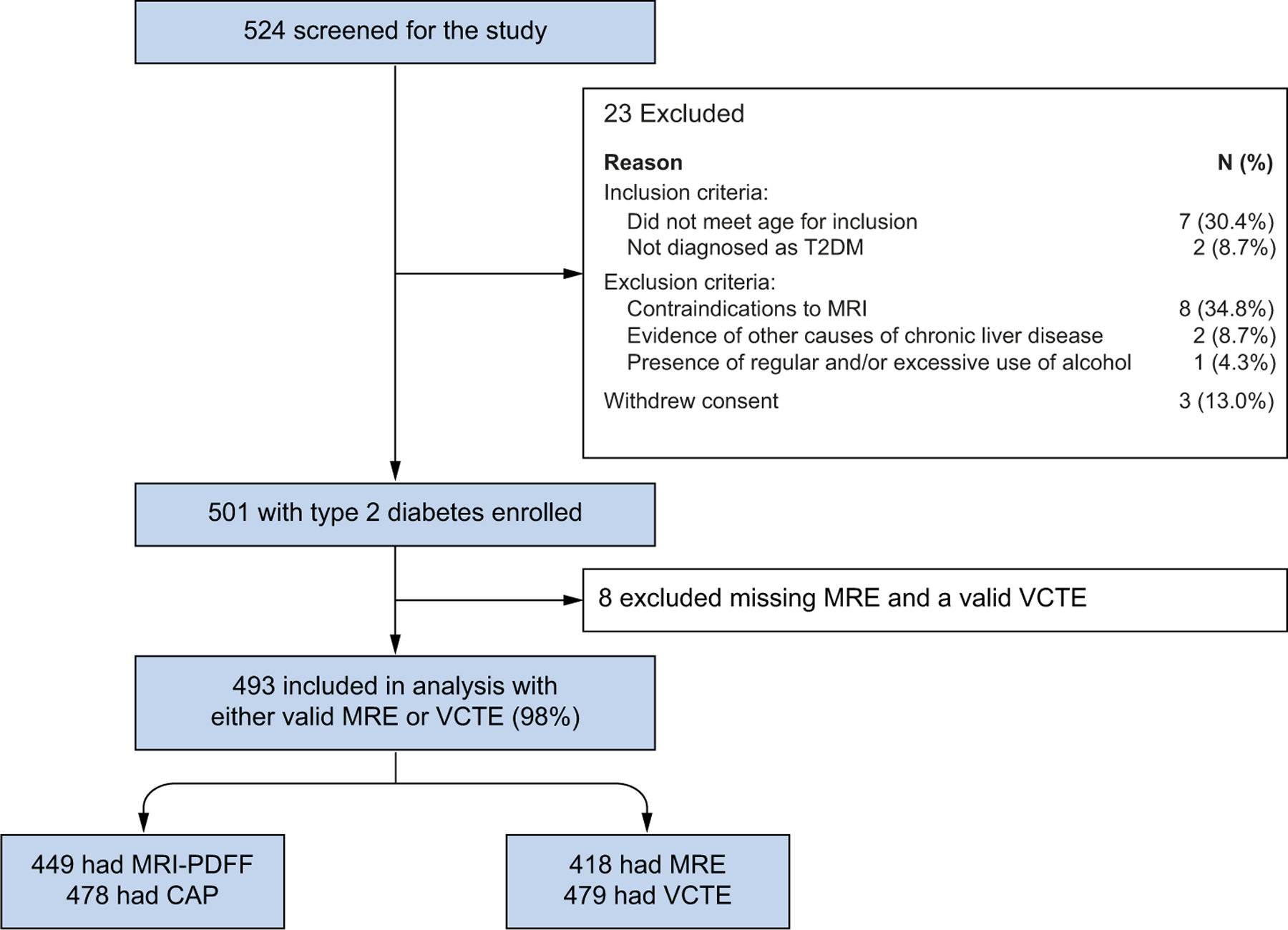
CAP, controlled-attenuation parameter; MRE, magnetic resonance elastography; MRI-PDFF, MRI-proton density fat fraction; T2DM, type 2 diabetes mellitus; VCTE, vibration-controlled transient elastography.
The mean (±SD) liver fat on MRI-PDFF was 9.8% (±7.9) and mean liver stiffness on MRE was 2.6 kPa (±1.1). The mean (±SD) CAP was 308 dB/m (±57) and mean liver stiffness on VCTE was 7.4 kPa (±6.2).
Prevalence of NAFLD in T2DM
The prevalence of NAFLD – defined as MRI-PDFF ≥5% or, if MRI-PDFF was not available, CAP ≥288 dB/m among individuals who consume little or no alcohol without any secondary cause for hepatic steatosis or other causes of liver disease – was 65.3% (n = 322). Patients with NAFLD were more likely to be younger, female, Asian, and to have higher BMI/obesity and the metabolic syndrome. NAFLD was associated with higher HbA1c, HOMA-IR, AST, ALT, triglycerides, LDL cholesterol, platelet count and INR, and lower HDL cholesterol. The mean (SD) CAP and MRI-PDFF were higher in the NAFLD vs. non-NAFLD population at 330 (45) dB/m vs. 265 (53) dB/m, p <0.001 and 13.8% (7) vs. 2.5% (1.4), p <0.001, respectively (Table 1).
Table 1.
Characteristics by NAFLD status.
| Total N = 493 |
No NAFLD N = 171 |
NAFLD N = 322 |
p value | |
|---|---|---|---|---|
| Demographic and clinical | ||||
| Age in years, mean (SD) | 64.4 (8.1) | 67.3 (7.7) | 63.0 (7.9) | <0.0001 |
| Female, n (%) | 311 (63.1) | 97 (56.7) | 214 (66.5) | 0.0330 |
| BMI (kg/m2), mean (SD) | 31.4 (5.9) | 29.8 (5.8) | 32.2 (5.8) | <0.0001 |
| Obesity (BMI ≥30 kg/m2) | 281 (57.4) | 77 (45.3) | 204 (63.8) | <0.0001 |
| Race, n (%) | 0.0047 | |||
| White | 181 (37.6) | 59 (35.5) | 122 (38.7) | |
| Hispanic | 198 (41.2) | 82 (49.4) | 116 (36.8) | |
| Asian | 71 (14.8) | 13 (7.8) | 58 (18.4) | |
| Other | 31 (6.4) | 12 (7.2) | 19 (6.0) | |
| Duration of T2DM (years), median (IQR) | 8.0 (12.0) | 10.0 (15.0) | 6.0 (12.0) | 0.0009 |
| Hypertension, n (%) | 304 (61.7) | 107 (62.6) | 197 (61.2) | 0.7621 |
| Hyperlipidemia, n (%) | 282 (57.2) | 105 (61.4) | 177 (55.0) | 0.1693 |
| Metabolic syndrome, n (%) | 304 (69.6) | 82 (55.4) | 222 (76.8) | <0.0001 |
|
| ||||
| Biochemical profile, median (IQR) | ||||
| HbA1c (%) | 6.8 (1.6) | 6.5 (1.5) | 6.9 (1.6) | 0.0004 |
| HOMA-IR, median (IQR) | 4.8 (5.5) | 3.7 (3.5) | 5.7 (6.1) | <0.0001 |
| AST (U/L) | 24.0 (16.0) | 21.0 (9.0) | 26.0 (17.0) | <0.0001 |
| ALT (U/L) | 25.0 (22.0) | 19.0 (10.0) | 30.5 (24.5) | <0.0001 |
| Alkaline phosphatase (U/L) | 79.0 (33.0) | 78.0 (34.0) | 80.5 (32) | 0.3089 |
| Total bilirubin (mg/dl) | 0.5 (0.2) | 0.4 (0.3) | 0.5 (0.2) | 0.9546 |
| Albumin (g/dl) | 4.4 (0.3) | 4.4 (0.4) | 4.5 (0.3) | 0.0098 |
| Triglycerides (mg/dl) | 144.0 (91.0) | 117.5 (68.0) | 162.0 (94.0) | <0.0001 |
| HDL (mg/dl) | 45.0 (16.0) | 48.0 (19.0) | 44.0 (14.0) | 0.0006 |
| LDL (mg/dl) | 87.0 (46.0) | 79.0 (39.0) | 89.0 (50.0) | 0.0048 |
| Platelet count (109/L) | 243.0 (84.0) | 229.0 (89.0) | 251.0 (90.0) | 0.0001 |
| INR | 1.0 (0.1) | 1 (0.1) | 1 (0.1) | 0.0201 |
|
| ||||
| Imaging, mean (SD) | ||||
| CAP (dB/m) | 307.7 (56.5) | 265.2 (52.8) | 329.7 (44.5) | <0.0001 |
| VCTE (kPa) | 7.4 (6.2) | 7.2 (8.1) | 7.5 (4.9) | 0.7273 |
| MRI-PDFF (%) | 9.8 (7.9) | 2.5 (1.4) | 13.8 (7.0) | <0.0001 |
| MRE (kPa) | 2.6 (1.1) | 2.6 (1.2) | 2.6 (1.1) | 0.9951 |
NAFLD defined as MRI-PDFF ≥5% or, if MRI was not available, as CAP ≥288 db/min t test performed on continuous variables presented as mean (SD), Wilcoxon rank sum test performed on all other continuous variables.
Chi-square or Fisher’s exact test as appropriate on all categorical variables.
Level of significance, p <0.05 n = 479 had VCTE data, n = 449 had MRI-PDFF data and n = 418 had MRE data available.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAP, controlled-attenuation parameter; FIB-4, fibrosis-4 index; HbA1c, hemoglobin A1c; HOMA-IR, homeostatic model assessment of insulin resistance; INR, international normalized ratio; MRE, magnetic resonance elastography; MRI-PDFF, MRI-proton density fat fraction; NAFLD, non-alcoholic fatty liver disease; T2DM, type 2 diabetes mellitus.
Prevalence of advanced fibrosis in T2DM
The prevalence of advanced fibrosis – defined as MRE ≥3.63 kPa or, if MRE was not available, VCTE ≥8.8 kPa – was 14% (Fig. 2). Advanced fibrosis was associated with higher BMI, HOMA-IR, AST, ALT, alkaline phosphatase, INR, FIB-4 score, NAFLD fibrosis score, VCTE and MRE and lower albumin and platelet count (Table 2). In addition, the prevalence of significant fibrosis (stage 2 or higher) – defined as MRE ≥3.0 kPa or, if MRE was not available, as VCTE ≥8.2 kPa – was 22.5%.
Fig. 2. Prevalence of NAFLD, advanced fibrosis, cirrhosis and hepatocellular carcinoma in adults aged ≥50 years with type 2 diabetes.
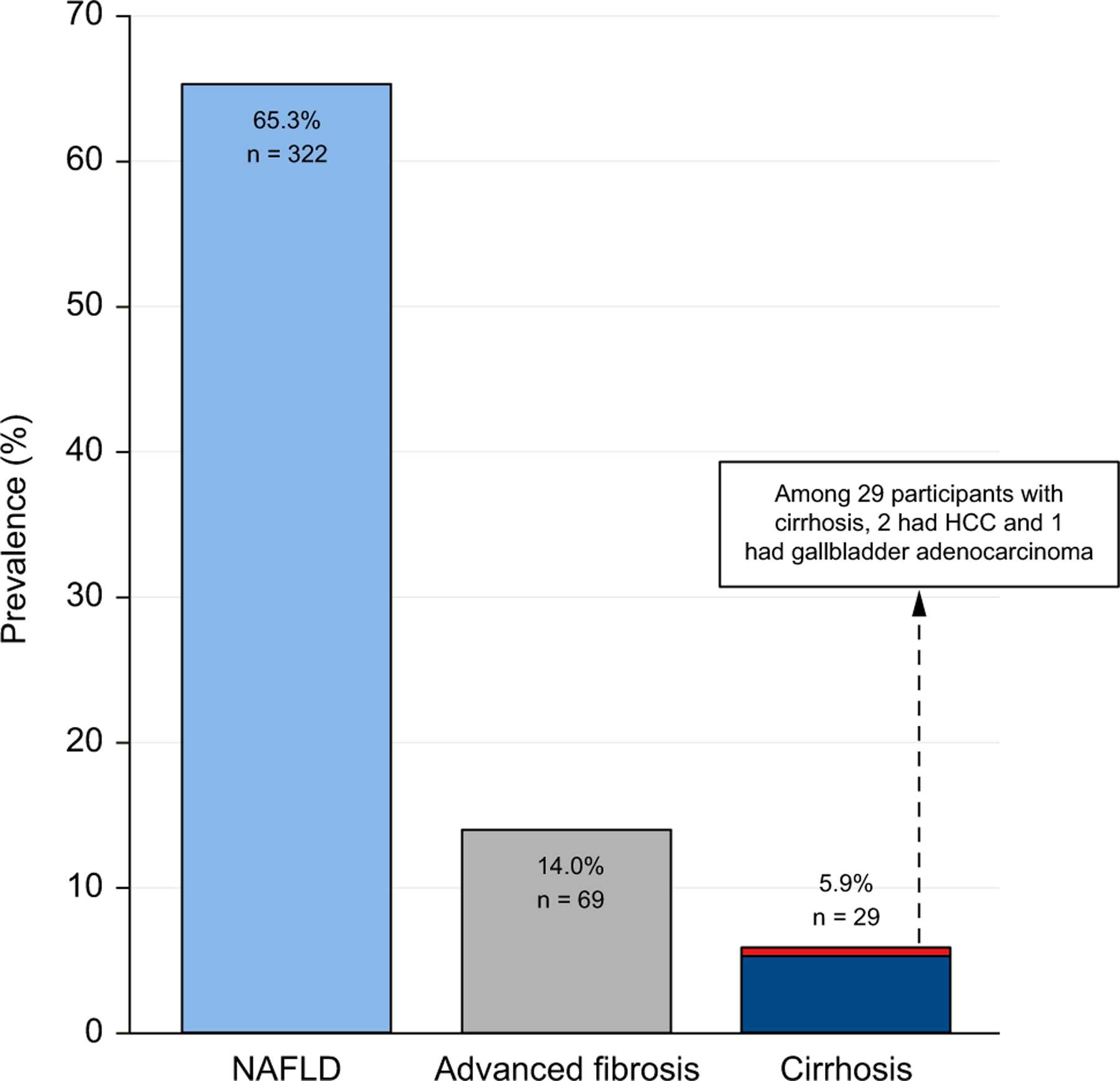
NAFLD, nonalcoholic fatty liver disease.
Table 2.
Clinical, demographic, and imaging characteristics by advanced fibrosis status.
| Total N = 493 |
No advanced fibrosis N = 424 |
Advanced fibrosis N = 69 |
p value | |
|---|---|---|---|---|
| Demographic and clinical | ||||
| Age in years, mean (SD) | 64.4 (8.1) | 64.5 (8.3) | 64.3 (7.2) | 0.8336 |
| Female, n (%) | 311 (63.1) | 263 (63.0) | 48 (69.6) | 0.2289 |
| BMI (kg/m2), mean (SD) | 31.4 (5.9) | 31.0 (5.6) | 33.9 (6.9) | 0.0013 |
| BMI categories, n (%) | ||||
| <25 kg/m2 | 58 (11.9) | 52 (12.3) | 6 (8.8) | 0.0027 |
| 25–30 kg/m2 | 152 (31.0) | 140 (33.2) | 12 (17.6) | |
| >30–35 kg/m2 | 176 (35.9) | 151 (35.8) | 25 (36.8) | |
| >35 kg/m2 | 104 (21.2) | 79 (18.7) | 25 (36.8) | |
| Obesity (BMI ≥30 kg/m2) | 281 (57.4) | 230 (54.5) | 51 (75.0) | 0.0015 |
| Race | 0.7843 | |||
| White, n (%) | 181 (37.6) | 153 (37.0) | 28 (41.8) | |
| Hispanic, n (%) | 198 (41.2) | 174 (42.0) | 24 (35.8) | |
| Asian, n (%) | 71 (14.8) | 60 (14.5) | 11 (16.4) | |
| Other, n (%) | 31 (6.4) | 27 (6.5) | 4 (6.0) | |
| Duration of DM (years), median (IQR) | 8.0 (12.0) | 8.0 (12.0) | 9.5 (11.0) | 0.6235 |
| Hypertension, n (%) | 304 (61.7) | 259 (61.1) | 45 (65.2) | 0.5126 |
| Hyperlipidemia, n (%) | 282 (57.2) | 241 (56.8) | 41 (59.4) | 0.6878 |
| Metabolic syndrome, n (%) | 304 (69.6) | 258 (69.0) | 46 (73.0) | 0.5200 |
|
| ||||
| Biochemical profile, median (IQR) | ||||
| HbA1c (%) | 6.8 (1.6) | 6.8 (1.6) | 6.8 (1.7) | 0.5791 |
| HOMA-IR | 4.8 (5.5) | 4.6 (4.7) | 9.0 (11.3) | <0.0001 |
| AST (U/L) | 24.0 (16.0) | 23.0 (12.0) | 41.5 (30.5) | <0.0001 |
| ALT (U/L) | 25.0 (22.0) | 23.0 (19.0) | 43.0 (35.0) | <0.0001 |
| Alkaline phosphatase (U/L) | 79.0 (33.0) | 78.0 (32.0) | 88.5 (41.5) | 0.0029 |
| Total bilirubin (mg/dl) | 0.5 (0.2) | 0.5 (0.2) | 0.5 (0.3) | 0.3139 |
| Albumin (g/dl) | 4.4 (0.3) | 4.5 (0.3) | 4.4 (0.4) | 0.0009 |
| Triglycerides (mg/dl) | 144.0 (91.0) | 145.0 (93.0) | 138.0 (93.0) | 0.8954 |
| HDL (mg/dl) | 45.0 (16.0) | 46.0 (15.0) | 41.0 (20.0) | 0.0423 |
| LDL (mg/dl) | 87.0 (46.0) | 87.0 (48.0) | 86.0 (47.0) | 0.2232 |
| Platelet count (109/L) | 243.0 (84.0) | 251.0 (85.0) | 222.0 (73.0) | <0.0001 |
| INR | 1.0 (0.1) | 1.0 (0.1) | 1.1 (0.2) | <0.0001 |
|
| ||||
| Clinical prediction rules | ||||
| FIB-4, median (IQR) | 1.3 (0.8) | 1.2 (0.7) | 1.9 (1.5) | <0.0001 |
| FIB-4 categories, n (%) | <0.0001 | |||
| <1.3 | 238 (49.0) | 223 (53.2) | 15 (22.4) | |
| 1.3–2.67 | 216 (44.4) | 182 (43.5) | 34 (50.7) | |
| >2.67 | 32 (6.6) | 14 (3.3) | 18 (26.9) | |
| NAFLD fibrosis score, median (IQR) | −0.3 (1.5) | −0.4 (1.5) | 0.4 (1.6) | <0.0001 |
| Agile 3+, median (IQR) | 0.04 (0.37) | 0.35 (0.30) | 0.90 (0.20) | <0.0001 |
| Agile 4, median (IQR) | 0.02 (0.06) | 0.02 (0.04) | 0.34 (0.46) | <0.0001 |
|
| ||||
| Imaging, mean (SD) | ||||
| CAP (dB/m) | 307.7 (56.5) | 306.7 (55.4) | 314.0 (62.7) | 0.3292 |
| VCTE (kPa) | 7.4 (6.2) | 5.7 (2.1) | 17.5 (11.3) | <0.0001 |
| MRI-PDFF (%) | 9.8 (7.9) | 9.8 (8.0) | 9.6 (7.0) | 0.8977 |
| MRE (kPa) | 2.6 (1.1) | 2.3 (0.5) | 4.9 (1.6) | <0.0001 |
t test performed on continuous variables presented as mean (SD), Wilcoxon rank sum test performed on all other continuous variables.
Chi-square or Fisher’s exact test as appropriate on all categorical variables.
Level of significance, p <0.05 Advanced fibrosis defined as MRE ≥3.63 kPa or if MRE not available, VCTE ≥8.8 kPa n = 479 had VCTE data, n = 449 had MRI-PDFF data and n = 418 had MRE data available.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAP, controlled-attenuation parameter; FIB-4, fibrosis-4 index; HbA1c, hemoglobin A1c; HOMA-IR, homeostatic model assessment of insulin resistance; INR, international normalized ratio; MRE, magnetic resonance elastography; MRI-PDFF, MRI-proton density fat fraction; NAFLD, nonalcoholic fatty liver disease; T2DM, type 2 diabetes mellitus.
In sensitivity analysis evaluating the prevalence of advanced fibrosis using a more conservative VCTE cut-off point of ≥9.7 kPa27 if MRE was not available, the results remained consistent with a prevalence of advanced fibrosis of 13%. Using only MRE for the definition of advanced fibrosis in 418 participants, the prevalence was 12.2%. Using only VCTE for the definition of advanced fibrosis in 479 participants, the prevalence was 19.4%. Finally, in sensitivity analysis limited to 404 participants with MRE and VCTE data, 10.2% of participants had both MRE ≥3.63 kPa and VCTE ≥8.8 kPa.
The strict definition of NAFLD may be a conservative estimate due to burnout of liver fat in NAFLD with advanced fibrosis/cirrhosis. Among 69 patients with advanced fibrosis, 58 had MRI-PDFF data and 36/58 (62.1%) patients had MRI-PDFF >5%. Conversely, 22/58 (37.9%) with liver fat <5% had advanced fibrosis.
In 418 participants with MRE and VCTE data, the diagnostic accuracy (AUROC) of VCTE for advanced fibrosis (cut-off point of ≥8.8 kPa) was 0.87, with a sensitivity of 83.7%, specificity of 90.4%, positive predictive value of 54.7% and a negative predictive value of 97.6%. The diagnostic accuracy of Agile 3+ for advanced fibrosis was similar to VCTE alone, AUROC 0.85 vs. 0.87, p = 0.60. Agile 4 had inferior performance to VCTE, AUROC 0.67 vs. 0.86, p <0.001. The FIB-4 cut-off point of 1.3 to exclude advanced fibrosis had a sensitivity of 81.6%. A lower FIB-4 cut-off point of 1.0 increased sensitivity to 95.9%.
Obesity enhances the risk of NAFLD and advanced fibrosis
When evaluating the population stratified by obesity, the prevalence of NAFLD increases from 55.5% in non-obese to 72.6% in obese participants, p = 0.002 (Fig. 3). Similarly, the prevalence of advanced fibrosis increases from 8.1% in non-obese to 18.2% in obese participants, p = 0.002. The prevalence of cirrhosis was 3.4% in non-obese and 7.5% in obese participants, p = 0.052.
Fig. 3. Prevalence of NAFLD, advanced fibrosis, cirrhosis in adults aged ≥50 years with type 2 diabetes stratified by obesity status.

Level of significance, p <0.05 (Chi-square test). NAFLD, non-alcoholic fatty liver disease.
In univariate logistic regression analysis, both obesity (odds ratio [OR] 2.50; 95% CI 1.40–4.48; p = 0.002) and insulin use (OR 2.57; 95% CI 1.27–5.19; p = 0.009) were associated with the presence of advanced fibrosis (Table 3). Sex, age, ethnicity, duration of diabetes and the presence of metabolic syndrome were not significantly associated with advanced fibrosis. Obesity and insulin use remained associated after multivariable adjustment for age and sex (OR 2.49; 95% CI 1.38–4.54; p = 0.003 and OR 2.71; 95% CI 1.33–5.50; p = 0.006, respectively).
Table 3.
Factors associated with advanced fibrosis on logistic regression (n = 493).
| Advanced fibrosis odds ratio (95% CI) | p value | |
|---|---|---|
| Demographic & biochemical | ||
| Female | 1.399 (0.808–2.423) | 0.2306 |
| Age (per 5 years) | 0.983 (0.839–1.151) | 0.8332 |
| Obesity (yes/no) | 2.504 (1.400–4.479) | 0.0020 |
| Hispanic ethnicity (yes/no) | 0.770 (0.450–1.316) | 0.3390 |
| Insulin (yes/no) | 2.569 (1.272–5.190) | 0.0085 |
| Duration of diabetes (per 5 years) | 1.025 (0.866–1.213) | 0.7739 |
| Metabolic syndrome (ATP III definition) | 1.217 (0.669–2.212) | 0.5205 |
|
| ||
| Multivariable adjustments | ||
| Obesity (age- and sex-adjusted) | 2.499 (1.377–4.535) | 0.0026 |
| Insulin use (age- and sex-adjusted) | 2.709 (1.334–5.501) | 0.0058 |
Level of significance, p <0.05 (Logistic Regression).
ATP III, Adult Treatment Panel III.
Prevalence of cirrhosis in T2DM
The prevalence of cirrhosis – defined as MRE ≥4.67 kPa or, if MRE was not available, as VCTE ≥15 kPa – was 5.9% (n = 29). The presence of cirrhosis was not associated with age, sex, ethnicity, duration of diabetes, insulin use and presence of metabolic syndrome. The average liver stiffness on MRE among those with cirrhosis compared to those without was 6.25 kPa vs. 2.46 kPa, p <0.001. The average FIB-4 among those with cirrhosis compared to those without was 2.96 vs. 1.42, p <0.001. In sensitivity analysis among 404 participants with both MRE and VCTE, 3.5% had both MRE ≥4.67 kPa and VCTE ≥15 kPa.
Prevalence of hepatobiliary malignancy in T2DM
Among the cirrhotic population, three patients were identified to have hepatobiliary malignancy within 6 months of the baseline visit. This included two patients with hepatocellular carcinoma and one with gallbladder adenocarcinoma. Both cases of hepatocellular carcinoma were stage T1 on initial diagnosis. VCTE data was available for all three patients and ranged from 27.4–75 kPa. MRE data was available for two out of three patients and ranged from 6.86–7.61 kPa. Both of these were consistent with a diagnosis of cirrhosis on elastography. However, FIB-4 >2.67 was only present in two patients and a third had a FIB-4 of 1.41.
Histologic characteristics of the cohort
Patients with elevated ALT, liver fat (MRI-PDFF ≥10%) or increased liver stiffness were offered a research liver biopsy, which was assessed by central histology read. Of 303 eligible patients, 134 patients underwent a liver biopsy (Fig. S1). The differences between those with and without liver biopsy are outlined in Table S2. The mean (±SD) NAFLD activity score was 3.8 (1.5). Steatosis, lobular inflammation, and hepatocyte ballooning were present in 90%, 95% and 66% of patients, respectively (Table S3). Among those who had a liver biopsy assessment, NASH was present in 61% (n = 80) and 30% (n = 39) had advanced fibrosis (Fig. S2). The diagnostic accuracies (AUROCs) of FIB-4, VCTE and MRE for the histologic diagnosis of advanced fibrosis were 0.72, 0.84 and 0.91, respectively. MRE was superior to VCTE for the diagnosis of histologic advanced fibrosis, p = 0.011 (Table S4).
Discussion
In this prospective, systematic assessment of older adults with T2DM, the prevalence of NAFLD, advanced fibrosis and cirrhosis was high and portends a significant risk of liver-related morbidity and mortality. Three patients identified with cirrhosis were diagnosed with hepatobiliary malignancy on liver cancer screening within 6 months of the baseline assessment, highlighting the potential impact of identifying this high-risk population with systematic screening. The presence of obesity amplified the risk of advanced fibrosis to 18% and this association persisted in multivariable adjusted models. Furthermore, insulin use was also associated with an increased risk of advanced fibrosis. In a subset of individuals with elevated liver tests, liver stiffness or high liver fat, who underwent liver biopsy, 61.1% had NASH, the progressive form of NAFLD. Overall, the high burden of undiagnosed liver disease assessed by the most accurate MRI-based biomarkers of NAFLD supports systematic screening of older patients with diabetes.
Multiple studies have demonstrated that the prevalence of NAFLD among patients with T2DM is 2–3-fold higher than that in the general population or between 60–70%,28 which was consistent with this study demonstrating a prevalence of 65% using the most accurate, MRI-based assessment of liver fat. Furthermore, the high rate of NASH among those who underwent liver biopsy (65.3%) is similar to published data on a middle-aged US cohort.29 Recent studies utilizing VCTE as a biomarker of liver fibrosis demonstrate a rate of advanced fibrosis of 9–15%.30,31 Given the lower specificity of VCTE compared to MRE and the potential for lower diagnostic accuracy among the morbidly obese,32 this study utilizing MRE confirms the high rate (14%) of advanced fibrosis in older adults with T2DM.
Furthermore, the presence of T2DM is associated with more severe NAFLD, progressive disease and liver-related morbidity4,5,33 and this study presents new data on the elevated risk of cirrhosis (6%) in this population using accurate MRE-based cut-off points. Identifying diabetes with undiagnosed cirrhosis has immediate implications for screening for hepatocellular carcinoma and gastroesophageal varices needing treatment, as well as for the implementation of cirrhosis healthcare maintenance. Importantly, T2DM also increases the risk of hepatocellular carcinoma34,35 and recommended screening implemented after diagnosing cirrhosis led to the early diagnosis of two patients with stage T1 hepatocellular carcinoma, offering the potential for treatment with curative intent.
This study also sheds light on the optimal use of non-invasive tests in this high-risk population. The FIB-4 threshold of 1.3 to rule out advanced fibrosis had 81% sensitivity, which improved to 96% when using a lower cut-off point of 1.0. In order to minimize false negative testing, this lower FIB-4 threshold may be considered in future screening strategies. Furthermore, Agile-3+ and Agile 4 did not have higher diagnostic accuracy compared to VCTE alone. Finally, the positive predictive value of VCTE remained limited (55%), suggesting that high-risk patients may still require confirmatory testing with MRE or liver biopsy.
Obesity and insulin use were key factors that amplified the risk of advanced fibrosis. The additional risk for advanced liver disease in the setting of obesity with T2DM in older adults further supports screening this population. Given GLP-1 agonists have demonstrated efficacy as a treatment for T2DM and obesity and emerging data supports their use in NASH,36 the systematic screening of this population could identify candidates for treatments that reduce metabolic and hepatic risk.
This prospective, systematic assessment of older adults with T2DM using the most accurate non-invasive biomarkers adds new information about the risk of advanced liver disease secondary to NAFLD. The study was performed at a single center, but the results are consistent with findings from other studies, supporting its generalizability. The current study is cross-sectional, limiting the ability to report on the risk of long-term liver-related morbidity and mortality in this cohort. However, the presence of advanced fibrosis has repeatedly demonstrated an association with future liver-related outcomes,37 highlighting the importance of its identification. This study included a diverse cohort with 41% of participants reporting Hispanic ethnicity; however, it may be insufficiently powered to detect racial or ethnic differences in the prevalence of advanced fibrosis. Finally, only a subset of patients underwent liver biopsy; however, it would be unethical to utilize an invasive test with a risk of significant complications in a screening study and this study leveraged the most accurate MRI-based non-invasive biomarkers of liver fat and fibrosis.
The optimal combination of non-invasive tests and their cost-effectiveness require further evaluation. Furthermore, additional longitudinal studies will be required to define the optimal frequency of screening assessments to capture potential disease progression. These data support the implementation of systematic screening for older adults with T2DM and provide much needed prospective data on the risk of advanced liver disease.
Supplementary Material
Financial support
VA is supported by NIDDK (K23DK119460). RL receives funding support from NCATS (5UL1TR001442), NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835), and NIAAA (U01AA029019). RL is also supported by an Investigator initiated study sponsored by Gilead Sciences.
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CAP
controlled-attenuation parameter
- INR
international normalized ratio
- MR
magnetic resonance
- MRE
magnetic resonance elastography
- MRI-PDFF
MRI-proton density fat fraction
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- T2DM
type 2 diabetes mellitus
- VCTE
vibration-controlled transient elastography
Footnotes
Conflict of interests
RL serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals and Viking Therapeutics. In addition his institutions received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Co-founder of LipoNexus Inc.
Please refer to the accompanying ICMJE disclosure forms for further.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2022.11.010.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request in deidentified form.
References
- [1].Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md) 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- [2].Loomba R, Wong R, Fraysse J, Shreay S, Li S, Harrison S, et al. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real world analysis of medicare data. Aliment Pharmacol Ther 2020;51:1149–1159. [DOI] [PubMed] [Google Scholar]
- [3].Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cel Metab 2022. Jul 5;34(7):969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 2015;62:1148–1155. [DOI] [PubMed] [Google Scholar]
- [5].Kleiner DE, Brunt EM, Wilson LA, Behling C, Guy C, Contos M, et al. Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw Open 2019;2:e1912565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md) 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- [7].Hsu C, Caussy C, Imajo K, Chen J, Singh S, Kaulback K, et al. Magnetic resonance vs. transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol : official Clin Pract J Am Gastroenterological Assoc 2019;17:630–637.e638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, et al. Magnetic resonance elastography vs. transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsyproven nonalcoholic fatty liver disease. Gastroenterology 2017;152:598–607.e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gidener T, Ahmed OT, Larson JJ, Mara KC, Therneau TM, Venkatesh SK, et al. Liver stiffness by magnetic resonance elastography predicts future cirrhosis, decompensation and death in NAFLD. Clin Gastroenterol Hepatol official Clin Pract J Am Gastroenterological Assoc 2020. Sep;19(9):1915–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Han MAT, Vipani A, Noureddin N, Ramirez K, Gornbein J, Saouaf R, et al. MR elastography-based liver fibrosis correlates with liver events in nonalcoholic fatty liver patients: a multicenter study. Liver Int 2020;40:2242–2251. [DOI] [PubMed] [Google Scholar]
- [11].Tamaki N, Kurosaki M, Takahashi Y, Itakura Y, Inada K, Kirino S, et al. Liver fibrosis and fatty liver are independently associated with cardiovascular disease risk. J Gastroenterol Hepatol 2021. Oct;36(10):2960–2966. [DOI] [PubMed] [Google Scholar]
- [12].Ajmera V, Nguyen K, Tamaki N, Sharpton S, Bettencourt R, Loomba R. Prognostic utility of magnetic resonance elastography and MEFIB index in predicting liver-related outcomes and mortality in individuals at risk of and with nonalcoholic fatty liver disease. Ther Adv Gastroenterol 2022;15:17562848221093869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Doycheva I, Cui J, Nguyen P, Costa EA, Hooker J, Hofflich H, et al. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther 2016;43:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 2012;56:922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015;61:1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Loomba R, Schork N, Chen CH, Bettencourt R, Bhatt A, Ang B, et al. Heritability of hepatic fibrosis and steatosis based on a prospective twin study. Gastroenterology 2015;149:1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Loomba R, Cui J, Wolfson T, Haufe W, Hooker J, Szeverenyi N, et al. Novel 3D magnetic resonance elastography for the noninvasive diagnosis of advanced fibrosis in NAFLD: a prospective study. Am J Gastroenterol 2016;111:986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology (Baltimore, Md) 2006;43:1317–1325. [DOI] [PubMed] [Google Scholar]
- [19].Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology (Baltimore, Md) 2007;45:846–854. [DOI] [PubMed] [Google Scholar]
- [20].Caussy C, Alquiraish MH, Nguyen P, Hernandez C, Cepin S, Fortney LE, et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology (Baltimore, Md) 2018;67:1348–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology (Baltimore, Md) 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- [22].Loomba R, Wolfson T, Ang B, Hooker J, Behling C, Peterson M, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology (Baltimore, Md) 2014;60:1920–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Siddiqui MS, Vuppalanchi R, Van Natta ML, Hallinan E, Kowdley KV, Abdelmalek M, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2019;17:156–163.e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md) 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- [25].Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood Institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- [26].Wong RJ, Liu B, Bhuket T. Significant burden of nonalcoholic fatty liver disease with advanced fibrosis in the US: a cross-sectional analysis of 2011–2014 National Health and Nutrition Examination Survey. Aliment Pharmacol Ther 2017;46:974–980. [DOI] [PubMed] [Google Scholar]
- [27].Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1717–1730. [DOI] [PubMed] [Google Scholar]
- [28].Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol 2019;71:793–801. [DOI] [PubMed] [Google Scholar]
- [29].Harrison SA, Gawrieh S, Roberts K, Lisanti CJ, Schwope RB, Cebe KM, et al. Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J Hepatol 2021;75:284–291. [DOI] [PubMed] [Google Scholar]
- [30].Lomonaco R, Godinez Leiva E, Bril F, Shrestha S, Mansour L, Budd J, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care 2021;44:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ciardullo S, Monti T, Perseghin G. High prevalence of advanced liver fibrosis assessed by transient elastography among U.S. Adults with type 2 diabetes. Diabetes Care 2021;44:519–525. [DOI] [PubMed] [Google Scholar]
- [32].Chen J, Yin M, Talwalkar JA, Oudry J, Glaser KJ, Smyrk TC, et al. Diagnostic performance of MR elastography and vibration-controlled transient elastography in the detection of hepatic fibrosis in patients with severe to morbid obesity. Radiology 2017;283:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Simon TG, Roelstraete B, Sharma R, Khalili H, Hagström H, Ludvigsson JF. Cancer risk in patients with biopsy-confirmed nonalcoholic fatty liver disease: a population-based cohort study. Hepatology (Baltimore, Md) 2021;74:2410–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, et al. Risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Gastroenterology 2018;155:1828–1837.e1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kanwal F, Kramer JR, Li L, Dai J, Natarajan Y, Yu X, et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology (Baltimore, Md) 2020;71:808–819. [DOI] [PubMed] [Google Scholar]
- [36].Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med 2021;384:1113–1124. [DOI] [PubMed] [Google Scholar]
- [37].Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med 2021;385:1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request in deidentified form.


